PURPOSE
Targeting the BCL-XL pathway has demonstrated the ability to overcome Janus kinase inhibitor resistance in preclinical models. This phase II trial investigated the efficacy and safety of adding BCL-XL/BCL-2 inhibitor navitoclax to ruxolitinib therapy in patients with myelofibrosis with progression or suboptimal response to ruxolitinib monotherapy (ClinicalTrials.gov identifier: NCT03222609).
METHODS
Thirty-four adult patients with intermediate-/high-risk myelofibrosis who had progression or suboptimal response on stable ruxolitinib dose (≥ 10 mg twice daily) were administered navitoclax at 50 mg once daily starting dose, followed by escalation to a maximum of 300 mg once daily in once in weekly increments (if platelets were ≥ 75 × 109/L). The primary end point was ≥ 35% spleen volume reduction (SVR35) from baseline at week 24. Secondary end points included ≥ 50% reduction in total symptom score (TSS50) from baseline at week 24, hemoglobin improvement, change in bone marrow fibrosis (BMF) grade, and safety.
RESULTS
High molecular risk mutations were identified in 58% of patients, and 52% harbored ≥ 3 mutations. SVR35 was achieved by 26.5% of patients at week 24, and by 41%, at any time on study, with an estimated median duration of SVR35 of 13.8 months. TSS50 was achieved by 30% (6 of 20) of patients at week 24, and BMF improved by 1-2 grades in 33% (11 of 33) of evaluable patients. Anemia response was achieved by 64% (7 of 11), including one patient with baseline transfusion dependence. Median overall survival was not reached with a median follow-up of 21.6 months. The most common adverse event was reversible thrombocytopenia without clinically significant bleeding (88%).
CONCLUSION
The addition of navitoclax to ruxolitinib in patients with persistent or progressive myelofibrosis resulted in durable SVR35, improved TSS, hemoglobin response, and BMF. Further investigation is underway to qualify the potential for disease modification.
INTRODUCTION
Myelofibrosis has a variable disease course characterized by anemia, bone marrow fibrosis (BMF), progressive splenomegaly, extramedullary hematopoiesis, and leukemic progression.1 The median overall survival (OS) ranges from < 2 to > 10 years.2
CONTEXT
Key Objective
To our knowledge, this phase II, multicenter, open-label trial is the first to report efficacy and safety of adding BCL-XL/BCL-2 inhibitor navitoclax to ruxolitinib for patients with primary or secondary myelofibrosis disease progression or suboptimal response to ruxolitinib monotherapy.
Knowledge Generated
The primary end point of the spleen volume reduction of ≥ 35% at week 24 was achieved in 26.5% of patients, and at any time on study, the spleen volume reduction of ≥ 35% and ≥ 50% reduction in total symptom score were achieved in 41% of patients each and bone marrow fibrosis improved by 1-2 grades in 33% of evaluable patients. Reversible thrombocytopenia without clinically significant bleeding was the most common adverse event (88%) but was manageable with dose reductions and interruptions.
Relevance
Combining navitoclax with ongoing ruxolitinib was manageable in this difficult-to-treat population, demonstrating encouraging and durable efficacy outcomes for this patient population who have limited treatment options.
Myelofibrosis is primarily driven by the constitutive activation of the Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway, leading to myeloproliferation, increased inflammatory cytokines, and overexpression of antiapoptotic B-cell lymphoma proteins, including BCL-XL, BCL-2, and MCL-1.1,3-8 This pathogenic maladaptation provides a rationale for exploring the novel therapeutic combination of JAK and BCL-XL/BCL-2 inhibition.9,10
The JAK1/2 inhibitor ruxolitinib and JAK2 inhibitor fedratinib are approved for the treatment of patients with intermediate- or high-risk myelofibrosis who are not candidates for stem-cell transplant.2,11-13 Although fedratinib has been shown to decrease BMF and JAK2 V617F–mutant allele burden in two phase I studies,14 ruxolitinib monotherapy exhibits a minimal impact on BMF and the impact of on driver mutation allele burden has not been clearly elucidated in phase II or III studies.15 After approximately 37 months, approximately half of patients treated with ruxolitinib discontinue, a third of whom report suboptimal response defined as lack or loss of spleen response.16 Data suggest that patients with three or more mutations or high molecular risk (HMR) mutations, defined as mutations in ASXL1, EZH2, SRSF2, IDH1, or U2AF1, have worse outcomes with JAK/STAT inhibitors.17-19
Currently, there is no standard of care beyond the class of JAK inhibitors approved for patients with suboptimal responses to JAK/STAT inhibitors.1,20 The phase III SIMPLIFY 2 trial of momelotinib in patients with myelofibrosis who had ruxolitinib treatment failure reported that momelotinib was not superior to best available therapy (spleen volume reduction of ≥ 35% [SVR35] rate of 7% for momelotinib compared with 6% for best available therapy).21 In another randomized trial with similar patients, pacritinib was more effective than best available therapy (SVR35 of 18% compared with 3%), but SVR35 rates were lower than that desired in this patient population that has few targeted treatment options after failure on JAK inhibitors.22 This highlights the unmet clinical need in myelofibrosis for novel therapeutic options after ruxolitinib.
Navitoclax (ABT-263) is an orally bioavailable, small-molecule BCL-2 homology 3 mimetic that binds with high affinity (Ki ≤ 1 nmol/L) to prosurvival BCL-2 family proteins (BCL-XL, BCL-2, and BCL-W), disrupting interactions with proapoptotic factors such as BIM, thereby promoting the apoptosis of malignant cells (Data Supplement, online only).23 Preclinical data indicate that ruxolitinib and navitoclax act synergistically; ruxolitinib suppresses the expression of BCL-XL and MCL-1, allowing navitoclax to inhibit the remaining BCL-XL and BCL-2 to promote apoptosis with a lower effective dose.9 Furthermore, navitoclax may overcome JAK2 inhibitor resistance through the resensitization of myeloid cells to JAK2 inhibition.9,24
The phase II REFINE trial (ClinicalTrials.gov identifier: NCT03222609) evaluated the efficacy and safety of navitoclax alone or in combination with ruxolitinib in patients with primary or secondary (postpolycythemia vera or postessential thrombocythemia myelofibrosis) myelofibrosis. Here, we report results of navitoclax added to ruxolitinib in patients with myelofibrosis in Cohort 1a who had progression or suboptimal response to ruxolitinib monotherapy.
METHODS
Study Design and Patients
This phase II, multicenter, open-label trial enrolled patients into four cohorts according to JAK inhibitor experience (Data Supplement). Here, we report the results of Cohort 1a, which was conducted across 11 sites globally, and enrolled patients between October 31, 2017, and April 10, 2019 (ClinicalTrials.gov identifier: NCT03222609).
Patients age ≥ 18 years with confirmed diagnosis of primary or secondary myelofibrosis were eligible for enrollment. Eligible patients had intermediate- or high-risk myelofibrosis as defined by Dynamic International Prognostic Scoring System25 and an Eastern Cooperative Oncology Group performance status26 of 0-2 and were unwilling or ineligible to undergo allogeneic stem-cell transplantation. Patients were required to have palpable splenomegaly (≥ 5 cm below the costal margin) or a spleen volume of ≥ 450 cm3, have received ≥ 12 weeks of ruxolitinib and been on a stable dose of ≥ 10 mg (twice a day) for ≥ 8 weeks before the first dose of navitoclax, and have a platelet count of ≥ 100 × 109/L.
