Abstract
Air pollution is a major environmental threat to public health; we know little, however, about its effects on adolescent brain development. Exposure to air pollution co-occurs, and may interact, with social factors that also affect brain development, such as early life stress (ELS). Here, we show that severity of ELS and fine particulate air pollution (PM2.5) are associated with volumetric changes in distinct brain regions, but also uncover regions in which ELS moderates the effects of PM2.5. We interviewed adolescents about ELS events, used satellite-derived estimates of ambient PM2.5 concentrations, and conducted longitudinal tensor-based morphometry to assess regional changes in brain volume over an approximately 2-year period (N = 115, ages 9–13 years at Time 1). For adolescents who had experienced less severe ELS, PM2.5 was associated with volumetric changes across several gray and white matter regions. Fewer effects of PM2.5 were observed for adolescents who had experienced more severe ELS, although occasionally they were in the opposite direction. This pattern of results suggests that for many brain regions, moderate to severe ELS largely constrains the effects of PM2.5 on structural development. Further theory and research is needed on the joint effects of ELS and air pollution on the brain.
Keywords: adolescence, air pollution, early life stress, fine particulate matter, PM2.5
Introduction
Air pollution is a major environmental threat to public health (World Health Organization 2016). Exposure to high levels of air pollution is associated with risk for respiratory infection, asthma, lung cancer, heart disease, stroke incidence, and Alzheimer’s and Parkinson’s disease (Kamppa and Castanas 2008; Block and Calderón-Garcidueñas 2009). Fine particulate matter (PM2.5), or air particles smaller than 2.5 μm in diameter, may be especially dangerous. PM2.5 particles can be composed of harmful mixtures of metals and chemicals that are easily inhaled deeply into the lungs and absorbed into the bloodstream, leading to local and systemic inflammation and oxidative stress which, in turn, can adversely affect brain development (see D’Angiulli 2018 for a review). Smaller PM2.5 particles can also reach the brain, potentially contributing to neuroinflammation and brain damage. Ultrafine particles originating from combustion processes can enter the brain directly via the olfactory nerve and bulb (Maher et al. 2016), as well as indirectly after entering the blood stream and translocating across the blood–brain barrier (Elder et al. 2006). Despite these findings, compared to our understanding of how PM2.5 affects cardiorespiratory functioning and physical health, we still know far less about the impact of PM2.5 on pediatric brain development in humans, especially beyond early childhood (D’Angiulli 2018).
Children and adolescents may be particularly vulnerable to the effects of ambient PM2.5 on brain development. Compared with adults, children and adolescents spend more time being physically active outdoors and breathe more air in proportion to their body weight; these factors increase their exposure to and intake of ambient air pollution (Sacks et al. 2011). Adolescence is also a period of rapid and dramatic brain development (Fuhrmann et al. 2015), which may confer greater vulnerability to environmental insults. A growing number of studies are reporting evidence that PM2.5 exposure has negative effects on cognitive and affective functioning in children and adolescents (Peterson et al. 2015; Sunyer et al. 2015; Jorcano et al. 2019; Miller et al. 2019; Miller, Gillette, et al. 2020a). Fewer studies, however, have investigated the impact of PM2.5 on the adolescent human brain. After controlling for age, sex, and socioeconomic status (SES), increased exposure to PM2.5 in youth in Mexico City has been associated with damage to the nasal epithelium and blood–brain barrier breakdown, potentially allowing more PM2.5 particles to pass into the brain (Calderón-Garciduenãs et al. 2015). These youth also show white matter damage associated with increased neuroinflammation (Calderón-Garcidueas et al. 2012), further suggesting that PM2.5 exposure has adverse effects on child and adolescent brain health. Another study that controlled for age, birthweight, IQ, SES, race, and intracranial volume found links between childhood exposure to higher levels of air pollution and greater cortical thinning and reduced gray matter volume (Beckwith et al. 2020). Similarly, Guxens and colleagues found that higher levels of prenatal air pollution were associated with cortical thinning in several brain regions (e.g., precuneus, middle frontal, and superior frontal regions) after controlling for factors such as SES, parental lifestyle, and ethnicity. Peterson et al. (2015) found that higher levels of prenatal air pollution were associated with reduced white matter. The structural alterations observed in both Guxens et al. (2018) and Peterson et al. (2015) were, in turn, associated with cognitive impairment. In the Adolescent Brain Cognitive Development (ABCD) study, regional PM2.5 estimates were associated with alterations in brain structure, but not with cognitive measures, in 9–10-year old participants after adjusting for various socio-demographic factors (e.g., age, sex, neighborhood safety) (Cserbik et al. 2020). Collectively, these findings suggest that exposure to PM2.5 adversely affects adolescent brain structures and networks that are critical for healthy cognitive and affective functioning.
Although PM2.5 may alter brain structure and function directly, it is important to recognize that exposure to air pollution co-occurs in the context of different social environments and events that are also implicated in adolescent brain development. Adverse, stressful events during childhood (i.e., early life stress; ELS), such as family violence, emotional abuse, poverty, and separation from family, have consistently been shown to have deleterious effects on the brain (Chen and Baram 2016) and to contribute to pathophysiology that could modify the impact of PM2.5 on risk for disease (McEwen and Tucker 2011; Olvera Alvarez et al. 2018). Indeed, findings of studies of child and adolescent respiratory health indicate that susceptibility to the adverse effects of air pollution may depend on exposure to ELS. Specifically, ELS has been related to increased susceptibility to the adverse effects of traffic-related air pollution on the development of asthma and lung functioning (Clougherty et al. 2007; Shankardass et al. 2009; Rancière et al. 2017). Similarly, Pagliaccio et al. (2020) recently reported that prenatal exposure to air pollution may magnify the effects of ELS on symptoms of psychopathology in childhood. In research with mice, maternal stress during pregnancy has been shown to increase offspring susceptibility to the adverse effects of air pollution on anxiety (Bolton et al. 2013). Taken together, these findings suggest a “double exposure” model in which ELS exacerbates risk related to increased air pollution. Alternatively, particularly high levels of stress or air pollution could exacerbate risk regardless of the level of the other factor (Clougherty and Kubzansky 2009). In fact, in a study of asthmatic children living in urban public housing, improving air quality by reducing allergen exposure was less effective in reducing symptoms for families who reported greater fear of violence in their communities (Clougherty et al. 2006). This finding fits with a saturation effect—that is, stress may overpower positive or negative contributions related to air quality (Clougherty and Kubzansky 2009). From a somewhat different perspective, ELS may organize or calibrate neurobiology in an adaptive way that reduces or inoculates against the impact of subsequent stressors, including those from the physical environment (Del Giudice et al. 2011; McEwen 2012). Thus, it is possible that youth exposed to less severe ELS will be more sensitive to the effects of air pollution. Theoretical and empirical work suggests that children exposed to moderate and severe ELS are less sensitive to social environmental influences on behavioral functioning than are their peers who are exposed to minimal ELS (Del Giudice et al. 2011). In research on nonhuman primates, squirrel monkeys exposed to ELS demonstrate reduced socioemotional and neurobiological sensitivity to subsequent stressors (Parker et al. 2004, 2006). These areas of research, however, have not considered whether ELS moderates sensitivity to air pollution exposure on the adolescent brain.
The present study was conducted to test whether ELS and PM2.5 interact to fit with a double exposure, a saturation effect, or a calibration model. Specifically, we examined whether individual differences in the severity of ELS moderate the association between community-level chronic PM2.5 and adolescent brain development. To date, studies of the effects of air pollution on brain structure and function have only included one assessment of the brain, and have not examined developmental changes (Herting et al. 2019). In the present study, in a sample of adolescents living in the San Francisco Bay Area we used longitudinal tensor-based morphometry (TBM) to assess expansion and contraction patterns across the brain that were associated with chronic PM2.5, ELS severity, and the interaction of these two factors. Given that no previous study has considered how PM2.5 and ELS jointly affect brain development in humans, we did not generate a priori hypotheses about, or constrain analyses to, specific brain regions. Rather, using longitudinal TBM allowed us to explore which brain structures were associated with PM2.5, ELS, and the interaction of PM2.5 and ELS. One advantage of longitudinal TBM over FreeSurfer-based cortical analyses that focus on cortical thickness and surface area is that TBM allows for whole-brain (including white-matter) voxel-wise analysis of brain volume. In addition, unlike voxel-based morphometry (VBM), which is another whole-brain analytic approach, TBM does not involve tissue segmentation or spatial smoothing steps that can limit resolution for small effects. TBM has been used to measure developmental changes in brain structure in both typically- and atypically-developing samples of adolescents (Hua et al. 2009, 2013; Dennis et al. 2017).
