Abstract
Context
The Study to Understand Fall Reduction and Vitamin D in You (STURDY), a randomized trial enrolling older adults with low 25-hydroxyvitamin D [25(OH)D], demonstrated vitamin D supplementation ≥ 1000 IU/day did not prevent falls compared with 200 IU/day, with doses ≥ 2000 IU/day potentially showing safety concerns.
Objective
To examine associations of achieved and change in 25(OH)D concentrations after 3 months of vitamin D supplementation with fall risk.
Design
Observational analysis of trial data.
Setting
General community.
Participants
A total of 637 adults aged ≥ 70 with baseline 25(OH)D concentrations 10 to 29 ng/mL and elevated fall risk. Three-month on-treatment absolute 25(OH)D; absolute and relative changes from baseline.
Main Outcome Measures
Incident first fall (primary) and first consequential fall (injury or sought medical care) up to 24 months. Cox models were adjusted for sociodemographics, season, Short Physical Performance Battery, and body mass index.
Results
At baseline, mean (SD) age was 77.1 (5.4) years and 25(OH)D was 22.1 (5.1) ng/mL; 43.0% were women and 21.5% non-White. A total of 395 participants experienced ≥ 1 fall; 294 experienced ≥ 1 consequential fall. There was no association between absolute achieved 25(OH)D and incident first fall (30-39 vs < 30 ng/mL hazard ratio [HR], 0.93; 95% CI, 0.74-1.16; ≥40 vs < 30 ng/mL HR, 1.09; 95% CI, 0.82-1.46; adjusted overall P = 0.67), nor absolute or relative change in 25(OH)D. For incident consequential first fall, the HR (95% CI) comparing absolute 25(OH)D ≥ 40 vs < 30 ng/mL was 1.38 (0.99-1.90).
Conclusion
Achieved 25(OH)D concentration after supplementation was not associated with reduction in falls. Risk of consequential falls may be increased with achieved concentrations ≥ 40 ng/mL.
Trial Registration
ClinicalTrials.gov: NCT02166333
Keywords: vitamin D, 25-hydroxvitamin D, fall risk
Falls are common among older adults and are a leading cause of morbidity and mortality in this population [1]. According to data from the Centers for Disease Control and Prevention, more than 1 in 4 US adults aged ≥ 65 years fall each year, resulting in more than 2.8 million injuries treated in the emergency department, more than 800 000 hospitalizations, and more than 27 000 deaths annually [2]. Therefore, strategies to prevent falls are of critical public health importance.
Vitamin D is a fat-soluble vitamin necessary for the maintenance of bone mineral density (BMD) [3, 4]. Achieving optimal serum concentrations of 25-hydroxyvitamin D [25(OH)D], the storage form of vitamin D, may be important for the prevention of falls. Proposed mechanisms of vitamin D and reduction of falls include maintenance of serum calcium levels, regulation of immune and inflammatory response, preservation of BMD, neuromuscular control and coordination, and muscle function, which together may improve balance and gait speed [5-7]. Indeed, several prospective observational studies have linked low baseline 25(OH)D concentrations with increased fall risk [8-10].
Nevertheless, randomized controlled trials (RCTs) evaluating the efficacy of vitamin D supplementation on risk of falls have been inconsistent, with some trials suggested that vitamin D supplementation of 800 to 1000 IU/day may reduce falls risk [11-13], whereas other studies suggested no benefit [14-16], and yet other trials testing relatively high doses of vitamin D documented an increased risk of falls [17-19]. Furthermore, there had also been heterogeneity across these trials in regard to whether 25(OH)D concentrations were even measured at baseline, at follow-up, or both [20].
In this context, the National Institute on Aging funded the STURDY (Study to Understand Fall Reduction and Vitamin D in You) trial, which tested the hypothesis that daily high-dose vitamin D supplementation would reduce the rate of falls among older adults with elevated falls risk and serum 25(OH)D concentrations of 10 to 29 ng/mL [5, 6]. The main STURDY trial results have recently been published [21]. The principal trial finding was that vitamin D3 supplementation ≥ 1000 IU/day did not prevent falls compared with 200 IU/day. In addition, several analyses raised safety concerns about vitamin D3 doses ≥ 1000 IU/day [21, 22].
Still, prior studies have suggested that the benefit of vitamin D supplementation may depend on not only baseline 25(OH)D concentrations but also achieved 25(OH)D concentration while supplementing [11]. Few studies have examined the relationship of falls risk with achieved or change in 25(OH)D concentrations from vitamin D supplementation. Therefore, in this prespecified observational analysis of the STURDY trial, we examined the association of achieved 25(OH)D concentrations with incident fall rates irrespective of treatment assignment. We hypothesized that the risk of falls would be lower among those with absolute achieved 25(OH)D concentrations of ≥ 30 ng/mL (the threshold of vitamin D status deemed to be adequate by the Endocrine Society [23]), compared with those whose concentrations remained < 30 ng/mL after 3 months of supplementation. In exploratory analyses, we hypothesized that those with greater absolute and relative change in 25(OH)D, compared with those with less change, would also have reduced falls risk.
Materials and Methods
Setting
The STURDY trial design and methods and main results have been published [5, 6, 21]. Briefly, STURDY was a double-masked, 2-stage, Bayesian, response-adaptive RCT designed to select the best dose of vitamin D supplementation for fall prevention from 3 candidate high vitamin D doses (dose-finding stage) and, if a best dose was selected, potentially confirm the efficacy of that dose for falls prevention compared with the control dose (confirmatory stage). A Johns Hopkins University institutional review board approved the protocol, and a data and safety monitoring board approved the protocol and monitored the trial. Each participant provided written informed consent.
Beginning on October 30, 2015, participants were randomized to 1 of 4 doses of vitamin D3 supplements: 200 IU/day (control), 1000 IU/day, 2000 IU/day, and 4000 IU/day and followed for incident falls for up to 2 years. During the dose-finding stage, the 1000 IU/day dose was found to be the dose associated with the lowest rate of falls among the 3 noncontrol doses and was selected as the best noncontrol dose. Enrolled participants who had been randomized to 2000 IU/day or 4000 IU/day were then switched to 1000 IU/day, and new enrollees were randomized 1:1 to 1000 IU/day or control (200 IU/day). In February 2019, after enrollment of 688 participants, the data and safety monitoring board recommended terminating the trial early for futility, after conditional power analysis indicated the trial had sufficient power to address its primary objective. Follow-up ended on May 31, 2019.
Study participants
Participants were recruited at 2 field centers in Maryland (the ProHealth Center located in west Baltimore and the George W Comstock Center in Hagerstown in Washington County), both at approximately 39 °C latitude. Eligible participants were community-dwelling adults aged ≥ 70 years with serum 25(OH)D concentrations of 10 to 29 ng/mL at baseline and elevated fall risk. Elevated fall risk was determined by self-report of 1 of the following: an injurious fall in the past year, 2 or more falls in the past year regardless of injury, fear of falling because of balance or walking problems, difficulty maintaining balance, or use of an assistive device when walking. Exclusion criteria included cognitive impairment (Mini-Mental State Examination score < 24); hypercalcemia (serum calcium levels > 10.5 mg/dL); kidney, bladder, or ureteral stone (1 recent or ≥ 2 lifetime); or use of personal supplements of vitamin D > 1000 IU/day or of calcium > 1200 mg/day.