Patients were excluded if they had received splenic irradiation ≤ 6 months before screening, had > 10% blasts in peripheral blood or bone marrow, were receiving medications that interfere with coagulation or platelet function (except aspirin ≤ 100 mg once daily and low-molecular-weight heparin), or had received prior therapy with any BCL-2 homology 3 mimetic.
Eligible patients continued their stable dose of ruxolitinib and initiated oral navitoclax at the starting dose of 50 mg once daily, with once weekly escalation to a maximum of 300 mg once daily. Navitoclax and ruxolitinib dose adjustments following platelet count are summarized in the Data Supplement. Navitoclax was interrupted or discontinued after any grade ≥ 2 bleeding event. Navitoclax and ruxolitinib were interrupted after grade 4 neutropenia (absolute neutrophil count < 0.5 × 109/L), febrile neutropenia, or grade ≥ 3 nonhematologic toxicity.
Treatment was continued until disease progression, unacceptable toxicity, initiation of alternative myelofibrosis treatment, other medical reasons, or withdrawal of consent. All patients had a safety follow-up visit 30 days after treatment discontinuation. Patients who discontinued treatment for reasons other than disease progression were followed approximately every 12 weeks until disease progression or initiation of another myelofibrosis therapy. Patients were followed for survival every 6 months and will be followed for up to 5 years after treatment discontinuation.
This study was conducted in accordance with the Protocol (online only), International Conference on Harmonisation guidelines,27 applicable regulations and guidelines governing clinical study conduct, and ethical principles that have their origin in the Declaration of Helsinki. The study Protocol, informed consent, and all other forms were approved by an Independent Ethics Committee or Institutional Review Board. All patients gave written informed consent for trial participation.
End Points and Assessments
The primary efficacy end point was SVR35 from baseline at week 24, measured by magnetic resonance imaging or computed tomography. Secondary efficacy end points included ≥ 50% reduction in total symptom score from baseline (TSS50) at week 24 as measured by the Myelofibrosis Symptom Assessment Form v4.0,28,29 anemia response per modified International Working Group criteria,25,30 and change in grade of BMF (assessed locally per European consensus grading system).31 Exploratory end points included SVR35 and TSS50 at any time on study, duration of SVR35 response, ≥ 50% reduction in palpable splenomegaly from baseline per modified Myeloproliferative Neoplasms Research and Treatment International Working Group criteria,25,30 and OS.
Blood samples for pharmacokinetic (PK) analysis were collected for navitoclax and ruxolitinib on day 1 (predose and 2, 4, 6, and 8 hours after drug administration), day 2 predose, and predose on days 8, 15, 22, 29, 43, 57, 85, 169, and 337. PK parameters included maximum observed plasma concentration (Cmax), time to Cmax (Tmax) for navitoclax and ruxolitinib and area under the plasma concentration-time curve (AUC) from time 0 to 24 hours postdose (AUC0-24) for navitoclax only and from time 0 to 12 hours postdose (AUC0--12) for ruxolitinib only.
Safety evaluations were performed throughout the study and ≤ 30 days after the last dose of study treatment. Adverse events (AEs) and laboratory evaluations were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events v4.03.32
Statistical Methods
A sample size of 34 was estimated to provide a percentage point estimate of 47.06 for SVR35 at week 24, with exact 95% CI within 17.55 percentage points from the point estimate under various assumptions about true SVR35 rate. In addition, if the true probability of experiencing a serious AE (SAE) because of the study treatment was 10%, then the probability of observing ≥ 1 SAE in 34 patients was > 97%, which was considered adequate.
Demographic, baseline, changes in BMF grade, and safety data are summarized using descriptive statistics. The length of follow-up was as observed and summarized using descriptive statistics. SVR35 and TSS50 were calculated as the proportion of patients who achieved SVR35 or TSS50 at week 24, with corresponding 95% CI derived by the exact method. Kaplan-Meier methodology was used to estimate time-to-event end points, and PK parameters were determined using noncompartmental methods. These analyses were conducted using SAS version 9.4 (SAS Institute, Inc, Cary, NC).
For the standardized effect size (SES), cross-sectional data for TSS at week 24 were stratified by anemia response. The within-group level of change in individual scores was expressed in SES, calculated as the mean change score from baseline and divided by the standard deviation at baseline.
RESULTS
Patient Demographics and Baseline Characteristics
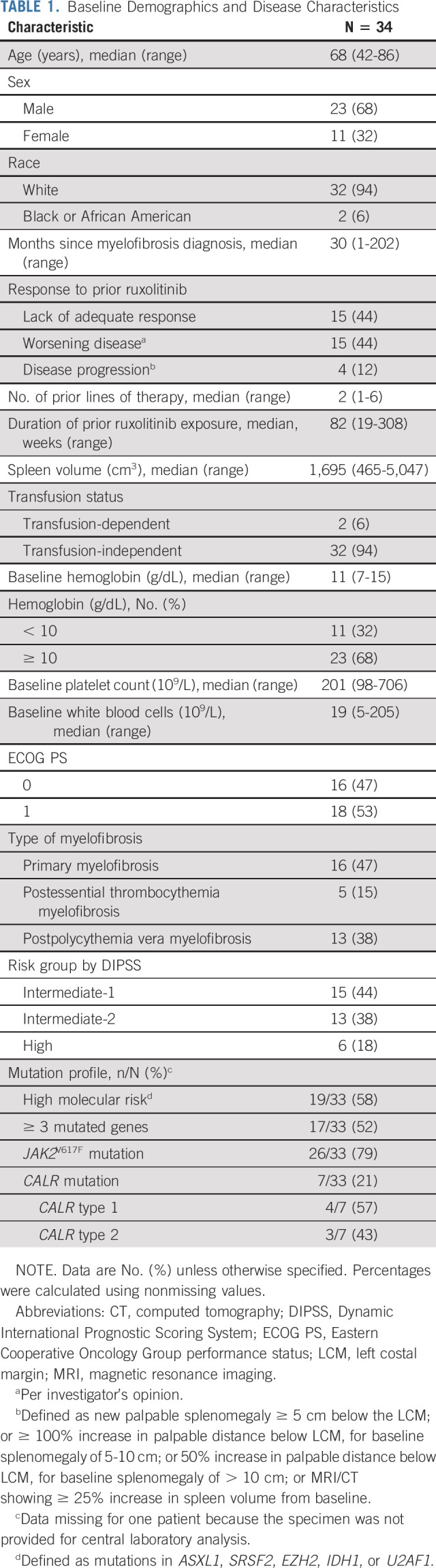
At the data cutoff (August 30, 2020), 34 patients with myelofibrosis had received ≥ 1 dose of navitoclax plus ruxolitinib. Most patients were male (n = 23, 68%), and the median age was 68 (range 42-86) years. The median duration of prior ruxolitinib exposure was 82 (range 19-308) weeks. Most patients lacked adequate response to prior ruxolitinib treatment or experienced worsening of the disease (n = 15, 44% each; Table 1); four patients (12%) had disease progression (spleen progression). Of 33 patients screened for mutations at enrollment, 26 (79%) had JAK2V617F mutations and the remaining seven (21%) had CALR mutations (four type 1 and three type 2 mutations); no patients had mutations in cMPL or TP53. Seventeen patients (52%) had ≥ 3 mutated genes, and 19 (58%) had HMR mutations; one of two patients with mutated U2AF1 had a mutation in the Q157 hotspot.
