Abstract
Background and Aims
The medical management of inflammatory bowel disease [IBD] has become increasingly targeted, through the identification of specific immune mediators involved in its pathogenesis. IL-23 is an inflammatory cytokine involved in both innate and adaptive immunity, which has been identified as a therapeutic target in Crohn’s disease [CD] and ulcerative colitis [UC] through its upstream inhibition of the T helper 17 [Th17] pathway. We sought to review available data on the efficacy of IL-23 inhibitors in the treatment of IBD and the potential for clinical and molecular predictors of response to facilitate a personalised medicine approach with these agents.
Methods
We reviewed and summarised available clinical trial data on the use of the IL-23 inhibitors risankizumab, brazikumab, mirikizumab, and guselkumab in the treatment of IBD, as well as the evidence from studies of these agents in IBD and other immune-mediated conditions which might inform prediction of response to IL-23 inhibition.
Results
Early clinical trials have demonstrated promising results following both induction and maintenance therapy with IL-23 inhibitors in CD and UC. Pre- and post-treatment levels of IL-22 and post-treatment levels of IL-17 have been identified as potential molecular predictors of response to therapy, in several studies. No significant clinical predictors of response have been identified thus far.
Conclusions
IL-23 antagonism is a promising therapeutic approach in IBD. Further exploration of molecular and clinical predictors of response may identify patients most likely to benefit from these medications.
Keywords: IL-23, p19, IBD
1. Introduction
The advent of biologic therapy in the management of inflammatory bowel disease [IBD] has had a dramatic impact on treatment strategies, clinical outcomes, and morbidity in patients with Crohn’s disease [CD] and ulcerative colitis [UC].1,2 While initially limited to anti-tumour necrosis factor [anti-TNF] agents such as infliximab, adalimumab, certolizumab, and golimumab, several additional therapeutic pathways have emerged over the past decade, with approved medications now including vedolizumab, an antibody to α 4β 7 integrin which specifically targets the gut immune response, ustekinumab, an inhibitor of the p40 subunits of interleukin [IL]-12 and -23, and tofacitinib, a small molecule inhibitor of the JAK-STAT pathway. These newer agents have allowed for an increasingly tailored approach to immunotherapy, and most are associated with preferable side effect profiles as compared with anti-TNF agents and are thus more appealing therapeutic agents for certain patient populations.2
The efficacy of ustekinumab in the treatment of both CD and UC3,4 underscores the importance of the IL-12 and IL-23 pathways in the pathogenesis of IBD and has spurred large-scale research efforts to develop medications specifically targeting these cytokines. In particular, several biologic agents targeting the p19 subunit of IL-23, a cytokine that shares the p40 subunit of IL-12,5 have been developed in recent years and are currently being studied in late-stage clinical trials. Beyond the promising efficacy of these highly targeted agents, it is worth considering whether their potential benefits can be further heightened by identifying specific patients with a higher likelihood of response to p19 blockade. Such an approach is one goal of personalised medicine, whereby the most effective drug, among a widening range of drugs with distinct mechanisms of action, is selected for a specific patient, thereby maximising efficacy. In order to address this concept, it is important to understand the mechanism of action of IL-23 blockers.
2. The IL-12 and IL-23 Pathways and Their Role in Inflammatory Bowel Disease
Both IL-12 and IL-23 are pro-inflammatory cytokines in the interleukin-6 [IL-6] family, which are produced by dendritic cells, monocytes, and macrophages in the intestinal mucosa.6,7 IL-12 is involved in the pathogenesis of several autoimmune processes [including IBD] through its effects on both innate and adaptive immunity. Innate immunity is targeted through the activation of natural killer [NK] cells, NK T cells, and group 1 innate lymphoid cells (ILC1s, which in turn produce TNF and interferon γ [IFNγ]). In contrast, adaptive immunity is influenced through activation of cytotoxic T cells, promotion of B cell class switching to immunoglobulins associated with T helper 1 [Th1] cells, and upregulation of the transcription factors T-bet and STAT4, which result in increased expression of Th1 cells and with additional downstream production of IFNγ. Many drugs that target IL-12 do so through its p40 subunit. Notably, the same p40 subunit can also combine with an additional subunit called p19, which leads to the creation of IL-23. It is through the inhibition of p40 that ustekinumab works to block both IL-12 and IL-23.5
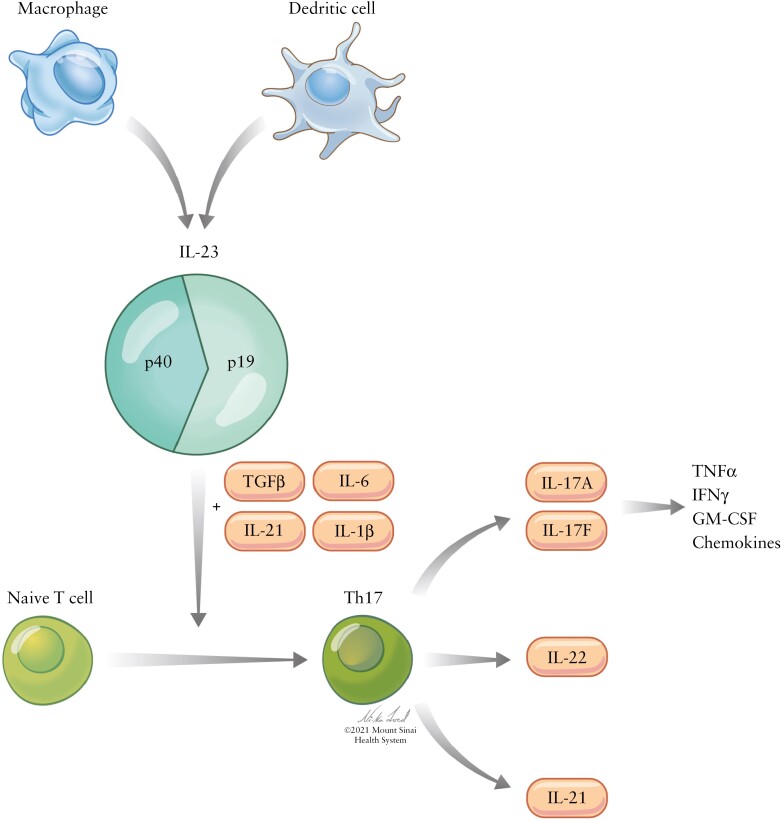
Like IL-12, IL-23 has been implicated in multiple autoimmune disease processes, also through effects on both innate and adaptive immune responses. Indeed, the IL-23 receptor [IL23-R] is expressed on T cells, ILCs, intraepithelial lymphocytes, NK cells, intestinal epithelial cells, and granulocytes, highlighting IL-23’s expansive role in the pro-inflammatory response.7 Most notable is its involvement in the T helper 17 [Th17] cell activation pathway in combination with transforming growth factor β [TGF-β] and cytokines IL-6, IL-1β, and IL-21, in turn increasing production of IL-17 and thus TNFα, IFNγ, granulocyte-macrophage colony-stimulating factor [GM-CSF], and other inflammatory chemokines through stimulation of endothelial cells and monocytes, as well as IL-21 and IL-22 [Figure 1].5–10 Several studies have demonstrated that patients with IBD have increased intestinal, serum, and plasma levels of IL-23 and Th17 cytokines. Moreover, studies examining the effect of IL-23 receptor loss of function and IL-23 inhibition have demonstrated decreased risk of IBD and less severe colitis, respectively.11
Figure 1.
