Abstract
Background
Coronavirus disease 2019 (COVID-19) is a manifestation of severe acute respiratory syndrome coronavirus 2, which results in many different complications including left ventricular (LV) thrombi.
Case summary
We present a 30-year-old female presenting with chest pain and shortness of breath. Patient had an extensive history including heart failure with an ejection fraction 15–20% and COVID-19 2 months ago. Echocardiogram revealed a 3.3 cm × 1.7 cm LV thrombus which was not present 4 months ago before her diagnosis of COVID-19. The LV thrombus embolized resulting in an embolus extending from the distal infrarenal abdominal aorta to the common iliac arteries bilaterally. Repeat COVID pre-procedure was positive. She underwent bilateral femoral artery cutdown, bilateral iliac artery embolectomy, superficial femoral artery embolectomy, and bilateral lower extremity fasciotomy. An extensive workup for the aetiology of the LV thrombus turned out to be negative and COVID-19 was deemed to be the aetiology of the thrombus. The patient was bridged from apixaban to warfarin and was successfully discharged within a few weeks.
Discussion
Hypercoagulability is a known complication of COVID-19 causing thrombi in various parts of the body including the LV. Early recognition with echocardiography, especially in patients with heart failure, and prompt treatment is key to avoid further complications such as embolization.
Keywords: Heart failure, COVID-19, Left ventricular thrombus, Embolization, Hypercoagulability, Case report
Learning points.
Coagulation abnormalities leading to a pro-coagulant state have been noted extensively in literature as one of the manifestations of Coronavirus disease 2019 (COVID-19), including the formation of left ventricular (LV) thrombus.
Early initiation of anticoagulation in patients with LV thrombus, exacerbated by heart failure, is key to the prevention of further clots. Patients with LV thrombi must be closely observed for signs and symptoms of embolization of LV thrombus.
Thromboembolism should still be on the differential diagnosis, especially in COVID-19, even if patients are on anticoagulant therapy.
Introduction
Since its emergence in late 2019, Coronavirus disease 2019 (COVID-19) has resulted in almost 490 million cases and over 6.1 million deaths worldwide as of April 2022.1 COVID-19 can involve multiple organs including the respiratory, cardiac, and immune systems.2 The most dreaded complication is acute respiratory distress syndrome, but it also leads to a hypercoagulable state causing multiple thrombi, including in the left ventricle. We present a case of left ventricular (LV) thrombus due to COVID-19 infection.
Timeline
| Timing | Events |
|---|---|
| 4 years before presentation |
|
| 4 months before presentation |
|
| 1 month before presentation |
|
| Initial presentation |
|
| Day 1 |
|
| Day 2 |
|
| Day 3 |
|
| Day 5 |
|
| Day 38 |
|
| Day 40 |
|
| 2 months post-discharge |
|
Case presentation
A 30-year-old female presented with chest pain and shortness of breath. The patient denies any complaints of palpitations or fevers. Her medical history was significant for non-ischemic cardiomyopathy, diagnosed 4 years ago, requiring an automatic implantable cardioverter-defibrillator, hypertension, diabetes mellitus, obstructive sleep apnoea, paroxysmal atrial fibrillation on apixaban, and recent COVID-19 infection. Her heart failure had been evaluated by her primary cardiologist and heart failure doctor by doing a cardiac catheterization showing normal coronary arteries, ever since her diagnosis 4 years ago. Previous transthoracic echocardiogram (TTE) done 4 months prior to admission showed a left ventricular ejection fraction (LVEF) of 15–20% with Grade 2 diastolic dysfunction and severe global hypokinesis of the LV, along with elevated right ventricular systolic pressure of 45–50 mmHg. Her medication history included carvedilol 37.5 mg/day twice a day (b.i.d.), sacubitril-valsartan 97–103 mg/day b.i.d., spironolactone 25 mg daily, hydralazine 25 mg three times a day (t.i.d.), isosorbide-dinitrate 10 mg t.i.d., torsemide 20 mg b.i.d., apixaban 5 mg b.i.d., and glipizide 5 mg b.i.d.. She was admitted to an outside hospital a month prior with COVID-19, not requiring mechanical ventilation, where she was treated with remdesivir and dexamethasone, which lead to her recovery, and continued her Eliquis due to normal renal function. She denies any smoking, or illicit drugs and drinks alcohol socially. She also has had multiple past admissions for heart failure.
