Abstract
Background and Aims
The centropogonid clade (Lobelioideae: Campanulaceae) is an Andean-centred rapid radiation characterized by repeated convergent evolution of morphological traits, including fruit type and pollination syndromes. While previous studies have resolved relationships of lineages with fleshy fruits into subclades, relationships among capsular species remain unresolved. This lack of resolution has impeded reclassification of non-monophyletic genera, whose current taxonomy relies heavily on traits that have undergone convergent evolution.
Methods
Targeted sequence capture using a probe-set recently developed for the centropogonid clade was used to obtain phylogenomic data from DNA extracted from both silica-dried and herbarium leaf tissue. These data were used to infer relationships among species using concatenated and partitioned species tree methods, and to quantify gene tree discordance.
Key Results
While silica-dried leaf tissue resulted in longer assembled sequence data, the inclusion of herbarium samples improved taxonomic representation. Relationships among baccate lineages are similar to those inferred in previous studies, although they differ for lineages within and among capsular clades. We improve the phylogenetic resolution of Siphocampylus, which forms ten groups of closely related species which we informally name. Two subclades of Siphocampylus and two individual species are rogue taxa whose placement differs widely across analyses. Gene tree discordance (including cytonuclear discordance) is rampant.
Conclusions
This first phylogenomic study of the centropogonid clade considerably improves our understanding of relationships in this rapid radiation. Differences across analyses and the possibility of additional lineage discoveries still hamper a solid and stable reclassification. Rapid morphological innovation corresponds with a high degree of phylogenomic complexity, including cytonuclear discordance, nuclear gene tree conflict and well-supported differences between analyses based on different nuclear loci. Together, these results point to a potential role of hemiplasy underlying repeated convergent evolution. This hallmark of rapid radiations is probably present in many other species-rich Andean plant radiations.
Keywords: Burmeistera, Campanulaceae, Centropogon, convergent evolution, cytonuclear discordance, gene tree conflict, Lobelioideae, museomics, Neotropics, phylogenomics, rapid radiation, Siphocampylus, taxonomy
INTRODUCTION
The centropogonid clade of Neotropical bellflowers (Campanulaceae: Lobelioideae) is a species-rich, phenotypically diverse lineage of ~550 species endemic to the Neotropics, with highest species richness in the Andean cordilleras of western South America. This clade comprises the genera Centropogon and Burmeistera, and mainland (i.e. non-Caribbean) species of Siphocampylus. Their high species richness and young age, with all species tracing back ~5 million years, make the centropogonids one of the fastest plant radiations documented to date (Lagomarsino et al., 2016).
The centropogonid clade is morphologically diverse. As in other rapid Andean radiations, including the iconic lupines (Hughes and Eastwood, 2006), growth forms in the clade are variable, including vines with woody bases, herbs, xerophytes, shrubs, and trees. Fruits are varied; ~40 % of the species produce dry capsules, while ~60 % of species produce fleshy berries, which have evolved multiple times independently (Lagomarsino et al., 2014). However, floral morphology is arguably the most impressive aspect of the clade’s phenotypic diversity: their tubular flowers range in length from less than 1 cm to more than 10 cm, with petals in nearly all colours of the rainbow, including white, green, yellow, red and orange. These floral forms have evolved under the selective pressure of two principal classes of pollinators: hummingbirds and bats. From an ancestral hummingbird pollination syndrome, bat pollination has evolved ~13 times independently (Lagomarsino et al., 2017). Pollination biology within the clade is well studied, including in the predominantly bat-pollinated genus Burmeistera (Muchhala, 2003, 2006a; Muchhala and Potts, 2007) and Centropogon nigricans, a species whose very long flowers are a product of a coevolutionary arms race with Anoura fistulata, a bat whose ribcage is modified to store its tongue, the longest among mammals relative to body size (Muchhala, 2006b). Many other species are adapted to hummingbird pollination, including the eucentropogonid clade of curved flowers that are largely pollinated by the specialist sicklebill hummingbird (Stein, 1992; Boehm et al., 2018).
The incredibly variable morphology in the centropogonid clade has been a challenge to taxonomists. Fruit type delimits genera, with Burmeistera and Centropogon producing fleshy berries and Siphocampylus producing dry capsules. In addition to their fleshy fruit, Burmeistera is further defined by its ebracteolate pedicels, isodiametric seeds and generally inflated corolla (Lammers, 1998). Subgeneric taxonomy predominantly relies on floral morphology (Wimmer, 1943, 1955), often with traits directly associated with pollination syndromes. Previous phylogenetic studies, which relied on a small number of plastid loci and the nuclear ribosomal DNA (nrDNA) internal transcribed spacer (ITS), have shown that the current classification of the centropogonid genera is non-monophyletic at both the generic and subgeneric levels (Antonelli, 2008; Knox et al., 2008; Lagomarsino et al., 2014), though support for the monophyly of Burmeistera within the centropogonid clade is strong (Bagley et al., 2020). The rampant non-monophyly is not surprising given the current classification scheme’s heavy reliance on traits that have undergone convergent evolution in response to selection from biotic or abiotic selective pressures. Despite the non-monophyly of taxa, there is strong support for five baccate (i.e. berry-producing) lineages (Lagomarsino et al., 2014), each with its own set of morphological tendencies and which correspond closely (though not exactly) to taxa previously proposed (McVaugh, 1965). However, resolution among capsular lineages (i.e. Siphocampylus) remains poor.
This poor resolution is probably due to the fact that the centropogonid clade is a rapid, recent radiation. Analyses that draw from loci from across the genome and model incomplete lineage sorting are likely to be better models of its evolutionary history than the concatenation analyses of plastid loci of previous analyses. Fortunately, the last decade has seen an increase in genome-scale datasets in diverse subfields of biology as high-throughput sequencing has become cheaper and more efficient (McKain et al., 2018). This has resulted in a profound change in the methodology of phylogenetics (Edwards, 2009; Bravo et al., 2019; Rothfels, 2021). As genomic-scale datasets become increasingly easy to generate, the number of loci and taxa sampled in individual phylogenetic studies is increasing. This has allowed the development and application of evolutionary models that incorporate information from the distinct evolutionary histories represented in different genes. Methods are now available that model incomplete lineage sorting (Vachaspati and Warnow, 2015; Zhang et al., 2017; Ogilvie et al., 2017), polyploidy (Jones et al., 2013) and introgression (Than et al., 2008; Solís-Lemus et al., 2017) into phylogenetic inference. In addition to taking advantage of the signal of diverse genes from the nuclear genome, deeper taxon sampling is now feasible due to the dual effect of decreasing costs of DNA sequencing and the development of methods that effectively isolate putatively homologous sequence regions from diverse taxa (McKain et al., 2018). It is uncontroversial to say that we are in the midst of a phylogenetic revolution.
This data revolution has led to an increased importance of genetic data from historical specimens (Lendemer et al., 2020). While the degraded DNA of their specimens was a major impediment to their inclusion in molecular phylogenies in the era of Sanger sequencing, natural history collections are now a major source of molecular phylogenetic data. Specimens from natural history collections, including herbaria, are commonly used in phylogenomic analyses (Bakker, 2017; Brewer et al., 2019; Alsos et al., 2020; Kates et al., 2021). Many of these studies use hybrid-enriched targeted sequence capture, a cost-effective method that is robust to low-quality, low-quantity DNA due to the high specificity of the RNA probes used to isolate targeted genomic regions (Faircloth et al., 2012; Lemmon et al., 2012; Weitemier et al., 2014)
Here we highlight the utility of sequence capture in incorporating historical specimens in the phylogenomic analysis of the centropogonid clade of Neotropical bellflowers. Using targeted sequence capture of loci recently developed for its subclade Burmeistera (Bagley et al., 2020), we infer the most data-rich phylogeny of the entire centropogonid clade to date, inferred with both species tree and concatenation methods. Despite a high degree of gene tree discordance and differences across separate analyses, we confirm the monophyly of previously named berry-producing lineages, and further identify allied groups of Siphocampylus species. We end with a discussion of the potential sources of gene tree discordance in the centropogonid clade, its implications for morphological evolution and the continued difficulty of resolving phylogenies of rapid radiations, even in the face of large phylogenomic datasets.
MATERIALS AND METHODS
Taxon sampling
We sampled 152 species from five genera of Lobelioideae; 108 of these were from field-collected tissue and 44 from herbarium specimens. Taxon sampling was densest in Burmeistera (74 spp., or 49 % of the sampling effort). A full taxon list and voucher information can be found in Supplementary Data Appendix S1.
There were two goals in sampling design: to have dense phylogenetic sampling within Burmeistera for species-level phylogenetics (Bagley et al., 2020), and broad representative sampling for Centropogon and Siphocampylus to resolve the phylogeny of the clade as a whole and facilitate future reclassification efforts. The broad-scale taxon sampling used the phylogeny of Lagomarsino et al. (2014) as a reference from which to include a full set of known subclades within the centropogonids. Species that represented the phylogenetic breadth of the centropogonid clade and its closest relatives were sampled from both field-collected tissue and herbarium collections. Field-collected tissues primarily came from previous work by A.A., L.P.L. and N.M. The herbarium at the Missouri Botanical Garden (MO; code following Index Herbariorum) was the primary source of herbarium tissue. This herbarium was most appropriate for this study due to its extensive collection of Neotropical specimens, including collections made by a taxonomic expert in Centropogon, Dr Bruce A. Stein, from the mid- to late 1980s. These specimens are both expertly identified and of an appropriate age to make effective DNA extraction likely. Across our sampled herbarium specimens, the range of ages at time of extraction was 4–77 years, with a mean age of 15.3 years. Multiple species that have never before been sampled in a phylogenetic analysis were included, especially from the polyphyletic genus Siphocampylus.