TABLE 1.
Baseline Demographics and Disease Characteristics
As of the data cutoff, 24 patients (71%) remained on study (Data Supplement). Five patients died (no deaths were deemed related to navitoclax or ruxolitinib), two patients withdrew consent from follow-up, two discontinued to undergo stem-cell transplant, and one discontinued because of progressive disease (Data Supplement).
Efficacy Assessments
The primary end point of SVR35 at week 24 was achieved in nine patients (26.5%; Fig 1A). Overall, 14 patients (41%) achieved SVR35 at any time on study, with six patients (18%) achieving this response at week 12 and eight at week 48; SVR35 was observed as late as week 72 (Table 2). The median spleen volume at week 24 was 1,069 cm3 (306-3,711) compared with 1,695 cm3 (465-5,047) at baseline (Data Supplement). The estimated median duration of SVR35 achieved at any time on study was 13.8 months (95% CI, 8.2 to not estimable [NE]), with no significant difference between patients with (n = 9) and without (n = 5) HMR (13.8 months [95% CI, 8.2 to NE] and 19.6 months [95% CI, 5.6 to NE], respectively). A palpable reduction of ≥ 50% in spleen length from baseline was achieved in 17 patients (50%) at week 24 and 20 patients (59%) at any time on study. The median study follow-up, as observed, was 21.6 (range 6.7-28.9) months. The median OS was not reached (NR) (95% CI, 26.1 to NE; Fig 2), and the estimated survival at 24 months was 84% (95% CI, 63.0 to 93.9). There was no significant difference in OS between patients with HMR (n = 19; NR [95% CI, 19.6 to NE]) and without HMR (n = 14; NR [95% CI, 26.1 to NE]) and OS by Dynamic International Prognostic Scoring System score, which is shown in the Data Supplement.
FIG 1.
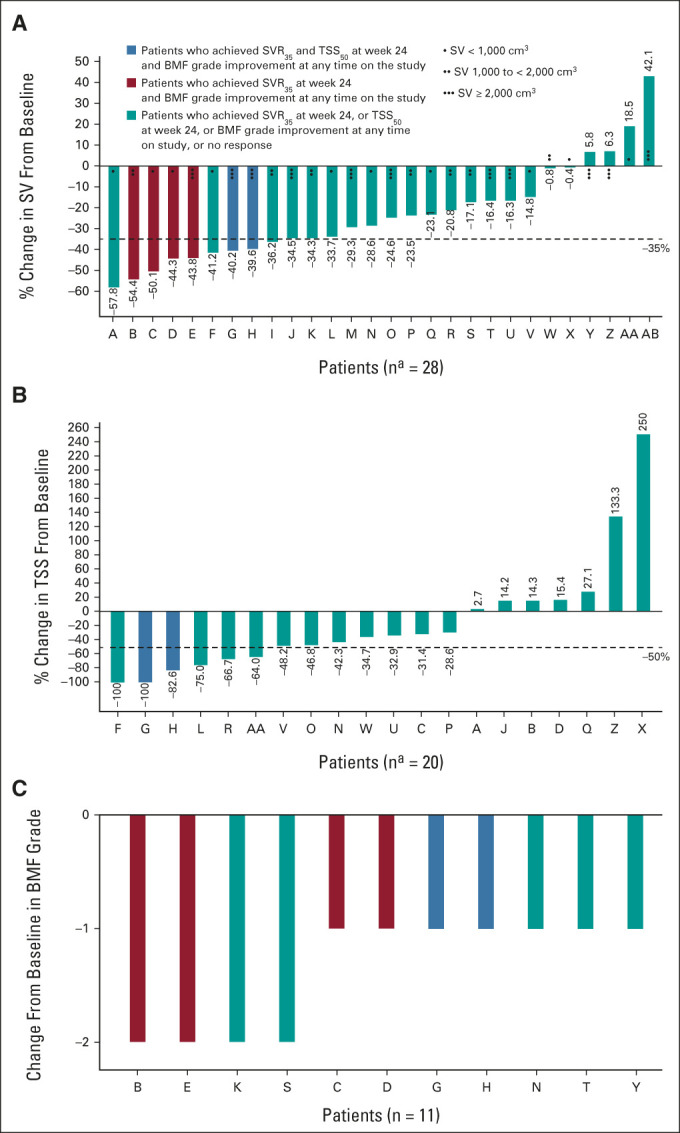
Percentage change from baseline in (A) spleen volume and (B) TSS at week 24 and (C) BMF improvement from baseline at any time on study. aPatients with nonmissing percent change from baseline at week 24. BMF, bone marrow fibrosis; SV, spleen volume; SVR35, spleen volume reduction of ≥ 35%; TSS, total symptom score; TSS50, ≥ 50% reduction in total symptom score.
TABLE 2.
Summary of Efficacy Assessments

FIG 2.
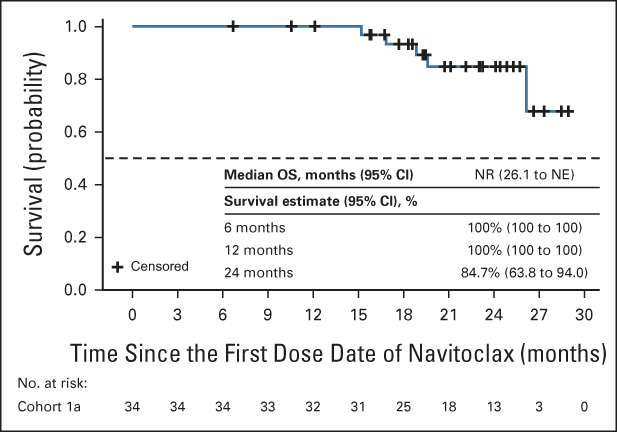
Kaplan-Meier curve of OS in all patients (N = 34). NE, not estimable; NR, not reached; OS, overall survival.
Of 20 patients evaluable for TSS at week 24, 6 (30%) reported ≥ 50% reduction of symptoms (Fig 1B), whereas TSS50 was achieved in 12 of 29 patients (41%) at any time on study. TSS50 was achieved in 50% of patients assessed at week 24 who were anemic (hemoglobin < 10 g/dL) at baseline compared with 18% of those who were not (Data Supplement). Of 33 patients with matched baseline and post-baseline data (one patient had missing grade at baseline), BMF improved from baseline by ≥ 1 grade in 11 of 33 patients (33%) at any time on study: one patient (3%) at week 12, 7 (21%) at week 24, 2 (6%) at week 48, and 1 (3%) at week 96. Of the 11 patients with improved BMF, seven patients improved by one grade and four patients by two grades (Fig 1C); the remaining 22 patients (67%) had equal or worsened BMF grades, with 13 patients having grade 3 fibrosis at baseline.