Vital signs showed tachycardia to 117 b.p.m. and elevated blood pressure at 181/136 mmHg; rest of the vitals including temperature were within normal limits. Physical examination was significant for a regular, tachycardic pulse but diminished breath sounds at the bases but no crackles. Cardiovascular examination was unremarkable, including no peripheral signs of endocarditis, including Roth spots, Osler nodes, Janeway lesions, and splinter haemorrhages. SpO2 was 102 mmHg on arterial blood gas and patient was not requiring supplemental oxygen.
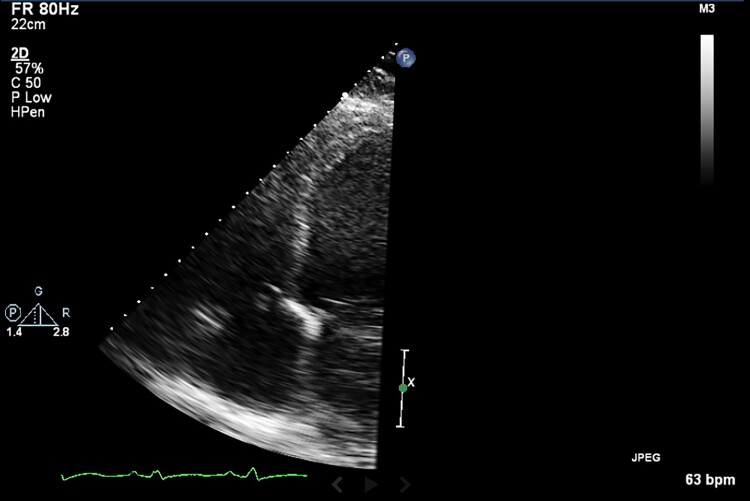
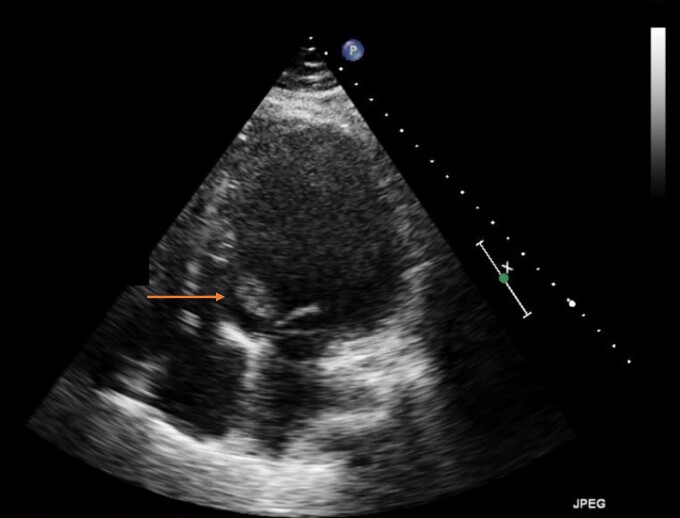
Initial workup was significant for brain natriuretic peptide of 1599 pg/mL (0–99 pg/mL), troponin I high sensitivity of 34 ng/L (<16ng/L), serum creatinine of 1.15 mg/dL (baseline 0.7 mg/dL) (0–1.11 mg/dL), D-dimer of 2442 ng/mL (0–230 ng/mL), ferritin 1852 ng/mL (5–204 ng/mL), fibrinogen 925 mg/dL (194–450 mg/dL), lactate dehydrogenase 1738 U/L (100–220 U/L), WBC 6.1 K/µL (4.0–12.0 K/µL), and thrombocytopenia at 103 000/mcL (150 000–400 000/mcL). Electrocardiogram showed sinus tachycardia, tall P- and QRS-waves suggestive of left atrial enlargement (with bifid P waves in lead II with >40 ms between the two peaks) and LV hypertrophy (R wave in aVL > 11 mm). Chest X-ray revealed cardiomegaly and central vascular prominence. A repeat COVID test was positive. The previous echo from 4 months prior to the current presentation did not show any LV thrombus (Figure 1). TTE during current admission showed decreased LVEF to 10–15%. and a new mobile mass in the LV outflow tract at the base of the mitral valve which measured 3.3 cm by 1.7 cm suggestive of a thrombus; however, vegetation could not be excluded (Figure 2, Supplemental data). LV internal dimension in systole and diastole were 7.1 cm and 7.3 cm, respectively.
Figure 1.
Transthoracic echocardiogram from 4 months prior to patient current presentation not showing any left ventricular thrombus.
Figure 2.
Apical four-chamber view on echocardiogram showing a 3.3 × 1.7 cm mobile mass concerning for thrombus during the current presentation.
