Abstract
Response assessment after immunotherapy remains a major challenge in glioblastoma due to an expected increased incidence of pseudoprogression. Gadolinium-enhanced magnetic resonance imaging (MRI) is the standard for monitoring therapeutic response, however, is markedly limited in characterizing pseudoprogression. Given that hypoxia is an important defining feature of glioblastoma regrowth, we hypothesized that [18F]-fluoromisonidazole (FMISO) positron emission tomography (PET) could provide an additional physiological measure for the diagnosis of immunotherapeutic failure. Six patients with newly diagnosed glioblastoma who had previously received maximal safe resection followed by Stupp protocol CRT concurrent with pembrolizumab immunotherapy were recruited for FMISO PET and Gd-MRI at the time of presumed progression. The hypoxic fraction was defined as the ratio of hypoxic volume to T1-weighted gadolinium-enhancing volume. Four patients diagnosed with pseudoprogression demonstrated a mean hypoxic fraction of 9.8 ± 10%. Two with recurrent tumor demonstrated a mean hypoxic fraction of 131 ± 66%. Our results, supported by histopathology, suggest that the noninvasive assessment of hypoxic fraction by FMISO PET/MRI is clinically feasible and may serve as a biologically specific metric of therapeutic failure.
Keywords: FMISO, glioblastoma, pembrolizumab, pseudoprogression, RANO
The standard of care, maximal safe resection followed by temozolomide (TMZ)-based chemoradiotherapy (CRT), for patients with newly diagnosed glioblastoma often results in neuroinflammatory changes, pseudoprogression (PSP), superimposed upon viable tumor. Pembrolizumab (anti-programmed cell death 1 [PD-1] antibody, Keytruda, Merck) has been FDA-approved for systemic tumors. Early reports in glioblastoma suggest that checkpoint blockade may improve overall survival in specific clinical settings and is expected to result in an increased incidence of PSP.1
Gadolinium-enhanced magnetic resonance imaging (Gd-MRI) is the standard for monitoring therapeutic response.2–7 Gd-MRI is a measure of neurovascular leakiness. As such, it is markedly limited in characterizing therapeutic failure. The differentiation of PSP from recurrent tumor is clinically meaningful as a survival benefit associated with PSP has been reported.8,9
Glioblastoma therapeutic failure is distinct from PSP and is in part biologically defined by hypoxia-mediated microvascular proliferation surrounding pseudopalisading necrosis.10 [18F]-fluoromisonidazole (FMISO) positron emission tomography (PET) imaging noninvasively quantifies tissue oxygen tension.11,12 Given that hypoxia is an important defining feature of glioblastoma regrowth, we hypothesized that FMISO PET would provide an additional physiological measure for the diagnosis of immunotherapeutic failure. Therefore, the aim of this study was to investigate the clinical utility of FMISO PET/MRI in patients treated with CRT combined with concurrent pembrolizumab for newly diagnosed glioblastoma. We report the preliminary findings of 6 patients with subsequent disease progression to demonstrate the feasibility of a novel FMISO PET/MRI-based hypoxic fraction metric to adjudicate treatment outcomes.
Case Presentation
Patient Population and Imaging Techniques
This prospective, institutional review board-approved study included the following inclusion criteria: (i) histologically confirmed diagnosis of glioblastoma (World Health Organization classification grade IV), (ii) documentation of IDH-1 mutational status, (iii) a Karnofsky performance score >50, (iv) maximal safe resection followed by Stupp protocol CRT, (v) concurrent pembrolizumab immunotherapy; 200 mg intravenous every 3 weeks (NCT03347617), and (vi) presumed disease progression based on modified RANO criteria.6 Six patients were recruited for FMISO PET and Gd-MRI at the time of presumed progression (NCT03649880). 3.7 MBq/kg (0.1 mCi/kg; up to 370 MBq or 10 mCi; OHSU Center for Radiochemistry Research, Portland, OR, USA) of FMISO was administered intravenously 90 minutes prior to PET imaging of the brain. FMISO PET and Gd-MRI were assessed using MIM V7.0.6 (MIM Software Inc., Cleveland, OH, USA). The hypoxic volume (HV) was defined as 1.2× of mean left cerebellum uptake within the T2-hyperintense lesion. Standard uptake values were measured as the mean (SUVmean) or maximum (SUVmax) voxel-wise values from the enhancing T2-hyperintense lesion. Standard uptake value ratios (SUVRmean or SUVRmax) were defined as the enhancing T2-hyperintense lesion SUV divided by the left cerebellum SUV. The hypoxic fraction was defined as the HV divided by the enhancing volume. All patients were either diagnosed as disease recurrence or PSP using surgical tissue sampling or according to modified RANO criteria using follow-up Gd-MRI.6 In the absence of tissue sampling, >25% increase in the lesion-enhancing sum product diameter defined recurrent tumor disease status. Table 1 summarizes the FMISO PET/Gd-MRI features of the cohort.
