Abstract
Background and Aims
Dormant resting buds are frequently regarded as static units, with protective cataphylls on the outside and embryonic foliage leaves on the inside. How the presence of cataphylls influences the dynamic, cyclical, annually repeating sequence of leaf forms that a resting bud gives rise to has rarely been interrogated. To examine the connection between dormant structure and growing-season development, we compare the complete seasonal heteroblastic sequence of leaf forms of six species of temperate Juglandaceae with distinctly different vegetative resting bud structures. These include buds with cataphylls; buds without cataphylls; and buds with caducous cataphylls that are lost before the onset of winter.
Methods
In a common garden setting over a 7-month growing season, the dimensions of 2249 individual vegetative metamers were tracked from first exposure to abscission along the shoots of saplings and mature trees. The timing of metamer initiation within terminal buds was investigated using micro-CT scanning. Character state transitions of resting bud types were estimated using a phylogenetic tree of Juglandaceae.
Key Results
The presence of cataphylls within a heteroblastic sequence is associated with a single cohort of foliage leaves that flush and abscise synchronously. This growing pattern is highly determinate, with next year’s terminal-bud cataphylls already initiated before spring leaf out. In contrast, in sequences without cataphylls, shorter-lived foliage leaves appear and abscise in a staggered fashion. Despite these differences in leaf demography, all examined heteroblastic sequences produce a series of small, caducous leaf forms that precede terminal bud set.
Conclusions
The ubiquity of caducous leaf forms in Juglandoideae may point to the importance of shoot tip protection far beyond the dormant season. In addition, the presence or absence of cataphylls in resting buds is indicative of distinct shoot ontogenetic patterns, and functional strategies, in summer.
Keywords: Bud scale, caducous leaves, cataphyll, Juglandaceae, leaf development, naked bud, neoformation, preformation, resting bud, seasonal heteroblasty, temperate trees, tree architecture
INTRODUCTION
Shakespeare’s The Winter’s Tale tells a story that plays out over two separate decades, separated by a large gap in time. The primary growth of temperate, woody perennials could be considered a botanical equivalent of the play. A shoot apical meristem generates a continuous sequence of vegetative metamers over two consecutive growing seasons, separated by a seasonal dormant period. In temperate trees, the leaf forms associated with each repeating unit can range from distinctly laminate foliage leaves to cataphylls (bud scales). Over the course of a growing season, leaf forms initiated in a previous season are being expanded while new embryonic metamers are added to the apex of the shoot.
During winter dormancy, the continuity of this developmental pattern can still be detected in the resting bud, despite active growth being paused. The tightly packaged organization of a terminal resting bud encompasses at once the final metamers of the primary growth of one season as well as the first leaves, in embryonic form, of the next. Yet, while the gap in The Winter’s Tale between Act III and Act IV has been extensively analysed by scholars in the context of the entire play, it is rare that we consider resting buds as a pause between two phases of a single developmental sequence. Rather, the resting bud tends to be characterized as a self-contained, static unit that is optimized for winter survival. This static interpretation is reinforced by the importance of buds as diagnostic traits for winter tree identification as well as by an imbalance in seasonal phenological research. Many more studies focus on buds as a spring phenophase than on the timing of bud set (Gallinat et al., 2015; Beil et al., 2021).
In many temperate, woody species, the appearance of a resting bud as a static unit is emphasized when the outermost leaf forms take the form of bud scales, or cataphylls, which create a layer that physically separates resting bud contents from the environment outside (Perry and Simons, 1967). Cataphylls are leaves that are serially homologous (Kaplan, 1984; Sattler, 1984) to foliage leaves but in which only the leaf base is expressed and not the petiole and lamina (Foster, 1928, 1929, 1931). The cataphyll form is thought to have evolved by acting primarily as a protective layer during the cold months in climates with a winter (Cooke et al., 2012; Alla et al., 2013). Cataphylls could potentially allow extraorgan ice formation (Kuprian et al., 2017; Villouta et al., 2020); elevate bud temperature through absorption of solar radiation (Wiegand, 1906; Vitasse et al., 2021); or prevent desiccation of developing foliage leaves in spring. However, data that shed light on how such protection may work mechanistically (and against which of the listed environmental challenges) remain sparse (Magnin et al., 2012; Jones, 2021). In addition, a significant number of temperate, woody species with naked buds (taxa that do not overwinter their foliage leaf primordia enveloped inside cataphylls) occur in over 40 angiosperm families. This surprising ubiquity of naked buds calls into question the idea that winter climatic variables are the sole selective agents that determine the presence of cataphylls (Schoonderwoerd and Friedman, 2021).
Instead, reports that the geographic distributions of temperate, woody species with naked buds might be limited by low precipitation in summer (Magnin et al., 2012; Schoonderwoerd and Friedman, 2021) imply a role for cataphylls in shoot tip protection during the growing season. Furthermore, some authors have proposed an association between naked buds and a seasonal growth pattern often described as indeterminate (Lubbock, 1899; Sakai, 1990; Wardle, 1991). Indeterminate shoots do not just flush preformed foliage leaves (those leaves that were initiated during the previous growing season and overwintered in the bud), but continue to initiate and expand additional neoformed foliage leaves as the growing season continues. This latter hypothesis suggests that a direct connection may exist between resting bud composition in the dormant phase on the one hand, and, on the other, the dynamic seasonal sequence of consecutive metamers to which the resting bud gives rise – and from which it develops.
To investigate how such a connection might be explained from a developmental perspective, a comparative study of seasonal primary growth that tracks leaf forms, leaf numbers and bud formation along shoot axes is required in a clade of temperate, woody angiosperm species with a diverse range of resting bud structures, including and excluding cataphylls. Of the angiosperm families that contain woody taxa with naked buds in the temperate zone, Juglandaceae is among the most diverse when it comes to resting bud structure (Schoonderwoerd and Friedman, 2021). The family contains ten extant genera with ~60 tree species (Stone, 1993; Manos and Stone, 2001; Manos et al., 2007; Song et al., 2020a), all characterized by large, dissected foliage leaves (Fig. 1M–R), but with various types of bud morphologies, including apparent instances of convergent evolution in resting bud type (Fig. 1A–F). This makes the family’s temperate subfamily Juglandoideae an ideal clade for investigating the relationship between seasonal metameric leaf sequences and resting bud structure.
Fig. 1.
A diversity of terminal resting bud morphologies occur within Juglandoideae among species with large distribution ranges that span similar winter climatic conditions. (A) The heteroblastic naked bud of C. cordiformis. (B) The imbricate scaly bud of C. ovata. (C) The imbricate scaly bud of Pl. strobilacea. (D) The heteroblastic naked bud of J. nigra. (E) The bud with caducous scales of a mature tree of Pt. rhoifolia, as is evident from cataphyll scars (left) and the archetypal naked bud of a Pt. rhoifolia sapling (right). (F) The archetypal naked bud of Pt. stenoptera. (G–L) Modern species distributions based on occurrence data from BIEN and GBIF. The colour gradient shows the minimum temperature of the coldest month for each geographic location according to Bioclim. (M–R) The mature foliage leaves of all six species are dissected and have a large surface area. Abbreviations: ab, axillary bud; c, cataphyll; cs, cataphyll scar; lam, lamina; p, petiole; EA, East Asia; NA, North America.
Juglandoideae includes species with terminal resting buds with true cataphylls and species with cataphyll-less terminal buds (Feist, 1887; Stone, 1993; Manos and Stone, 2001; eFloras, 2006; Schulz, 2014; Grauke et al., 2016), as well as interesting variation within both categories. Among those species with terminal resting buds bearing true cataphylls, most species have resting buds with an imbricate pattern of cataphylls during the dormant stage (Fig. 1B, C; ‘imbricate scaly bud’). Two species (Pterocarya macroptera and Pt. rhoifolia) have buds with caducous cataphylls [Fig. 1E (left); ‘bud with caducous scales’]. In buds with caducous cataphylls, embryonic foliage leaves are formed within bud scales, but the cataphylls are, somewhat puzzlingly from a winter-protective perspective, abscised at the onset of dormancy. This results in the direct exposure of the unexpanded foliage leaves to the environment during the winter. Among the cataphyll-less category, there are buds in which every leaf included in the bud expands a full lamina upon bud break [Fig. 1E (right), F; ‘archetypal naked bud’]. In other species, the laminae of the leaves that overwinter on the outside of the terminal bud do not expand to the same degree as the laminae of the leaves that overwinter inside the resting bud (Fig. 1A, D; ‘heteroblastic naked bud’). Frequently, dead, unexpanded, bud leaves, abscised at the petiole, can be found adhering to the outside of these heteroblastic naked buds in winter through contact between the laminae of individual leaf components (Supplementary Data Fig. S1). For these reasons, these outermost bud leaves are often morphologically misinterpreted as bud scales (Sargent, 1905; Trelease, 1918; Stone, 1993; Swanson, 1994; Grimm, 2002; Lance, 2004; eFloras, 2006, 2021; Stone et al., 2009; Jones and Wofford, 2013; Poland, 2018), despite their fully differentiated laminae with visible leaflets.
