PURPOSE
To examine cardiovascular disease (CVD) and mortality risk in women with breast cancer (BC) by cancer therapy received relative to women without BC.
METHODS
The study population comprised Kaiser Permanente Northern California members. Cases with invasive BC diagnosed from 2005 to 2013 were matched 1:5 to controls without BC on birth year and race/ethnicity. Cancer treatment, CVD outcomes, and covariate data were from electronic health records. Multivariable Cox proportional hazards models estimated hazard ratios (HRs) and 95% CIs of CVD incidence and mortality by receipt of chemotherapy treatment combinations, radiation therapy, and endocrine therapy.
RESULTS
A total of 13,642 women with BC were matched to 68,202 controls without BC. Over a 7-year average follow-up (range < 1-14 years), women who received anthracyclines and/or trastuzumab had high risk of heart failure/cardiomyopathy relative to controls, with the highest risk seen in women who received both anthracyclines and trastuzumab (HR, 3.68; 95% CI, 1.79 to 7.59). High risk of heart failure and/or cardiomyopathy was also observed in women with BC with a history of radiation therapy (HR, 1.38; 95% CI, 1.13 to 1.69) and aromatase inhibitor use (HR, 1.31; 95% CI, 1.07 to 1.60), relative to their controls. Elevated risks for stroke, arrhythmia, cardiac arrest, venous thromboembolic disease, CVD-related death, and death from any cause were also observed in women with BC on the basis of cancer treatment received.
CONCLUSION
Women with BC had increased incidence of CVD events, CVD-related mortality, and all-cause mortality compared with women without BC, and risks varied according to the history of cancer treatment received. Studies are needed to determine how women who received BC treatment should be cared for to improve cardiovascular outcomes.
INTRODUCTION
The United States has over 3.8 million female breast cancer (BC) survivors, with 281,550 new cases expected in 2021.1,2 The 5-year survival rate for women with invasive BC is 90%,1-3 resulting in a growing population of long-term BC survivors. Improvements in cancer treatments have reduced rates of BC-specific mortality, but deaths from other causes, including cardiovascular and cerebrovascular disease, have been increasing in BC survivors.4-7
CONTEXT
Key Objective
Many breast cancer (BC) treatments confer risk of cardiovascular disease (CVD), but risks by exposure to certain BC treatments alone or in specific clinically appropriate combinations have not been well quantified. This prospective study at Kaiser Permanente Northern California compared risk of incident CVD events and death in 13,642 women with BC diagnosed from 2005 to 2013 who received chemotherapy, radiation therapy, or endocrine therapy with 68,202 women without BC.
Knowledge Generated
Compared with age and race/ethnicity-matched controls without BC, women who received anthracyclines and/or trastuzumab, radiation therapy, or aromatase inhibitors had increased risk of developing heart failure/cardiomyopathy, stroke, arrhythmia, cardiac arrest, and venous thromboembolic disease, and of experiencing a CVD-related or all-cause death.
Relevance
Women with BC who receive specific treatment therapies may be at increased risk of incident CVD events. Development and testing of clinical pathways to manage these risks is needed.
Cardiovascular disease (CVD) is the leading cause of death among US women and is emerging as an important health concern of BC survivors.8 Multiple studies have shown that women with a history of BC are at higher risk of developing and dying of CVD compared with women without a history of BC.9-11 However, our understanding of why a higher CVD risk occurs in women with BC compared with women without BC is limited. Likely mechanisms have been proposed, including cancer-related treatment exposures, shared risk factors for cancer and CVD, and differential prevalence of risk factors before cancer diagnosis or differential incidence of risk factors after receipt of cancer therapies.8-14 Indeed, it is well established that many BC therapies are cardiotoxic. However, most research has focused on single BC treatment exposures within a sample of BC patients, thus precluding estimation of CVD risks relative to women without a history of BC and risk comparisons across treatments. Population-based research to help quantify the relative CVD burden risk for BC survivors is needed.
The Pathways Heart Study is well-poised to address this knowledge gap as a large cohort study within the Kaiser Permanente Northern California (KPNC) integrated health system. Herein, we quantify the risk of CVD incidence and death among women with BC relative to women without BC over a follow-up of up to 14 years overall and according to the cancer treatment they received.
METHODS
Study Population
The Pathways Heart Study is an ongoing, prospective cohort study within KPNC whose aim is to examine incident CVD and cardiometabolic risk factors in women with BC. KPNC electronic health records (EHRs) were used to identify eligible women and to extract their administrative and clinical data. Eligible cases were female patients diagnosed with stage I-IV BC from November 2005 to March 2013, at least age 21 years, enrolled in a KPNC health plan for at least 12 months before their BC diagnosis date, and had a history of chemotherapy, radiation therapy, or endocrine therapy. Cases were individually matched to controls 5:1 on year of birth, race, and ethnicity.
Data Collection and Measures
Covariate data from the EHR included sociodemographic and socioeconomic characteristics, health plan enrollment and utilization, health status and behaviors, and CVD risk factors and conditions at baseline, defined as the date of BC diagnosis or reference date for controls. Health plan utilization is the number of primary care outpatient or virtual visits in the year before diagnosis or reference date. The Comorbidity Point Score Version 2 (COPS2)15 is a measure of comorbidity calculated using diagnostic codes of inpatient and outpatient encounters occurring in the year before diagnosis or reference date. Baseline diabetes was identified as entry into the KPNC diabetes registry before diagnosis or reference date. Baseline dyslipidemia was defined using International Classification of Diseases (ICD) diagnostic codes, abnormal laboratory results, and lipid-lowering medication use within 3 years before diagnosis or reference date. Baseline hypertension was characterized using ICD diagnostic codes at primary care visits or hospitalizations and blood pressure medication use within 3 years before diagnosis or reference date.
