Abstract
Systemic lupus erythematosus (SLE) is a chronic inflammatory and representative autoimmune disease. Extremely complicated and multifactorial interactions between various genetic factors and individual susceptibility to environmental factors are involved in the pathogenesis of SLE. Several studies have reported that mutation and activation of toll-like receptor (TLR) 7 are involved in the onset of autoimmunity, including SLE. Thus, we investigated the response of SLE-prone mice to continuous environmental factors, particularly TLR7 agonist exposure, and changes in their phenotypes. Female and male NZBWF1 (BWF1) mice were treated from 20 weeks of age with a TLR7 agonist, imiquimod (IMQ), 3 times weekly for up to 12 weeks. IMQ-exposed female BWF1 mice showed worsened lupus nephritis. However, autoantibody production was not enhanced in IMQ-exposed female BWF1 mice. The Th1 cytokine expression was upregulated in the kidney of IMQ-treated mice. In IMQ-exposed BWF1 mice, neutralization of IFN-γ suppressed early-phase lupus nephritis. Additionally, in male BWF1 mice IMQ exposure induced minor aggravation of lupus nephritis. These results suggest that the induction of aggravated lupus nephritis by TLR7 agonist exposure was related to the expression of IFN-γ via acute TLR7 signal-induced renal inflammation, and that the involvement of genetic factors associated with a predisposition to SLE is also essential. Thus, the activation of TLR7 signaling by exposure to environmental factors may upset the balance of factors that maintain SLE remission. We hypothesize that the inhibition of TLR7 signaling and IFN-γ signaling is effective for preventing the onset and flare and maintaining remission of lupus nephritis.
Keywords: systemic lupus erythematosus, lupus nephritis, toll-like receptor 7, interferon-γ, environmental factors, Th1
We examined the phenotypes of imiquimod-induced SLE in female NZBWF1 mice. IFN-? signaling was involved in the early phase of development of imiquimod-induced lupus nephritis. However, in the later phase, IFN-? signaling no longer played a critical role.
Graphical Abstract
Graphical Abstract.
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease-associated with multiple organ failure and is characterized by the production of a wide variety of autoantibodies, immune complex deposits, and inflammatory cell infiltration in different tissues of the body. Among the damage to organs: lupus nephritis is one of the most serious complications [1].
Genetic and environmental factors trigger SLE. Although genetic factors are involved in the onset of SLE in the majority of cases, the frequency of SLE in both identical twins is ~25% [2]; i.e. the remaining cases occur due to environmental factors. In fact, many environmental triggers have been implicated in SLE, including ultraviolet light, infection, and other triggers [3].
In some reports, the overactivation of the intracellular nucleic acid sensor has been reported as a trigger for autoimmunity. Among them, toll-like receptor (TLR) 7 is an important virulence factor for SLE in humans and mouse lupus models. Pathogenicity due to the increased expression of TLR7 has been demonstrated in TLR7 transgenic mice [4] and Y-linked autoimmune acceleration (Yaa) mutation mice (these mice have the duplicate expression of the Tlr7 gene [5]). In humans, the increased expression of TLR7, which is associated with escape from X-chromosome inactivation, has been shown to be involved in the pathogenesis of SLE [6]. The overexpression of TLR7 causes excessive signal activation. Also, artificial overactivation of TLR7 signaling can elicit SLE-like autoimmunity [7]. TLR7 is a receptor that recognizes single-stranded RNA and is originally a sensor used to protect against foreign viruses [8]. However, the overactivation of TLR7 by environmental factors may lead to the exacerbation of SLE pathology. In fact, it has been clarified that wild-type mice develop lupus pathology through the forcible activation of TLR7 signaling by external factors [7]. Therefore, mice predisposed to SLE may have their SLE pathology exacerbated by exposure to TLR7 agonists compared to wild-type mice.
In the present study, we examined the effect of the activation of TLR7 signaling on the onset of SLE and its symptoms using NZBWF1 (BWF1) mice, a mouse model of lupus. BWF1 mice develop glomerulonephritis due to an increase in autoantibodies with aging [9–11]. Therefore, we investigated the possibility of SLE symptoms developing at an earlier age than normal and the physiological responses in BWF1 mice exposed to TLR7 agonists. By using and validating such a model, we hoped to address the question of what would occur if people at risk of developing SLE were exposed to TLR7 agonists and how they could avoid either the onset flare-ups of SLE.
Materials and methods
Mice and in vivo treatment
All mouse experiments were performed in accordance with the Institutional Animal Care and Use Committee of Juntendo University (approval number: 290043, 300100, 310237, and 2020094). Female and male NZBWF1 (BWF1) mice were purchased from Japan SLC (Hamamatsu, Japan). C57BL/6J mice were purchased from Charles River Laboratories Japan (Yokohama, Japan). Purchased mice were acclimatized to housing conditions at least 1 week before experiments. All mice were randomly assigned, and group was housed at 3–6 mice per cage. The number of animals in all experiments can be found in the figure legends.
Female or male BWF1 mice (age: 20 weeks) with a serum anti-double-stranded DNA (dsDNA) antibody (Ab) titer of <50 U/ml were measured for 1.25 μl (female; N = 7–15 mice per group) or 1.7 μl (male; N = 6 per group) of imiquimod (IMQ) cream (Beselna Cream; Mochida Pharmaceutical, Tokyo, Japan) using MICROMAN E (GILSON, Middleton, WI) and then the cream was applied topically to the skin on one side of the pinna three times a week, as previously described [7]. Female BWF1 mice rarely develop nephritis at around 20 weeks of age [12]; however, those with an excessive autoantibody level at a young age may spontaneously develop SLE symptoms independently of IMQ stimulation. Thus, we excluded these mice from the experiment.
For systemic treatment, the mice were injected intraperitoneally with 62.5 μg of R848 (resiquimod; Enzo Life Sciences, Farmingdale, NY) three times a week.
To neutralize IFN-γ, the mice were injected intraperitoneally with 300 μg of anti-mouse IFN-γ Ab (XMG1.2; BioLegend, San Diego, CA) every 7 days during the topical treatment with IMQ.
All mice were bred under specific pathogen-free conditions at the animal facilities of the Institute for Environment and Gender-Specific Medicine, Juntendo University Graduate School of Medicine, with ad libitum access to feed, and were treated humanely and with efforts made to alleviate suffering.
Enzyme-linked immunosorbent assay (ELISA)
The total IgM and IgG values in serum were determined with an ELISA kit (Thermo Fisher Scientific, Waltham, MA). Serum anti-dsDNA Ab levels (specific for IgG) were analyzed by a mouse anti-dsDNA antibody ELISA kit (FUJIFILM Wako Shibayagi, Shibukawa, Japan).
Flow cytometry
The spleen was crushed and passed through a 40-μm-pore nylon mesh. Kidney-infiltrating cells were prepared using a Multi Tissue Dissociation Kit 1 (Miltenyi Biotec, Bergisch Gladbach, Germany) with a gentleMACS Dissociator (Miltenyi Biotec), according to the manufacturer’s instructions. The obtained erythrocytes were lysed using a Lysing Buffer (BD Biosciences, San Jose, CA). Cells were stained with saturating concentrations of Abs (Supplementary Table S1) for 20–30 min at 4 °C and washed in FACS buffer (PBS containing 0.1% BSA and 0.09% sodium aside). If needed, cells were then stained with saturating concentrations of appropriate fluorochrome-conjugated streptavidin for 20 min at 4 °C. To detect intracellular IFN-γ, cells were incubated for ~5 h after the initiation of phorbol 12-myristate 13-acetate (Sigma–Aldrich, Tokyo, Japan) and ionomycin (Sigma–Aldrich) with brefeldin A (FUJIFILM Wako, Osaka, Japan). After incubation, cells were stained with surface antigen, then fixed with an IC fixation buffer (Invitrogen/eBioscience, Carlsbad, CA) for 30 min, permeabilized with a permeabilization buffer (Invitrogen/eBioscience), and stained with anti-IFN-γ Ab. Cells were analyzed using a FACSCalibur (BD Biosciences) and SH800 cell sorter (SONY, Tokyo, Japan), and the data were analyzed with the FlowJo software program (BD/Tree Star).
Serum type I IFN activity assay
B16 Blue IFNα/β reporter cells that produce secreted alkaline phosphatase (SEAP) under the control of type I IFN signaling were purchased from Invivogen (San Diego, CA). The reporter cells suspension in 100 μl RPMI medium (Sigma–Aldrich) containing 1% fetal bovine serum (FBS) were seeded in a 96-well plate, and 100 μl RPMI containing 1% FBS and 1% serum from BWF1 mice were added. After 20 h, culture media were collected and used for the SEAP assay. The SEAP activity in culture media was evaluated by a chemiluminescent method using a Great EscAPe SEAP Chemiluminescence Kit 2.0 (TaKaRa) according to the manufacturer’s instructions.
Monitoring proteinuria
Sufficient spot urine was subjected to a qualitative assessment for proteinuria, and measured using a dipstick (Uropaper III, EIKEN, Tokyo, Japan) and scored as – (<15 mg/dl), ± (15–30 mg/dl), 1+ (30–100 mg/dl), 2+ (100–300 mg/dl), 3+ (300–2000 mg/dl), or 4+ (>2000 mg/dl), according to the manufacturer’s scoring system. Severe nephritis was defined as a proteinuria score of ≥2+.
Renal histopathology and scoring
Mouse kidneys were fixed in 20% formalin and embedded in paraffin. Kidney sections were cut at a thickness of 2 μm for periodic acid-Schiff (PAS) and hematoxylin & eosin (HE) staining. For immunofluorescence staining, kidneys were embedded in OCT Compound (Sakura Finetek Japan, Tokyo, Japan) and frozen. Three-micrometer-thin sections were fixed in 4% paraformaldehyde. The sections were stained with the following antibodies: anti-mouse IgM (μ chain) (Invitrogen), anti-mouse IgG (H+L) (Invitrogen), anti-C3 (6A525; Santa Cruz, Santa Cruz, CA), and anti-C1q (JL-1; Abcam, Cambridge, UK) (Supplementary Table S1).