Here, we characterize the full seasonal sequence of leaf forms of consecutive metamers [hereafter seasonal heteroblastic sequence (Jones, 1999)] along a vegetative axis in six species of Juglandoideae. The species were selected based on the most important character transitions in resting bud structure (Fig. 1A–F) and similarity in their climatic distributions (Fig. 1G–L). In a common garden setting, the dimensions of each individual metamer on the shoots of saplings and mature trees were tracked from first exposure to maturation and finally abscission over a 7-month growing season. These periodic observations of developing shoots allowed us to directly compare leaf forms that do not usually temporally co-occur in their mature forms. Thus quantifying seasonal heteroblastic sequences in their entirety facilitated comparative analyses among species as well as between mature trees and saplings of the same species. In addition, using micro-CT scanning, we investigated the initiation of metamers within the terminal bud structure in the six species at various stages of bud development. Using these two methods, we show that resting bud structure and seasonal growth pattern in Juglandoideae are indeed related. On the level of the family phylogeny, we ask how one bud type, or growth pattern, may transition into another through evolution. With these data we aim to show that winter is not the only tale that explains the structure of the resting bud. Perhaps, in certain taxa, the necessity of cataphylls for bud survival during the dormant season can be considered a winter’s tale in the Shakespearean sense of the term: an urban legend.
MATERIALS AND METHODS
Study species
Six study species from eastern North America and eastern Asia – Carya cordiformis, Carya ovata, Juglans nigra, Platycarya strobilacea, Pterocarya rhoifolia and Pterocarya stenoptera – were selected as representatives of the terminal resting bud diversity present in Juglandoideae, the temperate clade of the walnut family (Fig. 1). Species occurrence data downloaded from the Botanical Information and Ecology Network (BIEN) database (Maitner et al., 2018) and the Global Biodiversity Network Facility (GBIF, 2021), combined with geographic climate data from Bioclim (Fick and Hijmans, 2017), indicate that the distributions of each of these species cover large areas that contain a variety of winter climates, including, in each case, localities with severe winter freezing (Fig. 1G–L). In other words, there are no great differences in the kinds of winter climates that the species experience in their modern, native ranges.
Study site
All developmental data in this study were recorded by repeatedly observing (1) mature trees accessioned in the living collections of the Arnold Arboretum of Harvard University (Boston, MA, USA) and (2) saplings grown in an outdoor experimental plot at the Arboretum’s on-site research facility. Two mature trees per species, each of which was at least 10 years old at the time of the study, were identified from the Arboretum’s living collections for surveying primary growth (C. cordiformis, accession numbers 12893*Q, 215-2008*A; C. ovata, 268-86*C, 318-89*B; J. nigra, 14761*A, 374*2012*A; Pl. strobilacea, 1781-77*A, 1781-77*D; Pt. rhoifolia, 1802-77*B, 1802-77*C; Pt. stenoptera, 1652-80*A, 1652-80*B). In this selection, preference was given to trees with known provenance and with branches that are easily accessible with an orchard ladder. All trees are situated in an open canopy setting, with partial shade from neighbouring trees during certain times of the day. Resting bud samples for structural analyses were collected from the same group of specimens and two additional accessions of J. nigra (22880*A) and Pl. strobilacea (1747-77*A).
Saplings were acquired as bare-root seedlings from commercial nurseries (Chief River Nursery, Grafton, WI, USA; Go Native Tree Farm, Lancaster, PA, USA; Pépinière Casse-Noisette, Maskinongé, Quebec, Canada) or grown from seed (Sheffield’s Seed Company, Locke, NY, USA) that we stratified and germinated under greenhouse conditions. All plants were moved outside to an experimental plot in full sun once roots had sufficiently developed (during either May 2017, October 2017 or May 2018) and planted in a grid with 1.5-m intervals between individuals for data collection in 2019. The trees were allowed to grow freely, without pruning or application of fertilizer. Because germination and establishment success of Pt. rhoifolia plants was low, two saplings of Pterocarya macroptera, which has the same bud type and growth pattern, kept at the Arboretum’s nursery facility were added to the study.
In 2019, the monthly total precipitation and mean average temperature recorded in Boston for the growing season months were close to the interannual mean monthly values for the area. The only anomalies occurred in April, when precipitation was higher than usual, and in July, which had a higher than usual mean average temperature. When short dry spells occurred, both the mature trees and saplings received additional irrigation.
Non-destructive survey of seasonal heteroblastic sequences over the growing season
Data collection. From April to October 2019, along selected shoots of 2- to 3-year-old saplings and of mature trees, every leaf form was tracked from first exposure to abscission to document the developmental changes in heteroblastic sequences of six species at seven discrete monthly timepoints. In April, terminal resting buds of monopodial shoots (soon to be primary growth shoots) were flagged and given a unique identifier for the reliable recapture of individual sequences. A mark was made at the height of the attachment point of the basal-most metamer with an unabscised leaf, whether foliage leaf or cataphyll, with a permanent marker. In this manner, the basal-most leaf in the dormant resting bud could consistently be identified at subsequent timepoints, even after its abscission. Successive leaves were numbered sequentially and could be identified by following the phyllotactic pattern from the leaf (scar) marked as the base of the sequence.
On mature trees, axes were evenly distributed between the two selected accessioned trees per species. We flagged terminal buds that could be accessed using a 3-m orchard ladder and that were situated near the edge of the crown so that the axes would develop in the highest possible light conditions upon leaf out of the canopy. Selected axes on saplings were leader shoots on trees selected using a random number generator. We flagged one bud per sapling, except in C. ovata, Pt. rhoifolia and Pl. strobilacea, where on occasion more than one terminal bud was marked per individual tree. If at any point during the growing season the growing tip of an axis was damaged, it was excluded from the analysis. Similarly, axes on mature trees that upon leaf out revealed termination in a female inflorescence were removed from the dataset.
At each monthly timepoint, we recorded the presence of all visible leaves in a resting bud or, upon internodal elongation, along the axis. The length of each visible leaf on the axis was measured (1) from the leaf base to the tip of the lamina and (2) from the petiole–lamina attachment point to the tip of the lamina in millimetres using a 50-cm ruler or in tenths of a millimetre with digital callipers for small scale leaves, small foliage leaves, small intermediate leaf forms or foliage leaf primordia.
Data analysis. For direct comparison between axes with different numbers of leaves, the leaf measurements for each axis were divided into ten bins according to relative metamer position in the entire documented seasonal ontogenetic sequence (Fig. 2). A 0–10 % category represents the earliest initiated leaf or leaf forms that overwintered in the most exposed position(s) in the resting bud. A 90–100 % category represents the distal-most leaf forms at the end of the growing season (that directly precede the equivalent 0–10 % form(s) of the subsequent seasonal cycle).
Fig. 2.
For accurate comparison across independent branches, and sequences of different species and age groups, leaf positions in a seasonal heteroblastic series were standardized. Leaf position was standardized by its relative position in the full seasonal heteroblastic sequence, from the earliest-initiated overwintering leaf in the dormant resting bud to the first repetition of that form. Leaves of an entire sequence were divided into ten bins of one or several neighbouring leaves. In this example, colour is an indicator of leaf size.
For each positional category, leaf dimensions were summarized at an early developmental stage, when leaves first became exposed (and observable), but were still folded, with unexpanded laminae. In addition, mean dimensions for mature leaves were calculated using the data collected at the timepoint with the longest measured leaf length. Leaf longevity per positional category was summarized using survival curves constructed from presence–absence data from all leaves across axes belonging to a certain species and age class.
Characterization of bud composition
Twigs bearing terminal resting buds or growing tips were collected from accessioned mature trees using an extendible pole pruner from the edge of the crown at time points (1) during dormancy (November/December), (2) in spring, just prior to leaf out (mid- to late April) and (3) in the summer months (June/July). A record was made of the number and types of leaf scars and attached leaves along the branches. Then, the terminal buds were removed and fixed in a 4 % acrolein solution in a modified PIPES buffer adjusted to pH 6.8 (50 mm PIPES; 1 mm MgSO4; 5 mm EGTA) for 24 h. Following fixation, the samples were rinsed with buffer and dehydrated through a graded ethanol series. Fully dehydrated samples in 100 % ethanol were critical-point dried (Autosamdri-815 Series A, Tousimis, Rockville, MD, USA) and mounted with epoxy adhesive on pipette tips stuffed with tissue paper. To reliably identify the bud contents without causing damage to the samples, the mounted buds were scanned at the Harvard Center for Nanoscale Systems with a micro-CT scanner (X-Tek HMX ST 225, Nikon Metrology, Brighton, MI, USA) using a molybdenum target. For each specimen, between 2000 and 3142 projections were recorded with the acceleration voltage set to 35–70 kV, the source current to 130–260 μA and the exposure time to 1.4–2.8 s. We performed 3-D reconstruction in CT Pro 3D (Nikon Metrology, Brighton, MI, USA) and the samples were optically sectioned in VG Studio MAX 3.0 (Volume Graphics, Heidelberg, Germany) to reveal their contents. The scan resolution was sufficient to identify the meristem and youngest leaf primordia in the buds.