Data on chemotherapy, radiation therapy, and endocrine therapy were obtained from the KPNC Cancer Registry and supplemented by procedure, infusion, and outpatient pharmacy data sources. Chemotherapy exposures focused on anthracyclines and trastuzumab on the basis of their known cardiotoxicity profiles,16,17 clinical applicability, and distribution of use within the study sample. Four mutually exclusive chemotherapy exposure groups were created: (1) anthracyclines without trastuzumab (group A), (2) anthracyclines with trastuzumab (group AT), (3) trastuzumab without anthracyclines (group T), and (4) neither anthracyclines nor trastuzumab (group N). Women in groups A, AT, and T may also have a history of cyclophosphamide, fluoropyrimidine, and/or taxane use. However, women in group N had a history of cyclophosphamide, fluoropyrimidine, and/or taxane use, either alone or in combination, but no history of anthracyclines or trastuzumab use. Radiation therapy included either side of the body and a subset who received radiation on the left side. Endocrine therapy was grouped according to receipt of either aromatase inhibitors or tamoxifen because of their likely opposing effects on the cardiovascular system.18
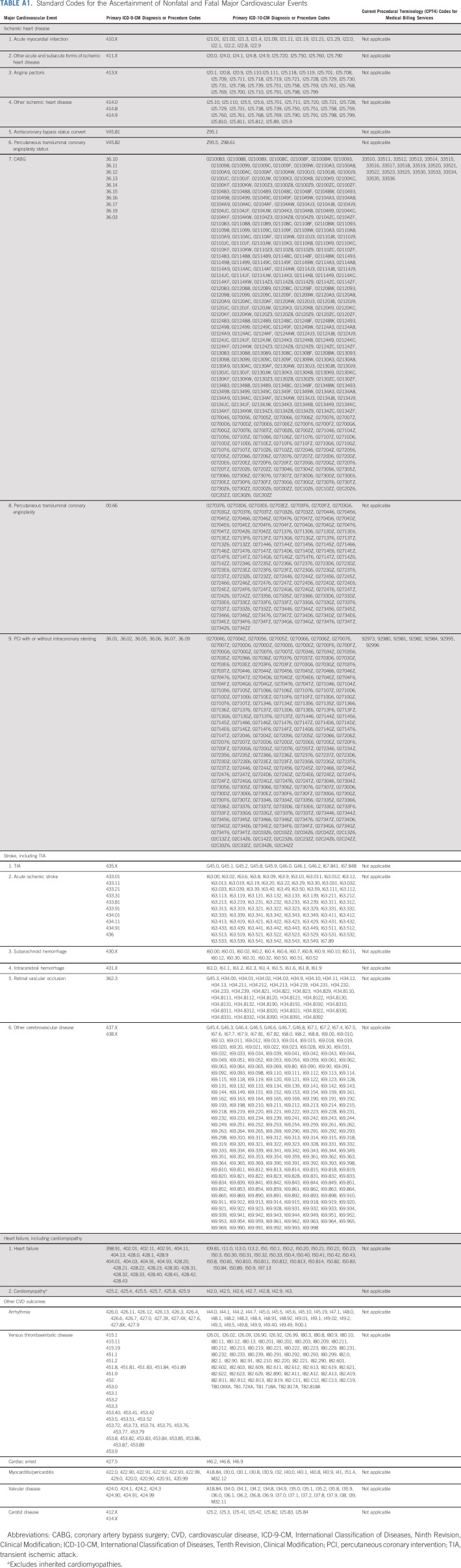
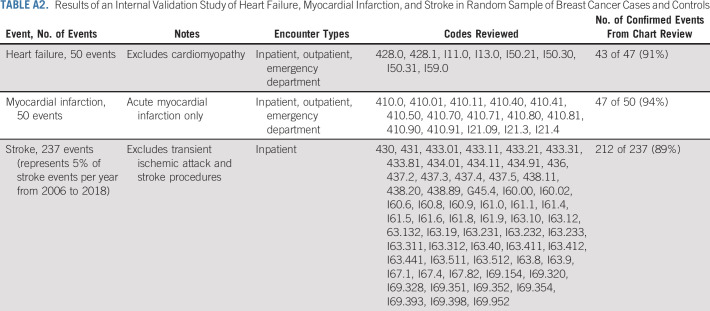
Cardiovascular events through December 31, 2018, were identified from the KPNC EHR according to the ICD diagnostic codes and Current Procedural Terminology codes from inpatient, ambulatory, and emergency department encounters and/or hospital discharge records (Appendix Table A1, online only). Primary CVD outcomes were ischemic heart disease, heart failure (HF), cardiomyopathy, and stroke. Secondary CVD outcomes included arrhythmia, cardiac arrest, carotid disease, myocarditis/pericarditis, transient ischemic attack, valvular disease, and venous thromboembolism (VTE). Cardiomyopathy excluded codes for inherited cardiomyopathies19 and was combined with HF events into a single outcome. A small physician-adjudicated validation study on the ICD diagnostic codes for HF, myocardial infarction, and stroke was completed in 337 KPNC patients with BC and controls and found positive predictive values of code ascertainment versus chart review validation ranging from 89% to 94% (Appendix Table A2, online only). Levels of agreement and discrepancy between the adjudicators were nondifferential.
Death data through December 31, 2018, were obtained from linkage to the KPNC mortality file, which is regularly updated with data from the California State Department of Vital Statistics, US Social Security Administration, National Death Index, and KPNC membership.
Statistical Analysis
Means and standard deviations for continuous variables and frequencies for categorical variables were calculated. Cumulative incidence rates (CIR) were estimated using 1 minus the Kaplan-Meier estimator of the survival probabilities for incident ischemic heart disease, HF and/or cardiomyopathy, and stroke. Women with the outcome within 2 years before study entry were excluded. Statistically significant differences comparing cases with controls were determined using the log-rank test.
