Abstract
Lifespan is the most straightforward surrogate measure of aging, as it is easily quantifiable. A common approach to measure Caenorhabditis elegans lifespan is to follow a population of animals over time and score viability based on movement. We previously developed an alternative approach, called the Replica Set method, to quantitatively measure lifespan of C. elegans in a high-throughput manner. The replica set method allows a single investigator to screen more treatments or conditions in the same amount of time without loss of data quality. The method requires common equipment found in most laboratories working with C. elegans and is thus simple to adopt. Unlike traditional approaches, the Replica Set method centers on assaying independent samples of a population at each observation point, rather than a single sample over time as with “traditional” longitudinal methods. The protocols provided here describe both the traditional experimental approach and the Replica Set method, as well as practical considerations for each.
Keywords: Aging, Lifespan, Survival analysis, C. elegans, High-throughput, Statistical analysis of quantitative phenotypes, Kaplan–Meier, Logistic regression
1. Introduction
Aging research was transformed by two major discoveries in Caenorhabditis elegans. The first was the discovery that single genetic changes could drastically alter C. eleganslifespan [1, 2]. However, lifespan is not a phenotype that is amenable to analysis by classic forward genetic approaches. Thus, the technological breakthrough of feeding-based RNAi, and subsequent development of comprehensive RNAi libraries, heralded a period of genetic discovery in aging research; to date over 900 genes are known to alter C. elegans lifespan after genetic perturbation (WormBase release WS270). Briefly, gene knockdown is achieved through the targeted degradation of endogenous mRNA via complementary dsRNA production within E. coli [3].
The most common method for assessing C. elegans lifespan is to follow relatively small populations of individual animals over time, scoring viability based on age-associated decrease in coordinated movement [4, 5]. This method has been widely used, as it provides straightforward, direct measurements of median and maximum lifespan. In this approach—here referred to as the traditional longitudinal method (TLM)—the same population of animals are scored at each observation, and the resulting data is analyzed with standard survival analysis tools such as the Kaplan–Meier estimator and the log-rank test [6, 7]. While this approach can scale to include more conditions by adding additional plates, throughput is limited. Furthermore, as the animals age they demonstrate progressively lower rates of spontaneous movement, necessitating mechanical stimulation with a light touch in order to accurately ascertain viability. In addition to requiring more time on the part of the investigator, manipulations involving touch can damage increasingly frail animals, which may lead to accidental mis-scoring or death of the animal.
The replica set method (RSM) is a high-throughput approach for measuring C. elegans lifespan [8-10]. Briefly, a population of isogenic, age-synchronized animals is divided into subpopulations (or “replicas”). Each replica covers a time point in the planned experiment. At each time point, one replica is scored, after which that replicate is discarded. Thus, over the expected lifespan for a given genetic background, a series of isogenic but independent populations are sampled and each individual animal is only ever scored once (see Note 1). Thus, there is no repeated handling of animals and minimal exposure to the outside environment, which minimizes environmental error. Finally, viability observed at one time point is completely independent of other observations, which minimizes the effect of experimental scoring error and increases throughput by an order of magnitude. This has allowed us to quantitate changes in lifespan over treatment with hundreds of RNAi clones simultaneously [8, 9].
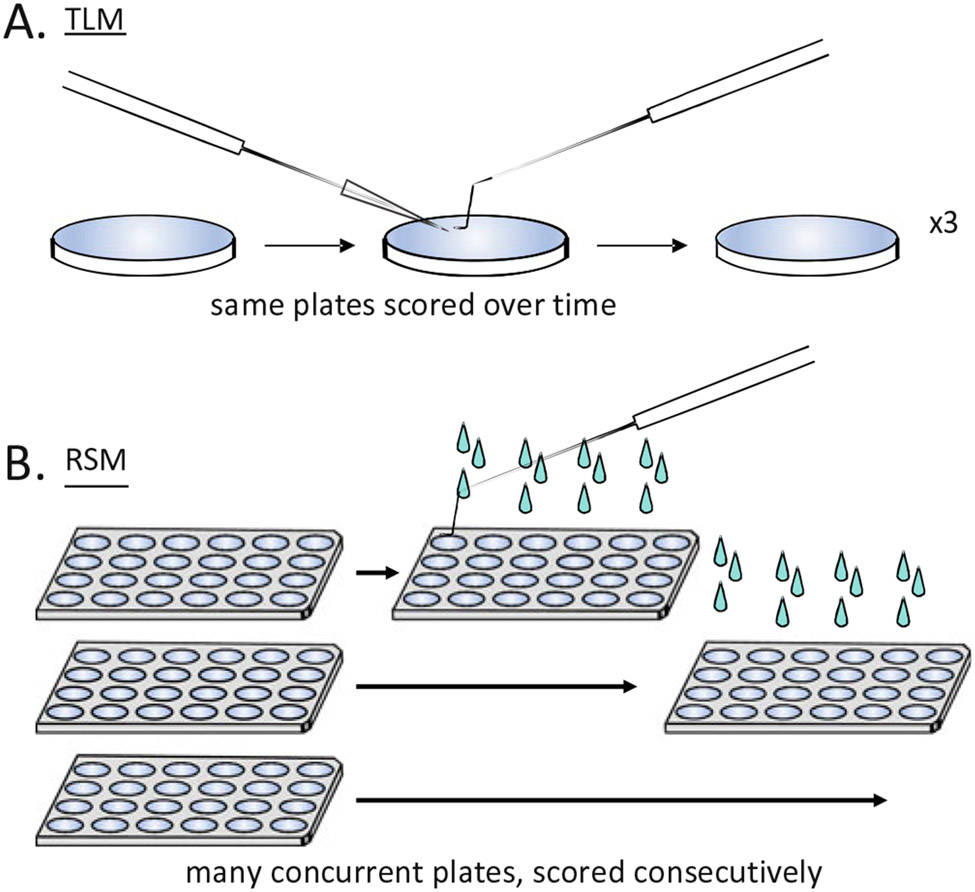
Here, we describe methods for both the TLM (see Subheading 3.5) and RSM (see Subheading 3.6) approaches to assaying lifespan in C. elegans. Figure 1 shows a schematic overview of some of the key differences in setup and scoring between TLM (1a) and RSM (1b).
Fig. 1. Traditional Longitudinal (TLM) and Replica Set (RSM) methods of assaying lifespan in C. elegans.
TLM involves scoring the same animals at multiple observation points during the course of an experiment, while with RSM an independent subpopulation of animals is scored at each time point. RSM improves throughput, enabling many more conditions to be tested by a single investigator in one experimental trial. (a) In scoring TLM plates, lethargic animals are touched to check vital status, dead animals are picked off, and cases such as low food or fungal growth on plates may require careful transfer of all the animals to new plates. (b) For RSM, each plate is only observed once—a plate is scored by flooding each well with M9 buffer, which stimulates movement of lethargic animals and frees them from sticky bacteria. Animals that do not move on their own are touched with a pick to determine their status. The plate is discarded after scoring at each time point (i.e., a replica is all of the conditions tested at one time point). The minimum number of replicate sets is equal to the total number of time points
1.1. General Considerations
There are six major considerations when setting up a lifespan experiment using either approach. Attention to these points will improve rigor and reproducibility both within and across experiments.