Table 1.
Cohort Clinical and Imaging Demographics
| Patient # | Age/Sex | Initial Diagnosis | Pembrolizumab Duration (Wks) | SUVmean | SUVmax | SUVR | HV (mL) | Enhancing Volume (mL) | HF (%) | Clinical Outcome at the Time of FMISO PET | Progression-Free Survival (Days) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39/M | IDH wildtype, MGMT unmethylated | 58 | 1.34 | 1.54 | 0.96 | 0 | 0.7 | 0 | PSP at day 450 based on mRANO | 574 days based on biopsy at the time of subsequent progression |
| 2 | 52/W | IDH mutated, MGMT methylated | 148 | 1.74 | 2.31 | 0.99 | 0.21 | 4.0 | 5 | PSP at day 973 based on mRANO | 1095 based on mRANO with subsequent progression |
| 3 | 73/M | IDH wildtype, MGMT methylated | 51 | 2.41 | 3.94 | 1.12 | 1.44 | 14.2 | 10 | PSP at day 393 based on biopsy | 429 days as a result of perioperative death |
| 4 | 59/M | IDH wildtype, MGMT unmethylated | 11 | 1.80 | 3.70 | 1.14 | 1.47 | 6.07 | 24 | PSP at day 92 based on biopsy | 231 days based on mRANO with subsequent progression |
| 5 | 73/M | IDH wildtype, MGMT methylated | 41 | 2.14 | 4.38 | 1.46 | 12.80 | 7.2 | 178 | Recurrent tumor at day 278 based on biopsy | 292 days based on biopsy |
| 6 | 43/M | IDH wildtype, MGMT methylated | 40 | 1.72 | 2.84 | 1.15 | 1.38 | 1.62 | 85 | Recurrent tumor at day 346 based on mRANO | 399 based on mRANO |
Wks, weeks; IDH, isocitrate dehydrogenase; MGMT, O-6-Methylguanine-DNA methyltransferase; SUVmean/max, mean or maximum standard uptake value within the region of interest; SUVR, standard uptake value ratio; HV, hypoxic volume is defined by values >1.2× the normal cerebellum; enhancing volume, the volume of gadolinium-induced T1 shortening; HF, hypoxic fraction—the ratio of hypoxic volume to T1-weighted gadolinium-enhancing volume. Clinical outcome at the time of presumed progression FMISO PET examination defined by surgical tissue sampling or modified RANO (mRANO) criteria. PSP, pseudoprogression. Progression-free survival, time from surgical resection date to date of clinical outcome; recurrent tumor.