Molecular lab work and sequence generation
DNA extraction followed a modified CTAB protocol (Doyle and Doyle, 1987). Approximately 100 mg of leaf tissue was used in these extractions for both herbarium specimens and field-collected tissue; due to tissue availability, a smaller amount of leaf tissue was used for a limited number of herbarium samples. No special treatment was given to tissue from herbarium specimens relative to silica-dried tissue. DNA concentration was assessed using a Qubit fluorometer, followed by agarose gel electrophoresis to determine whether high-molecular-weight DNA was present.
Sequence capture targeted a set of 745 nuclear loci that were developed from transcriptomes for phylogenetic utility within Burmeistera, a subclade of Neotropical bellflowers (Bagley et al., 2020). This probe set consists of 9998 120-bp probes at 2× tile density, resulting in a capture footprint of 599 880 bp (see Bagley et al., 2020 for additional details). Illumina library preparation, target enrichment sequence capture and sequencing were outsourced to RAPiD Genomics LLC (Gainesville, FL, USA). Degraded DNA, as inferred via gel imaging and common in our herbarium samples, was not sheared during library preparation. Sequencing was performed on an Illumina HiSeq 3000 (Illumina Inc., San Diego, CA, USA), generating 150-bp paired-end reads with a minimum sequencing depth of 20×.
Data processing, assembly and extraction into targeted loci
The program phyluce v1.5 (Faircloth, 2016) was used to process raw data into gene trees. This pipeline differs from other common sequence capture assemblers used in plants (e.g. HybPiper; Johnson et al., 2016) in that contigs are first assembled de novo and then mapped onto the target sequences. We first used illumiprocessor v2.0.8, a Trimmomatic wrapper built into phyluce (Faircloth, 2013; Bolger et al., 2014), to trim adapter contamination and low-quality bases from raw reads under default settings. We then assembled the clean reads into contigs within phyluce using the default settings of Trinity v2.6.6 (Grabherr et al., 2011). The newly assembled contigs were then matched to probes with 80 % minimum coverage and 80 % minimum identity required for a match.
Individual loci extracted with phyluce were aligned and edge-trimmed with MAFFT v7.130b (Katoh and Standley, 2013) under default settings. Gblocks v0.91b (Castresana, 2000) was used to internally trim poorly aligned regions under default settings.
To compare the assembly of DNA from herbarium specimens and silica-collected tissue, we calculated basic summary statistics of the phyluce assemblies between these two tissue types [e.g. average number of assembled base pairs, number of assembled contigs, average contig length and number of long loci (e.g. >1 kb)].
Gene tree inference and curation
Individual gene trees were inferred in RAxML v8.2.11 using rapid bootstrapping with a GTR model of molecular evolution and 78–130 bootstrap replicates. After exploratory species tree analyses, we filtered our data to improve phylogenetic inference (Molloy and Warnow, 2018). Using contig length as a proxy, we first filtered out taxa with low-copy data assemblies (i.e. all contigs <1000 bp). These 13 taxa (from an initial 152) were primarily from DNA extracted from herbarium specimens with low quantity of input DNA (Supplementary Data Appendix S2). Second, we filtered individual loci: we removed loci that had <75 % representation of the remaining 139 taxa, as well as loci that were shorter than 500 bp. These filtering steps left 96 loci for 139 taxa, which were used in all downstream analyses.
Phylogenomic analysis
To infer phylogenetic relationships, we performed a concatenated analysis on a combined, partitioned alignment of loci in RAxML using rapid bootstrapping. PartitionFinder2 (Lanfear et al., 2017) was used to determine the optimal partitioning scheme of loci. Subsequently, three different methods were then used to estimate coalescent-based species trees from: ASTRAL-III v5.6.2 (Zhang et al., 2017), ASTRID v1.4 (Vachaspati and Warnow, 2015) using the BioNJ method, and SVDQuartets implemented in PAUP v4.0a162 (Swofford, 2001; Chifman and Kubatko, 2014). ASTRAL-III and ASTRID used the individual gene trees as input; a concatenated alignment of all filtered loci was used to estimate the species tree with 100 bootstrap replicates in SVDQuartets.
We assessed gene tree discordance by mapping rooted gene trees to the RAxML topology using PhyParts (Smith et al., 2015) and subsequently visualized results using PhyPartsPieCharts (https://github.com/mossmatters/phyloscripts/tree/master/phypartspiecharts).
Comparison with previous analyses
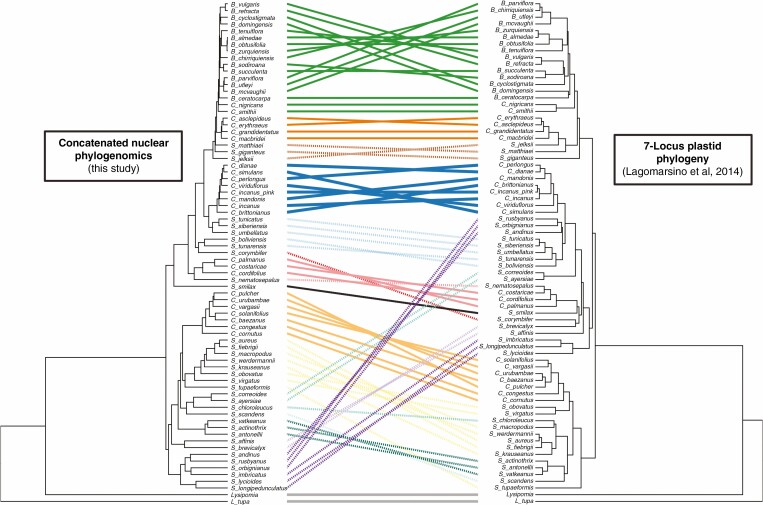
To explore differences with previous studies, we constructed a tanglegram of relationships among species included in both our RAxML concatenated phylogeny and the plastid phylogeny from Lagomarsino et al. (2014), using the tanglegram function of the dendextend R package (Galili, 2015).
RESULTS
Taxon sampling and target capture success
On average, ~6.3 million reads per sample (range: 1.8 million to 15.6 million reads) were generated via Illumina sequencing. Upon processing, sequence data for targeted loci were obtained for all included taxa, though the quantity was variable, ranging from only 6605 bp of assembled sequence in Burmeistera breviflora to 502 363 bp in Lysipomia pumila. The number of contigs recovered ranged from 28 to 669, with an average of 343. Median length of these ranged from 222 to 933 bp (with an average median length of 646 bp). It is possible that less-conservative data assembly pipelines (e.g. HybPiper; Johnson et al., 2016) would result in more or longer contigs (Herrando-Moraira et al., 2018), though we considered phyluce’s conservative nature in handling paralogues desirable for this rapid radiation (Siniscalchi et al., 2021). Our final dataset included 139 accessions, representing 68 species of Burmeistera, 26 species of Centropogon, 42 species of Siphocampylus, Lysipomia pumila and Lobelia tupa. A complete list of target capture summary statistics can be found in Supplementary Data Appendix S2.
Performance of herbarium vs. silica-dried tissue
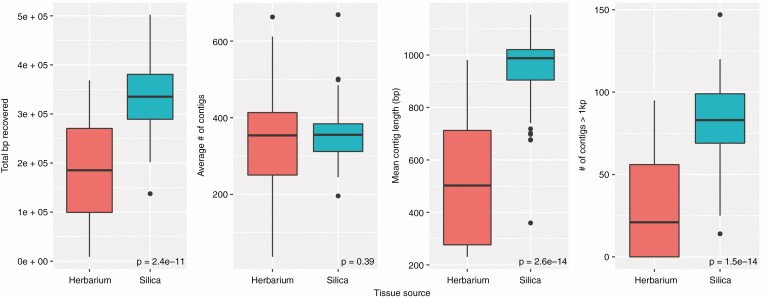
Across all of our samples, silica-dried tissue outperformed herbarium tissue in most summary statistics: the mean total assembled base pairs for silica tissue was ~332 kbp (vs. 181 kbp for herbarium specimens; t-test P < 0.0001); the mean average contig length was 951 bp for silica-dried tissue (vs. 525 bp; t-test P < 0.0001); and the mean number of contigs greater than 1 kbp was 83 in silica-dried tissue (vs. 30 from herbarium specimens; t-test P < 0.0001) (Fig. 1). However, herbarium and silica-dried tissue did not statistically significantly differ in the total number of contigs recovered (331 vs. 351 contigs; t-test P = 0.39).
Fig. 1.
Comparison of summary statistics for targeted locus assembly for herbarium (red) vs. silica-collected (blue) leaf tissue.
Based on preliminary analyses of our data, we considered any taxon lacking any contigs >1000 bp as insufficient quality for phylogenomic analysis. In preliminary phylogenetic analyses, these samples were attracted to the root of the phylogeny, forming grades successively sister to larger clades, a known artefact in phylogenomic analyses (Hosner et al., 2016). With this criterion, all field-collected specimens had enough data of sufficient quality to be included in phylogenetic analysis, while only 31 herbarium-collected specimens (representing 70.5 % of the total) could be included. Of those that failed, a majority (11/13) had <250 µg of input DNA. However, one sample that failed had >1300 µg of input DNA (i.e. C. tessmannii), and at the other extreme, our sample with the smallest amount of input DNA (i.e. B. curviandra) had efficient enough capture to be included.
