Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
In primary analysis, enzalutamide plus androgen deprivation therapy (ADT) improved radiographic progression-free survival (rPFS) in patients with metastatic hormone-sensitive prostate cancer (mHSPC); however, overall survival data were immature. In the phase III, double-blind, global ARCHES trial (ClinicalTrials.gov identifier: NCT02677896), 1,150 patients with mHSPC were randomly assigned 1:1 to enzalutamide (160 mg once daily) plus ADT or placebo plus ADT, stratified by disease volume and prior docetaxel use. Here, we report the final prespecified analysis of overall survival (key secondary end point) and an update on rPFS, other secondary end points, and safety. After unblinding, 180 (31.3%) progression-free patients randomly assigned to placebo plus ADT crossed over to open-label enzalutamide plus ADT. As of May 28, 2021 (median follow-up, 44.6 months), 154 of 574 patients randomly assigned to enzalutamide plus ADT and 202 of 576 patients randomly assigned to placebo plus ADT had died. Enzalutamide plus ADT reduced risk of death by 34% versus placebo plus ADT (median not reached in either group; hazard ratio, 0.66; 95% CI, 0.53 to 0.81; P < .001). Enzalutamide plus ADT continued to improve rPFS and other secondary end points. Adverse events were generally consistent with previous reports of long-term enzalutamide use. In conclusion, enzalutamide plus ADT significantly prolongs survival versus placebo plus ADT in patients with mHSPC.
INTRODUCTION
Enzalutamide in combination with androgen deprivation therapy (ADT) is approved for the treatment of metastatic hormone-sensitive prostate cancer (mHSPC [also referred to as metastatic castration-sensitive prostate cancer])1,2 on the basis of proven clinical benefits in the phase III ARCHES trial (ClinicalTrials.gov identifier: NCT02677896). At the time of the primary analysis, enzalutamide plus ADT significantly reduced the risk of radiographic disease progression or death in men with mHSPC; however, overall survival (OS) data were considered immature.3
Herein, we report the final prespecified OS analysis and an update on radiographic progression-free survival (rPFS), other secondary end points, and safety.
METHODS
Study Design
Details of the study design of ARCHES have been published previously.3
Enrolled patients were randomly assigned 1:1 to receive enzalutamide (160 mg once daily) plus ADT or placebo plus ADT, stratified by disease volume and prior docetaxel use. After the primary analysis, ARCHES was unblinded to allow patients randomly assigned to placebo plus ADT to cross over to enzalutamide plus ADT in an open-label extension.
End Points
OS (key secondary end point) was defined as the time from random assignment to death from any cause. We also report an update on rPFS and other key secondary end points. The data cutoff for this report was May 28, 2021.
Statistical analysis methodology is reported in the Data Supplement (online only).
RESULTS
Baseline Demographics and Patient History
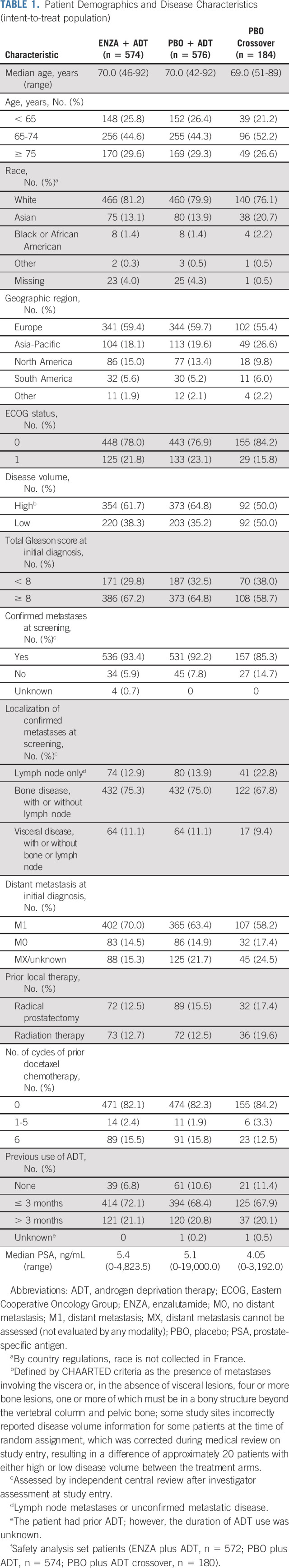
From March 21, 2016, to January 12, 2018, 1,150 patients were randomly assigned. Baseline demographics are presented in Table 1. Patient disposition is summarized in the Data Supplement.
TABLE 1.
