Abstract
Although some individuals with at-risk mental states (ARMS) develop overt psychosis, surrogate markers which can reliably predict a future onset of psychosis are not well established. The dorsal lateral prefrontal cortex (DLPFC) is thought to be involved in psychotic disorders such as schizophrenia. In this study, 73 ARMS patients and 74 healthy controls underwent 1.5-T 3D magnetic resonance imaging scans at three sites. Using labeled cortical distance mapping, cortical thickness, gray matter (GM) volume, and surface area of DLPFC were estimated. These measures were compared across the diagnostic groups. We also evaluated cognitive function among 36 ARMS subjects to clarify the relationships between the DLPFC morphology and cognitive performance. The GM volume of the right DLPFC was significantly reduced in ARMS subjects who later developed frank psychosis (ARMS-P) relative to those who did not (P = 0.042). There was a positive relationship between the right DLPFC volume and the duration prior to the onset of frank psychosis in ARMS-P subjects (r = 0.58, P = 0.018). Our data may suggest that GM reduction of the DLPFC might be a potential marker of future onset of psychosis in individuals with ARMS.
Keywords: at-risk mental state, dorsal lateral prefrontal cortex, magnetic resonance imaging, psychosis, schizophrenia
Introduction
Accumulating evidence suggests that individuals with psychotic disorders, such as schizophrenia, suffer not only from psychiatric symptoms but also from difficulty in achieving functional recovery (Jaaskelainen et al. 2013) and from various physical health problems (DE Hert et al. 2011). Many attempts have been made for the purpose of delaying or preventing frank psychosis in individuals with an at-risk mental state (ARMS) for psychotic disorders (Stafford et al. 2013). The establishment of surrogate makers to reliably predict a future onset of overt psychosis in ARMS individuals is crucial since the majority (>60%) of this population do not eventually develop psychosis (Fusar-Poli et al. 2012). Neurophysiological tests (Higuchi et al. 2014; Bodatsch et al. 2015), structural magnetic resonance imaging (sMRI) (Bois et al. 2015; Takahashi and Suzuki 2018), and functional magnetic resonance imaging (Allen et al. 2015) have been used to identify biological changes predating the transition to psychosis in ARMS. For instance, previous sMRI studies have shown gray matter (GM) reduction or cortical thinning of the anterior cingulate cortex (ACC) (Borgwardt et al. 2007; Fornito et al. 2008; Takayanagi et al. 2017), superior temporal gyrus (Borgwardt et al. 2007), and insular (Borgwardt et al. 2007; Takahashi et al. 2009) and parahippocampal gyrus (Mechelli et al. 2011) in ARMS subjects who later developed frank psychosis (ARMS-P).
Cognitive impairment in ARMS subjects has become well established in the past decade, as shown in two recent meta-analyses (Bora et al. 2014; Hauser et al. 2017) that also indicated that ARMS-P subjects performed lower in many neuropsychological domains, including attention and working memory, than those who did not develop psychosis (ARMS-NP), or healthy subjects. Likewise, a large-scale multisite study conducted in North America demonstrated attention and working memory deficits in ARMS-P group compared with ARMS-NP subjects (Seidman et al. 2016).
The dorsal lateral prefrontal cortex (DLPFC, Brodmann areas 46 and 9) is located in the midanterior part of the middle frontal gyrus (MFG) and is thought to be involved in various cognitive processes, particularly attention (Vossel et al. 2012; Bidet-Caulet et al. 2015) and working memory (Barbey et al. 2013; Weinberger et al. 1986), both of which are shown to be impaired in ARMS-P subjects as noted above. Although many sMRI studies have reported reduced GM volume or cortical thickness of the MFG in patients with schizophrenia, compared with healthy subjects (Kuperberg et al. 2003; Suzuki et al. 2005; Kasparek et al. 2007; Nesvag et al. 2008; Schultz et al. 2010; Takayanagi et al. 2011; Takayanagi et al. 2019), relatively few studies exclusively examined the DLPFC morphology in schizophrenia or ARMS subjects (Kikinis et al. 2010).