Broad phylogenetic patterns
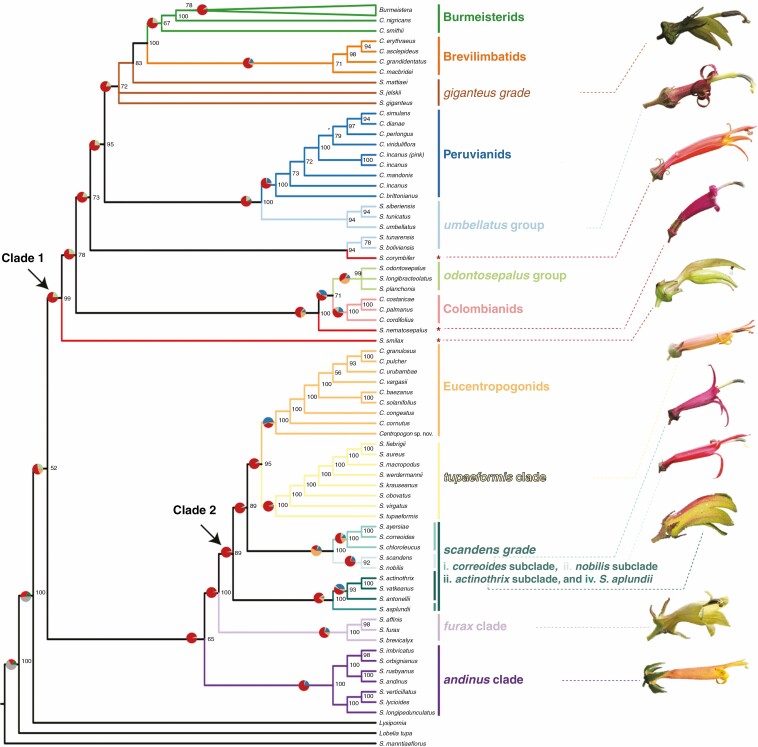
Building on recent results within Burmeistera (Bagley et al., 2020), we expand upon previous understanding of relationships within the broad centropogonid clade relative to previous analyses, the majority of which have relied on primarily or entirely plastid sequences (Knox et al., 2008; Antonelli, 2009; Lagomarsino et al., 2014; Bagley et al., 2020). This is the first nuclear phylogenomic study that targets the entire centropogonid clade. Phylogenetic relationships of Centropogon and Siphocampylus species are presented in Figs 2 and 3, while phylogenies that include Burmeistera are depicted in Supplementary Data Figs S1–S4. PhyParts results revealed a high degree of gene tree discordance in our dataset: most gene trees had conflicts other than the most common gene tree–species conflict at most nodes (red portion in the pie charts in Fig. 2; Fig. S5). A review of individual gene trees suggests that there are many conflicting phylogenies, not a small number of conflicts that are repeated throughout our dataset.
Fig. 2.
Concatenated RAxML phylogeny with informal names of closely allied species presented at the right, with the branches of the phylogeny corresponding to their members colour-coded accordingly. Names in bold text are from Lagomarsino et al. (2014) and correspond to the baccate lineages (i.e. species currently classified in Burmeistera and Centropogon). Names in italics correspond to capsular lineages (i.e. species currently classified in Siphocampylus). Individual rogue species are indicated by red asterisks. Numbers at nodes represent bootstrap support values. Pie charts above select branches illustrate gene tree discordance as estimated in PhyParts using all gene trees along the RAxML phylogeny and visualized using PhyPartsPieCharts; colours correspond to the proportion of gene trees that fall into different categories of concordance (blue: concordant genes; green: most common conflicting bipartition; red: other conflicting bipartitions; orange: gene trees with no information). Photos of living flowers from exemplar species of the Siphocampylus lineages are presented at the right. From top to bottom, these are: S. matthaei, S. tunarensis, S. corymbifer, S. nematosepalus, S. smilax, S. tupaeformis, S. correoides, S. scandens, S. actinothrix, S. affinis and S. andinus.
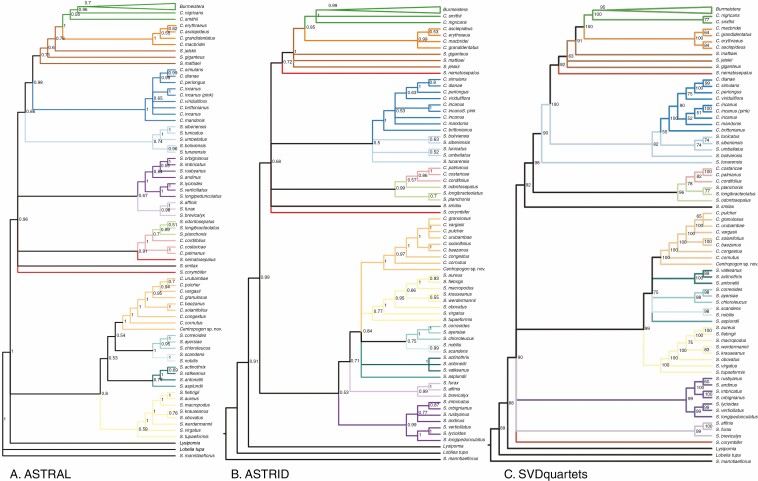
Fig. 3.
Results of species tree analyses, with consistently inferred species groups colour-coded as in Fig. 2. (A) ASTRAL-III analysis with local posterior probability values at nodes; (B) ASTRID analysis with bootstrap values at nodes; (C) SVDquartets phylogeny with bootstrap values at nodes.
Despite this, our results significantly advance our understanding of the relationships within and between subclades. At a broad scale, we identify two major clades of centropogonids that are supported in all our analyses: one that includes the burmeisterid, brevilimbatid, peruvianid and colombianid Centropogon species in addition to various lineages of Siphocampylus; and a second that includes the eucentropogonid clade and various lineages of Siphocampylus. In addition, two subclades and three individual species of Siphocampylus have inconsistent placement across our analyses.
The last comprehensive phylogenetic analysis of all three centropogonid genera (Lagomarsino et al., 2014) resolved the relationships among the berry-producing lineages (i.e. those taxa currently classified as Centropogon and Burmeistera) into five well-supported subclades (i.e. the burmeisterids, brevilimbatids, peruvianids, colombianids and eucentropogonids) using plastid sequences. We find strong support for the monophyly of four of those subclades (i.e. the burmeisterids, brevilimbatids, peruvianids, and eucentropogonids) in all of our analyses (Fig. 4). The Centropogon species of the remaining named subclade, the colombianids, are closely related in most of our analyses (i.e. monophyletic in ASTRID, SVDquartets, RAxML; Figs 2 and 3B, C and paraphyletic in ASTRAL; Fig. 3A); however, the unstable placement of S. nematosepalus, currently considered to belong to this clade, renders it non-monophyletic in every analysis. Siphocampylus nematosepalus is an outlier within the colombianid clade for its capsular fruit, which is hypothesized to represent a reversal from baccate ancestors (Lagomarsino et al., 2014).
Fig. 4.
Tanglegram illustrating the conflicts between the RAxML concatenated nuclear phylogenomic topology (left) and the seven-locus plastid phylogeny of Lagomarsino et al. (2014; right). Lines are colour-coded according to the scheme in Fig. 2, with baccate species represented by unbroken lines and capsular species represented by dashed lines.
While we largely confirm phylogenetic relationships within berry-producing taxa (i.e. Burmeistera and Centropogon), as outlined above, our results provide novel insights into the relationships of capsular taxa (i.e. ‘Siphocampylus’), which were largely unresolved in Lagomarsino et al. (2014). As in previous studies, we find strong support for the polyphyly of species currently classified in Siphocampylus, and we identify ten groups of Siphocampylus species that are consistently resolved as closely related. We have placed new informal names on these Siphocampylus lineages (Fig. 2), and include a description of them below in the context of the broader clades in which they occur.
Clade 1.
The first major clade includes at least nine distinct lineages (i.e. the burmeisterid, brevilimbatid, peruvianid and colombianid Centropogon subclades, and the giganteus, umbellatus and odontosepalus groups of Siphocampylus species, as well as S. nematosepalus and S. smilax). Two additional lineages of Siphocampylus (i.e. the furax and andinus clades) are inferred to belong in Clade 1 in the ASTRAL analysis only, and S. corymbifer is inferred to belong to it in all analyses except SVDquartets (Figs 2 and 3). While the membership of this clade differs across analyses based on the placement of these taxa, it is well supported in most analyses (ASTRAL/ASTRID/SVDQuartets/RAxML support: 0.96/0.68/92/99).
The burmeisterid subclade, which comprises Burmeistera and various bat-pollinated Centropogon species that share aspects of their gross floral morphology with Burmeistera (represented by C. nigricans and C. smithii here), is monophyletic in all analyses (0.55/0.85/100/67). Within the burmeisterid subclade, the monophyly of Burmeistera, represented by 68 taxa in our sampling, is moderately to well supported (0.7/0.99/95/78). The well-supported brevilimbatid subclade (1/0.99/100/71) is sister to the burmeisterid subclade in all analyses, consistent with results from Lagomarsino et al. (2014). This subclade comprises species of Centropogon with orange to red flowers generally with yellow corolla limbs and is represented by four taxa in our sampling (i.e. C. macbridei, C. grandidentatus, C. erythraeus and C. aslcepiadeus). Three robust, green-flowered species that we refer to as the giganteus grade are successively sister to the brevilimbatids + burmeisterids; together, these three groups have moderate support (0.71/0.72/63/72). The relationship between the burmeisterid, brevlimibatid and giganteus grades is consistent across analyses, and is further consistent with Lagomarsino et al. (2014).
Three remaining major groups (i.e. the peruvianid Centropogon clade and the umbellatus and odontosepalus groups of Siphocampylus) and two species (i.e. S. smilax and S. corymbifer) are always included in Clade 1; however, their relationship differs across analyses. In both the ASTRAL and ASTRID analyses, these groups form basal polytomies, with resolution within individual and between individual subclades.