We interpreted interaction effects through the lens of three different hypotheses. The double exposure model predicts that chronic PM2.5 will be associated with brain development most strongly in adolescents who experienced more severe ELS. In contrast, the saturation effect model predicts that more severe experiences of ELS will overpower any association with chronic PM2.5. Thus, potentially problematic patterns of brain development related to higher levels of chronic PM2.5 will be observed for adolescents who experienced less severe ELS, whereas adolescent who experienced more severe ELS will demonstrate potentially problematic patterns of brain development regardless of chronic PM2.5. This would be reflected by an ordinal interaction in which the mean brain volume change at high ELS is always higher or lower than the mean at low ELS, regardless of PM2.5. Finally, a calibration or inoculation perspective predicts that youth exposed to more severe ELS will be less sensitive to the negative effects of chronic PM2.5 on brain development than will their peers who are exposed to milder levels of ELS. From this perspective, moderate to more severe ELS would, to some degree, limit potentially problematic patterns of brain development related to chronic PM2.5. This would be reflected by an interaction in which extreme patterns of brain volume change related to PM2.5 would be observed for adolescents exposed to less severe ELS.
Materials and Methods
Sample
As part of a larger study on ELS and neurodevelopment, 115 adolescents (56% female) from the San Francisco and San Jose Bay Area completed an interview to assess ELS severity and provided useable TBM data at Time 1 (Mean age = 11.51; SD = 1.08) and again 2 years later at Time 2 (Mean age = 13.47; SD = 1.16) of the study. Participants were diverse in terms of race and ethnicity (51% White; 11% Asian; 8% Hispanic/Latinx; 5% Black; 18% More than one race or ethnicity; 4% Other; 2% Did not report) and family income (58% of families reported an annual income over $100 K; range from less than $5 k to greater than $150 K). Males and females were matched on pubertal stage at study entry. Given that females typically reach sexual maturity earlier than males, females were, on average, about 8 months younger than males in our sample (mean difference = 0.68 years, t(113) = 3.52, P < 0.001). Of the 214 adolescents who participated in the larger study, those who did not consent to be scanned at both time point (n = 35), did not provide useable TBM data due to motion (based on visual inspection) (n = 33), had braces (n = 16), or dropped out of the study after Time 1 (n = 15), were not included in our analyses. Participants who were included in our analyses did not differ significantly from participants who were excluded from analyses with respect to age at T1, sex, race and ethnicity, community-level socioeconomic disadvantage, and ELS (all P > 0.140). The data for the current analyses were collected from 2013 to 2018. Our estimates of cumulative PM2.5 for the 2 years prior to T1 came from satellite-derived PM2.5 data generated by van Donkelaar et al. (2019) (see below). Adolescents and their parents signed assent and consent forms, respectively. The Institutional Review Board of our university approved the study protocol.
PM2.5
We used satellite-derived estimates of PM2.5 concentrations that were compiled by the Atmospheric Composition Analysis Group at Dalhousie University (van Donkelaar et al. 2019). These estimates were based on aerosol optical depth (AOD) retrievals from NASA MODIS as well as other satellite instruments (MISR, SeaWIFS) combined with the GEOS-Chem model. AOD retrievals were calibrated to ground-based monitoring observations of PM2.5 using Geographically Weighted Regression. For each participant’s residential address, we used monthly data to calculate average PM2.5 over the 2 years prior to each participant’s T1 assessment month with a 0.01° × 0.01° spatial resolution (~1 km2). The PM2.5 data generated by van Donkelaar et al. (2016, 2019) are well-validated and widely used to characterize pollution exposure for health studies (Elten et al. 2020; Wu et al. 2020; Southerland et al. 2021).
Early Life Stress
As previously described (King et al. 2017; Miller, Ho, et al. 2020b), we operationalized ELS as stressful events occurring up to early adolescence. Adolescents completed an interview at Time 1 to assess exposure to 30 different types of stressful experiences using a modified version of the Traumatic Events Screening Inventory for Children (e.g., parental divorce, family mental illness/substance abuse, accidents, etc.) (Ribbe 1996). A panel of coders who were blind to the adolescents’ reactions during the interview rated interview responses for severity using a modified version of the UCLA Life Stress Interview coding system (Rudolph and Hammen 1999); the severity of each reported stressful event was rated on scale ranging from 0 (nonevent or no impact) to 4 (extremely severe impact) with half-point increments. Ratings were summed across stressful events that were rated at 0.5 or higher to create a cumulative measure of ELS severity for each adolescent.
Community Socioeconomic Disadvantage
Community-level disadvantage was assessed using the area deprivation index (ADI; https://www.neighborhoodatlas.medicine.wisc.edu; Kind and Buckingham 2018). The ADI maps socioeconomic disadvantage at the Census-block group level using Census data and 2018 American Community Survey data. Socioeconomic disadvantage is based on a factor analysis of measures of educational attainment, poverty, housing quality, and unemployment. The ADI converted these factor scores into deciles representing the amount of socioeconomic disadvantage in a given Census-block group relative to other Census-block groups in California.
Scan
Magnetic resonance imaging (MRI) scans were acquired using a 3T GE Discovery MR750 (GE Medical Systems) equipped with a 32-channel head coil (Nova Medical). Whole-brain T1-weighted images (T1w) were collected using the following spoiled gradient echo pulse sequence: 186 sagittal slices; TR (repetition time)/TE (echo time)/TI (inversion time) = 6.24/2.34/450 ms; flip angle = 12°; voxel size = 0.9 × 0.9 × 0.9 mm; scan duration = 5:15 min.
Tensor-Based Morphometry
Volumes were automatically skull-stripped using Brainsuite and brain masks were manually edited to remove extraneous skull or meninges by trained neuroanatomical experts. Advanced Normalization Tools (ANTs) N4 correction was used to correct for intensity inhomogeneities (Tustison et al. 2010). As described in earlier papers from this cohort, thirty participants, selected to be representative of the population, were used to make the minimal deformation target (MDT) (Dennis et al. 2019). The MDT serves as cohort-specific template for image registration, a critical step in examining alterations in brain structure and can lead to improved statistical power over generic templates (Leporé et al. 2007).
Each participant’s masked, nonuniformity-corrected T1-weighted image was aligned to the MDT using ANTs for rigid, affine, and nonlinear registration (symmetric normalization (SyN); Avants et al. 2008). Image similarity was measured using the ANTs implementation of mutual information (Avants et al. 2011). Image intensities were winsorized, excluding top and bottom 1% of voxels, and histogram matching was used. To assess changes between Time 1 and Time 2, we used ANTs unbiased_pairwise_registration (https://github.com/ANTsX/ANTs/blob/master/Scripts/unbiased_pairwise_registration.sh) which provides a less biased output than registering Time 1 and Time 2 images by creating a midpoint to which both scans are registered (Tustison et al. 2019).
The Jacobian determinant image, the warp field resulting from the midpoint registration, showed the direction and magnitude of the change between the participant’s Time 1 and Time 2 anatomical images. Output was visually checked for quality.
Statistical Analyses
In our voxel-wise linear regression testing for group differences, we included intracranial volume (ICV) as a covariate. The linear registration that is part of our processing protocol accounts for differences in overall brain scale, removing much of the effect of ICV; nevertheless, we included ICV, computed from the linearly registered image, as a covariate. In a prior analysis of this dataset, we found minimal differences between models with and without ICV as a covariate (Dennis et al. 2019). We examined one model to test unique associations with ELS and air pollution, and we examined a second model to test associations with the interaction between ELS and air pollution. TBM measurements could vary as a function of age, sex, ICV, and the length of the interval between Time 1 (T1) and Time 2 (T2) assessments. Further, being a person of color (POC) who experiences discrimination, or living in a disadvantaged community in terms of SES, could be additional sources of stress that impact TBM. Thus, we included age, sex, ICV, interval between Time 1 and Time 2, POC status, and community-level SES as covariates in both models. The two models that we tested were:
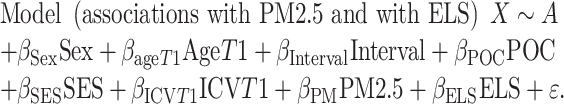 |
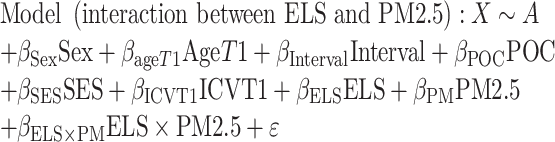 |
where X is the Jacobian determinant value at a given position, A is the constant Jacobian determinant term, the βs are the covariate regression coefficients, and ε is an error term. In longitudinal models, the Jacobian determinant is the difference between timepoints and thus is the change score. In the second model testing the interaction between PM2.5 and ELS, all predictors were centered prior to forming the continuous by continuous interaction term. For each model, results were corrected for multiple comparisons across all voxels tested using Searchlight FDR (q < 0.05) (Langers et al. 2007). We only conducted further analyses and report on those clusters that exceeded our criteria of 50 voxels. Given that our models controlled for ICV, observed clusters reflect localized areas of expansion and contraction in brain volume after accounting for global brain volume. Values representing average change in brain volume were extracted from each cluster and were inspected for extreme outlier values using boxplots (values outside of three times the interquartile above or below the third and first quartiles, respectively). In clusters containing extreme outliers, regression analyses were rerun with the cluster as the dependent variable after winsorizing these values to the next most extreme value (Erceg-Hurn and Mirosevich 2008). We have used these methods to detect and winsorize extreme outliers in prior analyses of PM2.5 and neurobiology (Miller et al. 2019; Miller, Gillette, et al. 2020a). Clusters that were no longer statistically significantly associated with ELS or PM2.5 in model 1, or with the ELS by PM2.5 interaction term in model 2, were dropped from our results.
