Abstract
Rheumatoid arthritis (RA) is an autoimmune disease characterized by proliferative synovitis with deterioration of cartilage and bone. Osteoclasts (OCs) are the active participants in the bone destruction of RA. Although with great advances, most current therapeutic strategies for RA have limited effects on bone destruction. Macrophage scavenger receptor A (SR-A) is a class of pattern recognition receptors (PRRs) involved in bone metabolism and OC differentiation. More recently, our study revealed the critical role of SR-A in RA diagnosis and pathogenesis. Here, we further demonstrated that serum SR-A levels were positively correlated with bone destruction in patients with RA. Anti-SR-A neutralizing antibodies significantly inhibited OC differentiation and bone absorption in vitro in patients with RA, but not in healthy individuals, dampening the expression of OC-specific genes such as tartrate-resistant acid phosphatase (TRAP), cathepsin K (CTSK), and matrix metalloproteinase-9 (MMP-9). Similar results were also seen in collagen-induced arthritis (CIA) mice in vitro. Moreover, the anti-SR-A neutralizing antibody could further ameliorate osteoclastogenesis in vivo and ex vivo in CIA mice, accompanied by decreased serum levels of C-terminal telopeptide and IL-6, exhibiting potential protective effects. These results suggest that blockade of SR-A using anti-SR-A neutralizing antibodies might provide a promising therapeutic strategy for bone destruction in the RA.
Keywords: scavenger receptor A, osteoclastogenesis, rheumatoid arthritis
The levels of serum SR-A were elevated in rheumatoid arthritis (RA) patients, potentially promoting osteoclastogenesis and bone destruction. Anti-SR-A neutralizing antibodies could significantly inhibit osteoclast differentiation and bone absorption both in RA patients and arthritis mice. Blockade of SR-A using anti-SR-A neutralizing antibodies might provide a promising therapeutic strategy for bone destruction in RA.
Graphical Abstract
Graphical Abstract.
Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory autoimmune disease with the typical characteristic of periarticular bone erosion [1]. The inflammation can lead to synovium proliferation, bone destruction, deformity, and function decline [2]. In 1971, Sharp et al. proposed a system including the observation and scoring of hands and wrists in patients with RA to assess structural change [3]. Van Der Heijde further modified the Sharp score for a better description of bone destruction, including bone erosion score and joint space narrowing (JSN) score [4]. Bone erosion is a critical outcome indicator in RA, predicting a more severe course of disease with a higher degree of disability [5].
Osteoclasts (OCs) are the main active participants in RA bone destruction [6]. They are multinucleated cells derived from hematopoietic precursors of the myeloid lineage with the potential of bone resorption. In RA, the generation, differentiation, and activation of OCs are increased as induced by proinflammatory cytokines such as TNF-α and IL-6 [7], and autoantibodies such as anti-citrullinated protein antibodies (ACPA) and rheumatoid factor (RF) [8], resulting in bone resorption and destruction. Therefore, inhibiting the formation and functions of OCs are ideal and effective approaches for RA targeted therapy. Although several inhibitors have been developed, including bisphosphonates [9], denosumab [10], and natural compounds such as xylitol [11], till now there are few therapeutic drugs targeting OCs available. Current drugs for RA treatment mainly consist of nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GC), and disease-modifying antirheumatic drugs (DMARDs) including conventional DMARDs (cDMARDs), biological DMARDs (bDMARDs), and targeted synthetic DMARDs (tsDMARDs). Although with great advances, their effects on inhibiting OCs and preventing joint and bone destruction are insufficient with limitations.
Macrophage scavenger receptor A (SR-A, CD204, MSR-1, SCARA1) is a kind of classical pattern recognition receptors (PRRs) primarily expressed on macrophages [12]. Recent studies have further indicated its critical involvement in T- and B-cell immune responses [13, 14]. Besides cancer, cardiovascular disease, and Alzheimer’s disease, the role of SR-A in autoimmune diseases, such as systemic lupus erythematosus, autoimmune hepatitis, and inflammatory bowel diseases have also been recognized [15]. Moreover, compared to WT mice, SR-A–/– mice revealed increased bone mass, bone thickness, bone density, and trabeculae number, which suggested the role of SR-A in bone metabolism and OC differentiation [16]. SR-A was then demonstrated to be able to directly promote OC differentiation [17]. SR-A may also activate the ERK and JNK signaling pathways and increase IL-6 production to further stimulate the formation of OCs [18]. In our previous large-scale, multicenter study, we unveiled a significant elevation of soluble SR-A (sSR-A) in the patient serum with RA, serving as a potential diagnostic marker [19]. Moreover, our group revealed that SR-A–/– mice were resistant to collagen-induced arthritis (CIA). Administration of SR-A recombinant protein exacerbated the incidence and progression of CIA, while SR-A neutralizing antibody ameliorated the severity of arthritis [19]. However, the therapeutic potential of SR-A blockade against bone destruction remains to be elucidated.
In this study, we aimed to characterize the correlation between sSR-A and bone destruction in RA and reveal the capacity of SR-A neutralizing antibody in inhibiting OC differentiation, trying to provide a potential approach for RA bone destruction therapy.
Materials and methods
Patients and samples
Serum samples were obtained from 80 patients with RA as well as 60 age- and sex-matched healthy volunteers, and peripheral blood (PB) samples were obtained from six patients with RA. All patients fulfilled the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification diagnostic criteria for RA. The detailed characteristics of patients with RA are provided in Table 1. The study was approved by Research Ethics Committee at Peking University People’s Hospital, Beijing, China. All patients and healthy donors provided informed consent.
Table 1.
Clinical and demographic characteristics of patients with RA
| Characteristics | Patients with RA and with radiographic images (n = 80) |
|---|---|
| Age, mean (range), yrs | 59 (26–84) |
| Gender, female/male | 66/14 |
| Duration, median (range), yrs | 10 (0.05–40) |
| ESR, median (range), mm/h | 48 (6–129) |
| CRP, median (range), mg/l | 16.2 (1–243) |
| ACPA, no. positive/no. negative/no. nd | 67/12/1 |
| RF, median (range), IU/ml | 139.5 (20–7330) |
| Tender joint count of 28 joints, median (range) | 6 (0–26) |
| Swollen joint count of 28 joints, median (range) | 4 (0–26) |
| DAS28, mean (range) | 5.24 (2.39–8.21) |
| SHARP score, median (range) | 82 (2–280) |
| Bone erosion score, median (range) | 44 (0–160) |
| Joint space narrowing score, median (range) | 44 (1–120) |
| Medication, no. (%) | |
| Steroids | 19 (23.75) |
| NSAIDs | 31 (38.75) |
| Methotrexate | 24 (30) |
| Other DMARDs | 54 (67.5) |
| Biologics | 4 (5) |
| No treatment | 8 (10) |
Abbreviations: ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; nd: not detected; DAS28: disease activity score 28.