To forecast the developmental fate of each of the metamers in a bud, we combined data from the collected bud samples and the non-destructive survey. The individual axis history of the collected samples was used to identify the number of nodes with mature leaves preceding the terminal bud sample. The median number of recorded nodes per axis on mature trees as identified by the non-destructive survey (Supplementary Data Table S1) was used as an estimate for the total number of leaves in a seasonal heteroblastic sequence. For each metamer contained within a bud, its position within a typical complete seasonal heteroblastic sequence could thus be projected.
Phylogenetic and ancestral state reconstruction
To construct a family-wide phylogenetic tree that includes a complete representation of the terminal bud-type diversity in Juglandaceae, we selected 47 taxa [of the 60 species circumscribed in the taxonomic treatment of Juglandaceae by Song et al. (2020a)] and identified four nuclear loci (ITS, phyA, ETS and LEAFY) and six plastid loci (matK, rbcL-atpB, rpoC1, rps16, trnH-psbA and trnL-trnF) from previous phylogenetic work on Juglandaceae (among others: Stanford et al., 2000; Manos and Stone, 2001; Aradhya et al., 2007; Manos et al., 2007; Stone et al., 2009; Zhang et al., 2013; Zhou et al., 2021). Nucleotide sequences were downloaded from GenBank (Supplementary Data Table S2) and aligned with MUSCLE v. 3.8.425 in Geneious Prime.
Dated phylogenetic trees were constructed using Bayesian inference in MrBayes v. 3.2 (Ronquist et al., 2012) with a partitioned (nuclear and plastid) data set. Two independent Markov chain Monte Carlo (MCMC) analyses with four chains each were run for 100 million generations, using a general time reversible (GTR) model with γ-distributed rate variation across sites, sampling every 1000 generations. Convergence was assessed using Tracer v. 1.7.2 (Rambaut et al., 2018). For dating, a relaxed clock model was used with date constraints placed on the Alfaroa + Oreomunnea clade (Paleooreomunnea stoneana; 37.8–47.8 Ma), on Juglans (Juglans clarnensis; 34–55 Ma) and on Pterocarya (Pterocarya smileyi; 5.3–23 Ma), as described in Zhang et al. (2013) and Song et al. (2020a), with fossil dates as described in Zhang et al. (2022).
Because no previous phylogenetic work has reliably reconstructed section Apocarya (pecan hickories) as a clade, but these species are generally believed to be monophyletic (Grauke et al., 2016), we chose to constrain Apocarya in order to avoid multiple bud character state changes due to phylogenetic ambiguities. In addition, Pt. macroptera and Pt. rhoifolia were constrained as sister species (Song et al., 2020b). To account for the variation in topologies of Juglandaceae reported in various systematic studies, the analysis was run once with a constraint placed on Platycarya + Juglandinae [as reported in Zhang et al. (2013), Song et al. (2020a) and Mu et al. (2020)], and once with Platycarya as sister to all other genera in Juglandoideae [as reported in Manos and Stone (2001), Manos et al. (2007) and the analysis of nuclear genomic data in Zhou et al. (2021)].
After observations of living specimens and/or review of published information, a morphological type of terminal resting bud was assigned to each of the taxa in the data set (Supplementary Data Table S3). To gain insight into the evolutionary gains and/or losses of cataphylls, Juglandaceae bud types were grouped into three categories: (1) archetypal naked buds, (2) heteroblastic naked buds, and (3) buds with cataphylls, including both imbricate and caducous bud types. When published bud descriptions used a different terminology from the bud type classification used in this study and described in Schoonderwoerd and Friedman (2021), this is noted in Supplementary Data Table S3. Ancestral character states and the number of character state transitions were estimated in Phytools v. 0.7-80 (Revell, 2012) with stochastic character mapping on the topologies generated by the two analyses with different placements of Platycarya. Per tree, 1000 simulations were generated, using an equal-rates transition model and no prior distribution on the root node.
RESULTS
Comparative development of seasonal heteroblastic sequences along vegetative shoot axes
From April to October 2019, 2249 leaves, ranging from cataphylls to foliage leaves and including intermediate forms, were tracked from first exposure to abscission along 93 axes. With the resulting data set, traits of leaves at distinct axis positions can be compared directly with each other, and dimensions of young, unexpanded leaves can be interpreted alongside their mature leaf proportions and leaf longevity properties (Fig. 3; Supplementary Data Fig. S2). Based on the heteroblastic sequences of lamina, petiole and leaf-base dimensions of young, unexpanded leaves (of mature trees) at first exposure, the six Juglandoideae species in this study were placed along a spectrum from Pt. stenoptera to C. ovata (Fig. 3A–F). This overview, expounded on below, facilitates identification of the differences between the full heteroblastic sequences of six related species. It also reveals several similarities between species with, ostensibly, widely differing seasonal heteroblastic patterns and terminal resting bud types.
Fig. 3.
Quantitative characterization of the seasonal heteroblastic sequences along shoots of mature trees in six Juglandoideae species. Leaf measurements for each species are summarized for each relative position, from base to tip (0–100 %), within the complete leaf sequence observed over a 7-month growing season. A 0–10 % category represents the earliest-initiated leaf or leaf forms that overwintered in the most exposed position(s) in the resting bud. A 90–100 % category represents the distal-most leaf form(s) at the end of the growing season [that directly precede(s) the equivalent 0–10 % form(s) of the subsequent seasonal cycle]. (A–F) Mean ratio of lamina length to total leaf length in unexpanded leaves at first exposure to the aerial environment. Lamina proportions are shown in colour; the proportions of leaf length occupied by the petiole and leaf base are represented in black. Bars that are (almost) completely black represent cataphylls. (G–L) Mean leaf length at full leaf expansion (maturity). Lamina proportions of laminar leaves are shown in colour; the proportions of leaf length occupied by the petiole and leaf base are shown in black. Mature cataphyll length is shown in blue. (M–R) Leaf lifespan from first exposure to the aerial environment to abscission expressed as the proportion of leaves at the same relative axis position present at a timepoint. Proportions <50 % not shown.
Extent of lamina differentiation in leaves upon first exposure, and variation thereof within the seasonal heteroblastic sequence
On one end of the spectrum, in Pt. stenoptera, a species with an archetypal naked bud, leaves show no specialization from one node to the next. At every node, the unexpanded lamina of a young leaf is the same relative size in relation to the entire unexpanded leaf length, without a change in proportion from one metamer to the next (Fig. 3A). In contrast, in the other five species, changes from one node to the next are evident in the relative size of the lamina of leaves upon first exposure (Fig. 3B–F).
In J. nigra and C. cordiformis, two species with heteroblastic naked buds, a gradual increase in the proportion of the unexpanded leaf allocated to the unexpanded lamina occurs in the first half of the seasonal ontogenetic sequence (0 until 40 or 50 %; Fig. 3B–C), followed by a decrease in the second half (>50 %). In these heteroblastic naked-budded species, the initiation of leaves along the seasonal axis is associated with laminar specialization that can be detected at an early developmental stage, but the lamina is never lost completely.
Interestingly, the heteroblastic sequences of the three cataphyll-bearing species Pt. rhoifolia, Pl. strobilacea and C. ovata are not strictly dimorphic (divided into cataphylls and foliage leaves alone). As in J. nigra and C. cordiformis, a gradual change in leaf form can be observed at multiple consecutive nodes, in this case of leaf forms intermediate between foliage leaves and cataphylls. In the cataphyll-bearing species, however, the pattern is more pronounced and the decrease of the unexpanded lamina proportion ultimately results in the full loss of the lamina in leaves initiated at some of the nodes. The three species differ in how common cataphylls are within their seasonal heteroblastic sequences, with ~20 % of nodes in Pt. rhoifolia lacking a lamina (Fig. 3D) and up to 50 % in C. ovata (Fig. 3F). Consequently, the two species that have an imbricate, persistent arrangement of cataphylls, Pl. strobilacea and C. ovata, have the least gradual nodal leaf form specialization of the six studied species, as well as the highest proportion of cataphylls. The terminal bud of Pt. rhoifolia, with caducous cataphylls, occupies an intermediate position between the heteroblastic naked buds and buds with imbricate, persistent cataphylls.
Leaf maturation patterns and leaf longevity.
The lack of seasonal differences in relative lamina differentiation at the unexpanded developmental stage in Pt. stenoptera does not result, however, in leaves at every node having the same fate over the growing season. The leaves that overwintered at the outermost, exposed positions of the archetypal naked bud reach a significantly smaller final size (~200-mm lengths; photosynthetic area ~90 cm2) than the leaves mid-axis (~400-mm lengths; photosynthetic area ~400 cm2; Fig. 3G). Furthermore, the leaves along the distal 30 % of the growing axis remain comparatively small (Fig. 3G). In addition, they have a much shorter lifespan (2–4 months) than the leaves that precede them, which typically remain on the axis for ~5 months (Fig. 3M).