Results
Descriptive statistics and correlations are presented in Table 1. In the supplement, Supplementary Table 1 shows descriptive statistics for ELS and PM2.5 estimates broken down by sex, race/ethnicity, and community disadvantage. Supplementary Figure 1 shows the study locations, the locations of Environmental Protection Agency ground monitors, and the distribution of PM2.5 estimates.
Table 1.
Descriptive statistics and correlations for ELS severity, fine particulate matter (PM2.5), and covariates
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. Sex (male = 1) | 1 | |||||||
| 2. Person of color status (POC = 1) | −0.05 | 1 | ||||||
| 3. T1 age | 0.31*** | −0.06 | 1 | |||||
| 4. T1–T2 interval (year) | −0.11 | −0.16 | −0.03 | 1 | ||||
| 5. T1 intracranial volume | −0.06 | −0.20* | 0.05 | −0.09 | 1 | |||
| 6. Community disadvantage (state-level decile ranking) | 0.07 | 0.20* | −0.16 | −0.00 | 0.04 | 1 | ||
| 7. ELS severity | 0.01 | 0.16 | 0.02 | 0.04 | 0.12 | 0.28** | 1 | |
| 8. PM2.5 (μg/m3) | 0.04 | 0.03 | −0.03 | 0.07 | 0.02 | 0.43*** | −0.04 | 1 |
| Mean or % | 44% | 48% | 11.51 | 1.96 | 1347.41 | 2.57 | 6.61 | 9.24 |
| SD | 1.08 | 0.45 | 15.02 | 2.04 | 5.20 | 0.98 | ||
| Range | 9.35–13.95 | 1.18–4.16 | 1298.14–1405.08 | 1–10 | .00–24.00 | 7.03–12.09 |
Notes: T1 = Time 1; T2 = Time 2; ELS = Early life stress; PM2.5 = particulate matter <2.5 μm. Females were coded as 0 and males were coded as 1.
* P < 0.05.
** P < 0.01.
*** P < 0.001.
Early Life Stress Effects on Brain Volume Changes
In our longitudinal whole-brain analyses we covaried for sex, age at Time 1, interval between Time 1 and Time 2, ICV at Time 1, POC status, and socioeconomic disadvantage. In our first model, we included both ELS and PM2.5 as predictors in order to control for each risk factor and to identify their independent effects. There were 22 clusters that were significantly associated with ELS that were larger than 50 voxels (see Table 2). ELS was positively associated with change in brain volume (expansion) in 13 of these clusters (β ranging from 0.32 to 0.45, all P < 0.001), which included white matter in the left cerebellum and bilateral superior frontal gyrus, and gray matter in the left caudate, right cuneus, right lateral and inferior occipital gyrus, and various frontal cortical regions (e.g., bilateral superior and inferior frontal gyrus, bilateral medial orbitofrontal cortex). ELS was negatively associated with volumetric change (contraction) in 9 clusters (β ranging from −0.31 to −0.46, all P < 0.003) that included gray matter in the right globus pallidus, right entorhinal cortex and fusiform gyrus, left posterior cingulate gyrus, and various frontal, temporal, and parietal cortical regions (e.g., right superior frontal gyrus, left supramarginal gyrus). One cluster that was associated with ELS contained an extreme outlier TBM value (left supramarginal gyrus); winsorizing this value did not change our findings.
Table 2.
Clusters that are uniquely associated with severity of ELS
| Voxels | Max. T-stat | X | Y | Z | Side | Structure | Tissue |
|---|---|---|---|---|---|---|---|
| Positive effects | |||||||
| 3426 | 4.39 | −22 | −53 | −33 | L | Cerebellum | WM |
| 2452 | 3.89 | 12 | 16 | 19 | R | Caudate | GM |
| 664 | 4.37 | −21 | 53 | −6 | L | Superior frontal gyrus | WM |
| 233 | 3.71 | 14 | −97 | 21 | R | Cuneus | GM |
| 213 | 3.53 | 40 | −83 | −5 | R | Lateral occipital gyrus | GM |
| 188 | 3.85 | 29 | 46 | 1 | R | Superior frontal gyrus | WM |
| 122 | 3.84 | 66 | −2 | 19 | R | Inferior frontal gyrus | GM |
| 119 | 4.71 | 0 | 41 | −18 | R | Medial orbitofrontal cortex | GM |
| 96 | 3.80 | 64 | 2 | 10 | R | Inferior frontal gyrus | GM |
| 86 | 3.63 | 40 | −68 | −3 | R | Inferior occipital gyrus | GM |
| 71 | 3.71 | −45 | 11 | −3 | L | Inferior frontal gyrus | GM |
| 56 | 4.00 | −16 | 55 | 4 | L | Superior frontal gyrus | WM |
| 55 | 3.28 | −5 | 22 | −19 | L | Medial orbitofrontal cortex | GM |
| Negative effects | |||||||
| 1340 | 3.84 | 18 | −8 | 1 | R | Globus pallidus | GM |
| 598 | 3.77 | 27 | 4 | −29 | R | Entorhinal cortex/fusiform gyrus | GM |
| 513 | 4.02 | −58 | 7 | −25 | L | Middle temporal gyrus | GM |
| 453 | 3.60 | −60 | −44 | 28 | L | Supramarginal gyrus | GM |
| 439 | 3.85 | −63 | −2 | 6 | L | Inferior frontal gyrus | GM |
| 243 | 3.73 | −52 | 8 | 35 | L | Precentral gyrus | GM |
| 236 | 3.40 | −7 | −50 | 12 | L | Posterior cingulate gyrus | GM |
| 61 | 3.42 | 20 | 6 | 48 | R | Superior frontal gyrus | GM |
| 53 | 3.22 | 26 | 4 | 61 | R | Superior frontal gyrus | GM |
PM2.5 Effects on Brain Volume Changes
PM2.5 was significantly associated with 38 clusters that were larger than 50 voxels (see Table 3). PM2.5 was positively associated with change in brain volume in 21 of these clusters (volume change per μg/m3 of PM2.5 ranging from 0.05% to 0.22%, β ranging from 0.29 to 0.48, all P < 0.007), which included white matter in the left caudate and corpus callosum, left cingulum, bilateral inferior fronto-occipital fasciculus, and right inferior frontal and temporal gyrus. PM2.5 was positively associated with change in gray matter in various regions, including the left precentral gyrus, left cerebellum, bilateral medial orbitofrontal cortex, and PM2.5 was negatively associated with volumetric change in brain volume in 17 clusters (volume change per μg/m3 of PM2.5 ranging from −0.05% to −0.11%, β ranging from to −0.29 to −0.41, all P < 0.006), including white matter in the left inferior temporal gyrus, left angular gyrus, left posterior thalamic radiation, left middle frontal gyrus, left hippocampal cingulum, and right postcentral gyrus. PM2.5 was negatively associated with gray matter clusters located in the left insula, right cingulate gyrus, right caudate, left cerebellum, and various cortical regions (e.g., bilateral fusiform gyrus, right precentral gyrus, bilateral middle frontal gyrus). Six clusters that were associated with PM2.5 contained an extreme outlier TBM value; winsorizing these values did not change our findings.
Table 3.