Pseudoprogression Case Presentation
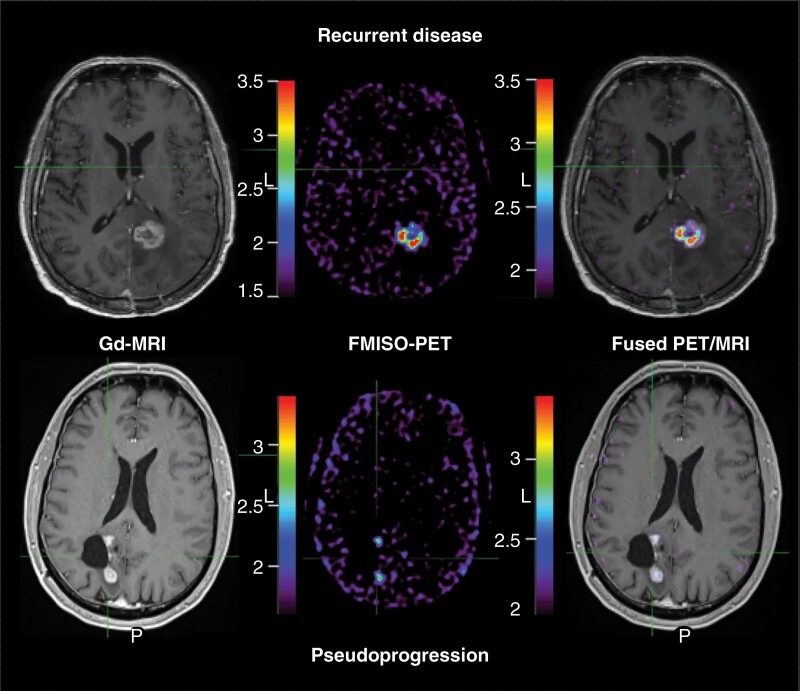
Four patients were diagnosed with PSP; two through histological tissue assessment. Minimal FMISO uptake was observed within the enhancing lesion (SUVmean 1.83 ± 0.44, SUVmax 2.87 ± 1.14, SUVR 1.05 ± 0.09; Figure 1). The HV was 0.78 ± 0.78 mL and the hypoxic fraction was 9.8 ± 10%.
Figure 1.
FMISO PET MRI at the time of glioblastoma presumed disease progression. Gadolinium-enhanced MRI (Gd-MRI; left) at the time of presumed disease progression in patients treated with Stupp protocol with concurrent pembrolizumab demonstrates similar appearing contrast-enhancing mass in patients with both recurrent tumor growth (top) and neuroinflammatory-based therapeutic changes; pseudoprogression (PSP, below). Unlike recurrent disease, patients with PSP demonstrated minimal hypoxic volume (middle column) and hypoxic fraction (right column; ratio of hypoxic volume to gadolinium-enhancing volume). In this example, the patient with recurrent disease demonstrated a hypoxic disease burden of 178%. Conversely, the example of PSP demonstrated a hypoxic fraction of 24%. Note: FMISO PET image (middle) window minimum is the mean cerebellar background and window maximum is 2× the mean cerebellar background. Fused PET/MRI image (right) window is the hypoxic volume of 1.2× the mean cerebellar background and window maximum is 2× the hypoxic volume SUV. PET intensity color scales represent the respective visualized SUV measures. Abbreviations: FMISO, [18F]-fluoromisonidazole; MRI, magnetic resonance imaging; PET, positron emission tomography; SUV, standard uptake values.
Recurrent Tumor Case Presentation
Two patients were diagnosed with recurrent tumor; one through image-guided tissue sampling. Marked FMISO uptake was observed within and about the enhancing lesion (SUVmean 1.93 ± 0.3, SUVmax 3.61 ± 1.09, SUVR 1.31 ± 0.22; Figure 1). The HV was 7.1 ± 8.08 mL and the hypoxic fraction was 131 ± 66%.
Discussion
We investigated the clinical utility of FMISO PET/MRI in defining glioblastoma therapeutic failure in 6 patients treated with CRT combined with concurrent pembrolizumab at the time of presumed disease progression. Our preliminary findings demonstrate the feasibility of FMISO PET/MRI-based hypoxic fraction as a noninvasive metric to adjudicate treatment outcomes. The presence of minimal hypoxic fraction supports the hypothesis that PSP is a reflection of therapeutically induced neuroinflammation which can be biologically differentiated from recurrent glioblastoma. Taken together, the FMISO PET/MRI hypoxic fraction technique presented here advances neuro-oncologic research and practice by presenting a new, biologically specific metric of therapeutic failure, which may eventually improve upon the response assessment of patients with glioblastoma.