Serum 25(OH)D assessment
Blood samples were collected in the nonfasting state through standard venipuncture technique at baseline and 3, 12, and 24 months after randomization. Serum concentrations of 25(OH)D3 and 25(OH)D2 were measured at the University of Maryland School of Medicine’s Clinical Core Research Laboratory (Baltimore, MD) using liquid chromatography-mass spectrometry calibrated to meet guidelines set for the National Institute of Standards and Technology [24]. Measurements from this core laboratory are certified by the Centers for Disease Control and Prevention Vitamin D Standardization-Certification Program. The coefficients of variation for 25(OH)D3 and 25(OH)D2 were 4.4% at a concentration of 10 ng/mL and 4.2% at concentrations of 20 ng/mL; bias was < 1.5%. The lower limit of detection for both analytes was 2 ng/mL and the limit of quantitation was 4 ng/mL. Concentrations of 25(OH)D3 and 25(OH)D2 were summed to determine the total 25(OH)D concentration. To convert ng/mL to nmol/L, multiply ng/mL by 2.5.
For this analysis, we evaluated 25(OH)D concentrations at baseline and 3 months after randomization to 1 of the 4 study doses of vitamin D3 supplements. The 3-month follow-up visit was chosen so it would allow sufficient time to reach a steady state of serum 25(OH)D concentrations after supplementation, and it maximized the number of individuals who had an on-treatment 25(OH)D concentration measured.
Fall Ascertainment
The trial used the World Health Organization definition of a fall—any fall, slip, or trip in which the participant lost his or her balance and landed on the floor or ground or at a lower level [25]. Falls were ascertained via 3 surveillance methods: fall calendars that the participants filled out daily and mailed monthly, interviews at scheduled clinic and telephone visits, and ad hoc telephone interviews (participants were instructed to call the clinic if they had a fall and staff called participants whose monthly calendar was not received). Falls data were collected throughout a participant’s follow-up, even after a first fall occurred. For each reported fall, the participant was interviewed about details of the fall. A fall was classified as “consequential” if the participant reported any injury or if medical care was sought. A participant’s first consequential fall, if it occurred, might not be the first fall reported by the participant; for example, if the first fall reported by the participant involved no injury or medical care being sought and the participant subsequently reported a fall with injury or medical care, then the later reported fall was the participant’s first consequential fall.
Participant Characteristics at Entry
Relevant participant characteristics at study entry were ascertained by interviews, questionnaires, physical examination, and functional testing. Dietary intake of vitamin D was estimated with the modified Calcium and Vitamin D Frequency Questionnaire [26]. Physical function was assessed with the Short Physical Performance Battery (SPPB) [27]. The SPPB includes 3 tests (standing balance, gait speed, chair rises), each scored 0 to 4; the SPPB score is calculated as the sum of the scores and ranges from 0 to 12, with higher score indicating better physical performance [5]. Frailty status was assessed using the criteria of Fried et al [28].
Statistical Methods
The primary exposure (independent) variable for this analysis was achieved 25(OH)D concentration at 3 months after start of supplementation defined as the absolute 25(OH)D concentration at that time and categorized into 3 strata (<30, 30-39, ≥40 ng/mL). In exploratory analyses, we also examined achieved 25(OH)D in 2 other ways. First, absolute change in 25(OH)D was calculated as follow-up concentration minus baseline concentration, and categorized into 3 strata (lowest 25%, middle 50%, highest 25%). Second, relative change in 25(OH)D was calculated as (follow-up concentration minus baseline concentration)/baseline concentration, and categorized into 3 strata (lowest 25%, middle 50%, highest 25%).
Participant baseline characteristics were examined by achieved 25(OH)D concentration (3 categorizations described previously) using means and SDs or medians and quartiles for continuous variables and frequency and percentage for categorical variables.
The primary outcome (dependent variable) for this paper was time to first fall; secondary outcomes included time to first consequential fall, rate of all falls, and rate of all consequential falls. Each outcome was evaluated on each of the 3 categorizations of achieved 25(OH)D concentration (absolute level, absolute change, relative change), resulting in 12 analyses (3 classifications × 4 outcomes).
A Cox proportional hazards regression model was used to compare the time from randomization to first fall for each higher achieved 25(OH)D category vs the lowest. Each hazard ratio (HR) and its 95% CI were derived from models adjusted for baseline covariates of age (continuous), race/ethnicity (other vs non-Hispanic White), sex (male vs female), education (more than high school vs high school or less), SPPB score (continuous), enrollment site location (Hagerstown vs Baltimore), body mass index (continuous), and month of randomization as proxy for season (11 indicator variables). Participants who did not fall were censored at their date of last observation or 2 years after randomization.
Negative binomial regression models were used to compare cumulative fall rate for each higher achieved 25(OH)D category vs the lowest; each model included an offset term for the participant’s observation time and adjusted for the covariates described previously. For these cumulative fall analyses, each participant’s observation time was their duration from randomization to date of last contact in the trial, and fall counts were top-coded at the value equal to the 99th percentile of the nonzero fall counts to reduce the influence of outliers.
Because our exposure categories used the serum 25(OH)D concentration at 3 months and our analyses included all falls observed from randomization through end of follow-up, we repeated all analyses of fall outcomes after excluding falls occurring in the first 90 days after randomization.
We used the Benjamini-Hochberg procedure to control the false discovery rate to below 1 of 12 (1/12 = 0.08), the number of overall analyses presented in this paper [29]. The unadjusted or nominal 2-sided overall P value and the Benjamini-Hochberg adjusted overall P value are shown for each analysis. An adjusted P value is statistically significant if < 0.08. Unadjusted or nominal P values are shown for each higher achieved 25(OH)D category vs the lowest.
All analyses were conducted in SAS version 9.4 (Cary, NC), Stata version 15 (College Station, TX), or R-v4.0.3 (https://www.r-project.org/).
Results
Of the 688 participants enrolled in STURDY, 637 (92.6%) completed a 3-month follow-up visit at which serum 25(OH)D was measured and thus were included in these analyses. Median observation time from randomization to end of follow-up for these 637 participants was 22.5 months (quartile 25 [Q25], Q75: 13.4, 24.1). A total of 395 participants experienced ≥ 1 fall and 294 experienced ≥ 1 consequential fall. A total of 1409 falls occurred during follow-up.
Achieved Serum 25(OH)D after 3 Months of Supplementation
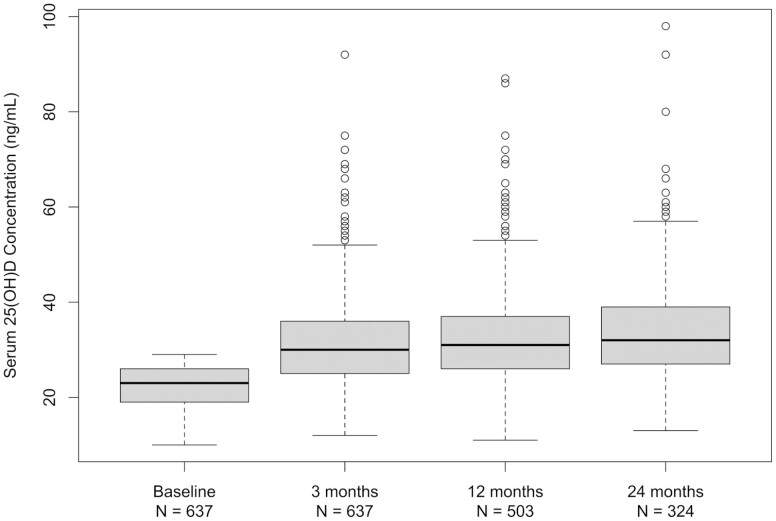
Box plots of absolute serum 25(OH)D concentration over time in Fig. 1 show that median 25(OH)D rose from 23 ng/mL at randomization to 30 ng/mL at 3 months, followed by maintenance of that level at 12 and 24 months (31 and 32 ng/mL, respectively). No participant had 25(OH)D concentration < 11 or > 98 ng/mL at any measurement time after randomization.
Figure 1.