Whole-brain analytic methods, such as voxel-based morphometry (VBM) and surface-based analysis (SBM), have widely been used for cortical morphometry in psychiatric disorders, such as schizophrenia (Kuperberg et al. 2003; Kasparek et al. 2007; Nesvag et al. 2008; Schultz et al. 2010; Takayanagi et al. 2011; Takayanagi et al. 2019). However, no research found the morphological abnormalities of MFG in ARMS-P subjects prior to the onset of frank psychosis by using VBM or SBM (Pantelis et al. 2003; Borgwardt et al. 2007; Koutsouleris et al. 2009; Mechelli et al. 2011; Tognin et al. 2014; Cannon et al. 2015). Labeled cortical distance mapping (LCDM) is a neuroimaging analytical tool that calculates the distances between labeled GM voxels and the GM/white matter (WM) cortical surface. LCDM can reliably characterize the morphometry of the laminar cortical mantle of cortical structures, such as cortical thickness and GM volume (Ceyhan et al. 2011). Although the boundary between WM and cerebrospinal fluid (CSF) is oftentimes obscure, LCDM can partly resolve this challenge since it treats the region of interest (ROI) as a laminar structure consisting of GM voxels and a local surface co-ordinate system based on an anatomically defined GM/WM cortical surface. Thus, this method is less susceptible to signal intensity inhomogeneity than whole-brain analyses. Using LCDM, we previously reported a reduced thickness of ACC in ARMS subjects who later develop full-blown psychosis when compared with healthy subjects (Takayanagi et al. 2017).
In this study, we examined DLPFC morphology based on our hypothesis that DLPFC structural anomalies may predate the onset of psychosis and underlie cognitive impairments (e.g., attention and working memory deficits) in ARMS subjects. We used ROI-based technique, namely LCDM, considering its advantage over whole-brain analyses (i.e., better tissue segmentation). We also assessed cognitive function in some of the ARMS participants by using the Japanese version of the brief assessment of cognition in schizophrenia (BACS-J) (Kaneda et al. 2007) to examine the relationship between the DLPFC morphology and cognitive performance.
Materials and Methods
Participants
Seventy-three ARMS subjects were recruited at three sites (Toho University Hospital, Tohoku University Hospital, and Toyama University Hospital), where specialized clinical services for ARMS are offered (Mizuno et al. 2009). The Comprehensive Assessment of At-Risk Mental State (CAARMS) (Yung et al. 2005) (University of Toyama and Tohoku University) or the Structured Interview for Prodromal Syndrome/the Scale of Prodromal Symptoms (SIPS/SOPS) (Miller, McGlashan, et al. 2003) (Toho University) were used for the diagnosis of ARMS. ARMS subjects were clinically monitored for at least 2 years after magnetic resonance imaging (MRI) scanning to see if they developed overt psychosis. Transition to psychosis was determined based on the CAARM or the SIPS criteria as detailed in our previous study (Takayanagi et al. 2017). Eighteen of 73 ARMS subjects developed frank psychosis (i.e., ARMS-P subjects) after MRI scanning. Based on the Diagnostic and Statistical Manual of Mental Disorders fourth edition (DSM-IV) (American Psychiatric Association 1994) criteria, the diagnoses of ARMS-P subjects comprised 12 schizophrenia cases, 1 delusional disorder case, 1 schizophreniform disorder case, and 4 cases with psychotic disorder not otherwise specified (NOS).
Seventy-four healthy controls (HC) were recruited from the community, hospital staff, and students at each site. HC were matched for age and gender with the ARMS subjects.
All subjects were physically healthy at the time of MRI scanning. Subjects were excluded if they 1) had a lifetime history of serious head injury, neurological illness, or other serious physical disease; 2) fulfilled the criteria for substance abuse/dependence; or 3) had previous psychotic episodes which met the criteria of DSM-IV. Most of the participants of this study (98.6%) overlap with those of our previous study (Takayanagi et al. 2017). All subjects provided written informed consent. If the participants were minors, written informed consent was provided by their parents. This study was approved by the Committee on Medical Ethics at each site.
MRI Data Acquisition
The MRI scanners and data acquisition parameters used at each site are detailed in the supplementary material. All three sites used scanners with field strength of 1.5 T.