Clusters that are uniquely associated with PM2.5
| Voxels | Max. T-stat | X | Y | Z | Side | Structure | Tissue |
|---|---|---|---|---|---|---|---|
| Positive effects | |||||||
| 4756 | 4.29 | −14 | 10 | 19 | L | Caudate/corpus callosum | GM/WM |
| 2679 | 4.88 | −31 | −10 | 61 | L | Precentral gyrus | GM |
| 839 | 3.51 | −14 | −19 | 36 | L | Cingulum | WM |
| 716 | 3.70 | −49 | −72 | 22 | L | Lateral occipital gyrus | GM |
| 677 | 3.88 | −48 | −68 | −32 | L | Cerebellum | GM |
| 590 | 4.70 | −42 | 2 | −16 | L | Planum polare | GM |
| 385 | 3.55 | 41 | −46 | 25 | R | Angular gyrus | GM |
| 363 | 3.46 | 37 | −23 | −4 | R | Inferior fronto-occipital fasciculus | WM |
| 327 | 4.26 | −13 | 47 | −15 | L | Medial orbitofrontal cortex | GM |
| 245 | 3.58 | −52 | −47 | −41 | L | Cerebellum | GM |
| 205 | 3.94 | −11 | 58 | 3 | L | Superior frontal gyrus | GM |
| 189 | 3.75 | −37 | −15 | −11 | L | Inferior fronto-occipital fasciculus | WM |
| 152 | 3.50 | 36 | −15 | −22 | R | Inferior temporal gyrus | WM |
| 122 | 3.33 | 48 | −45 | −2 | R | Middle temporal gyrus | WM |
| 85 | 4.55 | 1 | 42 | −18 | R | Medial orbitofrontal cortex | GM |
| 84 | 3.56 | 44 | 28 | 2 | R | Inferior frontal gyrus | WM |
| 77 | 3.57 | −11 | −35 | 11 | L | Splenium | WM |
| 67 | 3.44 | 29 | 25 | 9 | R | Inferior frontal gyrus | GM |
| 64 | 3.11 | −38 | −51 | 57 | L | Superior parietal lobule | GM |
| 62 | 3.55 | −19 | 18 | −17 | L | Medial orbitofrontal cortex | GM |
| 60 | 2.94 | −26 | −73 | −36 | L | Cerebellum | GM |
| Negative effects | |||||||
| 6043 | 5.22 | −37 | −19 | 8 | L | Insula | GM |
| 1599 | 4.31 | −43 | −9 | −25 | L | Inferior temporal gyrus | WM |
| 869 | 3.37 | 39 | −68 | −18 | R | Fusiform gyrus | GM |
| 815 | 4.16 | 28 | −12 | 66 | R | Precentral gyrus | GM |
| 744 | 4.01 | −16 | −44 | −9 | L | Fusiform gyrus | GM |
| 424 | 3.62 | 44 | 16 | −21 | R | Superior temporal gyrus | GM |
| 390 | 3.24 | 14 | 19 | 12 | R | Caudate | GM |
| 201 | 3.17 | −44 | −41 | 23 | L | Angular gyrus | WM |
| 144 | 3.70 | −35 | −36 | 10 | L | Posterior thalamic radiation | WM |
| 120 | 3.80 | 42 | 12 | 54 | R | Middle frontal gyrus | GM |
| 109 | 3.61 | 16 | −52 | 7 | R | Cingulate gyrus | GM |
| 95 | 3.48 | −1 | −47 | −10 | L | Cerebellum | GM |
| 83 | 4.06 | −30 | −18 | 38 | L | Precentral gyrus | GM |
| 75 | 2.99 | −29 | 13 | 30 | L | Middle frontal gyrus | GM |
| 73 | 3.29 | −30 | 6 | 38 | L | Middle frontal gyrus | WM |
| 63 | 3.47 | −26 | −17 | −25 | L | Hippocampal cingulum | WM |
| 52 | 3.42 | 52 | −21 | 30 | R | Postcentral gyrus | WM |
Interactive Effects of ELS and PM2.5 on Brain Volume Changes
There were significant interactions between ELS and PM2.5 on 32 clusters that were larger than 50 voxels. After identifying and winsorizing extreme outlier TBM values in five clusters (no more than five values winsorized in a single cluster), 31 clusters remained (see Fig. 1 and Table 4); the ELS by PM2.5 interaction term was positively and negatively associated with 19 and 12 of these clusters, respectively. We probed these interactions to determine whether PM2.5 was associated with volumetric changes (i.e., more expansion or contraction) for adolescents who had experienced less, average, or more severe ELS (i.e., less and more severe ELS defined as 1 SD below and above the mean, respectively). Figures 2 and 3 illustrate the estimated positive and negative interaction effects, respectively.
Figure 1.

Clusters showing a significant interaction between ELS and PM on regional brain volume. Note. T-maps are shown for significant clusters, with blue-purple for negative associations and red-yellow for positive associations. Left in image is right in brain. CR = corona radiata, IFG = inferior frontal gyrus, IOG = inferior occipital gyrus, mOFC = medial orbitofrontal cortex, MFG = middle frontal gyrus, MTG = middle temporal gyrus, SCR = superior corona radiata, SFG = superior frontal gyrus, SPL = superior parietal lobule, SS = sagittal stratum, STG = superior temporal gyrus, TTG = transverse temporal gyrus.
Table 4.
Clusters showing a significant interaction between ELS and PM2.5 on regional brain volume
| Voxels | Max. T-stat | X | Y | Z | Side | Structure | Tissue |
|---|---|---|---|---|---|---|---|
| Positive interactions | |||||||
| 4222 | 5.38 | −28 | 62 | 2 | L | Middle frontal gyrus | GM |
| 3726 | 4.41 | 17 | 30 | 18 | R | Corona radiata | WM |
| 3204 | 3.68 | 48 | −60 | −3 | R | Inferior occipital gyrus | GM |
| 2109 | 4.20 | 13 | 52 | 1 | R | Superior frontal gyrus | GM |
| 1978 | 4.62 | 40 | 49 | 7 | R | Middle frontal gyrus | GM |
| 866 | 4.25 | 53 | 8 | −19 | R | Superior temporal gyrus | GM |
| 860 | 3.27 | −7 | −82 | −19 | L | Cuneus | GM |
| 810 | 3.41 | 35 | −67 | −22 | R | Cerebellum | GM |
| 627 | 3.20 | −39 | −55 | 16 | L | Middle temporal gyrus | GM |
| 476 | 3.92 | −32 | 11 | 36 | L | Middle frontal gyrus | GM |
| 403 | 3.31 | −9 | 54 | 37 | L | Superior frontal gyrus | GM |
| 306 | 3.24 | −6 | −83 | 5 | L | Cuneus | GM |
| 228 | 3.51 | 20 | −45 | −40 | R | Cerebellum | WM |
| 196 | 2.97 | −55 | −38 | −14 | L | Middle temporal gyrus | GM |
| 165 | 3.35 | −23 | −80 | 14 | L | Cuneus | GM |
| 141 | 4.27 | 23 | −41 | −51 | R | Cerebellum | WM |
| 126 | 3.54 | −11 | −67 | 35 | L | Superior parietal lobule | GM |
| 95 | 3.52 | −14 | −41 | −26 | L | Cerebellum | WM |
| 57 | 3.89 | −12 | 24 | −14 | L | Medial orbitofrontal cortex | GM |
| Negative interactions | |||||||
| 13 842 | 5.76 | 14 | 2 | 57 | R | Superior frontal gyrus | GM/WM |
| 4737 | 4.25 | 3 | −62 | 9 | R | Cuneus | GM |
| 731 | 3.36 | −30 | −17 | 32 | L | Superior corona radiata | WM |
| 319 | 3.99 | −48 | −16 | 6 | L | Transverse temporal gyrus | GM |
| 317 | 3.49 | 46 | 35 | 10 | R | Inferior frontal gyrus | GM |
| 203 | 3.93 | 9 | −13 | −18 | R | Cerebral peduncle | WM |
| 179 | 3.31 | −38 | −42 | −11 | L | Sagittal stratum | WM |
| 144 | 3.65 | −19 | −38 | 8 | L | Lateral ventricle | CSF |
| 137 | 3.24 | −54 | −10 | 12 | L | Postcentral gyrus | GM |
| 94 | 3.28 | 9 | −63 | 17 | R | Cerebellum | GM |
| 55 | 3.22 | 35 | 33 | 16 | R | Middle frontal gyrus | WM |
| 54 | 3.63 | 4 | −80 | −32 | R | Cerebellum | GM |
Notes: Shown are the cluster size, peak regression statistic, coordinates (MNI), hemisphere, structure, and tissue type. R = right; L = left; GM = gray matter; WM = white matter; CSF = cerebrospinal fluid.
Figure 2.

Interactions between ELS severity and PM2.5 that were positively associated with volumetric regional changes. Note. For the y-axis in each plot, positive and negative values represent expansion and contraction, respectively. The y-axis scales vary across plots. L = left; R = right; GM = gray matter; WM = white matter; PM2.5 = particulate matter <2.5 μm.
Figure 3.

Interactions between ELS severity and PM2.5 that were negatively associated with volumetric regional changes. Note. For the y-axis in each plot, positive and negative values represent expansion and contraction, respectively. The y-axis scales vary across plots. L = left; R = right; GM = gray matter; WM = white matter; PM2.5 = particulate matter <2.5 μm.
As Table 5 shows, in 25 of 31 clusters PM2.5 was significantly associated with volumetric changes in adolescents who had experienced less severe ELS (volume change per μg/m3 of PM2.5 ranging from −0.06% to −0.15% for negative effects and 0.04% to 0.14% for positive effects, |β| ranging from 0.27 to 0.56, all P < 0.040). In contrast, in 18 of 31 clusters PM2.5 was unrelated to volumetric changes in adolescents who had experienced more severe ELS (|β| ranging from 0.01 to 0.19, all P > 0.937). The opposite pattern—that PM2.5 was associated with volumetric change in youth who had experienced more severe ELS and not in youth who had experienced less severe ELS—was detected in five clusters located in the left middle frontal gyrus, right superior frontal gyrus, left postcentral gyrus, right cerebellum (all gray matter), and in right corona radiata white matter. At average ELS severity, PM2.5 was only associated with volumetric change in four clusters located in left middle temporal gyrus and left superior frontal gyrus gray matter, in right cerebral peduncle white matter, and in left lateral ventricle cerebrospinal fluid. In 8 of 31 clusters, PM2.5 was significantly associated with TBM values at both low and high levels of ELS severity, but in opposite directions (i.e., crossover effects; see Table 5).
Table 5.