Across analyses, we find evidence for a close relationship between the peruvianid Centropogon subclade and the umbellatus group of Siphocampylus, both groups of generally green- to white-flowered species that are either sister to, are successively sister to or form a polytomy with the burmeisterid–giganteus clade. The peruvianids, a subclade of robust shrubs with large red or white flowers that is restricted to the Central Andes of Peru and Bolivia and represented by nine tips in our sampling (C. dianae, C. simulans, C. perlongus, C. viriduliflora, C. mandonis, C. brittonianus and three accessions of C. incanus), is strongly supported as monophyletic in all analyses (1/1/100/100). The umbellatus group, represented by five species in our sampling, is phenotypically similar to the peruvianid subclade, though these species produce capsules and not berries; these robust shrubs include species with both typically hummingbird-pollinated (e.g. S. boliviensis) and bat-pollinated (e.g. S. tunicatus) flowers (Lagomarsino and Santamaría-Aguilar, 2016). The relationships between species in the umbellatus group differ across analyses; it is monophyletic in the ASTRAL analysis (0.74) and a grade or unresolved in the remainder. Together, these two major groups form a clade [i.e. ASTRID and RAxML (the latter with the exception of S. tunarensis and S. boliviensis)] or grade (i.e. ASTRAL, SVDquartets).
We additionally find strong support for a close relationship between the colombianid subclade of Centropogon and a group of Siphocampylus species that we refer to as the odontosepalus group. Together, these species are strongly supported as monophyletic across all analyses (0.91/0.99/96/100). Both of these phenotypically similar groups are generally suffrutescent subshrubs with narrow, bright pink flowers found in the northern Neotropics; the colombianid subclade is distributed from southern Central America to northern South America, while the odontosepalus group comprises Siphocampylus species from northern South America (i.e. S. odontosepalus from Venezuela, S. longibracteolatus from Colombia and S. planchonis from Colombia and Venezuela). The relationship between these groups differs across analyses; they are either sister clades (RAxML; Fig. 2), or the colombianid species form a grade to a monophyletic odontosepalus group (ASTRAL; Fig. 3A), or the odontosepalus species form a grade to a monophyletic colombianid group (ASTRID, SVDQuartets; Fig. 3B, C). In the SVDquartets and RAxML analyses, these groups are sister to the clade that includes both the majority of Clade 1 species (i.e. the clade defined by the common ancestor of the burmeisterids and the umbellatus group). As previously noted, the monophyly of the colombianid clade sensuLagomarsino et al. (2014) is not supported due to the unstable placement of S. nematosepalus, a capsular species from montane Costa Rica (see discussion of taxa of uncertain placement below).
Finally, S. smilax is either sister to the remainder of Clade 1 or forms part of a basal polytomy with its other major subclades (Figs 2 and 3). This is a green-flowered species from Bolivia with a thickened, succulent woody base that is probably an adaptation for water storage in the relatively arid environments in which it occurs – an uncommon habitat for the centropogonid clade.
Clade 2.
The second major clade includes the eucentropogonid clade and five subclades of Siphocampylus species. Four of these subclades, together referred to as the scandens grade, are closely related in all analyses, which we collectively refer to as the scandens grade: (1) the correoides subclade (0.95/1/98/100), comprising S. correoides, S. ayersiae and S. chloroleucus; (2) the nobilis subclade (1/0.99/0.98/92), comprising S. scandens and S. nobilis; (3) the actinothrix subclade (0.77/1/100/100), comprising S. actinothrix, S. antonelli and S. vatkeanus; and (4) S. asplundii. We refer to the fifth group as the tupaeformis clade (0.59/0.77/100/100), which corresponds to S. fiebrigii, S. aureus, S. macropodus, S. werdermannii, S. krauseanus, S. obovatus, S. virgatus and S. tupaeformis in our sampling. Species in the tupaeformis clade tend to be erect, but short-statured shrubs with relatively small, hummingbird-pollinated flowers. While each of these subclades is resolved as monophyletic in all analyses, their relationships differ across analyses. In two analyses (i.e. ASTRAL and SVDquartets), the tupaeformis clade is sister to the remaining groups, while it is sister to the eucentropogonid clade in the RAxML analysis (Figs 2 and 3) and forms a basal polytomy with the rest of the Clade 2 subclades in the ASTRID analysis.
The eucentropogonid clade (which corresponds to Centropogon subgenus Eucentropogon) is monophyletic with highest possible support in all analyses (1/1/100/100). This is a group of about 55 species that are often pollinated by the sicklebill hummingbird (Stein, 1992; Boehm et al., 2018) and are characterized by extremely curved flowers with a cornute scale of fused trichomes at the apex of their ventral anthers. Relationships among eucentropogonid species are similar to those in previous analyses (Lagomarsino et al., 2014) and correspond to current taxonomic groups: we find that C. cornutus is sister to the group that includes subsections Amplifolii and Campylobotrys from Stein (1987), and that members of Campylobotrys are monophyletic. Only a single species of subsection Amplifolii, C. congestus, is included in our sampling, so we cannot evaluate its monophyly. However, there is one important difference from previous analyses: we find that a currently undescribed Centropogon species (whose morphology suggests that it belongs to the eucentropogonid clade as it shares the synapomorphic cornute scale and curved corolla) is sister to the rest of this clade.
While the subclades of the scandens grade lack resolution, relationships are consistent in the two analyses in which they do not form a polytomy (i.e. ASTRAL and RAxML analyses). In these analyses, there is strong support for a sister relationship between the correoides and nobilis subclades (1.0 and 100, respectively). All members of these clades are soft–woody vines often with coriaceous leaves, though floral morphology is notably variable, from typically hummingbird-pollinated flowers that are small, narrow, pink and relatively long (e.g. S. scandens) to typically bat-pollinated flowers that are large, wide, green and relatively short (e.g. S. ayersiae). The two additional subclades (i.e. the actinothrix subclade and S. asplundii) form a clade in the ASTRAL and RAxML analyses (0.77 and 100, respectively), and are sister the remaining members of the scandens grade and the eucentropogonid clade (ASTRAL) or the eucentropogonid + tupaeformis subclades (RAxML). The actinothrix subclade is composed of erect woody shrubs with a combination of flowers with hummingbird pollination (e.g. S. antonellii) and bat pollination (e.g. S. actinothrix) syndromes (Lagomarsino and Santamaría-Aguilar, 2016); S. asplundii is a scandent shrub with a narrow, pink flower.
Taxa of uncertain placement.
The membership of Clade 1 and Clade 2 is consistent across analyses with the exception of two subclades that are resolved in very different regions of the phylogeny across analyses: (1) the furax clade (0.98/0.99/99/100), comprising S. furax, S. affinis and S. brevicalyx; and (2) the andinus clade (1/0.99/99/100), comprising S. imbricatus, S. orbignianus, S. rusbyanus, S. andinus, S. verticillatus, S. lycioides and S. longipedunculatus. These two subclades tend to be closely related across analyses, forming a clade (ASTRAL; Fig. 3A) or grade (ASTRID, RAxML; Figs 2 and 3B), though they form part of a basal polytomy in the SVDquartets analysis (Fig. 3C). Their placement within the broader phylogeny differs across analyses. They are nested within Clade 1 in the ASTRAL analysis, are successively sister to Clade 2 in the ASTRID and RAxML analyses, and are part of a polytomy at the base of the centropogonid clade in the SVDquartets analysis.
Siphocampylus corymbifer is a scandent shrub with a corymb inflorescence that is atypical within the centropogonid clade and a similarly atypical widespread distribution that stretches from the Andean forests of Peru and Bolivia to the Atlantic Forest of Brazil. Its placement differs widely across analyses: ASTRAL and ASTRID place it in a polytomy at the base of Clade 1, SVDquartets places it in a polytomy at the base of the centropogonid clade, and RAxML places it sister to two other Central Andean species (S. tunarensis and S. boliviensis) within Clade 1. The second rogue species, S. nematosepalus, which was previously included in the colombianid clade (Lagomarsino et al., 2014), always belongs to Clade 1 but is placed differently across analyses: ASTRAL and RAxML place it sister to the odontosepalus group and colombianid clade with relatively strong support (0.91 and 100, respectively), while ASTRID and SVDquartets infer it to be sister to the burmeisterid–brevilimbatid–giganteus groups (with support of 0.72 and 92, respectively).
DISCUSSION
Building on recent progress within Burmeistera (Bagley et al., 2020), we present the first phylogenomic analysis of the entire centropogonid clade. Targeted sequence capture permitted herbarium specimens to be a major source of DNA, and despite the degraded quality of herbarium DNA, its inclusion did not present major barriers. After meeting certain minimum requirements for DNA quality, sequence capture was efficient for herbarium tissue, although it underperformed compared to field-collected tissues. This study presents the most data-rich phylogeny of this group to date, and is among the first nuclear phylogenomic studies of a species-rich cloud forest-centred Andean clade (Nevado et al., 2016; Vargas et al., 2017; Pouchon et al., 2018; Bagley et al., 2020; Acha et al., 2021). The inferred relationships represent significant departure from previous knowledge of phylogeny in the clade, particularly in increased resolution along the backbone of the phylogeny and among capsular species (currently recognized as the genus Siphocampylus). A high degree of gene tree discordance and complex patterns of morphological trait evolution characterize the centropogonid clade. These represent dual challenges to resolving phylogenies of rapid radiations and developing classification schemes in groups characterized by frequent morphological convergent evolution.