Similarly, in J. nigra and C. cordiformis, the species with heteroblastic naked buds, unexpanded lamina differentiation is not a reliable, linear predictor of mature leaf size (Fig. 3B, H; and C, I). In these species, the outermost, exposed leaves of the resting bud are both small at maturity and short-lived (Fig. 3H, N; and I, O). These traits recur in nodes along the distal half of the axes, as in the late-season leaves of Pt. stenoptera.
In fact, such small, laminate, short-lived leaf forms are not only observed along the axes of species with naked buds, but also in species with cataphylls. In Pt. rhoifolia and Pl. strobilacea, transitional, laminate, small and short-lived leaf forms occur between foliage leaves and cataphylls (Fig. 3P, Q, orange-coloured bars).
Patterns in timing of foliage leaf expansion: single-cohort versus staggered expansion
Within the seasonal heteroblastic sequences of the three species with cataphylls, Pt. rhoifolia, Pl. strobilacea and C. ovata, a single cohort of foliage leaves could be clearly identified. Leaves within such a cohort have a large and uniform mature leaf size (Fig. 3J–L, dark green bars). Their leaf longevity spans the entire growing season and starts and ends simultaneously, from first flush to autumn (Fig. 3P–R, dark green bars).
In contrast, in mature axes of Pt. stenoptera and J. nigra, the species with an archetypal naked bud and a heteroblastic naked bud, at the other end of the spectrum, foliage-type leaves are more variable in size (Fig. 3G, H). Therefore, in these species, it is more difficult to discern which nodes mark the transition from foliage leaves to leaves that are not primarily photosynthetic (and vice versa). Leaf longevity cannot be used to define a cohort either because leaves are typically present on the axis for 4–6 months of the growing season only and are expanded, and abscised, up to 3 months apart (Fig. 3M, N).
Both the single-cohort and staggered expansion patterns were shifted somewhat in the saplings, where all of the species appeared to expand more foliage-type leaves later into the season compared with their respective mature branch patterns (Supplementary Data Fig. S2M–R). This shift was accompanied by a reduction in the relative number of nodes with lamina reductions (cataphylls and intermediates) in saplings compared with mature trees (Supplementary Data Fig. S2A–F).
Identification of terminal bud components and comparative terminal bud development
In all six species, within a month after leaf out, a highly organized bud structure can be observed at the shoot apex. Micro-CT scanning of these early-season terminal bud organizations revealed that they are analogous both in appearance and in the approximate number of metameric components to their respective dormant resting bud structures (Fig. 4). Nonetheless, these early-season terminal bud components are not equivalent to those of the resting bud during the dormant phase. Our non-destructive survey established that late-season foliage leaves (in Pt. stenoptera and J. nigra) and caducous leaf forms (in all studied species) are removed from the shoot apex as the growing season progresses through internodal elongation or abscission (Fig. 3M–R). The formation of the ultimate dormant bud structure must therefore be a dynamic process in which metamers are added internally by the meristem as well as abscised externally over the growing season. To correctly identify when the first components of the dormant resting bud are initiated, we forecast the mature leaf size and leaf lifespan of the leaf components present within the developing terminal bud structures of summer (Figs 4A–L and 5A–F, S–X), dormant buds of the late autumn (Figs 4M–X and 5G–L) and swelling terminal buds of spring (Fig. 5M–R).
Fig. 4.
Comparison of summer terminal bud developmental stages with dormant resting bud morphologies of mature trees for six species in Juglandoideae. (A–F) Photographs of summer terminal bud stages in June, approximately a month after spring leaf out, shown alongside (G–L) optical micro-CT cross-sections, taken through the meristem, of buds of the same developmental stage. For Pt. rhoifolia (D), the terminal bud (right) is displayed next to a caducous leaf form (left) that, in June, is removed from the terminal bud by internodal elongation. The break in the axis stem is marked by an asterisk. (M–R) Photographs of resting terminal bud stages representative of the end of the growing season and the beginning of the dormant phase (and in the case of Pt. rhoifolia, prior to cataphyll loss), shown alongside (S–X) optical micro-CT cross-sections taken through the meristem of buds of the same developmental stage. For a detailed interpretation of scanned bud contents, see Fig. 5. Abbreviations: ab, axillary bud; bfl, basal foliage leaf (foliage leaf on the first half of the seasonal axis); bi, basal intermediate leaf form (intermediate leaf form on the first half of the seasonal axis); c, cataphyll; cdi, caducous distal intermediate (short-lived; intermediate leaf form on the second half of the seasonal axis); dfl, distal foliage leaf (foliage leaf on the second half of the seasonal axis); di, distal intermediate leaf form (intermediate leaf form on the second half of the seasonal axis).
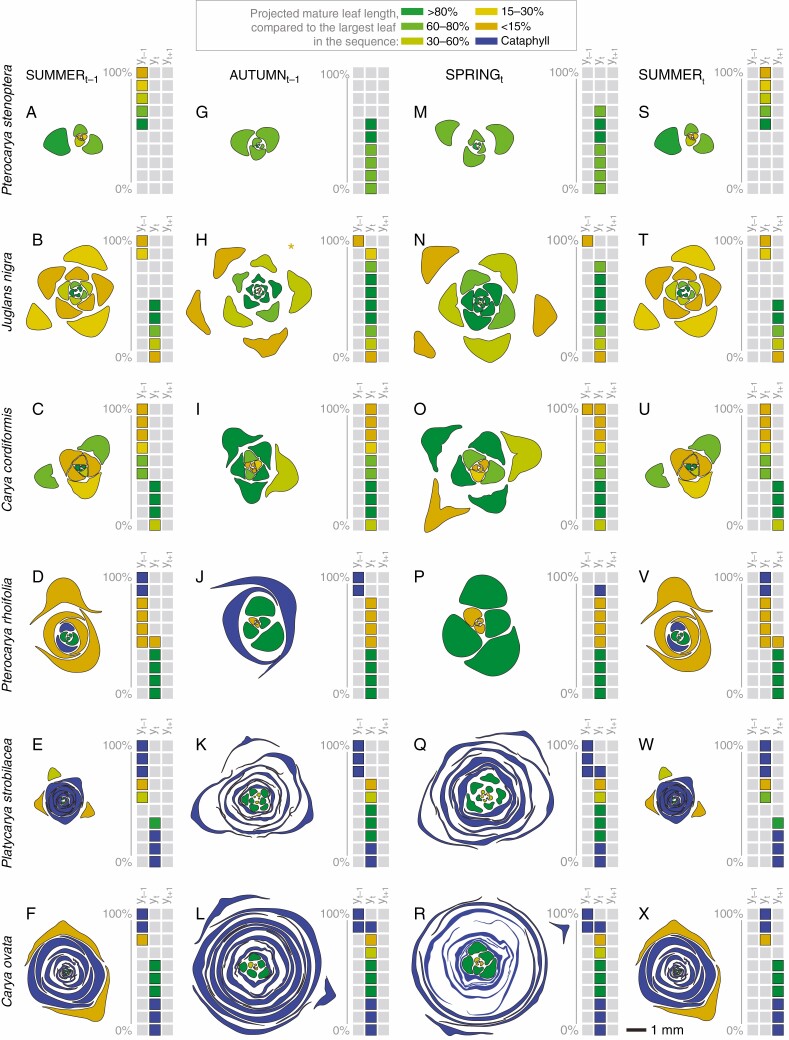
Fig. 5.
Projected fate of terminal bud components at three developmental stages throughout a year. Each row shows the progression of terminal bud organization in a species, from the early to mid-growing season (A–F; summert-1), to the end of the growing season/beginning of dormancy (G–L; autumnt-1), to spring bud swelling, just prior to leaf out (M–R; springt), and back to the early to mid-growing season (S–X; summert). Each bud is redrawn from an optical micro-CT cross-section (see Fig. 4), taken through the meristem, with each bud component artificially coloured. The artificial coloration, together with the schematic of three consecutive seasonal heteroblastic sequences (yt-1, yt and yt + 1), indicates the projected developmental fates of the bud components at maturation, as detailed in Fig. 3. Grey squares represent leaf positions that are not contained in the displayed bud structure. An asterisk marks the position of an abscised leaf. The youngest primordia and embryonic leaves are best viewed digitally, by zooming in on the individual bud structures.
In the three studied cataphyll-bearing species, Pt. rhoifolia, Pl. strobilacea and C. ovata, the most recently initiated primordium or primordia within the swelling, unexpanded spring bud, were identified as cataphylls (Fig. 5P–R). In other words, in these three species, the first component of the new resting bud is initiated before the resting bud in which it is contained has begun to leaf out.