Patient Demographics and Disease Characteristics (intent-to-treat population)
After study unblinding, 184 patients (31.9%) randomly assigned to placebo plus ADT remained progression-free and consented to cross over, 180 (31.3%) of whom received treatment with enzalutamide plus ADT (median time to crossover, 21.5 months). After a total of 356 deaths (enzalutamide plus ADT, n = 154; placebo plus ADT, n = 202), the data cutoff for the final OS analysis was May 28, 2021; the median follow-up time was 44.6 months.
After treatment discontinuation, 131 patients (23%) randomly assigned to enzalutamide plus ADT and 221 patients (38%) randomly assigned to placebo plus ADT received subsequent life-prolonging therapy; an additional 15 patients (8%) in the crossover group received subsequent life-prolonging therapy after discontinuing enzalutamide plus ADT (Data Supplement). Inclusive of crossover, 401 patients (70%) randomly assigned to placebo plus ADT received subsequent life-prolonging therapy, with 241 (42%) receiving enzalutamide as the first subsequent life-prolonging therapy.
OS
Patients randomly assigned to enzalutamide plus ADT had a 34% reduction in the risk of death versus placebo plus ADT (hazard ratio [HR], 0.66; 95% CI, 0.53 to 0.81; P < .001; Fig 1A); the median OS was not reached in either group. At 24, 36, and 48 months, 86%, 78%, and 71% of patients randomly assigned to enzalutamide plus ADT were estimated to be alive, respectively, compared with 82%, 69%, and 57% of patients randomly assigned to placebo plus ADT.
FIG 1.
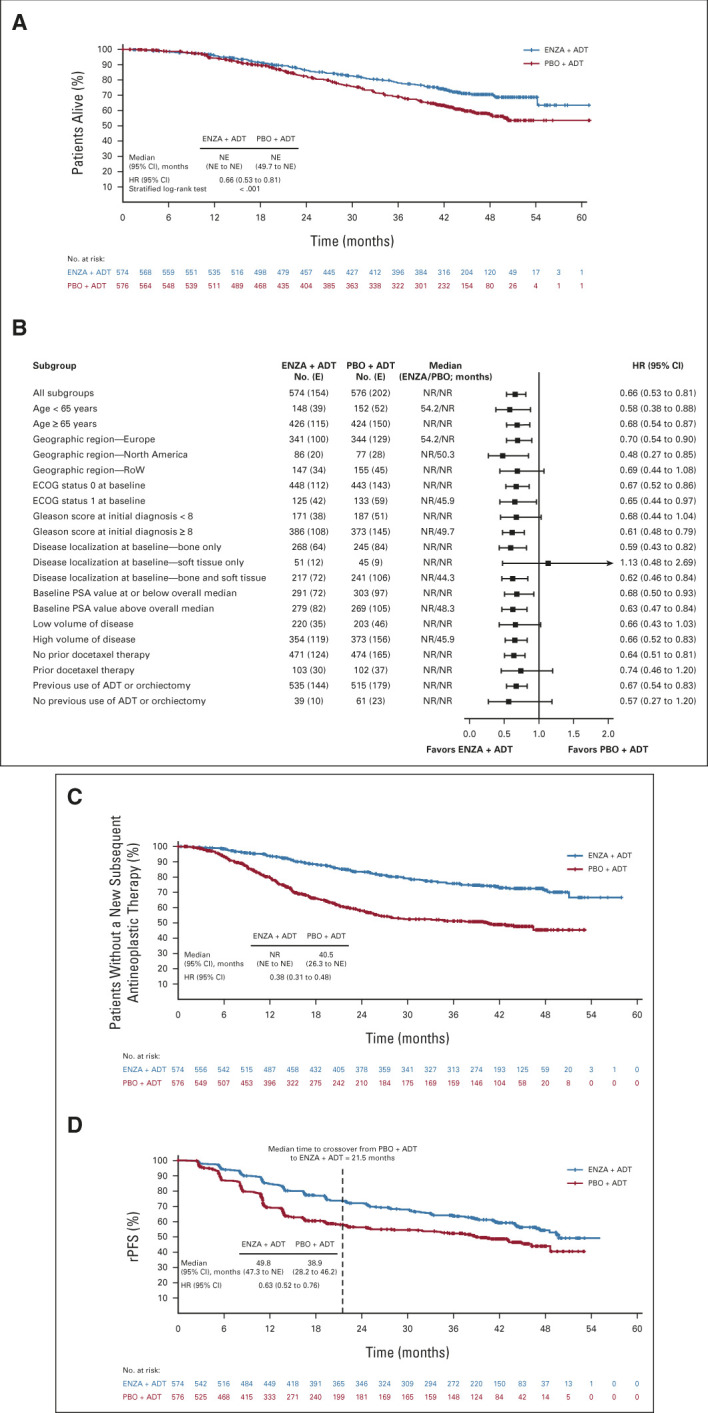
Efficacy analyses (intent-to-treat population) showing (A) Kaplan-Meier estimate of final OS analysis, (B) forest plot of OS subgroup analyses, (C) Kaplan-Meier estimates of time to first subsequent antineoplastic therapy, and (D) Kaplan-Meier estimates of rPFS (investigator assessed). ADT, androgen deprivation therapy; E, events; ECOG, Eastern Cooperative Oncology Group; ENZA, enzalutamide; HR, hazard ratio; NE, not evaluable; NR, not reached; OS, overall survival; PBO, placebo; PSA, prostate-specific antigen; RoW, rest of world; rPFS, radiographic progression-free survival.