Strain. Genetic background can substantially impact lifespan. Because self-fertilizing hermaphrodites tend to drive alleles to homozygosity, it is critical that strains obtained from different laboratories, knockout consortiums, or the Caenorhabditis Genetics Center (CGC, https://cgc.umn.edu/) be extensively backcrossed (6–10×) to internal wild-type control strains used within one’s laboratory. The ideal wild-type (N2) strain to use in C. elegans lifespan studies varies, and not all N2 stocks have the same lifespan [11] (see Note 2).
Population genetics. Despite the tendency of self-fertilizing hermaphrodites to drive alleles to homozygosity, C. elegans maintained under laboratory conditions are subject to the same population genetic forces as all living creatures (e.g., selective pressure). This is not a theoretical concern for C. elegans lifespan analysis, but can be minimized by keeping reference stock strains for all genotypes at −80 °C or in liquid nitrogen, and thawing a new stock every 3–6 months. An additional control we recommend every laboratory adopt to improve reproducibility and rigor is to freeze back leftover animals used in every lifespan experiment as a resource, which is stored at −80 °C or in liquid nitrogen (see Note 3).
Temperature. Generally, the lower the temperature, the longer C. elegans will live (between a range of approximately 16–25 °C) [4]. Notable exceptions are strains with temperature sensitive alleles, such as daf-2(e1370), which are maintained at the 16 °C permissive temperature during development but shifted to higher temperatures for genetic analysis. We have previously shown that animals at 25 °C are under mild heat stress [12] and have found genetic perturbations with differential effects on longevity at different temperatures (unpublished observations).
Amount of food and food choice. Animals that exhaust their food supply become dietarily restricted and long lived. Plates where the food supply has been exhausted can be distinguished by the loss of a bacterial lawn and burrowed animals, and such cases must be censored (see Note 4). One must also consider bacterial food source, as C. elegans lifespan varies dependent upon the bacteria being consumed as a food source due to varying nutrient content, pathogenicity, and other factors [13]. Genetic background may also increase sensitivity to diet with respect to lifespan; for example, lifespan of rict-1 mutants is shorter than N2 when fed OP50 but feeding HB101 bacteria substantially extends rict-1 lifespan while having only marginal impact on wild-type [14].
Reproduction. A single hermaphrodite can produce 300 self-progeny over the first few days of adulthood. Thus, it is essential to either prevent progeny production or separate parental animals from offspring. Several choices exist to deal with the production of progeny: (1) periodically move animals to new plates, (2) test lifespan in a “feminized” genetic background, or (3) add 5-fluoro-2′-deoxyuridine (FUdR) to late L4/young adult animals (i.e., after the completion of larval development but prior to the formation of internally fertilized progeny). The advantages and disadvantages of each are discussed in Notes 5-7 respectively.
Start with “clean” strains and reagents. Populations of C. elegans can pick up bacterial and fungal contaminants, which can be transferred between plates and even survive freezing and thawing. Contaminated reagents may necessitate early termination of an experiment. The RSM approach reduces exposure to airborne fungal contamination during the course of an experiment. However, there is no substitute for clean workspaces/incubators, use of sterile technique, and keeping stocks of C. elegans free of contamination. Alkaline hypochlorite treatment for synchronization prior to a lifespan trial kills many contaminants (see Subheading 3.4 and Note 24). However, some contaminants colonize the intestinal lumen and survive the procedure outlined in these methods. An alternative method to remove luminal contaminants is described in Note 8.
2. Materials
Use ultrapure water (filtered and UV sterilized) for preparing reagents. Practice sterile technique to prevent contamination of both C. elegans culture plates and bacterial growth media (see Note 9). Reagent solutions that cannot be autoclaved (e.g., FUdR and IPTG stocks) should be filter-sterilized with a 0.2-μm filter using a syringe or bottle-top filter system.
2.1. Agarose Growth Media for C. elegans
Round petri plates, 60 mm × 15 mm (used in the TLM).
Rectangular 24-well plate (used in the RSM, Fig. 2f).
Peristaltic dispensing pump, tubing.
Hot plate, stir bars.
55 °C large sterile water bath (see Note 10).
Parafilm.
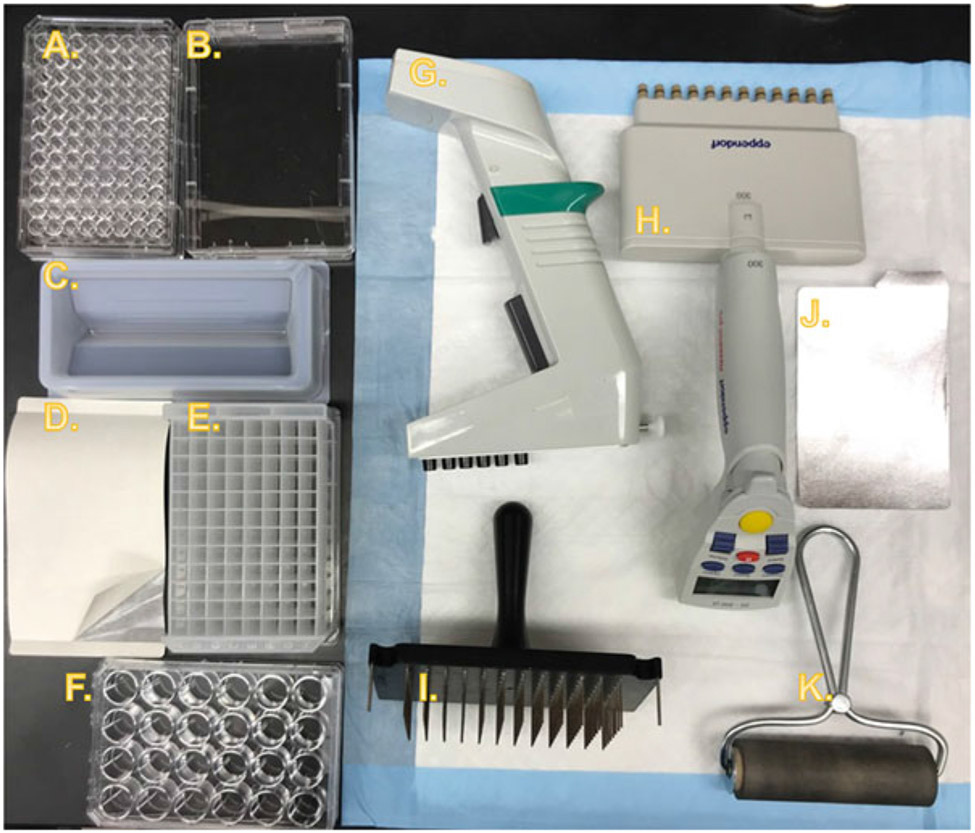
Fig. 2. Equipment and consumables for Replica Set experiments.