Herbariomic data substantially improved phylogeny despite yielding less sequence data
Overall, our results add to a growing body of literature showing that herbarium specimens are useful for increasing taxon sampling in phylogenomic analyses, although there are limitations to the use of this data source. The method that we used, sequence capture, does not require high-molecular-weight DNA, and instead is robust to low-quantity, low-quality input DNA (Hart et al., 2016; Brewer et al., 2019). Despite this, we found that sequence data derived from herbarium samples tend to be lower quality than data from silica-collected tissue. While similar numbers of contigs were recovered from both data types, those contigs tended to be shorter in herbarium specimen data, resulting in fewer long contigs (Fig. 1). Using the number of very long contigs (in our case, those >1 kbp) as a proxy for the quality of data, we removed 13 (31 %) herbarium specimens and no silica-collected tissues. However, it is likely that additional data curation would have allowed us to include these specimens in phylogenomic analysis, and this is a goal for future studies.
Despite dropping many samples derived from herbarium specimens from our phylogenomic analyses, the inclusion of those that remained greatly enhanced our understanding of relationships within the centropogonid clade. For example, this approach allowed us to include species known only from a single locality (e.g. Centropogon sp. nov. in the eucentropogonid clade) and species endemic to countries where it is not currently feasible to collect new material (e.g. S. odontosepalus from Venezuela). Further, the odontosepalus group, which forms either a clade or a grade closely related to the colombiand clade, is newly discovered and is currently exclusively composed of samples derived from herbarium specimens; for two of their three species (i.e. S. odontosepalus and S. planchonis), our targeted sequence capture data represent the first DNA sequences generated for their species. This points to an important role of herbarium specimens in phylogenetic lineage discovery, allowing us to continue to unravel branches of the tree of life even when it would otherwise not be feasible, whether due to logistical reasons (e.g. difficulty of additional fieldwork or lack of funding) or fundamental impossibility (e.g. extinction of taxa).
Herbarium specimens will continue to be an important potential source of phylogenomic data in the years to come. Highlighting this, many recent studies include a large proportion of herbarium specimens in sequence capture-based phylogenies (e.g. Silva et al., 2017; Schneider et al., 2021; Thomas et al., 2021). However, DNA extracted from herbarium specimens is not a panacea, with data quality generally being lower when derived from herbarium specimens than from silica-dried tissue. Additionally, herbarium specimens are physical objects that are permanently damaged each time tissue is sampled (especially if no material is available in the fragment packets), and many institutions have a limit on how many times a single specimen can be destructively sampled (Rabeler et al., 2019). For these reasons, inclusion of herbarium specimens in phylogenomic studies should be well justified and preferably only undertaken if no alternatives are available or if an individual specimen represents an important addition to a study (e.g. distinct morphology or locality).
Improved phylogenetic resolution of Neotropical bellflowers
At the deepest level, we resolve two major centropogonid clades – Clade 1 and Clade 2 in Fig. 2 – that are novel to this study (though two additional clades of Siphocampylus species, the furax and andinus clades, are variably placed within these major clades or in a basal polytomy across analyses). There are no apparent morphological synapomorphies that unite either Clade 1 or Clade 2. Both include species that span the majority of morphological variation within the centropogonid clade, including both baccate and capsular lineages; hummingbird and bat pollination syndromes; vining and erect habits; and a wide variety of pubescence types (Fig. 2).
Relationships among baccate lineages are consistent across analyses and similar to previous studies. The monophyly of Burmeistera and four separate clades of Centropogon are consistently well supported, as are the relationships between them. The fifth group of named Centropogon species, the colombianids (with the exception of S. nematosepalus), is monophyletic in all analyses except ASTRAL, and its placement within Clade 1 is consistent across phylogenies. Morphology within these baccate clades tends to be conserved beyond the fruit type that unites them, including many aspects of floral morphology, gross pollination syndrome, habit, pubescence type and, often, biogeography (Lagomarsino et al., 2014).
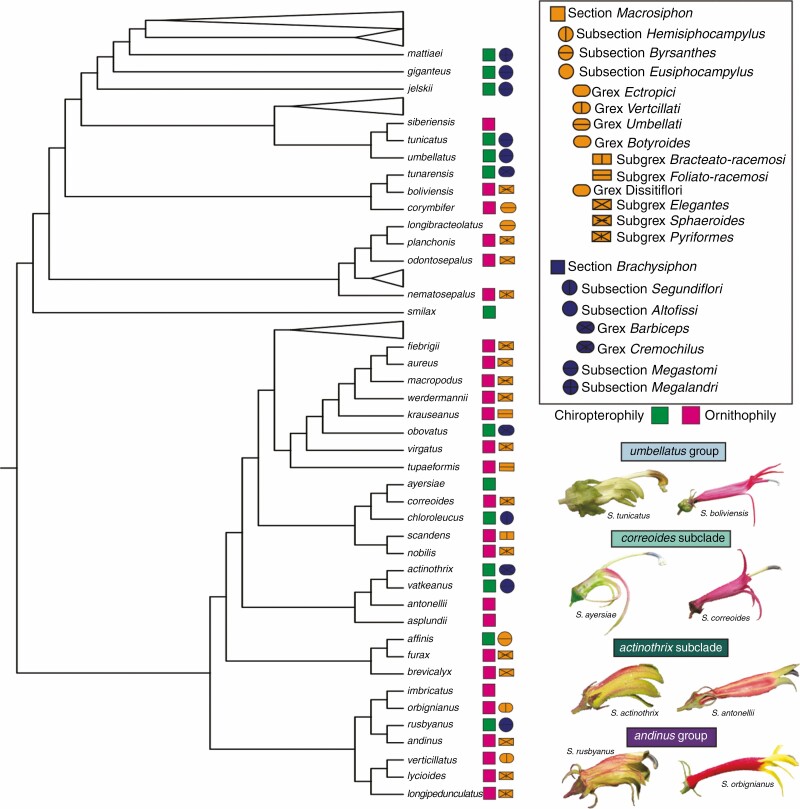
While relationships among Siphocampylus have improved support relative to previous analyses, this genus, defined by its plesiomorphic capsular fruits, is far from resolved. Support, membership and relationships between named groups of Siphocampylus species (as well as their relationship to baccate lineages) differ across analyses and tend to have lower support values than their baccate equivalents. Additionally, there are two rogue subclades (i.e. the andinus and furax subclades) and two rogue species of Siphocampylus (i.e. S. corymbifer and S. nematosepalus) that are unstable in their placement. Future research that tests for a potential hybrid origin or history of polyploidy in these lineages (e.g. implementing the program Dsuite; Malinsky et al., 2021) is likely to be fruitful. Despite these conflicts, we infer ten groups of closely allied species of Siphocampylus (these often forming either subclades or grades in different analyses) that are consistently identified across analyses. Contrasting with the pattern in Centropogon and Burmeistera, in which flowers tend to be similar among close relatives, subclades of Siphocampylus are marked by very variable floral morphology, often including multiple shifts in pollination syndromes (Figs 2 and 5).
Fig. 5.
The non-monophyly that characterizes the current classification of Siphocampylus is, in part, explained by convergent evolution of floral forms within pollination syndromes as infrageneric groups were circumscribed based on floral traits. The intrageneric classification scheme of Siphocampylus from Wimmer (1968) is presented in the key at top right, with section Macrosiphon in orange and section Brachysiphon in blue, with specific shapes and line patterns delimiting subsectional taxa. The taxa to which Siphocampylus species belong is denoted next to the tip of the RAxML topology, along with whether the species is hummingbird- or bat-pollinated. Siphocampylus species with no taxonomic symbol next to their name were not included in Wimmer (1968). Examples of closely related pollination shifts are shown at bottom right, with chiropterophilous species at left and ornithophilous species at right, with informal clade names above with colours corresponding to Fig. 2. Pictured species are S. tunicatus and S. boliviensis in the umbellatus group; S. ayersiae and S. correoides in the correoides group; S. actinothrix and S. antonellii in the actinothrix group; and S. rusybanus and S. orbignianus in the andinus group.
The discrepancy between relatively high constancy of relationships in Centropogon and Burmeistera lineages as compared to Siphocampylus lineages is probably because a derived character state, berry fruits, defines them. Capsular fruits are the ancestral state in the centropogonid clade, from which berries have been derived on multiple occasions (Lagomarsino et al., 2014; Crowl et al., 2016). A consequence of this history is that Centropogon subclades are relatively young relative to both the centropogonid clade as a whole and to many individual lineages of Siphocampylus. Their younger age also means there has been less time for phylogenetic conflicts due to introgression and hybridization to arise. While gene tree discordance is high at the base of both baccate and capsular lineages (Fig. 2), it is notable that the nodes with the highest portion of concordant genes are at the base of baccate subclades (i.e. the eucentropogonid and peruvianid subclades).
Finally, comparing our results to the plastid phylogeny of Lagomarsino et al. (2014), we observe a pattern consistent with extensive cytonuclear discordance. Cytonuclear discordance (i.e. when the phylogenetic signal of organelle genomes – in this case, the plastome – is significantly different from phylogenetic signal of the nuclear genome) is often considered evidence of reticulate evolution (Reiseberg and Soltis, 1991; Sloan et al., 2017), but can also be attributed to additional processes including selection and incomplete lineage sorting (Lee-Yaw et al., 2019).
While we find broad support for the same major baccate subclades as in the plastid phylogeny, relationships both within and between the capsular lineages differ substantially (Fig. 4). Given the extent of discordance among analyses and within nuclear gene trees, as well as the fact that the plastome is generally considered to be a single coalescent gene (Gonçalves et al., 2019; but see Doyle, 2021), it is very likely that at least some of the differences we observe are due to incomplete lineage sorting (Lee-Yaw et al., 2019; Firneno et al., 2020). However, much of the cytonuclear discordance we identify involves nodes deep in the phylogeny (e.g. members of the andinus clade of Siphocampylus), suggesting a potential for an ancient history of hybridization or introgression within the centropogonid clade. Given the clade’s extensive convergent evolution of morphological traits, the overlap of character states between subclades, and rapid rates of diversification, this would not be surprising and represents a promising area for future research.