Accordingly, and consistent with the observed single-cohort flush pattern (Fig. 3J–L, P–R), we found that all foliage leaves to be expanded in the coming growing season had been fully formed within the dormant bud. In fact, by the onset of bud dormancy, in these three species, the bud’s meristem had begun the transition to another terminal bud set, by the initiation of intermediate, caducous leaf forms (orange-coloured shapes in Fig. 5J–L), even if the new cataphylls themselves had not necessarily been initiated yet. In Pt. rhoifolia, this transition from foliage leaves to pre-terminal bud components occurred as early as the summer months of the growing season, not long after leaf out of the bud (Fig. 5D, V). This signifies that the entire complement of foliage leaves in Pt. rhoifolia is preformed almost a year before it is destined to become photosynthetically functional and that no further additions to the foliage leaves will be made after this time.
Mature shoots of C. cordiformis, despite lacking cataphylls, share with the three bud-scale-bearing species that the entire growing season’s sequence is predetermined in the dormant terminal buds (Fig. 5I). Neoformed metamers (that are both initiated and expanded during one growing season) are not typically a part of the seasonal heteroblastic leaf sequence in mature trees, but saplings were shown to neoform (Supplementary Data Table S1).
In the spring archetypal naked and heteroblastic naked resting buds of Pt. stenoptera and J. nigra, on the other hand, the transition to a new terminal bud had not yet been made (Fig. 5M, N). These terminal buds may contain most but not necessarily all of the foliage leaves that will have large photosynthetic surfaces during the growing season, particularly in younger trees (Supplementary Data Table S1). At the moment of bud flush in spring, the meristem in the buds of J. nigra and Pt. stenoptera has yet to initiate the primordia that will become the distal, late-season foliage-type leaves, or, if these leaf forms are present in the bud, they are in a primordial stage. This developmental organization results in a more staggered leaf developmental pattern over the growing season (Fig. 3M, N). In Pt. stenoptera, it is not until July that the neoformed leaves that complete the full heteroblastic seasonal sequence first become observable within the bud structure (Fig. 5S). At this time, the foliage leaves for the subsequent growing season have not yet been initiated, unlike in the other five species (Fig. 5T–X).
Macroevolutionary patterns of cataphyll presence
Our phylogenetic reconstruction of Juglandaceae that placed Platycarya as sister to Juglandinae was highly consistent with the most recent phylogenetic analyses of the family (Zhang et al., 2013; Song et al., 2020a; Mu et al., 2020; Zhang et al., 2022; Zhou et al., 2021). Most nodes were well supported, with the exception of the relationships among the North American hickories (Supplementary Data Fig. S3). Stochastic character mapping with three discrete terminal bud character states on this topology estimated an average of 10.25 character state changes, the majority of which consist of changes from an archetypal naked bud to either a heteroblastic naked bud or a cataphyll-bearing bud. The analysis infers that the last common ancestor of all Juglandaceae most likely had an archetypal naked terminal bud type and that this ancestral state was retained in Engelhardioideae and Rhoiptelea. The ancestral node of the temperate subfamily Juglandoideae was reconstructed as having archetypal naked terminal buds with a posterior probability of 0.86 (Fig. 6). Four independent transitions could be identified within Juglandoideae, from an ancestor with (heteroblastic) naked buds to a seasonal heteroblastic sequence with cataphylls: once in Platycarya, once in Juglans, once in Pterocarya and once in Carya (Fig. 6). Using an alternative topology did not influence the reconstruction of character state changes beyond minor differences in the posterior probabilities at certain nodes (Supplementary Data Fig. S4).
Fig. 6.
Ancestral state reconstruction and phylogenetic map of evolutionary transitions between terminal bud types that shows four independent transitions from naked buds to buds with cataphylls. Pie charts at the internal nodes of the time-calibrated phylogenetic tree show the posterior probability of three terminal bud character states as estimated by 1000 simulated stochastic character maps. Colour changes along the branches represent character state changes from a single simulated character history. Archetypal naked buds are shown in white and grey; heteroblastic naked buds are shown in orange and cataphyll-bearing buds (including terminal buds with caducous scales) are shown in blue.
DISCUSSION
Our study builds upon a rich tradition of investigations into resting bud contents and seasonally repeating patterns of leaf development and specialization in temperate trees (among others: Critchfield, 1960, 1970; Kozlowski and Clausen, 1966; Gill, 1971; Steingraeber, 1982; Remphrey, 1989; Sakai, 1990; Nitta and Ohsawa, 1998; Souza, 2000; Sabatier et al., 2003; Gordon et al., 2006; Guédon et al., 2006; Ohsawa et al., 2011; Magnin et al., 2012; Kukk and Sõber, 2015; Spriggs et al., 2018). Few of these studies have described seasonal heteroblastic patterns in a phylogenetic comparative context and addressed evolutionary transitions between species in the same clade. Equally few have considered direct relationships between summer growth pattern and the phenology and early formation of resting buds. In contradistinction, our comparative study starts from the premise that terminal resting buds are, by nature, formed in the context of a year-long shoot developmental sequence. With this work, we aim to show that the evolution of resting bud structures in some temperate trees has not been shaped by selective pressures of the winter environment, such as cold and freezing temperatures, alone.
Bud morphologies and seasonal heteroblastic sequences are inextricably connected beyond the dormant phase
Considering leaf initiation, expansion, and phenological timing of foliar development across a full growing season shows that, in Juglandoideae, resting bud morphologies and seasonal heteroblastic sequences are inextricably connected beyond the dormant phase. The six studied species could be placed on a gradient with, on the one hand, seasonal sequences of leaf forms that showed no or little variation in lamina differentiation in unexpanded leaves. On the other end of the spectrum, the seasonal sequence includes both foliage leaves with a highly differentiated lamina as well as leaf forms in which the lamina is completely lost (cataphylls). Within these six species, seasonal heteroblastic sequences with a higher degree of lamina reduction tend to also have more determinate annual shoot ontogenies. Species with cataphylls are in fact so determinate that surveyed unflushed terminal buds in spring included the primordial cataphylls of the next terminal bud, forming a structure reminiscent of a matryoshka doll set (Fig. 7A). Necessarily, such a bud structure results in a single preformed cohort of foliage leaves that expands, matures and senesces in synchrony, without any foliage leaves added to the sequence during the growing season (second flushes, or Lammas growth, aside; Fig. 7A). These findings confirm that Foster’s observations of early cataphyll formation, prior to leaf out, in Carya texana (Foster, 1931), are more broadly applicable within the wider family context.
Fig. 7.
Graphical representation of the development of (A) a determinate heteroblastic sequence with cataphylls and (B) an indeterminate heteroblastic sequence without cataphylls in Juglandoideae. (A) In Juglandoideae members with cataphyll-bearing terminal buds, the unflushed terminal bud in spring includes all foliage leaves to be expanded over the coming growing season and one or more primordial cataphylls of the subsequent terminal bud (shown in blue). As a result, a single preformed cohort of foliage leaves is expanded upon leaf out that matures and senesces in synchrony. The new terminal resting bud starts forming immediately after leaf out. (B) Naked buds may give rise to vegetative shoots with neoformation, in which foliage leaves additional to those contained within the resting bud are initiated as well as expanded during the growing season. Here, the earliest foliage leaves are often abscised before the end of the growing season and the new resting bud is not formed until neoformation is completed.
While such a highly determinate developmental pattern is known to occur in temperate, broadleaved species from other families (Gill, 1971; Remphrey, 1989), the nature and timing of bud set in cataphyll-bearing members of Juglandoideae contrasts starkly with that of some other taxa with cataphyll-bearing terminal buds, including the much-studied genus Populus. In Populus, the transition to bud set is dependent on photoperiod and is only initiated in response to a critical daylength fairly late in the growing season (Rohde and Boerjan, 2001; Rohde et al., 2011a). Moreover, neoformation of distinctly shaped foliage leaves is known to occur (Critchfield, 1960). Thus, the presence of cataphylls within a seasonal heteroblastic sequence need not be a constraint that prevents neoformation along shoots of temperate trees per se. Rather, our results indicate that there are likely one or several shared shoot developmental traits in Juglandoideae, not present in other clades, that cause cataphyll deployment in terminal buds to be linked to determinate growth [such as perhaps large foliage leaf size (Schoonderwoerd and Friedman, 2021)].
Species with a less pronounced lamina reduction along the seasonal shoot axis in Juglandoideae, such as Pt. stenoptera and J. nigra, tend to have a more indeterminate growth pattern with neoformed leaf forms, particularly in young trees (Fig. 7B). Along mature shoots of the 2019 growing season, the majority of these neoformed forms seemed to consist of the small, short-lived (caducous) transitional leaf forms that preceded terminal bud set. Importantly, however, both neoformed foliage leaf initiation and the timing of bud set in these species could plausibly respond to environmental conditions of the current growing season. In highly determinate species, on the other hand, changes in growing season conditions could only result in potential modifications to the initiation of developmental units that become functional in the subsequent year (Remphrey, 1989).