Radiological damage of patients with RA was evaluated using Sharp-van der Heijde (SHS) total score as reported previously [4].
Mice
Six to eight-week-old male DBA/1 mice were purchased from Huafukang Bioscience Company (Beijing, China). All mice were housed in a specific pathogen-free environment under controlled conditions (22 °C ambient temperature, 40% humidity). All animal procedures complied with relevant ethical regulations for animal research and were approved by the Institutional Animal Care and Use Committee (IACUC) of Peking University People’s Hospital.
ELISA analyses
The serum levels of sSR-A in patients with RA and healthy individuals were detected by ELISA as reported previously [19], using a commercially available human SR-A ELISA kit (Sino Biological Inc., Beijing, China). The levels of IL-6 in CIA mouse serum and the cell culture supernatant of patients with RA were detected by ELISA kit from Neobioscience (Beijing, China), while the levels of C-terminal telopeptide in CIA mouse serum were detected by ELISA kit from Chondrex (Redmond, WA). The results were obtained on a Synergy™ 4 Multi-Mode Microplate Reader with software GEN5CH 2.0 (BioTek, Winooski, VT).
CIA induction and treatment
Collagen-induced arthritis (CIA) models were established in DBA/1 mice by immunization on Day 1 with 200 μg bovine type II collagen (Chondrex Inc., Redmond, WA) emulsified in complete Freund adjuvant (CFA), and on Day 21 with 100 μg bovine type II collagen emulsified in incomplete Freund adjuvant (IFA) as a booster. The severity of arthritis was scored based on the level of inflammation as described previously [19].
After the arthritis initiation, the mice with similar scores of 2–3 were randomly divided into two groups. As reported in previous studies concerning blocking antibodies, the experimental group mice were intravenously administrated with anti-mouse SR-A neutralizing antibodies (AF1797, R&D Systems, Minneapolis, MN; 2 μg/mice in 200 μl PBS), while the control group mice were intravenously administrated with isotype control IgG every other day for a total of five times. The mice were sacrificed on Day 12 after the first treatment for further analysis.
OC differentiation
For human OC differentiation in vitro, freshly isolated PB mononuclear cells (PBMCs) from patients with RA or healthy individuals were suspended in complete α-MEM containing 10% FBS and planted in a 96-well plate at a density of 1 × 105 cells/well. After culturing for 4 h, the non-adherent cells were removed, remaining the adherent OC precursors. Then the cells were co-cultured with 2 μg/ml SR-A neutralizing antibody or isotype antibody along with RANKL (100 ng/ml) and M-CSF (50 ng/ml). Sometimes, the cells were co-cultured with RA serum pre-incubated with SR-A neutralizing antibody or isotype antibody in the presence of RANKL and M-CSF. The medium was changed every 3 days, and on Day 14, OC formation was measured through TRAP staining with leukocyte acid phosphatase kit (Sigma-Aldrich, St. Louis, MO). TRAP-positive multinucleated cells (MNCs) containing three or more nuclei in the entire single well of 96-well plate were observed and counted under an inverted fluorescence microscope (Olympus IX71-141, Tokyo, Japan).
For mouse OC differentiation in vitro, primary bone marrow cells (BMMs) from CIA or naïve mice were cultured in a 96-well plate at a density of 1 × 105 cells/well for 3 days in complete α-MEM with 10% FBS and 25 ng/ml M-CSF. Then non-adherent cells were discarded and the adherent cells were further cultured in the presence of 50 ng/ml RANKL, 25 ng/ml M-CSF, 2 μg/ml SR-A neutralizing antibody or isotype antibody. Six days later, tartrate-resistant acid phosphatase (TRAP) staining was performed for OC detection.
For mouse OC differentiation ex vivo, BMMs from CIA mice or naïve mice treated with SR-A neutralizing antibody or isotype antibody as described above were collected for OC formation accordingly.
Bone resorption assay
peripheral blood mononuclear cells (1 × 105 cells/well) from patients with RA were performed for OC differentiation as described above in 96-well plates coated with bovine cortical bone slices (6 mm in diameter and approx. 200 μd thick, IDS, Boldon, UK). On Day 14, the bone slices containing the OCs were collected and washed with distilled water. Then, the resorption pits were visualized by staining with 1% toluidine blue and detected under a polarized light microscope (Olympus BX51-P, Tokyo, Japan). The results were expressed as the area of resorption lacunae in the total plate area.
Mouse paw histopathology and micro-CT analyses
Mice were sacrificed and the hind paws were fixed in 4% buffered formaldehyde. The tissues were collected, fixed, paraffin-embedded, sectioned, stained with TRAP, and analyzed with NDP.view2 (Hamamatsu Photonics K.K., Japan).
Micro-CT images of the mouse paws were acquired on the Tri-Modality FLEX Triumph™ Pre-Clinical Imaging System (Gamma Medica-Ideas, Northridge, CA). CT image set acquisitions lasted 10 min and utilized beam parameters of 130 μA and 80 kVP. Analyze 10.0 (AnalyzeDirect, Overland Park, KS) was used to perform the image analysis.
RT-PCR and qPCR
RNA extraction, reverse transcription, and qPCR analyses were performed as described previously [20, 21]. Briefly, after the formation of OCs as described above, total RNA was extracted by RNAsimple Total RNA kit (Tiangen, Beijing, China), and reverse transcription was performed with the reverse transcriptase kit (Thermofisher Scientific, Waltham, MA) according to the manufacturer’s instructions. Then, qPCR was performed to analyze the OC-specific gene expression using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). The primers used were as follows:
Human GAPDH sense primer: 5ʹ-AAGGTGAAGGTCGGAGTCAA-3ʹ, antisense primer: 5ʹ-AATGAAGGGGTCATTGATGG-3ʹ;
Human TRAP sense primer: 5ʹ-GATCCTGGGTGCAGACTTCA-3ʹ, antisense primer: 5ʹ-GCGCTTGGAGATCTTAGAGT-3ʹ;
Human CTSK sense primer: 5ʹ-ACCGGGGTATTGACTCTGAA-3ʹ, antisense primer: 5ʹ- GAGGTCAGGCTTGCATCAAT-3ʹ;
Human MMP-9 sense primer: 5ʹ-CGCTACCACCTCGAACTTTG-3ʹ, antisense primer: 5ʹ-GCCATTCACGTCGTCCTTAT-3ʹ.