Hazard ratios (HRs) and 95% CIs were estimated by Cox proportional hazards models for the primary and secondary incident CVD outcomes among BC cases by relative to matched controls without BC. Analyses were stratified by receipt of chemotherapy combinations, radiation therapy, and endocrine therapy. The time scale was defined as time from BC diagnosis to the first of the following: incident CVD event, KPNC disenrollment, death, or end of study. The Cox proportional hazards model adjusted for factors determined a priori to influence the risk of both BC and CVD: baseline health characteristics (health care utilization, body mass index, menopausal status, smoking history, diabetes, dyslipidemia, hypertension, and COPS2 score) and socioeconomic status (neighborhood median household income and highest level of education). The model also adjusted for other BC therapies received. For example, the models examining chemotherapy combinations also included receipt of radiation therapy, aromatase inhibitor, and tamoxifen. Analyses were conducted using R (version 3.6.2) with the survival package.20
RESULTS
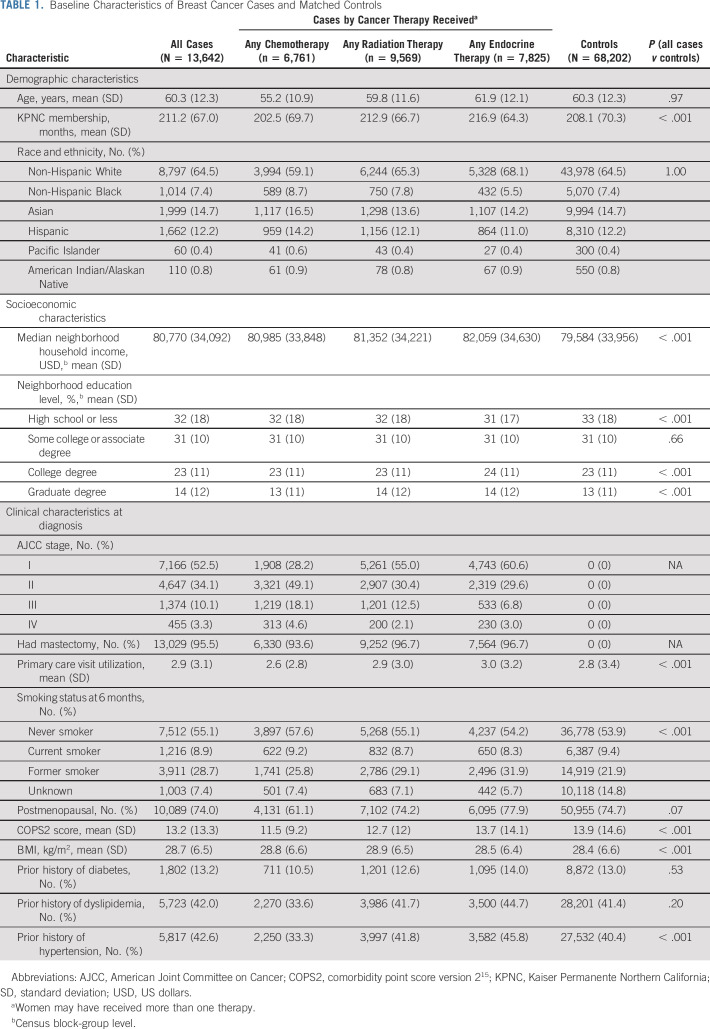
A total of 13,642 women with invasive BC and a history of receipt of chemotherapy, radiation therapy, or endocrine therapy were identified and matched to 68,202 women without BC (Fig 1). Mean age and race/ethnicity distributions were similar for cases and controls due to matching. All other characteristics, except for proportion with baseline diabetes and dyslipidemia, were significantly different between cases and controls (Table 1). Among cases, women who received any chemotherapy were younger and had a higher proportion of being a woman of color, never smoker, and diagnosed with stage II-IV disease compared with women who received any endocrine therapy or any radiation therapy. Women who received any endocrine therapy were more likely to have a history of diabetes, dyslipidemia, and hypertension.
FIG 1.
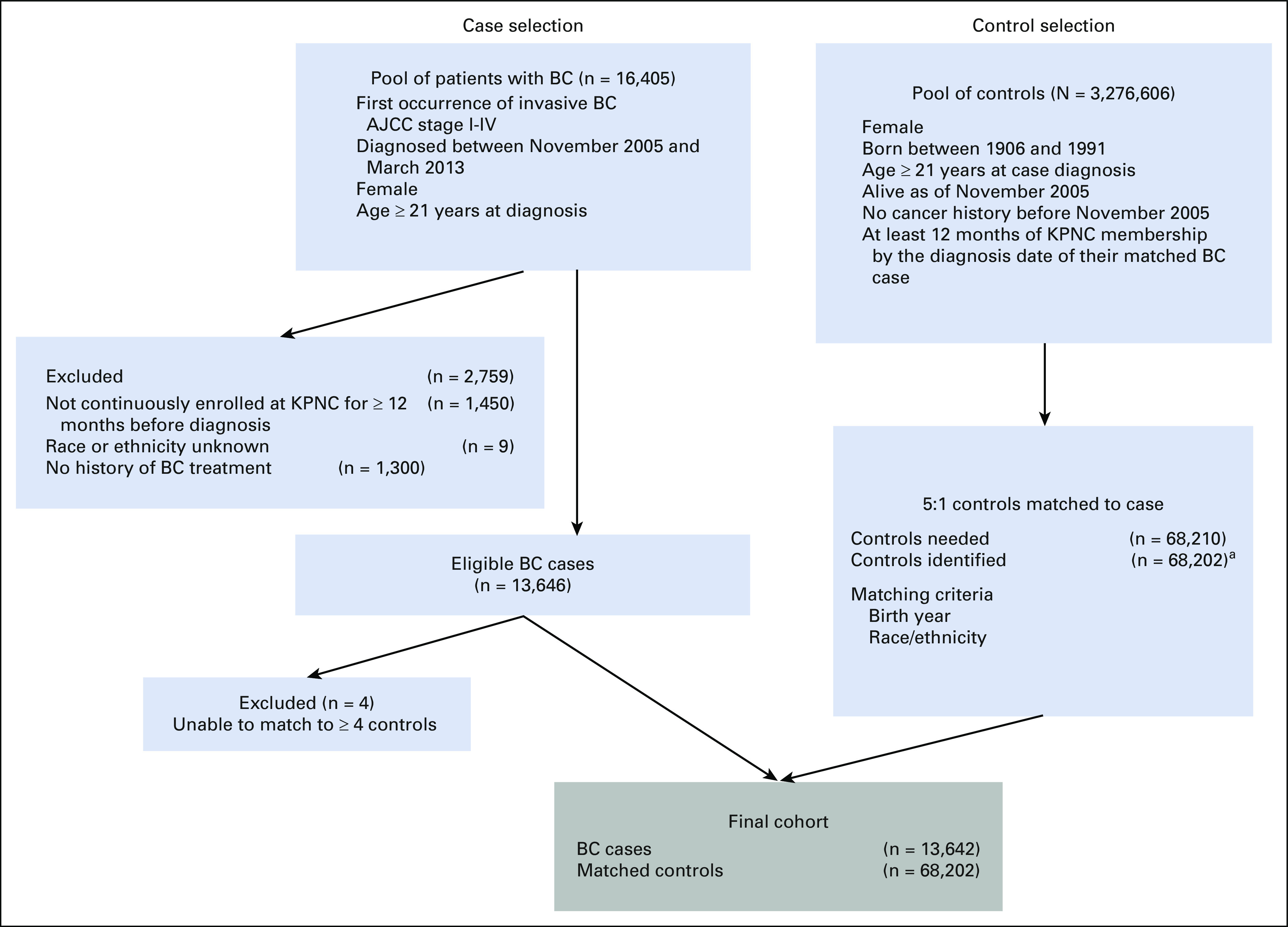
Selection of BC case and matched controls for the Pathways Heart Study. aEight cases matched to four controls; all remaining cases matched to five controls. AJCC, American Joint Committee on Cancer; BC, breast cancer; KPNC, Kaiser Permanente Northern California.