A prespecified rank-preserving structural failure time sensitivity analysis to adjust for a possible crossover effect demonstrated a 43% reduction in risk of death with enzalutamide plus ADT versus placebo plus ADT (HR, 0.57; 95% CI, 0.45 to 0.70; P < .001; Data Supplement). Median OS was not reached for enzalutamide plus ADT, but was 47.7 months (95% CI, 43.3 to not evaluable) for placebo plus ADT.
The clinical benefit of enzalutamide plus ADT was generally consistent across prespecified subgroups, except in patients with only soft tissue disease at baseline (n = 96; Fig 1B). Further exploratory post hoc subgroup analyses confirmed a survival benefit after enzalutamide plus ADT in all subgroups except for patients with lymph node metastases only and visceral metastases, most likely because of small patient numbers (Data Supplement).
rPFS and Secondary Efficacy End Points
Enzalutamide plus ADT delayed time to first subsequent antineoplastic therapy; median was not reached for enzalutamide plus ADT versus 40.5 months for placebo plus ADT (HR, 0.38; 95% CI, 0.31 to 0.48; Data Supplement; Fig 1C).
Compared with placebo plus ADT, enzalutamide plus ADT reduced the risk of radiographic progression or death by 37%, extending the median rPFS by approximately 11 months (Data Supplement; Fig 1D). A total of 117 patients (20%) randomly assigned to enzalutamide plus ADT had prostate-specific antigen (PSA) progression compared with 259 (45%) randomly assigned to placebo plus ADT, equating to a risk reduction of 72% (Data Supplement). After median time to crossover (21.5 months) was reached, the rate of radiographic and PSA progression slowed over time with placebo plus ADT (Fig 1D; Data Supplement).The reduced risk of radiographic progression or death and PSA progression observed with enzalutamide plus ADT, as compared with placebo plus ADT, was sustained after adjustment for crossover (Data Supplement). Enzalutamide plus ADT also delayed time to first symptomatic skeletal event (Data Supplement) and castration resistance (Data Supplement). Results of other secondary end point analyses are reported in the Data Supplement.
Safety
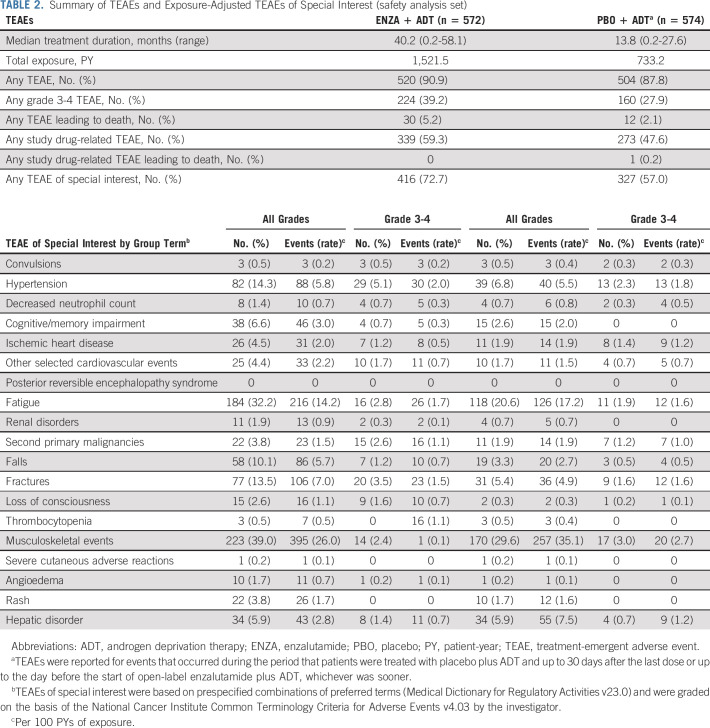
The median treatment duration was 40.2, 13.8, and 23.9 months in the enzalutamide plus ADT, placebo plus ADT, and crossover groups, respectively. Incidence of treatment-emergent adverse events was consistent with the primary analysis3 (Table 2; Data Supplement), and no new safety signals were identified.