While saplings in all of the six study species showed a more indeterminate growth pattern compared with their mature counterparts, as expected from patterns observed in other temperate trees (Cooke et al., 2012), the interspecific order of growth patterns was mostly preserved. Pterocarya stenoptera and J. nigra are the two most indeterminate species at both the sapling and mature stage and C. ovata the most determinate (Supplementary Data Fig. S2 and Table S1). This reveals that annual growing patterns are not infinitely flexible within a tree’s lifetime. We found that the heteroblastic naked bud structure of C. cordiformis, while resulting in determinate growth at maturity, allowed significant neoformation at the sapling stage and was considerably more indeterminate than that of C. ovata, its closest relative in this study with cataphyll-bearing terminal buds. Only in Pt. rhoifolia was the shift to a more determinate growing pattern with tree age accompanied by a change in terminal bud structure from archetypal naked to a bud with caducous cataphylls.
As the shifts in growing patterns from sapling to mature tree indicate, an indeterminate growing pattern with more neoformation is likely an opportunistic strategy that allows continued and rapid growth under optimal growing conditions. Apart from in saplings, which often experience strong light competition, this strategy could be advantageous in early successional species (Marks, 1975) or species that occur in climates in which late growing-season conditions are as favourable as, or more favourable than, the spring climate.
Cataphylls: winter protective structures, summer protective structures or developmental constraints?
The insight that resting bud organization may be tied to growing-season growth pattern, and ultimately ecological strategy, reframes the interpretation of the estimated four independent shifts in Juglandoideae from an ancestor with terminal naked buds to a lineage with cataphyll-bearing terminal resting buds. The three cataphyll-bearing taxa in this study with a determinate growth pattern (Pt. rhoifolia, Pl. strobilacea and C. ovata) represent three of the four estimated independent character-state changes. Consistently, Juglans regia, part of the fourth group with true cataphylls, is known to have entirely preformed shoots (Sabatier and Barthélémy, 2001). It should be noted, however, that, as in Pt. rhoifolia, saplings of J. regia have a terminal bud structure that differs from that of mature trees. In the case of the seasonal leaf sequence of J. regia saplings, a lamina reduction occurs over several nodes, but it never results in the complete lamina loss that would produce a true cataphyll (Sabatier et al., 2003), like in the heteroblastic naked buds discussed in this paper.
Combined evidence from the fossil record and phylogenetic reconstruction suggests a boreotropical origin for Juglandaceae (Manchester, 1987; Zhang et al., 2013; Song et al., 2020a; Zhang et al., 2022). Starting in the Oligocene, which marked the beginning of a period characterized by global cooling and the expansion of temperate biomes, the latitudinal range of the subfamily Engelhardioideae shifted towards the equator, while temperature preferences were maintained. Juglandoideae, on the other hand, appear to have moved into climates with colder winters and temperate seasonality between 23 Ma and the present (Zhang et al., 2022). Together, the independent character state changes and our current understanding of Juglandoideae climatic history suggest that the deployment of cataphylls in terminal buds is adaptive and possibly evolved in response to the transition to temperate seasonal climates. This scenario accommodates two interpretations. Firstly, a need for cataphyll protection during winter could have changed the structure of the terminal bud initially, which secondarily introduced a constraint on growing-season growth pattern and led to the loss of neoformation. Alternatively, shorter growing seasons could have selected for a more determinate growing pattern, which as a result allowed for cataphylls to be inserted into the seasonal heteroblastic sequence.
The most serious objection to the cataphylls-first hypothesis is the seasonal heteroblastic sequence of Pt. rhoifolia. In this species, which has a determinate growing pattern that includes caducous cataphylls, the foliage leaves eventually overwinter without enveloping cataphylls. As a result of the determinate growth pattern and cataphyll abscission, as many as 75 % of the photosynthetic leaves on a shoot of Pt. rhoifolia overwinter ‘unprotected’, directly exposed to the atmosphere (Fig. 5P, dark green shapes). This makes the caducous bud scales rather a puzzle from a traditional winter-adaptive perspective. The evolutionary transition, estimated by our analysis, from archetypal naked bud directly to bud with caducous cataphylls (Fig. 6) appears to refute that Pt. rhoifolia represents an evolutionary transitional stage in which cataphylls are in the process of being lost. A more plausible explanation appears to be that the true function of the caducous cataphylls is to protect developing embryonic foliage leaves from environmental pressures during the growing season, after which they are no longer needed. Similarly, when occasionally a single shoot of a J. regia sapling, which has a heteroblastic naked resting bud type during winter, goes through two bud flushes in a single season, the outermost leaf forms of the summer bud are (short-lived) true cataphylls (Sabatier et al., 1998, 2003). Both patterns seem to suggest that the insertion of cataphylls in the buds of these taxa did not evolve in response to cold winter climates.
The significance of caducous leaf forms
While perhaps most apparent in the resting buds of mature Pt. macroptera and Pt. rhoifolia, caducous leaf forms do not just occur in these taxa according to our data. In fact, a sequence of caducous, small leaves with limited photosynthetic surfaces that is present on the distal end of the axis during the mid- to late growing season appears to be conserved in all species surveyed here. The fact that the caducous leaf sequence is shared among all six species indicates that the abscissions are programmed events (independent of autumn foliage leaf abscission), rather than an opportunistic reaction to environmental circumstances. Furthermore, the caducous leaf forms are most likely part of a common conserved architecture present in the last common ancestor of Juglandoideae. Across Juglandoideae, this pattern of caducous, distal leaf forms offers a unifying explanation for the caducous cataphylls in Pt. rhoifolia, as well as the dead unexpanded leaves attached to some resting buds in J. nigra and C. cordiformis (Supplementary Data Fig. S1). With our non-destructive survey we showed that these attached leaves abscise prior to the end of the growing season and not as a result of winter conditions.
While this sequence of early-abscising, distal leaves unites the diversity of growing patterns in Juglandoideae, it sets the group apart from other temperate trees, such as Populus, in which the transition from foliage leaf to bud scale is described as immediate, with no transitional nodes (Goffinet and Larson, 1981; Rohde and Boerjan, 2001; Rohde et al., 2011b). Why such a clade-specific phyletic constraint might exist in Juglandoideae is a challenging question. One possible explanation is that nodes with caducous leaves are associated with the initiation of axillary buds containing male inflorescences, as observed in Pterocarya and Juglans. It could be hypothesized that the nodes themselves therefore are more important than the leaf forms associated with them, which have become susceptible to reduction over evolutionary time. However, in Carya (Grauke et al., 2016) and Platycarya (Fukuhara and Tokumaru, 2014), male inflorescences occur elsewhere in the shoot architecture, so this alone cannot explain the subfamily-wide caducous-sequence pattern. Alternatively, in Juglandoideae, the developmental programme at the terminal meristem might be insufficiently flexible to transition directly from initiating a foliage leaf to initiating a cataphyll and, instead, this transition happens over multiple, non-functional nodes. A counterargument to this hypothesis can be found in the species with archetypal naked buds, in which leaf forms do not undergo an observable (drastic) lamina reduction along the seasonal axis but short-lived leaf forms are still observed.
Another, more functional, explanation presents itself if we consider the morphology of terminal buds in midsummer. At these early developmental stages, the caducous leaf forms (shown in orange in Fig. 5T–X) occupy vulnerable, exposed positions, while the new resting bud is being formed. Consequently, it is possible to interpret these forms as short-term protective structures for the very earliest establishment of the terminal buds. Because such summertime protection would obviously not be associated with cold temperatures or freezing damage, this interpretation would also strengthen the hypothesis that (caducous) cataphylls, like reduced distal leaves, are protective prior to dormancy, during the early phases of bud set. Furthermore, such a hypothesis could provide a biological explanation for the previously detected association between the distribution of naked-budded temperate, woody angiosperm species and summer precipitation patterns (Schoonderwoerd and Friedman, 2021).
The evolutionary retention of naked buds in temperate environments
Perhaps, rather than asking how and why cataphylls became incorporated into terminal buds multiple times in Juglandoideae, it is more informative to consider why, according to the estimated character-state transitions, naked terminal buds were retained in many lineages, despite a presumed cooling of the climate relative to what ancestral taxa experienced. Since many broadleaved tree taxa produce some form of modified leaf without a petiole and a lamina at a location in their (reproductive) architecture, even if their buds lack cataphylls, the reduced cataphyll structure itself likely did not evolve de novo within Juglandoideae. If an evolutionary barrier exists to the incorporation of cataphylls within terminal buds, it is more plausibly a barrier to the heterotopic expression of a reduced, scale-like form within a terminal bud. In species in which neoformation provides an important advantage, this might plausibly prevent cataphylls from being incorporated into a terminal bud. After all, in Juglandoideae, the formation of a terminal bud with cataphylls requires a transition of the shoot apical meristem very early in the growing season to the initiation of several transitional leaf forms, subsequently followed by the initiation of cataphylls and embryonic foliage leaves. Thus, a shoot is prevented from initiating and expanding additional foliage leaves while the growing season is under way. In other words, in Juglandoideae, a trade-off may exist between the ability to utilize the full growing season for foliage leaf expansion – and actively respond to environmental cues of the current growing season – on the one hand, and the evolution of highly specialized shoot tip protection on the other. This view is supported by the axillary bud structures of taxa such as J. nigra and C. cordiformis, which contain cataphylls while the terminal buds of these taxa do not. The association detected in this study, between naked terminal buds and an indeterminate growth pattern, provides an evolutionary explanation for the retention of terminal naked buds specifically, which (unlike axillary buds) develop from (as well as give rise to) a seasonally cyclical leaf sequence.