TABLE 1.
Baseline Characteristics of Breast Cancer Cases and Matched Controls
Primary CVD Outcomes
Controls had higher 10-year CIRs for ischemic heart disease compared with cases, but differences were statistically significant only among women in chemotherapy groups A (2.4% v 1.7%, P = .04) and T (2.4% v 1.5%, P = .02; Fig 2). In contrast, women in chemotherapy groups A and AT had higher 10-year CIRs for HF/cardiomyopathy compared with controls (A: 4.1% v 2.3%; AT: 4.4% v 2.0%; both P < .01). Higher 10-year CIRs for HF and/or cardiomyopathy were similarly observed for women who received radiation therapy (left-sided only: 5.0% v 4.0%, P = .02; either side: 4.8% v 4.1%, P = .04) compared with controls (Fig 3). For stroke, controls for chemotherapy group A had higher 10-year CIRs compared with cases (3.7% v 2.6%, P = .01), but no other differences were noted.
FIG 2.
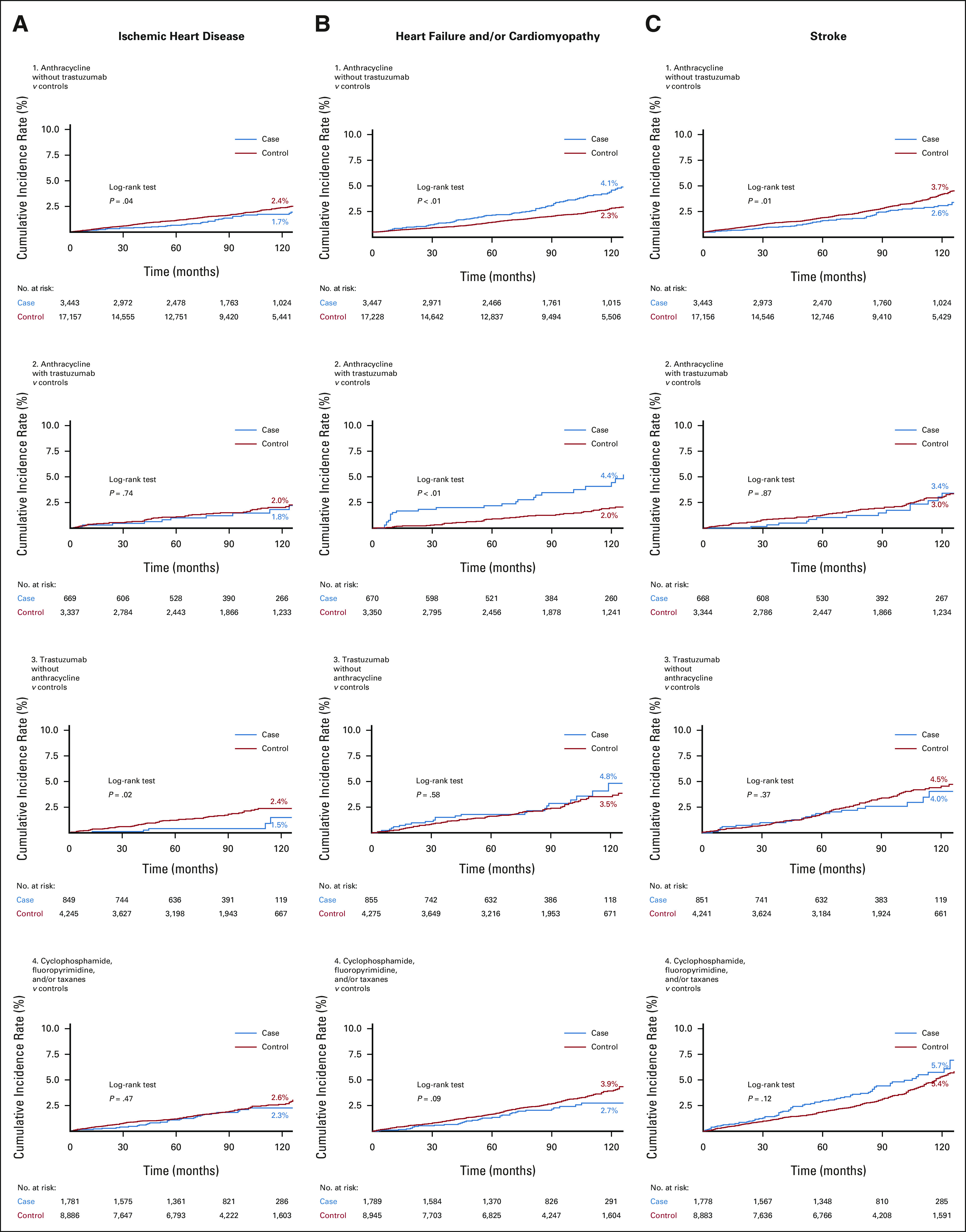
Ten-year cumulative incidence rate curves of (A, left column) ischemic heart disease, (B, middle column) heart failure and/or cardiomyopathy, and (C, right column) stroke in breast cancer cases according to combinations of chemotherapy drugs received compared with age and race/ethnicity-matched controls without breast cancer. Statistically significant differences were determined with the log-rank test.
FIG 3.