Future classification needs of Neotropical Lobelioideae
The classification of Neotropical Lobeloideae is in dire need of a thorough revision. This is especially true for Siphocampylus, for which none of the subgeneric taxa of Siphocampylus from the latest monograph (Wimmer, 1943, 1955, 1968) with more than a single species included in our sampling are monophyletic (Fig. 5).
The challenge of reclassification is amplified by the paraphyletic and polyphyletic nature of traits used to define named taxa in this clade. At the genus level, capsular fruits, the defining trait of Siphocampylus, are paraphyletic, while the multiple Centropogon and Burmeistera lineages represent independent evolution of berries (Lagomarsino et al., 2014). Similarly, taxa within Siphocampylus are largely defined by traits that have undergone repeated convergent evolution. For example, the two sections of Siphocampylus correspond largely to hummingbird pollination (section Macrosiphon) and bat pollination (section Brachysiphon) syndromes (Fig. 5). The former is defined by long, narrow, brightly coloured corollas with filaments that are adnate high on the corolla (a potential adaptation to reduce damage from hummingbird bills), while the latter is defined by short, wide, greenish or dull-coloured corollas with free filaments or filaments that are adnate near the base of the corolla. These traits are known to accurately predict pollinators in the centropogonid clade (Muchhala, 2003, 2006a; Muchhala and Thomson, 2009; Lagomarsino and Muchhala, 2019), and undergo strong selection pressure during the frequent shifts between pollination syndromes that characterize the history of the entire centropogonid clade (Lagomarsino et al., 2017). This reliance on traits that have been shown to be plesiomorphic (e.g. capsules for Siphcampylus and hummingbird pollination for section Macrosiphon) has resulted in a Russian doll of non-monophyletic classification that will require substantial reconfiguration to accurately reflect evolutionary relationships – a reconfiguration that will require a modification of classification at the level of the subfamily Lobelioideae, which is based on fruit type.
Rapid trait evolution and frequent convergence will make designating new taxa difficult, especially within paraphyletic Siphocampylus. There are no obvious floral, fruit or habit traits separating the various subclades of Siphocampylus that we have identified from all other lineages. Instead, traits that may experience less convergent selection pressure, including types of pubescence, aspects of seed morphology or anatomy, and micromorphology, are likely to be more useful. However, even with our substantial taxonomic expertise in this group, no characters are immediately obvious, suggesting that perhaps a combination of these characters may be necessary. Combining morphology and biogeography is likely to most accurately reflect relationships within the clade. Geography has already been shown to be informative: within the clade, the only monophyletic subsection of Siphocampylus (subsection Hemisiphocampylus), which is distantly related to all other species of the genus, is restricted to the Caribbean, while the peruvianid clade of Centropogon is restricted to the Central Andes of Peru and Bolivia (Lagomarsino et al., 2014).
Finally, determining how to best recircumscribe the three genera comprising the centropogonid clade (i.e. Centropogon, Burmeistera and Siphocampylus) requires a stable and well-supported phylogeny. While our new phylogeny is an important step in this direction, future phylogenetic research should aim for deeper taxon sampling, with a particularly important goal of increasing sampling of Siphocampylus species. Unlike Centropogon, for which species can be relatively easily placed into subclades that are unlikely to change with additional taxon sampling, it is possible that we will continue to identify novel lineages of Siphocampylus as additional species are added to the phylogeny – such as the odontosepalus group in this study, which comprises entirely newly sequenced species. Future reclassification efforts for Neotropical Lobelioideae represent a significant challenge, but one that can be met with additional phylogenetic data, further examination of morphology and a deep understanding of trait evolution.
Phylogenomic and systematic challenges innate to rapid, morphologically diverse rapid radiations
The difficulty in resolving relationships of rapid radiations is a well-known phenomenon in plant systematics, and is exemplified in many Andean plants radiations (Hughes and Eastwood, 2006; Nürk et al., 2013; Pease et al., 2016; Uribe-Convers et al., 2016; Vargas et al., 2017; Morales-Briones et al., 2018; Frost and Lagomarsino, 2021). The centropogonid clade, one of the fastest evolutionary radiations reported to date, is no exception. The extensive gene tree discordance we document is expected given that rates of incomplete lineage sorting are inversely proportional to time between speciation events (Pamilo and Nei, 1988). Furthermore, the history of this group is probably marked by frequent introgression, as suggested by the cytonuclear discordance and the unstable placement of several rogue species and lineages.
Because phylogenetic complexity is increasingly demonstrated in rapid plant radiations, including the centropogonid clade, data curation (including gene tree filtering) is a crucial first step in phylogenomic analyses. However, best practices in data curation are still being established, with many researchers demonstrating that subsampling of phylogenomic datasets results in more robust signal (Molloy and Warnow, 2018; Smith et al., 2018; Mongiardino Koch, 2021). In this study, we filtered out genes that had <75 % taxon sampling and those that were shorter than 500 bp. While this resulted in relatively few genes in our final dataset compared to the total sequenced (i.e. 96 of 745), it is a metric that has proven to result in higher phylogenetic performance compared to other filtering metrics in similar Andean plant radiations (Frost & Lagomarsino, 2021). Alternative gene filtering criteria (e.g. by clocklikeness or bipartition support) may result in different, potentially larger datasets that result in improved phylogenetic inference (Ferreira et al., 2022). However, more sequence data do not necessarily reflect a better quality dataset, and poorly curated phylogenomics datasets may result in inaccurate relationships (Brown and Thomson, 2017; Molloy and Warnow, 2018). A goal of empirical phylogenetics should be to interrogate phylogenetic performance across data subsets to reach an appropriate balance of data quality and quantity.
The phylogenomic complexity of the centropogonid clade corresponds to rapid morphological innovation (Lagomarsino et al., 2014, 2016, 2017), as has been observed in many distantly related groups of organisms (Parins-Fukuchi et al., 2020). It is possible that rampant convergent trait evolution in fruit and floral traits reflects hemiplasy (i.e. traits whose genetic basis is determined by a gene whose history does not match the species tree; Avise and Robinson, 2008; Guerrero and Hahn, 2018), rather than de novo convergent evolution. Hemiplasy as the main driver of fruit type evolution seems unlikely in the centropogonid clade, as fruit type is a complex character (Ortiz-Ramírez et al., 2018). In the centropogonid clade, baccate fruits tend to be very distinct and readily distinguishable across subclades, suggesting that even if hemiplasy is fully or partially responsible for repeated shifts in fruit type, the individual genes involved are probably different or there is a degree of de novo variation in addition to historical sorting. However, individual floral traits (including those related to pollination syndrome including colour, scent and overall size) seem more likely to have an extensive history of hemiplasy (Stankowski and Streisfeld, 2015). In fact, the paraphyletic phylogenetic distribution of floral traits in the centropogonid clade (vs. the synapomorphic distribution of fruit type) is similar to Jaltomata, a tomato relative in which de novo evolution seems to drive fruit traits (in this case, fruit colour), and evolution of floral traits probably represents hemiplasy (Wu et al., 2018).
Understanding the macroevolutionary history of rapid, species-rich radiations with extensive morphological variation remains an exciting challenge in evolutionary biology. Despite large datasets, support for phylogenomic relationships in these groups tends to be low, probably reflecting the complex, often non-bifurcating lineage splitting events and small populations that characterize these groups. This is true even in well-trodden systems that have the benefit of substantial genomic resources (e.g. Lake Victoria cichlids: Meier et al., 2017; Andean tomatoes: Pease et al., 2016). Andean cloud forest radiations, including the centropogonid clade, exemplify this phylogenomic challenge. It is likely that a full understanding of relationships of the understudied, but species-rich plant radiations of this biodiversity hotspot will remain a difficult problem in phylogenomics for years to come, even as datasets continue to expand in size and models improve. This exciting arena of evolutionary biology contrasts with the real threat to biodiversity in this region and ongoing extinction of species that belong to the many rapid radiations whose evolutionary histories we are just beginning to untangle (Antonelli, 2021).
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Appendix S1: Voucher Information for all samples included in study. Appendix S2: Summary statistics for sequence data for all samples included in study. Figure S1: Results of RAxML analysis without Burmeistera collapsed, as in Fig. 2. Figure S2: Results of ASTRAL analysis without Burmeistera collapsed, as in Fig. 3A. Figure S3: Results of ASTRID analysis without Burmeistera collapsed, as in Fig. 3B. Figure S4: Results of SVDquartets analysis without Burmeistera collapsed, as in Fig. 3C. Figure S5: Results of Phyparts analysis along the RAxML phylogeny, including discordant visualizations for every node in phylogeny.
Supplementary Material
ACKNOWLEDGEMENTS
The Muchhala Lab at UMSL, including Serena Achá, Camilo Calderón-Acevedo, Diana Gamba and Rosana Maguiña, provided assistance in the early stages of this research, and the Lagomarsino Lab at LSU provided feedback on an earlier draft. Brant Faircloth and Carl Oliveros provided assistance in implementing the phyluce pipeline. Portions of this research were conducted with high-performance computing resources provided by Louisiana State University (http://www.hpc.lsu.edu). Many herbaria, including the Missouri Botanical Garden (MO) and the University of Gothenburg (GB), allowed destructive sampling of their specimens for this research; and many other institutions, especially in Latin America (including COL, CR, LPB and QCA), provided important access to their collections that improved our understanding of this diverse, taxonomically complex plant clade.