Schematic of the IL-23 pathway. Following activation by innate immune cells, IL-23, in combination with TGFβ, IL-6, IL-21, and IL-1β, influences differentiation of naïve T cells into Th17 cells. These, in turn, produce several inflammatory cytokines, including IL17A and IL17F [which themselves produce TNFα, IFNγ, GM-CSF, and inflammatory chemokines], IL-22, and IL-21.
Given the complex and far-reaching effects of these immune pathways, it is reasonable to infer that a blockade of either cytokine could have profound effects on inflammation and thus serve as a promising therapeutic target in IBD. Notably, certain data have suggested that IL-23 has a more significant pro-inflammatory effect on peripheral tissue inflammation than IL-12, whereas some studies suggest that IL-12 may have a protective, anti-inflammatory effect,6,12 raising the possibility that blocking IL-12 may not be desirable in treating IBD. It is this line of thinking that has prompted the creation and study of medications explicitly directed at IL-23, specifically its p19 subunit.
2.1. Anti-IL-23 agents: data in IBD
A summary of IL-23 inhibitors with associated clinical trial details can be found in Table 1.
Table 1.
Summary of clinical trials of anti-IL-23 agents in IBD
| Agent | Mechanism of action | Trial [s] | Population studied | Study design | Primary outcome | Secondary outcomes/additional analyses |
|---|---|---|---|---|---|---|
| Risank izumab |
Monoclonal IgG1 antibody to IL23p19 | Randomised, double-blind, placebo-controlled Phase II clinical trial [NCT02031276, Feagan et al.13] with subsequent open-label extension14]; two randomised, double-blind, placebo-controlled Phase III clinical trials (ADVANCE [NCT03105128] and MOTIVATE [NCT03105128], D’Haens et al.15) | Feagan et al. 108 patients 18–75 years old, with minimum 3-month history of CD, with moderate-severely active disease based on CDAI 220–450, ulcers in the colon, ileum, or both, CDEIS-19 of at least 4 if isolated ileitis and at least 7 if ileocolitis Patients could have had prior exposure to anti-TNF agents or vedolizumab; 93% had prior exposure to anti-TNF; 79% had failed Patients excluded if: • prior ustekinumab exposure • exposure to any biologic within 8 weeks/5 half-lives of randomisation D’Haens et al. 850 patients [ADVANCE] and 569 patients [MOTIVATE] 16–80 years old with moderate-to-severe CD based on CDAI 220–450, average daily abdominal pain score [APS] ≥2 and average daily stool frequency [SF] ≥4, and endoscopic disease activity defined as SES-CD ≥6 [≥4 if ileal disease only] ADVANCE: patients had to have prior inadequate response to or intolerance of biologic therapy [Bio-IR] or conventional therapy [non-Bio-IR] MOTIVATE: patients had to have prior inadequate response to or intolerance of biologics [Bio-IR] |
Feagan et al. Treatment period one: patients randomised 1:1:1 as follows: • placebo • 200 mg IV risankizumab • 600 mg IV risankizumab, all patients dosed at Weeks 0, 4, 8 Analyses performed looking at each treatment group as well as the pooled risankizumab group [all patients who received the study drug] Treatment period two: following induction, patients received either 12 weeks of open-label 600 mg IV risankizumab [if not in deep remission] at Weeks 14, 18, and 22 or had a 12-week wash-out period [if in deep remission] Treatment period three: open-label extension with maintenance therapy [180 mg subcutaneously] up to 52 weeks in patients in deep remission at Week 26 D’Haen’s et al.: ADVANCE: randomised 2:2:1 as follows: • placebo • 600 mg IV • 1200 mg IV Dosed at Weeks 0, 4, 8. MOTIVATE: randomised 1:1:1 to receive: • placebo • 600 mg IV • 1200 mg IV |
Feagan et al. Treatment period one: clinical remission [defined as CDAI <150] at Week 12 met in 31% of risankizumab patients, vs 15% of placebo, with observed difference of 15%; p = 0.0489 Treatment period two: 26-week efficacy outcomes: clinical response or remission greater in all groups at Week 26 compared with the same groups at Week 12: • 55% of original placebo group, vs 18% at Week 12 • 59% of original group 200 mg group, vs 21% at Week 12 • 47% of the original 600-mg group, vs 26% at Week 12 Overall: clinical remission at Week 26: 53% of pooled treatment group clinical response at Week 26: 73% of pooled treatment group Total 61 patients [56%] in clinical remission at Week 26 Treatment period three: 52-week efficacy outcomes: clinical remission at Week 52: 71% of pooled treatment group clinical response at Week 52: 81% of pooled treatment group endoscopic remission at Week 52: 35% of pooled treatment group |
Feagan et al. Treatment period one: Clinical response [CDAI <150 or CDAI reduction from baseline ≥100]-met in 600mg group [p = 0.0366] and pooled group [p = 0.0273] Endoscopic remission [CDEIS ≤4; ≤2 in patients with ileitis only]-met in 200mg group [p = 0.0375], 600mg group [p = 0.0107], and pooled group [p = 0.0015] Endoscopic response [CDEIS reduced by >50% from baseline]-met in 600mg group [p = 0.0106] and pooled group [p = 0.0104] Deep remission [clinical and endoscopic remission]- not met in any group [p200mg = 0.97; p600mg = 0.31; ppooled = 0.50] Deep remission [both clinical and endoscopic remission]-met in 600mg group [p = 0.0164] and pooled group [p = 0.0107] Health-related quality of life based on changes in the Inflammatory Bowel Disease Questionnaire [IBDQ] -met in all groups, including placebo, with dose-dependent increase in score from baseline [p600mg = 0.0009] Reduction in median CRP from baseline-met in both 200mg and 600mg groups [p < 0.0001 for each] Reduction in median FC from baseline-met in 600mg group [p = 0.0003] Reduction in plasma IL-22 level from baseline-met in 600mg group [p = 0.0180] |
| Dosed at Weeks 0, 4, 8 | endoscopic response at Week 52: 55% of pooled treatment group mucosal healing at Week 52: 24% of pooled treatment group deep remission at Week 52: 29% of pooled treatment group D’Haen’s et al.: Clinical remission [defined as CDAI ≤150 [US] or SF ≤2.8 and APS ≤1, neither worse than baseline [outside USA] and endoscopic response [defined as >50% reduction in SES-CD from baseline, or at least 2-point reduction from baseline in patients with ileal disease only with baseline score of 4]: • CDAI <150: met in 45.2% of 600-mg group and 41.6% of 1200-mg group, vs 25.2% of placebo group, with treatment differences of 20.2% and 16.1%, respectively [p600mg ≤0.001, p1200mg ≤0.001] • APS ≤1 and SF ≤2.8 with no worsening from baseline: met in 43.5% of 600-mg group and 41% of 1200-mg group, vs 21.7% of placebo group, with treatment differences of 21.9% and 18.8%, respectively [p600mg ≤0.001, p1200mg ≤0.001] • endoscopic response: met in 40.3% of 600-mg group and 32.2% of 1200-mg group, vs 12% of placebo group, with treatment differences of 28.3% and 20.2%, respectively [p600mg ≤0.001, p1200mg ≤0.001] |
|||||
| Brazikumab | Monoclonal IgG2 antibody to IL23p19 | Double-blind, placebo-controlled Phase IIa clinical trial [NCT01714726, Sands et al.16] with open-label extension | Total of 119 patients 18–65 years old with minimum 6-month history of CD, with active disease based on CDAI 220–450, active inflammation based on CRP ≥5 mg/L, FC ≥250 ug/g, or endoscopic disease activity within 12 weeks of screening; 104 patients continued through the open-label period Patients had to have prior TNF exposure [primary or secondary loss of response or intolerance] |
Patients randomised 1:1 to receive placebo or 700 mg IV brazikumab at Weeks 0 and 4 during the 12-week induction period Open-label extension with maintenance therapy [brazikumab 210 mg subcutaneously every 4 weeks] in all patients for 100 weeks |