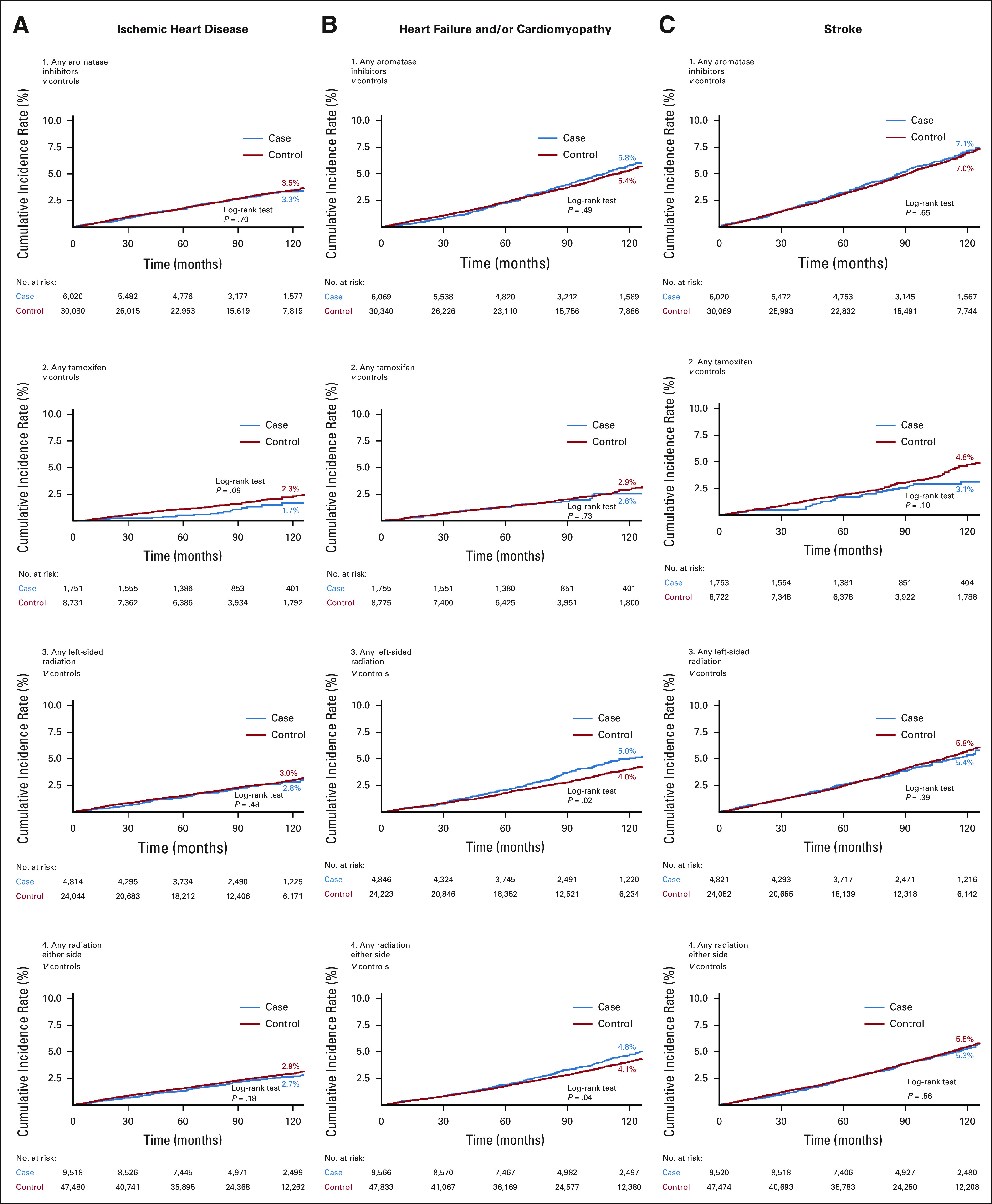
Ten-year cumulative incidence rate curves of (A, left column) ischemic heart disease, (B, middle column) heart failure and/or cardiomyopathy, and (C, right column) stroke in breast cancer cases by receipt of aromatase inhibitors, tamoxifen, left-sided radiation, and radiation (either side) treatment compared with age and race/ethnicity-matched controls without breast cancer. Statistically significant differences were determined with the log-rank test.
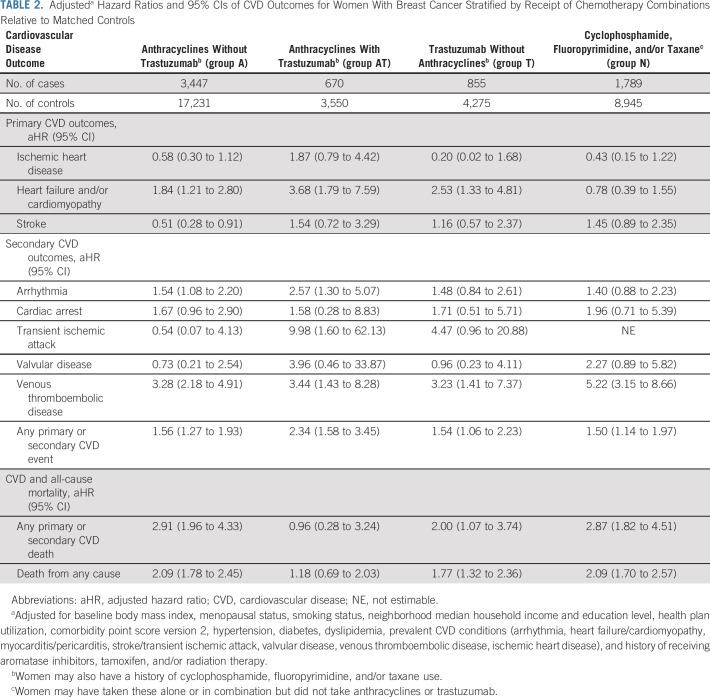
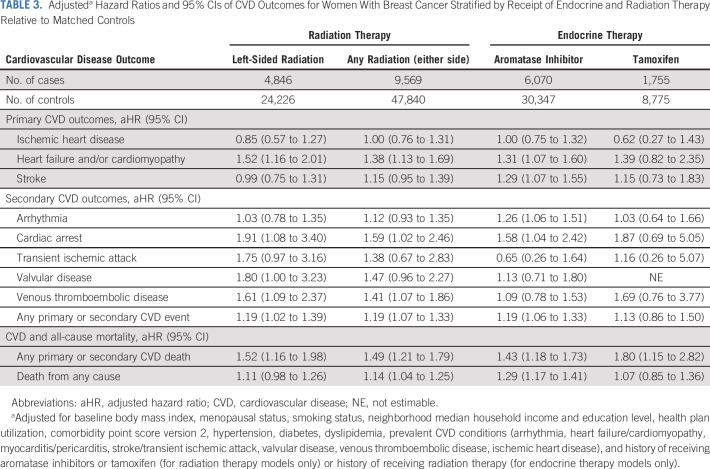
Over an average 7-year follow-up (range < 1 to 14 years), multivariable analyses showed that receipt of chemotherapy, radiation therapy, or endocrine therapy was not associated with incidence of ischemic heart disease. However, women in chemotherapy groups A, AT, and T had higher adjusted HR of HF and/or cardiomyopathy relative to their controls (Table 2). For groups A, AT, and T, the HR for HF/cardiomyopathy relative to matched controls without BC was 1.84 (95% CI, 1.21 to 2.80), 3.68 (95% CI, 1.79 to 7.59), and 2.53 (95% CI, 1.33 to 4.81), respectively. No risk associations for HF and/or cardiomyopathy were observed for women in chemotherapy group N. Left-sided radiation was associated with an HR of 1.52 (95% CI, 1.16 to 2.01), radiation on either side was associated with an HR of 1.38 (95% CI, 1.13 to 1.69), and aromatase inhibitors use was associated with an HR of 1.31 (95% CI, 1.07 to 1.60) of HF and/or cardiomyopathy relative to matched controls without BC (Table 3).