Anemia Responses
The mean hemoglobin level was stable over 120 weeks. Eleven patients had hemoglobin < 10 g/dL at baseline, and, of these 64% (7 of 11) had improvement in hemoglobin of ≥ 2 g/dL; at baseline, two of the 11 patients were transfusion-dependent, one of whom achieved transfusion independence in response to therapy. An exploratory analysis of SES suggested that patients who achieved an anemia response during the study experienced moderate improvement in TSS at week 24, whereas patients who did not achieve an anemia response showed no improvement in TSS at week 24 (SES33 = –0.68 and –0.15, respectively).
Pharmacokinetics
Navitoclax Cmax (0.6 μg/mL; 37%) was observed at a median Tmax of 4.0 hours (range 4.0-8.0) following navitoclax administration at 50 mg dose; AUC0-24 was 7.2 μg h/mL (45%, Data Supplement). Ruxolitinib PK parameters at doses 10 mg through 25 mg twice a day in combination with navitoclax are presented in the Data Supplement.
Safety
Median exposure to navitoclax and ruxolitinib was 81 weeks (4-126) and 83 weeks (10-124), respectively. In total, 24 patients (71%) reached the maximum navitoclax dose of 300 mg once daily and the remaining escalated to maximum 200 mg once daily (n = 8; 24%), 100 mg once daily (n = 1; 3%), and 50 mg once daily (n = 1; 3%). Navitoclax dose reduction because of AEs occurred in 26 (76%) patients, and dose interruptions in 22 (65%) patients, most commonly because of thrombocytopenia (n = 19, 56% each). Among the 24 patients who received navitoclax at 300 mg once daily, dose reduction because of AEs occurred in 19 patients (79%) with thrombocytopenia as the primary reason in 14 patients (58%). Five patients (15%) discontinued navitoclax because of an AE. Ruxolitinib dose was reduced because of AEs in 23 (68%) patients, was interrupted in 12 (35%) patients, and discontinued in two (6%) patients. The median dose of navitoclax was 200 mg/d, and the median dose of ruxolitinib was 10 mg twice a day.
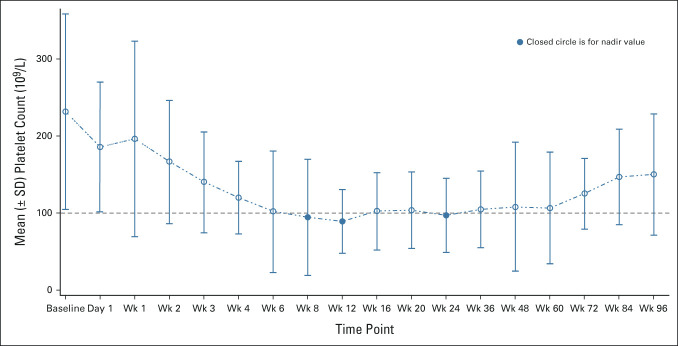
All 34 patients (100%) experienced at least one AE, 30 patients (88%) had grade ≥ 3 AEs, and 15 (44%) experienced SAEs (Table 3). The most common AEs of any grade were thrombocytopenia (n = 30, 88%), diarrhea (n = 24, 71%), fatigue (n = 21, 62%), and nausea (n = 13, 38%); the most common grade ≥ 3 AEs were thrombocytopenia (without clinically significant bleeding; n = 19, 56%), anemia (n = 11, 32%), and pneumonia (n = 4, 12%); the most common SAEs were pneumonia (n = 4, 12%) and splenic infarction (n = 2, 6%). Thrombocytopenia was manageable and reversible on dose reduction/interruption of navitoclax or ruxolitinib. The mean platelet count was < 100 × 109/L at 8, 12, and 24 weeks after the initiation of combined treatment (Fig 3). Of the five (15%) patients who died, four (12%) died ≤ 30 days after the last dose of navitoclax; none were deemed related to navitoclax or ruxolitinib.
TABLE 3.
Overview of Safety
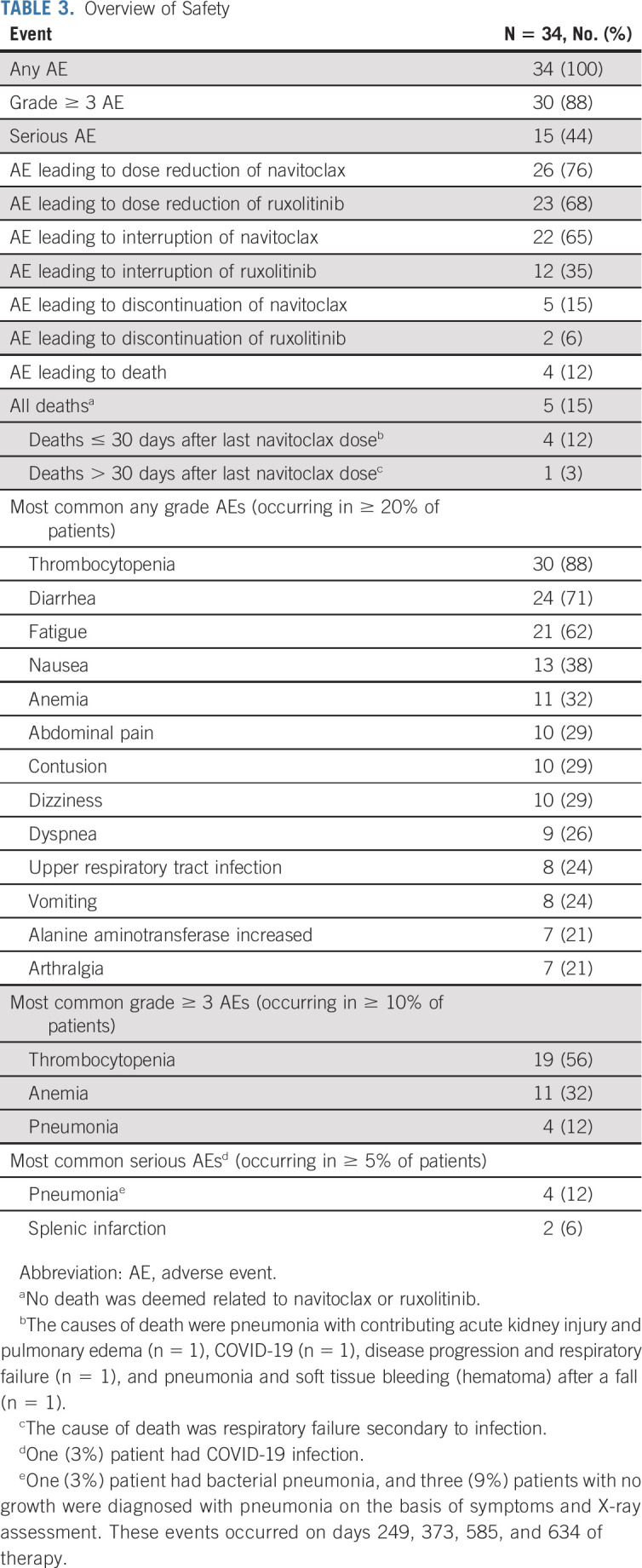
FIG 3.
