Supplemental Digital Content is Available in the Text.
In this systematic review and meta-analysis, ultrasound-guided lumbar medial branch blocks and facet joint injections were associated with significant risk of incorrect needle placement.
Keywords: Ultrasound, Medial branch block, Facet joint injection, Systematic review, Meta-analysis
Abstract
There is great interest in expanding the use of ultrasound (US), but new challenges exist with its application to lumbar facet–targeted procedures. The primary aim of this systematic review and meta-analysis was to determine the risk of incorrect needle placement associated with US–guided lumbar medial branch blocks (MBB) and facet joint injections (FJI) as confirmed by fluoroscopy or computerized tomography (CT). An a priori protocol was registered, and a database search was conducted. Inclusion criteria included all study types. Risk of bias was assessed using the Cochrane risk of bias tool for randomized controlled trials and the National Heart, Lung, and Blood tool for assessing risk bias for observational cohort studies. Pooled analysis of the risk difference (RD) of incorrect needle placement was calculated. Pooled analysis of 7 studies demonstrated an 11% RD (P < 0.0009) of incorrect needle placement for US-guided MBB confirmed using fluoroscopy with and without contrast. Pooled analysis of 3 studies demonstrated a 13% RD (P < 0.0001) of incorrect needle placement for US-guided FJI confirmed using CT. The time to complete a single-level MBB ranged from 2.6 to 5.0 minutes. The certainty of evidence was low to very low. Ultrasound-guided lumbar MBB and FJI are associated with a significant risk of incorrect needle placement when confirmed by fluoroscopy or CT. The technical limitations of US and individual patient factors could contribute to the risk of incorrect needle placement.
1. Introduction
Fluoroscopy is the most widely used imaging modality for performing lumbar medial branch nerve blocks (MBB) and facet joint injections (FJI).5,7,35 Current Procedural Terminology codes for ultrasound-guided paravertebral injections (0213T- 0218T) are considered investigational and experimental, and American Society of Interventional Pain Physicians guidelines mandate the use of fluoroscopy or computed tomography (CT) for facet interventions.29 However, there has been an effort to increase the use of ultrasound (US) for spine procedures, including sacroiliac joint injections, epidural steroid injections, MBB, and FJI.22,23 Proposed benefits of US include lower cost and avoidance of radiation exposure for patients and medical personnel.4,26 Although there is great interest in expanding the use of US, there are new challenges with its application to lumbar facet–targeted procedures including increased tissue depth in the lumbar region.26 The technological limitations of US combined with the tissue depth of lumbar facets may affect the accuracy of needle placement. This is critically important when facet-targeted procedures are used for diagnostic purposes.
The use of US to perform lumbar MBB and FJI and the associated risk of incorrect needle placement have not been previously summarized. The primary aim of this systematic review and meta-analysis was to determine the risk of incorrect needle placement associated with US-guided lumbar MBB and FJI as confirmed by fluoroscopy or CT. Secondary objectives include summarizing (1) the techniques used to perform US-guided lumbar MBB and FJI, (2) procedure time for performing US-guided lumbar MBB and FJI, and (3) complications.
2. Methods
2.1. Search strategy
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines,31 and the study protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42020172717) in April 2020.2 A comprehensive search of databases was conducted from inception to February 1, 2021, and there were no language restrictions. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Nonindexed Citations and Daily, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus.
The search strategy was conducted by an experienced librarian with input from the principal investigator. Controlled vocabulary supplemented with keywords was used to search for studies describing US-guided MBB and FJI for low back pain. The detailed strategy listing all search terms used and how they are combined is available in the supplemental materials document (available at http://links.lww.com/PR9/A160).
2.2. Study selection process
Study inclusion criteria included (1) evaluation of US-guided lumbar MBB and FJI, (2) all study designs including conference proceedings and abstracts, and (3) outcomes assessing feasibility, diagnosis, prognosis, or safety. Exclusion criteria included (1) human cadaver or animal studies.
In the first review phase, 2 pairs of reviewers independently screened all titles and abstracts identified by the search strategy. In the second phase, the 2 pairs of reviewers independently screened the full text of all studies and inclusion and exclusion criteria were applied. Any reviewer disagreements were resolved by a third party.
2.3. Data extraction
Data were extracted by 4 independent reviewers using a templated electronic database. Based on the a priori protocol, abstracted data included the year of publication; number of participants; type of intervention; imaging technique used to perform the intervention; and outcomes assessing feasibility, diagnosis, prognosis, or safety. The corresponding authors of selected studies were contacted if missing or incomplete data were reported.
2.4. Risk of bias assessment
Risk of bias was assessed using the Cochrane risk of bias tool for randomized controlled trials (RoB2).49 The National Heart, Lung, and Blood tools for assessing risk of bias were used for case series and observational cohort studies with and without controls.33
2.5. Grading of evidence
The various outcomes assessed in this review were evaluated according to the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.18,48 Domains of evaluation included risk of bias, imprecision, inconsistency, indirectness, and publication bias.
2.6. Evidence synthesis
For each study, the number of needles placed by US guidance for MBB or FJI was recorded and the number of needles confirmed by fluoroscopy or CT to be correctly placed by US guidance was also recorded. Using the inverse variance method, the risk difference of US-guided needle placement as confirmed by fluoroscopy or CT was pooled across all studies using a random effects model. Heterogeneity was expressed using the I2 statistic, and results were reported with 95% confidence intervals (95% CI). All statistical analyses were performed using RevMan (Reviewer Manager, version 5.3.5; the Cochran Collaboration, Copenhagen, Denmark).
3. Results
3.1. Characteristics of included studies
A flow diagram of the study selection process is depicted in Figure 1. A total of 22 studies met the inclusion and exclusion criteria (Table 1).3,9,13,14,16,17,19–21,24,25,32,37,40,41,43,47,54,55 Study designs included 6 randomized controlled trials (RCTs),16,19,25,51,54,55 1 controlled cohort study,43 9 cohort studies,3,13,14,21,24,28,32,37,47 1 retrospective comparative study,20 3 case series,9,17,40 and 2 case reports.6,41 Four studies were conference proceedings or abstracts.3,9,32,47
Figure 1.