Serum 25(OH)D concentration over time. Box plots of serum 25(OH)D concentration (ng/mL) before random assignment to vitamin D supplementation of 200, 1000, 2000, or 4000 IU/day and 3, 12, and 24 months later. To convert 25(OH)D ng/mL to nmol/L, multiply ng/mL by 2.5.
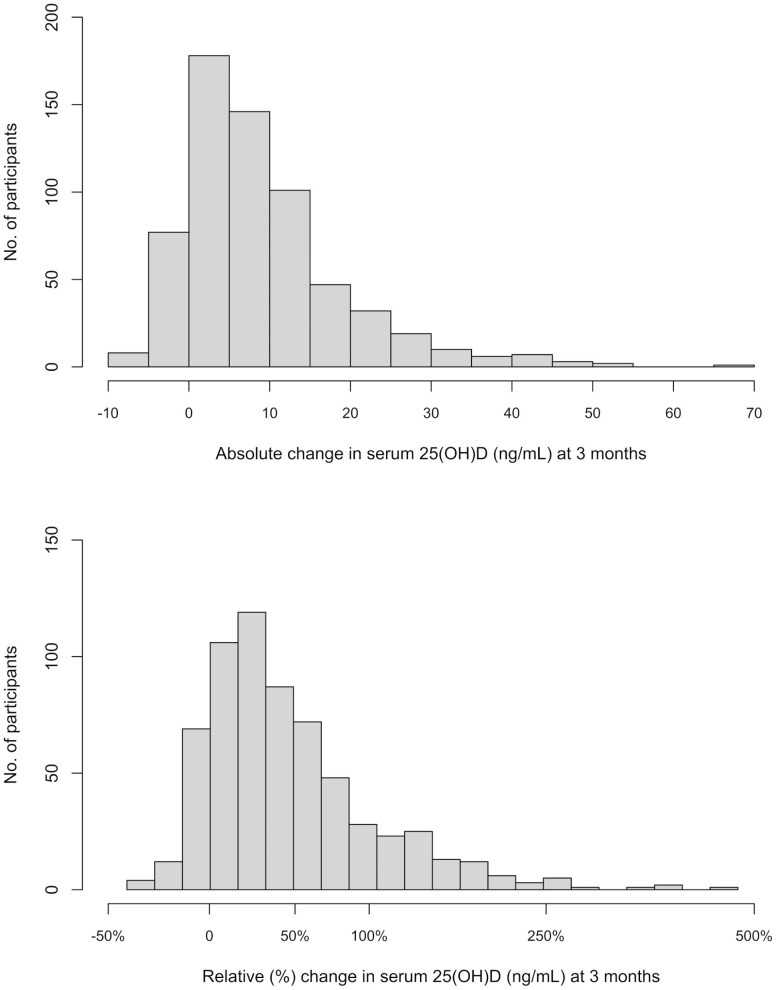
Histograms of the absolute change and relative change in 25(OH)D from baseline to 3 months are shown in Fig. 2A and 2B, respectively. Median absolute change at 3 months was 7 ng/mL and median relative change was 33%. Notably, 58 participants had a decrease in 25(OH)D concentration at 3 months; 27 participants had no change.
Figure 2.
Change in serum 25(OH)D concentration after 3 months of vitamin D supplementation. (A) Histogram of absolute change in serum 25(OH)D concentration in 637 participants 3 months after randomization to the vitamin D study pill (dose 200, 1000, 2000, or 4000 IU/day). Absolute change in serum 25(OH)D was calculated as concentration at 3 months minus concentration at baseline. Changes were sorted into bins of width 5 ng/mL. To convert 25(OH)D ng/ml to nmol/L multiply the ng/ml by 2.5. (B) Histogram of relative (%) change in serum 25(OH)D in 637 participants 3 months after randomization to the vitamin D study pill (dose 200, 1000, 2000, or 4000 IU/day). Relative change in serum 25(OH)D was calculated as (concentration at 3 months minus concentration at baseline) divided by concentration at baseline. Fifty-eight participants had a percent decrease (3% to 36%), 27 participants had no change, and 552 participants had a percent increase in serum 25(OH)D at 3 months relative to baseline. The x-axis uses a log scale.
After categorization of absolute 25(OH)D concentration at 3 months into 3 strata of < 30 ng/mL, 30 to 39 ng/mL, and ≥ 40 ng/mL and categorization of the 3-month absolute changes into the 3 ordered strata of lower 25%, middle 50%, and upper 25% (-9 to 2 ng/mL, 3-14 ng/mL, and 15-66 ng/mL, respectively), and similarly for the 3-month relative changes (-36% to 10%, 11%-70%, and 71%-455%, respectively), 380 individuals (59.7%) were in a stratum of the same order by all 3 categorizations, 28 (4.4%) were never in the same stratum, and 229 (35.9%) had 2 categorizations being concordant but not all 3.
Participant Characteristics at Study Entry
Baseline participant characteristics by achieved 25(OH)D concentrations, across each of the 3 categorizations of achieved level, are shown in Table 1. Overall, 43.0% of participants were female, 17.4% were Black race, and 3.8% were of other minority race/ethnicity. The mean age at baseline was 77.1 (SD 5.4) years and mean 25(OH)D concentration was 22.1 (SD 5.1) ng/mL
Table 1.
Characteristics of participants at baseline by strata of achieved serum 25(OH)D concentration
| Level at 3 mo (ng/mL) | Absolute change from BL to 3 mo (FU-BL; ng/mL) | Relative change from BL to 3 mo (FU-BL)/BL; %) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ≤29 (N = 299) |
30-39 (N = 242) |
>40 (N = 96) |
Lower 25%: -9 to 2 (N = 147) |
Middle 50%: 3-14 (N = 350) |
Upper 25%: 15-66 (N = 140) |
Lower 25%: -36 to 10 (N = 159) |
Middle 50%: 11-70 (N = 320) |
Upper 25%: 71-455 (N = 158) |
|
| Age, mean ± SD, y | 77.1 ± 5.3 | 76.9 ± 5.2 | 77.8 ± 6.0 | 77.0 ± 5.2 | 77.1 ± 5.3 | 77.4 ± 5.5 | 77.2 ± 5.4 | 76.8 ± 5.3 | 77.8 ± 5.3 |
| Female, no. | 121 (40.5%) | 106 (43.8%) | 47 (49.0%) | 58 (39.5%) | 147 (42.0%) | 69 (49.3%) | 65 (40.9%) | 132 (41.3%) | 77 (48.7%) |
| Race/ethnicitya, no. | |||||||||
| White, non-Hispanic | 234 (79.6%) | 183 (76.9%) | 76 (80.9%) | 118 (81.4%) | 268 (78.1%) | 107 (77.5%) | 128 (81.5%) | 244 (78.0%) | 121 (77.6%) |
| Black | 50 (17.0%) | 47 (19.7%) | 12 (12.8%) | 21 (14.5%) | 62 (18.1) | 26 (18.8%) | 23 (14.6%) | 57 (18.2%) | 29 (18.6%) |
| Other | 10 (3.4%) | 8 (3.4%) | 6 (6.4%) | 6 (4.1%) | 13 (3.8%) | 5 (3.6%) | 6 (3.8%) | 12 (3.8%) | 6 (3.8%) |
| No. missing | 5 | 4 | 2 | 2 | 7 | 2 | 2 | 7 | 2 |
| High school graduate or less, no. | 51 (17.1%) | 47 (19.4%) | 20 (20.8%) | 16 (10.9%) | 66 (18.9%) | 36 (25.7%) | 17 (10.7%) | 55 (17.2%) | 46 (29.1%) |
| Married or like relationship, no. | 176 (58.9%) | 124 (51.2%) | 48 (50.0%) | 91 (61.9%) | 197 (56.3%) | 60 (42.9%) | 97 (61.0%) | 178 (55.6%) | 73 (46.2%) |
| BMI (kg/m2), mean ± SD | 31.2 ± 6.0 | 30.1 ± 5.6 | 30.2 ± 6.8 | 31.0 ± 6.0 | 30.6 ± 6.1 | 30.0 ± 5.8 | 30.8 ± 6.0 | 30.8 ± 6.1 | 29.9 ± 5.8 |
| No. missing | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Current cigarette smoker, no. | 6 (2.0%) | 8 (3.3%) | 5 (5.2%) | 2 (1.4%) | 12 (3.4%) | 5 (3.6%) | 3 (1.9%) | 10 (3.1%) | 6 (3.8%) |
| Alcoholic drinks/wk, median (Q25, Q75) | 0 (0, 3) | 0 (0,4) | 0 (0, 5) | 0 (0, 4) | 0 (0, 3) | 0 (0, 4) | 0 (0, 4) | 0 (0, 3) | 0 (0, 4) |