FreeSurfer-Initialized Labeled Cortical Distance Mapping
First, all images were preprocessed by FreeSurfer software suite (version 5.3) working on a Macintosh workstation (MAC OS 10.7). The FreeSurfer pipeline is publicly available (https://surfer.nmr.mgh.harvard.edu/). FreeSurfer’s standard preprocessing steps consist of tissue intensity inhomogeneity normalization, nonbrain tissue removal, transformation to Talairach-like space, and segmentation of GM/WM tissue (Fischl 2012). The preprocessed images were carefully inspected, and errors were manually corrected by one trained researcher (D.S.) who was blinded to the subject’s identity. FreeSurfer extracts the surfaces of GM/WM and automatically parcellates 68 cortical ROIs using the Desikan-Killiany Atlas (Desikan et al. 2006), including the MFG.
The DLPFC surface was then cut from the MFG surface using an established automatic protocol (Al-Hakim et al. 2006; Kikinis et al. 2010): 1) The distance between the most anterior point of the frontal pole and most anterior point of the temporal pole was measured. 2) Cuts were made at 40% of the distance and 80% of the distance measured from the tip of the frontal pole. These anatomical rules to locate the DLPFC were established based on cytoarchitectonic data of BA46 from five human brains (Rajkowska and Goldman-Rakic 1995; Al-Hakim et al. 2006). For this automatic DLPFC cutting, we used own MATLAB (the MathWorks Inc.) script. Figure 1 shows an example of the extracted DLPFC on the left hemisphere.
Figure 1.
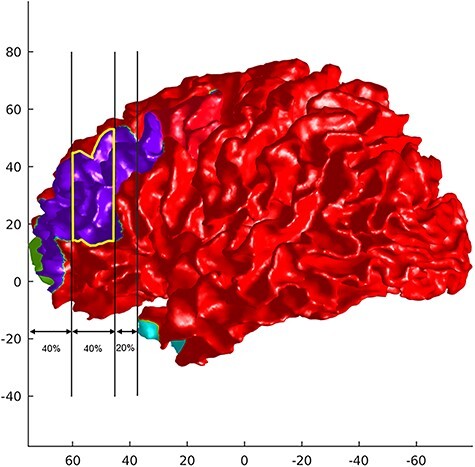
An example of DLPFC (yellow line) cut from the MFG (blue) on the left hemisphere.
Next, the cropped MRI was segmented into WM, GM, and CSF using a mixture model averaging method with alternating kernel mixture (AKM) (Lee et al. 2008; Wentz 2012). Compared with other segmentation methods using traditional Bayesian segmentation, AKM yields smaller errors, indicating the robustness and wide applicability of AKM across different structures (Lee et al. 2008). The GM/WM threshold from the segmentation was used to generate triangulated isosurfaces representing the 2D cortical surface. The previously developed method for generating LCDMs (Miller et al. 2000; Miller, Hosakere, et al. 2003; Ratnanather et al. 2004) was applied. To generate a distance map for the GM, the distance between each GM voxel and the closest GM/WM surface vertex was calculated at a 1 × 1 × 1 mm resolution. GM voxels associated with a vertex in the DLPFC surface were labeled DLPFC. Voxels in the range of −2 to 8 mm were used for the analysis. The result of LCDM is a probability distribution function of the GM distance from the DLPFC GM/WM surface. Finally, three DLPFC measures (i.e., cortical thickness, GM volume, and surface area) were estimated. Cortical thickness was determined using the distance at the 95th percentile of the distance distribution. Due to outlier voxels at distances >6 mm, the volume of voxels with distance ≤95th percentile was taken as the volume of the DLPFC. The area of the GM/WM boundary surface was calculated from the triangulated surface. By using LCDM, challenges stemming from the obscureness of the boundary between WM and CSF can partly be clarified. The algorithm for LCDM has been published several times (Miller et al. 2000; Miller, Hosakere, et al. 2003; Ratnanather et al. 2004) and is readily coded with the R statistical software package (https://www.r-project.org).
Neurocognitive Assessment
After the FreeSurfer-initialized labeled cortical distance mapping (FSLCDM) analyses, the availability of BACS-J (Kaneda et al. 2007) scores at baseline was retrospectively checked. Thirty-six ARMS subjects (49%) had undergone BACS-J assessment by trained psychiatrists or psychologists at baseline.