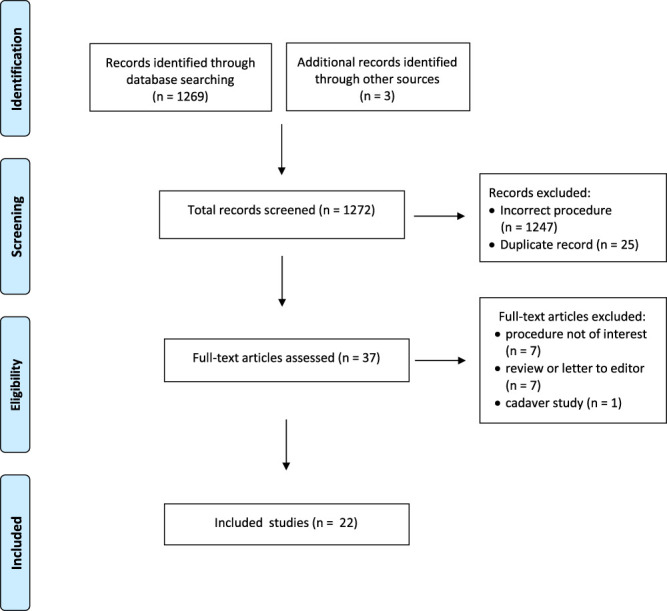
Preferred reporting items for systematic reviews and meta-analyses flowchart of study selection process.
Table 1.
Study characteristics.
| Author | Study design | No. of patients | No. US-guided blocks | Injectate | Technique | Levels blocked (number of blocks) | Confirmation method |
|---|---|---|---|---|---|---|---|
| Medial branch block | |||||||
| Batalov3 2013 | Single-arm cohort | 35 | 176 | 1 mL 0.25% bupivacaine and 5 mg methylprednisolone | US-guided “facet nerve block,” technique not specified | L2–L5 spinal levels; 17 unilateral, 18 bilateral | None |
| Chen6 2020 | Case report | 1 | 2 | 0.25 mL, content not described | Transverse view to determine target (junction of SAP and superior border of TP), lateral to medial in-plane injection, longitudinal view to confirm location | L2 MBB and L3 MBB | None |
| Etheridge14 2020 | Single-arm cohort | 115 | 100 (15 patients excluded due to inability to visualize target) | 0.5 mL 0.75% bupivacaine | Longitudinal view to determine level, transverse view to locate L4 MBB target (junction of the cephalad TP and SAP), lateral to medial in-plane injection; subsequent redirection of needle medially and caudally for L5 MBB while tracking progress in a sagittal view | L4 MBB (100), L5 MBB (100); all unilateral | Fluoroscopic needle position and contrast to validate position of L5 MBB only |
| Greher17 2004 | Case series | 5 | 28 | 1 mL 0.25% bupivacaine | Longitudinal view to determine level, transverse view to determine target (junction of the cephalad TP and SAP), in-plane injection, lateral to medial, verification with longitudinal view | L2 MBB (8), L3 MBB (10), L4 MBB (10); all bilateral | Fluoroscopic needle position |
| Han20 2017 | Retrospective comparative | 146 (US group: 68, FL group: 78) | 94 | 0.5 mL 1% lidocaine and 2.5 mg dexamethasone | Longitudinal scan to determine level, transverse view to determine target (junction of cephalad TP and SAP and junction of SAP and sacral ala); L5 MBB occasionally performed in out-of-plane fashion if sacral ala obstructed field of view | L3 MBB, L4 MBB, L5 MBB; number at each level not specified, number of unilateral and bilateral cases not specified | None |
| Hashemi21 2017 | Single-arm cohort | 30 | 89 | 1 mL 1% lidocaine and 40 mg triamcinolone | Longitudinal view to determine level, transverse view to determine target (junction of the cephalad TP and SAP), lateral to medial in-plane injection | L3 MBB (30), L4 MBB (31), L5 MBB (28); number of unilateral and bilateral cases not specified | Fluoroscopic needle position |
| Jung24 2012 | Single-arm cohort | 50 | 95 | 1 mL 2% lidocaine and 40 mg triamcinolone | Longitudinal view to determine level, transverse view to locate target (junction superior TP and SAP), lateral to medial in-plane injection | T12 MBB (1), L1 MBB (1), L2 MBB (3), L3 MBB (35), L4 MBB (48), L5 MBB (7); number of unilateral and bilateral cases not specified | Fluoroscopic needle position and contrast |
| Moon32 2013 | Single-arm cohort | 27 | 27 patients, total number of blocks not reported | 0.5% lidocaine | Transverse view to identify target (groove at root of TP and base of SAP) | Blocks performed at L1-L5; specific levels blocked are unclear; number of unilateral and bilateral cases not specified | None |
| Rauch37 2009 | Single-arm cohort | 20 | 84 | 0.3 mL mixture of 1% lidocaine and steroid | Longitudinal view to determine level, transverse to determine target, lateral to medial in-plane injection | L3 MBB (28), L4 MBB (29), L5 MBB (35); number of unilateral and bilateral cases not specified | Fluoroscopic needle position |
| Shim43 2006 | Self-controlled cohort | 20 | 101 | 1 mL 0.25% bupivacaine | Parasagittal view to determine level, transverse view to determine target (junction of cephalad TP and SAP), parasagittal view to confirm placement | T12 MBB (4), L1 MBB (22), L2 MBB (35), L4 MBB (31); number at L3 not reported but calculated to be 9 based on total number of blocks; number of unilateral and bilateral cases not specified | Fluoroscopic needle position and contrast |
| Soni47 2018 | Single-arm cohort | 30 | 74 | 0.5 mL 2% lidocaine | US-guided MBB, technique not specified | Levels and laterality not specified | Fluoroscopic needle position and contrast (contrast not specifically mentioned in text but is noted on included confirmatory imaging) |
| Facet joint injection | |||||||
| Constantinescu9 2017 | Case series | 3 | 3 patients, total number of blocks not reported | Local anesthetic and steroid | Intra-articular placement verified by US, views not specified | Not specified | None |
| Erdogan13 2019 | Single-arm cohort | 22 | 61 | 1 mL 2% lidocaine and 40 mg triamcinolone | Longitudinal view to determine level, transverse view with in-plane injection to superolateral corner of facet joint | Unilateral L3-4 (7), bilateral L3-4 (8), unilateral L4-5 (6), bilateral L4-5 (13), unilateral L5-S1 (4), bilateral L5-S1 (4); 6 levels could not be fully or partially visualized by US, although the specific levels were not specified | Fluoroscopic needle position and contrast |
| Galiano16 2007 | RCT | 40 (US group: 20, CT group: 20) | 20 | 1 mL 1% lidocaine, 1 mL 0.5% bupivacaine, and 4 mg betamethasone; 3 mL total volume | Parasagittal view to determine level, transverse view with in-plane injection to facet joint | L3-4 (1), L4-5 (6), L5-S1 (13); facet joints not able to be identified in 2 patients (level not specified), facets only partially identified in 2 other patients (level not specified) | CT needle position |
| Ha19 2010 | RCT | 105 (US group: 54, control group: 51) | 108 | 2% lidocaine and dexamethasone; 0.5 mL total volume | Parasagittal image to determine level, transverse view with in-plane injection | Bilateral L2-3 (3), bilateral L3-4 (15), bilateral L4-5 (28), bilateral L5-S1 (8) | None |
| Karkucak25 2020 | RCT | 49 (US group: 25, palpation-guided: 24) | 38 | 1% lidocaine and 10–20 mg triamcinolone per level; 1–2 mL total volume; 2nd injection performed at 2 wk | Parasagittal view to determine level, transverse view to determine target, lateral to medial in-plane injection | Unilateral L4-5 (18), unilateral L5-S1 (16), bilateral L5-S1 (2); 2 patients in US group did not complete the study | None |