Equipment necessary for RSM experiments is commonly available in laboratories that work with C. elegans, especially those which regularly utilize feeding-based RNAi. (a) 96-Well culture plate for storage of frozen libraries of bacterial RNAi clones as glycerol stocks. (b) Rectangular single-well plate for LB amp + tet agar slabs for creating intermediate-term working cultures of RNAi libraries on solid media. (c) Reagent reservoir for bulk reagent pipetting tasks with multichannel pipettes, such as adding fresh LB to a new 96-well plate. (d) Gas-permeable adhesive membrane for sealing deep-well plates during incubation while allowing gas exchange. Note that in the image a corner of the backing has been peeled back to reveal the membrane. (e) 2 mL deep-well culture plate for seeding replica plates. (f) 24-Well culture plate for use as replica plates. In a typical experiment, wells would be filled with solid RNAi growth media before seeding with bacteria and then C. elegans. (g) Electronic 1250 μL six-channel repeat pipette with adjustable tip spacing (sometimes referred to as an “equalizer” pipette). (h) Electronic 300 μL 12-channel repeat pipette (fixed spacing). (i) Pin plate replicator for 96-well plates with stainless-steel pins. Allows easy transfer of bacteria from glycerol stocks to liquid culture, or from liquid culture to solid media, while also being easy to sterilize. (j) Adhesive foil cover for frozen RNAi library glycerol stock plates. (k) Rubber roller for securing adhesive foil plate covers
2.1.1. Nematode Growth Media (NGM) for Culture of C. elegans on OP50 E. coli
Per 1 L: Combine 17 g bacteriological grade agar, 3 g NaCl (crystal), and 2.5 g Bacto peptone in a 2 L Erlenmeyer flask with stir bar. Add 1 L ultrapure water, stir. Cover and autoclave on a liquid program (~90 min).
Transfer to stir plate, add 1 mL 5 mg/mL cholesterol, 1 mL 1 M CaCl2, 1 mL 1 M MgSO4, and 25 mL 1 M KPO4.
Allow to cool to 55 °C, then optionally add 250 μL 40 mg/mL nystatin and 8 mL 25 mg/L streptomycin (see Note 11).
Use standard plate pouring procedures to dispense 12 mL media per 60 mm petri plate.
2.1.2. RNAi Growth Media for Culture of C. elegans on HT115 E. coli
Follow items 1 and 2 of Subheading 2.1.1.
Allow to cool to 55 °C. Add 1 mL 25 mg/mL carbenicillin and 6 mL 0.2 g/mL isopropyl β-d-1-thiogalactopyranoside (IPTG) (see Note 12).
Use standard plate pouring procedures to dispense 12 mL media per 60 mm petri plate, or 1.5 mL per well for 24-well plates.
2.2. E. coli and RNAi for Feeding-Based RNAi
2.3. Bacterial Cultures (Handling)
Luria broth (LB), with an appropriate antibiotic (see Note 15).
Bleach.
Ethanol, pure.
96-Well cultureplates, 340 μL wells (for glycerol stocks of RNAi collections, Fig. 2a).
Rectangular plate (Fig. 2b) (for colonies of RNAi clones, Fig. 3).
50 mL media reservoir (Fig. 2c).
Permeable membrane (e.g., Breathe-Easy microplate seals, Fig. 2d).
96-Well plates, deep well, 2 mL capacity wells (Fig. 2e).
96-Well plates, deep well, 600 μL capacity wells.
24-Well plates (RSM only) (Fig. 2f).
6-Well multichannel repeat pipette with adjustable tip spacing (Fig. 2g).
12-Well multichannel repeat pipette (Fig. 2h).
96-Pin plate replicator (Fig. 2i).
Adhesive aluminum foil cover (Fig. 2j).
Rubber roller to seal foil to plates (Fig. 2k).
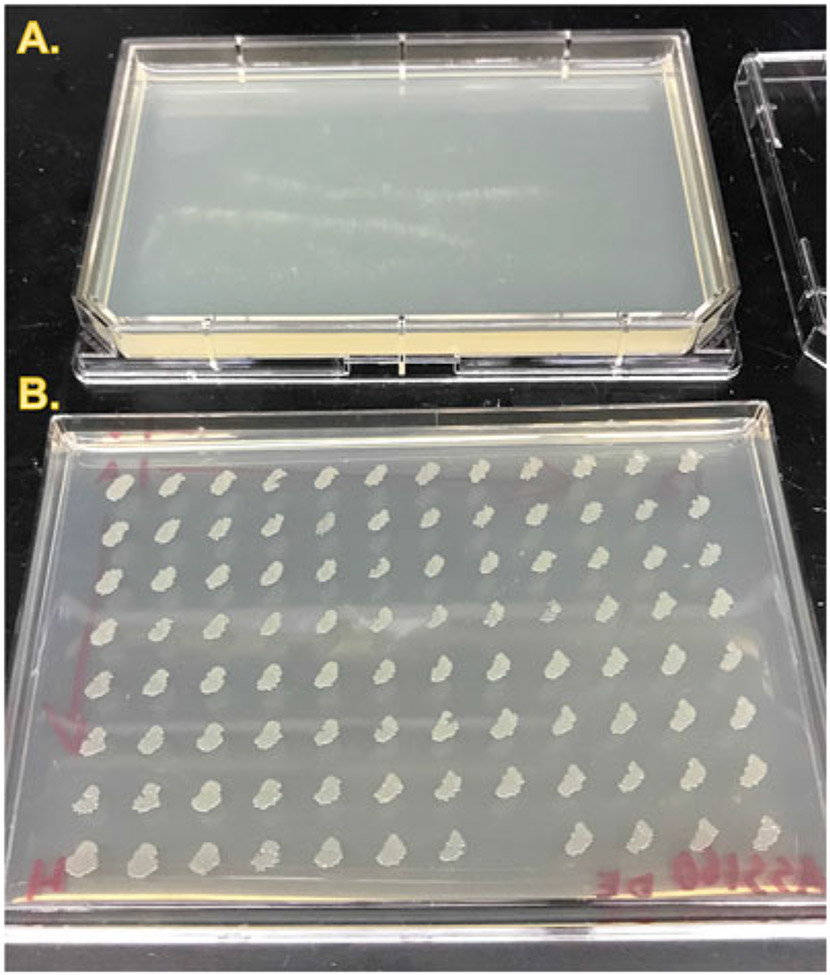
Fig. 3. Typical working stock of an RNAi library.
To minimize handling of frozen glycerol stocks, the frozen stocks are “stamped” to agar plates using a pin replicator (Fig. 2i) to create a “working plate” that will be good for 2–8 weeks when stored at 4 °C (see Note 18). The pin replicator or a multichannel pipette can then be used to inoculate liquid cultures. (a) Single-well rectangular plate with uninoculated LB amp + tet agar, lid removed. (b) An RNAi library plate after overnight incubation at 37 °C. Note that the spacing between spots is such that it is possible to pick from any spot without risk of cross-contamination
2.4. LB + Ampicillin + Tetracycline Agar Plate Recipe for HT115 Culture
Per 1 L: Add 32 g LB agar to 1 L of ultrapure water with a sterile stir bar in a 2 L flask. Stir on a stir plate. Once well-mixed, add 1.5 mL 2 N NaOH. Autoclave on a liquid program. Allow to cool with stirring until reaching 55 °C. Add 3 mL of 5 mg/mL tetracycline, and 1 mL of 50 mg/mL ampicillin. Allow to mix.