Clinical response [defined as reduction of ≥100 points on the CDAI from baseline] at Week 8 met in 49.2% of brazikumab patients, vs 26.7% of placebo, with absolute difference of 22.25% [p = 0.010] | Secondary outcomes: Clinical remission [defined as CDAI < 150] at Week 8 not statistically significant: met in 27.1% of brazikumab patients vs 15% of placebo, with absolute difference of 12.2% [p = 0.10] Clinical response at Week 12 not statistically significant:met in 37.3% of brazikumab patients vs 28.3% of placebo, with absolute difference of 9.1% [p = 0.29] Clinical remission at Week 12 not statistically significant: met in 20.3% of brazikumab patients, vs 13.3% of placebo, with absolute difference of 7% [p = 0.31] Exploratory analyses: sustained response and remission at Week 8 and Week 24: • Week 8: 42.8% of brazikumab patients, vs 23.1% of placebo • Week 24: 23.1% of brazikumab patients, vs 11.5% of placebo [similar rates of clinical remission and response and composite outcome in treatment and placebo groups at Week 24] |
| Composite outcome: clinical response and ≥50% reduction in either CRP or FC from baseline at Weeks 8, 12, 24 • Week 8 met, with 42.4% of brazikumab patients, vs 10% of placebo, absolute difference 12.2% [p <0.001] • Week 12 met, with 37.3% of brazikumab patients, vs 8.3% of placebo, absolute difference 29% [p <0.001] • Week 24 not met, with 46.2% of brazikumab patients, vs 48.1% of placebo Change from baseline in CRP, FC, IL-22: • CRP Week 8: significant reduction, p = 0.007 • CRP Week 12, significant reduction, p = 0.008 • FC Week 8, significant reduction, p = 0.027 • FC Week 12, significant reduction, p = 0.034 • IL-22 Week 8, significant reduction, p = 0.04 Value of baseline IL-22 as predictor of response: baseline median level of ≥15.6 pg/mL associated with greater rates of clinical response and remission at Week 8 |
||||||
| Mirikizumab | Monoclonal IgG4 antibody to IL23p19 | Randomised, double-blind, parallel arm, placebo-controlled Phase 2 clinical trial [I6T-MC-AMAC, Sandborn et al.,17 Sands et al.18 | Sandborn et al.: 249 patients 18–75 years old with UC for at least 3 months [based on endoscopy, pathology] with extension beyond the rectum [at least 15 cm of colon involved], with moderate-severely active disease based on total Mayo score of 6–12 with subscores of at least 2 [notably, total Mayo score used for inclusion, modified Mayo score used to assess efficacy] Patients could have prior biologic exposure, with discontinuation of anti-TNFs or experimental UC biologics ≥8 weeks before inclusion and discontinuation of vedolizumab ≥12 weeks before inclusion • >63% had prior biologic exposure Patients excluded if: • prior UC surgery • likely need for UC surgery during study • prior exposure to any anti-IL-23 agent [including ustekinumab] • prior ileostomy, colostomy, or fixed area of intestinal stenosis with associated symptoms |
Sandborn et al.: Patients randomised to 1:1:1:1 as follows, with stratification by prior biologic exposure: • placebo • 50 mg mirikizumab, with subsequent dosing by level checked at Weeks 2 and 6 [maximum dose 600 mg] • 200 mg mirikizumab, with subsequent dosing by level checked at Weeks 2 and 6 [maximum dose 600 mg] • 600 mg mirikizumab [fixed dose] All patients dosed at Weeks 0, 4, 8. Analyses performed looking at each treatment group as well as the combined mirikizumab group [all patients who received the study drug] At Week 12, mirikizumab responders then randomised 1:1 to receive maintenance therapy [200 mg subcutaneous mirikizumab] either every 4 or every 12 weeks until Week 52; placebo responders given subcutaneous sham injections every 4 weeks through Week 52 |
Sandborn et al.: Clinical remission [defined based on Mayo subscores: 0 for rectal bleeding, 0–1 for stool frequency [with decrease in 1 point from baseline], 0 for endoscopy] at Week 12 not statistically significant for 600 mg group vs placebo, p = 0.142. Other doses not compared with placebo as a result. Higher number of patients in remission across all treatment groups in biologic-naïve patients, compared with biologic-exposed. Sands et al.: Endoscopic response at Week 12, defined as reduction of >50% from baseline SES-CD score: met in all three treatment groups [statistical significance based on a p-value ≤0.1], with response achieved in 25.8% of the 200-mg group, 37.5% of the 600-mg group, and 43.8% of the 1000-mg group vs 10.9% of placebo [p200mg = 0.079, p600mg = 0.003, p1000mg <0.001] |
Sandborn et al.: Secondary outcomes: Clinical remission at Week 52 met in 53.7% of patients on every 4-week dosing and 39.7% of patients on every 12-week dosing Clinical response [defined as decrease in 9-point Mayo score of ≥2 points and ≥35% from baseline, with decrease in rectal bleeding ≥1 point or rectal bleeding score of 0 or 1] at Weeks 12 and 52: • Week 12-met in all treatment groups, regardless of prior biologic exposure [p50mg = 0.014, p200mg <0.001, p600mg = 0.001, pcombined <0.001] • Week 52 met in 80.9% of patients on every 4-week dosing and 76.1% of patients on every 12-week dosing Durable clinical remission [remission at Weeks 12 and 52] met in 61.1% of patients on every 4-week dosing and 38.5% of patients on every 12-week dosing Durable clinical response [response at Weeks 12 and 52]- met in 80.9% of patients on every 4-week dosing and 75% of patients on every 12-week dosing |
| Sands et al.: 191 patients ages 18–75 years, with at least 3-month history of moderate-to-severe CD (based on stool frequency ≥4 and/or abdominal pain ≥2 + SES-CD ≥7 [ileocolonic] or ≥4 [ileal disease only]) |
Sands et al.: Patients randomised 2:1:1:2 as follows, with stratification by prior biologic exposure: • placebo • 200 mg mirikizumab • 600 mg mirikizumab • 1000 mg mirikizumab All patients dosed at Weeks 0, 4, and 8. After Week 12, mirikizumab responders [defined as ≥1 point decrease in SES-CD from baseline] randomised to either continue their induction dosing regimen [200 mg IV, 600 mg IV, or 1000 mg IV every 4 weeks] + SC placebo every 4 weeks or 300 mg SC every 4 weeks + IV placebo every 4 weeks through Week 52 Patients in the mirikizumab induction groups without endoscopic improvement at Week 12 and those who were in the placebo group during induction were given 1000 mg IV + SC placebo every 4 weeks through Week 52 |
Endoscopic remission [Mayo endoscopic subscore of 0] at Weeks 12 and 52- • Week 12 no significant differences between treatment groups and placebo • Week 52 met in 14.9% of patients on every 4-week dosing and 28.3% of patients on every 12-week dosing Endoscopic improvement [Mayo endoscopic subscore of 0–1] at Week 12 met in 50-mg, 200-mg, and combined groups but not in 600-mg group [p50mg = 0.012, p200mg = 0.0007, p600mg = 0.215, pcombined = 0.006] Improvement in IBDQ from baseline at Weeks 12 and 52 • 12 weeks, met in 200-mg and 600-mg groups [p50mg = 0.062, p200mg = 0.0005, p600mg = 0.002, pcombined = not calculated] • 52 weeks, improved on average 61.7 points in every 4-week dosing group and 49.4 points in every 12-week dosing group |
||||
| Patients had to have a history of inadequate response or intolerance to 5-ASA, AZA, 6MP, steroids [or be steroid-dependent] and/or have prior biologic exposure • 67% of patients in placebo group and 60% of patients across all treatment groups had prior biologic exposure • 56.3% in the placebo group and 50% of patients across all treatment groups had a history of biologic failure Patients excluded if: • prior exposure to biologic agents targeting IL23-p19 • prior exposure to ustekinumab [exception: patients who received a single dose ≥12 weeks before inclusion] • exposure to natalizumab or B/T cell-depleting agents within 1 year of inclusion |