| Kullmer28 1997 | Single-arm cohort | 78 | 213 | 5 mL carbostesin in combination with corticosteroids | Transverse and longitudinal views to visualize facet joint; caudal to cranial in-plane injection | Bilateral L5-S1 (56), unilateral L5-S1 (2), unilateral L4-5 (1), bilateral L4-5 (46), bilateral L3-4 (3) | None |
| Sadeghian40 2018 | Case series | 10 | 18 | 5 mg bupivacaine and 40 mg methylprednisolone | Longitudinal view to determine level, transverse view with in-plane injection | L4-5 and L5-S1, number of blocks per level not specified | None |
| Santiago41 2014 | Case report | 3 | 3 | 0.25% bupivacaine and 10 mg methylprednisolone; 1 mL total volume | Longitudinal view to determine level, transverse view with out-of-plane injection | L1-2 (1), L2-3 (1), L3-4 (1) | Fluoroscopic needle position and contrast |
| Wen51 2014 | RCT | 20 (US group: 10, CT group: 10) | 37 | 0.5% lidocaine, 1–2 mL of analgesic solution | Facet joint identified with ultrasound in transverse plane, otherwise unspecified | Not specified | CT needle position |
| Ye54 2018 | RCT | 40 (US group: 20, CT group: 20) | 74 | 0.5 mL 2% lidocaine and 4 mg betamethasone; 2 mL total volume | Longitudinal view to determine level, transverse view to visualize facet joint | Not specified | CT needle position |
| Yun55 2012 | RCT | 57 (US group: 25, control group: 32) | 81 | 2 mL 1% lidocaine and 10 mg triamcinolone | Parasagittal view to identify level, transverse view with lateral to medial in-plane injection to midpoint of facet joint | Unilateral L4-5 (6), bilateral L4-5 (18), unilateral L5-S1 (5), bilateral L5-S1 (17) | None |
BMI, body mass index; FL, fluoroscopic; FJI, facet joint injection; MBB, medial branch block; SAP, superior articular process; TP, transverse process; RCT, randomized controlled trial; US, ultrasound.
3.2. Risk of bias assessment
The full risk of bias assessment is presented in the supplemental materials document (available at http://links.lww.com/PR9/A160). Five RCTs were graded as having some concerns,16,25,51,54,55 while 1 was graded as high risk of bias because of bias in reporting outcomes.19 In the nonrandomized studies, 1 was graded as having good quality,14 8 were graded as having fair quality,3,13,21,24,28,37,40,43 and 4 were graded as having poor quality.9,17,32,47 Significant risk of bias related to nonreporting of study data were identified, and most of the studies did not specify an a priori statistical plan.3,9,13,17,19–21,24,25,32,37,40,43,47,55 Three studies with high risk of bias because of nonreporting of information were conference abstracts.9,32,47 Some studies were susceptible to selection bias because of exclusion of patients with obesity.25,54 For all comparative studies, only 1 study reported that outcome assessors were blinded.14
3.3. Ultrasound-guided medial branch blocks
3.3.1. Technique for performing ultrasound-guided medial branch blocks
The included studies describe T12-L5 MBB (L5 dorsal ramus blocks are herein referred to as MBB) with varying laterality and injectate volumes as detailed in Table 1. For US-guided MBB, 7 studies described a sagittal approach to identify the spinal level for injection followed by a transverse view to identify the target for final needle placement.14,17,20,21,24,37,43 One study only described using the transverse view.32 All studies which specified the target for needle placement described the junction of the cephalad transverse process and the superior articular process14,17,20,21,24,32,43 which has been shown in a cadaveric and CT-confirmation study as being less specific than targeting a lower point midway between the upper border of the transverse process and the mamilloaccessory ligament.12 Two studies did not describe the technique for performing the US-guided MBB.3,47 Six studies specified injections performed in-plane from a lateral to medial direction.6,14,17,21,24,37 One study described a reorientation of needle direction after performing an L4 MBB, in which the needle was withdrawn and walked medially and caudally while observing progress towards the target for the L5 MBB (intersection of sacral ala and superior articular process) in an out-of-plane fashion.14
Placement of 4 needles was associated with suspected vascular uptake because of contrast spread only partially covering target area in one study14 and because of lack of dye visualization under fluoroscopy in another.43 One study used a total volume of 0.25 mL, while the other used a total volume of 1 mL. The specific level of suspected intravascular uptake was not described. The number of patients with these suspected findings was not specified.
3.3.2. Meta-analysis of ultrasound-guided medial branch blocks as confirmed by fluoroscopy
Seven studies confirmed needle placement with fluoroscopy (Table 2).14,17,21,24,37,43,47 Three of 7 studies confirmed needle placement using fluoroscopy with contrast.24,44,47 Forest plots of the meta-analysis confirming correct needle placement using fluoroscopy with and without contrast are depicted in Figure 2. Pooled analysis demonstrated a 17% RD (95% CI, −0.06 to 0.39, P = 0.15) of incorrect needle placement for US-guided MBB confirmed using fluoroscopy without contrast with high levels of heterogeneity identified (I2 = 95%). Pooled analysis demonstrated a 7% RD (95% CI, 0.04–0.10, P < 0.0001) of incorrect needle placement for US-guided MBB confirmed using fluoroscopy with contrast with low levels of heterogeneity observed (I2 = 24%). Pooled analysis of all 7 studies demonstrated an 11% RD (95% CI, 0.04–0.17, P < 0.0009) of incorrect needle placement for US-guided MBB confirmed using fluoroscopy with and without contrast with high levels of heterogeneity identified (I2 = 87%). Heterogeneity was investigated by conducting a sensitivity analysis. When the study by Rauch et al.37 was removed from the meta-analysis, the RD in the fluoroscopy without contrast subgroup declined to 4% (95% CI, −0.03 to 0.12, P = 0.18) and statistical heterogeneity was reduced (I2 = 45%). The RD in the pooled analysis of the remaining 6 studies declined to 7% (95% CI, 0.04–0.10, P = 0.0002), and heterogeneity was reduced (I2 = 46%). The study cohort of Rauch et al.37 comprised exclusively of patients with a body mass index (BMI) greater than 30.