FUNDING
This research was supported by a National Science Foundation Postdoctoral Research Fellowship in Biology to L.P.L. (Award 1523880), a Field Research for Conservation Grant from the WildCare Institute at the St. Louis Zoo to L.P.L. and N.M., and startup funds to L.P.L. from the LSU College of Science and Department of Biological Sciences. A.A. acknowledges financial support from the Swedish Research Council (2019-05191), the Swedish Foundation for Strategic Research (FFL15-0196) and the Royal Botanic Gardens, Kew. L.F. was supported by an Open Research Grant from the Grinnell College Center for Careers, Life, and Service.
LITERATURE CITED
- Acha S, Linan A, MacDougal J, Edwards C. 2021. The evolutionary history of vines in a neotropical biodiversity hotspot: Phylogenomics and biogeography of a large passion flower clade (Passiflora section Decaloba). Molecular Phylogenetics and Evolution 164: 107260. doi: 10.1016/j.ympev.2021.107260. [DOI] [PubMed] [Google Scholar]
- Alsos IG, Lavergne S, Merkel MKF, et al. . 2020. The treasure vault can be opened: Large-scale genome skimming works well using herbarium and silica gel dried material. Plants: 9: 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli A. 2008. Higher level phylogeny and evolutionary trends in Campanulaceae subfam. Lobelioideae: molecular signal overshadows morphology. Molecular Phylogenetics and Evolution 46: 1–18. doi: 10.1016/j.ympev.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Antonelli A. 2009. Have giant lobelias evolved several times independently? Life form shifts and historical biogeography of the cosmopolitan and highly diverse subfamily Lobelioideae (Campanulaceae). BMC Biology 7: 82. doi: 10.1186/1741-7007-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli A. 2021. The rise and fall of Neotropical biodiversity. Botanical Journal of the Linnean Society. doi: 10.1093/botlinnean/boab061. [DOI] [Google Scholar]
- Avise JC, Robinson TJ. 2008. Hemiplasy: a new term in the lexicon of phylogenetics. Systematic Biology 57: 503–507. doi: 10.1080/10635150802164587. [DOI] [PubMed] [Google Scholar]
- Bagley JC, Uribe-Convers S, Carlsen MM, Muchhala N. 2020. Utility of targeted sequence capture for phylogenomics in rapid, recent angiosperm radiations: Neotropical Burmeistera bellflowers as a case study. Molecular Phylogenetics and Evolution 152: 106769. [DOI] [PubMed] [Google Scholar]
- Bakker FT. 2017. Herbarium genomics: skimming and plastomics from archival specimens. Webbia 72: 35–45. doi: 10.1080/00837792.2017.1313383. [DOI] [Google Scholar]
- Boehm MM, Scholer MN, Kennedy JJ, et al. . 2018. The Manú Gradient as a study system for bird pollination. Biodiversity Data Journal 6: e22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo GA, Antonelli A, Bacon CD, et al. . 2019. Embracing heterogeneity: coalescing the Tree of Life and the future of phylogenomics. PeerJ 7: e6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GE, Clarkson JJ, Maurin O, et al. . 2019. Factors affecting targeted sequencing of 353 nuclear genes from herbarium specimens spanning the diversity of angiosperms. Frontiers in Plant Science 10: 1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Thomson RC. 2017. Bayes factors unmask highly variable information content, bias, and extreme influence in phylogenomic analyses. Systematic Biology 66: 517–530. [DOI] [PubMed] [Google Scholar]
- Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution 17: 540–552. [DOI] [PubMed] [Google Scholar]
- Chifman J, Kubatko L. 2014. Quartet inference from SNP data under the coalescent model. Bioinformatics 30: 3317–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowl AA, Miles NW, Visger CJ, et al. . 2016. A global perspective on Campanulaceae: Biogeographic, genomic, and floral evolution. American Journal of Botany 103: 233–245. [DOI] [PubMed] [Google Scholar]
- Doyle JJ. 2021. Defining coalescent genes: theory meets practice in organelle phylogenomics. Systematic Biology 71: 476–489. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. 1987. CTAB DNA extraction in plants. Phytochemical Bulletin 19: 11–15. [Google Scholar]
- Edwards SV. 2009. Is a new and general theory of molecular systematics emerging? Evolution; International Journal of Organic Evolution 63: 1–19. [DOI] [PubMed] [Google Scholar]
- Faircloth BC. 2013. Illumiprocessor: a trimmomatic wrapper for parallel adapter and quality trimming. Available at 10.6079/J9ILL (accessed 4 November 2016). [DOI]
- Faircloth BC. 2016. PHYLUCE is a software package for the analysis of conserved genomic loci. Bioinformatics 32: 786–788. [DOI] [PubMed] [Google Scholar]
- Faircloth BC, McCormack JE, Crawford NG, Harvey MG, Brumfield RT, Glenn TC. 2012. Ultraconserved elements anchor thousands of genetic markers spanning multiple evolutionary timescales. Systematic Biology 61: 717–726. [DOI] [PubMed] [Google Scholar]
- Ferreira PL, Batista R, Andermann T, Groppo M, Bacon CD, Antonelli A. 2022. Target sequence capture of Barnadesioideae (Compositae) demonstrates the utility of low coverage loci in phylogenomic analyses. Molecular Phylogenetics and Evolution 169: 107432. [DOI] [PubMed] [Google Scholar]
- Firneno TJ Jr, O’Neill JR, Portik DM, Emery AH, Townsend JH, Fujita MK. 2020. Finding complexity in complexes: assessing the causes of mitonuclear discordance in a problematic species complex of Mesoamerican toads. Molecular Ecology 29: 3543–3559. [DOI] [PubMed] [Google Scholar]
- Frost L, Lagomarsino L. 2021. More-curated data outperforms more data: Treatment of cryptic and known paralogs improves phylogenomic analysis and resolves a northern Andean origin of Freziera (Pentaphylacaceae). bioRxiv: 2021.07.01.450750. [Google Scholar]
- Galili T. 2015. dendextend: an R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 31: 3718–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves DJP, Simpson BB, Ortiz EM, Shimizu GH, Jansen RK. 2019. Incongruence between gene trees and species trees and phylogenetic signal variation in plastid genes. Molecular Phylogenetics and Evolution 138: 219–232. [DOI] [PubMed] [Google Scholar]
- Grabherr MG, Haas BJ, Yassour M, et al. . 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology 29: 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero RF, Hahn MW. 2018. Quantifying the risk of hemiplasy in phylogenetic inference. Proceedings of the National Academy of Sciences of the United States of America 115: 12787–12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart ML, Forrest LL, Nicholls JA, Kidner CA. 2016. Retrieval of hundreds of nuclear loci from herbarium specimens. Taxon 65: 1081–1092. [Google Scholar]
- Herrando-Moraira S; Cardueae Radiations Group. 2018. Exploring data processing strategies in NGS target enrichment to disentangle radiations in the tribe Cardueae (Compositae). Molecular Phylogenetics and Evolution 128: 69–87. [DOI] [PubMed] [Google Scholar]
- Hosner PA, Faircloth BC, Glenn TC, Braun EL, Kimball RT. 2016. Avoiding missing data biases in phylogenomic inference: An empirical study in the landfowl (Aves: Galliformes). Molecular Biology and Evolution 33: 1110–1125. [DOI] [PubMed] [Google Scholar]
- Hughes CE, Eastwood R. 2006. Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proceedings of the National Academy of Sciences of the United States of America 103: 10334–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MG, Gardner EM, Liu Y, et al. . 2016. HybPiper: Extracting coding sequence and introns for phylogenetics from high-throughput sequencing reads using target enrichment. Applications in Plant Sciences 4: 1600016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Sagitov S, Oxelman B. 2013. Statistical inference of allopolyploid species networks in the presence of incomplete lineage sorting. Systematic Biology 62: 467–478. [DOI] [PubMed] [Google Scholar]
- Kates HR, Doby JR, Siniscalchi CM, et al. . 2021. The effects of herbarium specimen characteristics on short-read NGS sequencing success in nearly 8000 specimens: Old, degraded samples have lower DNA yields but consistent sequencing success. Frontiers in Plant Science 12: 669064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox EB, Muasya AM, Muchhala N. 2008. The predominantly South American clade of Lobeliaceae. Systematic Botany 33: 462–468. [Google Scholar]
- Lagomarsino LP, Antonelli A, Muchhala N, Timmermann A, Mathews S, Davis CC. 2014. Phylogeny, classification, and fruit evolution of the species-rich Neotropical bellflowers (Campanulaceae: Lobelioideae). American Journal of Botany 101: 2097–2112. [DOI] [PubMed] [Google Scholar]
- Lagomarsino LP, Condamine FL, Antonelli A, Mulch A, Davis CC. 2016. The abiotic and biotic drivers of rapid diversification in Andean bellflowers (Campanulaceae). The New Phytologist 210: 1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagomarsino LP, Forrestel EJ, Muchhala N, Davis CC. 2017. Repeated evolution of vertebrate pollination syndromes in a recently diverged Andean plant clade. Evolution; International Journal of Organic Evolution 71: 1970–1985. [DOI] [PubMed] [Google Scholar]
- Lagomarsino LP, Muchhala N. 2019. A gradient of pollination specialization in three species of Bolivian Centropogon. American Journal of Botany 106: 633–642. [DOI] [PubMed] [Google Scholar]
- Lagomarsino LP, Santamaría-Aguilar D. 2016. Two new species of Siphocampylus (Campanulaceae, Lobelioideae) from the Central Andes. PhytoKeys 58: 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers TG. 1998. Review of the Neotropical endemics Burmeistera, Centropogon, and Siphocampylus (Campanulaceae: Lobelioideae), with description of 18 new species and a new section. Brittonia 50: 233–262. [Google Scholar]
- Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. 2017. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 34: 772–773. [DOI] [PubMed] [Google Scholar]