TABLE 2.
Summary of TEAEs and Exposure-Adjusted TEAEs of Special Interest (safety analysis set)
DISCUSSION
In ARCHES, enzalutamide plus ADT significantly reduced the risk of death in patients with mHSPC by 34% versus placebo plus ADT. The survival benefit of enzalutamide plus ADT became more apparent with additional follow-up. Enzalutamide plus ADT also delayed time to initiation of the first subsequent antineoplastic therapy. In total, 70% of patients who initially received placebo plus ADT went on to receive a life-prolonging treatment and, inclusive of those who crossed over, 42% went on to treatment with enzalutamide. Despite this, a statistically significant survival benefit was observed with enzalutamide plus ADT, highlighting the importance of early enzalutamide use in patients with mHSPC, rather than delaying initiation until the development of castration resistance. Importantly, improvement in OS with enzalutamide is unlikely to be the result of patients in the placebo plus ADT group receiving inadequate postprotocol therapy.
The survival benefit with early use of enzalutamide plus ADT was generally consistent across subgroups, with the exception of patients with lymph node metastases only and visceral metastases; however, both subgroups had relatively low patient numbers and statistical analyses were underpowered, as also reported in other large trials of mHSPC.4-6 Nevertheless, clinicians assessing and prescribing therapy for patients with mHSPC should feel reassured regarding survival benefit with enzalutamide for the majority of patients.
The superiority of enzalutamide plus ADT over placebo plus ADT for other efficacy end points was previously reported3 and maintained with additional follow-up. No new safety signals emerged. Taken together, these data indicate that longer-term use of enzalutamide was well tolerated and not associated with any new toxicity concerns, a key consideration for clinicians when choosing a systemic treatment for patients with advanced prostate cancer.
In conclusion, enzalutamide plus ADT significantly prolongs survival versus placebo plus ADT in patients with mHSPC, including across clinically important subgroups, and thus represents an effective and well-tolerated therapeutic option for patients with mHSPC.
ACKNOWLEDGMENT
We thank the patients who volunteered to participate in this trial and the investigators and trial staff who cared for them. Medical writing and editorial assistance were provided by Jake Stoddart, MRes, and Jane Beck, MA (Hons), from Complete HealthVizion, funded by the study sponsors.
Andrew J. Armstrong
Consulting or Advisory Role: Bayer, Dendreon, Pfizer, Astellas Scientific and Medical Affairs Inc, AstraZeneca, Merck, Bristol Myers Squibb, Janssen, FORMA Therapeutics, Novartis, Exelixis, Myovant Sciences, GoodRx
Research Funding: Dendreon (Inst), Bayer (Inst), Pfizer (Inst), Novartis (Inst), Janssen Oncology (Inst), Astellas Pharma (Inst), Gilead Sciences (Inst), Roche/Genentech (Inst), Bristol Myers Squibb (Inst), Constellation Pharmaceuticals (Inst), Merck (Inst), AstraZeneca (Inst), BeiGene (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), FORMA Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Circulating tumor cell novel capture technology (Inst)
Travel, Accommodations, Expenses: Astellas Scientific and Medical Affairs Inc
Arun A. Azad
Honoraria: Janssen, Astellas Pharma, Novartis, Tolmar, Amgen, Pfizer, Bayer, Telix Pharmaceuticals, Bristol Myers Squibb, Merck Serono, AstraZeneca, Sanofi, Ipsen, Merck Sharp & Dohme, Noxopharm, Aculeus Therapeutics
Consulting or Advisory Role: Astellas Pharma, Novartis, Janssen, Sanofi, AstraZeneca, Pfizer, Bristol Myers Squibb, Tolmar, Telix Pharmaceuticals, Merck Sharp & Dohme, Bayer, Ipsen, Merck Serono, Amgen, Noxopharm, Aculeus Therapeutics
Speakers' Bureau: Astellas Pharma, Novartis, Amgen, Bayer, Janssen, Ipsen, Bristol Myers Squibb, Merck Serono