Associations between PM2.5 and volumetric change for adolescents who experienced less, average, and more severe ELS
| Probing of interaction effects | ||||||||
|---|---|---|---|---|---|---|---|---|
| Interaction effect | Association with PM2.5 at lower ELS | Association with PM2.5 at moderate ELS | Association with PM2.5 at higher ELS | |||||
| β | P | β | P | β | P | β | P | |
| PM2.5-related contractions (negative effects) | ||||||||
| R Inferior occipital gyrus GM | 0.31 | 0.001 | −0.32 | 0.01 | −0.08 | 0.39 | 0.16 | 0.16 |
| R Superior temporal gyrus GM | 0.32 | 0.001 | −0.43 | 0.003 | −0.17 | 0.10 | 0.08 | 0.50 |
| L Cuneus GM | 0.28 | 0.004 | −0.30 | 0.03 | −0.007 | 0.50 | 0.16 | 0.15 |
| R Cerebellum GM | 0.34 | <0.001 | −0.34 | 0.01 | −0.07 | 0.46 | 0.19 | 0.09 |
| L Middle temporal gyrus GM | 0.31 | 0.001 | −0.54 | <0.001 | −0.30 | 0.003 | −0.06 | 0.58 |
| L Middle frontal gyrus GM | 0.30 | 0.002 | −0.38 | 0.006 | −0.15 | 0.16 | 0.09 | 0.44 |
| L Superior frontal gyrus GM | 0.32 | <0.001 | −0.56 | <0.001 | −0.31 | 0.003 | −0.06 | 0.61 |
| L Cuneus GM | 0.31 | 0.002 | −0.39 | 0.007 | −0.15 | 0.16 | 0.09 | 0.44 |
| R Cerebellum WM | 0.38 | <0.001 | −0.43 | 0.002 | −0.13 | 0.21 | 0.17 | 0.15 |
| L Cerebellum WM | 0.41 | <0.001 | −0.49 | <0.001 | −0.17 | 0.08 | 0.15 | 0.19 |
| L Postcentral gyrus GM | −0.37 | <0.001 | 0.21 | 0.13 | −0.07 | 0.49 | −0.36 | 0.003 |
| R Cerebellum GM | −0.29 | <0.007 | 0.25 | 0.05 | −0.11 | 0.23 | −0.48 | <0.001 |
| PM2.5-related expansions (positive effects) | ||||||||
| R Superior frontal gyrus GM/WM | −0.34 | <0.001 | 0.37 | 0.009 | 0.11 | 0.32 | −0.16 | 0.18 |
| R Cuneus GM | −0.30 | 0.002 | 0.32 | 0.02 | 0.08 | 0.43 | −0.15 | 0.20 |
| L Superior corona radiata WM | −0.28 | 0.002 | 0.33 | 0.01 | 0.11 | 0.24 | −0.11 | 0.33 |
| L Transverse temporal gyrus GM | −0.32 | 0.002 | 0.30 | 0.04 | 0.05 | 0.64f | −0.20 | 0.10 |
| R Cerebral peduncle WM | −0.38 | <0.001 | 0.54 | <0.001 | 0.29 | 0.009 | 0.01 | 0.94 |
| L Sagittal stratum WM | −0.37 | <0.001 | 0.37 | 0.007 | 0.09 | 0.39 | −0.20 | 0.09 |
| L Lateral ventricle CSF | −0.34 | <0.001 | 0.48 | <0.001 | 0.21 | 0.04 | −0.05 | 0.68 |
| R Cerebellum GM | −0.28 | 0.005 | 0.28 | 0.05 | 0.06 | 0.60 | −0.17 | 0.17 |
| L Middle frontal gyrus GM | 0.35 | <0.001 | −0.25 | 0.07 | 0.02 | 0.82 | 0.30 | 0.01 |
| R Corona radiata WM | 0.32 | 0.001 | −0.21 | 0.12 | 0.04 | 0.72 | 0.28 | 0.02 |
| R Superior frontal gyrus GM | 0.34 | <0.001 | −0.21 | 0.13 | 0.06 | 0.56 | 0.32 | 0.006 |
| PM2.5-related expansions and contractions (crossover effects) | ||||||||
| R Middle frontal gyrus GM | 0.33 | <0.001 | −0.28 | 0.03 | −0.03 | 0.79 | 0.23 | 0.04 |
| R Cerebellum WM | 0.36 | <0.001 | −0.32 | 0.02 | −0.03 | 0.75 | 0.25 | 0.04 |
| L Middle temporal gyrus GM | 0.40 | <0.001 | −0.33 | 0.02 | −0.02 | 0.87 | 0.30 | 0.01 |
| L Cuneus GM | 0.42 | <0.001 | −0.31 | 0.02 | 0.01 | 0.89 | 0.34 | 0.003 |
| L Medial orbitofrontal cortex GM | 0.48 | <0.001 | −0.47 | <0.001 | −0.10 | 0.33 | 0.28 | 0.01 |
| L Superior parietal lobule GM | 0.36 | <0.001 | −0.27 | 0.04 | 0.00 | 0.97 | 0.28 | 0.01 |
| R Inferior frontal gyrus GM | −0.44 | <0.001 | 0.31 | 0.02 | −0.03 | 0.75 | −0.38 | 0.001 |
| R Middle frontal gyrus WM | −0.44 | <0.001 | 0.46 | <0.001 | 0.12 | 0.20 | −0.22 | 0.04 |
Notes: Slope estimates represent the association between fine particulate matter (PM2.5) and volumetric regional change (within clusters) at lower (−1 SD), moderate (mean), and higher (+1 SD) values of ELS severity. These estimates are adjusted for age, sex, time interval from Time 1 to Time 2, intracranial volume at Time 1, POC status, and community socioeconomic disadvantage. Statistically significant effects of PM2.5 at different levels of ELS are presented in bold text. L = left; R = right; GM = gray matter; WM = white matter; CSF = cerebrospinal fluid.
At lower levels of ELS severity, PM2.5 was associated with contraction in 16 clusters and with expansion in 13 clusters. Specifically, PM2.5 was associated with contraction of white matter clusters in the cerebellum and gray matter in clusters located in, among other regions, the bilateral middle frontal and bilateral superior frontal gyrus, left medial orbitofrontal cortex, left cuneus, and left superior parietal lobule. At lower levels of ELS severity, PM2.5 was associated with expansion of white matter in the right superior frontal gyrus, right superior corona radiata, right cerebral peduncle, left sagittal stratum, and right middle frontal gyrus, and expansion of gray matter in the right superior frontal gyrus, right cuneus, right inferior frontal gyrus, and left transverse temporal gyrus.
At higher levels of ELS severity, PM2.5 was associated with contraction in four clusters and expansion in nine clusters. Specifically, PM2.5 was associated with contraction of white matter in the right middle frontal gyrus and gray matter in clusters located in right inferior frontal gyrus, left postcentral gyrus, and right cerebellum. At higher levels of ELS severity, PM2.5 was associated with expansion of white matter in the right cerebellum and gray matter in clusters located in the bilateral middle frontal gyrus, right superior frontal gyrus, left middle temporal gyrus, left superior parietal lobule, left cuneus, and left medial orbitofrontal cortex.
In summary, PM2.5 was associated primarily with change in volume in several gray and white matter clusters at lower levels of ELS severity—nearly twice as many clusters were significantly associated with PM2.5 at low than at high ELS severity. Fewer clusters were significantly associated with PM2.5 at average levels of ELS severity. At low levels of ELS, PM2.5 was associated with contractions across various frontal, temporal, and parietal gray matter clusters and white matter clusters in the cerebellum, but also with expansion of white matter in, among areas, the left superior corona radiata. At high levels of ELS, statistically significant clusters related to PM2.5 primarily reflected expansion in gray matter in specific frontal, temporal and parietal regions. These findings were robust to our analytical decisions concerning which covariates to include versus exclude from the model, and to sensitivity analyses conducted with participants who lived at the same residential location at both T1 and T2 (see Supplementary Material).
Discussion
A growing body of work is recognizing the importance of examining how stress and air pollution interact to affect health (Clougherty et al. 2007; Clougherty and Kubzansky 2009; Shankardass et al. 2009; McEwen and Tucker 2011; Rancière et al. 2017; Olvera Alvarez et al. 2018; Pagliaccio et al. 2020). To date, however, this research has focused primarily on respiratory health or behavioral outcomes; in contrast, human neuroimaging studies have considered the independent contributions of psychosocial and environmental risk factors to brain development but have not examined these two types of factors simultaneously. Here, we identify patterns of regional brain expansion and contraction that are uniquely associated with ELS or PM2.5. In addition, our findings integrate and extend previous studies by showing that the impact of fine particulate air pollution—an environmental risk factor—on volumetric change in various white and gray matter regions depends on the severity of ELS—a psychosocial risk factor. Adolescents with a history of less severe ELS were more sensitive to the effects of PM2.5 in terms of volumetric expansion and contraction across a number of brain regions involved in cognitive and affective functioning, including prefrontal and temporal regions, the cerebellum, and central white matter. There were far fewer links between PM2.5 and brain development in youth exposed to moderate to more severe ELS. These findings are consistent with the perspective that ELS determines openness to the effects of other experiences, including those related to the physical environment (McEwen 2012). Thus, ELS may constrain sensitivity to the effects of air pollution on the brain. Although these findings could also be interpreted as being consistent with a saturation effect model, ELS did not appear to have a robust effect on brain development in these regions that overpowered any additional effect of pollution. If this were the case, we should have observed ordinal interaction effects in which the mean brain volume change at high ELS was always higher (or lower when PM2.5 was associated with contractions) than the mean brain change at low ELS, regardless of PM2.5. Our interaction effects did not follow this pattern and therefore were inconsistent with an important prediction of the saturation effect model.