TABLE 2.
Adjusteda Hazard Ratios and 95% CIs of CVD Outcomes for Women With Breast Cancer Stratified by Receipt of Chemotherapy Combinations Relative to Matched Controls
TABLE 3.
Adjusteda Hazard Ratios and 95% CIs of CVD Outcomes for Women With Breast Cancer Stratified by Receipt of Endocrine and Radiation Therapy Relative to Matched Controls
Inverse associations of 0.51 (95% CI, 0.28 to 0.91) for stroke were found among group A relative to controls (Table 2). However, risk associations for stroke were not observed among groups AT, T, and N women compared with controls. Finally, women with a history of aromatase inhibitor use had an HR of 1.29 (95% CI, 1.07 to 1.55) for stroke, but no risk associations were observed for tamoxifen or radiation therapy recipients (Table 3).
Secondary CVD Outcomes
Statistically significant elevated HRs were observed for arrhythmia among chemotherapy groups A (HR, 1.54; 95% CI, 1.08 to 2.20) and AT (HR, 2.57; 95% CI, 1.30 to 5.07) and aromatase inhibitor (HR, 1.26; 95% CI, 1.06 to 1.51) users compared with controls. Similarly, 1.91 (95% CI, 1.08 to 3.40), 1.59 (95% CI, 1.02 to 2.46), and 1.58 (95% CI, 1.04 to 2.42) higher risks were observed for cardiac arrest among left-sided radiation, any-sided radiation, and aromatase inhibitor recipients, respectively, relative to controls (Table 3), but no associations were seen for the chemotherapy groups (Table 2). All treatment groups had increased risks for VTE, but statistically significant risks were only observed for the chemotherapy and radiation groups only (Tables 2 and 3). Because of few carotid disease and myocarditis/pericarditis events, these outcomes were excluded in the tables.
CVD and All-Cause Mortality
Except for women in chemotherapy group AT, risk of cardiovascular death was higher among all BC therapy groups compared with their matched controls without BC. Risk of all-cause death was higher relative to controls for women in chemotherapy groups A, T, and N (Table 2) and for women who received radiation on either side or aromatase inhibitors (Table 3).
DISCUSSION
Our access to comprehensive KPNC EHR data in a large cohort of 13,642 women with and 68,202 women without BC enabled a detailed investigation of incident CVD outcomes and deaths across distinct and clinically appropriate groups of BC therapies. Over an average follow-up of 7 years, women with BC had an increased risk of developing HF and/or cardiomyopathy, stroke, arrhythmia, cardiac arrest, and VTE, and of dying from a CVD-related event that varied by the history of BC treatment. We examined CVD outcomes in women according to receipt of anthracyclines and/or trastuzumab, which are known to confer the greatest risk of cardiotoxicity.16,17 We also estimated incident CVD risks associated with aromatase inhibitor and tamoxifen use as well as radiation therapy, including a subgroup exposed to left-sided radiation.
We observed that women with a history of anthracycline use had consistently higher cumulative incidence of HF and/or cardiomyopathy, which was not apparent in women who received only trastuzumab or received neither anthracyclines nor trastuzumab. Anthracyclines and trastuzumab are cancer treatments both recognized as cardiotoxic, but damage from anthracyclines is generally considered irreversible and dose-dependent while toxicity from trastuzumab is often reversible and dose-independent.21,22
Our multivariable analysis showed a hierarchy of HF and/or cardiomyopathy risk with lowest elevated risk in women on anthracyclines without trastuzumab, followed by women on trastuzumab without anthracyclines, and finally, the highest risk in women with a history of using both drugs. This is consistent with other studies.17,23-29 A study of 613 BC survivors reported a 1.02 risk for cardiotoxicity within 2 years of doxorubicin initiation among women on doxorubicin only that increased four-fold among women on doxorubicin and concomitant trastuzumab.24 A cohort study of nearly 9,000 patients with BC followed for a median 5.4 years reported a nearly two-fold risk for HF at least 18 months post-treatment associated with chemotherapy and trastuzumab compared with chemotherapy alone.27 In a retrospective study of US insurance claims data in approximately 16,500 female BC survivors followed for up to 6 years, trastuzumab use had a two-fold risk and anthracycline use had a 1.5-fold risk of incident HF relative to nonusers, but no associations were seen for taxane or radiation exposure.23 A Canadian study using administrative data from over 78,000 patients with BC and nearly 235,000 age-matched controls reported a multivariable adjusted HR of 1.81 for hospitalized HF among those exposed to either anthracyclines or trastuzumab.30 Similar to our own analysis, this study used a control group without BC, thus illustrating the general burden of these exposures on HF and/or cardiomyopathy in BC survivors.
Our analysis identified higher risk of arrhythmia among BC cases with anthracycline exposure and risks three-fold and higher for VTE in all chemotherapy groups, with the highest observed in women with neither anthracycline nor trastuzumab use. The aforementioned Canadian study reported an adjusted 1.89 risk of arrhythmia among BC patients with anthracycline and/or trastuzumab use compared with age-matched controls.30 Arrhythmia can be a consequence of HF and/or cardiomyopathy and vice versa in a bidirectional manner; thus, the same cause of cardiovascular damage may affect risks for both outcomes. Numerous studies and reviews have identified chemotherapy exposure in many cancers to be a risk factor for venous thrombosis31-34 including alkylating agents such as cyclophosphamide.35
We observed a higher risk of CVD-related and all-cause mortality associated with all chemotherapy groups, except the anthracycline with trastuzumab group. It is unclear why this combination group showed no associations, although it had the lowest number of women with BC and may not have been as well-powered as the other groups. Of note, women in the cyclophosphamide, fluoropyrimidine, and/or taxane group had nearly three-fold and two-fold risks of CVD-related and all-cause mortality, respectively. This group also had high risk of VTE, which might be driving the higher risk of death since VTE is a leading cause of CVD-related mortality among patients with cancer.36,37
We found that BC cases with a history of radiation therapy on either side had higher risks of HF and/or cardiomyopathy, cardiac arrest, VTE, CVD-related death, and all-cause mortality, and those with left-sided radiation exposure had higher risk of HF and/or cardiomyopathy, cardiac arrest, valvular disease, VTE, and CVD-related death. Our findings are generally consistent with the literature.38-41 Our analysis of radiation therapy adjusted for chemotherapy exposures but did not account for the specific timing or order of therapy, as other studies have been able to do.23,42 More work is needed to understand the possible additive effects of various BC treatments and the implications of the timing of receipt of these therapies.