Table 2.
Number of correct and incorrect needles placed by ultrasound for medial branch blocks and facet joint injections.
| Author | Number of needles placed by US | Number confirmed as incorrect |
|---|---|---|
| US-guided MBB confirmed by fluoroscopy without contrast | ||
| Greher17 2004 | 28 | 3 |
| Hashemi21 2017 | 84 | 2 |
| Rauch37 2009 | 52 | 32 |
| US-guided MBB confirmed by fluoroscopy with contrast | ||
| Etheridge14 2020a | 100 | 5 |
| Jung24 2012 | 95 | 8 |
| Shim43 2006 | 101 | 5 |
| Soni47 2018 | 74 | 10 |
| US-guided FJI confirmed by computerized tomography | ||
| Galiano16 2007 | 18 | 1 |
| Wen51 2014 | 42 | 5 |
| Ye54 2018 | 74 | 10 |
| US-guided FJI confirmed by fluoroscopy with contrast | ||
| Erdogan13 2019 | 61 | 4 |
FJI, facet joint injection; MBB, medial branch block; US, ultrasound.
Figure 2.
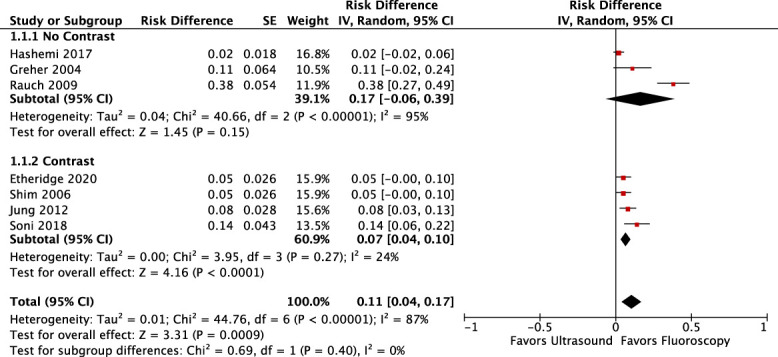
Risk difference forest plots for ultrasound-guided medial branch blocks confirmed by fluoroscopy with and without contrast.
3.3.3. Procedure time for a single-level ultrasound-guided medial branch blocks
Three studies reported the time needed to perform a single-level MBB.21,37,43 These studies reported that the average time ranged from 4.0 to 5.0 minutes.21,37,43 One of these studies also reported a total procedure time of 5.9 (SD 1) minutes, which may have included additional time to perform adjacent-level injections.21 Another study reported the time to perform L5 MBB in-plane and out-of-plane after reorientation of the needle from its position immediately after L4 MBB. Time for completion of this technique was reported as 153.93 (SD 41.56) seconds.14 An additional study reported that the procedure time for performing a US-guided MBB was significantly shorter compared with fluoroscopy (323 vs 430 seconds, P < 0.001).20 It was not clear from the methods of this study whether performance time was for a single-level or multilevel procedure. Another study reported that the maximum procedure time for multiple blocks at multiple levels was 40 minutes.17
3.3.4. Complications associated with ultrasound-guided medial branch blocks
Complications were reported in 3 studies. Dizziness and bilateral lower extremity weakness were reported in 1 patient immediately after US-guided MBB.32 A vasovagal reaction was noted in 4 patients.20 Procedure level and laterality were not defined in these cases. Transient headache was noted in 2 patients.20 A small superficial hematoma was noted in 1 patient who underwent unilateral L4 and L5 MBB.14
3.4. Ultrasound-guided intra-articular facet joint injections
3.4.1. Technique for performing ultrasound-guided facet joint injections
The included studies describe L1-2, L2-3, L3-4, L4-5, and L5-S1 FJI with varying laterality and injectate volumes as detailed in Table 1. Eight studies described a sagittal view to determine the spinal level followed by a transverse view to identify the target facet joint.13,16,19,25,40,41,54,55 One study described using both longitudinal and transverse views to identify the target with injection performed in-plane in a caudal to cranial trajectory.28 One study described confirmation of intra-articular injection with US but did not describe the particular views that were used.9 Eight studies described an in-plane approach,13,16,19,25,28,40,54,55 and 1 case report described an out-of-plane approach.41
3.4.2. Ultrasound-guided facet joint injections confirmed by fluoroscopy with contrast
In a single study of US-guided FJI, correct needle position was confirmed using fluoroscopy with contrast (Table 2). A 7% RD was observed (95% CI, −0.00 to 0.13, P = 0.06).13
3.4.3. Meta-analysis of ultrasound-guided facet joint injections confirmed by computerized tomography
Three studies confirmed US-guided needle placement with CT (Table 2). A forest plot of the meta-analysis confirming correct needle placement using CT is depicted in Figure 3. Pooled analysis demonstrated a 13% RD (95% CI, 0.06–0.19, P < 0.0001) of incorrect needle placement for US-guided FJI confirmed using CT, and no heterogeneity was identified (I2 = 0%).
Figure 3.
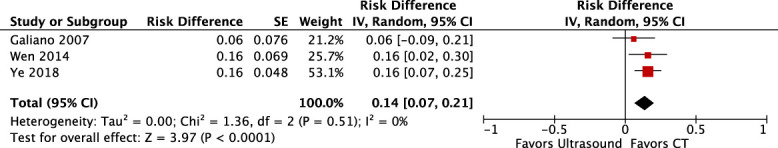
Risk difference forest plots for ultrasound-guided facet joint injections confirmed by computerized tomography.