Mean platelet count over time. Platelet nadir was defined as < 100 × 109 cells/L as indicated by the dashed line. SD, standard deviation; Wk, week.
DISCUSSION
To our knowledge, this phase II, multicenter, open-label trial is the first to report results from the combination of navitoclax and ruxolitinib for patients with primary or secondary myelofibrosis with prior ruxolitinib experience. The primary end point of SVR35 was reported in 26.5% of patients at week 24 and in 41% at any time on study, with an estimated median duration of 13.8 months overall. Although 52% of patients harbored ≥ 3 mutated genes and 58% had HMR, the median OS was NR. Moreover, the combination of navitoclax and ruxolitinib was tolerable with dose adjustment in response to thrombocytopenia.
The addition of navitoclax to ruxolitinib was associated with improvement in spleen volume and total symptom control in patients with suboptimal response to ruxolitinib monotherapy after prolonged prior exposure (median of 82 weeks). Although an SVR35 rate of 26.5% at week 24 is lower than that observed in trials with JAKi-naïve patients with myelofibrosis,34,35 our findings demonstrate encouraging SVR35 rates for patients despite suboptimal response to prior ruxolitinib monotherapy; in trials of momelotinib (7%) and pacritinib (18%) after ruxolitinib discontinuation in patients with advanced myelofibrosis, SVR35 rates were similarly low.21,22
In some patients, SVR35 was achieved as late as week 72, suggesting that this combination therapy may have a positive impact that may take longer than 24 weeks to manifest, and therefore, extended follow-up may be required. Furthermore, previous studies of treatments for patients with myelofibrosis have described associations between improvements in SVR, TSS, or spleen volume with myelofibrosis-associated cytokines after treatment,36-38 and further analyses are ongoing to elucidate these relationships. In addition, this trial was designed before the understanding that disease modification in myelofibrosis could be more adequately informed by surrogate end points like driver allele burden and BMF improvement. Given that 16% of patients with frontline ruxolitinib treatment had ≥ 1 grade reduction in BMF after a median of 2.2 years in COMFORT-II,35 the BMF reduction of 33% in this phase II trial provides evidence that the combination of navitoclax and ruxolitinib after prior ruxolitinib may exert early disease-modifying activity. Interestingly, patients who achieved anemia response also demonstrated improvement in TSS at week 24.
It is encouraging that, although this cohort comprises patients who had suboptimal response to ruxolitinib, median OS was NR at a median follow-up of 21.6 months. This suggests that the addition of navitoclax to ruxolitinib may result in increased OS compared with conventional (eg, danazol, hydroxyurea, etc) or targeted (eg, investigational JAK2 or non-JAK2 inhibitors, etc) therapies received after ruxolitinib discontinuation (median OS of up to 14 months reported for both conventional and targeted therapies).16,39,40 Although the inclusion of intermediate-1–risk patients and the short length of follow-up may confound conclusions regarding survival outcomes, the proportion of intermediate-1–risk patients included in this trial is consistent with previous studies.16,39 These findings are potentially significant for this difficult-to-treat population, as a majority of patients (58%) had HMR mutations in addition to their suboptimal ruxolitinib monotherapy response. However, small patient numbers, the open-label study design, and lack of comparator group limit the interpretation of findings from this study.
It is hypothesized that because navitoclax and ruxolitinib have distinct mechanisms of action and noncompetitive elimination,9,41,42 drug–drug interaction and cross-potentiation of AEs may be avoided. The lower ruxolitinib Cmax and moderately lower AUC0-12 compared with historical data43 can likely be attributed to limited PK samples collected in the absorption phase, resulting in underestimation of Cmax and AUC0-12. Navitoclax exposures were similar to historical monotherapy data,44 suggesting that ruxolitinib did not affect navitoclax PK, which is consistent with ruxolitinib not being an inhibitor or inducer of major metabolizing enzymes.43
The safety profile observed for navitoclax plus ruxolitinib was similar to previous studies of patients with myelofibrosis treated with single-agent ruxolitinib although the rate of thrombocytopenia was higher in patients receiving this combination therapy compared with ruxolitinib alone.34,35,45 The most frequent hematologic AEs were thrombocytopenia (without clinically significant bleeding) and anemia, which were reversible on navitoclax and/or ruxolitinib dose hold or reduction. The most common gastrointestinal events of any grade were abdominal pain, diarrhea, nausea, and vomiting; most were grade 1/2. No death was deemed related to navitoclax or ruxolitinib.
In summary, the combination of navitoclax with ruxolitinib was manageable in this difficult-to-treat population and demonstrated encouraging and durable efficacy outcomes. The findings suggest disease-modifying activity in a population with limited therapeutic options after ruxolitinib unresponsiveness or resistance.2,12,20 Further studies, including allelic burden and biomarker modification analyses, are underway to fully evaluate the potential of this novel combination for disease modification. The combination of navitoclax with ruxolitinib is being further investigated in two global phase III clinical trials which compare navitoclax plus ruxolitinib with: placebo plus ruxolitinib in patients who are JAK2i-naïve (TRANSFORM-1; ClinicalTrials.gov identifier: NCT04472598); and best available therapy in patients have experienced progression or suboptimal response after ruxolitinib treatment (TRANSFORM-2; ClinicalTrials.gov identifier: NCT04468984).
ACKNOWLEDGMENT
Medical writing support was provided by Marta Rossi, PhD, and Hayley Ellis, PhD, of Fishawack Communications Ltd.