Use standard plate pouring procedures to dispense about 45 mL sterilized liquid LB agar mix per rectangular single-well plate.
2.5. Caenorhabditis elegans
C. elegans strains; typically obtained from the Caenorhabditis Genetics Center (CGC) (see Note 16).
Motorized tube rotator.
Plastic lidded storage boxes (to hold plates).
Plastic bags (to hold boxes).
C. elegans M9 buffer (3 g KH2PO4, 6 g Na2HPO4, 5 g NaCl. Add H2O to 1 L. Autoclave. Allow to cool; add 1 mL sterile 1 M MgSO4).
Hypochlorite egg prep solution (6 mL bleach, 3.2 mL 5 M NaOH, 800 μL H2O), store for a maximum of 2 weeks.
5-Fluoro-2′-deoxyuridine (FUdR), 1000× stock: dissolve 1 g FUdR into 10 mL ultrapure water, filter-sterilize with a 0.2-μm filter. Aliquot into sterile 1.5 mL tubes. Store at −20 °C.
3. Methods
Here we describe the necessary preparatory steps and both the TLM and RSM in detail. Examples of actual lifespan data generated by each approach using similar conditions are shown in Fig. 4. For each approach, C. elegans populations and experiment plates with animals should always be stored in a dark temperature-controlled incubator at a temperature appropriate to the strain(s) under study when not being prepared, inspected, or scored.
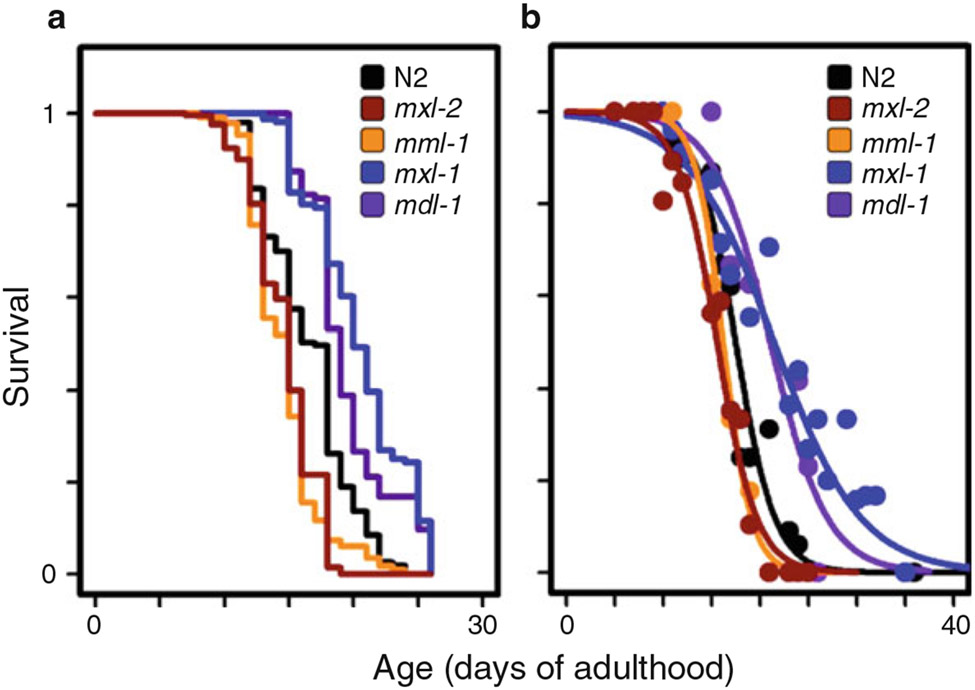
Fig. 4. Results from the Replica Set method are similar to those of the traditional approach.
An example of lifespan results from both the RSM and TLM for the same set of experimental conditions: perturbation of either gene of the MML-1::MXL-2 heterodimeric complex shortens lifespan compared to N2 (WT), while perturbation of either component of the opposing MDL-1::MXL-1 complex extends lifespan. While the approach to plotting the data between the RSM and TLM is different, the conclusions reached are the same. (a) Traditional lifespan assay, Kaplan–Meier plot. (b) Replica set data, fit to a logit curve. (This figure is reprinted from [31] with permission via a Creative Commons Attribution (CC BY) license)
3.1. RNAi Library Preparation for RSM
Remove adhesive foil cover (Fig. 2j) from previously prepared frozen 96-well glycerol sock library plate containing RNAi clones (see Note 14).
Sterilize plate replicator (Fig. 2i) by immersing the pins in 50% bleach, ultrapure H2O, and ethanol (sequentially). Immerse pins for 30 s when in bleach and ethanol. Flame the tips briefly after ethanol immersion. Repeat (see Note 17).
Gently—but firmly—grind plate replicator into the wells of the still-frozen glycerol stocks. Inoculate 200 μL LB + Amp cultures and seal the inoculated plate with a permeable membrane (such as Breathe-Easy microplate seals, Fig. 2d). Allow cultures to grow overnight at 37 °C with optional shaking. Reseal the glycerol stock plate with an adhesive foil cover.
Sterilize plate replicator as in step 1 and immerse tips into the overnight culture. Apply the tips with even pressure to a rectangular LB amp + tet agar plate, and transfer bacteria to the plate gently with a small circular motion without allowing the pins to dig into the agar. Ensure adequate space is left between adjacent spots. Allow colonies to grow overnight at 37 °C (Fig. 3).
Store agar plate at 4 °C inverted (lid side down), wrapped in Parafilm. Plates can be stored for 2–8 weeks (see Note 18).
3.2. Preparation and Seeding of Agar Nematode Plates: 60 mm Plates for TLM
Prepare enough liquid LB media to grow cultures for seeding at least three to six 60 mm plates per test condition (see Note 19). Each plate requires 3 mL of culture which will be 10× concentrated before seeding (see Note 20). Include the appropriate antibiotics, if applicable (see Note 15).
Grow cultures of E. coli overnight (16–20 h at 37 °C in a shaking incubator at 220 rpm).
Concentrate bacteria to 10× by centrifugation at 3000 × g for 15–20 min in a benchtop centrifuge, aspirate the supernatant, and resuspend the pellet at 1/tenth starting volume of LB.
Aliquot 200–300 μL of bacteria to each 60 mm plate. To increase experimental rigor, label plates so the experimenter will be blind to the test conditions.
Allow open plates to dry in a clean environment, such as a laminar flow hood/bench, until all liquid has been absorbed or allow covered plates to dry on the bench overnight. Drying may take several hours. Monitor plates while drying to ensure that the agar is dry without overdrying (see Note 21). For seeded RNAiplates dried in a hood, store dried plates within a plastic worm box overnight (up to 24 h) at room temperature to allow the induction of dsRNA production.
Store unused seeded plates at 4 °C (see Note 22).