Research Funding: Astellas Pharma, AstraZeneca, Merck Serono, Merck Serono (Inst), Novartis (Inst), Pfizer (Inst), Bristol Myers Squibb (Inst), Sanofi (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Aptevo Therapeutics (Inst), MedImmune (Inst), Bionomics (Inst), Synthorx (Inst), Astellas Pharma (Inst), Ipsen (Inst), Lilly (Inst), Gilead Sciences (Inst), Janssen (Inst), Exelixis (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Sanofi, Merck Serono, Amgen, Janssen, Tolmar, Pfizer
Taro Iguchi
Consulting or Advisory Role: Astellas Pharma, Bayer
Speakers' Bureau: Astellas Pharma, Bayer Yakuhin, Janssen, Sanofi, AstraZeneca, Takeda
Research Funding: Astellas Pharma, Bayer Yakuhin
Russell Z. Szmulewitz
Honoraria: Astellas Pharma
Consulting or Advisory Role: AstraZeneca, AbbVie, Exelixis, Merck, Amgen, Janssen Oncology, Sanofi, Astellas Pharma, Pfizer
Research Funding: AbbVie (Inst), Astellas Pharma (Inst), MacroGenics (Inst), Janssen Oncology (Inst), Plexxikon (Inst), Harpoon Therapeutics (Inst), Merck (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Patent licensed by The University of Chicago, of which I am a coinventor to Corcept Therapeutics for combination AR/GR inhibition in prostate cancer
Travel, Accommodations, Expenses: Corcept Therapeutics
Daniel P. Petrylak
Stock and Other Ownership Interests: Bellicum Pharmaceuticals, TYME
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Roche, Astellas Pharma, AstraZeneca, Lilly, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Clovis Oncology, Incyte, Janssen, Pharmacyclics, Seattle Genetics, UroGen Pharma, Advanced Accelerator Applications, Ipsen, Bicycle Therapeutics, Mirati Therapeutics, Monopteros Therapeutics, Regeneron, Gilead Sciences
Research Funding: Progenics (Inst), Sanofi (Inst), Endocyte (Inst), Genentech (Inst), Merck (Inst), Astellas Medivation (Inst), Novartis (Inst), AstraZeneca (Inst), Bayer (Inst), Lilly (Inst), Innocrin Pharma (Inst), MedImmune (Inst), Pfizer (Inst), Roche (Inst), Seattle Genetics (Inst), Clovis Oncology (Inst), Bristol Myers Squibb (Inst), Advanced Accelerator Applications (Inst), Agensys (Inst), BioXcel Therapeutics (Inst), Eisai (Inst), Mirati Therapeutics (Inst), Replimune (Inst), Medivation (Inst), Gilead Sciences (Inst)
Expert Testimony: Celgene, Sanofi
Jeffrey Holzbeierlein
Consulting or Advisory Role: Basilea, KDx Diagnostics
Research Funding: MDxHealth (Inst)
Uncompensated Relationships: Astellas Medivation
Arnauld Villers
Research Funding: Astellas Pharma (Inst), Janssen Oncology (Inst), Ipsen (Inst)
Antonio Alcaraz
Consulting or Advisory Role: Astellas
Travel, Accommodations, Expenses: Olympus, Ipsen, Janssen, Bayer
Boris Alexeev
Honoraria: AstraZeneca, Astellas Pharma, Ferring, Eisai, Janssen, Bayer, MSD, Merck, Pfizer, Roche, Sanofi, Bristol Myers Squibb
Consulting or Advisory Role: AstraZeneca, Astellas Pharma, Bayer, Bristol Myers Squibb, Ferring, Janssen, Merck, Sanofi, Pfizer, MSD, Roche, Eisai
Speakers' Bureau: Janssen, Sanofi, Ferring, Astellas Pharma, Pfizer, AstraZeneca, Bayer, Merck, Bristol Myers Squibb, MSD, Eisai, Roche
Research Funding: AstraZeneca, Merck, Sanofi, Bayer, Astellas Pharma, Janssen, Bristol Myers Squibb, Bavarian Nordic, Pfizer, ICON Clinical Research, Eisai, MSD, Roche
Travel, Accommodations, Expenses: AstraZeneca, Astellas Pharma, Bayer, Bristol Myers Squibb, Janssen, MSD, Pfizer, Sanofi
Neal D. Shore
Consulting or Advisory Role: Bayer, Janssen Scientific Affairs, Dendreon, Tolmar, Ferring, Medivation/Astellas, Amgen, Pfizer, AstraZeneca, Myovant Sciences, Astellas Pharma, AbbVie, Merck, Bristol Myers Squibb/Sanofi, Boston Scientific, Clovis Oncology, Exact Imaging, FerGene, Foundation Medicine, CG Oncology, Invitae, MDxHealth, Myriad Genetics, Nymox, Propella Therapeutics, Genzyme, Sanofi, Sesen Bio, CG Oncology, Exact Sciences, Genesis Cancer Care, Pacific Edge Biotechnology, Phosphorus, UroGen Pharma, Speciality Networks, PreView
Speakers' Bureau: Janssen, Bayer, Dendreon, Astellas Pharma, AstraZeneca, Clovis Oncology, Pfizer, Guardant Health, Merck, Foundation Medicine