Renal pathology was classified into six types using the revised International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification of lupus nephritis [13, 14]. In brief, Class I, minimal mesangial glomerulonephritis; Class II, mesangial proliferative glomerulonephritis, showing purely mesangial hypercellularity of any degree and/or mesangial matrix expansion; Class III, focal glomerulonephritis involving <50% of the total number of glomeruli; Class IV, diffuse segmental or global glomerulonephritis involving ≥50% of the total number of glomeruli either segmentally or globally. Class V is membranous glomerulonephritis. Class VI is advanced sclerotic glomerulonephritis with >90% of glomeruli globally sclerosed without residual activity. Renal tissue injury was evaluated using the modified National Institutes of Health lupus nephritis activity and chronicity indices [14]. Briefly, the activity indices were the sum of scores (on a scale of 1–3) for endocapillary hypercellularity, neutrophils/karyorrhexis, fibrinoid necrosis, hyaline deposits, cellular/fibrocellular crescents, and interstitial inflammation. The fibrinoid necrosis and cellular/fibrocellular crescents scores were double-weighted. The chronicity indices were the sum of scores (on a scale of 1–3) for total glomerulosclerosis sore, fibrous crescents, tubular atrophy, and interstitial fibrosis. All histological evaluations were assessed by three independent investigators, but at least two researchers were unaware of the group allocation.
RNA extraction and quantitative polymerase chain reaction (qPCR)
Total RNA extraction and reverse transcription-qPCR were performed as described previously [15, 16]. Briefly, total RNA was extracted using an Isogen II (Nippon Gene, Tokyo, Japan), RNeasy Mini kit, RNeasy micro kit, or miRNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The extracted RNA was reverse transcribed with a PrimeScript RT reagent Kit (TaKaRa, Kusatsu, Japan). qPCR was performed using a TB Green Premix Ex Taq (TaKaRa) and the QuantStudio 5 (Thermo Fisher Scientific) or LightCycler 480 System II (Roche, Basel, Switzerland). The results were normalized to the β-actin expression or calculated by the comparative Ct (ΔΔCt) method. Various specific primer sequences are listed in Supplementary Table S2.
Primary mesangial cell culture
Kidneys from 7-week-old female C57BL/6J mice were harvested. Glomeruli were isolated by a differential sieving method through serial steel meshes (63, 106, and 180 μm pore size) [17]. These purified glomeruli were collected and seeded to Dulbecco’s Modified Eagle’s Medium/Ham’s F-12 (FUJIFILM Wako) supplemented with penicillin (100 U/ml)/streptomycin (100 μg/ml) (Sigma–Aldrich) and 10% FBS. Outgrowing cells were passed to subcultures. Cells were identified as mesangial cells based on their growth pattern and the staining pattern for α-smooth muscle actin [18, 19]. We used the mesangial cells between the eighth and twentieth passages and a medium containing 1% FBS was generally used for studies. Trypsinized-mesangial cells were seeded in 12-well plates. After incubating overnight, the cells were treated with recombinant human IL-1β (10 ng/ml; R&D Systems, Minneapolis, MN) or TNF-α (10 ng/ml; R&D Systems) and recombinant murine IFN-γ (50 ng/ml; BioLegend) with or without R848 (resiquimod, 1 μg/ml) for 24 h and then subjected to qPCR.
MACS purification
For purification of CD4+ T cells from C57BL/6J mice (age: 7–16 weeks, N = 2–4 mice per experiment), splenocytes were harvested and using the CD4+ T cell Isolation Kit (Miltenyi Biotec) according to the manufacturer’s instructions and cells were separated using an autoMACS Pro Separator (Miltenyi Biotec) (Negative selection). The cell purity was ≥90%.
Western blotting
MACS-purified CD4+ T cells were seeded onto an anti-CD3ε Ab (BioLegend) immobilized plate and stimulated with IFN-α (BioLegend) or IFN-γ (BioLegend) for 24 h. Cells were lysed with 0.5% NP-40 lysis buffer and SDS–PAGE and western blotting were performed [20]. Abs for western blotting were against TLR7 (NBP2-24906; Novus, Centennial, CO) and β-actin (AC-15; Sigma–Aldrich). Horseradish peroxidase-conjugated anti-IgG secondary Abs against rabbit IgG (Dako, Glostrup, Denmark) or mouse IgG (Cell Signaling, Danvers, MA) were used with Chemi-Lumi One substrate (Nacalai Tesque, Kyoto, Japan).
Th1 skewing
For the sorting of naïve CD4+ T cells, MACS-purified splenic CD4+ T cells from C57BL/6J mice were stained with CD4 and CD25, and CD4+ CD25- cells were sorted with an SH800 cell sorter. Sorted naïve CD4+ T cells were seeded into anti-CD3ε Ab (BioLegend)-coated wells in the presence of anti-CD28 Ab (1 μg/ml; BioLegend), anti-IL-4 Ab (10 μg/ml; BioLegend) plus recombinant murine IL-2 (10 ng/ml; BioLegend) and recombinant murine IL-12 (p70) (10 ng/ml; BioLegend) with or without R848 (1 μg/ml) in complete RPMI medium (RPMI medium supplemented with 10% FBS, sodium pyruvate (1 mM; FUJIFILM Wako), nonessential amino acid (FUJIFILM Wako), 2-mercaptoethanol (50 μM; FUJIFILM Wako), HEPES (10 mM; Nacalai Tesque) and penicillin/streptomycin). After 7 days, cells were restimulated with phorbol 12-myristate 13-acetate (10 ng/ml) and ionomycin (1 μg/ml) with brefeldin A (1 μg/ml) for five hours. At the end of incubation, cells were stained with CD4 and intracellularly stained with IFN-γ.
Co-culture of mesangial cells and the Th1 cell migration assay
C57BL/6J mice derived-mesangial cells were co-cultured with nonpolarized (Th0) cells or Th1 cells using Millicell inserts (Millipore, Burlington, MA) or cell culture inserts (Corning, Corning, NY) with an anti-CD3ε Ab pre-coated 0.4-μm porous membrane and then treated with IL-1β or R848 and exposed to anti-IFN-γ Ab (10 μg/ml; BioLegend) or control IgG (BioLegend) for 24 h. After incubation, mesangial cells and CD4+ T cells were subjected to qPCR.
An in vitro migration assay was performed using cell culture inserts (Corning) with an anti-CD3ε Ab pre-coated 3-μm porous membrane. Mesangial cells were seeded into a bottom chamber, and carboxyfluorescein diacetate succinimidyl ester (CFSE; BioLegend) labeled-skewed Th1 cells were seeded on the top chamber of each insert. Cells were then treated with IL-1β or R848, and control IgG or anti-IFN-γ Ab for 24 h. After incubation, CFSE labeled Th1 cells that had migrated into the bottom chamber were counted using a fluorescence microscope (BZ-810; Keyence, Osaka, Japan).
Statistical analyses
This study was neither officially pre-registered nor were any a priori power calculations completed to determine the sample size, as this was an exploratory study. Statistical analyses were performed using the GraphPad Prism 6 software program (GraphPad Software, La Jolla, CA). In the animal study, differences between groups were compared using Student’s t-test (two groups) or a one-way ANOVA followed by a post-Tukey or Dunnett test (multiple groups). In the comparison of renal pathology scores, differences between groups were compared using the Mann-Whitney U test (two groups) or Kruskal–Wallis test followed by Dunn’s post hoc test (multiple groups). In co-culture studies, statistical analyses were performed using Student’s paired t-test. P values of < 0.05 were considered to indicate statistical significance.