Statistical Analysis
One-way analysis of variance, independent two-sample t-tests, or a chi-squared test were used for the comparison of clinical measures across the diagnostic groups. DLPFC measures (i.e., cortical thickness, volume, and surface area) were compared among the groups (i.e., controls, ARMS-NP, and ARMS-P) by using repeated measures analysis of covariance (ANCOVA), with diagnosis as the between-subject factor; hemisphere as the within-subject factor; and age, sex, intracranial volume (ICV), antipsychotic use, and scanning sites as nuisance covariates. Sex, antipsychotic use, and each scanning site were entered as binary variables. For the post hoc pairwise comparison, we used Bonferroni’s correction. Furthermore, for the purpose of better management of intersite variance, we performed a meta-analytic calculation of overall effect sizes for a random effect model (Han and Eskin 2011) by using Review Manager version 5.4. (the Nordic Cochrane Centre, Cochrane Collaboration) based on previous studies (van Erp et al. 2016; Sasabayashi et al. 2020). The associations between DLPFC measures and neurocognitive functions (i.e., BACS scores) in ARMS subjects were evaluated by calculating partial correlation coefficients adjusted for age, sex, ICV, antipsychotic use, and scanning sites. Bonferroni’s correction was again adopted for these correlational analyses. When a significant DLPFC structural change was found between ARMS-P and ARMS-NP/healthy subjects, the correlation of the DLPFC measure with the duration (weeks) between MRI scanning and the onset of psychosis in ARMS-P subjects was evaluated by calculating partial correlation coefficients controlling only for sites, considering the small sample size of this group (n = 18). All statistical analyses were conducted using SPSS (ver.18) (IBM Corp.). The significance level was set at P < 0.05 (two-tailed).
Results
Clinical Characteristics
Table 1 summarizes the clinical characteristics of the diagnostic groups. HC, ARMS-NP, and ARMS-P groups were similar in terms of age, gender distribution, and parental educational level. Thirty of 73 ARMS subjects (41%) were taking antipsychotics at baseline. Self-reported educational attainment was significantly higher in HC than in ARMS-NP (P < 0.001) and ARMS-P (P < 0.001) groups. The BACS subscores did not differ among ARMS-NP and ARMS-P groups.
Table 1.
Demographic and clinical characteristics
| Variables | Group | ||||
|---|---|---|---|---|---|
| HC | ARMS-NP | ARMS-P | Statistics | P | |
| Total number of subjects | 74 | 55 | 18 | ||
| Site | |||||
| Toyama | 52 | 11 | 5 | ||
| Toho | 5 | 19 | 4 | ||
| Tohoku | 17 | 25 | 9 | ||
| Age (mean ± SD) | 22.6 ± 4.3 | 22.3 ± 6.5 | 20.1 ± 4.3 | F = 1.7 | 0.19 |
| Sex (male/female) | 37/37 | 21/34 | 5/13 | X2 = 3.7 | 0.16 |
| Handednessa (right/both/left) | 58/0/0 | 34/8/2 | 10/2/2 | ||
| Education yearsb (mean ± SD) | 14.8 ± 1.9 | 12.2 ± 2.6 | 12.3 ± 2.3 | F = 23.8 | <0.001 |
| Parental education yearsc (mean ± SD) | 12.9 ± 2.2 | 13.5 ± 2.0 | 13.4 ± 1.6 | F = 0.86 | 0.43 |
| Weeks between scanning and onset of psychosis (mean ± SD) | 40.1 ± 32.6 | ||||
| On antipsychotics (n, %) | 21, 38% | 9, 50% | X2 = 0.64 | 0.78 | |
| Antipsychotics dose (mean mg ± SD, chlorpromazine equivalent)d | 145 ± 102 | 196 ± 130 | t = 1.1 | 0.26 | |
| BACS-J subscorese | |||||
| Verbal memory (mean ± SD) | 46.4 ± 10.1 | 50.7 ± 12.6 | t = 1.0 | 0.32 | |
| Digit sequencing task (mean ± SD) | 18.3 ± 5.0 | 21.0 ± 5.4 | t = 1.4 | 0.18 | |
| Token motor task (mean ± SD) | 70.9 ± 14.1 | 70.0 ± 8.1 | t = 0.18 | 0.86 | |
| Category/letter fluency (mean ± SD) | 40.2 ± 12.8 | 44.3 ± 10.2 | t = 0.87 | 0.39 | |
| Symbol coding (mean ± SD) | 63.3 ± 12.9 | 64.9 ± 19.2 | t = 0.28 | 0.78 | |
| Tower of London (mean ± SD) | 17.8 ± 2.5 | 18.1 ± 2.4 | t = 0.28 | 0.73 | |
NP, did not develop psychosis; P, developed psychosis.