3.3. Preparation of Bacterial Cultures and Seeding of Agar Nematode Plates: Multiwell Replicate RNAi Plates for RSM
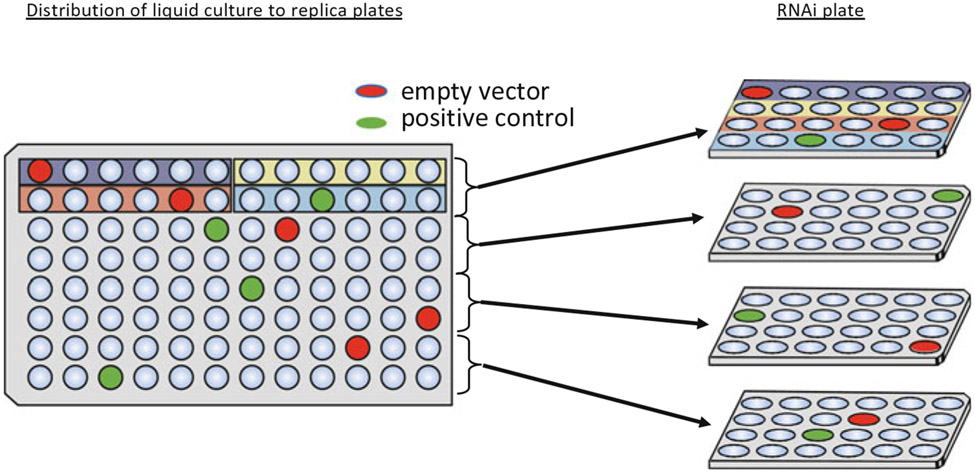
Determine the necessary number of 24-well replicate plate sets, and the number of plates per replicate set to calculate the total number of necessary plates (see Note 23 for considerations). Figure 5 illustrates how the liquid culture grown in a 96-well plate is allocated to the 24-well replicate plates. Replicate plates in each 24-well block should be distinguished by a colored stripe or other label.
For every ten replicate sets, inoculate 1.5 mL LB + Amp cultures in 96-well deep-well plates (Fig. 2e) using the plate replicator (Fig. 2i) and the bacterial colonies previously prepared on an LB amp + tet agar slab plate (Fig. 3b). Cover with breathable membrane seal (Fig. 2d) and grow 16–20 h at 37 °C with shaking.
Using a 6-well multichannel repeat pipette with adjustable tip spacing (Fig. 2g), seed 120 μL of an overnight culture to each well. Allow plates to dry uncovered in laminar flow hood until all liquid has been absorbed. Do not over-dry plates (see Note 21). To allow for induction of dsRNA in HT115 E. coli, store dried plates overnight at room temperature (see Note 12).
Store unused seeded plates at 4 °C (see Note 22).
Fig. 5. Creating a “replicate set” from a 96-well RNAi library plate.
A 96-well RNAi library plate is divided into four blocks of 24 wells, where the four unique 24-well plates make up part of one “replicate set.” The library should be set up such that each block of 24 wells has at least one randomly placed empty-vector control (L4440 RNAi) and positive control. Well A1 of the 96-well library is usually reserved for an empty vector control
3.4. Obtaining a Synchronized Population of Animals
A synchronized population of C. elegans may be obtained from gravid adults by methods including (A) an “egg lay,” or (B) alkaline hypochlorite treatment (“egg prep”) (see Note 24). Use hypochlorite treatment for RSM experiments. Either technique will provide enough animals for most TLM experiments (see Note 25).
(A) For an egg lay:
Move 5–10 gravid adult animals to new plates (with food) that will be used for the lifespan experiment. Use only animals that have not starved for at least three generations (see Note 24).
Allow the adults to lay eggs for 4 h or until there are about 50 eggs on each plate.
Remove the adults, and move the plates to incubator.
(B) For hypochlorite treatment:
Using M9, collect animals into 15 mL centrifuge tube using a glass Pasteur pipette with a plug. A strain may be pooled from multiple plates.
Centrifuge tubes for 15 s at approximately ~650 × g. Aspirate supernatant.
Wash with M9: add 10 mL fresh M9, centrifuge 30 s, aspirate supernatant. Repeat.
Resuspend in 4 mL M9 and add 2 mL hypochlorite egg prep solution. After adding the hypochlorite solution, the timing of the remaining steps is critical to optimize yield.
Vortex for 3 min, with occasional shaking (every 30–60 s). After the 3 min have elapsed, check the tube under a dissecting microscope—eggs should be visible and depending on the density may appear as a “cloud.” If no eggs are yet visible and adults are still intact, vortex for an additional 10–20 s and check again.
Centrifuge for 30 s, aspirate the supernatant, and wash twice with M9 as in step 3. The first wash in particular should be performed as quickly as possible, to minimize the additional exposure of embryos to bleach.
Resuspend the eggs in 3 mL M9, and transfer to a new tube. Allow hatching overnight with rotation at 20 °C (or other temperature, if necessary). The embryos with hatch and arrest at L1, yielding a synchronized population.
Determine the density of L1 animals per μL: pipette 10 μL of resuspended L1s onto a 60 mm plate and count (3×) to determine the average number of L1s per μL. Calculating the density of animals is necessary for the distribution of the appropriate number of animals. The “L1 solution” may need to be diluted.
3.5. Traditional Longitudinal Method (TLM; Low-Throughput)
Identify necessary C. elegans strains and RNAi clones. OP50 E. coli can be used if feeding-based RNAi is not needed. Transformed HT115 E. coli is used for feeding-based RNAi. Prepare 3–6 60 mm plates per condition using RNAi growth media for RNAi experiments, or NGM media for OP50 (see Subheading 2.1). If not immediately preparing bacteria, unseeded plates can be stored at 4 °C. Choice of approach in handling progeny may influence the selection of C. elegans strains for the experiment (see Notes 5-7 for considerations).
Prepare and seed bacterial cultures as described in Subheading 3.2.
Prepare C. elegans as described in Subheading 3.4. For the TLM use at least 50 animals per plate (i.e., each technical replicate).
If using FUdR to control progeny production, when animals reach the L4 stage dilute filter-sterile 1000× FUdR stock to 160× with ultrapure water and add 50 μL of 160× FUdR to the center of each 60 mm plate.
Inspect plates and remove males. Males can be distinguished on a dissecting stereomicroscope from hermaphrodites at the L4 stage. When studying hermaphrodites, it is important that males be removed before the adult stage, see Note 26.
Score viability daily. Animals that are not moving spontaneously can be touched gently on the head with a platinum wire (or alternatively with an eyelash). Animals that fail to move are scored as dead and removed from the plate. Record the number of dead animals observed for each plate/condition for each time point observed. As animals age they become increasingly lethargic and are eventually paralyzed, such that older live animals may only be able to be distinguished by a subtle movement of the head in response to touch.
Animals that die for reasons unrelated to aging should be censored, examples include: overt developmental defects, desiccation on the side of the dish, and intestinal rupturing through the vulva.
Any males observed during the course of the experiment should be removed and the number recorded.
If a plate appears likely to starve before the end of the experiment, or if fungal contamination is observed, move all remaining animals to a backup plate with the relevant bacteria (and FUdR, if appropriate).
Repeat from step 6 until no live animals remain.
Additional experiment trials should be conducted independently and recorded separately.
See Subheading 3.7 for data analysis.
3.6. Replica Set Method (RSM; High-Throughput)
Prepare RNAi library plates and create LB amp + tet agar plates for inoculating the bacterial cultures that will be used for seeding the replicate plates. See Subheading 3.1.