3.4.4. Procedure time for ultrasound-guided facet joint injections
Two studies reported the procedure time for performing a single intra-articular FJI at L3-4, L4-5, and L5-S1.16,51 One of these studies found a nonsignificant difference for the US-guided group (14.3 minutes, SD 6.6) compared with the CT-guided group (22.3 minutes, SD 6.3).16 Notably, in this study, the time recorded for the US-guided group also included the time expended obtaining CT control images.16 The other study reported a time of 206 seconds (SD 27 seconds) to perform a single-level FJI.51 Ha et al.19 measured the time to complete bilateral L2-3, L3-4, L4-5, and L5-S1 FJI, with no significant difference in procedure time observed between the US-guided (265 seconds) and fluoroscopy groups (247 seconds).19 Yun et al.55 measured time to complete multiple-level FJI.55 In this study, 25 patients underwent US-guided L4-5 and L5-S1 FJI for a total of 81 injections, while 32 patients underwent fluoroscopically guided L4-5 and L5-S1 FJI for a total of 104 injections. The procedure time in the US-guided group (263.4 seconds, SD 6.5) was significantly longer compared with the fluoroscopy group (248.7 seconds, SD 5.9, P = 0.023).55 In the study by Constantinescu et al.,9 which did not have a comparison group, the total US-guided procedure time ranged between 20 and 30 minutes. The number and levels of the injections were not reported.9 The definition and measurement of procedure time varied across studies.
3.4.5. Complications associated with ultrasound-guided intra-articular facet joint injections
Complications were reported in 2 studies. Fluid retention in the upper and lower extremities was reported in 1 patient, although it was unclear whether this patient was in the US-guided or CT-guided group.16 Other details including level of the injection or time course of the symptoms were not reported. In the study by Ha et al.,19 a superficial infection that improved within a few days was reported. Whether antibiotics were administered was not reported. In the same study, an episode of transient lower motor neuron weakness that improved within 1 day was reported.19 The distribution of weakness was not reported. Several minor complications were reported in 4 patients in the US-guided FJI group and 3 patients in the fluoroscopically guided FJI group, but specific details about which complication occurred in each treatment group were not reported.19 These minor complications included aggravation of LBP, paresthesia, headache, brief chest pain, and an allergic reaction. All symptoms attributed to the minor complications resolved within a few hours.19
3.5. Grading of evidence
Certainty in evidence was assessed as low to very low primarily because of factors related to risk of bias, inconsistency, and imprecision.18 The complete assessment is presented in Table 3. Imprecision was primarily due to small sample sizes. Indirectness was noted because the image-guided interventions required highly specialized skills that may not be easily translated to health care personnel with less experience.14,16,20,21,25,32,37,40,41,54,55
Table 3.
Grading of recommendations, assessment, development, and evaluation (GRADE) of evidence.
| Quality Assessment | Certainty in outcomes | |||||
|---|---|---|---|---|---|---|
| Risk of bias | Imprecision | Inconsistency | Indirectness | Publication bias | ||
| Medial Branch Blocks | ||||||
| Accuracy of injection | Moderate risk of bias primarily from selection bias | Imprecision because of relatively small sample sizes | Some inconsistency from lack of a priori statistics | Some concern of indirectness because of a specialized skill set required to perform the procedure that may not be widely available | Moderate risk given results that studies nearly universally favor US-guided MBB as feasible and many studies with only a single proceduralist | Low |
| Procedure time | Moderate risk of bias primarily from selection bias | Imprecision because of relatively small sample sizes | High inconsistency from lack of a priori statistics | Some concern of indirectness because of a specialized skill set required to perform the procedure that may not be widely available | Moderate risk given many studies with only a single proceduralist | Very low |
| Facet Joint Injections | ||||||
| Accuracy of injection | Moderate risk of bias primarily from selection bias | Imprecision because of relatively small sample sizes | Some inconsistency from lack of a priori statistics | Some concern of indirectness because of a specialized skill set required to perform the procedure that may not be widely available | Moderate risk given results that nearly universally favor US-guided FJI as feasible and many studies with only a single proceduralist | Low |
| Procedure time | Moderate risk of bias primarily from selection bias | Imprecision because of relatively small sample sizes | High inconsistency from variability of effects and lack of a priori statistics | Some concern of indirectness because of a specialized skill set required to perform the procedure that may not be widely available | Moderate risk given many studies with only a single proceduralist | Very low |
4. Discussion
The key findings of this systematic review include the following: (1) The pooled RD of US-guided MBB confirmed by fluoroscopy with or without contrast was 11%, and no significant group differences were observed; (2) the RD of US-guided FJI confirmed by fluoroscopy with contrast was 7%; and (3) the pooled RD of US-guided FJI confirmed by CT was 13%. The time to complete a single-level US-guided MBB ranged from 2.6 to 5.0 minutes, and a single study reported a significantly shorter procedure time for US-guided MBB compared with fluoroscopic guidance.20 However, the time to complete a single or multilevel US-guided FJI varied widely. Few complications were reported for US-guided, fluoroscopically guided, or CT-guided procedures. Important sources of heterogeneity and bias were identified, and the certainty in evidence was low to very low.
The RD of US-guided MMB and FJI as confirmed by fluoroscopy or CT warrants further consideration. Ultrasound technology is based on the piezoelectric principle, whereby electrical current passing through crystals in the US transducer are converted into pulsed sound waves.1,30,53 These ultrasonic waves are transmitted into the targeted tissues and reflected back to the transducer.1,53 High frequency transducers with shorter pulse length yield a higher resolution image. However, resolution is substantially limited when visualizing deeper structures because of attenuation of sound waves through the intervening tissues.1,27,45,46 Depth gain compensation can correct for the loss of acoustic energy through attenuation,36,45 but for deeper structures, depth gain compensation is inadequate for optimal visualization. Individual patient factors such as increased BMI and variations in adipose tissue distribution can contribute to suboptimal resolution.10,34 Thus, it can be posited that the technological limitations of US and individual patient factors are key contributors to the lower accuracy of US-guided MBB and FJI.
Despite the lower accuracy of US-guided needle placement, a previous meta-analysis reported the effectiveness of US-guided FJI were comparable with fluoroscopy-guided and CT-guided FJI.52 In this study, immediate postprocedural outcomes were assessed including pain scores, Modified Oswestry Disability (MOD) scores, and procedure time. Inclusion criteria included randomized and nonrandomized trials. The meta-analysis involved 2 fluoroscopy-guided trials19,55 and 1 CT-guided trial16; these 3 trials were included in our systematic review. In the meta-analysis, the weighted mean difference in pain scores, MOD scores, and procedure time did not differ significantly between the US-guided group and the combined fluoroscopy-guided and CT-guided group. However, high levels of statistical heterogeneity were identified for the pain score and procedure time analyses. No statistical heterogeneity was identified for the MOD analysis, but this comparison only included the 2 fluoroscopy trials.19,55 The findings of this systematic review and meta-analysis extend the findings of the meta-analysis of Wu et al.52 by including trials of US-guided MBB and reporting the RD of inaccurate needle placement. The findings of this review suggest that although the immediate postprocedural pain scores of US-guided FJI were similar to conventional imaging modalities, the risk of inaccurate needle placement could have deleterious effects on the diagnostic accuracy of MBB.