Research Funding: AbbVie, Amgen, Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb/Pfizer, Boston Scientific, Clovis Oncology, Dendreon, Exact Imaging, Ferring, Foundation Medicine, Invitae, Janssen, MDxHealth, Merck, Myovant Sciences, Myriad Genetics, Nymox, Pfizer, Sanofi, Sesen Bio, Tolmar
Francisco Gomez-Veiga
Honoraria: AbbVie, Astellas, AstraZeneca, Bayer, Ferring, GE, GlaxoSmithKline, Ipsen, Janssen, Sanofi
Consulting or Advisory Role: AbbVie, Astellas, AstraZeneca, Bayer, Ferring, GE, GlaxoSmithKline, Ipsen, Janssen, Sanofi
Speakers' Bureau: AbbVie, Astellas, AstraZeneca, Bayer, GE, Janssen, Orion
Research Funding: AbbVie, Astellas, AstraZeneca, Ipsen, Janssen
Travel, Accommodations, Expenses: AbbVie, Astellas, Bayer, Janssen, Orion
Brad Rosbrook
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Fabian Zohren
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer, AlloVir Inc (I)
Patents, Royalties, Other Intellectual Property: AlloVir Inc (I)
Shunsuke Yamada
Employment: Astellas Pharma
Stock and Other Ownership Interests: Astellas Pharma
Gabriel P. Haas
Employment: Astellas Pharma
Arnulf Stenzl
Consulting or Advisory Role: Ipsen, Roche, Janssen, Alere, Bristol Myers Squibb, Steba Biotech, Synergo, Ferring, Bayer, Astellas Pharma
Research Funding: Karl Storz (Inst), Astellas Pharma, AstraZeneca, Medivation, Janssen, Johnson & Johnson (Inst), Roche (Inst), Cepheid (Inst), Immatics (Inst), Bayer (Inst), Novartis (Inst), Amgen (Inst), GenomeDx (Inst)
Patents, Royalties, Other Intellectual Property: Patent A290/99 Implantable incontinence device, AT00/0001:C-Trap, implantable device to treat urinary incontinence, 2018/6579 Gene expression signature for subtype and prognostic prediction of renal cell carcinoma
Expert Testimony: GBA Pharma
Travel, Accommodations, Expenses: Janssen, Ipsen, Sanofi/Aventis, CureVac, Ferring, Astellas Pharma, Amgen, AstraZeneca
No other potential conflicts of interest were reported.
See accompanying editorial on page 1599
PRIOR PRESENTATION
Presented in part at the European Society for Medical Oncology Virtual Congress, September 16-20, 2021.
SUPPORT
Supported by Astellas Pharma Inc and Pfizer Inc, the codevelopers of enzalutamide.
CLINICAL TRIAL INFORMATION
NCT02677896 (ARCHES)
DATA SHARING STATEMENT
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
AUTHOR CONTRIBUTIONS
Conception and design: Andrew J. Armstrong, Arun A. Azad, Taro Iguchi, Francisco Gomez-Veiga, Shunsuke Yamada, Gabriel P. Haas
Administrative support: Arnauld Villers, Neal D. Shore
Provision of study materials or patients: Taro Iguchi, Russell Z. Szmulewitz, Arnulf Stenzl
Collection and assembly of data: Andrew J. Armstrong, Taro Iguchi, Russell Z. Szmulewitz, Jeffrey Holzbeierlein, Arnauld Villers, Antonio Alcaraz, Boris Alekseev, Fabian Zohren, Arnulf Stenzl
Data analysis and interpretation: Andrew J. Armstrong, Arun A. Azad, Taro Iguchi, Russell Z. Szmulewitz, Daniel P. Petrylak, Jeffrey Holzbeierlein, Arnauld Villers, Neal D. Shore, Francisco Gomez-Veiga, Brad Rosbrook, Fabian Zohren, Shunsuke Yamada, Gabriel P. Haas
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Improved Survival With Enzalutamide in Patients With Metastatic Hormone-Sensitive Prostate Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Andrew J. Armstrong
Consulting or Advisory Role: Bayer, Dendreon, Pfizer, Astellas Scientific and Medical Affairs Inc, AstraZeneca, Merck, Bristol Myers Squibb, Janssen, FORMA Therapeutics, Novartis, Exelixis, Myovant Sciences, GoodRx
Research Funding: Dendreon (Inst), Bayer (Inst), Pfizer (Inst), Novartis (Inst), Janssen Oncology (Inst), Astellas Pharma (Inst), Gilead Sciences (Inst), Roche/Genentech (Inst), Bristol Myers Squibb (Inst), Constellation Pharmaceuticals (Inst), Merck (Inst), AstraZeneca (Inst), BeiGene (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), FORMA Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Circulating tumor cell novel capture technology (Inst)