Exploratory objectives: Reduction in CRP, FC, IL-22 and IL-17A from baseline at Week 12: • FC lower in all treatment groups vs placebo [pcombined = 0.018] • CRP lower in all treatment groups vs placebo [pcombined = 0.004] • IL-22 lower in all treatment groups vs placebo [pcombined <0.0001] • IL-17A lower in all treatment groups vs placebo [pcombined <0.0001] Histological remission at Weeks 12 and 52 • Week 12 met in 200-mg, 600-mg and combined groups [p50mg = 0.632, p200mg = 0.001, p600mg = 0.028, pcombined = 0.006] • Week 52 met in 66% of patients on every 4-week dosing and 37% of patients on every 12-week dosing Symptomatic remission [stool frequency score 0–1, rectal bleeding score 0] at Weeks 12 and 52 |
|||||
| • exposure to any CD drug under investigation within 8 weeks of inclusion or 5 drug half-lives • exposure to interferon within 8 weeks of inclusion • complicated CD, defined as history of strictures, stenoses, or other complications that could require operative intervention • history of bowel resection or diversion within 6 months of inclusion • history of any kind of intra-abdominal surgery within 3 months of inclusion • presence of a stoma |
• Week 12- met in all treatment groups [p50mg = 0.054, p200mg <0.001, p600mg = 0.003, pcombined =<0.001] • Week 52 [with sustained symptomatic remission from Weeks 16–52]- met in 77.5% of patients on every 4-week dosing and 75.2% of patients on every 12-week dosing Sands et al.: Secondary outcomes: Endoscopic response at Week 52 [defined as ≥50% reduction in SES-CD from baseline]: re-randomised cohorts: • among patients with endoscopic improvement [reduction in SES-CD by at least 1 point from baseline] following induction: met in 58.5% of patients in IV group and 58.7% of patients in SC group • among patients with endoscopic response following induction: met in 69.6% of IV group and 66.7% of SC group |
|||||
| non-randomised cohorts: • among treatment group patients without endoscopic response following induction: met in 20% of patients • among placebo patients without endoscopic response following induction: met in 42.4% of patients Endoscopic remission at Weeks 12 and 52 [defined as SES-CD <4 for ileocolonic CD or <2 for isolated ileal CD, with no subscore >1]: • Week 12 met in the 600-mg and 1000-mg groups [p600mg = 0.01, p1000mg <0.001] |
||||||
| • Week 52: re-randomised cohorts: • among patients with endoscopic improvement following induction: met in 19.5% of IV group and 32.6% of SC group • among patients with endoscopic remission following induction: met in 50% of IV group and 64.3% of SC group non-randomised cohorts: • among treatment group patients without endoscopic response following induction: met in 13.3% of patients • among placebo patients without endoscopic response following induction: met in 18.6% of patients |
||||||
| Patient-reported outcome [PRO] of remission at Weeks 12 and 52 [defined as average daily stool frequency ≤2.5and average daily abdominal pain <1]: • Week 12 met in the 600-mg and 1000-mg groups [p600mg = 0.004, p1000mg = 0.013] • Week 52 re-randomised cohorts: • overall met in 46.3% of patients in IV group and 45.7% of patients in SC group • among patients with PRO remission at Week 1 met in 71.4% of patients in IV group and 66.7% of patients in SC group non-randomised cohorts: • Among treatment group patients without endoscopic response following induction: met in 36.7% of patients • among placebo patients without endoscopic response following induction: met in 40.7% of patients Change from baseline IBD-Q score: • Week 12 met in all treatment groups [p200mg < 0.001, p600mg <0.001, p1000mg <0.001] • Week 52 re-randomised cohorts: • overall- change in baseline score of 64.3 points in IV group and 66.4 points in SC group |
||||||
| non-randomised cohorts: Change from baseline IBD-Q • among treatment grouppatients without endoscopic response following i nduction: change from baseline 44.5 points • among placebo patients without endoscopic response following induction: change from baseline 53.6 points Exploratory objectives: Change in CRP and FC from baseline: • Week 12 median percent change in hsCRP from baseline significantly greater in all treatment groups [p200mg <0.001, p600mg <0.001, p1000mg <0.001]; greatest change in 1000-mg group • Week 12 median percent change in FC from baseline significantly greater in 600-mg and 1000-mg groups [p600mg <0.05, p1000mg <0.001]; greatest change in 1000-mg group • Week 52 median percent change in hsCRP similar across all groups, ranging from -45.9% to -59.5% • Week 52 median percent change in FC similar across all groups, ranging from -72.5% to -81% |
||||||
| CDAI response at Weeks 12 and 52 [defined as CDAI reduction of ≥100 points from baseline score or CDAI <150]: • Week 12 met in all treatment groups [p200mg = 0.015, p600mg = 0.001, p1000mg = 0.026] • Week 52 re-randomised cohorts: •met in 53.7% of patients in IV group and 69.6% of patients in SC group non-randomised cohorts: • among treatment group patients without endoscopic response following induction: met in 46.7% of patients • among placebo patients without endoscopic response following induction: met in 52.5% of patients CDAI remission at Weeks 12 and 52 [defined as CDAI <150]: • Week 12 met in the 600-mg and 1000-mg groups [p600mg <0.001, p1000mg = 0.013] • Week 52 |
||||||
| re-randomised cohorts: • overall- met in 39% of patients in IV group and 56.5% of patients in SC group • among patients with CDAI remission at Week 12 met in 69.2% of patients in IV group and 86.7% of patients in SC group non-randomised cohorts: • among treatment group patients without endoscopic response following induction: met in 23.3% of patients • among placebo patients without endoscopic response following induction: met in 40.7% of patients PRO response [defined as ≥30% reduction in stool frequency and/or abdominal pain; not worse than baseline]: • Week 12 met in all treatment groups [p200mg = 0.023, p600mg = 0.003, p1000mg = 0.006] • Week 52 re-randomised cohorts: • met in 68.3% of patients in IV group and 71.7% of patients in SC group non-randomised cohorts: • among treatment group patients without endoscopic response following induction: met in 60% of patients • among placebo patients without endoscopic response following induction: met in 61% of patients |
||||||
| Guselkumab | Monoclonal IgG1 lambda antibody to IL23p19 | Phase II, double-blind, placebo-controlled, multicentre clinical trial [NCT0346641, Sands et al.,19, D’Haens et al.20] | 250 patients with moderate-severe CD with failure of other therapies (biologics, i.e. anti-TNFs, vedolizumab; ‘conventional therapies’, i.e. corticosteroids, other immunosuppressants [not explicitly defined]); 50% had failed biologics previously |
Patients randomised 1:1:1:1:1 as follows: • 200 mg IV guselkumab at 0, 4, and 8 weeks • 600 mg IV guselkumab at 0, 4, and 8 weeks • 1200 mg IV guselkumab at 0, 4, and 8 weeks • ~6 mg/kg IV ustekinumab at Week 0 and 90 mg subcutaneously at Week 8[reference group] • Placebo [IV] Analyses performed looking at each treatment group as well as the combined guselkumab group [all patients who received the study drug] |
