Every cell in our bodies is encoded with pathways of programmed cell death so that useless or harmful cells can be eliminated for the benefit of the organism as a whole. Among these, the mitochondrial pathway of apoptosis is prominent for its role in oncogenesis and response to cancer therapy. The selective activation of apoptosis in cancer cells has been an important goal in oncology for decades. Recently, a class of drugs called BH3 mimetics has emerged. In the original report that accompanies this article, Harrison et al1 present another novel application of BH3 mimetic therapy with navitoclax in the context of myelofibrosis.
The point of commitment to cell death in the mitochondrial apoptotic pathway is mitochondrial outer membrane permeabilization (MOMP). MOMP is directly regulated by the BCL-2 family of proteins, named after the first protein characterized in this family. The BCL-2 family contains both proapoptotic and antiapoptotic proteins. BAX and BAK, and perhaps less certainly BOK, are so-called effectors.2,3 Following allosteric activation, they can homo-oligomerize and form large pores in the mitochondrial outer membrane, permitting egress of intermembrane space contents. Among these contents are cytochrome c and second mitochondria-derived activator of caspase which facilitate the activation of a family of cysteine proteases called caspases.4,5 Caspases are required for many of the characteristics of apoptotic cell death, including widespread proteolysis, oligonucleosomal DNA fragmentation, and tagging of the cell with signals that facilitate phagocytosis (Fig 1).6
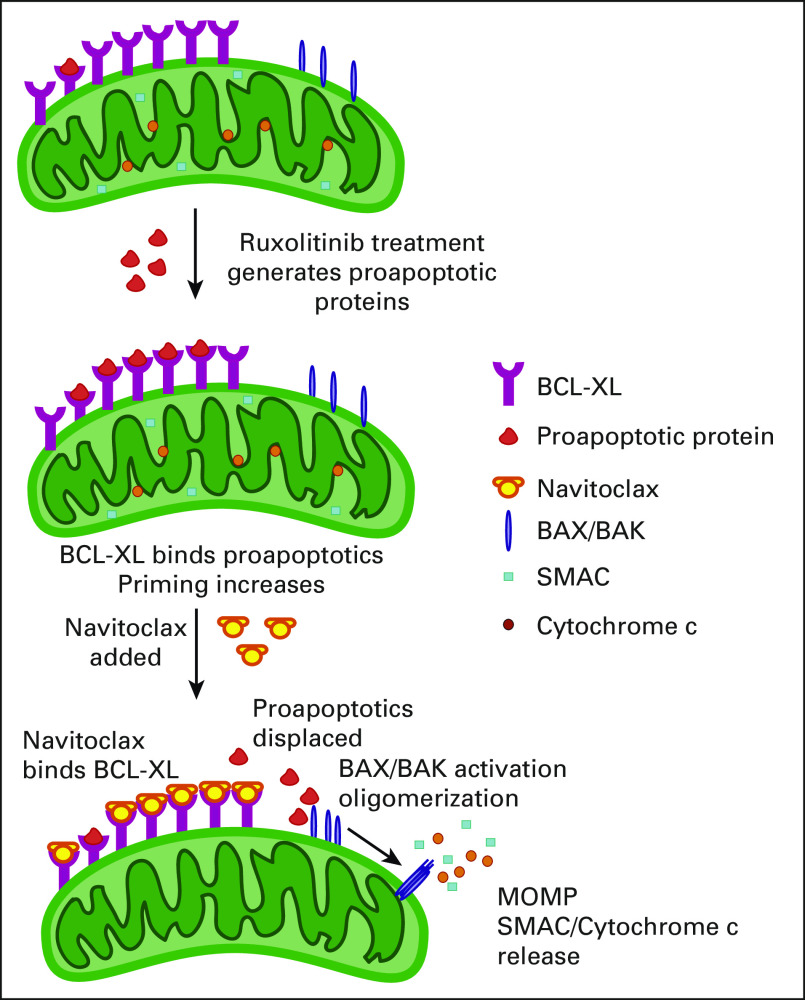
FIG 1.
Mitochondrial apoptotic priming drives sensitivity to BH3 mimetics. At top, ruxolitinib treatment generates proapoptotic proteins that are sequestered by antiapoptotic BCL-XL at the mitochondrial outer membrane (middle), priming BCL-XL and rendering the cell more dependent on BCL-XL on survival. Treatment with a BH3 mimetic such as navitoclax, which competes for the BH3 binding site in BCL-XL, displaces proapoptotic proteins from BCL-XL. Some of these displaced proteins, such as BIM, can activate BAX or BAK, initiating their homo-oligomerization and MOMP. MOMP allows egress of cytochrome c and SMAC, which facilitate widespread activation of caspases, which in turn cleave many proteins. These cleavage events lead to DNA endonuclease activation and tagging of the cell with eat-me signals to facilitate phagocytosis, completing the process of apoptosis. Figure credit: Jeremy Ryan. MOMP, mitochondrial outer membrane permeabilization; SMAC, second mitochondria-derived activator of caspase.