Travel, Accommodations, Expenses: Astellas Scientific and Medical Affairs Inc
Arun A. Azad
Honoraria: Janssen, Astellas Pharma, Novartis, Tolmar, Amgen, Pfizer, Bayer, Telix Pharmaceuticals, Bristol Myers Squibb, Merck Serono, AstraZeneca, Sanofi, Ipsen, Merck Sharp & Dohme, Noxopharm, Aculeus Therapeutics
Consulting or Advisory Role: Astellas Pharma, Novartis, Janssen, Sanofi, AstraZeneca, Pfizer, Bristol Myers Squibb, Tolmar, Telix Pharmaceuticals, Merck Sharp & Dohme, Bayer, Ipsen, Merck Serono, Amgen, Noxopharm, Aculeus Therapeutics
Speakers' Bureau: Astellas Pharma, Novartis, Amgen, Bayer, Janssen, Ipsen, Bristol Myers Squibb, Merck Serono
Research Funding: Astellas Pharma, AstraZeneca, Merck Serono, Merck Serono (Inst), Novartis (Inst), Pfizer (Inst), Bristol Myers Squibb (Inst), Sanofi (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Aptevo Therapeutics (Inst), MedImmune (Inst), Bionomics (Inst), Synthorx (Inst), Astellas Pharma (Inst), Ipsen (Inst), Lilly (Inst), Gilead Sciences (Inst), Janssen (Inst), Exelixis (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Sanofi, Merck Serono, Amgen, Janssen, Tolmar, Pfizer
Taro Iguchi
Consulting or Advisory Role: Astellas Pharma, Bayer
Speakers' Bureau: Astellas Pharma, Bayer Yakuhin, Janssen, Sanofi, AstraZeneca, Takeda
Research Funding: Astellas Pharma, Bayer Yakuhin
Russell Z. Szmulewitz
Honoraria: Astellas Pharma
Consulting or Advisory Role: AstraZeneca, AbbVie, Exelixis, Merck, Amgen, Janssen Oncology, Sanofi, Astellas Pharma, Pfizer
Research Funding: AbbVie (Inst), Astellas Pharma (Inst), MacroGenics (Inst), Janssen Oncology (Inst), Plexxikon (Inst), Harpoon Therapeutics (Inst), Merck (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Patent licensed by The University of Chicago, of which I am a coinventor to Corcept Therapeutics for combination AR/GR inhibition in prostate cancer
Travel, Accommodations, Expenses: Corcept Therapeutics
Daniel P. Petrylak
Stock and Other Ownership Interests: Bellicum Pharmaceuticals, TYME
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Roche, Astellas Pharma, AstraZeneca, Lilly, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Clovis Oncology, Incyte, Janssen, Pharmacyclics, Seattle Genetics, UroGen Pharma, Advanced Accelerator Applications, Ipsen, Bicycle Therapeutics, Mirati Therapeutics, Monopteros Therapeutics, Regeneron, Gilead Sciences
Research Funding: Progenics (Inst), Sanofi (Inst), Endocyte (Inst), Genentech (Inst), Merck (Inst), Astellas Medivation (Inst), Novartis (Inst), AstraZeneca (Inst), Bayer (Inst), Lilly (Inst), Innocrin Pharma (Inst), MedImmune (Inst), Pfizer (Inst), Roche (Inst), Seattle Genetics (Inst), Clovis Oncology (Inst), Bristol Myers Squibb (Inst), Advanced Accelerator Applications (Inst), Agensys (Inst), BioXcel Therapeutics (Inst), Eisai (Inst), Mirati Therapeutics (Inst), Replimune (Inst), Medivation (Inst), Gilead Sciences (Inst)
Expert Testimony: Celgene, Sanofi
Jeffrey Holzbeierlein
Consulting or Advisory Role: Basilea, KDx Diagnostics
Research Funding: MDxHealth (Inst)
Uncompensated Relationships: Astellas Medivation
Arnauld Villers
Research Funding: Astellas Pharma (Inst), Janssen Oncology (Inst), Ipsen (Inst)
Antonio Alcaraz
Consulting or Advisory Role: Astellas
Travel, Accommodations, Expenses: Olympus, Ipsen, Janssen, Bayer
Boris Alexeev
Honoraria: AstraZeneca, Astellas Pharma, Ferring, Eisai, Janssen, Bayer, MSD, Merck, Pfizer, Roche, Sanofi, Bristol Myers Squibb
Consulting or Advisory Role: AstraZeneca, Astellas Pharma, Bayer, Bristol Myers Squibb, Ferring, Janssen, Merck, Sanofi, Pfizer, MSD, Roche, Eisai
Speakers' Bureau: Janssen, Sanofi, Ferring, Astellas Pharma, Pfizer, AstraZeneca, Bayer, Merck, Bristol Myers Squibb, MSD, Eisai, Roche
Research Funding: AstraZeneca, Merck, Sanofi, Bayer, Astellas Pharma, Janssen, Bristol Myers Squibb, Bavarian Nordic, Pfizer, ICON Clinical Research, Eisai, MSD, Roche