Results
Exposure of female BWF1 mice to IMQ
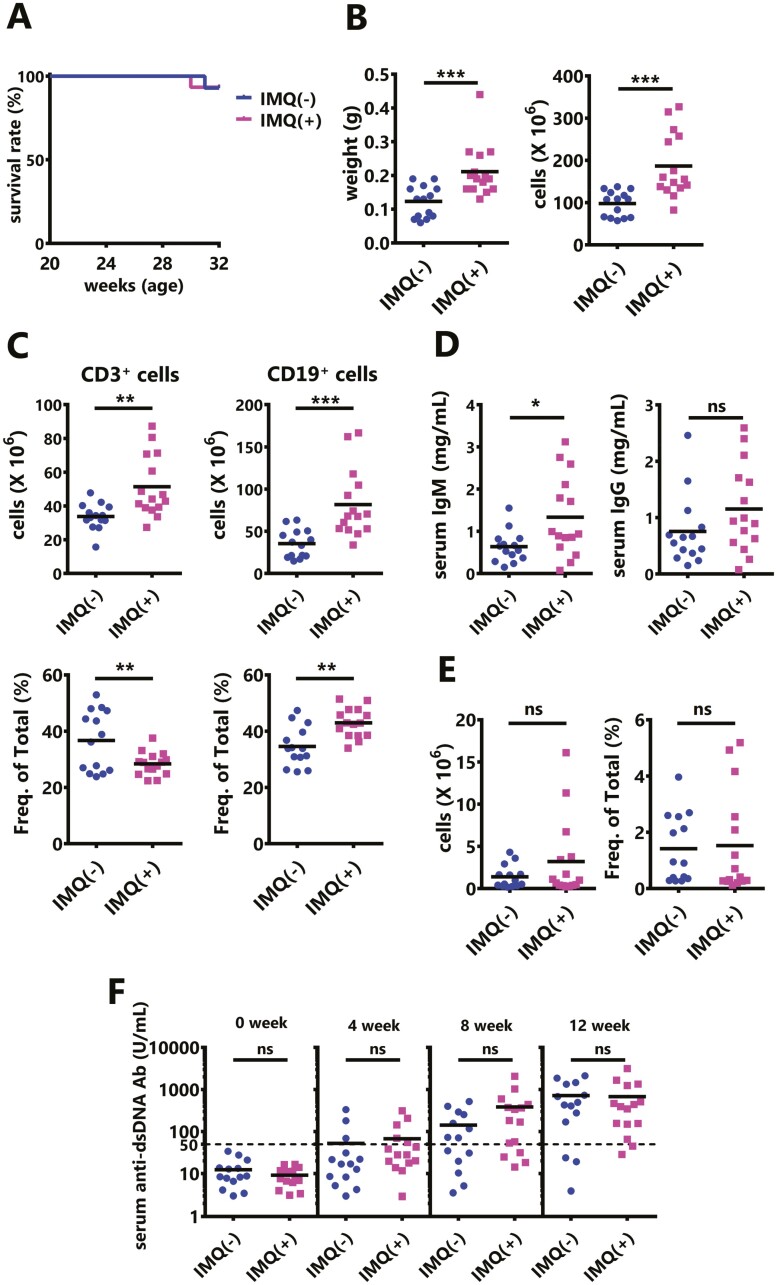
To examine the phenotype of exposure to TLR7 agonists in SLE model mice, we performed the continuous transdermal administration of IMQ to BWF1 mice. Repeated treatment with IMQ could cause severe systemic inflammation [7] but did not significantly affect the survival at our facility (Fig. 1A). However, IMQ treatment induces splenomegaly (Fig. 1B), and CD3+ T cells and CD19+ B cells were significantly increased in the IMQ-treated mouse spleen (Fig. 1C upper panel). In contrast, the percentage of T cells was significantly decreased, while the percentage of B cells was significantly increased in splenocytes (Fig. 1C lower panel). The serum IgM levels were significantly elevated, while the serum IgG levels were only slightly increased, despite inducing inflammation (Fig. 1D). Furthermore, there was no significant increase in the number or percentage of follicular helper T (Tfh) cells, which are helper T cells that regulate B cell maturation and activation as well as antibody production, in the spleens of IMQ-treated mice (Fig. 1E). Additionally, the serum anti-dsDNA Ab levels were not enhanced by IMQ treatment (Fig. 1F). Although the Type I IFN activity in serum was measured, it did not increase in IMQ-exposed mice at 8 weeks (Supplementary Fig. S1). Taken together, IMQ stimulation of BWF1 mice did not promote autoimmunity, although there was excessive immune cell activation.
Figure 1.
Continuous transcutaneous sensitization of imiquimod (IMQ) does not accelerate autoimmunity in NZBWF1 (BWF1) mice. IMQ was topically administered on one ear to 20-week-old female BWF1 mice three times weekly for 12 weeks. (A) The survival curves are shown (IMQ[−]: N = 14, IMQ[+]: N = 15). (B) Spleen weight (left) and the total number of splenocytes (right) from untreated BWF1 mice, treated with IMQ for 12 weeks, or moribund. (C) The numbers and percentages of CD3+ T cells and CD19+ B cells in splenocytes from untreated mice, treated with IMQ for 12 weeks, or moribund were obtained through a flow cytometric analysis (IMQ[−]: N = 14, IMQ[+]: N = 15). (D) Total IgM and IgG in the serum of BWF1 mice that were treated with IMQ for 12 weeks or moribund (IMQ[−]: N = 14, IMQ[+]: N = 15). (E) The percentages and numbers of follicular helper T cells (Tfh: CD4+ TCRβ+ CXCR5hi PD-1hi) in splenocytes from untreated mice, treated with IMQ for 12 weeks, or moribund were obtained through a flow cytometric analysis (IMQ[−]: N = 14, IMQ[+]: N = 15). (F) Serum was collected before, and after 4, 8, and 12 weeks of treatment, and the anti-dsDNA antibody (Ab) level was measured by an ELISA (IMQ[−]: N = 14, IMQ[+]: N = 15). The results were analyzed by a log-rank test (A) or a two-tailed Student’s t-test (B–F). Each dot represents an individual mouse. Asterisks indicate statistically significant differences (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001) (ns: not significant).
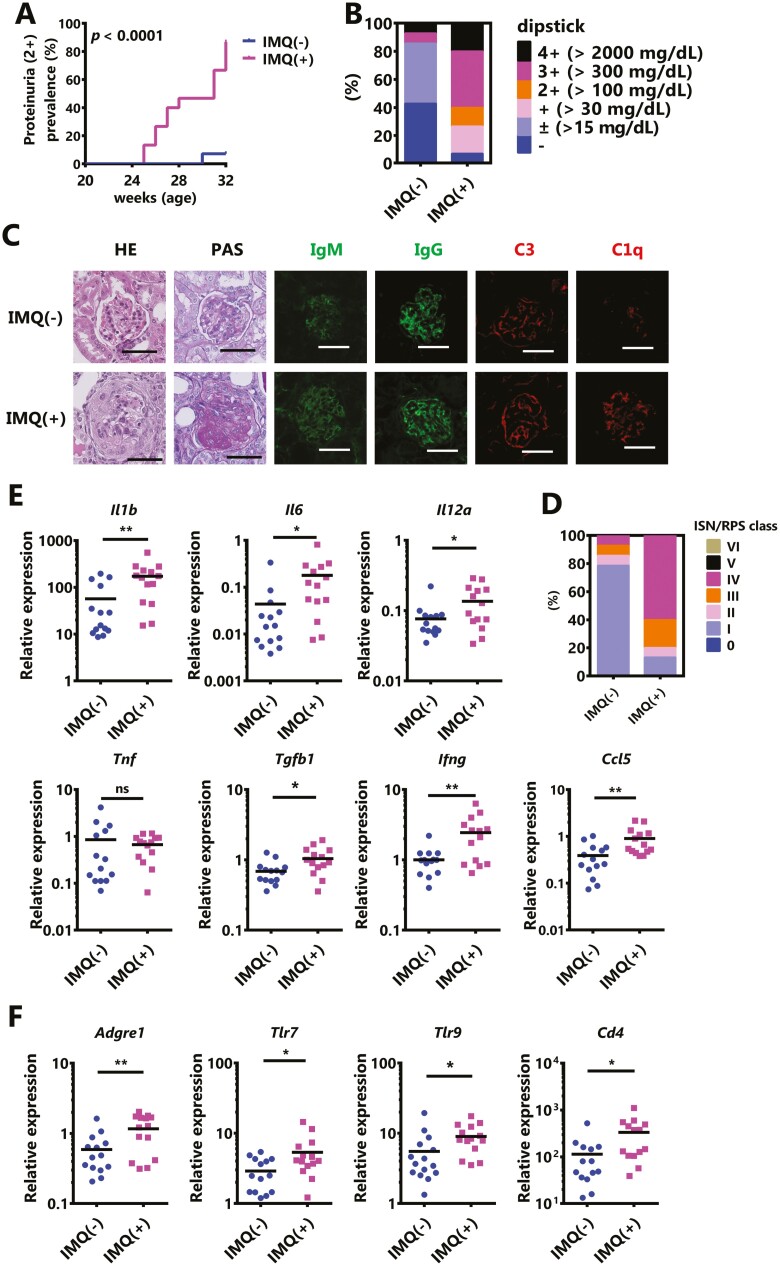
On the other hand, the monitoring of renal damage revealed that IMQ treatment significantly accelerated the onset of severe proteinuria (Fig. 2A). In a qualitative test of proteinuria at 12 weeks after the initiation of IMQ treatment (32 weeks of age), IMQ-treated mice showed more severe proteinuria (≥2+) in comparison to spontaneous onset (Fig. 2B: IMQ(−); 2/14, IMQ(+); 11/15). A pathological analysis of the kidneys showed that IMQ-treatment promoted endocapillary hypercellularity, crescent formation, and immune complex deposition (Fig. 2C). The severity of glomerulonephritis was evaluated based on the revised ISN/RPS classification of lupus nephritis, and IMQ treatment increased the frequency of severe glomerulonephritis (Fig. 2D, Table 1).
Figure 2.
IMQ stimulation leads to the dominant development of glomerulonephritis in BWF1 mice. Twenty-week-old female BWF1 mice were treated with IMQ for 12 weeks. (A) The chronological progression of proteinuria. The rate of severe proteinuria (≥2+ in a qualitative test) is shown (IMQ[−]: N = 14, IMQ[+]: N = 15). The P value shows differences in Kaplan–Meier curves between IMQ(−) and IMQ(+). (B) Proteinuria was measured by dipstick at sampling. The ratio of each score is shown. (C) Representative images of kidney sections BWF1 mice without treatment or with 12 weeks of topical treatment with IMQ. Sections were stained with hematoxylin & eosin (HE), periodic acid–Schiff (PAS), anti-IgM, anti-IgG, anti-C3, and anti-C1q. Bar=50 μm. (D) Lupus nephritis was characterized based on the revised International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003 classification. The ratio of each score is shown (IMQ[−]: N = 14, IMQ[+]: N = 15). (E, F) Whole kidneys were harvested from these mice and RNA was extracted. The expression of (E) Il1b, Il6, Il12a, Tnf, Tgfb1, Ifng, and Ccl5, and (F) Cd4, Adgre1 (also known as F4/80), Tlr7, and Tlr9 was determined by quantitative polymerase chain reaction (qPCR). The expression of the indicated genes was normalized to that of Actb (IMQ[−]: N = 14, IMQ[+]: N = 14). A statistical analysis was performed using a log-rank test (A) or a two-tailed Student’s t-test (E, F). Asterisks indicate statistically significant differences (∗P < 0.05, ∗∗P < 0.01) (ns: not significant).
Table 1.