We assert that the noninvasive quantification of post-therapeutic hypoxic fraction is a feasible imaging approach for defining glioblastoma therapeutic efficacy. Hypoxia is a disease driving malignant biological feature of glioblastoma. FMISO PET HV has been explored as a response assessment metric of antiangiogenic therapy.13–16 However, HV may fail to account for overall size of malignant biology observed with tumor recurrence. As such, its utility within the heterogeneous glioblastoma microenvironment may be misleading with respect to the clinical significance. A small HV within a large volume of therapeutically treated tumor may have a very different clinical significance when compared with a case of small volume of therapeutically treated tumor. Therefore, we propose the use of FMISO PET/MRI-derived hypoxic fraction, as a metric of both hypoxic and lesion volume, may improve upon the clinical use of HV alone in assessing immunotherapeutic efficacy. An alternative hypothesis may be that a large hypoxic fraction prior to or at the completion of radiation therapy may result in early disease progression. We are actively addressing the mechanism of hypoxic fraction in our prospective study through longitudinal FMISO PET/MRI.
The use of biologically specific imaging metrics for assessing glioblastoma therapeutic response is likely to provide improved noninvasive diagnostic capabilities. Unfortunately, contrast-enhanced MRI response criteria lack specificity in characterizing therapeutic failure because local regional tumor regrowth cannot be prospectively distinguished from therapy-mediated neuroinflammation. Prior investigators have attempted to improve diagnostic criteria by assessing neoangiogenic features. Dynamic susceptibility weighed cerebral blood volume (CBV) is a widely used physiologic MRI metric of glioblastoma response assessment. However, this methodology fails to account for CRT-induced vasodilatory effects observed with PSP. Several recent meta-analyses provide evidence that the use of CBV has a pooled sensitivity and specificity that are both ~80%.17–19 Radiolabeled amino acid-based PET molecular imaging has also been explored for the assessment of brain tumor response. This approach relies upon the intracellular accumulation of [11C]-methionine (MET), 6-[18F]-l-fluoro-l-3,4-dihydroxyphenylalanine (FDOPA), or O-(2-[18F]-fluoroethyl)-l-tyrosine (FET) through l-type amino acid transporter (LAT). Upregulation of LAT1 has been correlated with glioma cellular proliferative index.20 The diagnostic utility of amino acid PET imaging is still being prospectively adjudicated, but preliminary studies suggest a diagnostic accuracy of FET PET of ~85% for differentiating glioblastoma PSP from true progression.21 While these imaging capabilities improve upon the use of T1 enhancement, there remains room for improved diagnostic utility. The clinical utilization of FMISO PET/MRI-derived hypoxic fraction may improve upon current imaging metrics by directly assessing biological features of glioblastoma therapeutic failure.
The small sample size of our study precludes any interpretation beyond the demonstration of preliminary clinical feasibility. We continue to prospectively enroll patients to determine the diagnostic capability of FMISO PET/MRI to differentiate glioblastoma recurrence from PSP.
Conclusion
In a cohort of 6 patients with newly diagnosed glioblastoma treated with CRT combined with concurrent pembrolizumab, we demonstrate the clinical feasibility of FMISO PET/MRI to distinguish recurrent tumor from PSP at the time of presumed disease progression. Our results, supported by histopathology, suggest that the noninvasive assessment of hypoxic fraction may serve as a biologically specific metric of therapeutic failure.
Acknowledgments
R.F.B. thanks Bethany Barajas, RN, MSN, CNL and Jana Ivanidze MD, PhD for their helpful comments regarding this article; the many clinical collaborators, including Adam Brown, Lauren Drake, and Libby Mirande, for their helpfulness; and the many wonderful patients included in this study who selflessly contributed their time to undergo research medical imaging while confronting a deadly disease. R.F.B. dedicates this research to the memory of Rachel Dawn Gabani.
Funding
This research was funded by the National Institutes of Health/National Cancer Institute [1K08CA237809-01 and 2L30CA220897-02A1].