Travel, Accommodations, Expenses: AstraZeneca, Astellas Pharma, Bayer, Bristol Myers Squibb, Janssen, MSD, Pfizer, Sanofi
Neal D. Shore
Consulting or Advisory Role: Bayer, Janssen Scientific Affairs, Dendreon, Tolmar, Ferring, Medivation/Astellas, Amgen, Pfizer, AstraZeneca, Myovant Sciences, Astellas Pharma, AbbVie, Merck, Bristol Myers Squibb/Sanofi, Boston Scientific, Clovis Oncology, Exact Imaging, FerGene, Foundation Medicine, CG Oncology, Invitae, MDxHealth, Myriad Genetics, Nymox, Propella Therapeutics, Genzyme, Sanofi, Sesen Bio, CG Oncology, Exact Sciences, Genesis Cancer Care, Pacific Edge Biotechnology, Phosphorus, UroGen Pharma, Speciality Networks, PreView
Speakers' Bureau: Janssen, Bayer, Dendreon, Astellas Pharma, AstraZeneca, Clovis Oncology, Pfizer, Guardant Health, Merck, Foundation Medicine
Research Funding: AbbVie, Amgen, Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb/Pfizer, Boston Scientific, Clovis Oncology, Dendreon, Exact Imaging, Ferring, Foundation Medicine, Invitae, Janssen, MDxHealth, Merck, Myovant Sciences, Myriad Genetics, Nymox, Pfizer, Sanofi, Sesen Bio, Tolmar
Francisco Gomez-Veiga
Honoraria: AbbVie, Astellas, AstraZeneca, Bayer, Ferring, GE, GlaxoSmithKline, Ipsen, Janssen, Sanofi
Consulting or Advisory Role: AbbVie, Astellas, AstraZeneca, Bayer, Ferring, GE, GlaxoSmithKline, Ipsen, Janssen, Sanofi
Speakers' Bureau: AbbVie, Astellas, AstraZeneca, Bayer, GE, Janssen, Orion
Research Funding: AbbVie, Astellas, AstraZeneca, Ipsen, Janssen
Travel, Accommodations, Expenses: AbbVie, Astellas, Bayer, Janssen, Orion
Brad Rosbrook
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Fabian Zohren
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer, AlloVir Inc (I)
Patents, Royalties, Other Intellectual Property: AlloVir Inc (I)
Shunsuke Yamada
Employment: Astellas Pharma
Stock and Other Ownership Interests: Astellas Pharma
Gabriel P. Haas
Employment: Astellas Pharma
Arnulf Stenzl
Consulting or Advisory Role: Ipsen, Roche, Janssen, Alere, Bristol Myers Squibb, Steba Biotech, Synergo, Ferring, Bayer, Astellas Pharma
Research Funding: Karl Storz (Inst), Astellas Pharma, AstraZeneca, Medivation, Janssen, Johnson & Johnson (Inst), Roche (Inst), Cepheid (Inst), Immatics (Inst), Bayer (Inst), Novartis (Inst), Amgen (Inst), GenomeDx (Inst)
Patents, Royalties, Other Intellectual Property: Patent A290/99 Implantable incontinence device, AT00/0001:C-Trap, implantable device to treat urinary incontinence, 2018/6579 Gene expression signature for subtype and prognostic prediction of renal cell carcinoma
Expert Testimony: GBA Pharma
Travel, Accommodations, Expenses: Janssen, Ipsen, Sanofi/Aventis, CureVac, Ferring, Astellas Pharma, Amgen, AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1.US Food and Drug Administration : XTANDI Highlights of Prescribing Information. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/203415s015lbl.pdf [Google Scholar]
- 2.European Medicines Agency : Xtandi Summary of Product Characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/xtandi-epar-product-information_en.pdf [Google Scholar]
- 3.Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. : ARCHES: A randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol 37:2974-2986, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyriakopoulos CE, Chen YH, Carducci MA, et al. : Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: Long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol 36:1080-1087, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fizazi K, Tran N, Fein L, et al. : Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): Final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol 20:686-700, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Chi KN, Chowdhury S, Bjartell A, et al. : Apalutamide in patients with metastatic castration-sensitive prostate cancer: Final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol 39:2294-2303, 2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.