It should be noted, furthermore, that the shoot apical meristem and earliest leaf primordia in the naked buds of Juglandoideae are never unprotected, even if specialized cataphylls are not present. The highly organized terminal bud structures observed in this study are indicative of protection of the internal, developing structures in summer as well as winter (Figs 4 and 5). In addition, in the naked buds of Pt. stenoptera, J. nigra and C. cordiformis, the leaves that overwinter on the outside of the bud, while indeed capable of expanding a photosynthetic lamina, provide but a small part of the total light-capture surface that is expanded by the heteroblastic sequence over the growing season (Figs 2, 3G–I and 5). This means that even if the position of the outermost leaf could be considered ‘risky’, any actual physical damage to the exterior of the resting bud is probably of little consequence for the total photosynthetic area expanded along a shoot over an entire season. Consequently, in each of the cataphyll-less species studied here, the protective function of these outermost leaves could be interpreted as equal to or greater than their ultimate photosynthetic function in the context of the entire heteroblastic sequence, even though they are not specialized cataphylls.
Together, a phylogenetically conserved developmental constraint that links cataphyll-bearing terminal buds and determinate growth plus evidence that cataphylls are not required for bud survival in winter imply that naked buds in Juglandoideae should not be considered less optimal for surviving in temperate climates. While winter is the season during which cataphylls in many taxa of temperate, woody species are most noticeable to human eyes, studying heteroblastic sequences over the growing season shows that the evolution of cataphylls is not merely a winter’s tale. In addition to winter protection, in certain clades, shoot tip protection in the spring, summer and autumn as well as the developmental flexibility to neoform and respond directly to growing season conditions may need to be cast in leading roles as determining factors of resting bud structure and evolution. Not only do these insights help to decipher the existence of species with naked buds in temperate zones, they also pose interesting new questions regarding the connection between the seasonal shoot developmental programme in temperate, woody species and their functional ecology.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: dormant terminal buds of C. cordiformis and J. nigra showing bud leaves that have abscised at the petiole, but are still attached to the terminal bud. Figure S2: quantitative characterization of the seasonal heteroblastic sequences along leader shoots of saplings of six Juglandoideae species. Figure S3: majority-rule consensus tree based on Bayesian analysis of four nuclear and six plastid loci. Figure S4: ancestral state reconstruction of terminal bud types in Juglandaceae using an alternative phylogenetic topology. Table S1: median number of recorded nodes per axis. Table S2: GenBank accession numbers of gene sequences for phylogenetic reconstruction. Table S3: character matrix for ancestral state reconstruction.
ACKNOWLEDGEMENTS
We thank the Arnold Arboretum of Harvard University for access to the living collections and invaluable advice and plant care during the establishment of a sapling common garden, as well as Kaitlyn Degroot, Amelia Keyser-Gibson, Nathan Oalican and Daniel Mindich for their enthusiastic support in the timely collection of field measurements. Recommendations for phylogenetic comparative methods were generously provided by Jacob Suissa and we thank Pamela Diggle, Andrew Knoll and two reviewers for their helpful comments on the manuscript.
FUNDING
The micro-CT scanning for this work was supported by the Dean’s Competitive Fund for Promising Scholarship (Harvard University Faculty of Arts and Sciences) and performed at the Harvard Center for Nanoscale Systems.
LITERATURE CITED
- Alla AQ, Camarero JJ, Montserrat-Martí G. 2013. Seasonal and inter-annual variability of bud development as related to climate in two coexisting Mediterranean Quercus species. Annals of Botany 111: 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aradhya MK, Potter D, Gao F, Simon CJ. 2007. Molecular phylogeny of Juglans (Juglandaceae): a biogeographic perspective. Tree Genetics & Genomes 3: 363–378. [Google Scholar]
- Beil I, Kreyling J, Meyer C, Lemcke N, Malyshev AV. 2021. Late to bed, late to rise—warmer autumn temperatures delay spring phenology by delaying dormancy. Global Change Biology 27: 5806–5817. [DOI] [PubMed] [Google Scholar]
- Cooke JEK, Eriksson ME, Junttila O. 2012. The dynamic nature of bud dormancy in trees: environmental control and molecular mechanisms. Plant, Cell & Environment 35: 1707–1728. [DOI] [PubMed] [Google Scholar]
- Critchfield WB. 1960. Leaf dimorphism in Populus trichocarpa. American Journal of Botany 47: 699–711. [Google Scholar]
- Critchfield WB. 1970. Shoot growth and heterophylly in Ginkgo biloba. Botanical Gazette 131: 150–162. [Google Scholar]
- eFloras. 2006. Flora of China. http://www.efloras.org/flora_page.aspx?flora_id=2 (14 January 2021, date last accessed).
- eFloras. 2021. Flora of North America. http://www.efloras.org/flora_page.aspx?flora_id=1 (14 January 2021, date last accessed).
- Feist A. 1887. Die Schutzeinrichtungen der Laubknospen dicotyler Laubbäume während ihrer Entwickelung. Nova Acta Academiae Caesareae Leopoldino-Carolinae Germanicae Naturae Curiosorum 51: 303–344. [Google Scholar]
- Fick SE, Hijmans RJ. 2017. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. International Journal of Climatology 37: 4302–4315. [Google Scholar]
- Foster AS. 1928. Salient features of the problem of bud-scale morphology. Biological Reviews 3: 123–164. [Google Scholar]
- Foster AS. 1929. Investigations on the morphology and comparative history of development of foliar organs I. The foliage leaves and cataphyllary structures in the horsechestnut (Aesculus hippocastanum L.), cont’d. American Journal of Botany 16: 475–501. [Google Scholar]
- Foster AS. 1931. Investigations on the morphology and comparative history of development of foliar organs III. Cataphyll and foliage-leaf ontogeny in the black hickory (Carya buckleyi var. arkansana). American Journal of Botany 18: 864–887. [Google Scholar]
- Fukuhara T, Tokumaru S. 2014. Inflorescence dimorphism, heterodichogamy and thrips pollination in Platycarya strobilacea (Juglandaceae). Annals of Botany 113: 467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat AS, Primack RB, Wagner DL. 2015. Autumn, the neglected season in climate change research. Trends in Ecology & Evolution 30: 169–176. [DOI] [PubMed] [Google Scholar]
- GBIF.org. 2021. GBIF Occurrence Download. 10.15468/dl.kecqp3 (22 August 2021, date last accessed). [DOI]
- Gill AM. 1971. The formation, growth and fate of buds of Fraxinus america L. in central Mass. Harvard Forest Paper 20: 1–16. [Google Scholar]
- Goffinet MC, Larson PR. 1981. Structural changes in Populus deltoides terminal buds and in the vascular transition zone of the stems during dormancy induction. American Journal of Botany 68: 118–129. [Google Scholar]
- Gordon D, Damiano C, DeJong TM. 2006. Preformation in vegetative buds of Prunus persica: factors influencing number of leaf primordia in overwintering buds. Tree Physiology 26: 537–544. [DOI] [PubMed] [Google Scholar]
- Grauke LJ, Wood BW, Harris MK. 2016. Crop vulnerability: Carya. HortScience 51: 653–663. [Google Scholar]
- Grimm WC. 2002. Illustrated book of trees: the comprehensive field guide to more than 250 trees of eastern North America. Mechanicsburg, PA: Stackpole Books. [Google Scholar]
- Guédon Y, Puntieri JG, Sabatier S, Barthélémy D. 2006. Relative extents of preformation and neoformation in tree shoots: analysis by a deconvolution method. Annals of Botany 98: 835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CS. 1999. An essay on juvenility, phase change, and heteroblasty in seed plants. International Journal of Plant Sciences 160: S105–S111. [DOI] [PubMed] [Google Scholar]
- Jones CS. 2021. ‘… we really don’t know [buds] at all …’. New Phytologist 232: 461–463. [DOI] [PubMed] [Google Scholar]
- Jones RL, Wofford BE. 2013. Woody plants of Kentucky and Tennessee: the complete winter guide to their identification and use. Lexington: University Press of Kentucky. [Google Scholar]
- Kaplan DR. 1984. The concept of homology and its central role in the elucidation of plant systematic relationships. In: Duncan T, Stuessy TF, eds. Cladistics: perspectives on the reconstruction of evolutionary history. New York: Columbia University Press, 51–70. [Google Scholar]