Sands et al.: Change in CRP from baseline, median change in combined guselkumab group, -2.17, vs -1.68 in ustekinumab group and 0 in placebo group Normalisation of CRP [≤3 mg/L] in patients with abnormal CRP at baseline, 35.4% in combined guselkumab group, vs 25% in ustekinumab group and 19.4% in placebo group Change in FC from baseline -176 in combined guselkumab group, vs -135.5 in ustekinumab group and +20 in placebo group Normalisation of FC [≤250 ug/g] in patients with abnormal FC at baseline, 33.3% in combined guselkumab group, vs 25% in ustekinumab group and 27.3% in placebo group Change in ‘clinical-biomarker response’ [defined as ≥100-point reduction in CDAI or CDAI <150 and ≥50% reduction in FC or CRP compared with baseline] • Overall 48% of combined guselkumab group, vs 7.8% in placebo group • Patients with history of biologic failure, 46.1% of combined guselkumab group, vs 8.7% of placebo • Patients with history of conventional treatment failure, 50% of combined guselkumab group, vs 7.1% of placebo |
D’Haens et al.: Dose-response or exposure-response relationship between guselkumab and endoscopic response, remission or healing, no significant relationships appreciated |
| D’Haens et al.: SES-CD change from baseline, mean reduction of -4.6 in combined guselkumab group, vs -4.1 in ustekinumab group and -0.5 in placebo group Endoscopic response [≥50% improvement from baseline or SES-CD <2] • Overall- 37.3% in combined guselkumab group, vs 30.6% in ustekinumab group and 11.8% in placebo group • Patients with history of biologic failure, 30.3% in combined guselkumab group, vs 19.6% in ustekinumab group and 13% in placebo group • Patients with history of conventional treatment failure, 44.6% in combined guselkumab group, vs 43.5% in ustekinumab group and 10.7% in placebo group Endoscopic healing [absence of mucosal ulcerations] • Overall: 17.3% in combined guselkumab group, vs 18.4% in ustekinumab group and 3.9% in placebo group • Patients with history of biologic failure, 11.8% in combined guselkumab group, vs 7.7% in usekinumab group and 8.7% in placebo group |
||||||
| • Patients with history of conventional treatment failure, 23% in combined guselkumab group, vs 30.4% in ustekinumab group and 0% in placebo group Endoscopic remission [SES-CD <2] • Overall, 14% in combined guselkumab group, vs 14.3% in ustekinumab group and 3.9% in placebo group • Patients with a history of biologic failure, 10.5% in guselkumab group vs 3.8% in ustekinumab group and 8.7% in placebo group • Patients with history of conventional treatment failure-17.6% in combined guselkumab group, vs 26.1% in ustekinumab group and 0% in placebo group |
IBD, inflammatory bowel diseaase; CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; CDEIS, Crohn’s Disease Endoscopic Index of Severity; SES-CD, Simple Endoscopic Score-CD; SC, subcutaneous; IV, intravenous; CRP, C-reactive protein; FC, faecal calprotectin; TNF, tumour necrosis factor; 5-ASA, 5-aminosalicylic acid; AZA, azathioprine; 6MP, 6-mercaptopurine; IBDQ, Inflammatory Bowel Disease Questionnaire.
2.2. Risankizumab
Risankizumab is a monoclonal IgG1 antibody to the IL23 p19 subunit which has delivered promising results in early studies. In a Phase II, blinded, randomised, placebo-controlled trial in patients with moderate-to-severe Crohn’s disease, risankizumab was found to be superior to placebo at all tested treatment doses in the induction phase for the primary outcome of clinical remission, defined as Crohn’s Disease Activity Index [CDAI] <150 at Week 12. Most secondary outcomes were also met, with significantly more patients in the pooled risankizumab group achieving clinical response [CDAI <150 or CDAI reduction from baseline ≥100], endoscopic remission or response based on reduction in Crohn’s Disease Endoscopic Index of Severity [CDEIS], and deep remission characterised by both clinical and endoscopic response to therapy. Notably, these findings were largely driven by results from the patients receiving higher-dose risankizumab, suggesting that a higher dose of medication during induction was associated with increased efficacy. Additional promising findings were seen in the open-label extension of this study, which revealed a higher proportion of patients in clinical remission at Week 26 compared with Week 12 in all treatment groups, as well as 71% of patients on maintenance dosing remaining in clinical remission at Week 52, though notably with lower rates of sustained endoscopic remission and healing. More favourable endoscopic outcomes were observed in patients who had been in the higher-dose induction group, though rates of clinical response and remission were similar at Week 52 regardless of induction dosing group.13,14,21
Additional support for the use of risankizumab in the treatment of moderate-to-severe CD was found in the Phase III ADVANCE and MOTIVATE trials, in which authors observed that patients treated with 600 mg or 1200 mg of risankizumab during the 12-week induction period were significantly more likely to achieve the co-primary endpoints of clinical remission [defined as CDAI <150 or average daily stool frequency of ≤2.8 and average daily abdominal pain score of <1] and endoscopic response (defined as reduction in Simple Endoscopic Score for Crohn’s Disease [SES-CD] >50% from baseline or at least a two-point reduction from baseline in patients with ileal disease only) as compared with placebo. These results were appreciated regardless of prior biologic exposure, though numerically higher rates of response were observed in patients who were biologic-naïve.15 No data are yet available on the efficacy of risankizumab in UC; however, Phase II/III randomised, double-blind, placebo-controlled studies examining induction and maintenance therapy in patients with moderate to severe UC are under way.7,13 Additionally, although there have not yet been trials comparing the efficacy of risankizumab with ustekinumab in IBD, data from Phase II and III clinical trials in patients with moderate-to-severe plaque psoriasis have demonstrated superior efficacy of risankizumab in this population, supporting the hypothesis that explicit targeting of IL-23 may be more effective than a combined blockade of IL-12 and IL-23.22,23
2.3. Brazikumab
Brazikumab, a monoclonal IgG2 antibody targeting the p19 subunit of IL-23, has also produced positive results in clinical trials. Findings from a Phase IIa clinical trial of patients with moderate-to-severe CD with objective evidence of active disease, who had either failed or were unable to tolerate anti-TNF medications, demonstrated a significant difference in clinical response [defined as reduction in ≥100 points on CDAI from baseline] at Week 8 in patients who received IV brazikumab at Weeks 0 and 4 as compared with placebo. A significant number of patients also achieved a composite outcome of either clinical response or clinical remission [CDAI <150] and a 50% decrease in either C-reactive protein [CRP] or faecal calprotectin [FC] from baseline at Weeks 8 and 12. Moreover, results from the open-label extension portion of the study [Weeks 12 to 112, during which all participants received subcutaneous maintenance dosing every 4 weeks] showed that patients who received brazikumab in the induction period and as maintenance therapy had similar rates of response to those who received placebo during induction and then started on open-label therapy after Week 12, suggesting that the medication may be effective even in the absence of intravenous induction.16
2.4. Mirikizumab
Mirikizumab is a humanised IgG4-isotype monoclonal antibody that also targets the p19 subunit of IL-23. Phase II trials for use in both moderate-to-severe UC and moderate-to-severe CD have yielded promising results.