- Lee-Yaw JA, Grassa CJ, Joly S, Andrew RL, Rieseberg LH. 2019. An evaluation of alternative explanations for widespread cytonuclear discordance in annual sunflowers (Helianthus). The New Phytologist 221: 515–526. [DOI] [PubMed] [Google Scholar]
- Lemmon AR, Emme SA, Lemmon EM. 2012. Anchored hybrid enrichment for massively high-throughput phylogenomics. Systematic Biology 61: 727–744. [DOI] [PubMed] [Google Scholar]
- Lendemer J, Thiers B, Monfils AK, et al. . 2020. The Extended Specimen Network: a strategy to enhance US biodiversity collections, promote research and education. Bioscience 70: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinsky M, Matschiner M, Svardal H. 2021. Dsuite - Fast D-statistics and related admixture evidence from VCF files. Molecular Ecology Resources 21: 584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKain MR, Johnson MG, Uribe-Convers S, Eaton D, Yang Y. 2018. Practical considerations for plant phylogenomics. Applications in Plant Sciences 6: e1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVaugh R. 1965. South American Lobelioideae new to science. Annals of the Missouri Botanical Garden 52: 399. [Google Scholar]
- Meier JI, Marques DA, Mwaiko S, Wagner CE, Excoffier L, Seehausen O. 2017. Ancient hybridization fuels rapid cichlid fish adaptive radiations. Nature Communications 8: 14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy EK, Warnow T. 2018. To include or not to include: the impact of gene filtering on species tree estimation methods. Systematic Biology 67: 285–303. [DOI] [PubMed] [Google Scholar]
- Mongiardino KN. 2021. Phylogenomic subsampling and the search for phylogenetically reliable loci. Molecular Biology and Evolution 38: 4025–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Briones DF, Liston A, Tank DC. 2018. Phylogenomic analyses reveal a deep history of hybridization and polyploidy in the Neotropical genus Lachemilla (Rosaceae). The New Phytologist 218: 1668–1684. [DOI] [PubMed] [Google Scholar]
- Muchhala N. 2003. Exploring the boundary between pollination syndromes: Bats and hummingbirds as pollinators of Burmeistera cyclostigmata and B. tenuiflora (Campanulaceae). Oecologia 134: 373–380. [DOI] [PubMed] [Google Scholar]
- Muchhala N. 2006a. The pollination biology of Burmeistera (Campanulaceae): Specialization and syndromes. American Journal of Botany 93: 1081–1089. [DOI] [PubMed] [Google Scholar]
- Muchhala N. 2006b. Nectar bat stows huge tongue in its rib cage. Nature 444: 701–702. [DOI] [PubMed] [Google Scholar]
- Muchhala N, Potts MD. 2007. Character displacement among bat-pollinated flowers of the genus Burmeistera: analysis of mechanism, process and pattern. Proceedings of the Royal Society B: Biological Sciences 274: 2731–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchhala N, Thomson JD. 2009. Going to great lengths: selection for long corolla tubes in an extremely specialized bat-flower mutualism. Proceedings of the The Royal Society B: Biological Sciences 276: 2147–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevado B, Atchison GW, Hughes CE, Filatov DA. 2016. Widespread adaptive evolution during repeated evolutionary radiations in New World lupins. Nature Communications 7: 12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nürk NM, Scheriau C, Madriñán S. 2013. Explosive radiation in high Andean Hypericum—rates of diversification among New World lineages. Frontiers in Genetics 4: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie HA, Bouckaert RR, Drummond AJ. 2017. StarBEAST2 brings faster species tree inference and accurate estimates of substitution rates. Molecular Biology and Evolution 34: 2101–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Ramírez CI, Plata-Arboleda S, Pabón-Mora N. 2018. Evolution of genes associated with gynoecium patterning and fruit development in Solanaceae. Annals of Botany 121: 1211–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamilo P, Nei M. 1988. Relationships between gene trees and species trees. Molecular Biology and Evolution 5: 568–583. [DOI] [PubMed] [Google Scholar]
- Parins-Fukuchi C, Stull GW, Smith SA. 2020. Phylogenomic conflict coincides with rapid morphological innovation. Cold Spring Harbor: Cold Spring Harbor Laboratory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease JB, Haak DC, Hahn MW, Moyle LC. 2016. Phylogenomics reveals three sources of adaptive variation during a rapid radiation. PLoS Biology 14: e1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouchon C, Fernández A, Nassar JM, et al. . 2018. Phylogenomic analysis of the explosive adaptive radiation of the Espeletia complex (Asteraceae) in the Tropical Andes. Systematic Biology 67: 1041–1060. [DOI] [PubMed] [Google Scholar]
- Rabeler RK, Svoboda HT, Thiers B, et al. . 2019. Herbarium practices and ethics, III. Systematic Botany 44: 7–13. [Google Scholar]
- Reiseberg L, Soltis D. 1991. Phylogenetic consequences of cytoplasmic gene flow in plants. Evolutionary Trends in Plants 5: 65–84. [Google Scholar]
- Rothfels CJ. 2021. Polyploid phylogenetics. The New Phytologist 230: 66–72. [DOI] [PubMed] [Google Scholar]
- Schneider JV, Jungcurt T, Cardoso D, et al. . 2021. Phylogenomics of the tropical plant family Ochnaceae using targeted enrichment of nuclear genes and 250+ taxa. Taxon 70: 48–71. [Google Scholar]
- Silva C, Besnard G, Piot A, Razanatsoa J, Oliveira RP, Vorontsova MS. 2017. Museomics resolve the systematics of an endangered grass lineage endemic to north-western Madagascar. Annals of Botany 119: 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniscalchi CM, Hidalgo O, Palazzesi L, et al. . 2021. Lineage-specific vs. universal: a comparison of the Compositae1061 and Angiosperms353 enrichment panels in the sunflower family. Applications in Plant Sciences 9: 1. doi: 10.1002/aps3.11422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DB, Havird JC, Sharbrough J. 2017. The on-again, off-again relationship between mitochondrial genomes and species boundaries. Molecular Ecology 26: 2212–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Brown JW, Walker JF. 2018. So many genes, so little time: a practical approach to divergence-time estimation in the genomic era. PLoS One 13: e0197433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Moore MJ, Brown JW, Yang Y. 2015. Analysis of phylogenomic datasets reveals conflict, concordance, and gene duplications with examples from animals and plants. BMC Evolutionary Biology 15: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solís-Lemus C, Bastide P, Ané C. 2017. PhyloNetworks: a package for phylogenetic networks. Molecular Biology and Evolution 34: 3292–3298. [DOI] [PubMed] [Google Scholar]
- Stankowski S, Streisfeld MA. 2015. Introgressive hybridization facilitates adaptive divergence in a recent radiation of monkeyflowers. Proceedings of The Royal Society B: Biological Sciences 282: 20151666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BA. 1987. Systematics and evolution of Centropogon subgenus Centropogon (Campanulaceae: Lobelioideae). Raven PH, ed. PhD Thesis. https://www.proquest.com/docview/303612520?pq-origsite=gscholar&fromopenview=true. [Google Scholar]
- Stein BA. 1992. Sicklebill hummingbirds, ants, and flowers: Plant-animal interactions and evolutionary relationships in Andean Lobeliaceae. Bioscience 42: 27–33. [Google Scholar]
- Swofford DL. 2001. PAUP*: Phylogenetic Analysis Using Parsimony (and other methods) 4.0.b5. Sunderland: Sinauer Associates. [Google Scholar]
- Than C, Ruths D, Nakhleh L. 2008. PhyloNet: a software package for analyzing and reconstructing reticulate evolutionary relationships. BMC Bioinformatics 9: 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AE, Igea J, Meudt HM, Albach DC, Lee WG, Tanentzap AJ. 2021. Using target sequence capture to improve the phylogenetic resolution of a rapid radiation in New Zealand Veronica. American Journal of Botany 108: 1289–1306. [DOI] [PubMed] [Google Scholar]
- Uribe-Convers S, Settles ML, Tank DC. 2016. A phylogenomic approach based on PCR target enrichment and high throughput sequencing: Resolving the diversity within the South American Species of Bartsia L. (Orobanchaceae). PLoS One 11: e0148203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachaspati P, Warnow T. 2015. ASTRID: Accurate Species TRees from Internode Distances. BMC Genomics 16: S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas OM, Ortiz EM, Simpson BB. 2017. Conflicting phylogenomic signals reveal a pattern of reticulate evolution in a recent high-Andean diversification (Asteraceae: Astereae: Diplostephium). The New Phytologist 214: 1736–1750. [DOI] [PubMed] [Google Scholar]
- Weitemier K, Straub SCK, Cronn RC, et al. . 2014. Hyb-Seq: Combining target enrichment and genome skimming for plant phylogenomics. Applications in Plant Sciences 2: 1400042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer FE. 1943. Campanulaceae-Lobelioideae. I. Teil. In: Mansfeld R, ed. Das Pflanzenreich IV.276b. Leipzig: Wilhem Engelmann, 1–260. [Google Scholar]
- Wimmer FE. 1955. Lobeliacearum species Novae Austro-Americanae. Brittonia 8: 107–111. [Google Scholar]
- Wimmer FE. 1968. Campanulaceae–Lobelioideae supplementum et Campanulaceae–Cyphiodeae. In: Danert S, ed. Das Pflanzenreich. Berlin: Akademie-Verlag, 815–1024. [Google Scholar]
- Wu M, Kostyun JL, Hahn MW, Moyle LC. 2018. Dissecting the basis of novel trait evolution in a radiation with widespread phylogenetic discordance. Molecular Ecology 27: 3301–3316. [DOI] [PubMed] [Google Scholar]
- Zhang C, Sayyari E, Mirarab S. 2017. ASTRAL-III: Increased scalability and impacts of contracting low support branches. In: Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). Cham: Springer, 53–75. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.