| SPPBb score, median (Q25, Q75) | 9 (8, 10) | 9 (8,11) | 9 (7, 10) | 9 (8, 10) | 9 (8, 11) | 9 (7.5, 10) | 9 (8, 11) | 9 (8, 11) | 9 (7, 10) |
| Gait speed (m/s), mean ± SD | 0.86 ± 0.24 | 0.88 ± 0.22 | 0.86 ± 0.24 | 0.88 ± 0.23 | 0.87 ± 0.24 | 0.85 ± 0.23 | 0.88 ± 0.23 | 0.88 ± 0.24 | 0.84 ± 0.23 |
| No. missing | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| No. of morbid conditions, median (Q25, Q75)c | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) |
| Frailty statusd, no. (%) | |||||||||
| Robust | 88 (29.5%) | 71 (29.3%) | 31 (32.3%) | 43 (29.3%) | 105 (30.1%) | 42 (30.0%) | 44 (27.7%) | 103 (32.3%) | 43 (27.2%) |
| Prefrail | 175 (58.7%) | 144 (59.5%) | 55 (57.3%) | 91 (61.9%) | 201 (57.6%) | 82 (58.6%) | 101 (63.5%) | 177 (55.5%) | 96 (60.8%) |
| Frail | 35 (11.7%) | 27 (11.2%) | 10 (10.4%) | 13 (8.8%) | 43 (12.3%) | 16 (11.4%) | 14 (8.8%) | 39 (12.2%) | 19 (12.0%) |
| No. missing | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Study site location, no. | |||||||||
| Hagerstown, MD | 137 (45.8%) | 113 (46.7%) | 48 (50.0%) | 62 (42.2%) | 164 (46.9%) | 72 (51.4%) | 60 (37.7%) | 155 (48.4%) | 83 (52.5%) |
| Baltimore, MD | 162 (54.2%) | 129 (53.3%) | 48 (50.0%) | 85 (57.8%) | 186 (53.1%) | 68 (48.6%) | 99 (62.3%) | 165 (51.6%) | 75 (47.5%) |
| 25(OH)D (ng/mL) | |||||||||
| Median (Q25, Q75) | 22 (17, 25) | 24.5 (21, 28) | 23 (18.5, 27) | 25 (23, 27) | 23 (19, 26) | 20 (15, 24) | 26 (23, 28) | 23 (20, 26) | 17 (14, 21) |
| Mean ± SD | 20.9 ± 5.1 | 23.6 ± 4.6 | 22.3 ± 5.1 | 24.6 ± 3.5 | 22.1 ± 5.0 | 19.6 ± 5.4 | 25.1 ± 3.4 | 22.8 ± 4.4 | 17.9 ± 5.0 |
| Dietary vitamin De (IU/d), median (Q25, Q75) | 182 (118, 276) |
178 (104, 286) |
162 (108, 260) |
194 (126, 301) |
180.5 (103.5, 280.5) |
146 (101, 260) |
201 (127, 304) |
182.5 (106, 285) |
147 (95, 237) |
| No. missing | 2 | 0 | 1 | 0 | 2 | 1 | 0 | 2 | 1 |
| Personal supplemental vitamin D (IU/d) | |||||||||
| Any, no. | 93 (31.1%) | 113 (46.7%) | 33 (34.4%) | 69 (46.9%) | 134 (38.3%) | 36 (25.7%) | 78 (49.1%) | 128 (40.0%) | 33 (20.9%) |
| For those with any, median (Q25, Q75) | 700 (400, 1000) | 800 (500, 1000) | 600 (429, 1000) | 643 (400, 1000) | 800 (500, 1000) | 586 (400, 900) | 700 (400, 1000) | 800 (500, 1000) | 571 (400, 800) |
| Month randomized, no.f | |||||||||
| January, February, or March | 47 (15.7%) | 33 (13.6%) | 18 (18.8%) | 23 (15.7%) | 49 (14.0%) | 26 (18.6%) | 28 (17.6%) | 46 (14.4%) | 24 (15.2%) |
| April, May, June | 111 (37.1%) | 109 (45.0%) | 43 (44.8%) | 47 (32.0%) | 152 (43.4%) | 64 (45.7%) | 49 (30.8%) | 137 (42.8%) | 77 (48.7%) |
| July, August, September | 89 (29.8%) | 61 (25.2%) | 27 (28.1%) | 45 (30.6%) | 93 (26.6%) | 39 (27.9%) | 45 (28.3%) | 87 (27.2%) | 45 (28.5%) |
| October, November, December | 52 (17.4%) | 39 (16.1%) | 8 (8.3%) | 32 (21.8%) | 56 (16.0%) | 11 (7.9%) | 37 (23.3%) | 50 (15.6%) | 12 (7.6%) |
To convert 25(OH)D ng/ml to nmol/L, multiply ng/mL by 2.5.
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BL, baseline; BMI, body mass index; FU, follow-up; Q, quartile; SPPB, Short Physical Performance Battery.
a Race/ethnicity was self-reported.
b The SPPB is a 3-part assessment of physical functioning: balance testing, timed 4-m walk at usual pace, and demonstration of ability to complete 5 chair stands; each part is scored 0 to 4, and the total SPPB score (range, 0-12) is the sum of the 3 subscores. Higher scores indicate better physical function.
c Participant was asked about 14 morbid conditions (“have you ever been told by a physician that you had”): cancer other than basal cell skin cancer, heart disease, high cholesterol, hypertension, stroke, peripheral vascular disease, chronic lung disease, diabetes, kidney disease, liver disease, connective tissue disease, arthritis, Parkinson disease, multiple sclerosis.
d Physical frailty phenotype was determined using the criteria of Fried et al (2001); frailty status is determined based on conditions of weight loss, exhaustion, slow gait speed, low grip strength, and low physical activity; frail (3 conditions present), prefrail (1 or 2 conditions), and robust (no conditions).
e Dietary vitamin D intake was estimated from the modified Calcium and Vitamin D Frequency Questionnaire (Taylor et al, 2009).
f Counts of persons randomized are pooled over 3 mo; however, ≥ 2 persons in each stratum of each classification were randomized during each month.
Achieved Serum 25(OH)D by Randomized Dose
Table 2 shows the distribution of participants in each randomized dose group across the 3 strata of each definition of achieved 25(OH)D at 3 months. The percentage of participants in the lowest stratum of achieved 25(OH)D decreased as dose increased and the percentage of participants in the highest stratum of achieved 25(OH)D increased as dose increased, regardless of definition of achieved 25(OH)D.
Table 2.