The findings of this systematic review have important implications for research and clinical practice. First, in a summary by Cohen et al.,7 the false-positive rate of fluoroscopically guided MBBs based on placebo-controlled blocks in randomized trials varied from 16% to 30%.8,38,39,42 The false-negative rate may be magnified by imaging modalities that miss the target nerve or cannot reliably detect intravascular uptake.11 The findings of this meta-analysis suggest that US-guided MBB could further impede the ability to accurately identify patients for radiofrequency denervation. However, use of US may be indicated in austere environments or select clinical scenarios where avoiding radiation exposure is a key outcome. The use of US may also be considered when diagnostic accuracy is a secondary concern. For example, as suggested by the findings of the meta-analysis of Wu et al.,52 the therapeutic effects of US-guided FJI may not be affected by inaccurate needle placement; thus, US may be an acceptable imaging modality for these injections. Further research using cadaver dissection models and prospective clinical trials are needed to drive development of techniques aimed at reducing the risk and understanding the clinical effects of incorrectly placed needles. Second, in the study by Rauch et al. that involved adults with a BMI >30 undergoing US-guided MBB, the RD was 38%. This finding is consistent with numerous studies where BMI >30 was associated with an increased risk of failed nerve blocks for regional anesthesia.10,15,34,50
This study has limitations. Details about how the US-guided procedures were performed varied between studies which could have influenced the findings of this systematic review. Training in fluoroscopically guided spine procedures is more extensive than US training. As a result, the outcomes of studies conducted by practitioners with expertise in performing US-guided procedures may not be generalizable to the general population of pain specialty physicians. Potential variations in how fluoroscopy was used without contrast to confirm needle placement could have affected the study findings. More specifically, no significant RD was observed for the US and fluoroscopy without contrast comparison (Fig. 2). The lack of significance could be due, in part, to high levels of heterogeneity which could be related to undefined variations in how fluoroscopy was used without contrast to confirm needle placement.
In conclusion, the risk of incorrect needle placement associated with US-guided MMB and FJI is high when needle position is confirmed using fluoroscopy or CT (Fig. 4). The technical limitations of US and individual patient characteristics, particularly elevated BMI, could be important determinants of incorrect needle placement associated with US-guided MBB and FJI. Further research is needed to identify optimal procedural techniques aimed at reducing the risk of incorrect needle placement for US-guided facet interventions.
Figure 4.
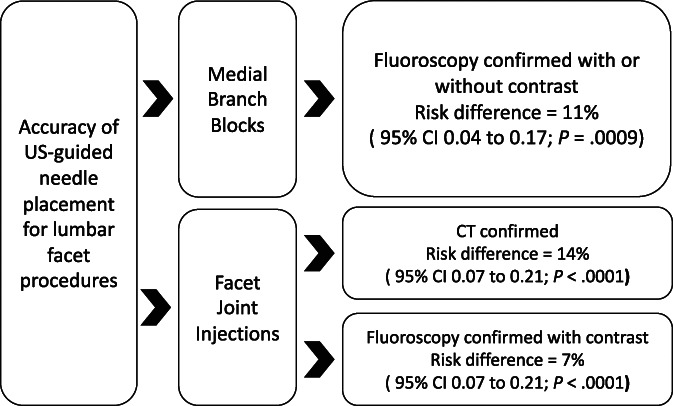
Summary of key study findings.
Disclosures
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A160.
Supplementary Material
Acknowledgements
The study was performed without funding, and the authors have no conflicts of interest to report.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
Contributor Information
Zachary M. Ashmore, Email: ashmore.zachary@mayo.edu.
Michael M. Bies, Email: bies.michael@mayo.edu.
James B. Meiling, Email: meiling.james@mayo.edu.
Rajat N. Moman, Email: rnm05c@gmail.com.
Leslie C. Hassett, Email: hassett.leslie@mayo.edu.
Christine L. Hunt, Email: hunt.christine@mayo.edu.
Steven P. Cohen, Email: scohen40@jhmi.edu.
References
- [1].Aldrich JE. Basic physics of ultrasound imaging. Crit Care Med 2007;35:S131–137. [DOI] [PubMed] [Google Scholar]
- [2].Ashmore Z, Bies M. Ultrasound vs fluoroscopically guided medial branch block for low back pain, 2020 CRD42020172717.PROSPERO. Available at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020172717. Accessed December 15, 2021. [Google Scholar]
- [3].Batalov A, Todorov P, Sheitanov I. Ultrasound-guided facet joint nerve blocks in the management of chronic low back pain due to lumbar osteoarthritis. Osteoporos Int 2013;1:S152–3. [Google Scholar]
- [4].Bertini L, Baciarello M. Ultrasound and facet blocks: a review. Eur J Pain Suppl 2009;3:139–43. [Google Scholar]
- [5].Boswell MV, Trescot AM, Datta S, Schultz DM, Hansen HC, Abdi S, Sehgal N, Shah RV, Singh V, Benyamin RM, Patel VB, Buenaventura RM, Colson JD, Cordner HJ, Epter RS, Jasper JF, Dunbar EE, Atluri SL, Bowman RC, Deer TR, Swicegood JR, Staats PS, Smith HS, Burton AW, Kloth DS, Giordano J, Manchikanti L, Physicians ASoIP. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician 2007;10:7–111. [PubMed] [Google Scholar]
- [6].Chen CPC, Chih-Kuang C, Chen JL, Chen HM, Suputtitada A. Ultrasound-guided lumbar spine medial branch blocks for the treatment of low back pain. Am J Phys Med Rehabil 2021;100:e73–e74. [DOI] [PubMed] [Google Scholar]