Mice GAPDH sense primer: 5ʹ-GGTGAAGGTCGGTGTGAACG-3ʹ, antisense primer: 5ʹ-CTCGCTCCTGGAAGATGGTG-3ʹ;
Mice TRAP sense primer: 5ʹ-CGACCATTGTTAGCCACATACG-3ʹ, antisense primer: 5ʹ-TCGTCCTGAAGATACTGCAGGTT-3ʹ;
Mice CTSK sense primer: 5ʹ-GCTTGGCATCTTTCCAGTTTTAC-3ʹ, antisense primer: 5ʹ-TATCCAGTGCTTGCTTCCCTTCT-3ʹ;
Mice MMP-9 sense primer: 5ʹ-CGTGTCTGGAGATTCGACTTGA-3ʹ, antisense primer: 5ʹ-TTGGAAACTCACACGCCAGA-3ʹ.
Statistical analysis
GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA) was used for statistical analysis. Differences between various groups were evaluated by the Spearman’s rank correlation test, Mann–Whitney U test, Wilcoxon matched-paired signed-rank test, paired Student’s t-test, and one-way ANOVA test. All data are expressed as mean ± SEM. A confidence level above 95% (P < 0.05) was considered statistically significant (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ns, not significant).
Results
Elevated serum sSR-A correlates with bone destruction in patients with RA
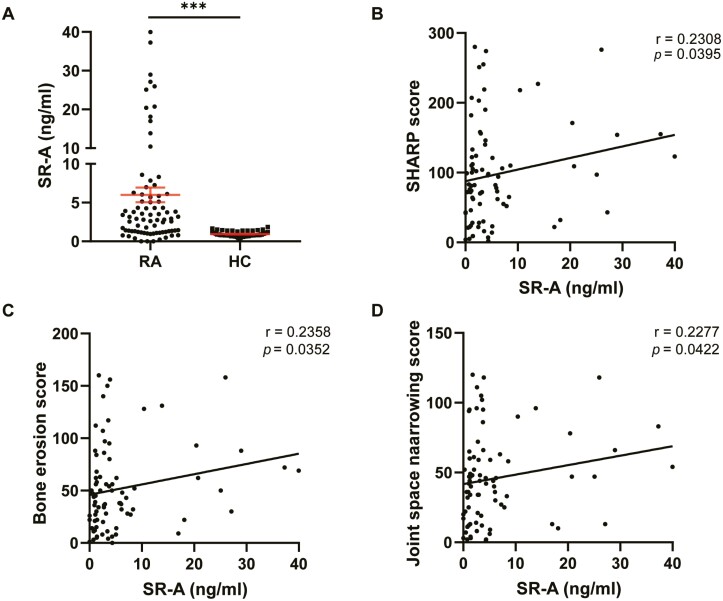
A previous study by our group revealed the diagnostic value of sSR-A and its pathogenic activity in RA. In the present study, we further analyzed the correlation between sSR-A and bone destruction in patients with RA. Serum samples from patients with RA with radiographic images (n = 80) and healthy controls (HC, n = 60), were collected for detection of the levels of sSR-A. The result showed that sSR-A in patients with RA was significantly higher than that in HC (median 2.906 ng/ml, mean 6.006 ng/ml, SD 8.441 ng/ml, ∗∗∗P < 0.001, Fig. 1A). Sharp/van der Heijde score (SHS) was then performed and simple linear regression showed that the level of sSR-A positively correlated with the SHARP score (Fig. 1B). Further analysis showed that the serum sSR-A level in patients with RA correlated positively with both erosion score and JSN score (Fig. 1C, D).
Figure 1.
Correlation analysis of the level of sSR-A with bone destruction in patients with RA. (A) The serum level of sSR-A was higher in patients with RA than that in healthy individuals (RA, n = 80; HC, n = 60; ∗∗∗P < 0.001). (B–D) The level of sSR-A in patients with RA was positively correlated with the SHARP score (B; r = 0.2308, ∗P = 0.0395), the erosion score (C; r = 0.2358, ∗P = 0.0352), and the joint space narrowing score (D; r = 0.2277, ∗P = 0.0422). The results are presented as mean ± SEM (two-tailed Mann–Whitney U test (A) and two-tailed Spearman’s rank correlation test (B–D)).
SR-A neutralizing antibody inhibits OC differentiation and bone destruction in patients with RA
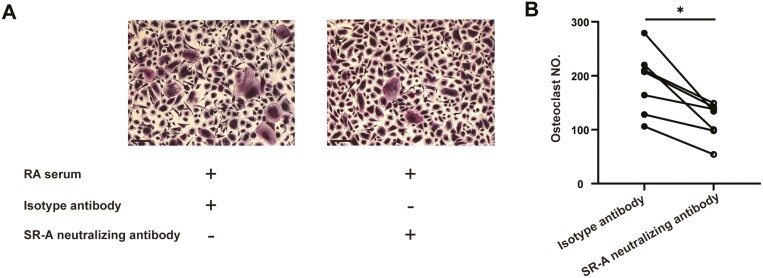
We first tried to reveal the potential involvement of sSR-A in RA bone destruction. As shown in Fig. 2, treatment of healthy individual monocytes with serum of the patient with RA fundamentally stimulated OC formation in vitro. However, the stimulatory effects of serum of the patients with RA were aborted when pre-incubated with SR-A neutralizing antibody, which indicated the role of sSR-A in osteoclastogenesis.
Figure 2.
The potential involvement of sSR-A in RA bone destruction. Monocytes from healthy individuals (n = 6) were co-cultured with the serum of the patient with RA pre-incubated with 2 μg/ml anti-human SR-A neutralizing antibody or isotype antibody, 100 ng/ml RANKL, and 50 ng/ml M-CSF. After 14 days, the cells were fixed and set for TRAP staining, and multinucleated cells (more than three nuclei) with TRAP positivity were counted. The representative figures (A) and the statistical result (B) were shown, respectively (two-tailed Wilcoxon matched-paired signed-rank test, ∗P < 0.05). Scale bar = 50 μm.