Distribution of participants in each randomized dose group across strata of achieved serum 25(OH)D concentration
| 200 IU/d (N = 314) |
1000 IU/d (N = 195) |
2000 IU/d (N = 64) |
4000 IU/d (N = 64) |
|
|---|---|---|---|---|
| Level at 3 mo, ng/mL | ||||
| ≤ 29 | 214 (68.2%) | 67 (34.4%) | 15 (23.4%) | 3 (4.7%) |
| 30-39 | 96 (30.6%) | 101 (51.8%) | 33 (51.6%) | 12 (18.8%) |
| > 40 | 4 (1.3%) | 27 (13.8%) | 16 (25.0%) | 49 (76.6%) |
| Absolute change from BL to 3 mo (FU-BL, ng/mL) | ||||
| Lower 25%: -9 to 2 | 119 (37.9%) | 22 (11.3%) | 5 (7.8%) | 1 (1.6%) |
| Middle 50%: 3-14 | 179 (57.0%) | 129 (66.2%) | 33 (51.6%) | 9 (14.1%) |
| Upper 25%: 15-66 | 16 (5.1%) | 44 (22.6%) | 26 (40.6%) | 54 (84.4%) |
| Relative change from BL to 3 months ((FU-BL)/BL, (%)) | ||||
| Lower 25%: -36 to 10 | 127 (40.4%) | 26 (13.3%) | 5 (7.8%) | 1 (1.6%) |
| Middle 50%: 11-70 | 152 (48.4%) | 125 (64.1%) | 32 (50.0%) | 11 (17.2%) |
| Upper 75%: 71-455 | 35 (11.1%) | 44 (22.6%) | 27 (42.2%) | 52 (81.3%) |
To convert 25(OH)D ng/ml to nmol/L, multiply ng/mL by 2.5.
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BL, baseline; FU, follow-up.
Fall Outcomes by Achieved 25(OH)D
The fall outcomes by categories of achieved 25(OH)D at 3 months are shown in Table 3 for all 3 definitions of achieved 25(OH)D. Incident first fall rates per 100 person-years were 78.2, 67.4, and 81.8, respectively for the 3 strata defined by absolute 25(OH)D concentration. The HRs (95% CI) for incident first fall were 0.93 (0.74-1.16) and 1.09 (0.82-1.46) for absolute achieved concentrations of 30 to 39 and ≥ 40 ng/mL, respectively, each vs < 30 ng/mL. These HRs were not statistically different (unadjusted overall P = 0.55; adjusted overall P = 0.67). Similarly, there was no difference in time to first fall among the 3 strata when achieved 25(OH)D at 3 months was defined as absolute change, nor when defined as relative change.
Table 3.
Fall outcomes by 3 definitions of achieved serum 25(OH)D concentration
| Level at 3 mo, ng/mL | Absolute change from BL to 3 mo (FU-BL), ng/mL) | Relative change from BL to 3 mo ((FU-BL)/BL), % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ≤ 29 (N = 299) |
30-39 (N = 242) |
> 40 (N = 96) |
Lower 25%: -9 to 2 (N = 147) |
Middle 50%: 3-14 (N = 350) |
Upper 25%: 15-66 (N = 140) |
Lower 25%: -36 to 10 (N = 159) |
Middle 50%: 11-70 (N = 320) |
Upper 25%: 71-455 (N = 158) |
|
| First fall | |||||||||
| No. of events | 188 | 141 | 66 | 97 | 205 | 93 | 104 | 188 | 103 |
| No. of PYa | 240.3 | 209.2 | 80.7 | 102.6 | 305.5 | 122.1 | 112.5 | 281.5 | 136.3 |
| Rate per 100 PY | 78.2 | 67.4 | 81.8 | 94.5 | 67.1 | 76.2 | 92.5 | 66.8 | 75.6 |
| HRb, vs reference | Reference | 0.93 | 1.09 | Reference | 0.83 | 0.94 | Reference | 0.84 | 0.95 |
| 95% CIb | 0.74-1.16 | 0.82-1.46 | 0.64-1.06 | 0.70-1.26 | 0.66-1.08 | 0.71-1.27 | |||
| P, vs reference unadjustedb | 0.51 | 0.55 | 0.14 | 0.67 | 0.17 | 0.74 | |||
| P, overall unadjusted (adjusted)c | 0.55 (0.67) | 0.29 (0.60) | 0.34 (0.60) | ||||||
| First consequential falld | |||||||||
| No. of events | 133 | 106 | 55 | 67 | 152 | 75 | 71 | 143 | 80 |
| No. of PYa | 312.5 | 255.0 | 97.6 | 147.1 | 374.1 | 143.9 | 159.3 | 339.3 | 166.5 |
| Rate per 100 PY | 42.6 | 41.6 | 56.4 | 45.5 | 40.6 | 52.1 | 44.6 | 42.1 | 48.1 |
| HRb, vs reference | Reference | 1.02 | 1.38 | Reference | 0.99 | 1.28 | Reference | 1.05 | 1.20 |
| 95% CIb | 0.79-1.33 | 0.99-1.90 | 0.73-1.33 | 0.90-1.81 | 0.79-1.42 | 0.85-1.69 | |||
| P vs reference unadjustedb | 0.87 | 0.05 | 0.92 | 0.17 | 0.72 | 0.30 | |||
| P, overall unadjusted (adjusted)c | 0.15 (0.60) | 0.20 (0.60) | 0.56 (0.67) | ||||||
| All falls | |||||||||
| Total no. of eventse | 567 | 494 | 230 | 338 | 631 | 322 | 351 | 575 | 365 |
| Total no. of PYf | 466.4 | 380.4 | 167.2 | 223.5 | 550.0 | 240.5 | 239.7 | 506.6 | 267.7 |
| Rate per 100 PYg | 121.6 | 129.8 | 137.6 | 151.2 | 114.7 | 133.9 | 146.4 | 113.5 | 136.3 |
| Rate ratio vs referenceh | Reference | 1.12 | 1.15 | Reference | 0.75 | 0.93 | Reference | 0.78 | 0.94 |
| 95% CIh | 0.89-1.40 | 0.85-1.56 | 0.58-0.96 | 0.69-1.26 | 0.60-1.00 | 0.70-1.27 | |||
| P vs reference unadjustedh | 0.33 | 0.36 | 0.02 | 0.66 | 0.05 | 0.70 | |||
| P overall (unadjusted) adjustedi | 0.53 (0.67) | 0.05 (0.60) | 0.12 (0.60) | ||||||
| All consequential fallsd | |||||||||
| Total no. of eventse | 261 | 228 | 113 | 134 | 319 | 149 | 138 | 301 | 163 |
| Total no. of PYf | 466.4 | 380.4 | 167.2 | 223.5 | 550.0 | 240.5 | 239.7 | 506.6 | 267.7 |
| Rate per 100 PYg | 56.0 | 59.9 | 67.6 | 60.0 | 58.0 | 62.0 | 57.6 | 59.4 | 60.9 |
| Rate ratio vs referenceh | Reference | 1.15 | 1.24 | Reference | 0.99 | 1.08 | Reference | 1.07 | 1.05 |
| 95% CIh | 0.90-1.48 | 0.90-1.71 | 0.74-1.31 | 0.77-1.52 | 0.80-1.42 | 0.75-1.46 | |||
| P vs reference unadjustedh | 0.26 | 0.20 | 0.92 | 0.64 | 0.66 | 0.78 | |||
| P overall unadjusted (adjusted)i | 0.35 (0.60) | 0.81 (0.88) | 0.91 (0.91) |
To convert 25(OH)D ng/mL to nmol/L, multiply ng/mL by 2.5. These analyses include the 637 participants (92.6% of 688 randomized) who had serum 25(OH)D measured at the 3-month follow-up visit.