Data missing for 30 subjects.
Data missing for 7 subjects.
Data missing for 38 subjects.
Calculated in all ARMS subjects
36 ARMS subjects (27 ARMS-NP and 9 ARMS-P subjects) underwent BACS-J.
DLPFC Measures
Repeated measures ANCOVA adjusted for age, gender, ICV, antipsychotic use, and scanning site demonstrated a significant hemisphere × diagnosis interaction for the volume of the DLPFC (F2,138 = 3.799, P = 0.025). Post hoc testing showed that the volume of the right DLPFC was smaller in the ARMS-P group relative to the ARMS-NP (Pcorrected = 0.042) and HC (Pcorrected = 0.028) groups (Fig. 2, Table 2). The GM volume reduction of the right DLPFC in ARMS-P subjects compared with ARMS-NP was replicated in the meta-analysis (Cohen’s d = −0.66, P = 0.02) (Supplementary Fig. S1), while the difference between ARMS-P group and HCs did not reach the statistically significant level (P = 0.24) (Supplementary Fig. S2). In HC, a rightward laterality was confirmed (Pcorrected = 0.003), while such asymmetry was not detected in ARMS-NP (Pcorrected = 0.28) and ARMS-P groups (Pcorrected = 0.09) (Table 2). We found a significant positive correlation of right DLPFC volume with weeks between MRI scanning and onset of psychosis in the ARMS-P group (r = 0.58, P = 0.018) (Fig. 3). There was a trend-level positive association between the left DLPFC thickness and BACS symbol coding score (r = 0.541, 0.05/36 = 0.0014 < P = 0.0017 < 0.1/36 = 0.0028) in the 36 ARMS subjects who underwent BACS.
Figure 2.
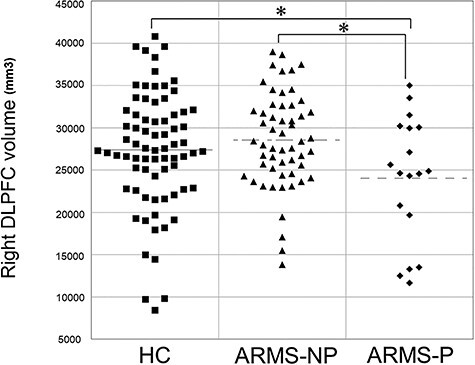
Comparisons of volume of the right DLPFC among HC, ARMS-NP, and ARMS-P patients. *P < 0.05.
Table 2.
Comparisons of DLPFC measures among diagnostic groups
| HC (n = 74) | ARMS-NP (n = 55) | ARMS-P (n = 18) | ANCOVAa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | Hemisphere | Hemisphere × diagnosis | ||||||||||
| Measures | Mean | SD | Mean | SD | Mean | SD | F | P | F | P | F | P |
| Left DLPFC thickness (mm) | 3.50 | 0.72 | 3.14 | 0.62 | 3.42 | 0.64 | 2.133 | 0.122 | 0.010 | 0.921 | 1.557 | 0.214 |
| Right DLPFC thickness (mm) | 3.65 | 0.69 | 3.35 | 0.65 | 3.30 | 0.79 | ||||||
| Left DLPFC volume (mm3) | 25 479 | 6231 | 27 500 | 5311 | 26 119 | 6782 | 1.983 | 0.142 | 0.774 | 0.380 | 3.799 * , ** | 0.025 |
| Right DLPFC volume (mm3) | 27 383 | 6905 | 28 546 | 5605 | 24 048 | 7400 | ||||||
| Left DLPFC area (mm2) | 3167 | 1008 | 2994 | 929 | 3026 | 1032 | 2.137 | 0.122 | 0.089 | 0.766 | 0.337 | 0.715 |
| Right DLPFC area (mm2) | 2979 | 1197 | 3177 | 944 | 2804 | 996 | ||||||
DLPFC, dorsolateral prefrontal cortex.