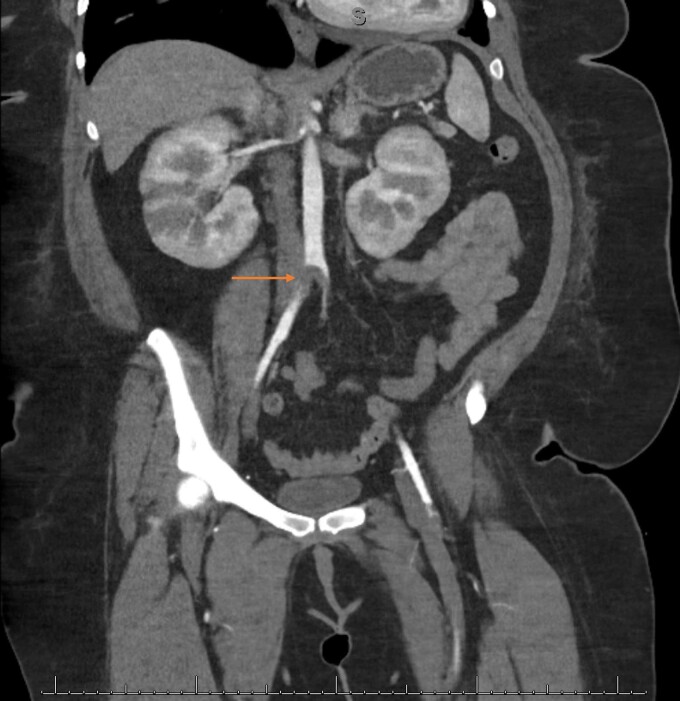
The patient was admitted, and a heparin infusion was started for the LV thrombus. Patient continued to be afebrile and blood cultures were drawn for concerns for the possibility of endocarditis. Apixaban was discontinued. Cardiothoracic (CT) surgery was consulted for evaluation for a LV embolectomy; however, medical management was favoured. On Day 2, the patient felt heaviness in her lower extremities and physical examination revealed absent pulses bilaterally. Cardiothoracic angiogram aorta with bilateral run-off (Figure 3) revealed a near-occlusive embolus within the distal infrarenal abdominal aorta extending into the common iliac arteries bilaterally. She underwent a bilateral femoral artery cutdown, bilateral iliac artery embolectomy, superficial femoral artery embolectomy, and bilateral lower extremity fasciotomy. A repeat TTE, on Day 3, did not reveal the original LV thrombus. However, there were two new mobile, echogenic structures in the LV apex and at the basal antero-lateral wall suggestive of further LV thrombus.
Figure 3.
Cardiothoracic angiogram showing a near-occlusive thrombus within the distal infrarenal abdominal aorta extending into the common iliac arteries bilaterally.
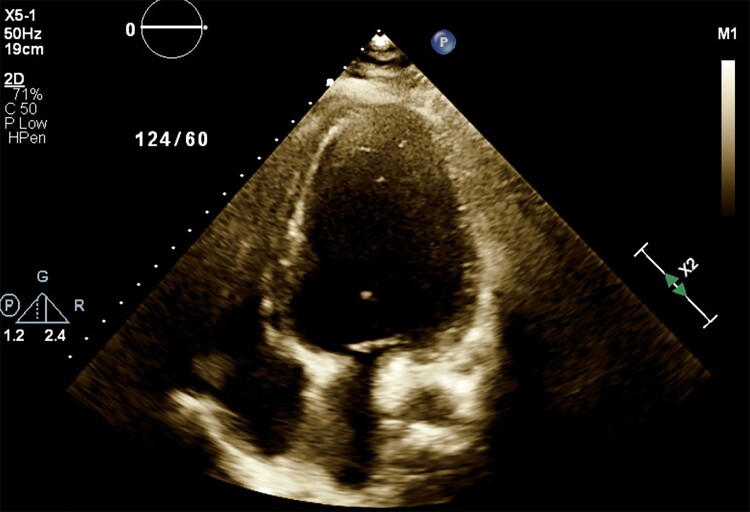
To identify the aetiology of the thrombi, a hypercoagulable workup including antinuclear antibodies, beta-2-glycoprotein, and anticardiolipin antibody was conducted; however, all were negative. Blood cultures that were previously drawn on admission, continued to be negative for any pathogen making endocarditis and a septic embolus unlikely. COVID-19 was deemed the aetiology for the LV thrombus. Heparin was bridged to warfarin. Repeat echocardiogram before discharge did not show any apical thrombus with a LVEF of 15%. After concerns for surgical wound healing, pain management due to bilateral fasciotomies, successful bridging from heparin to warfarin and titration of her heart failure therapy were addressed, the patient was successfully discharged after 6 weeks. Patient was discharged on carvedilol 25 mg b.i.d., Entresto 49–51 mg b.i.d., spironolactone 25 mg daily, torsemide 20 mg daily, and warfarin 9 mg daily, until the next follow-up with her cardiologist. A follow-up echocardiogram, 2 months later post-discharge, showed an ejection fraction of 25% with no recurrence of the LV thrombi (Figure 4).