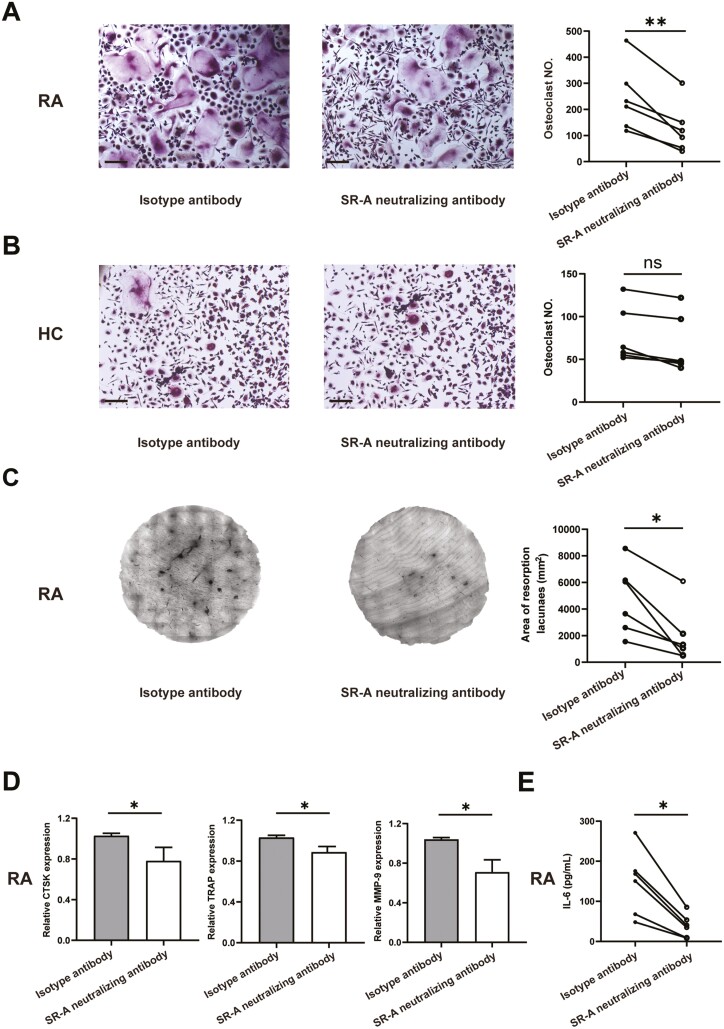
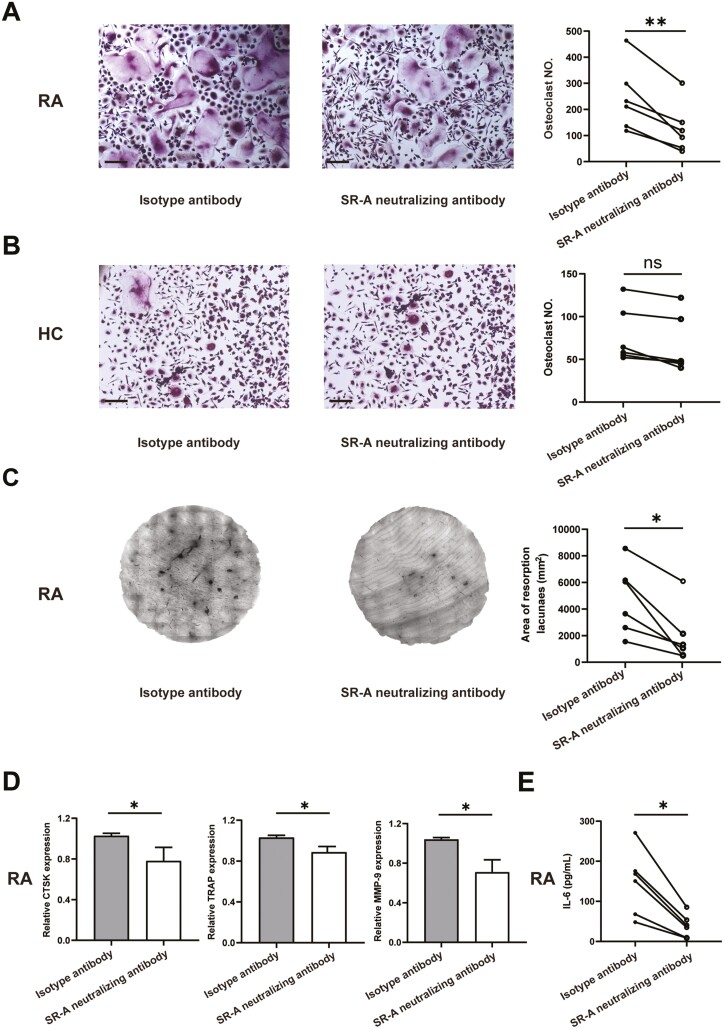
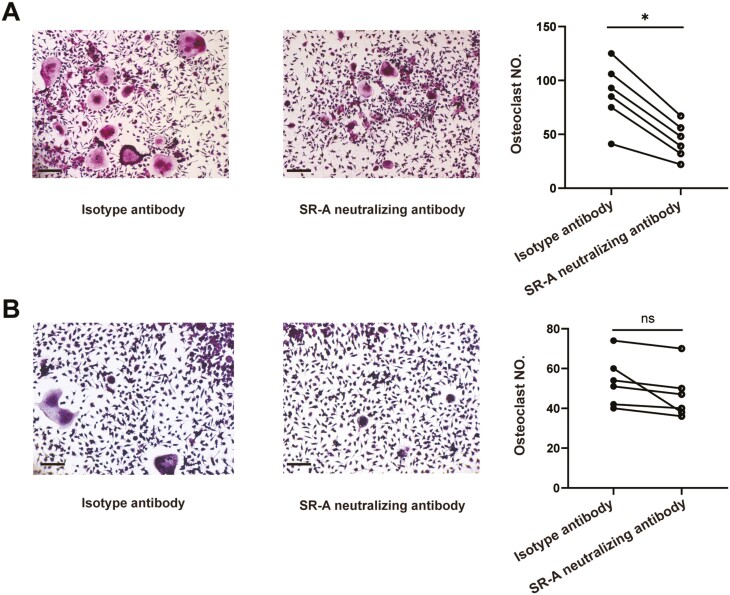
We then examined the effects of blockade of SR-A on osteoclastogenesis in patients with RA. Monocytes from patients with RA and healthy individual peripheral blood were cultured with SR-A neutralizing antibody or isotype antibody for OC differentiation. Tartrate-resistant acid phosphatase (TRAP) staining showed that compared with isotype antibody, SR-A neutralizing antibody could substantially inhibit the OC formation and decrease the number of OCs in patients with RA (Fig. 3A). Nevertheless, only a minimal inhibitory effect of SR-A neutralizing antibody on OC differentiation was seen in healthy individuals (Fig. 3B), indicating the disease-specific pathologic role of SR-A. Bone resorption assay further showed that SR-A neutralizing antibody could functionally dampen the bone erosion and destruction in patients with RA (Fig. 3C). Moreover, the expression of TRAP, cathepsin K (CTSK), and matrix metalloproteinase-9 (MMP-9), marker genes for OCs, were significantly down-regulated after SR-A neutralizing antibody treatment in RA (Fig. 3D). The levels of IL-6 in the cell culture supernatants were also fundamentally decreased after the treatment (Fig. 3E).
Figure 3.