- [7].Cohen SP, Bhaskar A, Bhatia A, Buvanendran A, Deer T, Garg S, Hooten WM, Hurley RW, Kennedy DJ, McLean BC, Moon JY, Narouze S, Pangarkar S, Provenzano DA, Rauck R, Sitzman BT, Smuck M, van Zundert J, Vorenkamp K, Wallace MS, Zhao Z. Consensus practice guidelines on interventions for lumbar facet joint pain from a multispecialty, international working group. Reg Anesth Pain Med 2020;45:424–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cohen SP, Doshi TL, Constantinescu OC, Zhao Z, Kurihara C, Larkin TM, Griffith SR, Jacobs MB, Kroski WJ, Dawson TC, Fowler IM, White RL, Verdun AJ, Jamison DE, Anderson-White M, Shank SE, Pasquina PF. Effectiveness of lumbar facet joint blocks and predictive value before radiofrequency denervation: the facet treatment study (facts), a randomized, controlled clinical trial. Anesthesiology 2018;129:517–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Constantinescu G, Menon S, King R, Gulati M, Tavakolizadeh A. Ultrasound-guided lumbar facet joint injections as an alternative to fluoroscopic-guided injections—preliminary results and perspective. Ultrasound 2017;25:NP30–NP31. [Google Scholar]
- [10].Cotter JT, Nielsen KC, Guller U, Steele SM, Klein SM, Greengrass RA, Pietrobon R. Increased body mass index and asa physical status iv are risk factors for block failure in ambulatory surgery—an analysis of 9,342 blocks. Can J Anaesth 2004;51:810–16. [DOI] [PubMed] [Google Scholar]
- [11].Derby R, Melnik I, Choi J, Lee JE. Indications for repeat diagnostic medial branch nerve blocks following a failed first medial branch nerve block. Pain Physician 2013;16:479–88. [PubMed] [Google Scholar]
- [12].Dreyfuss P, Schwarzer AC, Lau P, Bogduk N. Specificity of lumbar medial branch and l5 dorsal ramus blocks. A computed tomography study. Spine 1997;22:895–902. [DOI] [PubMed] [Google Scholar]
- [13].Erdogan S, Okur SC, Atici A, Gokcen HB, Polat B, Atici Y. Accuracy of the anatomic placement in ultrasonography guided facet joint blockage with supervising of c-arm fluoroscopy. Iranian J Radiol 2019;16:e84389. [Google Scholar]
- [14].Etheridge JPB, De Villiers F, Venter J, Squire P, Farnquist B, Finlayson RJ. Ultrasound-guided l5 dorsal ramus block: validation of a novel technique. Reg Anesth Pain Med 2020;45:176–9. [DOI] [PubMed] [Google Scholar]
- [15].Franco CD, Gloss FJ, Voronov G, Tyler SG, Stojiljkovic LS. Supraclavicular block in the obese population: an analysis of 2020 blocks. Anesth Analg 2006;102:1252–4. [DOI] [PubMed] [Google Scholar]
- [16].Galiano K, Obwegeser AA, Walch C, Schatzer R, Ploner F, Gruber H. Ultrasound-guided versus computed tomography-controlled facet joint injections in the lumbar spine: a prospective randomized clinical trial. Reg Anesth Pain Med 2007;32:317–22. [DOI] [PubMed] [Google Scholar]
- [17].Greher M, Scharbert G, Kamolz LP, Beck H, Gustorff B, Kirchmair L, Kapral S. Ultrasound-guided lumbar facet nerve block: a sonoanatomic study of a new methodologic approach. Anesthesiology 2004;100:1242–8. [DOI] [PubMed] [Google Scholar]
- [18].Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. Grade guidelines: 1. Introduction-grade evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. [DOI] [PubMed] [Google Scholar]
- [19].Ha DH, Shim DM, Kim TK, Kim YM, Choi SS. Comparison of ultrasonography- and fluoroscopy-guided facet joint block in the lumbar spine. Asian Spine J 2010;4:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Han SH, Park KD, Cho KR, Park Y. Ultrasound versus fluoroscopy-guided medial branch block for the treatment of lower lumbar facet joint pain: a retrospective comparative study. Medicine 2017;96:e6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hashemi M, Jazayeri SM, Niaki AS, Nikooseresht M, Hosseinpanah A, Razavi SS, Farivar F, Shahandeh F. Ultrasound guidance for interventional pain management of lumbar facet joint pain: an anatomical and clinical study. Iranian J Radiol 2017;14:e28297. [Google Scholar]
- [22].Hofmeister M, Dowsett LE, Lorenzetti DL, Clement F. Ultrasound- versus fluoroscopy-guided injections in the lower back for the management of pain: a systematic review. Eur Radiol 2019;29:3401–9. [DOI] [PubMed] [Google Scholar]
- [23].Hurdle MFB. Ultrasound-guided spinal procedures for pain: a review. Phys Med Rehabil Clin N Am 2016;27:673–86. [DOI] [PubMed] [Google Scholar]
- [24].Jung H, Jeon S, Ahn S, Kim M, Choi Y. The validation of ultrasound-guided lumbar facet nerve blocks as confirmed by fluoroscopy. Asian Spine J 2012;6:163–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Karkucak M, Batmaz I, Kerimoglu S, Ayar A. Comparison of clinical outcomes of ultrasonography-guided and blind local injections in facet syndrome: a 6-week randomized controlled trial. J Back Musculoskelet Rehabil 2020;33:431–6. [DOI] [PubMed] [Google Scholar]
- [26].Korbe S, Udoji EN, Ness TJ, Udoji MA. Ultrasound-guided interventional procedures for chronic pain management. Pain Manag 2015;5:465–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kremkau FW, Taylor KJ. Artifacts in ultrasound imaging. J Ultrasound Med 1986;5:227–37. [DOI] [PubMed] [Google Scholar]
- [28].Küllmer K, Rompe JD, Löwe A, Herbsthofer B, Eysel P. [ultrasound image of the lumbar spine and the lumbosacral transition. Ultrasound anatomy and possibilities for ultrasonically-controlled facet joint infiltration]. Z Orthop Ihre Grenzgeb 1997;135:310–14. [DOI] [PubMed] [Google Scholar]
- [29].Manchikanti L, Kaye AD, Soin A, Albers SL, Beall D, Latchaw R, Sanapati MR, Shah S, Atluri S, Abd-Elsayed A, Abdi S, Aydin S, Bakshi S, Boswell MV, Buenaventura R, Cabaret J, Calodney AK, Candido KD, Christo PJ, Cintron L, Diwan S, Gharibo C, Grider J, Gupta M, Haney B, Harned ME, Helm S, II, Jameson J, Jha S, Kaye AM, Knezevic NN, Kosanovic R, Manchikanti MV, Navani A, Racz G, Pampati V, Pasupuleti R, Philip C, Rajput K, Sehgal N, Sudarshan G, Vanaparthy R, Wargo BW, Hirsch JA. Comprehensive evidence-based guidelines for facet joint interventions in the management of chronic spinal pain: American society of interventional pain physicians (asipp) guidelines facet joint interventions 2020 guidelines. Pain Physician 2020;23:S1–S127. [PubMed] [Google Scholar]