The observed patterns of regional brain contraction and expansion related to the main effects and interaction of ELS and PM2.5 may reflect cumulative allostatic load related to psychosocial and/or environmental stressors. Interestingly, PM2.5 and ELS affect many of the same biological pathways that have been implicated in apoptosis and neurogenesis, allostatic load, and inflammation (McEwen and Tucker 2011; Koutmani and Karalis 2015; Bolton et al. 2017; Peixoto et al. 2017; Thomson 2019). In the present study, the brain regions that showed greater contraction, or shrinking, related to moderate to severe ELS, increased PM2.5, or increased PM2.5 in the context of a specific ELS history (i.e., less or more severe ELS), may be experiencing accelerated cell death related to neuroinflammation (Brockmeyer and D’Angiulli 2016). Conversely, brain regions that showed greater expansion related to these factors may be undergoing neural reorganization. For example, moderate to high levels of ELS or high PM2.5 may support the consistent activation and strengthening of pathways that are atypical in the absence of psychosocial or environmental risk factors. Brain regions that showed greater expansion related to PM2.5 in the context of higher levels of ELS may reflect strengthening of pathways that are atypical in the absence of both psychosocial and environmental risk factors. Further research is needed to elucidate the specific biological mechanisms that underlie regional brain expansion and contraction.
Although there were more effects of PM2.5 at low than at high levels of ELS, it should be noted that we observed eight clusters in which PM2.5 was significantly associated with volumetric changes at both low and high levels of ELS, but in opposite directions (i.e., crossover effects). For example, in adolescents with a history of less severe ELS, PM2.5 was associated with volumetric contractions in the right middle frontal gyrus and cerebellum, and left middle temporal gyrus, cuneus, superior parietal lobule, and medial orbitofrontal cortex. Conversely, in adolescents with a history of more severe ELS, PM2.5 was associated with volumetric expansions in these same regions. One possible explanation for these crossover effects was that our analysis was statistically powered to detect this specific pattern of interaction between ELS and PM2.5 in these particular clusters. Another possible explanation is that PM2.5-related developmental processes in these clusters are differentially affected at low versus high levels of severity of ELS. For example, PM2.5-related contractions in the context of less severe ELS history may reflect cell death related to air pollution-specific neuroinflammation. Conversely, in the context of a history of more severe ELS, PM2.5-related expansions in these same clusters may reflect regions that undergo strengthening of pathways that is atypical in the absence of both PM2.5 and ELS. Another possible explanation for the observed crossover effects, and for our interaction effects in general, is that these clusters may have different developmental timelines following exposure to high versus low ELS that alter the impact of chronic exposure to PM2.5. For example, some studies suggest that ELS leads to accelerated or earlier development of specific brain regions and circuits, potentially reflecting reduced developmental plasticity (Callaghan and Tottenham 2016). Different levels or stages of developmental plasticity may dictate how PM2.5 is related to changes in brain volume, although the specific biological processes that might lead to PM2.5 related contractions at earlier stages but expansions at later stages (or vice versa) are not yet clear.
Our finding that, overall, adolescents exposed to less severe ELS are more susceptible to the effects of PM2.5 on brain development stands in contrast to much of the literature on respiratory health, which has largely supported a double exposure model. There are three possible explanations for this inconsistency. First, our study focused on brain development rather than on respiratory health as the outcome of interest. The brain plays a central role in the biological embedding of early social and physical experiences that shape responses and sensitivity to subsequent input (Del Giudice et al. 2011; McEwen 2012). Thus, the interaction between ELS and PM2.5 may have a different effect on the brain than it does on other neurobiological systems that are less centrally involved in orchestrating responses to stress and air pollution. Second, the extent to which the interaction effects of ELS and PM2.5 are specific to the overall range of PM2.5 in our study is unclear. Our findings are consistent with prior research suggesting that even relatively low levels of PM2.5 affect structural brain development (Cserbik et al. 2020). Nevertheless, it is possible that much higher levels of PM2.5 will affect brain development in youth regardless of ELS history, or will change interaction effects such that ELS and PM2.5 will compound risk for adverse effects on brain development. As a related point, we assessed ELS in a community sample of adolescents in the Bay Area who, on average, resided in socioeconomically advantaged communities relative to other communities in California. Although these adolescents reported a wide range of ELS exposures (Miller, Ho, et al. 2020b), certain types of ELS, such as family financial problems, are less prevalent in these communities. It is possible that considering these types of ELS, or severely traumatic forms of ELS that were less common in our sample (e.g., sexual abuse), could provide support for a saturation effect model. Third, it is possible that our findings are in part due to our study design. We considered PM2.5 over the 2 years prior to our first neuroimaging assessment, and we used a retrospective measure of ELS. Prior work suggests that there are developmental windows of increased sensitivity to the adverse effects of PM2.5 (Rosa et al. 2020), and that prospective and objective measures of ELS may be related to different risk pathways (Baldwin et al. 2019). Thus, examining a different developmental window of PM2.5 exposure, using a prospective measure of ELS, or both, may lead to different findings. As a related point, we conducted the first neuroimaging assessment with this sample in early adolescence; however, ELS and PM2.5 likely also contribute to brain development that occurs in childhood. Conducting neuroimaging earlier in development, and conducting repeating assessments over a longer time window, would help to further clarify whether the joint effects of ELS and PM2.5 on adolescent brain development are different from or similar to their joint effects on other types of outcomes.
Given that many of the brain regions that were associated with ELS and PM2.5 are involved in affective and cognitive functioning, our findings may have implications for adolescent well-being. For example, the orbitofrontal cortex and insula play key roles in processes such as emotion regulation and salience detection (Davidson et al. 2000; Menon and Uddin 2010). Previous studies suggest that morphology in these regions can indicate risk for psychopathology and is sensitive to ELS and PM2.5 (Hanson et al. 2010; Saleh et al. 2017; Cserbik et al. 2020). Our study is the first, however, to show that PM2.5 is associated with volumetric contractions over time in insula gray matter, and that PM2.5 and ELS are associated with volumetric expansions over time in distinct gray matter regions of the medial orbitofrontal cortex, volumetric changes that may have implications for altered affective processes and mental health problems in adolescence. We also found that ELS, PM2.5, and their interaction were associated with volumetric changes in prefrontal regions such as the inferior and superior frontal gyrus, and central white matter such as the corona radiata; these regions are involved in higher cognitive processes, including executive function and attention (Hampshire et al. 2010; Urger et al. 2014). Further, structural variation in these regions has been implicated in difficulties that emerge during adolescence, including depression, risk-taking, and substance abuse (Ersche et al. 2012; Schmaal et al. 2017; Herzberg et al. 2018). Interestingly, recent research has found that increased PM2.5 is also associated with decreased cognitive development and greater risk for emotional and behavioral problems (Sunyer et al. 2015; Guxens et al. 2018; Jorcano et al. 2019), although it is important to note that PM2.5 was not associated with performance on cognitive measures in the ABCD study (Cserbik et al. 2020). Future prospective studies should test whether volumetric changes in the brain regions observed in our study might be mechanisms by which ELS, PM2.5, and their interaction influence cognitive, affective, and behavioral functioning in adolescence.
We should note several limitations of this study. First, we do not know whether our findings are specific to cumulative PM2.5 exposure and/or to PM2.5 exposure during a particular developmental window. We used PM2.5 estimates for the 2 years prior to the first assessment, but did not have a complete history of participants’ residential addresses. Conversely, the time scale for our assessment of ELS events spanned the entirety of childhood. Nevertheless, we should note that individual differences in air pollution exposure may be stable across development, given that youth tend to grow up in similar communities over the course of their childhood and adolescence, although this assumption also depends on the temporal stability of other factors such as the locations of major sources of pollution. Thus, differentiating timing from duration effects is a challenge for air pollution research in general (Newbury et al. 2019). Second, we used satellite-derived estimates of PM2.5 concentrations, but did not measure PM2.5 exposure directly (Steinle et al. 2015; Pagliaccio et al. 2020). Capturing individual differences in exposure and dose of PM2.5 will be important for determining the degree to which effects are specific to this form of air pollution. As a related point, our observational findings do not identify the mechanisms by which increased PM2.5 may lead to regional brain expansion and contraction, and do not causally link PM2.5 to brain development. Although PM2.5 directly affects the developing brain, it is also possible that our observed effects are due to unmeasured factors related to air pollution. We controlled for variables that have been posited to be correlated with ambient air pollution exposure, including POC status and socioeconomic disadvantage, but we cannot definitively rule out all possible confounds or selection variables that might lead individuals to reside in certain communities that have more or less air pollution (Oakes 2004). Randomized trials are necessary to determine whether changing PM2.5 exposure affects brain measures differently for youth exposed to more versus less severe ELS. Third, our sample size may have limited our ability to detect significant interaction effects in other brain clusters. This possibility should be tested in larger, longitudinal studies. Fourth, urban environments likely contain substantial spatial variation in PM2.5 levels, as well as composition, which is not captured by our resolution of 1 km2 and general PM2.5 measure. Fifth, we did not assess indoor PM2.5. Recent estimates suggest that more than 60% of PM2.5 indoors originates from outdoor sources (Bi et al. 2021), but indoor sources certainly make significant contributions, particularly at lower PM2.5 concentrations. Given that most people spend the majority of their time indoors, future work should consider whether the links between exposure to indoor PM2.5 and adolescent brain development are comparable with what we observed in our study of outdoor PM2.5. Lastly, we should note that we were interested in examining chronic exposure to PM2.5, but that it is possible that short-term fluctuations in PM2.5 could also be related to brain development during adolescence.