The renal pathological score (modified National Institutes of Health (NIH) activity and chronicity indices) in female NZBWF1 (BWF1) mice with or without imiquimod (IMQ) treatment for 12 weeks
| IMQ(−) (n = 14) | IMQ(+) (n = 15) | P value | |
|---|---|---|---|
| Mean±SD | Mean±SD | Mann–Whitney test | |
| Activity index | 2.452 ± 2.809 | 7.667 ± 5.111 | 0.0001 |
| Chronicity index | 0.548 ± 1.21 | 1.778 ± 1.975 | 0.0032 |
Next, to identify cytokines and cells type that promote IMQ-induced nephritis, the gene expression of renal inflammatory mediators and various cell surface proteins was measured by qPCR. The results, it was suggested that IMQ treatment increased the expression of many inflammatory cytokines and caused renal injury (Fig. 2E). Furthermore, since the expression levels of Ardgre1 (encoding F4/80), Tlr7/9, and Cd4 were increased in the kidney (Fig. 2F) may indicate that IMQ treatment triggered glomerulonephritis by infiltrating these cell surface marker-expressing cells into the kidney. Therefore, IMQ treatment did not promote autoimmunity in BWF1 mice; however, it preferentially exacerbated their nephritis.
In a previous study using MRL-lpr mice, another lupus-prone mouse, continuous intraperitoneal administration of IMQ exacerbated glomerulonephritis [21]. We, therefore, verified the systemic administration of R848, which is a TLR7/8 agonist and more soluble in water than IMQ, with BWF1 mice by intraperitoneal administration. However, unlike the transdermal administration of IMQ and findings in previous studies, the BWF1 mice fell into a moribund state after 4 weeks of R848 systemic exposure. The anti-dsDNA Abs level in these mice did not increase (Supplementary Fig. S2A); however, they showed a marked increase in liver weight (Supplementary Fig. S2B). On the other hand, there was almost no kidney damage (Supplementary Fig. S2C).
Influence of IFN-γ and Th1 cells on renal mesangial cell activation
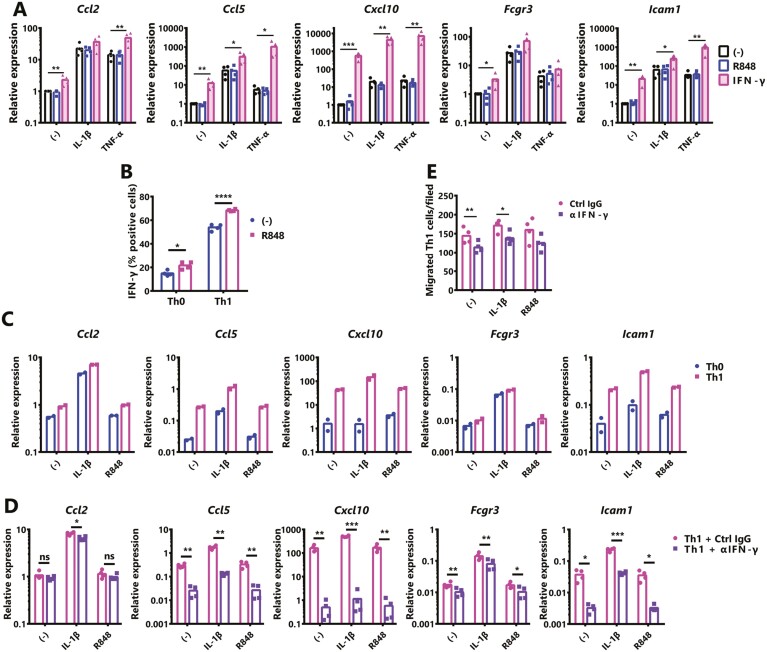
The activation of TLR7 signaling increased the renal expression of Th1 cytokines, especially Ifng (Fig. 2E). One of the roles of IFN-γ in lupus nephritis is to induce the expression of MHC class II in mesangial cells and renal tubular epithelial cells. It has been suggested that the expression of MHC class II allows these cells to act as antigen-presenting cells, amplifying the immune response and enhancing nephritis [22]. Therefore, we examined the effects of IFN-γ and TLR7 signaling on glomerular mesangial cells under inflammatory conditions. In the development of glomerulonephritis, mesangial cells enhance the expression of chemokines and adhesion molecules by inflammatory stimuli and promote the infiltration of leukocytes into the glomerulus [23]. Primary mesangial cells were stimulated with IL-1β or TNF-α and exposed to IFN-γ with or without R848 for 24 h. Although there are slight differences between IL-1β and TNF-α stimulation, the presence of IFN-γ enhanced both in the inflammatory response of mesangial cells. On the other hand, R848 remained unchanged in the inflammatory response of mesangial cells (Fig. 3A). Hence, it was suggested that renal cells are indirectly inflamed by the TLR7 signaling in vivo and are indirectly affected by other cell-derived factors.
Figure 3.
Enhancement of the inflammatory response in renal mesangial cells by Th1-derived IFN-γ. (A) Primary normal mouse mesangial cells were exposed to IL-1β (10 ng/ml), TNF-α (10 ng/ml), IFN-γ (50 ng/ml) and IL-1β+IFN-γ, TNF-α+IFN-γ with or without TLR7 agonist R848 (1 μg/ml) for 24 h. The expression of indicated genes was examined by qPCR. (B) Percentages of intracellular IFN-γ production from polarized Th1 cells in the presence or absence of R848 (1 μg/ml), assessed after 5 h of restimulation with phorbol 12-myristate 13-acetate (10 ng/ml) + ionomycin (1 μg/ml). (C) Mesangial cells were co-cultured with a T-cell receptor (TCR)-stimulated Th0 or Th1 cells using a Transwell system (0.4-μm pore). Cells were treated with IL-1β or R848 for 24 h. The expression of indicated genes was examined by qPCR. Assays were performed in duplicate. (D, E) Mesangial cells were co-cultured with TCR-stimulated Th1 cells using a cell culture insert ((D); 0.4-μm pore Millicell, or (E); 3-μm pore Transwell). Cells were treated with IL-1β or R848 and exposed to control IgG (Ctrl) or anti-IFN-γ Ab (αIFN-γ; 10 μg/ml) for 24 h. (D) Mesangial cells were subjected to qPCR. (E) Th1 cells that migrated to the bottom chamber were counted. Values represent the means, and each dot represents an individual experiment (N = 2 or 4). A one-way ANOVA followed by Dunnett’s multiple-comparison test (A), a two-tailed unpaired Student’s t-test (B), or paired Student’s t-test (D, E) was used to analyze the results. Asterisks indicate statistically significant differences (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001) (ns: not significant).
T cells, group 1 innate lymphoid cells (including γδT, NK, and NKT cells), and part of group 3 innate lymphoid cells are known as IFN-γ producing cells [24]. As shown in Fig. 2F, more Cd4 mRNA was detected in the kidneys of IMQ-treated mice than non-treated mice and may be increased CD4+ cells. Therefore, it was considered that the increased expression of Ifng in the kidney may be related to CD4+ T cells, especially Th1 cells, and we, therefore, examined whether TLR7 signaling promotes Th1 differentiation. First, we examined whether TLR7 was expressed on CD4+ T cells. Although TLR7 was slightly expressed in steady-state CD4+ T cells, IFN-α or IFN-γ stimulation slightly enhanced the TLR7 expression, and the activation of T-cell receptor (TCR) markedly increased the TLR7 expression (Supplementary Fig. S3A and B). Sorted naïve CD4+ T cells were cultured in Th1 conditions with or without R848 for 7 days, then intrasellar IFN-γ was stained. A FACS analysis revealed that the induction of Th1 cells was significantly increased by R848 treatment (Fig. 3B). Interestingly, the presence of R848 in Th0 condition culture enhanced the IFN-γ expression.
Next, to examine the effect of Th1 cells on the inflammatory response of mesangial cells, we co-cultured mesangial cells and TCR-activated Th0 or Th1 cells with IL-1β or R848 stimulation, and the expression of inflammatory mediators in mesangial cells was analyzed by qPCR. Co-culturing with Th1 cells markedly enhanced the inflammatory response of mesangial cells in comparison to co-culturing with Th0 cells (Fig. 3C) because the TNF-α expression level was higher in TCR-activated Th1 cells (Supplementary Fig. S3C). At the same time, the inflammatory responses of mesangial cells and the activation of Th cells were unaffected by the presence of R848.
TCR-activated CD4+ T cells prominently produce TNF-α, while only Th1 produces high IFN-γ. Therefore, the increased inflammatory response of Th1-derived mesangial cells may be due to Th1-derived IFN-γ. To investigate this hypothesis, mesangial cells and activated Th1 cells were co-cultured with or without anti-IFN-γ Ab, as a result, the expression of chemokines that attract activated T cells (e.g. Ccl5 and Cxcl10) in mesangial cells was significantly suppressed by the neutralization of IFN-γ (Fig. 3D). This suppression was also similar for the presence of IL-1β and R848. Next, we examined whether Th1-derived IFN-γ is involved in the migration of Th1 by mesangial cells. Mesangial cells were seeded in the lower chamber and Th1 was added to the upper chamber on which anti-CD3ε Ab was immobilized and cultured in the presence of IL-1β or R848 with or without anti-IFN-γ Ab for 24 h, and the number of Th1 cells that infiltrated into the lower chamber was counted. As shown in Fig. 3E, neutralization of IFN-γ reduced the infiltration of Th1 cells to the mesangial cells. Therefore, IFN-γ enhances mesangial cell activation by inflammatory stimuli, increased adhesion molecules and immune complex deposition, and cell migration. It was suggested that the increase of Th1 by the enhancement of TLR7 signaling was partly responsible for this increase in IFN-γ.