Conflict of interest statement. R.F.B., P.A., J.L., K.A.K., A.R., N.M., and E.A.N.: None declared.
References
- 1. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 3. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 4. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 5. Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology (iRANO): a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017;14(2): 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wen PY, Chang SM, Van den Bent MJ, et al. Response assessment in neuro-oncology clinical trials. J Clin Oncol. 2017;35(21):2439–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gahramanov S, Varallyay C, Tyson RM, et al. Diagnosis of pseudoprogression using MRI perfusion in patients with glioblastoma multiforme may predict improved survival. CNS Oncol. 2014;3(6):389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barajas RF Jr, Hamilton BE, Schwartz D, et al. Combined iron oxide nanoparticle ferumoxytol and gadolinium contrast enhanced MRI defines glioblastoma pseudo-progression. Neuro Oncol. 2019;21(4):517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rong Y, Durden DL, Van Meir EG, Brat DJ. ‘Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol. 2006;65(6):529–539. [DOI] [PubMed] [Google Scholar]
- 11. Jerabek PA, Patrick TB, Kilbourn MR, Dischino DD, Welch MJ. Synthesis and biodistribution of 18F-labeled fluoronitroimidazoles: potential in vivo markers of hypoxic tissue. Int J Rad Appl Instrum A. 1986;37:599–605. [DOI] [PubMed] [Google Scholar]
- 12. Rasey JS, Nelson NJ, Chin L, Evans ML, Grunbaum Z. Characteristics of the binding of labeled fluoromisonidazole in cells in vitro. Radiat Res. 1990;122:301–308. [PubMed] [Google Scholar]
- 13. Ratai EM, Zhang Z, Fink J, et al. ACRIN 6684: multicenter, phase II assessment of tumor hypoxia in newly diagnosed glioblastoma using magnetic resonance spectroscopy. PLoS One. 2018;13(6):e0198548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang S, Michalek JE, Reardon DA, et al. Assessment of tumor hypoxia and perfusion in recurrent glioblastoma following bevacizumab failure using MRI and 18F-FMISO PET. Sci Rep. 2021;11(1):7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barajas RF, Krohn KA, Link JM, et al. Glioma FMISO PET/MR imaging concurrent with antiangiogenic therapy: molecular imaging as a clinical tool in the burgeoning era of personalized medicine. Biomedicines. 2016;4(4):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muzi M, Wolsztynski E, Fink JR, et al. Assessment of the prognostic value of radiomic features in 18F-FMISO PET imaging of hypoxia in postsurgery brain cancer patients: secondary analysis of imaging data from a single-center study and the multicenter ACRIN 6684 trial. Tomography. 2020;6(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Dijken BRJ, van Laar PJ, Holtman GA, van der Hoorn A. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol. 2017;27(10):4129–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsakiris C, Siempis T, Alexiou GA, et al. Differentiation between true tumor progression of glioblastoma and pseudoprogression using diffusion-weighted imaging and perfusion-weighted imaging: systematic review and meta-analysis. World Neurosurg. 2020;144:e100–e109. [DOI] [PubMed] [Google Scholar]
- 19. Suh CH, Kim HS, Jung SC, et al. Multiparametric MRI as a potential surrogate endpoint for decision-making in early treatment response following concurrent chemoradiotherapy in patients with newly diagnosed glioblastoma: a systematic review and meta-analysis. Eur Radiol. 2018;28(6):2628–2638. [DOI] [PubMed] [Google Scholar]
- 20. Haining Z, Kawai N, Miyake K, et al. Relation of LAT1/4F2hc expression with pathological grade, proliferation and angiogenesis in human gliomas. BMC Clin Pathol. 2012;12:4. doi: 10.1186/1472-6890-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galldiks N, Law I, Pope WB, Arbizu J, Langen KJ. The use of amino acid PET and conventional MRI for monitoring of brain tumor therapy. Neuroimage Clin. 2016;13:386–394. doi: 10.1016/j.nicl.2016.12.020. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]