Data from a Phase II study of patients with moderate-to-severe UC receiving mirikizumab induction therapy were largely positive. Although the primary outcome of the study was not achieved, as none of the three induction dose regimens of mirikizumab was significantly associated with clinical remission at Week 12 [defined as Mayo subscore of 0 for rectal bleeding, 0 or 1 for stool frequency with reduction of ≥1 point from baseline, and 0 or 1 for endoscopic findings], the trend in results was positive, with a numerically higher number of patients in all treatment arms demonstrating the desired clinical response compared with placebo. Treatment groups were also numerically more likely to achieve endoscopic improvement as well as symptomatic improvement (reflected by higher Inflammatory Bowel Disease Questionnaire [IBDQ] scores and lower Mayo Clinic scores) at Week 12, and patients in the two higher induction dosing groups were more likely to achieve histological response as compared with placebo. It is important to highlight that two of the three mirikizumab induction dosing regimens in this study were designed to be adjusted based on patient drug levels, and that the patients in these groups did not have a significantly better response than those receiving the standard, unadjusted treatment dose of 600 mg.
Following induction, a large percentage of patients in both maintenance treatment groups [with dosing either every 4 or every 12 weeks] had sustained clinical remission or response at Week 52, with more responders in the group receiving shorter interval dosing. Of note, most of the endpoints were achieved in patients regardless of prior biologic exposure, but several findings were more appreciable in patients who were biologic-naïve. Thus, whereas mirikizumab could be an appropriate option in patients with or without prior treatment with biologics, it is possible that this medication may be most effective as a first-line biologic agent in UC.17
Similarly promising results were observed in a Phase II study of mirikizumab for treatment of moderate-to-severe CD.18 Authors found that patients in all three treatment groups achieved the primary outcome of endoscopic response at Week 12 [defined as a reduction of 50% from baseline SES-CD score], with the most significant response appreciated in the group receiving the highest dose of the drug. Patients in all treatment groups were also significantly more likely to achieve endoscopic remission, patient-reported outcome [PRO] response or remission, and percent change in median high-sensitivity CRP [hsCRP] following induction as compared with placebo. Patients in the higher-dosing groups [600 mg or 1000 mg] also had significantly higher rates of remission based on CDAI score as well as percent change in FC following induction, versus patients receiving placebo. For both hsCRP and FC changes, the greatest improvements were appreciated in patients receiving the highest dose of the medication. Notably, clinical improvement [based on CDAI, PRO, and IBDQ] was observed in patients receiving mirikizumab as early as 4 weeks into the trial.
During the maintenance period [through Week 52 of therapy], patients who achieved an endoscopic response following induction were re-randomised to receive either IV or SC maintenance therapy every 4 weeks [while simultaneously receiving the placebo version of the form they were not being treated with, in order to maintain blinding], whereas those who did not achieve endoscopic response or received placebo during induction were all given IV therapy every 4 weeks. A notable proportion of patients in all maintenance groups achieved the desired endoscopic and symptomatic outcomes, with more appreciable results observed in patients who had achieved endoscopic or clinical response or remission during induction. Importantly, there did not appear to be a notable difference in outcomes between patients who received IV versus SC maintenance therapy, suggesting that these formulations may be similarly efficacious following induction.18
2.5. Guselkumab
Guselkumab, an IgG1-lambda monoclonal antibody to the p19 subunit of IL23, is currently being investigated as a potential therapy for moderate-to-severely active Crohn’s disease in Phase II/III clinical trials [GALAXI 1, 2, and 3].24 Interim analyses following 12-week induction in GALAXI 1 revealed a greater reduction in CRP and FC in patients treated with any dose of guselkumab compared with placebo. Additionally, combined clinical response [defined as ≥100-point reduction in baseline CDAI score or CDAI <150] and improvement in FC and CRP was found in more patients treated with guselkumab than placebo.19 Interim analyses of endoscopic improvement after treatment with guselkumab produced similarly positive results, with more patients in any guselkumab treatment group achieving endoscopic response, healing, or remission compared with patients who received placebo.20 Additionally, both studies revealed that patients treated with guselkumab were slightly numerically more likely to achieve some of the desired outcomes than those treated with ustekinumab [used as a reference group], which may further support the hypothesis that explicit targeting of IL-23 is more desirable than a combined IL-12/IL-23 blockade19,20. Longer-term outcomes from GALAXI will be important in determining the efficacy of guselkumab beyond the induction period.
3. Predictors of Response to IL-23 Targeted Therapy
3.1. Clinical predictors of response to IL-23 antagonists
Several of the aforementioned studies performed subgroup analyses to identify potential clinical predictors of therapeutic response to IL-23 inhibition. Some potentially promising patterns were observed [Table 2], but none of these was found to be significant and thus further research in this area is warranted. For example, Feagan et al. observed that patients who received the highest dose of risankizumab [600 mg] during induction had higher rates of mucosal healing and endoscopic remission and response during maintenance as compared with patients in other induction groups. However, no baseline characteristics [including CDAI score, disease duration, disease location, previous steroid or anti-TNF use, symptoms including stool frequency and abdominal pain, or active fistulising disease] were found to be significant predictors of sustained medication response in post-hoc analyses.14 Additionally, although not statistically significant, Sandborn et al. found that biologic-naïve UC patients in all mirikizumab treatment groups had numerically higher rates of clinical remission as compared with patients with previous biologic exposure.17 D’Haens et al. also appreciated this pattern in CD patients treated with any dose of risankizumab during induction, with numerically more biologic-naïve patients achieving endoscopic response and clinical remission at Week 12.15 Sands et al., however, found inconsistent patterns in response to mirikizumab, based on prior biologic exposure: authors observed numerically higher endoscopic response and remission rates with low-dose [200 mg] mirikizumab in biologic-naïve CD patients compared with biologic-exposed; however, this difference did not persist with higher doses of medication. Clinical markers of response based on previous biologic exposure also varied by dose in an unpredictable pattern; for example, greater rates of CDAI remission were observed in biologic-naïve patients at the 200 mg and 1000 mg doses, but not at the 600 mg dose. As such, a history of biologic exposure did not appear to be a reliable predictor of response in this study. Importantly, Sands et al. did observe that CD patients who achieved endoscopic and/or clinical response or remission following induction with mirikizumab were more likely to have sustained response to maintenance therapy,18 a finding which could aid in clinical decision making in patients without a significant response after 12 weeks of therapy. In summary, additional data are needed to better identify significant clinical predictors of response to IL-23 therapy.