Inhibition of IFN-γ signaling in IMQ-exposed female BWF1 mice
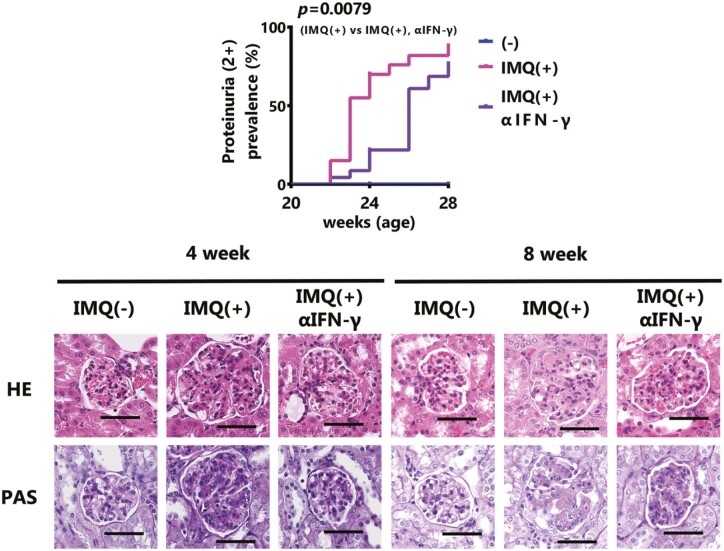
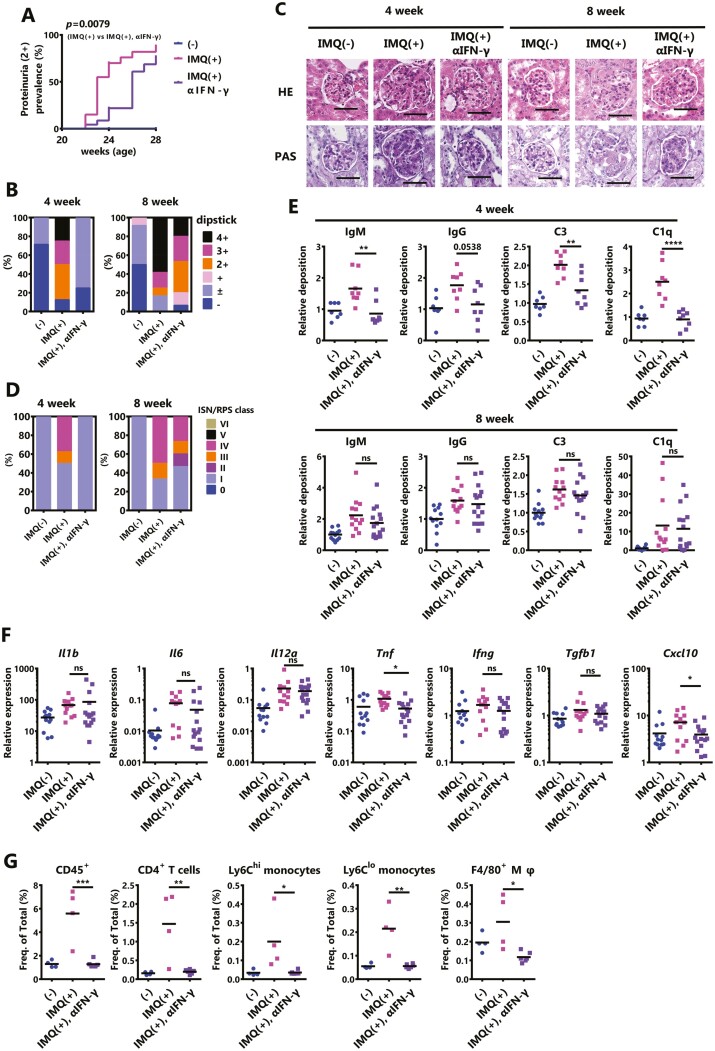
Based on the results of in vitro experiments, IFN-γ, which is increased by the activation of TLR7 signaling, may exacerbate IMQ-induced lupus nephritis. Therefore, we examined whether IFN-γ is involved in the development of IMQ-induced lupus nephritis using neutralizing antibodies. The development of severe proteinuria (≥2+) promoted by IMQ-treatment was significantly reduced by the neutralization of IFN-γ, but the effect disappeared at the late phase (Fig. 4A and B). A histopathological analysis showed that the progression of nephritis tended to be inhibited by the neutralization of IFN-γ 4 weeks after the beginning of IMQ stimulation, but at 8 weeks, the effect of IFN-γ neutralization was markedly attenuated (Fig. 4C and D, Table 2). Focusing on the renal tubulointerstitium, four weeks after the start of IMQ stimulation, the accumulation of lymphocytes tended to be reduced by IFN-γ neutralization, a condition that was further maintained after 8 weeks (Supplementary Fig. S4A). In contrast, the immune complex deposition in the glomerulus was the neutralization of IFN-γ can suppress the deposition in the early phase but has little effect in the late phase (Fig. 4E). Concurrently, the serum anti-dsDNA Ab levels did not change with the administration of anti-IFN-γ (Supplementary Fig. S4B). The expression of inflammatory mediators in the kidney at 8 weeks after starting IMQ stimulation remained almost unchanged except for Tnf and Cxcl10 which decreased after neutralizing IFN-γ (Fig. 4F). It was, therefore, suggested that IFN-γ plays an important role only in the progression of early-phase IMQ-induced lupus nephritis. In particular, IFN-γ may contribute to the exacerbation of IMQ-induced lupus nephritis by promoting early-phase leukocyte infiltration into the kidney (Fig. 4G, Supplementary Fig. S4C).
Figure 4.
IFN-γ aggravates IMQ-induced nephritis in the early phase. Twenty-week-old female BWF1 mice were neutralized with IFN-γ monoclonal Ab during 8 weeks of treatment with IMQ. (A) The rate of severe proteinuria (≥2+ on a qualitative test) is shown (IMQ[−]: N = 12–19, IMQ[+]: N = 12–20, IMQ[+], αIFN-γ: N = 15–23). The P value shows the differences in Kaplan–Meier curves between IMQ(+) versus IMQ(+) and αIFN-γ. (B) Proteinuria was measured by dipstick at sampling. The ratio of each score is shown (4 week; IMQ[−]: N = 7, IMQ[+]: N = 8, IMQ[+], αIFN-γ: N = 8, 8 week; IMQ[−]: N = 12, IMQ[+]: N = 12, IMQ[+], αIFN-γ: N = 15). (C) Representative images of kidney sections from mice treated with IMQ for 4 weeks or 8 weeks. Sections were stained with HE and PAS. Bar = 50 μm (D) Lupus nephritis was characterized based on the revised ISN/RPS 2003 classification. The ratio of each score is shown (4 week; IMQ[−]: N = 7, IMQ[+]: N = 8, IMQ[+], αIFN-γ: N = 8, 8 week; IMQ[−]: N = 12, IMQ[+]: N=12, IMQ[+], αIFN-γ: N = 15). (E) Kidney sections from mice treated with IMQ for 4 weeks or 8 weeks. Sections were stained with anti-IgM, anti-IgG, anti-C3, and anti-C1q, and were observed by fluorescence microscopy. The deposition of IgM, IgG, C3, and C1q in the kidney was shown using the mean fluorescence intensity (4 week; IMQ[−]: N = 7, IMQ[+]: N = 8, IMQ[+], αIFN-γ: N = 8, 8 week; IMQ[−]: N = 12, IMQ[+]: N = 12, IMQ[+], αIFN-γ: N = 15). (F) Total RNA was extracted from the whole kidney of mice treated for 8 weeks. The expression level of the indicated genes was examined by qPCR. The expression of the indicated genes was normalized to that of β-actin. (IMQ[−]: N = 12, IMQ[+]: N = 12, IMQ[+], αIFN-γ: N = 15). (G) The changes in the populations of indicated infiltrating lymphocytes in the kidney after 4 weeks of IMQ treatment were measured by FACS (IMQ[−]: N = 4, IMQ[+]: N = 4, IMQ[+], αIFN-γ: N = 6). CD4+ T cells; CD45+ CD4+ CD11b− CD11c−, Ly6Chi monocytes; CD45+ CD11b+ Ly6G- F4/80− Ly6C+ CD43hi, Ly6Clo monocytes; CD45+ CD11b+ Ly6G- F4/80- Ly6C− CD43lo, F4/80+ macrophages (Mφ); CD45+ CD11b+ Ly6G− F4/80+. The results were analyzed by a log-rank test (A) or one-way ANOVA followed by Tukey’s multiple-comparison test (E–G). Asterisks indicate statistically significant differences (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001) (ns: not significant) and numbers indicate P values.
Table 2.