Figure 4.
Echocardiogram 2 months post-discharge showing resolution of left ventricular thrombus.
Discussion
The virus SARS-CoV-2 belongs to the coronavirus family, which is enveloped positive-stranded RNA viruses. This virus is presumed to act via viral structural spike (S) protein by binding with the angiotensin-converting enzyme (ACE) II receptor, which is present in many organs of the body including the pulmonary, cardiac, and endothelial cells of the blood vessels.2 Of the clinical manifestations of COVID-19, coagulation abnormalities leading to a hypercoagulable state have been noted extensively in evolving literature. The exact mechanism for the hypercoagulability is not completely understood. However, in vitro studies of SARS-CoV-1 and data collected regarding COVID-19 suggests that SARS-CoV-2, due to its near-similarity in its genome with SARS-CoV-1, acts by causing hyperinflammation due to increased pro-inflammatory cytokines, reducing innate and adaptive responses to the virus, and interfering with the renin–angiotensin–aldosterone system by decreasing ACE II activity and hence increasing Ang II, resulting in vasoconstriction and pro-inflammatory and pro-oxidative state.3
Endothelial injury is a hallmark of the SARS-CoV-2. Inflammatory cells attached to the endothelial cells of the blood vessels in different organs including the lungs and heart as well as the elevated levels of von Willebrand factor and P-selectin, that are released by endothelial cells when they are compromised, in patients with COVID-19, lead to hypercoagulability.4 COVID-19 also leads to a hypercoagulable state by increasing pro-thrombotic factors such as fibrinogen, D-dimer, Factor VIII, and many others as seen in our patient as well.5 Finally, our patient had several risk factors that could lead to stasis including heart failure.
Left ventricular thrombus is a dreaded complication arising from cardiac pathologies including myocardial infarction and heart failure. In a study at Brigham and Women’s Hospital, heart failure was found to be the main cause of LV thrombi after evaluating over 140 000 echocardiograms.6 One of the many complications of LV thrombus is embolic events. A cohort study comparing patients with LV thrombus with patients without LV thrombus showed that the former had four times the long term incidence of embolic events.7
COVID-19 manifests in several haematologic complications including thrombotic complications in the venous system such as deep venous thrombosis, and the arterial system manifesting as myocardial infarction, stroke, and limb ischaemia.8,9 This patient had a severely depressed ejection fraction that led to stasis and had added risk factors of being hypercoagulable due to COVID-19, making her at risk for the formation of a LV thrombus and the subsequent embolization to the lower limbs.
There have been a few reports of patient developing intracardiac thrombi in patients with COVID-19.10,11 However, in medical literature, there has not been a reported incident of such LV intracardiac thrombi, resulting in subsequent embolization into the lower limb causing ischaemia. Since COVID-19 is a recent entity, there have been no studies to date regarding the incidence of LV thrombi or subsequent embolization in patients who have COVID-19.
Due to the lack of randomized trials showing the efficacy of direct oral anticoagulants (DOACs) in patients with LV thrombi, warfarin is the mainstay therapy to inhibit clot progression. American Heart Association guidelines recommend 3–6 months of therapy.12 However, there have been case series and retrospective studies that have shown increased efficacy of DOACs in the resolution of LV thrombi13,14 including a quicker resolution of LV thrombi when comparing to warfarin.13 DOACs hold the advantage of having a safer profile in terms of bleeding risk13 and more infrequent monitoring.
Unfractionated heparin (UFH) or low molecular weight heparin (LMWH) are the most common methods for venous thromboembolism prophylaxis.14 In case of COVID-19, early reports suggest that UFH and LMWH have antiviral and anti-inflammatory properties as well as antithrombotic activities.15 Treatment failure with apixaban in our patient can be attributed to many factors, including medication non-compliance. Another reason for apixaban failure in our patient could be due to the ability of SARS-CoV-2 to alter the activity of multiple cytochrome P450 enzymes that also play a role in the metabolism of apixaban. However, the clinical significance of these interactions at this point is unknown. One of the many ways to transition to another anticoagulation in such patients is to transition to warfarin with parenteral anticoagulation, such as UFH, as bridging therapy.16 As in our patient, a heparin drip was started, and she was transitioned to warfarin. Echocardiogram done 2 months later after discharge (Figure 4) showed resolution of the LV thrombus.