Age, gender, ICV, use of antipsychotics, and scanning site were entered as covariates.
*Post hoc tests showed that volume was reduced on the right hemisphere in ARMS-P subjects compared with ARMS-NP (P = 0.042) and HC (P = 0.028) groups.
**A rightward laterality (left < right) was seen only in HC (P = 0.003).
Figure 3.
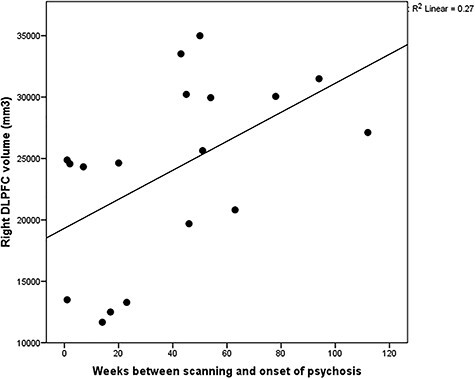
Correlation of the right DLPFC volume with weeks between scanning and onset of psychosis in ARMS-P subjects.
Discussion
We found a GM reduction of the right DLPFC in ARMS individuals who later developed frank psychosis as compared with those who did not using LCDM. We also found a positive relationship between the right DLPFC volume and the duration prior to the onset of frank psychosis (i.e., a smaller DLPFC volume at baseline was associated with earlier onset of subsequent psychosis). Taken together, our findings may suggest that the DLPFC volume change might be useful as a prognostic marker of future psychosis in individuals with ARMS. Our finding of altered DLPFC morphology preceding the onset of psychosis collaborates with previous studies that demonstrated GM volume or cortical thickness reductions in the ACC (Fornito et al. 2008; Takayanagi et al. 2017), parahippocampal gyrus (Mechelli et al. 2011), superior temporal gyrus (Borgwardt et al. 2007), and insula (Borgwardt et al. 2007; Takahashi et al. 2009) among ARMS-P subjects.
Previous studies using whole-brain analyses, such as VBM or SBM, did not detect cortical changes in the middle frontal regions among ARMS-P subjects prior to the onset of overt psychosis (Pantelis et al. 2003; Borgwardt et al. 2007; Koutsouleris et al. 2009; Mechelli et al. 2011; Tognin et al. 2014; Cannon et al. 2015), and thus our finding is not consistent with these works. The discrepancy may be due to differences in the methodology (e.g., ROI analysis vs. whole-brain analysis or the difference in the tissue segmentation method) and the sample size between studies.
GM reductions and cortical thinning of the MFG in schizophrenia patients relative to HC have been demonstrated in a number of MRI studies (Kuperberg et al. 2003; Suzuki et al. 2005; Kasparek et al. 2007; Nesvag et al. 2008; Kikinis et al. 2010; Schultz et al. 2010; Takayanagi et al. 2011; Takayanagi et al. 2019). Taken together with our data, the GM reduction in MFG, or more specifically the DLPFC, may exist prior to the onset of frank psychosis.
Our data showed that a smaller volume of the right DLPFC at baseline was associated with earlier onset of frank psychosis. This positive relationship may suggest that DLPFC volume could be useful for identifying ARMS individuals at an imminent risk for psychosis. On the other hand, we are unable to determine whether such DLPFC volume change in ARMS-P subjects is progressive or static since we lack follow-up MRI scanning.
We demonstrated a rightward asymmetry of DLPFC volume in healthy subjects but not in ARMS subjects. GM volume reduction of the left DLPFC in established schizophrenia patients has been shown by the VBM meta-analysis (Bora et al. 2012). The previous study which used the same DLPFC cutting method (Kikinis et al. 2010) demonstrated 1) the rightward laterality both in schizophrenia patients and HCs and 2) the left dominancy of MFG volume reduction. Take together with our results, the lack of rightward asymmetry of DLPFC volume in both ARMS-NP and ARMS-P subjects might be associated with the vulnerability to psychosis. Previously reported volume reduction of the left MFG/DLPFC in schizophrenia may occur during/after the manifestation of psychotic symptoms, while the alteration on the right hemisphere may predate the onset of psychosis.