Renal pathological score (modified NIH activity and chronicity indices) in IMQ-exposed female BWF1 mice with or without anti-IFN-γ antibody treatment for 4 or 8 weeks
| 4 weeks | IMQ(−) (n = 7) | IMQ(+) (n = 8) | IMQ(+) αIFN-γ (n = 8) |
P value IMQ(+) vs. IMQ(+), αIFN-γ |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Kruskal–Wallis and Dunn tests | |
| Activity index | 0.476 ± 0.424 | 3.125 ± 2.702 | 1.208 ± 0.689 | >0.9999 |
| Chronicity index | 0 ± 0 | 0.833 ± 1.054 | 0.0833 ± 0.154 | 0.0698 |
| 8 weeks | IMQ(−) (n = 12) | IMQ(+) (n = 12) | IMQ(+), αIFN-γ (n = 15) |
P value IMQ(+) vs. IMQ(+), αIFN-γ |
| Mean ± SD | Mean ± SD | Mean ± SD | Kruskal–Wallis and Dunn tests | |
| Activity index | 0.694 ± 0.577 | 5.583 ± 4.171 | 3.667 ± 3.703 | 0.56 |
| Chronicity index | 0.0556 ± 0.130 | 1.528 ± 1.636 | 0.8 ± 1.542 | 0.2858 |
Exposure of male BWF1 mice to IMQ
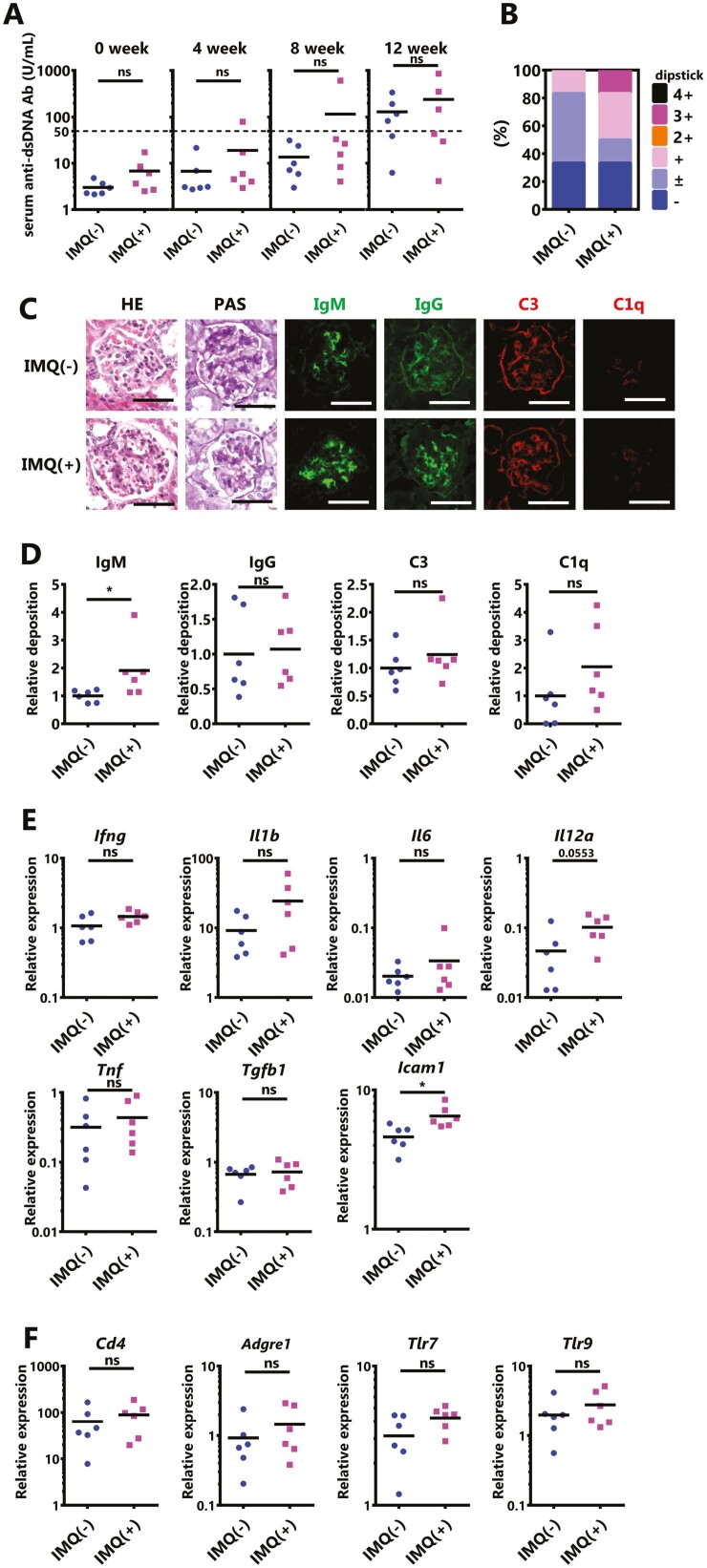
Since IFN-γ only played a role in the exacerbation of IMQ-induced lupus nephritis in the early phase, we verified what contributed to the worsening in the late phase. Indeed, in normal mice, the administration of recombinant IFN-γ alone does not cause death in chronic nephritis [25]; thus, events of some kind in female BWF1 mice may support the exacerbation of nephritis. In our study, the antibody levels of female BWF1 were not increased, with or without IMQ treatment, and autoantibodies increased spontaneously with aging (Fig. 1E). That is, in the early phase, nephritis progressed with the support of IFN-γ in addition to conferring predisposition to SLE, including autoantibodies; however, in the late phase, this may have switched to the progression of nephritis, which is caused by the deposition of immune complexes without the need for IFN-γ support. In particular, an increase in anti-DNA Ab is directly involved in the exacerbation of lupus nephritis [26]. Therefore, in mice with a low predisposition to SLE, especially those with a low level of autoantibodies, nephritis may not be severe, even if the IFN-γ expression is enhanced by IMQ treatment. To test this hypothesis, 20-week-old male BWF1 mice, which have slower spontaneous production of autoantibodies than females [26] were treated with IMQ for 12 weeks. In contrast to findings in females, there was no excessive activation of immune cells upon IMQ stimulation (Supplementary Fig. S5). The anti-dsDNA Ab was not increased by IMQ stimulation as in females (Fig. 5A). Only one mouse developed severe proteinuria (≥2+) during the 12-weeks IMQ stimulation (Fig. 5B). The histopathological analysis revealed that nephritis was slightly but significantly worsened in the IMQ-treated mice (Fig. 5C, Table 3). In addition, the deposition of immune complexes, albeit only in IgM, was significantly increased, suggesting that glomerulonephritis had slightly progressed (Fig. 5C and D). However, IMQ-treated male mice nephritis were much less injured in comparison to IMQ-treated female mice (Fig. 2C vs. Fig. 5C, Table 1 vs. Table 3). The expression of inflammatory mediators in the kidney was measured by qPCR, which revealed that most genes remained unchanged or tended to slightly increase, only the Icam1expression was significantly increased by IMQ treatment for 12 weeks (Fig. 5E). In addition, as inferred from the results of the qPCR analysis of the cell surface marker expression, IMQ stimulation was not associated with a significant increase in renal cell infiltration (Fig. 5F). In summary, the IMQ-induced development of lupus nephritis was reduced in male BWF1 mice, which had a lower predisposition to SLE than females. These data suggested that a predisposition towards SLE supports the development of lupus nephritis in IMQ-treated female BWF1 mice.
Figure 5.
Worsening of nephritis by IMQ requires a predisposition to SLE. IMQ was topically administered to 20-week-old male BWF1 mice three times weekly, similarly to females, on one ear for 12 weeks. (A) The anti-dsDNA Ab level in serum was measured by an ELISA before treatment, and after 4, 8, or 12 weeks of IMQ treatment. (N = 6 mice per group). (B) Proteinuria was measured by dipstick at sampling. The ratio of each score is shown. (N = 6 mice per group). (C) Representative images of kidney sections from untreated BWF1 mice or BWF1 mice treated with topical IMQ for 12 weeks. Sections were stained with HE, PAS, anti-IgM, anti-IgG, anti-C3, and anti-C1q. Bar = 50 μm. (D) The deposition of IgM, IgG, C3, and C1q in the kidney is shown using the mean fluorescence intensity (N = 6 mice per group). (E, F) Whole kidneys were harvested from these mice, and RNA was extracted. The expression of (E) Ifng, Il1b, Il6, Il12a, Tnf, Tgfb1, and Icam1. (F) Cd4, Adgre1 (also known as F4/80), Tlr7, and Tlr9 was determined by qPCR. The expression of the indicated genes was normalized to that of Actb. All graphs show the mean values, and each dot shows an individual mouse (N = 6 mice per group). The results were analyzed by a two-tailed Student’s t-test. Asterisks indicate statistically significant differences (∗P < 0.05, ∗∗P < 0.01, ns: not significant) and numbers indicate P values.
Table 3.
Renal pathological score (modified NIH activity and chronicity indices) in male BWF1 mice with or without IMQ treatment for 12 weeks.
| IMQ(−) (n = 6) | IMQ(+) (n = 6) | P value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mann–Whitney test | |
| Activity index | 1.056 ± 0.328 | 2.444 ± 0.981 | 0.0238 |
| Chronicity index | 0.056 ± 0.136 | 0.491 ± 0.200 | 0.197 |
Discussion
In individuals with a genetic predisposition to SLE, certain environmental factors can lead to the development, exacerbation, or flare of the pathological condition. UV irradiation is a well-known factor that causes SLE, and is associated with flare-ups, and exposure to pesticides and heavy metals has been reported as another trigger of SLE [27–29]. Furthermore, catching a cold is considered to be a trigger for SLE. The influenza virus and human coronavirus that causes the common cold are the single-stranded RNA virus. These are recognized by TLR7 in infected individuals and the innate immune response is activated. At this time, innate immune cells activate TLR7 signaling and produce type I IFN. As a result, it exerts an antiviral effect [8]. Generally, these responses are tightly controlled and exposure to the virus does not easily cause SLE. However, the chronic activation of TLR7 signaling can trigger SLE [4, 5]. We, therefore, investigated the phenotype of female BWF1 mice with a predisposition to SLE when TLR7 signaling is chronically activated due to viral infection or other triggers; i.e. we investigated the possibility of early onset of SLE following exposure to TLR7 agonists. Based on the findings concerning the previous systemic administration of IMQ to MRL-lpr mice [21] and topical IMQ administration to wild-type mice [7], it was expected that autoimmunity would be enhanced, and glomerulonephritis would worsen earlier in BWF1 mice. However, our data revealed that not all phenotypes deteriorated as they did in aged female BWF1 mice, but that the kidneys were significantly injured from the early phase of IMQ exposure (Fig. 1, 2). Chronic activation of TLR7 signaling resulted in the significant development of splenomegaly and lymphocyte activation but did not promote autoantibody production (Fig. 1E). One of the factors that increased the production of autoantibodies was thought to be an increase in IFN-α; however, there was no change when stimulated under the conditions of our study (Supplementary Fig. S1). Indeed, type I IFN is a cytokine that is profoundly involved in the pathophysiology of SLE, while recent studies suggested that the chronic production of type I IFN is predominant in the cGAS-STING pathway rather than the activation of TLR7 signaling [30, 31].