Despite these limitations, this study provides novel evidence that psychosocial risk factors moderate the impact of environmental risk factors on adolescent brain development. These effects were consistent across different brain regions and suggest that PM2.5 is associated with a greater number of volumetric alterations for adolescents who are exposed to less severe ELS. These findings provide a nuanced, rich understanding of which adolescents may be most sensitive to PM2.5 in terms of brain development and which brain regions are most sensitive to ELS, PM2.5, and their interactive effects on volumetric changes. Further, these findings serve as a call for more research and development of theory that considers the joint effects of air pollution and ELS on the brain. In addition, despite living in communities with PM2.5 levels that are considered safe by the EPA, elevated PM2.5 was nevertheless associated with altered patterns of regional brain volumetric expansion and contraction. Thus, even at relatively lower levels, PM2.5 may affect adolescent brain development.
Supplementary Material
Contributor Information
Jonas G Miller, Department of Psychology, Stanford University, Stanford, CA 94305, USA.
Emily L Dennis, Department of Neurology, University of Utah School of Medicine, Salt Lake City, UT 84132, USA.
Sam Heft-Neal, Center for Food Security and the Environment, Stanford University, Stanford, CA 94305, USA.
Booil Jo, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA 94305, USA.
Ian H Gotlib, Department of Psychology, Stanford University, Stanford, CA 94305, USA.
Funding
National Institute of Mental Health (R37-MH101495 to I.H.G., and T32-MH019908 to Allan L. Reiss (funding J.G.M.)) and the Stanford Center on Longevity (funding J.G.M.).
Notes
We thank the research staff who made this work possible, and the families who participated in this study. Conflict of Interest: None declared.
References
- Avants BB, Epstein CL, Grossman M, Gee JC. 2008. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 12(1):26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. 2011. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 54:2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin JR, Reuben A, Newbury JB, Danese A. 2019. Agreement between prospective and retrospective measures of childhood maltreatment: a systematic review and meta-analysis. JAMA Psychiat. 76:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith T, Cecil K, Altaye M, Severs R, Wolfe C, Percy Z, Maloney T, Yolton K, LeMasters G, Brunst K, et al. 2020. Reduced gray matter volume and cortical thickness associated with traffic-related air pollution in a longitudinally studied pediatric cohort. PLoS One. 15:e0228092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi J, Wallace LA, Sarnat JA, Liu Y. 2021. Characterizing outdoor infiltration and indoor contribution of PM2.5 with citizen-based low-cost monitoring data. Environ Pollut. 276:116763. [DOI] [PubMed] [Google Scholar]
- Block ML, Calderón-Garcidueñas L. 2009. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 32(9):506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Huff NC, Smith SH, Mason SN, Foster WM, Auten RL, Bilbo SD. 2013. Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice. Environ Health Perspect. 121:1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Marinero S, Hassanzadeh T, Natesan D, Le D, Belliveau C, Mason SN, Auten RL, Bilbo SD. 2017. Gestational exposure to air pollution alters cortical volume, microglial morphology, and microglia-neuron interactions in a sex-specific manner. Vol 9. Lausanne: Frontiers in Synaptic Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmeyer S, D’Angiulli A. 2016. How air pollution alters brain development: the role of neuroinflammation. Transl Neurosci. 7:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueas L, Mora-Tiscareño A, Styner M, Gómez-Garza G, Zhu H, Torres-Jardón R, Carlos E, Solorio-López E, Medina-Cortina H, Kavanaugh M, et al. 2012. White matter hyperintensities, systemic inflammation, brain growth, and cognitive functions in children exposed to air pollution. J Alzheimers Dis. 31(1):183–191. [DOI] [PubMed] [Google Scholar]
- Calderón-Garciduenãs L, Vojdani A, Blaurock-Busch E, Busch Y, Friedle A, Franco-Lira M, Sarathi-Mukherjee P, Martínez-Aguirre X, Bin PS, Torres-Jardón R, et al. 2015. Air pollution and children: neural and tight junction antibodies and combustion metals, the role of barrier breakdown and brain immunity in neurodegeneration. J Alzheimers Dis. 43(3):1039–1058. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Tottenham N. 2016. The stress acceleration hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr Opin Behav Sci Development Behav. 7:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Baram TZ. 2016. Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology. 41(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty J, Levy J, Hynes H, Spengler J. 2006. A longitudinal analysis of the efficacy of environmental interventions on asthma-related quality of life and symptoms among children in urban public housing. J Asthma. 43(5):335–343. [DOI] [PubMed] [Google Scholar]
- Clougherty JE, Kubzansky LD. 2009. A framework for examining social stress and susceptibility to air pollution in respiratory health. Environ Health Perspect. 117(9):1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty JE, Levy JI, Kubzansky LD, Ryan PB, Suglia SF, Canner MJ, Wright RJ. 2007. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ Health Perspect. 115(8):1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserbik D, Chen J-C, McConnell R, Berhane K, Sowell ER, Schwartz J, Hackman DA, Kan E, Fan CC, Herting MM. 2020. Fine particulate matter exposure during childhood relates to hemispheric-specific differences in brain structure. Environ Int. 143:105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angiulli A. 2018. Severe urban outdoor air pollution and children’s structural and functional brain development, from evidence to precautionary strategic action. Lausanne: Frontiers in Public Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. 2000. Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science. 289:591–594. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. 2011. The adaptive calibration model of stress responsivity. Neurosci Biobehav Rev. 35(7):1562–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Faskowitz J, Rashid F, Babikian T, Mink R, Babbitt C, Johnson J, Giza CC, Jahanshad N, Thompson PM, Asarnow RF. 2017. Diverging volumetric trajectories following pediatric traumatic brain injury. NeuroImage: Clinical. 15:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Humphreys KL, King LS, Thompson PM, Gotlib IH. 2019. Irritability and brain volume in adolescents: cross-sectional and longitudinal associations. Soc Cogn Affect Neurosci. 14(7):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, Potter R, Maynard A, Ito Y, Finkelstein J, et al. 2006. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 114(8):1172–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elten M, Donelle J, Lima I, Burnett RT, Weichenthal S, Stieb DM, Hystad P, van Donkelaar A, Chen H, Paul LA, et al. 2020. Ambient air pollution and incidence of early-onset paediatric type 1 diabetes: a retrospective population-based cohort study. Environ Res. 184:109291. [DOI] [PubMed] [Google Scholar]
- Erceg-Hurn DM, Mirosevich VM. 2008. Modern robust statistical methods: an easy way to maximize the accuracy and power of your research. Am Psychol. 63:591–601. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. 2012. Abnormal brain structure implicated in stimulant drug addiction. Science. 335(6068):601–604. [DOI] [PubMed] [Google Scholar]
- Fuhrmann D, Knoll LJ, Blakemore SJ. 2015. Adolescence as a sensitive period of brain development. Trends Cogn Sci. 19(10):558–566. [DOI] [PubMed] [Google Scholar]
- Guxens M, Lubczyńska MJ, Muetzel RL, Dalmau-Bueno A, Jaddoe VWV, Hoek G, van der Lugt A, Verhulst FC, White T, Brunekreef B, et al. 2018. Air pollution exposure during fetal life, brain morphology, and cognitive function in school-age children. Biol Psychiatry. 84(4):295–303. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. 2010. The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage. 50(3):1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD. 2010. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci. 30:7466–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Younan D, Campbell CE, Chen J-C. 2019. Outdoor air pollution and brain structure and function from across childhood to young adulthood: a methodological review of brain MRI studies. Vol 7. Lausanne: Front Public Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg MP, Hodel AS, Cowell RA, Hunt RH, Gunnar MR, Thomas KM. 2018. Risk taking, decision-making, and brain volume in youth adopted internationally from institutional care. Neuropsychologia. 119:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Hibar DP, Ching CRK, Boyle CP, Rajagopalan P, Gutman BA, Leow AD, Toga AW, Jack CR, Harvey D, et al. 2013. Unbiased tensor-based morphometry: improved robustness and sample size estimates for Alzheimer’s disease clinical trials. NeuroImage. 66:648–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Leow AD, Levitt JG, Caplan R, Thompson PM, Toga AW. 2009. Detecting brain growth patterns in normal children using tensor-based morphometry. Hum Brain Mapp. 30(1):209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorcano A, Lubczyńska MJ, Pierotti L, Altug H, Ballester F, Cesaroni G, El Marroun H, Fernández-Somoano A, Freire C, Hanke W, et al. 2019. Prenatal and postnatal exposure to air pollution and emotional and aggressive symptoms in children from 8 European birth cohorts. Environ Int. 131:104927. [DOI] [PubMed] [Google Scholar]