Claire N. Harrison
Honoraria: Celgene, Novartis, CTI BioPharma Corp, Roche/Genentech, Geron, AOP Orphan Pharmaceuticals, BMSi, Constellation Pharmaceuticals
Consulting or Advisory Role: Promedior, Celgene, AOP Orphan Pharmaceuticals, Galectin Therapeutics, Sierra Oncology
Speakers' Bureau: Novartis, CTI BioPharma Corp, Gilead Sciences, Incyte, Celgene, Janssen Oncology
Research Funding: Novartis (Inst), Celgene (Inst), Constellation Pharmaceuticals (Inst)
Jacqueline S. Garcia
Consulting or Advisory Role: AbbVie, Takeda, Astellas Pharma
Research Funding: AbbVie (Inst), Pfizer (Inst), Genentech/Roche (Inst), Lilly (Inst), AstraZeneca (Inst), Prelude Therapeutics (Inst)
Tim C.P. Somervaille
Honoraria: Novartis, Celgene/Bristol Myers Squibb
Consulting or Advisory Role: Novartis
Research Funding: Imago Biosciences, Cellcentric
James M. Foran
Consulting or Advisory Role: SERVIER, Bristol Myers Squibb/Celgene, Stemline Therapeutics, Taiho Pharmaceutical, Syros Pharmaceuticals, Sanofi, Certara Inc, Novartis, Pfizer, Revolution Medicines, Revolution Medicines
Research Funding: AbbVie (Inst), Actinium Pharmaceuticals (Inst), Aprea Therapeutics (Inst), Aptose Biosciences (Inst), Boehringer Ingelheim (Inst), H3 Biomedicine (Inst), Kura Oncology (Inst), Sellas Life Sciences (Inst), Takeda (Inst), Trillium Therapeutics (Inst), Xencor (Inst)
Srdan Verstovsek
Consulting or Advisory Role: Constellation Pharmaceuticals, Sierra Oncology, Incyte, Novartis, Celgene
Research Funding: Incyte (Inst), Celgene (Inst), Protagonist Therapeutics (Inst), Sierra Oncology (Inst), PharmaEssentia (Inst), Telios (Inst), Constellation Pharmaceuticals (Inst), AbbVie (Inst), Geron (Inst), Galecto Biotech (Inst), Kartos Therapeutics (Inst)
Catriona Jamieson
Stock and Other Ownership Interests: Aspera Biomedicines, Inc
Patents, Royalties, Other Intellectual Property: Patent Royalties—Methods for Manipulating Phagocytosis Mediated by CD47, Patent #US20090191202A1
Ruben Mesa
Honoraria: Novartis, shire, AOP Orphan Pharmaceuticals, Sierra Oncology, AbbVie, Geron, BMS
Consulting or Advisory Role: Baxalta, Galena Biopharma, Incyte, Novartis
Research Funding: Incyte (Inst), Genentech (Inst), CTI (Inst), Promedior (Inst), NS Pharma (Inst), Gilead Sciences (Inst), Pfizer (Inst), PharmaEssentia (Inst), Celgene (Inst), AbbVie (Inst), Imago Pharma (Inst)
Travel, Accommodations, Expenses: Novartis, Incyte, AOP Orphan Pharmaceuticals, PharmaEssentia
Ellen K. Ritchie
Consulting or Advisory Role: Incyte, Celgene, Pfizer, Novartis, Bristol Myers Squibb
Speakers' Bureau: Celgene, Incyte
Research Funding: Astellas Pharma (Inst), Novartis (Inst), Pfizer (Inst), Jazz Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Pfizer
Srinivas K. Tantravahi
Honoraria: Novartis, Karyopharm Therapeutics
Consulting or Advisory Role: Karyopharm Therapeutics, Novartis
Research Funding: Karyopharm Therapeutics
Pankit Vachhani
Consulting or Advisory Role: Blueprint Medicines, Incyte, AbbVie, Jazz Pharmaceuticals, CTI BioPharma Corp, Novartis, Amgen, Pfizer
Speakers' Bureau: Incyte
Research Funding: Seattle Genetics (Inst), Amgen (Inst), Astex Pharmaceuticals (Inst), Incyte (Inst), Blueprint Medicines (Inst), Kartos Therapeutics (Inst), Gilead/Forty Seven (Inst), Constellation Pharmaceuticals (Inst), AbbVie (Inst), CTI BioPharma Corp (Inst), Takeda (Inst)
Casey L. O'Connell
Research Funding: Astex Pharmaceuticals (Inst), Genentech (Inst)
Rami S. Komrokji
Stock and Other Ownership Interests: AbbVie
Consulting or Advisory Role: Novartis, Bristol Myers Squibb, Jazz Pharmaceuticals, AbbVie, Geron, Acceleron Pharma
Speakers' Bureau: Jazz Pharmaceuticals, Bristol Myers Squibb, Agios
Travel, Accommodations, Expenses: Jazz Pharmaceuticals, Bristol Myers Squibb, Agios
Jason Harb
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Jessica E. Hutti
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Leanne Holes
Employment: AbbVie, Bristol Myers Squibb/Celgene/Juno
Stock and Other Ownership Interests: AbbVie
Abdullah A. Masud
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Silpa Nuthalapati
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Jalaja Potluri
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Naveen Pemmaraju
Employment: MD Anderson Cancer Center
Leadership: ASH, ASCO.
Honoraria: Incyte, Novartis, LFB Biotechnologies, Stemline Therapeutics, Celgene, AbbVie, MustangBio, Roche Molecular Diagnostics, Blueprint Medicines, DAVA Pharmaceuticals, Springer, Aptitude Health, Neopharm, CareDX
Consulting or Advisory Role: Blueprint Medicines, Pacylex, Immunogen, Bristol Myers Squibb, Clearview Healthcare Partners, Astellas Pharma US Inc, Protagonist Therapeutics, Inc, Triptych Health Partners, CTI BioPharma Corp
Research Funding: Novartis, Stemline Therapeutics, Samus Therapeutics, AbbVie, Cellectis, Affymetrix/Thermo Fisher Scientific, Daiichi Sankyo, Plexxikon, MustangBio
Travel, Accommodations, Expenses: Stemline Therapeutics, Celgene, AbbVie, DAVA Oncology, MustangBio
(OPTIONAL) Uncompensated Relationships: Dan's House of Hope
(OPTIONAL) Uncompensated Relationships: Oncology Times
No other potential conflicts of interest were reported.
See accompanying article on page 1693
DISCLAIMER
All the authors had access to relevant data and participated in the drafting, review, and approval of this manuscript.
PRIOR PRESENTATION
Presented as poster presentation at the 62nd ASH virtual annual meeting and exposition, December 5-8, 2020, and as an e-poster presentation at the EHA virtual congress 2021, June 9-17, 2021.
SUPPORT
Navitoclax is being developed by AbbVie. AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the manuscript. T.C.P.S. received salary support from Cancer Research UK grant no. C5759/A20971.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
AbbVie is committed to responsible data sharing regarding the clinical trials that we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets). This includes requests for clinical trial data for unlicensed products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
AUTHOR CONTRIBUTIONS
Conception and design: Claire N. Harrison, Jacqueline S. Garcia, Catriona Jamieson, Ruben Mesa, Pankit Vachhani, Jason Harb, Leanne Holes, Silpa Nuthalapati, Jalaja Potluri, Naveen Pemmaraju