Table 2.
Potential methods to predict and/or assess response to IL-23 inhibition
| Biomarker/predictor | Observed pattern[s] |
|---|---|
| Patient-based factors | • Higher induction dosing of risankizumab was associated with higher rates of endoscopic response/remission during maintenance therapy in patients with CD13 • Biologic-naïve UC patients had numerically higher rates of clinical remission with mirikizumab compared with patients with prior biologic exposure [though not statistically significant]17 • Biologic-naïve CD patients had numerically higher rates of clinical remission and endoscopic response with risankizumab compared with patients with prior biologic exposure [though not statistically significant]15 • CD patients with endoscopic and/or clinical response or remission following induction therapy with mirikizumab were more likely to have sustained response at Week 5218 |
| TNFR2+IL23+ T cells | • Patients with TNFR2+IL23+ T cells were significantly less likely to respond to anti-TNF therapy25 |
| IL-22 following therapy | • IL-22 levels decreased following mirikizumab induction therapy for treatment of UC17 • IL-22 gene expression in ileal tissue decreased following rizankizumab induction therapy for treatment of CD13 • IL-22 levels decreased following brazikumab induction therapy for treatment of CD16 • IL-22 levels remained suppressed after withdrawal of guselkumab therapy in psoriasis patients with sustained clinical response; IL-22 levels increased following recurrence of symptoms26 |
| IL-17 following therapy | • IL-17 levels decreased with mirikizumab induction therapy for treatment of UC17 • IL-17A and IL17F levels remained suppressed after withdrawal of guselkumab therapy in psoriasis patients with sustained clinical response; IL-17A and IL-17F levels increased following recurrence of symptoms26 |
| IL-22 prior to therapy | • Patients with IL-22 levels greater than the median threshold of 15.6 pg/mL were significantly more likely to achieve clinical response and remission following brazikumab induction therapy for treatment of CD16 • Patients with elevated IL-22 levels at baseline were significantly more likely to respond to direct IL-22 inhibition with fezakinumab for treatment of atopic dermatitis27 |
CD, Crohn’s disease; UC, ulcerative colitis.
3.2. Molecular predictors of response to IL-23 antagonists
Recent data have demonstrated that differences in the expression of certain T cell-associated surface proteins may be associated with an increased likelihood of response to IL-23 targeted therapy [Table 2]. In their study of patients with Crohn’s disease before and after treatment with anti-TNF agents, Schmitt et al. found that patients who did not respond to anti-TNF agents [i.e. did not achieve an SES-CD <5 with treatment] had significantly more activity in genes associated with IL23-R dependent pathways compared with TNF responders. Additionally, non-responders demonstrated significant upregulation of IL23p19, IL23-R, and IL17A, and associated downstream transcription factor pSTAT3 during treatment compared with responders. Further analyses revealed that non-responders had significantly more T cells expressing IL23-R in combination with TNF receptor 2 [TNFR2, a receptor that on its own was found to be associated with increased responsiveness to ant-TNF agents], and that expression of these particular TNFR2+IL23R+ T cells was increased in non-responders as they were exposed to anti-TNF agents. Medication-induced apoptosis was also reduced in patients with TNFR2+IL23R+ T cells. These findings identify an important potential predictor of response to IL-23 targeted therapy [as well as non-response to anti-TNF therapy].25
3.3. IL-17 and IL-22 as potential predictors of disease response
Several studies have also identified IL-22 and IL-17, both cytokines downstream of IL-23 [Figure 1], as predictors of response to IL-23 inhibition [Table 2]. In their trial comparing mirikizumab with placebo in UC patients, Sandborn et al. observed a reduction in IL-22 and IL-17 levels in all patients who received mirikizumab as compared with placebo. Although the direct relationship between disease activity and these cytokine levels was not evaluated, authors did find that patients who received any dose of mirikizumab during induction also had higher rates of symptomatic remission, lower Mayo scores, and reductions in CRP and FC at Week 12 compared with placebo.17 Feagan et al. appreciated a similar result when they compared IL-22 gene expression in ileal biopsies after 12 weeks of treatment with rizankizumab as compared with placebo.13 Consistent findings were also observed by Gordon et al. in the VOYAGE-2 study, a randomised controlled trial comparing guselkumab with adalimumab and placebo for the treatment of moderate-to-severe psoriasis. Authors monitored levels of IL-17A, IL-17F, and IL-22 after withdrawal of guselkumab therapy in certain randomised patients at Week 28 of the study, and found that patients who maintained clinical response despite medication withdrawal had ongoing suppression of IL-17A, IL-17F, and IL-22, whereas loss of response was associated with increased serum levels of IL-17A from Week 40, IL-17F from Week 36, and IL-22 from Week 44 as compared with the time of medication withdrawal. Notably, elevations in these markers occurred several weeks after patients developed recurrent symptoms, which began as early as Week 32 [4 weeks before elevation in any studied cytokine]. Additionally, results from logistic regression models demonstrated that cytokine levels were weak predictors of disease recurrence. As such, and given that cytokine levels tended to lag behind clinical symptoms, these data do not support the use of proactive monitoring of these cytokines to predict disease recurrence or flare while being treated with an anti-IL-23 agent.26 It is important to note that comparable studies have not yet been reported in IBD and may result in different findings, as the pathophysiology of gut inflammation in IBD differs from that of psoriasis in the skin.
Preliminary findings suggest that pre-treatment levels of IL-22 may also predict response to IL-23 inhibition [Table 2]. In their Phase IIa trial comparing brazikumab with placebo, Sands et al. found that IL-22 levels declined following treatment. Additionally, patients who had pre-treatment levels of IL-22 that were greater than or equal to the median threshold concentration of the population studied [≥15.6 pg/mL], were more likely to achieve clinical response [reduction in CDAI ≥100] and remission [CDAI <150] as compared with patients with baseline levels below 15.6 pg/mL.16 Brunner et al. found similar results when assessing IL-22 levels before and following direct IL-22 inhibition with fezakinumab in patients with atopic dermatitis. Like Sands et al., authors of this study found that patients with higher IL-22 levels at baseline were significantly more likely to respond to treatment.16,27 By contrast, Powell et al. assayed IL-22 in the colon tissue of patients with Crohn’s disease. In their analyses of data from the UNITI study [a Phase III trial assessing the efficacy of ustekinumab], the authors did not find that the expression of IL-22 responsive transcripts in colon tissue before initiation of ustekinumab could be used to predict response to treatment.28 These findings must be interpreted in the context of ustekinumab’s mechanism of action, which includes inhibition of both the IL-12 and IL-23 pathways, whereas IL-22 is a downstream cytokine of only the IL-23 pathway.
4. Application in Clinical Practice
Clinical trial data on the use of anti-IL23 agents for the treatment in IBD as well as other immune-mediate diseases have generated impressive results, with some data suggesting possible superiority to anti-TNFs.29 Additionally, the identification of possible predictors of disease response creates the potential for a tailored approach to therapy; if there is a point when testing for the presence of TNFR2+IL23R+ T cells and tissue IL-22 levels before initiation of therapy becomes readily available, the pathway for choosing therapy [i.e., whether or not to start with an anti-TNF agent versus an IL-23 inhibitor] may become much more clearly defined on an individual basis. The ability to trend downstream cytokines [IL-22, IL-17] in addition to usual inflammatory markers as a method for non-invasive disease activity monitoring also holds promise. Ultimately, additional data on both treatment efficacy and predictors of response [on both a clinical and a molecular level] are needed to understand how to most effectively employ IL-23 inhibitors in clinical practice.