- Kozlowski TT, Clausen JJ. 1966. Shoot growth characteristics of heterophyllous woody plants. Canadian Journal of Botany 44: 827–843. [Google Scholar]
- Kukk M, Sõber A. 2015. Bud development and shoot morphology in relation to crown location. AoB Plants 7: plv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuprian E, Munkler C, Resnyak A, et al. 2017. Complex bud architecture and cell-specific chemical patterns enable supercooling of Picea abies bud primordia. Plant, Cell & Environment 40: 3101–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance R. 2004. Woody plants of the southeastern United States: a winter guide. Athens, GA: University of Georgia Press. [Google Scholar]
- Lubbock J. 1899. On buds and stipules. London: Kegan Paul, Trench and Trübner. [Google Scholar]
- Magnin A, Grosfeld J, Barthélémy D, Puntieri J. 2012. Bud and shoot structure may relate to the distribution area of South American Proteaceae tree species. Flora 207: 599–606. [Google Scholar]
- Maitner BS, Boyle B, Casler N, et al. 2018. The BIEN R package: a tool to access the Botanical Information and Ecology Network (BIEN) database. Methods in Ecology and Evolution 9: 373–379. [Google Scholar]
- Manchester S. 1987. Fossil history of the Juglandaceae. Monographs in Systematic Botany from the Missouri Botanic Gardens 21: 1–137. [Google Scholar]
- Manos PS, Stone DE. 2001. Evolution, phylogeny, and systematics of the Juglandaceae. Annals of the Missouri Botanical Garden 88: 231–269. [Google Scholar]
- Manos PS, Soltis PS, Soltis DE, et al. 2007. Phylogeny of extant and fossil Juglandaceae inferred from the integration of molecular and morphological data sets. Systematic Biology 56: 412–430. [DOI] [PubMed] [Google Scholar]
- Marks PL. 1975. On the relation between extension growth and successional status of deciduous trees of the northeastern United States. Bulletin of the Torrey Botanical Club 102: 172–177. [Google Scholar]
- Mu X-Y, Tong L, Sun M, et al. 2020. Phylogeny and divergence time estimation of the walnut family (Juglandaceae) based on nuclear RAD-Seq and chloroplast genome data. Molecular Phylogenetics and Evolution 147: 106802. [DOI] [PubMed] [Google Scholar]
- Nitta I, Ohsawa M. 1998. Bud structure and shoot architecture of canopy and understorey evergreen broad-leaved trees at their northern limit in East Asia. Annals of Botany 81: 115–129. [Google Scholar]
- Ohsawa M, Shumiya T, Nitta I, Wildpret W, del Acro M. 2011. Comparative structure, pattern, and tree traits of laurel cloud forests in Anaga, northern Tenerife (Canary Islands) and in lauro-fagaceous forests of central Japan. In: Tropical montane cloud forests: science for conservation and management. Cambridge: Cambridge University Press, 147–155. [Google Scholar]
- Perry TO, Simons RW. 1967. Growth of bud scales and leaves during the winter. Forest Science 13: 400–401. [Google Scholar]
- Poland J. 2018. The field key to winter twigs: a guide to native and planted deciduous trees, shrubs and woody climbers xylophytes) found in the British Isles. Southampton: Privately published. [Google Scholar]
- Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. 2018. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67: 901–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remphrey WR. 1989. Shoot ontogeny in Fraxinus pennsylvanica (green ash). I. Seasonal cycle of terminal meristem activity. Canadian Journal of Botany 67: 1624–1632. [Google Scholar]
- Revell LJ. 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Rohde A, Boerjan W. 2001. Insights into bud development and dormancy in poplar. In: Huttunen S, Heikkilä H, Bucher J, Sundberg B, Jarvis P, Matyssek R, eds. Trends in European forest tree physiology research. Dordrecht: Springer Netherlands, 33–52. [Google Scholar]
- Rohde A, Bastien C, Boerjan W, Thomas S. 2011a. Temperature signals contribute to the timing of photoperiodic growth cessation and bud set in poplar. Tree Physiology 31: 472–482. [DOI] [PubMed] [Google Scholar]
- Rohde A, Storme V, Jorge V, et al. 2011. b. Bud set in poplar – genetic dissection of a complex trait in natural and hybrid populations. New Phytologist 189: 106–121. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier S, Barthélémy D. 2001. Bud structure in relation to shoot morphology and position on the vegetative annual shoots of Juglans regia L. (Juglandaceae). Annals of Botany 87: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier S, Barthélémy D, Ducousso I, Germain É. 1998. Modalités d’allongement et morphologie des pousses annuelles chez le noyer commun, Juglans regia L. ‘Lara’ (Juglandaceae). Canadian Journal of Botany 76: 1253–1264. [Google Scholar]
- Sabatier S, Barthélémy D, Ducousso I. 2003. Periods of organogenesis in mono- and bicyclic annual shoots of Juglans regia L. (Juglandaceae). Annals of Botany 92: 231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S. 1990. The relationship between bud scale morphology and indeterminate and determinate growth patterns in Acer (Aceraceae). Canadian Journal of Botany 68: 144–148. [Google Scholar]
- Sargent CS. 1905. Manual of the trees of North America. Boston, MA: Houghton, Mifflin and Company. [Google Scholar]
- Sattler R. 1984. Homology – a continuing challenge. Systematic Botany 9: 382–394. [Google Scholar]
- Schoonderwoerd KM, Friedman WE. 2021. Naked resting bud morphologies and their taxonomic and geographic distributions in temperate, woody floras. New Phytologist 232: 523–536. [DOI] [PubMed] [Google Scholar]
- Schulz B. 2014. Gehölzbestimmung im Winter: mit Knospen und Zweigen. Stuttgart: Ulmer. [Google Scholar]
- Song Y-G, Fragnière Y, Meng H-H, et al. 2020. a. Global biogeographic synthesis and priority conservation regions of the relict tree family Juglandaceae. Journal of Biogeography 47: 643–657. [Google Scholar]
- Song Y-G, Li Y, Meng H-H, et al. 2020. b. Phylogeny, taxonomy, and biogeography of Pterocarya (Juglandaceae). Plants 9: 1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza M. 2000. Bud content and its relation to shoot size and structure in Nothofagus pumilio (Poepp. et Endl.) Krasser (Nothofagaceae). Annals of Botany 85: 547–555. [Google Scholar]
- Spriggs EL, Schmerler SB, Edwards EJ, Donoghue MJ. 2018. Leaf form evolution in Viburnum parallels variation within individual plants. American Naturalist 191: 235–249. [DOI] [PubMed] [Google Scholar]
- Stanford AM, Harden R, Parks CR. 2000. Phylogeny and biogeography of Juglans (Juglandaceae) based on matK and ITS sequence data. American Journal of Botany 87: 872–882. [PubMed] [Google Scholar]
- Steingraeber DA. 1982. Heterophylly and neoformation of leaves in sugar maple (Acer saccharum). American Journal of Botany 69: 1277–1282. [Google Scholar]
- Stone DE. 1993. Juglandaceae. In: Kubitzki K, Rohwer JG, Bittrich V, eds. The families and genera of vascular plants, Vol. II. Berlin: Springer, 348–359. [Google Scholar]
- Stone DE, Oh S-H, Tripp EA, Ríos G LE, Manos PS. 2009. Natural history, distribution, phylogenetic relationships, and conservation of Central American black walnuts (Juglans sect. Rhysocaryon). Journal of the Torrey Botanical Society 136: 1–25. [Google Scholar]
- Swanson RE. 1994. A field guide to the trees and shrubs of the southern Appalachians. Baltimore: Johns Hopkins University Press. [Google Scholar]
- Trelease W. 1918. Winter botany: a companion volume to the author’s plant materials of decorative gardening. Urbana: privately published. [Google Scholar]
- Villouta C, Workmaster BA, Bolivar-Medina J, Sinclair S, Atucha A. 2020. Freezing stress survival mechanisms in Vaccinium macrocarpon Ait. terminal buds. Tree Physiology 40: 841–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitasse Y, Baumgarten F, Zohner CM, et al. 2021. Impact of microclimatic conditions and resource availability on spring and autumn phenology of temperate tree seedlings. New Phytologist 232: 537–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle P. 1991. Vegetation of New Zealand. Cambridge: Cambridge University Press. [Google Scholar]
- Wiegand KM. 1906. Some studies regarding the biology of buds and twigs in winter. Botanical Gazette 41: 373–424. [Google Scholar]
- Zhang J-B, Li R-Q, Xiang X-G, et al. 2013. Integrated fossil and molecular data reveal the biogeographic diversification of the eastern Asian-eastern North American disjunct hickory genus (Carya Nutt.). PLoS ONE 8: e70449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Ree RH, Salamin N, Xing Y, Silvestro D. 2022. Fossil-informed models reveal a boreotropical origin and divergent evolutionary trajectories in the walnut family (Juglandaceae). Systematic Biology 71: 242–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Hu Y, Ebrahimi A, et al. 2021. Whole genome based insights into the phylogeny and evolution of the Juglandaceae. BMC Ecology and Evolution 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.