SR-A neutralizing antibody inhibits OC differentiation and bone destruction in patients with RA. (A) Monocytes from patients with RA (n = 6) were co-cultured with isotype antibody or anti-human SR-A neutralizing antibody at 2 μg/ml for 14 days in the presence of 100 ng/ml RANKL and 50 ng/ml M-CSF. Then the cells were fixed and set for TRAP staining, and multinucleated cells (more than three nuclei) with TRAP positivity were counted. The representative figures and the statistical result were shown, respectively. Scale bar = 50 μm. (B) SR-A neutralizing antibody showed minimal effects on OC differentiation in healthy individual monocytes. OC differentiation was performed as described above (n = 6), and the representative figures and statistical results were shown, respectively. Scale bar = 50 μm. (C) SR-A neutralizing antibody inhibited bone destruction in patients with RA. OC differentiation was performed as describe above with the bone slices at the bottom of the 96-well plates (n = 6). Then the resorption area was stained and analyzed. (D) The differentiated OCs from patients with RA as described in (A) were set for qPCR analysis of the expression of the OC maker genes, including CTSK, TRAP, and MMP-9. (E) The level of IL-6 in the cell culture supernatants of patients with RA was also detected by ELISA. The statistical results are presented as mean ± SEM (two-tailed Wilcoxon matched-paired signed-rank test, ∗P < 0.05, ∗∗P < 0.01, ns, not significant).
Although SR-A neutralizing antibody blocks both soluble and membrane-bound SR-A, our preliminary results revealed that the expression of membrane-bound SR-A on monocytes of the patient with RA were significantly decreased as compared with healthy individuals, while the levels of SR-A mRNA as well as soluble SR-A in the serum were significantly elevated (data not shown). Therefore, we speculated that SR-A neutralizing antibodies inhibited osteoclastogenesis in patients with RA mainly through blocking soluble SR-A. Nevertheless, the detailed functions and underlying mechanisms need to be further studied.
SR-A neutralizing antibody dampens OC differentiation in arthritis mice in vitro
We next detected the effects of SR-A neutralizing antibody on OC differentiation in collagen-induced arthritis (CIA) mice in vitro. Bone marrow-derived monocytes (BMMs) from CIA mice and naïve mice were cultured with SR-A neutralizing antibody or isotype antibody for 9 days in the presence of M-CSF and RANKL. As shown in Fig. 4A, TRAP staining revealed that SR-A neutralizing antibody could markedly alleviate the OC formation in CIA mice. However, only a faintest effect was detected in naïve mice (Fig. 4B), similar to that in healthy individuals. These results further demonstrated the function of SR-A neutralizing antibodies in compromising OC differentiation in RA.
Figure 4.
SR-A neutralizing antibody dampens OC differentiation in collagen-induced arthritis (CIA) mice in vitro. Bone marrow-derived monocytes (BMMs) from CIA mice (A, n = 6) and naïve mice (B, n = 6) were co-cultured with isotype antibody or anti-mouse SR-A neutralizing antibody at 2 μg/ml in the presence of 100 ng/ml RANKL and 50 ng/ml M-CSF. After 9 days, the cells were fixed for tartrate-resistant acid phosphatase (TRAP) staining. The typical figures and the statistical results were shown, respectively (two-tailed paired Student’ s t test, ∗P < 0.05, ns, not significant). Scale bar = 50 μm.
SR-A neutralizing antibody ameliorates osteoclastogenesis in arthritis mice in vivo
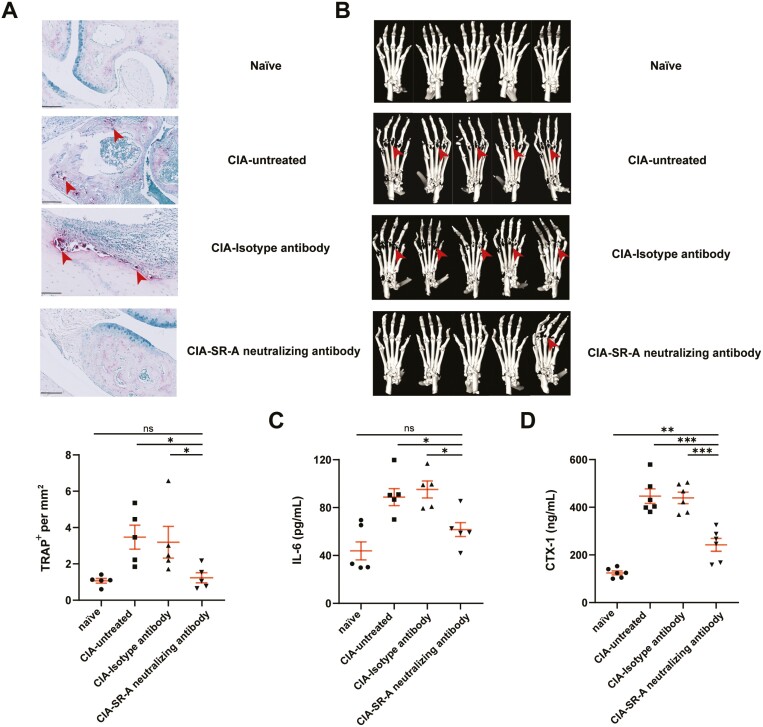
We further revealed whether blockade of SR-A by SR-A neutralizing antibodies could affect OC formation and bone destruction in CIA mice in vivo. Established CIA mice with similar scores of 2–3 was injected with SR-A neutralizing antibody or isotype antibody intravenously once every 2 days for five injections before sacrifice. As shown in Fig. 5A, TRAP staining revealed that SR-A neutralizing antibodies could decrease the number of OCs in CIA mice. Micro-CT imaging further demonstrated that compared with isotype antibody, SR-A neutralizing antibody also significantly alleviated bone destruction (Fig. 5B). Unfortunately, we failed to provide the quantification result because the equipment we used could not provide parameters for quantification of bone erosions and destruction. Moreover, the serum levels of IL-6 that promote osteoclastogenesis, as well as the serum levels of C-terminal telopeptide, a biomarker for bone remodeling, were significantly decreased in CIA mice receiving SR-A neutralizing antibody treatment (Fig. 5C, D). All these results indicated the function of SR-A neutralizing antibody in ameliorating arthritis severity and osteoclastogenesis in vivo.
Figure 5.
SR-A neutralizing antibody ameliorates osteoclastogenesis in CIA mice in vivo. After the initiation of disease, CIA mice with similar scores of 2–3 were injected with anti-mouse SR-A neutralizing antibody or isotype antibody (2 μg/mouse) intravenously every 2 days for a total of five injections (n = 5 per group). (A) After sacrifice, the paws from SR-A neutralizing antibody-, or isotype antibody-treated CIA mice, or untreated CIA mice, as well as naïve mice were fixed, paraffin-embedded, sectioned, stained with TRAP, and analyzed by NDP.view2. Arrows indicate osteoclastogenesis. Scale bar = 100 μm. (B) Micro-CT imaging of paws from different group mice as in (A) was also performed. Arrows indicate the bone erosion and destruction. (C–D) The serum levels of IL-6 (C) and C-terminal telopeptide (D) in different group mice were detected by ELISA. The statistical results are presented as mean ± SEM (One-way ANOVA test followed by Dunnett’s posttest for multiple comparisons, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ns, not significant).