Determine the necessary number of 24-well agar C. elegans plates for the replicate sets (see Note 23) and prepare as outlined in Subheading 2.1.
Seed bacterial cultures to prepared 24-well replica plates according to Subheading 3.3.
Determine the minimum number of animals needed and expand parental populations accordingly. The number needed can be calculated based on 15–20 animals per well, 24 wells per plate, for the number of plates determined in step 2. Depending on the density of the starting population, one 100 mm plate with many gravid adults can provide 20,000–50,000 L1s from a hypochlorite treatment egg prep (see Note 25).
Prepare populations of synchronized L1s by hypochlorite treatment as described in Subheading 3.4.
Add 15–20 L1 animals to each well of the 24-well replica plates. This can be done efficiently with a reagent reservoir and a 6-well electronic pipette, with the volume based on the previously calculated density of the L1 solution. Be sure to gently mix the L1s in the reservoir occasionally, to prevent settling.
When the animals have reached L4, add 25 μL of 50× FUdR (dilute filter-sterile 1000× FUdR stock with ultrapure water) to each well using a 6-channel electronic pipette or repeat pipettor (see Note 7).
For each observation timepoint, take one complete set of plates (i.e., 1 replicate) from the incubator and flood each well with M9. Record the total number of animals, and then touch any nonmoving animals on the head with a platinum wire. Animals that do not move are counted as dead; at later time points movement may be limited to subtle head motion. Animals that die for reasons unrelated to aging should be censored, examples include: overt developmental defects, desiccation on the side of the dish, and intestinal rupturing through the vulva.
Do not score wells that have been starved or are contaminated—make a note of the event but do not record the total.
Discard plates after scoring. For each well, repeat from step 8 daily until no live animals have been observed for two consecutive time points.
Additional experimental trials should be conducted independently.
See Subheading 3.7 for data analysis.
3.7. Plotting and Analysis of Lifespan Data
TLM data can be plotted and analyzed with any one of the many software tools that support survival analysis with the nonparametric Kaplan–Meier estimator [6] and the log-rank test [7] (see Note 27), Different software tools vary in their requirements for data formats. Be sure to consider censored animals appropriately—in this case as right-censored—as including this information avoids bias [15].
For RSM experiment analysis, data is fit to a logit curve to estimate median and maximum lifespan, as logit curves are most appropriate for modelling C. elegans mortality data [16]. As most tools for survival analysis do not support logit curve modeling, we developed a tool, WormLife, that handles both logit (for Replica Set) and Kaplan–Meier (for traditional lifespan) survival analyses. Seehttps://github.com/samuelsonlab-urmc/wormlife for documentation and to find the latest version (see also Note 28).
4. Notes
For the RSM, one must determine how long a strain on a control RNAi will survive prior to any experiment, then plan the number of time points to best measure how an RNAi clone will impact lifespan (i.e., 15 time points requires a minimum of 15 sets).
N2 is the wild-type strain, and the Caenorhabditis Genetics Center (CGC, https://cgc.umn.edu/) has three different stocks available: “N2” (often referred to as “N2H,” https://cgc.umn.edu/strain/N2), “N2 Male” (referred to as “N2M,” https://cgc.umn.edu/strain/N2%20Male), and “N2 (ancestral)” (https://cgc.umn.edu/strain/N2%20(ancestral)). Variations in lifespan have been found between these three stocks, with “N2 (ancestral)” being the shortest lived and “N2M” the longest lived under standard laboratory conditions [11]. For years the “N2M” stock was recommended as the ideal reference wild-type stock [11]. However, recently a mutation in fln-2 has been found that alters longevity of N2M, suggesting “N2H” may be a more appropriate wild-type reference strain (see “‘N2 male’ is a long-lived fln-2 mutant,” http://wbg.wormbook.org/2018/12/11/n2-male-is-a-long-lived-fln-2-mutant/).
Leftover arrested L1 animals can be readily frozen back to create a “snap shot” of the specific genetic profile of the strain used in each lifespan experiment. Carefully annotating frozen stocks that were actually used in specific experiments provides a critical resource: at a later date it could be compared to other strains that are at least superficially of an identical genetic background (either within or between laboratories). This greatly improves experimental reproducibility and rigor, as direct comparison allows assessment of the effect of genetic background.
It is possible to reseed with concentrated bacteria or move animals to a new plate with food, but this must occur prior to exhaustion of food. Note, RNAi clones require induction of dsRNA production via IPTG prior to reseeded (i.e., directly adding to the bacterial culture).
Picking animals to new plates every other day or so during the reproductive period is an option only applicable to traditional lifespan analysis (TLM). However, there are also some strain-specific considerations. Some strains (e.g., daf-2(e1370)) have much longer “reproductive spans,” necessitating additional days on which parents would have to be picked to new plates. Strains with an egl phenotype (egg-laying defect) cannot be analyzed by this method and require FUdR as they undergo high rates of premature death due to internal hatching. For example, the link between TGF-β signaling and lifespan was not initially appreciated due to such defects, and was only realized when FUdR was used [17].
Feminized genetic mutants fail to produce sperm and therefore cannot produce progeny in the absence of males. One must inspect plates for males and remove them to prevent mating. Fem mutations, such as fer-15(b26);fem-1(hc17) which is a temperature-dependent sterile strain, have been utilized used in aging studies and do not appear to cause any overt aging-phenotypes [18, 19]. However, the use of fem alleles requires the creation of double mutant animals for every genetic background to be examined. Note that strains with mutations that ablate the germ line are long-lived (e.g., glp-1) and are not equivalent to fem backgrounds.
FUdR (5-fluoro-2′-deoxyuridine) is a nucleotide base analog that blocks DNA synthesis. Since adult C. elegans somatic cells are postmitotic, treatment with FUdR does not affect the soma, but prevents germline proliferation. FUdR is commonly added directly to plates, but care must be taken with timing, as addition must occur at the L4 stage, after somatic development but before egg production. Addition of FUdR after animals have become gravid adults will result in progeny that will arrest at the L1 stage but will feed, thereby exhausting the food from the plates. Addition of FUdR prior to L4 will disrupt development, especially at the vulva, which will eventually cause animals to rupture between day 7 and 14 of adulthood. Higher concentrations of FUdR can also lead to rupturing. It is worth noting that FUdR treatment influences lifespan in some genetic backgrounds [20, 21].
Here is a brief method for cleaning up strains with luminal contamination: (1) Pick 5–10 gravid animals to an NGM plate with no food and let them crawl around a few minutes. (2) Pipet 10–15 μL of hypochlorite egg prep solution close to the edge of a different NGM plate (away from the bacterial lawn). (3) Pick up the animals from the unseeded intermediate plate by sliding the pick under them (“spatula style”) and transfer them directly into the spot of hypochlorite solution. Adults will die; internally fertilized eggs will hatch overnight and crawl to the bacteria. (4) Transfer L1 animals farthest from the hypochlorite spot to a new plate.