- [30].Manwar R, Kratkiewicz K, Avanaki K. Overview of ultrasound detection technologies for photoacoustic imaging. Micromachines (Basel) 2020;11:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [32].Moon SH, Lee S, Kim KH, Rho JH. Effect of ultrasound-guided lumbar medial branch block in chronic low back pain. Skeletal Radiol 2013;42:879. [Google Scholar]
- [33].National Heart, Lung, and Blood Institute. Study quality assessment tools, 2014. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed March 3, 2022. [Google Scholar]
- [34].Nielsen KC, Guller U, Steele SM, Klein SM, Greengrass RA, Pietrobon R. Influence of obesity on surgical regional anesthesia in the ambulatory setting: an analysis of 9,038 blocks. Anesthesiology 2005;102:181–7. [DOI] [PubMed] [Google Scholar]
- [35].Peckham ME, Hutchins TA, Shah LM. Conventional image-guided procedures for painful spine. Neuroimaging Clin N Am 2019;29:539–51. [DOI] [PubMed] [Google Scholar]
- [36].Pye SD, Wild SR, McDicken WN. Adaptive time gain compensation for ultrasonic imaging. Ultrasound Med Biol 1992;18:205–12. [DOI] [PubMed] [Google Scholar]
- [37].Rauch S, Kasuya Y, Turan A, Neamtu A, Vinayakan A, Sessler DI. Ultrasound-guided lumbar medial branch block in obese patients: a fluoroscopically confirmed clinical feasibility study. Reg Anesth Pain Med 2009;34:340–2. [DOI] [PubMed] [Google Scholar]
- [38].Revel M, Poiraudeau S, Auleley GR, Payan C, Denke A, Nguyen M, Chevrot A, Fermanian J. Capacity of the clinical picture to characterize low back pain relieved by facet joint anesthesia. Proposed criteria to identify patients with painful facet joints. Spine 1998;23:1972–6. discussion 1977. [DOI] [PubMed] [Google Scholar]
- [39].Rocha ID, Cristante AF, Marcon RM, Oliveira RP, Letaif OB, Barros Filho TE. Controlled medial branch anesthetic block in the diagnosis of chronic lumbar facet joint pain: the value of a three-month follow-up. Clinics (Sao Paulo) 2014;69:529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sadeghian H, Motiei-Langroudi R. Sonography guided lumbar nerve and facet blocks: the first report of clinical outcome from Iran. Radiography (London) 2018;24:52–6. [DOI] [PubMed] [Google Scholar]
- [41].Santiago AEQ, Leal PC, Bezerra EHM, Giraldes ALA, Ferraro LC, Rezende AH, Sakata RK. Ultrasound-guided facet block to low back pain: a case report. Braz J Anesthesiol 2014;64:278–80. [DOI] [PubMed] [Google Scholar]
- [42].Schutz U, Cakir B, Dreinhofer K, Richter M, Koepp H. Diagnostic value of lumbar facet joint injection: a prospective triple cross-over study. PLoS One 2011;6:e27991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shim J-K, Moon J-C, Yoon K-B, Kim W-O, Yoon D-M. Ultrasound-guided lumbar medial-branch block: a clinical study with fluoroscopy control. Reg Anesth Pain Med 2006;31:451–4. [DOI] [PubMed] [Google Scholar]
- [44].Sim J, Madden S. Illness experience in fibromyalgia syndrome: a metasynthesis of qualitative studies. Soc Sci Med 2008;67:57–67. [DOI] [PubMed] [Google Scholar]
- [45].Smith J, Finnoff JT. Diagnostic and interventional musculoskeletal ultrasound: Part 1. Fundamentals. PM R 2009;1:64–75. [DOI] [PubMed] [Google Scholar]
- [46].Smith J, Finnoff JT. Diagnostic and interventional musculoskeletal ultrasound: Part 2. Clinical applications. PM R 2009;1:162–77. [DOI] [PubMed] [Google Scholar]
- [47].Soni L, Mohan VK, Garg B, Punj J, Bhoi D. Fluoroscopic validation and technical feasibilityof ultrasound guided lumbar medial branch block in facet jointarthropathy. Reg Anesth Pain Med 2018;43:e56. [Google Scholar]
- [48].Spencer FA, Iorio A, You J, Murad MH, Schünemann HJ, Vandvik PO, Crowther MA, Pottie K, Lang ES, Meerpohl JJ, Falck-Ytter Y, Alonso-Coello P, Guyatt GH. Uncertainties in baseline risk estimates and confidence in treatment effects. BMJ 2012;345:e7401. [DOI] [PubMed] [Google Scholar]
- [49].Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- [50].Todd MM. A lesson learned. Can J Anaesth 2005;52:770–1. [DOI] [PubMed] [Google Scholar]
- [51].Wen CB, Li YZ, Sun L, Xiao H, Yang BX, Song L, Liu H. [a clinical trial of ultrasound-guided facet joint block in the lumbar spine to treat facet joint related low back pain]. Sichuan Da Xue Xue Bao Yi Xue Ban 2014;45:712–16. [PubMed] [Google Scholar]
- [52].Wu T, Zhao WH, Dong Y, Song HX, Li JH. Effectiveness of ultrasound-guided versus fluoroscopy or computed tomography scanning guidance in lumbar facet joint injections in adults with facet joint syndrome: a meta-analysis of controlled trials. Arch Phys Med Rehabil 2016;97:1558–63. [DOI] [PubMed] [Google Scholar]
- [53].Wu WT, Chang KV, Hsu YC, Hsu PC, Ricci V, Özçakar L. Artifacts in musculoskeletal ultrasonography: from physics to clinics. Diagnostics (Basel) 2020;10:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ye L, Wen C, Liu H. Ultrasound-guided versus low dose computed tomography scanning guidance for lumbar facet joint injections: same accuracy and efficiency. BMC Anesthesiol 2018;18:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yun DH, Kim HS, Yoo SD, Kim DH, Chon JM, Choi SH, Hwang DG, Jung PK. Efficacy of ultrasonography-guided injections in patients with facet syndrome of the low lumbar spine. Ann Rehabil Med 2012;36:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A160.