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BL, baseline; FU, follow-up; HR, hazard ratio; PY, person-years.
a For the first fall analysis, data from participants who did not fall were censored at their date of last contact for fall follow-up; for the first consequential fall analysis, data from participants who did not have a consequential fall were censored at their date of last contact for fall follow-up, with the exception that any participant who reported a fall that could not be categorized yes or no as to consequential was censored at that date if they had not previously reported a consequential fall. Thirteen participants who had not previously reported a consequential fall were censored at the date of their first fall that could not be categorized yes or no as to consequential.
b Each HR and its 95% CI were derived from a Cox proportional hazards regression model including serum 25(OH)D level group (2 indicator variables) and adjustment covariates (all at baseline) of age (continuous), race/ethnicity (other vs non-Hispanic White), sex (male vs female), education (more than high school vs high school or less), enrollment site location (Hagerstown vs Baltimore), Short Physical Performance Battery score (continuous), body mass index (continuous), and month of randomization as proxy for season (11 indicator variables); P vs reference tests whether the HR differs from 1 and is from the log-rank test and not adjusted for multiple comparisons.
c P overall is a 2 degrees of freedom analysis of variance for the overall effect of serum 25(OH)D group on time to first fall and tests whether the HRs differ. The Benjamini-Hochberg procedure was used to control the false discovery rate to less than 1/12 (1 out of the number of overall comparisons in the manuscript); an adjusted P value (shown in parentheses next to the unadjusted P) is statistically significant if < 0.08.
d A consequential fall is a fall that resulted in any injury or medical care sought.
e Fall counts have been top-coded at the value equal to the 99th percentile of nonzero fall counts.
f Observation time for a participant is the duration from randomization to date of last contact in the trial.
g Rate of all events is calculated as the total number of events summed across all participants divided by the total person-time observed summed across all participants.
h Each rate ratio, its 95% CI, and its P vs reference are derived from a negative binomial model with a term for treatment, an offset term for the participant’s observation time from randomization to date of last contact in the trial, and adjustment covariates as above. P vs reference is not adjusted for multiple comparisons.
i P overall is a 2 degree of freedom analysis of variance for the overall effect of serum 25(OH)D on total number of falls. The Benjamini-Hochberg procedure was used to control the false discovery rate to less than 1/12 (1 out of the number of overall comparisons in the manuscript); an adjusted P value (shown in parentheses next to the unadjusted P) is statistically significant if < 0.08.
There was no difference in time to first consequential fall among the 3 strata for any definition of achieved 25(OH)D. However, although the test of overall difference between strata defined by absolute concentration was not significant (unadjusted overall P = 0.15; adjusted overall P = 0.60), the adjusted HR for ≥ 40 vs < 30 ng/mL was 1.38 (95% CI, 0.99-1.99, P = 0.05). This HR was the most extreme observed (no observed HR was smaller than 0.75).
No difference in rates of all falls was seen when achieved 25(OH)D was defined as absolute level or relative change. The rates of all falls did not differ statistically across strata defined by absolute change in 25(OH)D after adjustment for multiple comparisons (unadjusted overall P = 0.05; adjusted overall P = 0.60). For the middle 50% of absolute change in concentration vs lower 25%, the rate ratio (95% CI) was 0.75 (0.58-0.96), and for the upper 25% vs lower 25%, the rate ratio (95% CI) was 0.93 (0.69-1.26).
The rates of all consequential falls were not statistically different between strata regardless of whether strata were defined by absolute 25(OH)D level, absolute change 25(OH)D, or relative change in 25(OH)D. After multiple comparisons adjustment, there was no association between any fall outcome and absolute 25(OH)D, nor absolute or relative change in 25(OH)D when falls occurring in the first 90 days after randomization were excluded from analysis (data not shown).
Discussion
In this observational analysis of the STURDY trial, we found no evidence for association of achieved concentration of 25(OH)D with risk of incident or cumulative falls, nor for incident or cumulative consequential falls. This finding was evident regardless of how we defined achieved concentration (absolute level at 3 months, absolute change, or relative change). Notably, there was no apparent higher risk of falls for those at the lowest achieved concentrations of 25(OH)D.
Although no statistically significant associations were found after adjustment for multiple comparisons, 1 analysis hints at possible increased risk of consequential falls with achieved concentrations of ≥ 40 ng/mL compared with achieved concentrations of < 30 ng/mL, and another analysis suggests a possible reduction in total (all) falls for the middle 50% of absolute change in 25(OH)D compared with the bottom quartile of change.
The present findings are consistent with the main trial results, which found no benefit of falls prevention with vitamin D3 supplementation of ≥ 1000 IU/day and a suggestion of harm, with first serious fall and first fall with hospitalization occurring more frequently, with higher doses of supplementation ≥ 2000 IU/day compared with 200 IU/day [21]. Because higher doses of supplementation would be expected to achieve higher serum concentrations of 25(OH)D, our findings suggestive of more consequential falls with higher achieved serum 25(OH)D concentrations would align with concerns raised from STURDY [21, 22] and other trials [17-19] that high-dose vitamin D supplementation is potentially harmful.
In aggregate, these data should prompt public health officials to reconsider their advice regarding the maximal tolerable vitamin D threshold, which is currently set at 4000 IU/day per the National Academy of Medicine (formerly the Institute of Medicine) [30]. In contrast, it should be noted that the VITAL trial did not demonstrate any harm with supplementation doses of 2000 IU/day for outcomes of cardiovascular disease or cancer, nor was hypercalcemia seen [31]. The National Academy of Medicine states that the recommended daily allowance of vitamin D intake for adults aged 19 to 70 years is 600 IU and for adults aged > 70 years old is 800 IU for the purposes of optimizing bone health and overall health [30]. However, a considerable proportion of US adults use vitamin D supplementation in excess of these recommended allowances. Between 1999 and 2014, the proportion of US adults using vitamin D supplements > 1000 IU/day increased more than 60-fold (from 0.3% to 18.2%) and those using vitamin D supplements > 4000 IU/day increased by over 30-fold (from 0.1% to 3.2%) [32].
In light of the growing evidence showing lack of benefit with vitamin D supplementation [16] or potential for harm [17-19, 33, 34] for musculoskeletal outcomes, in 2018 the US Preventive Services Task Force reversed its recommendations from 2012, which had previously advised vitamin D supplementation for falls prevention in older adults [35], and it currently does not recommend vitamin D supplementation for falls prevention in older persons without osteoporosis or vitamin D deficiency [1]. Additionally, an updated US Preventive Services Task Force recommendation found insufficient evidence to balance the benefits vs harms of screening for vitamin D deficiency among asymptomatic adults [36, 37].
Furthermore, although vitamin D supplementation has been advised to treat deficiency or insufficiency across guidelines, there has been ongoing controversy about what actually constitutes an “adequate” 25(OH)D concentration [38]. The Endocrine Society has defined 25(OH)D concentrations < 20 ng/mL as deficient, with 20 to 29.9 ng/mL considered insufficient, and ≥ 30 ng/mL considered optimal [23]. In contrast, the National Academy of Medicine considers serum 25(OH)D concentrations < 12 ng/mL as deficient, with 12 to 20 ng/mL at risk for inadequacy, >20 to 50 ng/mL considered adequate for bone health and overall health for most Americans, and concentrations > 50 ng/mL at potential risk for adverse events [30]. At 3 months after randomization in STURDY, no participant had achieved concentration < 12 ng/mL and only 30 people had achieved levels > 50 ng/mL, limiting our ability to examine fall outcomes for these extreme categories of achieved 25(OH)D concentrations. Our highest category of absolute 25(OH)D at 3 months ranged from 40 to 92 ng/mL, and our findings align with this concern for harm at elevated serum 25(OH)D concentrations.