The infiltration of monocytes/macrophages into the glomerulus is essential for the onset and progression of glomerulonephritis. Under pathological conditions, activated monocytes/macrophages secrete various inflammatory mediators and activate resident cells. Activated resident cells express chemokines and adhesion molecules, promote leukocyte infiltration, and promote glomerular injury [22]. Our data showed that the infiltration of leukocytes, especially monocytes/macrophages and CD4+ T cells, was significantly enhanced in the kidneys of IMQ-stimulated mice by confirming the RNA expression of cell-specific surface markers in the kidney (Fig. 2F). It is suggested that TLR7 agonists activate monocytes, enhance the expression of TNF-α and IL-1β, and enhance the activation of mesangial cells, which promotes the above-mentioned cycle of exacerbation of glomerulonephritis. In addition, the higher expression of IFN-γ was a characteristic in the kidneys of IMQ-treated mice (Fig. 2E). Since IFN-γ affects the increasing activation of mesangial cells (Fig. 3A), it may directly contribute to the promotion of leukocyte infiltration into the kidney. Interestingly, the activation of TLR7 signaling in CD4+ T cells promoted differentiation into Th1 cells, which produce IFN-γ (Fig. 3B). TCR-activated CD4+ T cells had higher TLR7 expression levels in comparison to non-activated CD4+ T cells (Supplementary Fig. S3A), suggesting that the TLR7 stimulation directly activated CD4+ T cells and altered their differentiation. Taken together, the internal environment may be in a state where the differentiation of Th1 is easily promoted. Furthermore, since the expression of Il12a in the kidney was also enhanced by IMQ stimulation (Fig. 2E), the differentiation of Th1 may have been promoted in tertiary lymphoid tissues of the kidney [32] in addition to the secondary lymphoid tissues around the kidney. With an increase in Th1, renal cells are activated by pro-inflammatory cytokines along with IFN-γ, and Th1 is more likely to infiltrate the kidney. Subsequently, Th1 is also activated in the kidneys, which can further exacerbate glomerulonephritis [22, 25]. In particular, TCR-activated Th1 also expresses TNF-α (Supplementary Fig. S3B) and is one of the factors that further activates the resident cells in cooperation with IFN-γ derived from Th1 and IL-1β, which are derived from monocytes/macrophages.
Our data showed that the neutralization of IFN-γ could block the inflammatory cycle above-described for several weeks after the start of IMQ stimulation (Fig. 4). IFN-γ is involved in the development of IMQ-induced lupus nephritis, especially in the promotion of leukocyte infiltration (Fig. 4G). In addition, the infiltration and accumulation of monocytes activated by TLR7 agonists exacerbate glomerulonephritis in an immune complex-independent manner [33]. Focusing only on the renal tubulointerstitium, lymphocyte infiltration tended to be inhibited by neutralization of IFN-γ, even after 8 weeks of IMQ treatment (Supplementary Fig. S4A). However, the lymphocytic infiltration throughout the whole kidney was observed to be comparable to that with or without IFN-γ after 8 weeks of IMQ treatment (Supplementary Fig. S4C). Furthermore, the deposition of immune complexes on the glomeruli rarely differed, regardless of the IFN-γ status (Fig. 4E). These data suggest that the increase in IFN-γ is not the only factor involved in the exacerbation of IMQ-induced lupus nephritis. A possible factor is a spontaneous increase in autoantibodies with aging. It is possible that the increase in autoantibodies over time promoted the deposition of immune complexes on glomeruli and the exacerbation of late-phase IMQ-induced lupus nephritis. In our experiment, the production of anti-dsDNA Ab was not suppressed by the administration of anti-IFN-γ Ab in IMQ-treated mice (Supplementary Fig. S4B). Thus, it was suggested that IFN-γ only contributes to the promotion of cell infiltration into the kidney in the early phase of IMQ stimulation and not to the promotion of autoimmunity and immune complex deposition on the glomeruli. In addition, Jacob et al. showed that NZW mice that are not predisposed to the development of SLE do not die from the administration of IFN-γ, whereas female BWF1 mice die early. Also, the administration of IFN-γ did not affect anti-DNA antibody levels [25]. This report supports our results. Furthermore, in our results, focusing on the importance of SLE predisposition in IMQ-induced lupus nephritis, it was revealed that in male BWF1 mice, IMQ stimulation slightly increased nephritis; however, the symptoms were much less severe in comparison to those in female mice (Fig. 2 vs. Fig. 5). These data may be based on the fact that male BWF1 mice have a low genetic predisposition to SLE, such as anti-dsDNA antibodies, in comparison to female mice (Fig. 5A vs. Fig. 1E). In fact, the presence of autoantibodies injures the kidneys of patients with SLE [34]. In addition, the intake of low-dose bisphenol A, a type of endocrine-disrupting chemical, into female BWF1 mice inhibited the production of IFN-γ and IgG2a. In this case as well, nephritis was significantly suppressed [35]. Therefore, it was suggested that the activation of TLR7 signaling in female BWF1 mice significantly induces renal injury by promoting the deposition of immune complexes in the glomerulus through the effect of IFN-γ.
In this study, TLR7 signaling was activated percutaneously. In the present study, TLR7 agonist caused activation of various TLR7-positive resident cells and immune cells around the skin, which in turn may have spilled over into systemic inflammation. A characteristic feature of female BWF1 mice is that autoantibodies increase with age. The increase in IFN-γ also leads to the enhanced expression of Fc receptors in glomerular mesangial cells (Fig. 3A). Therefore, it is likely that TLR7 signaling-induced IFN-γ created an environment that facilitated the deposition of increased autoantibodies on the glomeruli, thus accelerating the worsening of glomerulonephritis. When considering a viral infection, it may be better to activate TLR7 signaling by intratracheal stimulation. Unlike the skin, the airway and lungs are rich in innate immune cells, which leads to different results. However, because there is a complication of renal injury due to SARS-CoV-2 infection [36], nephritis may be more likely to occur if there is a predisposition to SLE, even if the stimulation method is different. This may be due to epithelial tissue damage, and the self-RNA released from the damaged cells may have contributed to the amplification of inflammation as an autoantigen [37]. Although, it should be noted that the induction of strong inflammation can cause cytokine storms and lead to death. In fact, in our experiment, the intraperitoneal administration of R848 to female BWF1 mice (age: 20 weeks) probably caused severe hepatitis or myocarditis with almost no or little increase in the anti-dsDNA Ab level and renal injury (Supplementary Fig. S2). At least, Yokogawa et al. reported that the topical administration of R848 induced an autoimmune response in normal mice, whereas systemic treatment with R848 did not induce an autoimmune response [7]. In contrast, continuous intraperitoneal injection of a weaker stimulus (IMQ: 25 μg) in MRL-lpr mice than in our systemic administration experiments resulted in increased autoantibodies and the development of nephritis [21]. Therefore, exposure to chronic stimuli that are never too strong may be one of the factors that led to the results of this study.
In conclusion, IMQ-induced lupus nephritis appeared earlier in comparison to spontaneous lupus nephritis in female BWF1 mice. The promotion of the early phase of IMQ stimulation of IFN-γ signaling was important for this mechanism. However, the effect of IFN-γ signaling was masked by an increase in SLE predisposition, probably due to autoantibodies. In other words, it was suggested that the reduction of the initial cytokine response was important for lupus nephritis, which is exacerbated by environmental factors. Therefore, the inhibition of TLR7 and IFN-γ signaling may maintain remission and prevent flares of SLE. Among existing drugs, hydroxychloroquine may be useful for inhibiting TLR7 signaling [38, 39], and JAK inhibitors [15, 40] or ustekinumab, an anti-IL-12/23 (p40) Ab that helps inhibit Th1 differentiation [41, 42], may be useful for the inhibition of IFN-γ signaling.
Supplementary Material
Acknowledgments
The authors thank the members of our laboratory for their technical support and their helpful discussions. In particular, the authors thank M. Kurosawa and R. Kosuge for their support in breeding the animals.
Glossary
Abbreviations
- Ab
antibody
- BWF1
NZBWF1
- dsDNA
double-stranded DNA
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- HE
hematoxylin & eosin
- IMQ
imiquimod
- ISN/RPS
International Society of Nephrology/Renal Pathology Society
- PAS
periodic acid–Schiff
- qPCR
quantitative polymerase chain reaction
- SEAP
secreted alkaline phosphatase
- SLE
systemic lupus erythematosus
- TCR
T-cell receptor
- TLR
toll-like receptor
Contributor Information
Kunihiro Hayakawa, Institute for Environment and Gender-Specific Medicine, Juntendo University Graduate School of Medicine, Chiba 279-0021, Japan.
Maki Fujishiro, Institute for Environment and Gender-Specific Medicine, Juntendo University Graduate School of Medicine, Chiba 279-0021, Japan.
Yuko Yoshida, Institute for Environment and Gender-Specific Medicine, Juntendo University Graduate School of Medicine, Chiba 279-0021, Japan.
Yuko Kataoka, Institute for Environment and Gender-Specific Medicine, Juntendo University Graduate School of Medicine, Chiba 279-0021, Japan.
Shota Sakuma, Institute for Environment and Gender-Specific Medicine, Juntendo University Graduate School of Medicine, Chiba 279-0021, Japan.
Takuya Nishi, Institute for Environment and Gender-Specific Medicine, Juntendo University Graduate School of Medicine, Chiba 279-0021, Japan.
Keigo Ikeda, Department of Internal Medicine and Rheumatology, Juntendo University Urayasu Hospital, Chiba 279-0021, Japan.
Shinji Morimoto, Department of Internal Medicine and Rheumatology, Juntendo University Urayasu Hospital, Chiba 279-0021, Japan.
Kenji Takamori, Institute for Environment and Gender-Specific Medicine, Juntendo University Graduate School of Medicine, Chiba 279-0021, Japan.
Iwao Sekigawa, Institute for Environment and Gender-Specific Medicine, Juntendo University Graduate School of Medicine, Chiba 279-0021, Japan; Department of Internal Medicine and Rheumatology, Juntendo University Urayasu Hospital, Chiba 279-0021, Japan.