- Kamppa M, Castanas E. 2008. Human health effects on air pollution. Environ Pollut. 151(2):362–367. [DOI] [PubMed] [Google Scholar]
- Kind AJH, Buckingham WR. 2018. Making neighborhood-disadvantage metrics accessible—the neighborhood atlas. N Engl J Med. 378:2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Colich NL, LeMoult J, Humphreys KL, Ordaz SJ, Price AN, Gotlib IH. 2017. The impact of the severity of early life stress on diurnal cortisol: the role of puberty. Psychoneuroendocrinology. 77:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutmani Y, Karalis KP. 2015. Neural stem cells respond to stress hormones: distinguishing beneficial from detrimental stress. Vol 6. Lausanne: Frontiers in Physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langers DRM, Jansen JFA, Backes WH. 2007. Enhanced signal detection in neuroimaging by means of regional control of the global false discovery rate. NeuroImage. 38(1):43–56. [DOI] [PubMed] [Google Scholar]
- Leporé N, Brun C, Pennec X, Chou Y-Y, Lopez OL, Aizenstein HJ, Becker JT, Toga AW, Thompson PM. 2007. Mean template for tensor-based morphometry using deformation tensors. Med Image Comput Comput Assist Interv. 10:826–833. [DOI] [PubMed]
- Maher BA, Ahmed IAM, Karloukovski V, MacLaren DA, Foulds PG, Allsop D, Mann DMA, Torres-Jardón R, Calderon-Garciduenas L. 2016. Magnetite pollution nanoparticles in the human brain. Proc Natl Acad Sci USA. 113(39):10797–10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. 2012. Brain on stress: How the social environment gets under the skin. Proc Natl Acad Sci USA. 109(Supplement_2):17180–17185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Tucker P. 2011. Critical biological pathways for chronic psychosocial stress and research opportunities to advance the consideration of stress in chemical risk assessment. Am J Public Health. 101(S1):S131–S139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JG, Gillette JS, Kircanski K, LeMoult J, Gotlib IH. 2020a. Air pollution is associated with elevated HPA-Axis response to stress in anxious adolescent girls. Comprehensive Psychoneuroendocrinology. 4:100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JG, Gillette JS, Manczak EM, Kircanski K, Gotlib IH. 2019. Fine particle air pollution and physiological reactivity to social stress in adolescence. Psychosom Med. 81(7):641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JG, Ho TC, Humphreys KL, King LS, Foland-Ross LC, Colich NL, Ordaz SJ, Lin J, Gotlib IH. 2020b. Early life stress, frontoamygdala connectivity, and biological aging in adolescence: a longitudinal investigation. Cereb Cortex. 30:4269–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury JB, Arseneault L, Beevers S, Kitwiroon N, Roberts S, Pariante CM, Kelly FJ, Fisher HL. 2019. Association of air pollution exposure with psychotic experiences during adolescence. JAMA Psychiat. 76(6):614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes JM. 2004. The (mis)estimation of neighborhood effects: causal inference for a practicable social epidemiology. Soc Sci Med. 58(10):1929–1952. [DOI] [PubMed] [Google Scholar]
- Olvera Alvarez HA, Kubzansky LD, Campen MJ, Slavich GM. 2018. Early life stress, air pollution, inflammation, and disease: an integrative review and immunologic model of social-environmental adversity and lifespan health. Neurosci Biobehav Rev. 92:226–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, Herbstman JB, Perera F, Tang D, Goldsmith J, Peterson BS, Rauh V, Margolis AE. 2020. Prenatal exposure to polycyclic aromatic hydrocarbons modifies the effects of early life stress on attention and thought problems in late childhood. J Child Psychol Psychiatry. 61:1253–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. 2004. Prospective investigation of stress inoculation in young monkeys. Arch Gen Psychiatry. 61:933–941. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. 2006. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. PNAS. 103:3000–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto MS, de Oliveira Galvão MF, Batistuzzo de Medeiros SR. 2017. Cell death pathways of particulate matter toxicity. Chemosphere. 188:32. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Rauh VA, Bansal R, Hao X, Toth Z, Nati G, Walsh K, Miller RL, Arias F, Semanek D, et al. 2015. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiat. 72(6):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancière F, Bougas N, Viola M, Momas I. 2017. Early exposure to traffic-related air pollution, respiratory symptoms at 4 years of age, and potential effect modification by parental allergy, stressful family events, and sex: a prospective follow-up study of the Paris birth cohort. Environ Health Perspect. 125(4):737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbe D. 1996. Psychometric review of traumatic event screening instrument for children (TESI-C). In: Stamm BH, editor. Measurement of stress, trauma, and adaptation. Lutherville, MD: Sidran, pp. 386–387.
- Rosa MJ, Hair GM, Just AC, Kloog I, Svensson K, Pizano-Zárate ML, Pantic I, Schnaas L, Tamayo-Ortiz M, Baccarelli AA, et al. 2020. Identifying critical windows of prenatal particulate matter (PM2.5) exposure and early childhood blood pressure. Environ Res. 182:109073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C. 1999. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: a transactional perspective. Child Dev. 70(3):660–677. [DOI] [PubMed] [Google Scholar]
- Sacks JD, Stanek LW, Luben TJ, Johns DO, Buckley BJ, Brown JS, Ross M. 2011. Particulate matter-induced health effects: who is susceptible? Environ Health Perspect. 119(4):446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A, Potter GG, McQuoid DR, Boyd B, Turner R, MacFall JR, Taylor WD. 2017. Effects of early life stress on depression, cognitive performance, and brain morphology. Psychol Med. 47:171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, Cheung JW, Van Erp TGM, Bos D, Ikram MA, et al. 2017. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 22(6):900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, Berhane K. 2009. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc Natl Acad Sci USA. 106(30):12406–12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southerland VA, Anenberg SC, Harris M, Apte J, Hystad P, van Donkelaar A, Martin RV, Beyers M, Roy A. 2021. Assessing the distribution of air pollution health risks within cities: a neighborhood-scale analysis leveraging high-resolution data sets in the bay area, California. Environ Health Perspect. 129:37006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinle S, Reis S, Sabel CE, Semple S, Twigg MM, Braban CF, Leeson SR, Heal MR, Harrison D, Lin C, et al. 2015. Personal exposure monitoring of PM2.5 in indoor and outdoor microenvironments. Sci Total Environ. 508:383–394. [DOI] [PubMed] [Google Scholar]
- Sunyer J, Esnaola M, Alvarez-Pedrerol M, Forns J, Rivas I, López-Vicente M, Suades-González E, Foraster M, Garcia-Esteban R, Basagaña X, et al. 2015. Association between traffic-related air pollution in schools and cognitive development in primary school children: a prospective Cohort study. PLoS Med. 12(3):e1001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson EM. 2019. Air pollution, stress, and allostatic load: linking systemic and central nervous system impacts. J Alzheimers Dis. 69(3):597–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC. 2010. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 29:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Holbrook AJ, Avants BB, Roberts JM, Cook PA, Reagh ZM, Duda JT, Stone JR, Gillen DL, Yassa MA, Alzheimer's Disease neuroimaging initiative. 2019. Longitudinal mapping of cortical thickness measurements: an alzheimer’s disease neuroimaging initiative-based evaluation study. J Alzheimers Dis. 71:165–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urger SE, De Bellis MD, Hooper SR, Woolley DP, Chen SD, Provenzale J. 2014. The superior longitudinal fasciculus in typically developing children and adolescents: diffusion tensor imaging and neuropsychological correlates. J Child Neurol. 30(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Donkelaar A, Martin RV, Brauer M, Hsu NC, Kahn RA, Levy RC, Lyapustin A, Sayer AM, Winker DM. 2016. Global estimates of fine particulate matter using a combined geophysical-statistical method with information from satellites, models, and monitors. Environ Sci Technol. 50:3762–3772. [DOI] [PubMed] [Google Scholar]
- van Donkelaar A, Martin RV, Li C, Burnett RT. 2019. Regional estimates of chemical composition of fine particulate matter using a combined geoscience-statistical method with information from satellites, models, and monitors. Environ Sci Technol. 53:2595–2611. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2016. Ambient air pollution: a global assessment of exposure and burden of disease. Geneva: World Health Organization. [Google Scholar]
- Wu X, Nethery RC, Sabath MB, Braun D, Dominici F. 2020. Air pollution and COVID-19 mortality in the United States: strengths and limitations of an ecological regression analysis. Sci Adv. 6:eabd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