BAX and BAK can be activated by another subset of proapoptotic BCL-2 family proteins, the BH3-only activators. Activators, which include BIM and BID, can interact with BAX and BAK and induce an allosteric change that permits BAX and BAK homo-oligomerization and MOMP.7,8 Antiapoptotic proteins, which include BCL-2, BCL-XL, MCL-1, BCL-w, and BFL-1, block apoptosis by binding the BH3 domains of monomeric activators or effectors, sequestering and neutralizing them.9 Another class of proapoptotic proteins, the BH3-only sensitizers, exert their proapoptotic function by competing for the hydrophobic BH3 binding site in the antiapoptotic proteins, displacing effectors bound there, driving progression to MOMP.10
BCL-2 homology 3 (BH3) domains are amphipathic alpha-helices possessed by all members of the BCL-2 family. Although there are four such BH domains, BH1-4, the BH3-only proteins contain only the BH3. The BH3 domains of BH3-only proteins act as natural inhibitors of the function of the antiapoptotic proteins. Some are broadly inhibitory, interacting with all the antiapoptotic proteins, whereas others act more narrowly, in some cases inhibiting only a single antiapoptotic protein.11-14 Thus, the natural oligopeptide BH3 domains served as prototypes of cancer therapeutics that could inhibit BCL-2 or BCL-XL or MCL-1, albeit ones with very poor pharmacologic properties.10
A team at AbbVie was responsible for ground-breaking strategies to produce the first pharmacologically useful small molecule mimetics of the sensitizer BH3 domains.15 They used rapid NMR-based screening of ligands for two different aspects of the hydrophobic BH3 binding site in BCL-XL to create the first effective BH3 mimetic inhibitor, ABT-737. ABT-737 antagonized BCL-2, BCL-XL, and BCL-w, mimicking the selectivity of the BH3 domain of the BAD protein. Building on this program, AbbVie later developed ABT-263 (navitoclax),16 with specificity similar to ABT-737, and later ABT-199 (venetoclax),17 a selective BCL-2 antagonist. Venetoclax, the first effective drug directly targeting an apoptotic pathway, has received regulatory approval so far in acute myelomonocytic leukemia (AML) and chronic lymphatic leukemia (CLL) by regulatory bodies worldwide.18,19 Moreover, the success of venetoclax has spawned an entire new class of BH3 mimetic drugs made by several companies targeting BCL-2, BCL-XL, and MCL-1 with varying degrees of specificity. These are currently being tested in more than 100 clinical trials internationally.
Given the expanding number of BH3 mimetics, one might ask, how might one determine which drug is best for an individual patient or type of cancer? First, we should understand that cellular sensitivity to a BH3 mimetic requires that cell to be dependent on the antiapoptotic protein targeted by that BH3. Thus, cells that are sensitive to the BCL-2 selective venetoclax are those that are dependent on BCL-2. Dependence on an individual antiapoptotic protein requires that protein to be primed with a proapoptotic protein: mere expression of the antiapoptotic protein is not sufficient.20 For example, in CLL, a disease broadly sensitive to venetoclax, abundant BCL-2 is expressed, but critically a large proportion of that BCL-2 is sequestering the activator protein BIM. When venetoclax competes for the BH3-binding domain of BCL-2, some BIM is displaced, freeing it to activate BAX and induce MOMP and commitment to cell death.21 This can happen very rapidly in CLL, where a patient with an elevated WBC can see it normalize within hours of starting venetoclax.
However, quantitative measurement of the protein complexes necessary to know the degree of priming of an individual antiapoptotic protein in a patient tumor is technically very challenging. One alternative is to use BH3 profiling, an assay in which mitochondria of patient cancer cells are exposed to a standardized set of BH3 peptides to functionally gauge dependence on individual antiapoptotic proteins.21,22 Another alternative is to simply expose patient tumor cells ex vivo directly to the BH3 mimetics in question and measure a property indicative of cell death.15,23 Both approaches were critical to correctly identify the BCL-2 dependence in CLL and AML, which led to successful clinical trials and regulatory approvals in both those diseases for venetoclax.
In the myelofibrosis trial reported in the Journal of Clinical Oncology,1 the choice of navitoclax was made primarily because it can target BCL-XL. Dependence on BCL-XL was identified in myelofibrosis by several lines of evidence, including BH3 profiling.24,25 The combination with ruxolitinib was chosen because it is a drug with demonstrated activity in myelofibrosis. This is in concordance with a principle that has already been supported clinically, with the activity of venetoclax with anti-CD20 and BTK inhibitors in CLL,26 or venetoclax with hypomethylating agents in AML27: the right BH3 mimetic makes drugs that already work in a particular disease work better.