Funding
This study was supported, in part, by a Grant-in-Aid (S1311011) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT)-supported Program for the Strategic Research Foundation at Private University and by a Japan Society for the Promotion of Science KAKENHI grant number 20K07428 to KH, 19K09068 to KI and 18K08415 to SM.
Conflicts of interest
The authors declare no conflicts of interest in association with the present study.
Author contributions
Conceptualization, K.H.; Methodology, K.H., and M.F.; Formal analysis, K.H., and M.F., Investigation, K.H., M.F., Y.Y., S.S., T.N., and Y.K., Writing – original draft, K.H.; Writing – review and editing, M.F., Y.Y., S.S., T.N., Y.K., K.I., S.M., K.T., and I.S.; Supervision, K.T., and I.S.; Project administration, K.H.; Funding acquisition, K.H., K.I., S.M., K.T., and I.S.
Ethics approval
All mouse experiments followed the Animal Research In Vivo Experiment Report (ARRIVE) guidelines.
Data availability
The data that support the findings of this study are available from the corresponding author (K.H.) upon reasonable request.
References
- 1. Manson JJ, Rahman A. Systemic lupus erythematosus. Orphanet J Rare Dis 2006, 1, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Margery-Muir AA, Bundell C, Nelson D, Groth DM, Wetherall JD. Gender balance in patients with systemic lupus erythematosus. Autoimmun Rev 2017, 16, 258–68. [DOI] [PubMed] [Google Scholar]
- 3. Gulati G, Brunner HI. Environmental triggers in systemic lupus erythematosus. Semin Arthritis Rheum 2018, 47, 710–7. [DOI] [PubMed] [Google Scholar]
- 4. Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM.et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity 2007, 27, 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J.et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A 2006, 103, 9970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Souyris M, Cenac C, Azar P, Daviaud D, Canivet A, Grunenwald S.et al. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol 2018, 3, eaap8855. [DOI] [PubMed] [Google Scholar]
- 7. Yokogawa M, Takaishi M, Nakajima K, Kamijima R, Fujimoto C, Kataoka S.et al. Epicutaneous application of toll-like receptor 7 agonists leads to systemic autoimmunity in wild-type mice: a new model of systemic Lupus erythematosus. Arthritis Rheumatol 2014, 66, 694–706. [DOI] [PubMed] [Google Scholar]
- 8. Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 2004, 303, 1529–31. [DOI] [PubMed] [Google Scholar]
- 9. Dubois EL, Horowitz RE, Demopoulos HB, Teplitz R. NZB/NZW mice as a model of systemic lupus erythematosus. JAMA 1966, 195, 285–9. [PubMed] [Google Scholar]
- 10. de Vries MJ, Hijmans W. Pathological changes of thymic epithelial cells and autoimmune disease in NZB, NZW and (NZB x NZW)F1 mice. Immunology 1967, 12, 179–96. [PMC free article] [PubMed] [Google Scholar]
- 11. Haraldsson MK, dela Paz NG, Kuan JG, Gilkeson GS, Theofilopoulos AN, Kono DH. Autoimmune alterations induced by the New Zealand Black Lbw2 locus in BWF1 mice. J Immunol 2005, 174, 5065–73. [DOI] [PubMed] [Google Scholar]
- 12. Seegal BC, Accinni L, Andres GA, Beiser SM, Christian CL, Erlanger BF.et al. Immunologic studies of autoimmune disease in NZB-NZW F1 mice. I. Binding of fluorescein-labeled antinucleoside antibodies in lesions of lupus-like nephritis. J Exp Med 1969, 130, 203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB.et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 2004, 15, 241–50. [DOI] [PubMed] [Google Scholar]
- 14. Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT.et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 2018, 93, 789–96. [DOI] [PubMed] [Google Scholar]
- 15. Ikeda K, Hayakawa K, Fujishiro M, Kawasaki M, Hirai T, Tsushima H.et al. JAK inhibitor has the amelioration effect in lupus-prone mice: the involvement of IFN signature gene downregulation. BMC Immunol 2017, 18, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hayakawa K, Kawasaki M, Hirai T, Yoshida Y, Tsushima H, Fujishiro M.et al. MicroRNA-766-3p contributes to anti-inflammatory responses through the indirect inhibition of NF-kappaB signaling. Int J Mol Sci 2019, 20, 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schlondorff D. Preparation and study of isolated glomeruli. Methods Enzymol (Academic Press) 1990, 191, 130–40. [DOI] [PubMed] [Google Scholar]
- 18. Kitamura M, Mitarai T, Maruyama N, Nagasawa R, Yoshida H, Sakai O. Mesangial cell behavior in a three-dimensional extracellular matrix. Kidney Int 1991, 40, 653–61. [DOI] [PubMed] [Google Scholar]
- 19. Meng Y, Kasai A, Hiramatsu N, Hayakawa K, Yamauchi K, Takeda M.et al. Continuous, noninvasive monitoring of local microscopic inflammation using a genetically engineered cell-based biosensor. Lab Invest 2005, 85, 1429–39. [DOI] [PubMed] [Google Scholar]
- 20. Yoshida Y, Hayakawa K, Fujishiro M, Ikeda K, Tsushima H, Hirai T.et al. Social defeat stress exacerbates atopic dermatitis through downregulation of DNA methyltransferase 1 and upregulation of C-C motif chemokine receptor 7 in skin dendritic cells. Biochem Biophys Res Commun 2020, 529, 1073–9. [DOI] [PubMed] [Google Scholar]
- 21. Pawar RD, Patole PS, Zecher D, Segerer S, Kretzler M, Schlöndorff D.et al. Toll-like receptor-7 modulates immune complex glomerulonephritis. J Am Soc Nephrol 2006, 17, 141–9. [DOI] [PubMed] [Google Scholar]
- 22. Kuroiwa T, Lee EG. Cellular interactions in the pathogenesis of lupus nephritis: the role of T cells and macrophages in the amplification of the inflammatory process in the kidney. Lupus 1998, 7, 597–603. [DOI] [PubMed] [Google Scholar]
- 23. Meng XM, Nikolic-Paterson DJ, Lan HY. Inflammatory processes in renal fibrosis. Nat Rev Nephrol 2014, 10, 493–503. [DOI] [PubMed] [Google Scholar]
- 24. Artis D, Spits H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [DOI] [PubMed] [Google Scholar]
- 25. Jacob CO, van der Meide PH, McDevitt HO. In vivo treatment of (NZB X NZW)F1 lupus-like nephritis with monoclonal antibody to gamma interferon. J Exp Med 1987, 166, 798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lambert PH, Dixon FJ. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med 1968, 127, 507–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Achtman JC, Werth VP. Pathophysiology of cutaneous lupus erythematosus. Arthritis Res Ther 2015, 17, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sobel ES, Gianini J, Butfiloski EJ, Croker BP, Schiffenbauer J, Roberts SM. Acceleration of autoimmunity by organochlorine pesticides in (NZB x NZW)F1 mice. Environ Health Perspect 2005, 113, 323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Via CS, Nguyen P, Niculescu F, Papadimitriou J, Hoover D, Silbergeld EK. Low-dose exposure to inorganic mercury accelerates disease and mortality in acquired murine lupus. Environ Health Perspect 2003, 111, 1273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kato Y, Park J, Takamatsu H, Konaka H, Aoki W, Aburaya S.et al. Apoptosis-derived membrane vesicles drive the cGAS-STING pathway and enhance type I IFN production in systemic lupus erythematosus. Ann Rheum Dis 2018, 77, 1507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murayama G, Chiba A, Kuga T, Makiyama A, Yamaji K, Tamura N.et al. Inhibition of mTOR suppresses IFNα production and the STING pathway in monocytes from systemic lupus erythematosus patients. Rheumatology (Oxford) 2020, 59, 2992–3002. [DOI] [PubMed] [Google Scholar]
- 32. Sato Y, Boor P, Fukuma S, Klinkhammer BM, Haga H, Ogawa O.et al. Developmental stages of tertiary lymphoid tissue reflect local injury and inflammation in mouse and human kidneys. Kidney Int 2020, 98, 448–63. [DOI] [PubMed] [Google Scholar]
- 33. Kuriakose J, Redecke V, Guy C, Zhou J, Wu R, Ippagunta SK.et al. Patrolling monocytes promote the pathogenesis of early lupus-like glomerulonephritis. J Clin Invest 2019, 129, 2251–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med 2008, 358, 929–39. [DOI] [PubMed] [Google Scholar]
- 35. Sawai C, Anderson K, Walser-Kuntz D. Effect of bisphenol A on murine immune function: modulation of interferon-gamma, IgG2a, and disease symptoms in NZB X NZW F1 mice. Environ Health Perspect 2003, 111, 1883–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y.et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol 2020, 31, 1959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lou F, Sun Y, Xu Z, Niu L, Wang Z, Deng S.et al. Excessive polyamine generation in keratinocytes promotes self-RNA sensing by dendritic cells in psoriasis. Immunity 2020, 53, 204–216.e10. [DOI] [PubMed] [Google Scholar]
- 38. Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN.et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019, 78, 736–45. [DOI] [PubMed] [Google Scholar]
- 39. Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol 2011, 186, 4794–804. [DOI] [PubMed] [Google Scholar]
- 40. Jamilloux Y, El Jammal T, Vuitton L, Gerfaud-Valentin M, Kerever S, Sève P. JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun Rev 2019, 18, 102390. [DOI] [PubMed] [Google Scholar]
- 41. van Vollenhoven RF, Hahn BH, Tsokos GC, Wagner CL, Lipsky P, Touma Z.et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet 2018, 392, 1330–9. [DOI] [PubMed] [Google Scholar]
- 42. Wada Y, Cardinale I, Khatcherian A, Chu J, Kantor AB, Gottlieb AB.et al. Apilimod inhibits the production of IL-12 and IL-23 and reduces dendritic cell infiltration in psoriasis. PLoS One 2012, 7, e35069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (K.H.) upon reasonable request.