Our analysis of endocrine therapies distinguished aromatase inhibitor and tamoxifen exposures because of opposing effects on the cardiovascular system.18 Many prior studies examining the role of endocrine therapies on CVD compared women receiving aromatase inhibitors with those receiving tamoxifen,43-45 making it challenging to understand the independent impacts of these exposures. We identified a higher risk of CVD-related death associated with tamoxifen use compared with controls without BC, but no associations with any other CVD outcome. We observed that the risk of cardiac arrest was not statistically significant among tamoxifen users, a finding consistent with two other published studies, although those studies compared tamoxifen with aromatase inhibitor use, as opposed to women without BC.46,47
Previous studies reported an increased risk of VTE associated with tamoxifen use,18,43,48 especially in comparison with aromatase inhibitor users. However, we observed risks that were not statistically significant in tamoxifen and aromatase inhibitor users relative to controls without BC. In contrast to tamoxifen, aromatase inhibitor use was associated with increased risk of HF and/or cardiomyopathy, stroke, arrhythmia, cardiac arrest, CVD-related death, and all-cause death. Similar to the models testing receipt of radiation therapy, these risks included adjustment with other BC treatments, prompting the need for further investigation of sequential or concomitant associations of various BC treatments.
Study strengths include a large sample of BC cases with statistical power to stratify across combinations of various treatments, a racially and ethnically diverse patient population from an integrated health care system with robust EHR data, and a large pool of matched controls without BC. Limitations include reduced generalizability because women in our study had higher household median incomes and education levels compared with the general US population. However, KPNC provides care to over 30% of the population in counties with a physical service presence and, aside from having health insurance, is generally representative of this catchment area. Finally, our analysis did not include data about treatment combinations across different therapy types, dosage, and duration, but future analyses to examine these details are planned.
In this prospective cohort study, women with BC compared with those without BC had higher risk of developing HF and/or cardiomyopathy, stroke, arrhythmia, cardiac arrest, and VTE, and of dying. Importantly, risks varied by the history of chemotherapy, radiation therapy, or endocrine therapy. These findings support the need to develop effective clinical strategies to reduce CVD incidence in BC survivors.
ACKNOWLEDGMENT
We thank the KPNC patients who provided the data for this study.
APPENDIX
TABLE A1.
Standard Codes for the Ascertainment of Nonfatal and Fatal Major Cardiovascular Events
TABLE A2.
Results of an Internal Validation Study of Heart Failure, Myocardial Infarction, and Stroke in Random Sample of Breast Cancer Cases and Controls
Carlos Iribarren
Consulting or Advisory Role: Gen in CODE, LLC
Research Funding: Genentech/Roche
Dawn L. Hershman
Consulting or Advisory Role: AIM Specialty Health
Romain Neugebauer
Employment: Kaiser Permanente
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2020 ASCO annual meeting, virtual, May 29-31, 2020.
SUPPORT
Supported by NCI R01CA214057 and U01CA195565.
AUTHOR CONTRIBUTIONS
Conception and design: Heather Greenlee, Dawn L. Hershman, Lawrence H. Kushi, Romain Neugebauer, Marilyn L. Kwan
Financial support: Heather Greenlee, Lawrence H. Kushi, Marilyn L. Kwan
Administrative support: Heather Greenlee, Marilyn L. Kwan
Provision of study materials or patients: Lawrence H. Kushi, Marilyn L. Kwan
Collection and assembly of data: Zaixing Shi, Cecile A. Laurent, Janise M. Roh, Margarita Santiago-Torres, Hanjie Shen, Marilyn L. Kwan
Data analysis and interpretation: Heather Greenlee, Carlos Iribarren, Jamal S. Rana, Richard Cheng, Mai Nguyen-Huynh, Eileen Rillamas-Sun, Zaixing Shi, Cecile A. Laurent, Valerie S. Lee, Hanjie Shen, Dawn L. Hershman, Lawrence H. Kushi, Marilyn L. Kwan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Risk of Cardiovascular Disease in Women With and Without Breast Cancer: The Pathways Heart Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Carlos Iribarren
Consulting or Advisory Role: Gen in CODE, LLC
Research Funding: Genentech/Roche
Dawn L. Hershman
Consulting or Advisory Role: AIM Specialty Health
Romain Neugebauer
Employment: Kaiser Permanente
No other potential conflicts of interest were reported.