Administrative support: Ellen K. Ritchie
Provision of study materials or patients: Jacqueline S. Garcia, James M. Foran, Ruben Mesa, Ellen K. Ritchie, Srinivas K. Tantravahi, Rami S. Komrokji, Naveen Pemmaraju
Collection and assembly of data: Claire N. Harrison, Jacqueline S. Garcia, Tim C.P. Somervaille, James M. Foran, Catriona Jamieson, Ruben Mesa, Ellen K. Ritchie, Pankit Vachhani, Rami S. Komrokji, Leanne Holes, Jalaja Potluri, Naveen Pemmaraju
Data analysis and interpretation: Claire N. Harrison, Jacqueline S. Garcia, Tim C.P. Somervaille, James M. Foran, Srdan Verstovsek, Catriona Jamieson, Ruben Mesa, Srinivas K. Tantravahi, Pankit Vachhani, Rami S. Komrokji, Jason Harb, Jessica E. Hutti, Leanne Holes, Abdullah A. Masud, Silpa Nuthalapati, Jalaja Potluri, Naveen Pemmaraju
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Addition of Navitoclax to Ongoing Ruxolitinib Therapy for Patients With Myelofibrosis With Progression or Suboptimal Response: Phase II Safety and Efficacy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Claire N. Harrison
Honoraria: Celgene, Novartis, CTI BioPharma Corp, Roche/Genentech, Geron, AOP Orphan Pharmaceuticals, BMSi, Constellation Pharmaceuticals
Consulting or Advisory Role: Promedior, Celgene, AOP Orphan Pharmaceuticals, Galectin Therapeutics, Sierra Oncology
Speakers' Bureau: Novartis, CTI BioPharma Corp, Gilead Sciences, Incyte, Celgene, Janssen Oncology
Research Funding: Novartis (Inst), Celgene (Inst), Constellation Pharmaceuticals (Inst)
Jacqueline S. Garcia
Consulting or Advisory Role: AbbVie, Takeda, Astellas Pharma
Research Funding: AbbVie (Inst), Pfizer (Inst), Genentech/Roche (Inst), Lilly (Inst), AstraZeneca (Inst), Prelude Therapeutics (Inst)
Tim C.P. Somervaille
Honoraria: Novartis, Celgene/Bristol Myers Squibb
Consulting or Advisory Role: Novartis
Research Funding: Imago Biosciences, Cellcentric
James M. Foran
Consulting or Advisory Role: SERVIER, Bristol Myers Squibb/Celgene, Stemline Therapeutics, Taiho Pharmaceutical, Syros Pharmaceuticals, Sanofi, Certara Inc, Novartis, Pfizer, Revolution Medicines, Revolution Medicines
Research Funding: AbbVie (Inst), Actinium Pharmaceuticals (Inst), Aprea Therapeutics (Inst), Aptose Biosciences (Inst), Boehringer Ingelheim (Inst), H3 Biomedicine (Inst), Kura Oncology (Inst), Sellas Life Sciences (Inst), Takeda (Inst), Trillium Therapeutics (Inst), Xencor (Inst)
Srdan Verstovsek
Consulting or Advisory Role: Constellation Pharmaceuticals, Sierra Oncology, Incyte, Novartis, Celgene
Research Funding: Incyte (Inst), Celgene (Inst), Protagonist Therapeutics (Inst), Sierra Oncology (Inst), PharmaEssentia (Inst), Telios (Inst), Constellation Pharmaceuticals (Inst), AbbVie (Inst), Geron (Inst), Galecto Biotech (Inst), Kartos Therapeutics (Inst)
Catriona Jamieson
Stock and Other Ownership Interests: Aspera Biomedicines, Inc
Patents, Royalties, Other Intellectual Property: Patent Royalties—Methods for Manipulating Phagocytosis Mediated by CD47, Patent #US20090191202A1
Ruben Mesa
Honoraria: Novartis, shire, AOP Orphan Pharmaceuticals, Sierra Oncology, AbbVie, Geron, BMS
Consulting or Advisory Role: Baxalta, Galena Biopharma, Incyte, Novartis
Research Funding: Incyte (Inst), Genentech (Inst), CTI (Inst), Promedior (Inst), NS Pharma (Inst), Gilead Sciences (Inst), Pfizer (Inst), PharmaEssentia (Inst), Celgene (Inst), AbbVie (Inst), Imago Pharma (Inst)
Travel, Accommodations, Expenses: Novartis, Incyte, AOP Orphan Pharmaceuticals, PharmaEssentia
Ellen K. Ritchie
Consulting or Advisory Role: Incyte, Celgene, Pfizer, Novartis, Bristol Myers Squibb
Speakers' Bureau: Celgene, Incyte
Research Funding: Astellas Pharma (Inst), Novartis (Inst), Pfizer (Inst), Jazz Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Pfizer
Srinivas K. Tantravahi
Honoraria: Novartis, Karyopharm Therapeutics
Consulting or Advisory Role: Karyopharm Therapeutics, Novartis
Research Funding: Karyopharm Therapeutics
Pankit Vachhani
Consulting or Advisory Role: Blueprint Medicines, Incyte, AbbVie, Jazz Pharmaceuticals, CTI BioPharma Corp, Novartis, Amgen, Pfizer
Speakers' Bureau: Incyte
Research Funding: Seattle Genetics (Inst), Amgen (Inst), Astex Pharmaceuticals (Inst), Incyte (Inst), Blueprint Medicines (Inst), Kartos Therapeutics (Inst), Gilead/Forty Seven (Inst), Constellation Pharmaceuticals (Inst), AbbVie (Inst), CTI BioPharma Corp (Inst), Takeda (Inst)
Casey L. O'Connell
Research Funding: Astex Pharmaceuticals (Inst), Genentech (Inst)
Rami S. Komrokji
Stock and Other Ownership Interests: AbbVie
Consulting or Advisory Role: Novartis, Bristol Myers Squibb, Jazz Pharmaceuticals, AbbVie, Geron, Acceleron Pharma
Speakers' Bureau: Jazz Pharmaceuticals, Bristol Myers Squibb, Agios
Travel, Accommodations, Expenses: Jazz Pharmaceuticals, Bristol Myers Squibb, Agios
Jason Harb
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Jessica E. Hutti
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Leanne Holes
Employment: AbbVie, Bristol Myers Squibb/Celgene/Juno
Stock and Other Ownership Interests: AbbVie
Abdullah A. Masud
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Silpa Nuthalapati
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Jalaja Potluri
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Naveen Pemmaraju
Employment: MD Anderson Cancer Center
Leadership: ASH, ASCO.
Honoraria: Incyte, Novartis, LFB Biotechnologies, Stemline Therapeutics, Celgene, AbbVie, MustangBio, Roche Molecular Diagnostics, Blueprint Medicines, DAVA Pharmaceuticals, Springer, Aptitude Health, Neopharm, CareDX
Consulting or Advisory Role: Blueprint Medicines, Pacylex, Immunogen, Bristol Myers Squibb, Clearview Healthcare Partners, Astellas Pharma US Inc, Protagonist Therapeutics, Inc, Triptych Health Partners, CTI BioPharma Corp
Research Funding: Novartis, Stemline Therapeutics, Samus Therapeutics, AbbVie, Cellectis, Affymetrix/Thermo Fisher Scientific, Daiichi Sankyo, Plexxikon, MustangBio
Travel, Accommodations, Expenses: Stemline Therapeutics, Celgene, AbbVie, DAVA Oncology, MustangBio
(OPTIONAL) Uncompensated Relationships: Dan's House of Hope
(OPTIONAL) Uncompensated Relationships: Oncology Times
No other potential conflicts of interest were reported.