SR-A neutralizing antibody attenuates OC differentiation in arthritis mice ex vivo
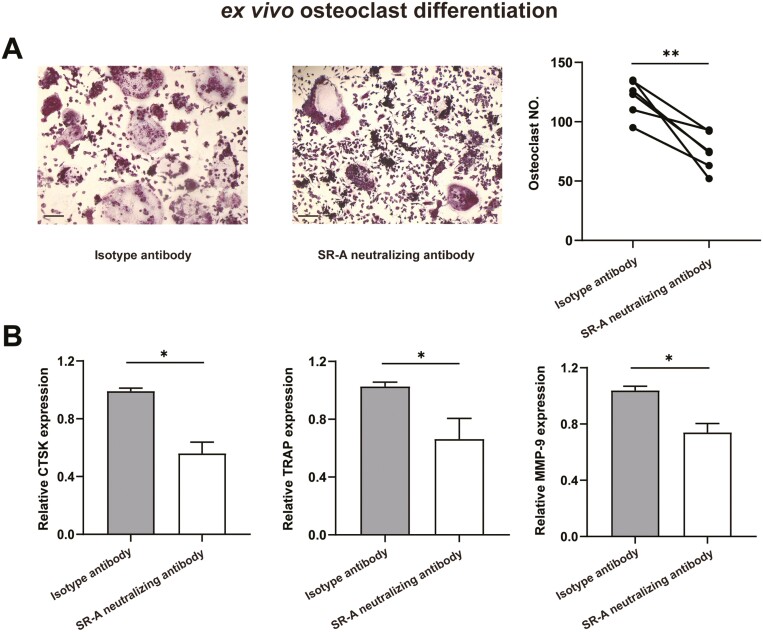
To confirm the direct effects of SR-A neutralizing antibody on osteoclastogenesis in CIA mice in vivo, BMMs from CIA mice receiving SR-A neutralizing antibody or isotype antibody treatment as described above were harvested for OC differentiation ex vivo. TRAP staining showed that the number of OCs in CIA mice treated with SR-A neutralizing antibody was significantly lower than that in CIA mice treated with isotype antibody (Fig. 6A). Moreover, the expression of OC-specific-marker genes, CTSK, TRAP, MMP-9, was also down-regulated after SR-A neutralizing antibody treatment (Fig. 6B).
Figure 6.
SR-A neutralizing antibody attenuates osteoclastogenesis in CIA mice ex vivo. BMMs from CIA mice treated with SR-A neutralizing antibody or isotype antibody as described in Figure 5 were harvested for osteoclast differentiation ex vivo. After 9 days, the cells were fixed and set for TRAP staining, and multinucleated cells (more than three nuclei) with TRAP positivity were counted. The typical figures and the statistical result were shown, respectively (A). Scale bar = 50 μm. The relative expression of CTSK, TRAP, and MMP-9 was also evaluated by qPCR (B). The statistical results are presented as mean ± SEM (two-tailed paired Student’ s t test, ∗P < 0.05, ∗∗P < 0.01).
Taken together, these results revealed the direct function of SR-A neutralizing antibodies in ameliorating arthritis severity and osteoclastogenesis in vivo.
Discussion
Our previous study has revealed the diagnostic value and pathogenic activity of SR-A in patients with RA and mice with experimental arthritis [19]. In this study, we further described the positive correlation between sSR-A and bone destruction in patients with RA. Moreover, we revealed the therapeutic potential of blocking SR-A by neutralizing antibodies in ameliorating OCs differentiation and bone destruction both in patients with RA and CIA mice. In addition, SR-A neutralizing antibody down-regulated the expression of OC-specific genes such as TRAP, CTSK, and MMP-9, and attenuated the production of inflammation cytokine IL-6 and bone remodeling biomarker C-terminal telopeptide. Therefore, SR-A neutralizing antibodies may provide a promising strategy for RA therapy especially in dampening bone destruction.
Ideal treatment in RA aims for both reductions of chronic inflammation and structural protection of the impaired bones and joints. However, current therapeutic regimens hardly achieve this perfect goal. Clinical observations indicated that compared with biologic agents, cDMARDs such as methotrexate (MTX), sulfasalazine (SSZ), leflunomide (LEF), and hydroxychloroquine (HCQ) display minimal protective effect on bone destruction in RA [22]. As a bDMARDs, tumor necrosis factor (TNF)-blocking reagents, such as infliximab, could virtually arrest the progression of bone destruction in RA. Emerging studies have provided evidence that TNF-α may potentiate RANKL-induced OC differentiation, and impair the function of Treg cells which are potential suppressors of OC formation [23]. Moreover, Abatacept, which can mimic the effect of cytotoxic T-lymphocyte-associated antigen 4 (CTLA4), could directly target cell surface receptor CD80/CD86 on precursor cells of OCs [24]. tsDMARDs such as Janus-associated kinase (JAK) inhibitor were also reported to directly affect osteoclastogenesis [25]. But these existing b/tsDMARDs are far from perfect due to the potential adverse effects, such as the higher risk of reactivation of latent tuberculosis and other serious opportunistic infections, malignancy, and the inadequate response in a proportion of patients [26, 27].
In this study, we demonstrated that SR-A neutralizing antibodies could significantly inhibit OC differentiation and bone destruction in patients with RA and CIA mice in vivo and in vitro. Given the anti-inflammatory effects of blocking SR-A in CIA mice as proved in our previous study [19], SR-A neutralizing antibodies may provide a latent and supplementary strategy for RA therapy with dual protective functions. This also partially explained the fast effect of SR-A neutralizing antibodies administration against osteoclastogenesis observed in the study.
Nevertheless, currently, few SR-A neutralizing antibodies are available. In this study, we used two SR-A neutralizing antibodies from R&D systems, anti-mouse SR-A neutralizing polyclonal antibody (pAb) and anti-human monoclonal antibody (mAb). However, pAbs are not the ideal reagents for therapeutic application in the clinic. Novel anti-mouse SR-A mAbs for neutralization need to be further designed for therapeutics. Moreover, anti-human SR-A neutralizing antibodies targeting the predominant epitopes, especially the pathogenic domain of soluble SR-A, need to be further developed, since our results indicate that soluble SR-A and membrane-bound SR-A might demonstrate different functions [19]. In addition, further modification of SR-A neutralizing antibodies, such as galactosylation and sialylation that enhances the anti-inflammatory activity, as well as partially or fully humanization that minimizes the immunogenicity, would further facilitate their potential clinical application [28, 29].