REFERENCES
- 1.American Cancer Society : Breast Cancer Facts & Figures 2019-2020. Atlanta, GA, American Cancer Society, 2019 [Google Scholar]
- 2.American Cancer Society : Cancer Facts and Figures 2021. Atlanta, GA, American Cancer Society, 2021 [Google Scholar]
- 3.Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 71:7-33, 2021 [DOI] [PubMed] [Google Scholar]
- 4.Afifi AM, Saad AM, Al-Husseini MJ, et al. : Causes of death after breast cancer diagnosis: A US population-based analysis. Cancer 126:1559-1567, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Ye Y, Otahal P, Marwick TH, et al. : Cardiovascular and other competing causes of death among patients with cancer from 2006 to 2015: An Australian population-based study. Cancer 125:442-452, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Sturgeon KM, Deng L, Bluethmann SM, et al. : A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J 40:3889-3897, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel-Qadir H, Austin PC, Lee DS, et al. : A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol 2:88-93, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Blaes AH, Konety SH: Cardiovascular disease in breast cancer survivors: An important topic in breast cancer survivorship. J Natl Cancer Inst 113:105-106, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradshaw PT, Stevens J, Khankari N, et al. : Cardiovascular disease mortality among breast cancer survivors. Epidemiology 27:6-13, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armenian SH, Xu L, Ky B, et al. : Cardiovascular disease among survivors of adult-onset cancer: A community-based retrospective cohort study. J Clin Oncol 34:1122-1130, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramin C, Schaeffer ML, Zheng Z, et al. : All-cause and cardiovascular disease mortality among breast cancer survivors in CLUE II, a long-standing community-based cohort. J Natl Cancer Inst 113:137-145, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park NJ, Chang Y, Bender C, et al. : Cardiovascular disease and mortality after breast cancer in postmenopausal women: Results from the Women's Health Initiative. PLoS One 12:e0184174, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gernaat SAM, Ho PJ, Rijnberg N, et al. : Risk of death from cardiovascular disease following breast cancer: A systematic review. Breast Cancer Res Treat 164:537-555, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau ES, Paniagua SM, Liu E, et al. : Cardiovascular risk factors are associated with future cancer. JACC CardioOncol 3:48-58, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escobar GJ, Gardner MN, Greene JD, et al. : Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care 51:446-453, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Volkova M, Russell R III: Anthracycline cardiotoxicity: Prevalence, pathogenesis and treatment. Curr Cardiol Rev 7:214-220, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seidman A, Hudis C, Pierri MK, et al. : Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 20:1215-1221, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Matthews A, Stanway S, Farmer RE, et al. : Long term adjuvant endocrine therapy and risk of cardiovascular disease in female breast cancer survivors: Systematic review. BMJ 363:k3845, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen LA, Yood MU, Wagner EH, et al. : Performance of claims-based algorithms for identifying heart failure and cardiomyopathy among patients diagnosed with breast cancer. Med Care 52:e30-e38, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Core Team : R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing [Google Scholar]
- 21.Gabani M, Castañeda D, Nguyen QM, et al. : Association of cardiotoxicity with doxorubicin and trastuzumab: A double-edged sword in chemotherapy. Cureus 13:e18194, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shakir DK, Rasul KI: Chemotherapy induced cardiomyopathy: Pathogenesis, monitoring and management. J Clin Med Res 1:8-12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry ML, Niu J, Zhang N, et al. : Cardiotoxicity and cardiac monitoring among chemotherapy-treated breast cancer patients. JACC Cardiovasc Imaging 11:1084-1093, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho H, Lee S, Sim SH, et al. : Cumulative incidence of chemotherapy-induced cardiotoxicity during a 2-year follow-up period in breast cancer patients. Breast Cancer Res Treat 182:333-343, 2020 [DOI] [PubMed] [Google Scholar]
- 25.Drafts BC, Twomley KM, D'Agostino R Jr, et al. : Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging 6:877-885, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu AF, Mukku RB, Verma S, et al. : Cardiac safety of non-anthracycline trastuzumab-based therapy for HER2-positive breast cancer. Breast Cancer Res Treat 166:241-247, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banke A, Fosbøl EL, Ewertz M, et al. : Long-term risk of heart failure in breast cancer patients after adjuvant chemotherapy with or without trastuzumab. JACC Heart Fail 7:217-224, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Goldhar HA, Yan AT, Ko DT, et al. : The temporal risk of heart failure associated with adjuvant trastuzumab in breast cancer patients: A population study. J Natl Cancer Inst 108:djv301, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Jacobse JN, Schaapveld M, Boekel NB, et al. : Risk of heart failure after systemic treatment for early breast cancer: Results of a cohort study. Breast Cancer Res Treat 185:205-214, 2021 [DOI] [PubMed] [Google Scholar]
- 30.Abdel-Qadir H, Thavendiranathan P, Austin PC, et al. : The risk of heart failure and other cardiovascular hospitalizations after early stage breast cancer: A matched cohort study. J Natl Cancer Inst 111:854-862, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hassan SA, Palaskas N, Kim P, et al. : Chemotherapeutic agents and the risk of ischemia and arterial thrombosis. Curr Atheroscler Rep 20:10, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Mosaad M, Elnaem MH, Cheema E, et al. : Cancer-associated thrombosis: A clinical scoping review of the risk assessment models across solid tumours and haematological malignancies. Int J Gen Med 14:3881-3897, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brand JS, Hedayati E, Bhoo-Pathy N, et al. : Time-dependent risk and predictors of venous thromboembolism in breast cancer patients: A population-based cohort study. Cancer 123:468-475, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Khorana AA, Dalal M, Lin J, et al. : Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 119:648-655, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Zahir MN, Shaikh Q, Shabbir-Moosajee M, et al. : Incidence of venous thromboembolism in cancer patients treated with Cisplatin based chemotherapy—A cohort study. BMC Cancer 17:57, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farge D, Frere C, Connors JM, et al. : 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol 20:e566-e581, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Khorana AA, Francis CW, Culakova E, et al. : Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 5:632-634, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Mitchell JD, Cehic DA, Morgia M, et al. : Cardiovascular manifestations from therapeutic radiation: A multidisciplinary expert consensus statement from the International Cardio-Oncology Society. JACC CardioOncol 3:360-380, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng YJ, Nie XY, Ji CC, et al. : Long-term cardiovascular risk after radiotherapy in women with breast cancer. J Am Heart Assoc 6:e005633, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamood R, Hamood H, Merhasin I, et al. : Risk of cardiovascular disease after radiotherapy in survivors of breast cancer: A case-cohort study. J Cardiol 73:280-291, 2019 [DOI] [PubMed] [Google Scholar]
- 41.Sardar P, Kundu A, Chatterjee S, et al. : Long-term cardiovascular mortality after radiotherapy for breast cancer: A systematic review and meta-analysis. Clin Cardiol 40:73-81, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boekel NB, Duane FK, Jacobse JN, et al. : Heart failure after treatment for breast cancer. Eur J Heart Fail 22:366-374, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu X, Chlebowski RT, Shi J, et al. : Aromatase inhibitor and tamoxifen use and the risk of venous thromboembolism in breast cancer survivors. Breast Cancer Res Treat 174:785-794, 2019 [DOI] [PubMed] [Google Scholar]
- 44.Pineda-Moncusí M, Garcia-Giralt N, Diez-Perez A, et al. : Thromboembolic, cardiovascular and overall mortality risks of aromatase inhibitors, compared with tamoxifen treatment: An outpatient-register-based retrospective cohort study. Ther Adv Med Oncol 12:1758835920909660, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi SH, Kim KE, Park Y, et al. : Effects of tamoxifen and aromatase inhibitors on the risk of acute coronary syndrome in elderly breast cancer patients: An analysis of nationwide data. Breast 54:25-30, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdel-Qadir H, Amir E, Fischer HD, et al. : The risk of myocardial infarction with aromatase inhibitors relative to tamoxifen in post-menopausal women with early stage breast cancer. Eur J Cancer 68:11-21, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Kamaraju S, Shi Y, Smith E, et al. : Are aromatase inhibitors associated with higher myocardial infarction risk in breast cancer patients? A Medicare population-based study. Clin Cardiol 42:93-100, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matthews AA, Peacock Hinton S, Stanway S, et al. : Endocrine therapy use and cardiovascular risk in postmenopausal breast cancer survivors. Heart 107:1327-1335, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]