Sterile technique is essential when preparing for lifespan experiments. Sterilize work areas with a 10% bleach solution followed with an ultrapure water rinse, then 70% ethanol. This includes lab benches and other surfaces such as hoods. Work over a flame when possible, and wear gloves, a surgical mask, and a clean lab coat. Minimize the time plates are left open to the air and outside of the incubator. Never store boxes, plates, lab coats, and so on on the floor. Anything that touches the floor should be considered as contaminated.
As it is often difficult to maintain sterility of a water bath, we use Laboratory Armor Beads (e.g., Fisher Scientific #A1254301) instead of water in the bath. We have found the “bead bath” to be both cleaner and lower maintenance.
Streptomycin and nystatin can optionally be added to prevent undesired growth of contaminating organisms on plates, which is important for long experiments, such as for lifespan assays. However, not all laboratories typically work with an OP50 stock that is streptomycin-resistant. The CGC has both variants available, OP50 (not streptomycin resistant) and OP50-1 (resistant).
Unseeded RNAi agar plates can be stored at 4 °C for several months before seeding with bacteria. Some laboratories add fresh IPTG (concentration) at the same time as bacterial seeding, to ensure adequate dsRNA production in case of degradation of the IPTG in the agar over time. Do not add IPTG to bacterial cultures too early, as this can severely reduce the bacterial growth rate.
For experiments using RNAi, dsRNA production is induced in transformed HT115 E. coli [22] on RNAi plates, and for lifespan studies without RNAi, OP50 E. coli on standard NGM plates can be used. HT115 is derived from K12 E. coli and is used for feeding based RNAi, as it lacks RNase III, the enzyme normally responsible for degradation of double-stranded RNA in E. coli [23]. In contrast, the typical food source to maintain C. elegans is OP50 which is E. coli B.
Collections of RNAi clones are commonly maintained as glycerol stocks in a 96-well format and stored at −80 °C. Always keep frozen—work on dry ice when at the bench—and minimize exposure to even slight warming, as this greatly reduces E. coli viability. Custom RNAi sublibraries (i.e., a group of RNAi clones selected from a larger library) can be easily assembled for convenience. Within a collection, each RNAi clone is represented at least once. In principle, this approach could be similarly applied for different chemical treatments, different animal strains, and so on. When assembling sublibraries in a 96-well format, typically, an empty vector negative control is included in the first well of a collection of 96. Specifically, for RSM assays, as the clones from each 96-well plate are split into blocks of 24 wells on different plates, an additional empty vector is inserted randomly within every 24 wells of the library, so that every resulting 24-well plate has an empty vector well as a negative control (Fig. 5). Similarly, a positive control should be randomly inserted within every group of 24-wells (Fig. 5). For example, when looking at a collection of RNAi clones expected to increase lifespan, daf-2(RNAi) is frequently included. Daf-2 encodes the C. elegans insulin/IGF-1 receptor, and daf-2 inactivation via feeding-based RNAi robustly increases lifespan at least two-fold in wild-type animals [1]. As of the time of writing, both the Ahringer [24] and Vidal (ORFeome) [25] RNAi libraries or individual clones are available from two commercial vendors, Source Bioscience (https://www.sourcebioscience.com/products/life-sciences-research/clones/rnai-resources/) and Horizon Dharmacon (https://dharmacon.horizondiscovery.com/cdnas-and-orfs/non-mammalian-cdnas-and-orfs/c-elegans/). Note that the former provides library plates in a 384-well format, whereas the latter utilizes 96-well plates.
HT115 E. coli is grown in LB with ampicillin (50 μg/mL). Standard OP50 E. coli is not ampicillin resistant.
Strain selection may influence the design of the assay, and is necessary to consider prior to preparing other reagents, as different strains may have substantial differences in lifespan even without additional treatment. Additionally, larger populations of animals are required for RSM than TLM experiments, which may be difficult to obtain for very slow-growing strains, or for those with extrachromosomal transgenes or balanced mutations. Some strains may also have temperature-sensitive alleles, requiring growth development at a temperature different from the typical 20 °C; as temperature can have a dramatic effect on C. elegans lifespan, it is necessary to treat all strains in an experiment similarly in order to achieve comparable results.
Be sure to remove all bleach from the pins of the plate replicator, as it could inadvertently kill bacteria. Do not leave the pins in bleach for extended periods, it will corrode the stainless-steel pins. Avoid overly long exposure of the pin replicator to the flame after ethanol immersion so that the pins cool fast enough before transferring bacteria without affecting viability.
Bacterial colonies on the stamped agar plate are now ready for subsequent use. Always store plates lid side down; lid side up will allow condensation to cross-contaminate RNAi clones! Colonies of E. coli can be stored for 2–8 weeks at 4 °C, depending on particular RNAi clones, as RNAi clones retain efficacy in dsRNA production after IPTG induction for variable lengths of time. For small collections of RNAi clones, RNAi efficiency should be empirically determined by qRT-PCR to confirm gene expression knockdown.
At least three technical replicate plates should be set up per condition. When using FUdR, set up two extra backup plates in case animals need to be moved off of contaminated plates to a backup plate. When not using FUdR, three additional plates will be needed per condition to transfer parental animals away from progeny every 2 days, until the cessation of progeny production—additional plates may be handy in case of contamination issues.
Concentration of bacteria is optional but recommended to decrease incidence of starvation during the course of the experiment. If not concentrating, adjust starting volumes of culture appropriately.
Cracking of the agar is a symptom of overdry plates/wells. Dry agar and cracking can lead to C. elegans burrowing. Wet plates or wells can also lead to burrowing behavior. As burrowed animals should be censored when scoring lifespan experiments, monitoring of plates during drying is important.
Do not leave dried plates at room temperature for more than 24 h. After 1 day at RT, plates can be stored at 4 °C for up to 2 weeks in a sealed zip-lock bag (to prevent plates from drying out). Before use, return plates stored at 4 °C to room temperature within the zip-lock bag to prevent condensation from introducing airborne fungal contaminants.
As RSM requires one set of replicate plates per observation, it is necessary to estimate the number of necessary observations at the beginning of the experiment. This is usually based on preliminary knowledge of the lifespan for a given set of experimental conditions of interest (strain, RNAi, etc.). Observation frequency is also important to consider; we will usually score a set for a given strain every other day until deaths are observed, at which point we change to daily scoring. Thus, the number of plates necessary for one strain in an RSM trial can be calculated as R * (O + E), where R is the number of plates in each replicate set (usually four if using 24-well replicate plates and a full 96-well library), O is the estimated number of observations, and E is a number of extra replicate sets allocated for cases such as contamination.
The “egg lay” method is a relatively quick way to obtain small synchronized populations, which does not necessarily involve synchronization by larval arrest, which is worth considering in the context of possible synthetic interactions with larval arrest. Alternatively, alkaline hypochlorite treatment could be used to isolate eggs, which are directly seeded for subsequent lifespan analysis. However, some eggs will not survive (leading to a smaller number of animals than anticipated) and synchronizing L1 animals via arrest yields a population with a tighter developmental window between animals. As many more L1s are often obtained than are needed for the experiment, the remaining animals can be frozen (see Note 3).
Starvation leads to transgenerational changes in gene expression that can influence lifespan [26, 27]. When preparing for an experiment, ensure that the parent population has not starved for at least three generations.