Besides SR-A neutralizing antibodies, several other regents targeting SR-A, including small-molecule inhibitors (SMIs) of SR-A and inhibitory peptides, could also be developed for RA bone destruction treatment. Currently, tannic acid and rhein were identified as SMIs of SR-A [30–32]. However, only tannic acid has been revealed the ability to ameliorate inflammation and joint damage in arthritis mice [30]. Moreover, through phage-displayed peptide library, a novel peptide antagonist of SR-A, PP1, was selected to target the carriers to atherosclerotic aortic artery lesions [33]. Nevertheless, its effect against RA bone destruction remains unclear. Due to their unique mechanism of action and the simultaneous effect on multiple mediators, screening for more specific and competent SMIs and inhibitory peptides may offer promising and supplementary strategies for the treatment of RA inflammation and bone destruction.
In conclusion, here we revealed that the SR-A neutralizing antibodies could inhibit OCs differentiation both in patients with RA and CIA mice, protecting against bone destruction. Developing more specific and optimal SR-A monoclonal antibodies and designing SMIs or inhibitory peptides will provide novel therapeutic strategies for treating bone destruction in RA.
Acknowledgements
None.
Glossary
Abbreviations
- ACPA
anti-citrullinated protein antibodies
- BMMs
bone marrow-derived monocytes
- CFA
complete Freund adjuvant
- CIA
collagen-induced arthritis
- CTSK
cathepsin K
- IFA
incomplete Freund adjuvant
- JSN
joint space narrowing
- MMP-9
matrix metalloproteinase-9
- NSAIDs
nonsteroidal anti-inflammatory drugs
- OCs
osteoclasts
- PB
peripheral blood
- PBMCs
peripheral blood mononuclear cells
- PRRs
pattern recognition receptors
- RA
rheumatoid arthritis
- RF
rheumatoid factor
- SHS
Sharp/van der Heijde score
- SMIs
small-molecule inhibitors
- SR-A
scavenger receptor A
- TRAP
tartrate-resistant acid phosphatase
Contributor Information
Yang Xie, Department of Rheumatology and Immunology, Peking University People’s Hospital & Beijing Key Laboratory for Rheumatism Mechanism and Immune Diagnosis (BZ0135), Beijing, China.
Xiang Jiang, Department of Rheumatology and Immunology, Peking University People’s Hospital & Beijing Key Laboratory for Rheumatism Mechanism and Immune Diagnosis (BZ0135), Beijing, China; Peking-Tsinghua Center for Life Sciences, Peking University, Beijing, China.
Ping Wang, Department of Rheumatology and Immunology, Peking University People’s Hospital & Beijing Key Laboratory for Rheumatism Mechanism and Immune Diagnosis (BZ0135), Beijing, China.
Xi Zheng, Department of Rheumatology and Immunology, Peking University People’s Hospital & Beijing Key Laboratory for Rheumatism Mechanism and Immune Diagnosis (BZ0135), Beijing, China; Peking-Tsinghua Center for Life Sciences, Peking University, Beijing, China.
Jing Song, Department of Rheumatology and Immunology, Peking University People’s Hospital & Beijing Key Laboratory for Rheumatism Mechanism and Immune Diagnosis (BZ0135), Beijing, China; Peking-Tsinghua Center for Life Sciences, Peking University, Beijing, China.
Mingxin Bai, Department of Rheumatology and Immunology, Peking University People’s Hospital & Beijing Key Laboratory for Rheumatism Mechanism and Immune Diagnosis (BZ0135), Beijing, China.
Yundi Tang, Department of Rheumatology and Immunology, Peking University People’s Hospital & Beijing Key Laboratory for Rheumatism Mechanism and Immune Diagnosis (BZ0135), Beijing, China.
Xiangyu Fang, Department of Rheumatology and Immunology, Peking University People’s Hospital & Beijing Key Laboratory for Rheumatism Mechanism and Immune Diagnosis (BZ0135), Beijing, China.
Yuan Jia, Department of Rheumatology and Immunology, Peking University People’s Hospital & Beijing Key Laboratory for Rheumatism Mechanism and Immune Diagnosis (BZ0135), Beijing, China.
Zhanguo Li, Department of Rheumatology and Immunology, Peking University People’s Hospital & Beijing Key Laboratory for Rheumatism Mechanism and Immune Diagnosis (BZ0135), Beijing, China; Peking-Tsinghua Center for Life Sciences, Peking University, Beijing, China; State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing, China.
Fanlei Hu, Department of Rheumatology and Immunology, Peking University People’s Hospital & Beijing Key Laboratory for Rheumatism Mechanism and Immune Diagnosis (BZ0135), Beijing, China; State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing, China; Department of Integration of Chinese and Western Medicine, School of Basic Medical Sciences, Peking University, Beijing, China.
Funding
The National Natural Science Foundation of China (grants 82171773, 81971523, 81671604, and 81302554 to Dr F. Hu, U1903210 to Dr Z. Li, and 81871281 to Dr Y. Jia), the Beijing Nova Program (grants Z181100006218044 and Z211100002121163 to Dr F. Hu), the Fundamental Research Funds for the Central Universities: Peking University Clinical Medicine Plus X-Young Scholars Project (grant PKU2021LCXQ014 to Dr F. Hu), and the Peking University People’s Hospital (grant RDX2020-01 to Dr F. Hu).
Conflict of interests
The authors declare that they have no competing interests.
Authors’ contributions
Y.X., X.J., P.W., X.Z. performed the experiments and analyzed the data. J.S., MX.B., YD.T., XY.F. provided reagents, materials and analysis tools. Y.X. wrote the original draft of the manuscript. Y.J., ZG.L. and FL.H. revised the manuscript. FL.H. designed the study and defined the final version of the manuscript.
Ethical approval
The study protocol was approved by the ethics committees of Peking University People’s Hospital.
Data availability
The data underlying this article are available in the article.