Both low and high concentrations of 25(OH)D may be adverse for health. Indeed, a U-shaped relationship of vitamin D supplement doses with fracture risk has been suggested [39]. For the outcome of all falls, a reduction in fall risk seen with middle 50% of achieved 25(OH)D concentration but not with highest achieved levels also supports the concept that higher concentrations are not necessarily better for musculoskeletal health. Prior other studies have also suggested a U-shaped relationship of serum 25(OH)D concentrations with mortality, cardiovascular diseases, and cancer, with both low and high 25(OH)D concentrations being associated with adverse events [40-43].
Prior meta-analyses have generally focused on comparing doses of vitamin D supplementation with fall risk [16]. However, there is variable response to an achieved 25(OH)D concentration for a given vitamin D supplementation dose based on age, bioavailability, obesity status, racial factors, and genetic polymorphisms related to vitamin D metabolism [44, 45]. Thus, we undertook these analyses because we anticipated the purported benefit of vitamin D supplementation may depend on achieved blood concentration and not the dose per se. In a 2009 meta-analysis incorporating 8 RCTs, supplemental vitamin D in the dose range of 700 to 1000 IU/day reduced the risk of falls by 19%, but falls were not reduced unless a blood concentration of at least 24 ng/mL was achieved [11]. However, in this present analysis, we did not confirm any benefit for falls reduction with higher achieved 25(OH)D concentrations or see an increased risk of falls at lower concentrations.
Rather, we found a suggestion for harm of consequential falls with higher achieved concentrations ≥ 40 ng/mL even after adjusting for potential confounding factors. The mechanisms underlying this association are uncertain. One possibility is that individuals with higher 25(OH)D concentrations may be healthier and more active, and thus engaging in more at-risk outdoor activities where they may be more likely to be injured by a fall; however, our analyses were adjusted for baseline functional status (SPPB score). On the other hand, it may be that high-dose vitamin D supplementation (and thus, high achieved 25(OH)D concentrations) actually cause bone resorption, placing these individuals at increased vulnerability of a fracture with their fall. In an RCT of healthy adults without osteoporosis, high-dose vitamin D supplementation ≥ 4000 IU/day (compared to 400 IU/day) actually decreased BMD rather than increased it [34]. High serum concentrations of 25(OH)D have been associated with increased concentrations of 1,25-dihydroxyvitamin D (calcitriol), the active form of vitamin D. Although activated vitamin D is generally thought to promote bone mineralization by decreasing PTH through negative feedback mechanisms, overstimulation of the calcitriol pathway may actually lead to bone resorption. Calcitriol increases blood calcium levels mostly by increasing uptake of calcium from intestines, but it also can promote release of calcium from bones by stimulating osteoclasts, which break down bone tissue [46, 47]. Although high-dose vitamin D can lead to hypercalcemia, there were no safety concerns of hypercalcemia during the STURDY trial, even with higher doses of vitamin D supplementation [21].
Our findings should be considered in the context of several limitations. First, individuals with severe vitamin D deficiency—25(OH)D concentrations < 10 ng/mL—were excluded from the trial and thus we cannot make inference about whether a relative change in 25(OH)D concentrations might be beneficial for falls prevention in this at-risk population. However, only 23 individuals were excluded because of a baseline level < 10 ng/mL. Second, this is an observational analysis, and as such, we cannot make any causal inferences. Although we adjusted for a number of potential confounders including functional status (SPPB), there may be residual confounding of achieved serum 25(OH)D concentrations by health status. Generally, low 25(OH)D concentrations are indicative of a poorer health status [48-50], where we could presume that individuals with low achieved 25(OH)D might be at greater falls risk from poorer underlying health and less outdoor physical activity. But rather, we found an opposite trend with 25(OH)D concentrations ≥ 40 ng/mL being associated with a possible greater risk of consequential fall and no evidence of higher fall risk at lower levels of achieved 25(OH)D. Third, the study findings were limited to a population of adults aged 70 years or older, at risk of falling, with low baseline 25(OH)D concentrations, and thus results cannot be extrapolated to other populations such as younger individuals and those who are not at elevated fall risk. Fourth, the follow-up period of the study was relatively short with approximate follow-up of 24 months; however, the number of falls was large, thereby providing substantial statistical power. Finally, there were multiple analyses. Hence, our findings of a possible increased risk of consequential falls with absolute concentrations of ≥ 40 ng/mL, and another analysis of a possible reduction in total (all) falls for the middle 50% of absolute change in 25(OH)D might be the result of type 1 errors.
In conclusion, this observational analysis of the STURDY RCT did not find any association of achieved 25(OH)D concentrations after 3 months on supplementation with reduction in falls. A potential for harm of consequential falls with achieved concentrations ≥ 40 ng/mL cannot be excluded. These findings from observational analyses of the STURDY trial, in conjunction with primary trial results, support reevaluation of the upper tolerable dose of vitamin D intake.
Acknowledgments
The authors thank the participants, the field center staff, and the entire STURDY Collaborative Research Group for their invaluable contributions to the STURDY trial.
Glossary
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- BMD
bone mineral density
- HR
hazard ratio
- RCT
randomized controlled trial
- SPPB
Short Physical Performance Battery
- STURDY
Study to Understand Fall Reduction and Vitamin D in You
Financial Support
The STURDY trial was funded by the National Institute on Aging (U01AG047837) with support from the Office of Dietary Supplements, the Mid-Atlantic Nutrition Obesity Research Center (P30DK072488), and the Johns Hopkins Institute for Clinical and Translation Research (UL1TR003098). E.D.M. is supported by the Amato Fund for Women’s Cardiovascular Health Research at Johns Hopkins. S.P.J. was supported by a National Institute of Diabetes and Digestive and Kidney Diseases training grant (T32DK007732) and a National Heart, Lung, and Blood Institute career development award (K23HL135273). J.A.S. was supported by a National Institute on Aging career development award (K01AG048765). A.A.W. was supported by the Johns Hopkins Older Americans Independence Center (P30AG059298) and the Johns Hopkins Alzheimer’s Disease Resource Center for Minority Aging Research (P30AG021334). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This paper is subject to the NIH Public Access Policy.
Disclosures
The authors do not report any disclosures related to the topic of the submitted work. Outside of this work, E.D.M. has served on advisory board for AstraZeneca, Amarin, Bayer, Boehringer Ingelheim, Esperion, Novartis, Novo Nordisk, Novartis, and Pfizer. J.A.S. acts as a consultant for EMD Serono. R.H.C. is a consultant for Roche Diagnostics, Siemens Healthcare Diagnostics, and Becton Dickinson Medical Technology. L.J.A. receives payments from Wolters Kluwer for chapters in UpToDate. No other authors report any disclosures. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data Availability Statement
Deidentified participant data and data dictionary will be available at https://archive.data.jhu.edu/, contingent upon institutional review board approval.
References
- 1. Grossman DC, Curry SJ, Owens DK; US Preventive Services Task Force. Interventions to Prevent Falls in Community-Dwelling Older Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2018;319(16):1696-1704. doi: 10.1001/jama.2018.3097. [DOI] [PubMed] [Google Scholar]
- 2.National Council on Aging. https://www.ncoa.org/article/get-the-facts-on-falls-prevention. Accessed November 28, 2020.