Even with FUdR treatment, hermaphrodites that have mated live shorter than those that have not which can skew the results of a TLM experiment [28-30]. As such, it is critical to check plates and remove any males at the L4 stage.
The Kaplan–Meier estimator and associated plots, as well as the log-rank test are supported by many software tools, available both online and as part of statistical packages. A popular free option is the web-based OASIS2 (https://sbi.postech.ac.kr/oasis2/). Other tools supporting such analysis that might already be available to investigators include Graphpad Prism (https://www.graphpad.com/scientific-software/prism/) and JMP (https://www.jmp.com/). Be sure to use tools which support right-censored data and enter your recorded information on censored animals. Check for data quality issues such as missing observations and unexpected results for control conditions before running statistical analysis.
We developed WormLife for analysis of Replica Set experiments; support is also included for Kaplan–Meier-style plots. The primary user interface includes features for plotting survival curves from both methods, comparing curves between trials, and obtaining the estimated median survival values. Features are included for automating plotting for large-scale experiments with many different conditions. An R script is also included for permutation-based statistical testing between conditions. As detailed discussion is beyond the scope of this chapter, see https://github.com/samuelsonlab-urmc/wormlife for current documentation, and our previously published protocol for getting started with the software in [10].
Acknowledgments
This work was supported by NIH grants R01AG043421 and R01AG62593.
Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
References
- 1.Kenyon C, Chang J, Gensch E et al. (1993) A C. elegans mutant that lives twice as long as wild type. Nature 366:461–464. 10.1038/366461a0 [DOI] [PubMed] [Google Scholar]
- 2.Johnson T (1990) Increased life-span of age-1 mutants in Caenorhabditis elegans and lower Gompertz rate of aging. Science 249:908–912. 10.1126/science.2392681 [DOI] [PubMed] [Google Scholar]
- 3.Timmons L, Fire A (1998) Specific interference by ingested dsRNA [10]. Nature 395:854. 10.1038/27579 [DOI] [PubMed] [Google Scholar]
- 4.Klass MR (1977) Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev 6:413–429. 10.1016/0047-6374(77)90043-4 [DOI] [PubMed] [Google Scholar]
- 5.Raj F, Amrit G, Ratnappan R et al. (2014) The C. elegans lifespan assay toolkit. Methods 68:465–475. 10.1016/j.ymeth.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 6.Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 5318910:457–481. 10.2307/2281868 [DOI] [Google Scholar]
- 7.Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50:163–170 [PubMed] [Google Scholar]
- 8.Samuelson AV, Carr CE, Ruvkun G (2007) Gene activities that mediate increased life span of C. elegans insulin-like signaling mutants. Genes Dev 21:2976–2994. 10.1101/gad.1588907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuelson AV, Klimczak RR, Thompson DB et al. (2007) Identification of Caenorhabditis elegans genes regulating longevity using enhanced RNAi-sensitive strains. Cold Spring Harb Symp Quant Biol 72:489–497. 10.1101/sqb.2007.72.068 [DOI] [PubMed] [Google Scholar]
- 10.Cornwell AB, Llop JR, Salzman P et al. (2018) The replica set method: a high-throughput approach to quantitatively measure Caenorhabditis elegans lifespan. J Vis Exp (136):e57819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gems D, Riddle DL (2000) Defining wild-type life span in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci 55:215–219 [DOI] [PubMed] [Google Scholar]
- 12.Das R, Melo JA, Thondamal M et al. (2017) The homeodomain-interacting protein kinase HPK-1 preserves protein homeostasis and longevity through master regulatory control of the HSF-1 chaperone network and TORC1-restricted autophagy in Caenorhabditis elegans. PLoS Genet 1:1–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim DH (2013) Bacteria and the aging and longevity of Caenorhabditis elegans. Annu Rev Genet 47:233–246. 10.1146/annurev-genet-111212-133352 [DOI] [PubMed] [Google Scholar]
- 14.Soukas AA, Kane EA, Carr CE et al. (2009) Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev 23:496–511. 10.1101/gad.1775409.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark TG, Bradburn MJ, Love SB, Altman DG (2003) Survival analysis part I: basic concepts and first analyses. Br J Cancer 89(2):232–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanfleteren JR, De Vreese A, Braeckman BP (1998) Two-parameter logistic and Weibull equations provide better fits to survival data from isogenic populations of Caenorhabditis elegans in axenic culture than does the Gompertz model. J Gerontol Ser A Biol Med Sci 53A:B393–B403. 10.1093/gerona/53A.6.B393 [DOI] [PubMed] [Google Scholar]
- 17.Shaw WM, Luo S, Landis J et al. (2007) The C. elegans TGF-B Dauer pathway regulates longevity via insulin signaling. Curr Biol 17:1635–1645. 10.1016/j.cub.2007.08.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen M, Hsu AL, Dillin A, Kenyon C (2005) New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet 1:0119–0128. 10.1371/journal.pgen.0010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen M, Chandra A, Mitic LL et al. (2008) A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet 4:e24. 10.1371/journal.pgen.0040024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson EN, Corkins ME, Li J-C et al. (2016) C. elegans lifespan extension by osmotic stress requires FUdR, base excision repair, FOXO, and sirtuins. Mech Ageing Dev 154:30–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucanic M, Plummer WT, Chen E et al. (2017) Impact of genetic background and experimental reproducibility on identifying chemical compounds with robust longevity effects. Nat Commun 8:14256. 10.1038/ncomms14256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamath RS, Martinez-Campos M, Zipperlen P et al. (2000) Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol 2:research0002.1–10. 10.1186/gb-2000-2-1-research0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timmons L, Court DL, Fire A (2001) Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263:103–112 [DOI] [PubMed] [Google Scholar]
- 24.Kamath RS, Fraser AG, Dong Y et al. (2003) Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421:231–237. 10.1038/nature01278 [DOI] [PubMed] [Google Scholar]
- 25.Ceron J, Koreth J, Hao T et al. (2004) Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res 14:2162–2168. 10.1101/gr.2505604.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rechavi O, Houri-Ze’Evi L, Anava S et al. (2014) Starvation-induced transgenerational inheritance of small RNAs in C. elegans. Cell 158(2):277–287. 10.1016/j.cell.2014.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larance M, Pourkarimi E, Wang B et al. (2015) Global proteomics analysis of the response to starvation in C. elegans. Mol Cell Proteomics 14:1989–2001. 10.1074/mcp.M114.044289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi C, Murphy CT (2014) Mating induces shrinking and death in Caenorhabditis mothers. Science 343:536–540. 10.1126/science.1242958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maures TJ, Booth LN, Benayoun BA et al. (2014) Males shorten the life span of C. elegans hermaphrodites via secreted compounds. Science 343:541–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gems D, Riddle DL (1996) Longevity in Caenorhabditis elegans reduced by mating but not gamete production. Nature 379:723. [DOI] [PubMed] [Google Scholar]
- 31.Johnson DW, Llop JR, Farrell SF et al. (2014) The Caenorhabditis elegans Myc-Mondo/Mad complexes integrate diverse longevity signals. PLoS Genet 10:e1004278. 10.1371/journal.pgen.1004278 [DOI] [PMC free article] [PubMed] [Google Scholar]