Conclusion
Thromboembolism should be considered in the differential diagnosis of the patients with COVID-19 who develop new vascular or neurological symptoms. Early initiation of anticoagulation is key.
Supplementary Material
Lead author biography

Hamza Zahid Ullah Muhammadzai is an Internal Medicine resident at Abington Jefferson Health in Abington, PA, USA.
Supplementary material
Supplementary material is available at European Heart Journal—Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
References
- 1. John Hopkins Coronavirus Resource Center. John Hopkins University & Medicine. https://coronavirus.jhu.edu/(02 April 2022).
- 2. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020;324:782–793. [DOI] [PubMed] [Google Scholar]
- 3. Henry BM, Vikse J, Benoit S, Favaloro EJ, Lippi G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta 2020;507:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lowenstein CJ, Solomon SD. Severe COVID-19 is a microvascular disease. Circulation 2020;142:1609–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost 2020;18:1738–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCarthy CP, Murphy S, Venkateswaran RV, Singh A, Chang LL, Joice MG, Rivero JM, Vaduganathan M, Januzzi JL Jr, Bhatt DL. Left ventricular thrombus: contemporary etiologies, treatment strategies, and outcomes. J Am Coll Cardiol 2019;73:2007–2009. [DOI] [PubMed] [Google Scholar]
- 7. Velangi PS, Choo C, Chen KH, Kazmirczak F, Nijjar PS, Farzaneh-Far A, Okasha O, Akçakaya M, Weinsaft JW, Shenoy C. Long-term embolic outcomes after detection of left ventricular thrombus by late gadolinium enhancement cardiovascular magnetic resonance imaging: a matched cohort study. Circ Cardiovasc Imaging 2019;12:e009723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med 2020;26:1017–1032. [DOI] [PubMed] [Google Scholar]
- 9. Ali Z, Ullah W, Saeed R, Ashfaq A, Lashari B. Acute COVID-19 induced fulminant systemic vascular thrombosis: a novel entity. Int J Cardiol Heart Vasc 2020;30:100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaki A, Singh H, Cohen G, Schreiber T. A case report of a large intracardiac thrombus in a COVID-19 patient managed with percutaneous thrombectomy and right ventricular mechanical circulatory support. Eur Heart J Case Rep 2020;4:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Imaeda S, Kabata H, Shiraishi Y, Kamata H, Tsuruta H, Yuasa S, Ishii M, Fukuda K, Fukunaga K. Left ventricular thrombus with COVID-19 complication in a patient with dilated cardiomyopathy. CJC Open 2021;3:124–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCarthy CP, Vaduganathan M, McCarthy KJ, Januzzi JL, Bhatt DL, McEvoy JW. Left ventricular thrombus after acute myocardial infarction: screening, prevention, and treatment. JAMA Cardiol 2018;3:642–649. [DOI] [PubMed] [Google Scholar]
- 13. Fleddermann AM, Hayes CH, Magalski A, Main ML. Efficacy of direct acting oral anticoagulants in treatment of left ventricular thrombus. Am J Cardiol 2019;124:367–372. [DOI] [PubMed] [Google Scholar]
- 14. Jones DA, Wright P, Alizadeh MA, Fhadil S, Rathod KS, Guttmann O, Knight C, Timmis A, Baumbach A, Wragg A, Mathur A, Antoniou S. The use of Novel Oral Anti-Coagulant’s (NOAC) compared to vitamin K antagonists (warfarin) in patients with left ventricular thrombus after Acute Myocardial Infarction (AMI). Eur Heart J Cardiovasc Pharmacother 2021;7:398–404. [DOI] [PubMed] [Google Scholar]
- 15. Bikdeli B, Madhavan MV, Gupta A, Jimenez D, Burton JR, Der Nigoghossian C, Chuich T, Nouri SN, Dreyfus I, Driggin E, Sethi S, Sehgal K, Chatterjee S, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Bertoletti L, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Tafur AJ, Francese DP, Batra J, Falanga A, Clerkin KJ, Uriel N, Kirtane A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Leon MB, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH. Pharmacological agents targeting thromboinflammation in COVID-19: review and implications for future research. Thromb Haemost 2020;120:1004–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McIlroy G, Smith N, Lokare A, Beale K, Kartsios C. Treatment failure in patients receiving direct oral anticoagulants: clinical management and outcomes from a single-center review of 59 consecutive patients. Blood 2018;132:5058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.