- 3. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4. Hannan MT, Litman HJ, Araujo AB, et al. Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab. 2008;93(1):40-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Michos ED, Mitchell CM, Miller ER 3rd, et al. Rationale and design of the Study To Understand Fall Reduction and Vitamin D in You (STURDY): A randomized clinical trial of Vitamin D supplement doses for the prevention of falls in older adults. Contemp Clin Trials. 2018;73:111-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Michos ED, Mitchell CM, Miller ER, et al. Corrigendum to “Rationale and design of the Study To Understand Fall Reduction and Vitamin D in You (STURDY): A randomized clinical trial of Vitamin D supplement doses for the prevention of falls in older adults” [Contemp Clin Trials. 73 (2018) 111-122]. Contemp Clin Trials. 2020;90:105936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rejnmark L. Effects of vitamin d on muscle function and performance: a review of evidence from randomized controlled trials. Ther Adv Chronic Dis 2011;2(1):25-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arima K, Mizukami S, Nishimura T, et al. Epidemiology of the association between serum 25-hydroxyvitamin D levels and musculoskeletal conditions among elderly individuals: a literature review. J Physiol Anthropol. 2020;39(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rothenbacher D, Klenk J, Denkinger MD, et al. ; ActiFE Study Group. Prospective evaluation of renal function, serum vitamin D level, and risk of fall and fracture in community-dwelling elderly subjects. Osteoporos Int. 2014;25(3):923-932. [DOI] [PubMed] [Google Scholar]
- 10. Shimizu Y, Kim H, Yoshida H, Shimada H, Suzuki T. Serum 25-hydroxyvitamin D level and risk of falls in Japanese community-dwelling elderly women: a 1-year follow-up study. Osteoporos Int. 2015;26(8):2185-2192. [DOI] [PubMed] [Google Scholar]
- 11. Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ 2009;339:b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalyani RR, Stein B, Valiyil R, Manno R, Maynard JW, Crews DC. Vitamin D treatment for the prevention of falls in older adults: systematic review and meta-analysis. J Am Geriatr Soc. 2010;58(7):1299-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Broe KE, Chen TC, Weinberg J, Bischoff-Ferrari HA, Holick MF, Kiel DP. A higher dose of vitamin d reduces the risk of falls in nursing home residents: a randomized, multiple-dose study. J Am Geriatr Soc. 2007;55(2):234-239. [DOI] [PubMed] [Google Scholar]
- 14. Uusi-Rasi K, Patil R, Karinkanta S, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med 2015;175(5):703-711. [DOI] [PubMed] [Google Scholar]
- 15. Bolland MJ, Grey A, Gamble GD, Reid IR. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2(7):573-580. [DOI] [PubMed] [Google Scholar]
- 16. Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018;6(11):847-858. [DOI] [PubMed] [Google Scholar]
- 17. Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815-1822. [DOI] [PubMed] [Google Scholar]
- 18. Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, et al. . Monthly High-Dose Vitamin D Treatment for the Prevention of Functional Decline: a randomized clinical trial. JAMA Intern Med 2016;176(2):175-183. [DOI] [PubMed] [Google Scholar]
- 19. Smith LM, Gallagher JC, Suiter C. Medium doses of daily vitamin D decrease falls and higher doses of daily vitamin D3 increase falls: A randomized clinical trial. J Steroid Biochem Mol Biol. 2017;173:317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tang O, Juraschek SP, Appel LJ. Design features of randomized clinical trials of vitamin d and falls: a systematic review. Nutrients 2018;10:964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Appel LJ, Michos ED, Mitchell CM, et al. The Effects of Four Doses of Vitamin D Supplements on Falls in Older Adults: a response-adaptive, randomized clinical trial. Ann Intern Med. 2021;174(2):145-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wanigatunga AA, Sternberg AL, et al. The effects of vitamin D supplementation on types of falls. J Am Geriatr Soc. 2021;69(10):2851-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930. [DOI] [PubMed] [Google Scholar]
- 24. Phinney KW, Bedner M, Tai SS, et al. Development and certification of a standard reference material for vitamin D metabolites in human serum. Anal Chem. 2012;84(2):956-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization. Ageing and Life Course Unit. WHO global report on falls prevention in older age. Geneva, Switzerland: World Health Organization.2008. https://www.who.int/publications-detail-redirect/9789241563536
- 26. Taylor C, Lamparello B, Kruczek K, Anderson EJ, Hubbard J, Misra M. Validation of a food frequency questionnaire for determining calcium and vitamin D intake by adolescent girls with anorexia nervosa. J Am Diet Assoc. 2009;109(3):479-485, 485 e471-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-94. [DOI] [PubMed] [Google Scholar]
- 28. Fried LP, Tangen CM, Walston J, et al. Cardiovascular Health Study Collaborative Research G. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-156. [DOI] [PubMed] [Google Scholar]
- 29. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Methodol 1995;57(1):289-290. [Google Scholar]
- 30. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manson JE, Cook NR, Lee IM, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rooney MR, Harnack L, Michos ED, et al. . Trends in use of high-dose vitamin D supplements exceeding 1000 or 4000 international units daily, 1999-2014. JAMA 2017;317(23):2448-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women--a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology. 2007;46(12):1852-1857. [DOI] [PubMed] [Google Scholar]
- 34. Burt LA, Billington EO, Rose MS, Raymond DA, Hanley DA, Boyd SK. Effect of High-Dose Vitamin D Supplementation on Volumetric Bone Density and Bone Strength: a randomized clinical trial. JAMA 2019;322(8):736-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moyer VA. US Preventive Services Task Force. Prevention of falls in community-dwelling older adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(3):197-204. [DOI] [PubMed] [Google Scholar]
- 36. Krist AH, Davidson KW, Mangione CM, et al. ; US Preventive Services Task Force. Screening for Vitamin D Deficiency in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325(14):1436-1442. [DOI] [PubMed] [Google Scholar]
- 37. Michos ED, Kalyani RR, Segal JB. Why USPSTF still finds insufficient evidence to support screening for vitamin D deficiency. JAMA Netw Open 2021;4(4):e213627. [DOI] [PubMed] [Google Scholar]
- 38. Rosen CJ, Abrams SA, Aloia JF, et al. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab. 2012;97(4):1146-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anagnostis P, Bosdou JK, Kenanidis E, Potoupnis M, Tsiridis E, Goulis DG. Vitamin D supplementation and fracture risk: evidence for a U-shaped effect. Maturitas 2020;141:63-70. [DOI] [PubMed] [Google Scholar]
- 40. Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008;117(4):503-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Freedman DM, Looker AC, Abnet CC, Linet MS, Graubard BI. Serum 25-hydroxyvitamin D and cancer mortality in the NHANES III study (1988-2006). Cancer Res. 2010;70(21):8587-8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Michaelsson K, Baron JA, Snellman G, et al. Plasma vitamin D and mortality in older men: a community-based prospective cohort study. Am J Clin Nutr. 2010;92(4):841-848. [DOI] [PubMed] [Google Scholar]
- 44. Levin GP, Robinson-Cohen C, de Boer IH, et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA 2012;308(18):1898-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim SM, Lutsey PL, Michos ED. Vitamin D and cardiovascular disease: can novel measures of vitamin D status improve risk prediction and address the vitamin D racial paradox? Cur Cardiovasc Risk Rep. 2017;11(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Suda T, Takahashi N, Abe E. Role of vitamin D in bone resorption. J Cell Biochem. 1992;49(1):53-58. [DOI] [PubMed] [Google Scholar]
- 47. Nakamichi Y, Udagawa N, Suda T, Takahashi N. Mechanisms involved in bone resorption regulated by vitamin D. J Steroid Biochem Mol Biol. 2018;177:70-76. [DOI] [PubMed] [Google Scholar]
- 48. Heravi AS, Michos ED. Vitamin D and calcium supplements: helpful, harmful, or neutral for cardiovascular risk? Methodist DeBakey Cardiovasc J 2019;15(3):207-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chin K, Appel LJ, Michos ED, Vitamin D. Calcium, and cardiovascular disease: A“D”vantageous or “D”etrimental? An era of uncertainty. Curr Atherosclerosis Rep. 2017;19(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Michos ED, Cainzos-Achirica M, Heravi AS, Appel LJ. Vitamin D, calcium supplements, and implications for cardiovascular health: JACC Focus Seminar. J Am Coll Cardiol. 2021;77(4):437-449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified participant data and data dictionary will be available at https://archive.data.jhu.edu/, contingent upon institutional review board approval.