References
- 1. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011, 365, 2205–19. [DOI] [PubMed] [Google Scholar]
- 2. Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res 2018, 6, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharp JT, Lidsky MD, Collins LC, Moreland J. Methods of scoring the progression of radiologic changes in rheumatoid arthritis. Correlation of radiologic, clinical and laboratory abnormalities. Arthritis Rheum 1971, 14, 706–20. [DOI] [PubMed] [Google Scholar]
- 4. van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 2000, 27, 261–3. [PubMed] [Google Scholar]
- 5. Ødegård S, Landewé R, van der Heijde D, Kvien TK, Mowinckel P, Uhlig T. Association of early radiographic damage with impaired physical function in rheumatoid arthritis: a ten-year, longitudinal observational study in 238 patients. Arthritis Rheum 2006, 54, 68–75. [DOI] [PubMed] [Google Scholar]
- 6. Tanaka Y, Nakayamada S, Okada Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr Drug Targets Inflamm Allergy 2005, 4, 325–8. [DOI] [PubMed] [Google Scholar]
- 7. Rolph D, Das H. Transcriptional regulation of osteoclastogenesis: the emerging role of KLF2. Front Immunol 2020, 11, 937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steffen U, Schett G, Bozec A. How autoantibodies regulate osteoclast induced bone loss in rheumatoid arthritis. Front Immunol 2019, 10, 1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cui Y, Zhu T, Li D, Li Z, Leng Y, Ji X.et al. Bisphosphonate-functionalized scaffolds for enhanced bone regeneration. Adv Healthcare Mater 2019, 8, e1901073. [DOI] [PubMed] [Google Scholar]
- 10. Chiu YG, Ritchlin CT. Denosumab: targeting the RANKL pathway to treat rheumatoid arthritis. Expert Opin Biol Ther 2017, 17, 119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Syng-Ill L, Seung-Ho O, Hyunjoo J, Jong-Pill K, Yun-Jung Y, Jeong-Taeg S.et al. Xylitol down-regulates 1α,25-dihydroxy vitamin D3-induced osteoclastogenesis via in part the inhibition of RANKL expression in osteoblasts. Int J Oral Biol 2013, 38, 127–34. [Google Scholar]
- 12. Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol 2013, 13, 621–34. [DOI] [PubMed] [Google Scholar]
- 13. Yi H, Zuo D, Yu X, Hu F, Manjili MH, Chen Z.et al. Suppression of antigen-specific CD4+ T cell activation by SRA/CD204 through reducing the immunostimulatory capability of antigen-presenting cell. J Mol Med (Berlin, Germany) 2012, 90, 413–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haasken S, Auger JL, Taylor JJ, Hobday PM, Goudy BD, Titcombe PJ.et al. Macrophage scavenger receptor 1 (Msr1, SR-A) influences B cell autoimmunity by regulating soluble autoantigen concentration. J Immunol (Baltimore, Md: 1950) 2013, 191, 1055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zani IA, Stephen SL, Mughal NA, Russell D, Homer-Vanniasinkam S, Wheatcroft SB.et al. Scavenger receptor structure and function in health and disease. Cells 2015, 4, 178–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin YL, de Villiers WJS, Garvy B, Post SR, Nagy TR, Safadi FF.et al. The effect of class a scavenger receptor deficiency in bone. J Biol Chem 2007, 282, 4653–60. [DOI] [PubMed] [Google Scholar]
- 17. Takemura K, Sakashita N, Fujiwara Y, Komohara Y, Lei X, Ohnishi K.et al. Class A scavenger receptor promotes osteoclast differentiation via the enhanced expression of receptor activator of NF-kappaB (RANK). Biochem Biophys Res Commun 2010, 391, 1675–80. [DOI] [PubMed] [Google Scholar]
- 18. Guo S, Ni Y, Ben J, Xia Y, Zhou T, Wang D.et al. Class A scavenger receptor exacerbates osteoclastogenesis by an interleukin-6-mediated mechanism through ERK and JNK signaling pathways. Int J Biol Sci 2016, 12, 1155–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu F, Jiang X, Guo C, Li Y, Chen S, Zhang W.et al. Scavenger receptor-A is a biomarker and effector of rheumatoid arthritis: a large-scale multicenter study. Nat Commun 2020, 11, 1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu F, Liu H, Liu X, Zhang X, Xu L, Zhu H.et al. Pathogenic conversion of regulatory B10 cells into osteoclast-priming cells in rheumatoid arthritis. J Autoimmun 2017, 76, 53–62. [DOI] [PubMed] [Google Scholar]
- 21. Hu F, Mu R, Zhu J, Shi L, Li Y, Liu X.et al. Hypoxia and hypoxia-inducible factor-1α provoke toll-like receptor signalling-induced inflammation in rheumatoid arthritis. Ann Rheum Dis 2014, 73, 928–36. [DOI] [PubMed] [Google Scholar]
- 22. Schett G, Stach C, Zwerina J, Voll R, Manger B. How antirheumatic drugs protect joints from damage in rheumatoid arthritis. Arthritis Rheum 2008, 58, 2936–48. [DOI] [PubMed] [Google Scholar]
- 23. Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J.et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest 2000, 106, 1229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bozec A, Zaiss MM, Kagwiria R, Voll R, Rauh M, Chen Z.et al. T cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med 2014, 6, 235ra–60. [DOI] [PubMed] [Google Scholar]
- 25. van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S.et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012, 367, 508–19. [DOI] [PubMed] [Google Scholar]
- 26. Morinobu A. JAK inhibitors for the treatment of rheumatoid arthritis. Immunol Med 2020, 43, 148–55. [DOI] [PubMed] [Google Scholar]
- 27. Atzeni F, Nucera V, Gerratana E, Cirillo M, Marino F, Miceli G.et al. Concerns about the safety of anti-TNF agents when treating rheumatic diseases. Expert Opin Drug Saf 2020, 19, 695–705. [DOI] [PubMed] [Google Scholar]
- 28. Krinner EM, Raum T, Petsch S, Bruckmaier S, Schuster I, Petersen L.et al. A human monoclonal IgG1 potently neutralizing the pro-inflammatory cytokine GM-CSF. Mol Immunol 2007, 44, 916–25. [DOI] [PubMed] [Google Scholar]
- 29. Pagan JD, Kitaoka M, Anthony RM. Engineered sialylation of pathogenic antibodies in vivo attenuates autoimmune disease. Cell 2018, 172, 564–77.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shukla M, Gupta K, Rasheed Z, Khan KA, Haqqi TM. Consumption of hydrolyzable tannins-rich pomegranate extract suppresses inflammation and joint damage in rheumatoid arthritis. Nutrition (Burbank, Los Angeles County, Calif) 2008, 24, 733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raycroft MT, Harvey BP, Bruck MJ, Mamula MJ. Inhibition of antigen trafficking through scavenger receptor A. J Biol Chem 2012, 287, 5310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yuan Y, Li X, Zaidi SA, Arnatt CK, Yu X, Guo C.et al. Small molecule inhibits activity of scavenger receptor A: lead identification and preliminary studies. Bioorganic Med Chem Lett 2015, 25, 3179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Segers FM, Yu H, Molenaar TJ, Prince P, Tanaka T, van Berkel TJ.et al. Design and validation of a specific scavenger receptor class AI binding peptide for targeting the inflammatory atherosclerotic plaque. Arterioscler Thromb Vasc Biol 2012, 32, 971–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.