We used 1.5-T scans which may not be state of the art. With lower resolution, there is the potential for a larger standard deviation (SD) in thickness measures, and thus larger sample size may be required for showing group differences. In addition, there is also potential for deep folds (with only a thin layer of WM or CSF) to be mischaracterized as GM, which can lead to overestimates of thickness. On this point, we performed quality control of segmentations and reperformed segmentation with AKM in failed cases. AKM has been shown to have greater accuracy (Lee et al. 2008). For 1.5-T images, LCDMs have been shown to be robust to different cortical regions, such as cingulate gyrus and ventromedial prefrontal cortex (Miller et al. 2000).
The later versions of FreeSurfer (e.g., version 6.0) can be applied for the FSLCDM pipeline, though we use version 5.3. FreeSurfer 6.0 updated the mappings for subcortical structures, added features to handle more error cases in registration (e.g., when ventricles are very large), and introduced some speed-ups. We believe these changes do not significantly affect the FSLCDM pipeline.
For vertex-wise analyses, cortical thickness might be more sensitive for detecting focal GM changes when compared with volume or surface area (Pereira et al. 2012). We, however, used the ROI approach with LCDM. Our previous studies using the ROI-based LCDM analyses yielded various results, such as thickness/volume reductions of ACC in deficit-schizophrenia patients (Takayanagi et al. 2013), thickness reduction of ACC in ARMS-P group (Takayanagi et al. 2017), and no significant changes of planum temporale in ARMS-P/ARMS-NP subjects (Takayanagi et al. 2020). Thus, the imaging methods used in our study and the regional specificity may have affected our results (i.e., volume was more meaningful than thickness/area).
We failed to replicate previous works that have shown attention or working memory deficits in ARMS-P groups (Bora et al. 2014; Hauser et al. 2017). Although it might be difficult to speculate the reasons for the negative finding regarding attention/working memory function among ARMS cases in our study, the small number of ARMS subjects who underwent BACS may have influenced this result.
Our finding might have been confounded by the mixed use of different criteria for ARMS (i.e., SIPS/SOPS or CAARMS) among sites. As the CAARMS criteria is less restrictive than that of SIPS though they largely overlap (Miller, McGlashan, et al. 2003; Yung et al. 2005), some subjects may not meet the criteria of one of these instruments. Fusar-Poli et al. (2016) demonstrated a high CAARMS-versus-SIPS agreement in the identification of ARMS subjects (overall agreement = 86%; kappa = 0.781). Therefore, we believe most of the ARMS subjects meet the criteria of both CAARMS and SIPS.
We should consider several limitations. First, MRI scanners and acquisition parameters were different among sites. In addition, the disproportion of comprising controls and ARMS subjects across research sites may have confounded the results. Particularly, the number of the HC recruited at the Toho site (n = 5) was significantly smaller than those of other sites. However, we believe that our finding was not merely a consequence of these site differences because our statistical models accounted for site differences. Furthermore, there were no diagnosis × site or hemisphere × diagnosis × site interactions. Second, some ARMS subjects took antipsychotics; the effects of antipsychotic medications on brain morphology cannot be excluded (Vita et al. 2015), although we treated usage of antipsychotics as a nuisance covariate in each statistical model. Third, the sample size of ARMS-P group (n = 18) was relatively small. Likewise, only about half of the ARMS subjects underwent BACS measurement as noted above. Thus, the statistical power of some comparison/correlation analyses may not be sufficient. Fourth, we were unable to consider handedness in the statistical models due to missing data (n = 30). Finally, due to the cross-sectional design of this study, we were unable to see whether the DLPFC alteration (i.e., GM reduction) is static or progressive. Longitudinal analyses are warranted in future studies.