Acknowledgements
The authors would like to thank Ni-ka Ford for her assistance with Figure 1, which has been printed with permission from ©Mount Sinai Health System.
Contributor Information
Zoë S Gottlieb, Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Bruce E Sands, Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Funding
This paper was published as part of a supplement financially supported by AbbVie.
Disclosures
BES discloses research grants from Takeda, Pfizer, Theravance Biopharma R&D, Janssen; consulting fees from 4D Pharma, Abbvie, Allergan, Amgen, Arena Pharmaceuticals, AstraZeneca, Bacainn Therapeutics, Boehringer-Ingelheim, Boston Pharmaceuticals, Capella Bioscience, Celgene, Celltrion Healthcare, F.Hoffmann-La Roche, Ferring, Gilead, Immunic, Index Pharmaceuticals, Ironwood Pharmaceuticals, Janssen, Lilly, Morphic Therapeutic, Oppilan Pharma, OSE Immunotherapeutics, Otsuka, Palatin Technologies, Pfizer, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Redhill Biopharma, Rheos Medicines, Salix Pharmaceuticals, Seres Therapeutics, Shire, Sienna Biopharmaceuticals, Surrozen, Takeda, Target PharmaSolutions, Theravance Biopharma R&D, USWM Enterprises, Viela Bio, Vivelix Pharmaceuticals.
Author Contributions
ZSG and BES reviewed the available literature and conceptualised the paper. ZSG drafted the manuscript, which was then edited by BES. Figure 1 was conceptualised by ZSG and BES and created by NF [see Acknowledgements].
References
- 1. Viscido A, Papi C, Latella G, Frieri G. Has infliximab influenced the course and prognosis of acute severe ulcerative colitis? Biologics 2019;13:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berg DR, Colombel JF, Ungaro R. The role of early biologic therapy in inflammatory bowel disease. Inflamm Bowel Dis 2019;25:1896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sands BE, Sandborn WJ, Panaccione R, et al. UNIFI Study Group. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019;381:1201–14. [DOI] [PubMed] [Google Scholar]
- 4. Feagan BG, Sandborn WJ, Gasink C, et al. UNITI–IM-UNITI Study Group. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–60. [DOI] [PubMed] [Google Scholar]
- 5. Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol 2019;16:185–96. [DOI] [PubMed] [Google Scholar]
- 6. Tang C, Chen S, Qian H, Huang W. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology 2012;135:112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Noviello D, Mager R, Roda G, Borroni RG, Fiorino G, Vetrano S. The IL23-IL17 immune axis in the treatment of ulcerative colitis: successes, defeats, and ongoing challenges. Front Immunol 2021;12:611256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol 2009;129:1339–50. [DOI] [PubMed] [Google Scholar]
- 9. Morishima Y, Ano S, Ishii Y, et al. Th17-associated cytokines as a therapeutic target for steroid-insensitive asthma. Clin Dev Immunol 2013;2013:609395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Truchetet ME, Mossalayi MD, Boniface K. IL-17 in the rheumatologist’s line of sight. Biomed Res Int 2013;2013:295132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allocca M, Furfaro F, Fiorino G, Gilardi D, D’Alessio S, Danese S. Can IL-23 be a good target for ulcerative colitis? Best Pract Res Clin Gastroenterol 2018;32–3:95–102. [DOI] [PubMed] [Google Scholar]
- 12. Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med 2003;198:1951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feagan BG, Sandborn WJ, D’Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 2017;389:1699–709. [DOI] [PubMed] [Google Scholar]
- 14. Feagan BG, Panés J, Ferrante M, et al. Risankizumab in patients with moderate to severe Crohn’s disease: an open-label extension study. Lancet Gastroenterol Hepatol 2018;3:671–80. [DOI] [PubMed] [Google Scholar]
- 15. D’Haens G, Panaccione R, Colombel JF, et al. Risankizumab induction therapy in patients with moderate-to-severe Crohn’s disease: results from the advance and motivate phase 3 studies, in Digestive Disease Week, Virtual, May 22–25. 2021. [Google Scholar]
- 16. Sands BE, Chen J, Feagan BG, et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: a phase 2a study. Gastroenterology 2017;153:77–86.e6. [DOI] [PubMed] [Google Scholar]
- 17. Sandborn WJ, Ghosh S, Panes J, et al. Efficacy of upadacitinib in a randomised trial of patients with active ulcerative colitis. Gastroenterology 2020;158:2139–49.e14. [DOI] [PubMed] [Google Scholar]
- 18. Sands BE, Sandborn WJ, Peyrin-Biroulet PD, et al. Efficacy and safety of mirikizumab after 52-weeks maintenance treatment in patients with moderate-to-severe Crohn’s disease. Gastroenterology 2021;160:S37. [Google Scholar]
- 19. Sands BE, Danese S, Andrews JM, et al. The effect of guselkumab induction therapy on inflammatory biomarkers in patients with moderately to severely active Crohn’s disease: week 12 results from the phase 2 GALAXI 1 study. Gastroenterology 2021;160:S-350–1. [Google Scholar]
- 20. D’Haens G, Rubin T, Panes J, et al. The effect of guselkumab induction therapy on endoscopic outcome measures in patients with moderately to severely active Crohn’s disease: week 12 results from the phase 2 GALAXI 1 study. Gastroenterology 2021;160:S-91. [Google Scholar]
- 21. Ma C, Panaccione R, Khanna R, Feagan BG, Jairath V. IL12/23 or selective IL23 inhibition for the management of moderate-to-severe Crohn’s disease? Best Pract Res Clin Gastroenterol 2019;38–9:101604. [DOI] [PubMed] [Google Scholar]
- 22. Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis [UltIMMa-1 and UltIMMa-2]: results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018;392:650–61. [DOI] [PubMed] [Google Scholar]
- 23. Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med 2017;376:1551–60. [DOI] [PubMed] [Google Scholar]
- 24. Janssen Research & Development. A Study of the Efficacy and Safety of Guselkumab in Participants With Moderately to Severely Active Crohn’s Disease [GALAXI]. 2021. https://clinicaltrials.gov/ct2/show/NCT03466411 Accessed June 9, 2021.
- 25. Schmitt H, Billmeier U, Dieterich W, et al. Expansion of IL-23 receptor bearing TNFR2+ T cells is associated with molecular resistance to anti-TNF therapy in Crohn’s disease. Gut 2019;68:814–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol 2019;139:2437–46.e1. [DOI] [PubMed] [Google Scholar]
- 27. Brunner PM, Pavel AB, Khattri S, et al. Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J Allergy Clin Immunol 2019;143:142–54. [DOI] [PubMed] [Google Scholar]
- 28. Powell N, Pantazi E, Pavlidis P, et al. Interleukin-22 orchestrates a pathological endoplasmic reticulum stress response transcriptional programme in colonic epithelial cells. Gut 2020;69:578–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol 2017;76:405–17. [DOI] [PubMed] [Google Scholar]