The future of BH3 mimetics in cancer lies in identifying active combinations. To identify the right BH3 mimetic for a distinct histology or even an individual patient will likely require continued dependence on functional strategies such as direct exposure of tumor to drugs or BH3 profiling. Static precision medicine tools such as next-generation sequencing have not been of much utility in this regard. There are many creative ideas working their way from the laboratory to the clinic in identifying novel, mechanistic synergy–based combinations with BH3 mimetics. In the meantime, however, identifying the right combination partners for BH3 mimetics might be as simple as selecting what is already given in a particular disease.
Anthony Letai
Stock and Other Ownership Interests: Zentalis, Flash Therapeutics, Dialectic
Consulting or Advisory Role: Zentalis, Flash Therapeutics, Anji Onco, Dialectic Therapeutics
Research Funding: Novartis Institutes for BioMedical Research (Inst), AbbVie (Inst)
Patents, Royalties, Other Intellectual Property: I am an inventor on several patents involving BH3 profiling technology. These are owned by my employer, Dana-Farber Cancer Institute (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/174250
No other potential conflicts of interest were reported.
See accompanying article on page 1671
SUPPORT
Supported by National Cancer Institute (NCI R35CA242427).
AUTHOR'S DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Apoptosis: Directly Targeted at Last
The following represents disclosure information provided by the author of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Anthony Letai
Stock and Other Ownership Interests: Zentalis, Flash Therapeutics, Dialectic
Consulting or Advisory Role: Zentalis, Flash Therapeutics, Anji Onco, Dialectic Therapeutics
Research Funding: Novartis Institutes for BioMedical Research (Inst), AbbVie (Inst)
Patents, Royalties, Other Intellectual Property: I am an inventor on several patents involving BH3 profiling technology. These are owned by my employer, Dana-Farber Cancer Institute (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/174250
No other potential conflicts of interest were reported.
REFERENCES
- 1.Harrison CN, Garcia JS, Somervaille TCP, et al. : Addition of navitoclax to ongoing ruxolitinib therapy for patients with myelofibrosis with progression or suboptimal response: Phase II safety and efficacy. J Clin Oncol 40:1671-1680, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei MC, Zong WX, Cheng EH, et al. : Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 292:727-730, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llambi F, Wang YM, Victor B, et al. : BOK is a non-canonical BCL-2 family effector of apoptosis regulated by ER-associated degradation. Cell 165:421-433, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du C, Fang M, Li Y, et al. : Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102:33-42, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Li P, Nijhawan D, Budihardjo I, et al. : Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479-489, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Nagata S: Apoptosis and clearance of apoptotic cells. Annu Rev Immunol 36:489-517, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Korsmeyer SJ, Wei MC, Saito M, et al. : Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ 7:1166-1173, 2000 [DOI] [PubMed] [Google Scholar]
- 8.O'Connor L, Strasser A, O'Reilly LA, et al. : Bim: A novel member of the Bcl-2 family that promotes apoptosis. EMBO J 17:384-395, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oltvai ZN, Milliman CL, Korsmeyer SJ: Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609-619, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Letai A, Bassik MC, Walensky LD, et al. : Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2:183-192, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Opferman JT, Letai A, Beard C, et al. : Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426:671-676, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Willis SN, Wei A, et al. : Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17:393-403, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Kuwana T, Bouchier-Hayes L, Chipuk JE, et al. : BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 17:525-535, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Certo M, Del Gaizo Moore V, Nishino M, et al. : Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9:351-365, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Oltersdorf T, Elmore SW, Shoemaker AR, et al. : An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435:677-681, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Tse C, Shoemaker AR, Adickes J, et al. : ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 68:3421-3428, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Souers AJ, Leverson JD, Boghaert ER, et al. : ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19:202-208, 2013 [DOI] [PubMed] [Google Scholar]
- 18.DiNardo CD, Jonas BA, Pullarkat V, et al. : Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 383:617-629, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Seymour JF, Ma S, Brander DM, et al. : Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: A phase 1b study. Lancet Oncol 18:230-240, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng J, Carlson N, Takeyama K, et al. : BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell 12:171-185, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Del Gaizo Moore V, Brown JR, Certo M, et al. : Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest 117:112-121, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vo TT, Ryan J, Carrasco R, et al. : Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell 151:344-355, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konopleva M, Contractor R, Tsao T, et al. : Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 10:375-388, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Winter PS, Sarosiek KA, Lin KH, et al. : RAS signaling promotes resistance to JAK inhibitors by suppressing BAD-mediated apoptosis. Sci Signal 7:ra122, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo J, Roberts L, Chen Z, et al. : JAK2V617F drives Mcl-1 expression and sensitizes hematologic cell lines to dual inhibition of JAK2 and Bcl-xL. PLoS One 10:e0114363, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davids MS, Lampson BL, Tyekucheva S, et al. : Acalabrutinib, venetoclax, and obinutuzumab as frontline treatment for chronic lymphocytic leukaemia: A single-arm, open-label, phase 2 study. Lancet Oncol 22:1391-1402, 2021 [DOI] [PubMed] [Google Scholar]
- 27.DiNardo CD, Pratz K, Pullarkat V, et al. : Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133:7-17, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]