REFERENCES
- 1.Harrison CN, Schaap N, Mesa RA: Management of myelofibrosis after ruxolitinib failure. Ann Hematol 99:1177-1191, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vannucchi A, Barbui T, Cervantes F, et al. : Philadelphia chromosome-negative chronic myeloproliferative neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26:v85-v99, 2015 [DOI] [PubMed] [Google Scholar]
- 3.de Freitas RM, da Costa Maranduba CM: Myeloproliferative neoplasms and the JAK/STAT signaling pathway: An overview. Rev Bras Hematol Hemoter 37:348-353, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pikman Y, Lee BH, Mercher T, et al. : MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 3:e270, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James C, Ugo V, Le Couédic J-P, et al. : A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434:1144-1148, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Wernig G, Mercher T, Okabe R, et al. : Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood 107:4274-4281, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klampfl T, Gisslinger H, Harutyunyan AS, et al. : Somatic mutations of calreticulin in myeloproliferative neoplasms. New Engl J Med 369:2379-2390, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Tognon R, Gasparotto EP, Neves RP, et al. : Deregulation of apoptosis-related genes is associated with PRV1 overexpression and JAK2 V617F allele burden in Essential Thrombocythemia and Myelofibrosis. J Hematol Oncol 5:1-11, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo J, Roberts L, Chen Z, et al. : JAK2 V617F drives Mcl-1 expression and sensitizes hematologic cell lines to dual inhibition of JAK2 and Bcl-xL. PLoS One 10:e0114363, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winter PS, Sarosiek KA, Lin KH, et al. : RAS signaling promotes resistance to JAK inhibitors by suppressing BAD-mediated apoptosis. Sci Signal 7:ra122, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benites BD, Lima CSC, Lorand-Metze I, et al. : Primary myelofibrosis: Risk stratification by IPSS identifies patients with poor clinical outcome. Clinics 68:339-343, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerds A, Gotlib J, Bose P, et al. : NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Version 1.2020: Myeloproliferative Neoplasms, 2020 [Google Scholar]
- 13.Deisseroth A, Kaminskas E, Grillo J, et al. : US Food and Drug Administration approval: Ruxolitinib for the treatment of patients with intermediate and high-risk myelofibrosis. Clin Cancer Res 18:3212-3217, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Jamieson C, Hasserjian R, Gotlib J, et al. : Effect of treatment with a JAK2-selective inhibitor, fedratinib, on bone marrow fibrosis in patients with myelofibrosis. J Transl Med 13:294, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bose P, Verstovsek S: JAK inhibition for the treatment of myelofibrosis: Limitations and Future perspectives. Hemasphere 4:e424, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palandri F, Breccia M, Bonifacio M, et al. : Life after ruxolitinib: Reasons for discontinuation, impact of disease phase, and outcomes in 218 patients with myelofibrosis. Cancer 126:1243-1252, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Patel KP, Newberry KJ, Luthra R, et al. : Correlation of mutation profile and response in patients with myelofibrosis treated with ruxolitinib. Blood 126:790-797, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiegel JY, McNamara C, Kennedy JA, et al. : Impact of genomic alterations on outcomes in myelofibrosis patients undergoing JAK1/2 inhibitor therapy. Blood Adv 1:1729-1738, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettit K, Gerds AT, Yacoub A, et al. : A Phase 2a Study of the LSD1 Inhibitor Img-7289 (Bomedemstat) for the Treatment of Myelofibrosis, Washington, DC, American Society of Hematology, 2019 [Google Scholar]
- 20.Pardanani A, Tefferi A: How I treat myelofibrosis after failure of JAK inhibitors. Blood 132:492-500, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Harrison CN, Vannucchi AM, Platzbecker U, et al. : Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): A randomised, open-label, phase 3 trial. Lancet Haematol 5:e73-e81, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Mascarenhas J, Hoffman R, Talpaz M, et al. : Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: A randomized clinical trial. JAMA Oncol 4:652-659, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tse C, Shoemaker AR, Adickes J, et al. : ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 68:3421-3428, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Waibel M, Solomon VS, Knight DA, et al. : Combined targeting of JAK2 and Bcl-2/Bcl-xL to cure mutant JAK2-driven malignancies and overcome acquired resistance to JAK2 inhibitors. Cell Rep 5:1047-1059, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Passamonti F, Cervantes F, Vannucchi AM, et al. : A dynamic prognostic model to predict survival in primary myelofibrosis: A study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 115:1703-1708, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Oken MM, Creech RH, Tormey DC, et al. : Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649-656, 1982 [PubMed] [Google Scholar]
- 27.Branch SK: Guidelines from the International Conference on Harmonisation (ICH). J Pharm Biomed Anal 38:798-805, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Mesa RA, Schwager S, Radia D, et al. : The Myelofibrosis Symptom Assessment Form (MFSAF): An evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res 33:1199-1203, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mesa RA, Kantarjian H, Tefferi A, et al. : Evaluating the serial use of the myelofibrosis symptom assessment form for measuring symptomatic improvement: Performance in 87 myelofibrosis patients on a JAK1 and JAK2 inhibitor (INCB018424) clinical trial. Cancer 117:4869-4877, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tefferi A, Cervantes F, Mesa R, et al. : Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood 122:1395-1398, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiele J, Kvasnicka HM, Facchetti F, et al. : European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica 90:1128-1132, 2005 [PubMed] [Google Scholar]
- 32.National Cancer Institute : Common Terminology Criteria for Adverse Events v4.0 3. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen J: Statistical Power Analysis for the Behavioral Sciences. Hillsdle, NJ, Erlbaum. Conner BE: The Box in the Barn. Columbus, Highlights for Children Inc, 1988 [Google Scholar]
- 34.Verstovsek S, Mesa RA, Gotlib J, et al. : A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. New Engl J Med 366:799-807, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrison CN, Vannucchi AM, Kiladjian J-J, et al. : Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia 30:1701-1707, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dueck AC, Cleeland CS, Dantzer R, et al. : Cytokine Profile Changes in 309 Myelofibrosis Patients: Comparison of JAK1/JAK2 Inhibitor Therapy vs. Placebo-Correlative Analysis From the COMFORT-I Trial, Washington, DC, American Society of Hematology, 2013 [Google Scholar]
- 37.Pardanani A, Tefferi A, Jamieson C, et al. : A phase 2 randomized dose-ranging study of the JAK2-selective inhibitor fedratinib (SAR302503) in patients with myelofibrosis. Blood Cancer J 5:e335, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pemmaraju N, Garcia JS, Potluri J, et al. : The addition of navitoclax to ruxolitinib demonstrates efficacy within different high-risk populations in patients with relapsed/refractory myelofibrosis. Blood, 136:49-50, 2020 [Google Scholar]
- 39.Kuykendall AT, Shah S, Talati C, et al. : Between a rux and a hard place: Evaluating salvage treatment and outcomes in myelofibrosis after ruxolitinib discontinuation. Ann Hematol 97:435-441, 2018 [DOI] [PubMed] [Google Scholar]
- 40.Newberry KJ, Patel K, Masarova L, et al. : Clonal evolution and outcomes in myelofibrosis after ruxolitinib discontinuation. Blood, 130:1125-1131, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J, Pradhan R, Rosen L, et al. : Effect of rifampin on the pharmacokinetics, safety and tolerability of navitoclax (ABT‐263), a dual inhibitor of Bcl‐2 and Bcl‐XL, in patients with cancer. J Clin Pharm Ther 39:680-684, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Incyte Corporation : Ruxolitinib Prescribing Information. 2020. https://www.jakafi.com/pdf/prescribing-information.pdf [Google Scholar]
- 43.Food and Drug Administration WU : Ruxolitinib (Jakafi), Application Number: 202192Orig1s000, Clinical Pharmacology and Biopharmaceutics Review(s). 2011. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202192Orig1s000ClinPharmR.pdf [Google Scholar]
- 44.Xiong H, Pradhan RS, Nada A, et al. : Studying navitoclax, a targeted anticancer drug, in healthy volunteers—Ethical considerations and risk/benefit assessments and management. Anticancer Res 34:3739-3746, 2014 [PubMed] [Google Scholar]
- 45.Verstovsek S, Mesa RA, Gotlib J, et al. : Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: Results of a median 3-year follow-up of COMFORT-I. Haematologica 100:479, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials that we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets). This includes requests for clinical trial data for unlicensed products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.