In summary, this multisite study using LCDM suggested that GM alteration of the DLPFC predates the onset of frank psychosis in individuals with ARMS. On the other hand, previously reported attention or working memory deficits before the onset of psychosis were not detected. The DLPFC morphological change might be useful as an objective marker for the prediction of future transition to psychosis in the clinical high-risk population.
Funding
Japanese Society for the Promotion of Science (Kiban C No. 18K07549 to Y.T., Wakate No.18K15509 and Kiban B No.19H03579 to D.S., Kiban C No.18K07550 to T.T., Kiban B No. 20H03598 to M.S.); Japan Agency for Medical Research and Development (AMED) (JP19dk0307029 to K.M., M.M., and M.S.); National Institutes of Health (P41-EB015909 to J.T.R. and S.K.).
Notes
Conflict of Interest: None declared.
Supplementary Material
Contributor Information
Yoichiro Takayanagi, Department of Neuropsychiatry, University of Toyama Graduate School of Medicine and Pharmaceutical Sciences, Toyama 9300194, Japan; Arisawabashi Hospital, Toyama 9392704, Japan.
Sue Kulason, Center for Imaging Science and Institute for Computational Medicine, Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD 21211, USA.
Daiki Sasabayashi, Department of Neuropsychiatry, University of Toyama Graduate School of Medicine and Pharmaceutical Sciences, Toyama 9300194, Japan; Research Center for Idling Brain Science, University of Toyama, Toyama 9300194, Japan.
Tsutomu Takahashi, Department of Neuropsychiatry, University of Toyama Graduate School of Medicine and Pharmaceutical Sciences, Toyama 9300194, Japan; Research Center for Idling Brain Science, University of Toyama, Toyama 9300194, Japan.
Naoyuki Katagiri, Department of Neuropsychiatry, Toho University School of Medicine, Tokyo 1438541, Japan.
Atsushi Sakuma, Department of Psychiatry, Tohoku University Hospital, Sendai, Miyagi 9808574, Japan.
Noriyuki Ohmuro, Department of Psychiatry, Tohoku University Hospital, Sendai, Miyagi 9808574, Japan; Osaki Citizen Hospital, Sendai, Miyagi 9896183, Japan.
Masahiro Katsura, Department of Psychiatry, Tohoku University Hospital, Sendai, Miyagi 9808574, Japan.
Shimako Nishiyama, Department of Neuropsychiatry, University of Toyama Graduate School of Medicine and Pharmaceutical Sciences, Toyama 9300194, Japan; Health Administration Center, University of Toyama, Toyama 9308555, Japan.
Mikio Kido, Department of Neuropsychiatry, University of Toyama Graduate School of Medicine and Pharmaceutical Sciences, Toyama 9300194, Japan; Research Center for Idling Brain Science, University of Toyama, Toyama 9300194, Japan.
Atsushi Furuichi, Department of Neuropsychiatry, University of Toyama Graduate School of Medicine and Pharmaceutical Sciences, Toyama 9300194, Japan; Research Center for Idling Brain Science, University of Toyama, Toyama 9300194, Japan.
Kyo Noguchi, Department of Radiology, University of Toyama Graduate School of Medicine and Pharmaceutical Sciences, Toyama 9300194, Japan.
Kazunori Matsumoto, Department of Psychiatry, Tohoku University Hospital, Sendai, Miyagi 9808574, Japan; Kokoro no Clinic OASIS, Sendai, Miyagi 9800802, Japan.
Masafumi Mizuno, Department of Neuropsychiatry, Toho University School of Medicine, Tokyo 1438541, Japan.
J Tilak Ratnanather, Center for Imaging Science and Institute for Computational Medicine, Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD 21211, USA.
Michio Suzuki, Department of Neuropsychiatry, University of Toyama Graduate School of Medicine and Pharmaceutical Sciences, Toyama 9300194, Japan; Research Center for Idling Brain Science, University of Toyama, Toyama 9300194, Japan.
References
- Al-Hakim R, Fallon J, Nain D, Melonakos J, Tannenbaum A. 2006. A dorsolateral prefrontal cortex semi-automatic segmenter. Proc SPIE. 6144:170–177. [Google Scholar]
- Allen P, Chaddock CA, Egerton A, Howes OD, Barker G, Bonoldi I, Fusar-Poli P, Murray R, McGuire P. 2015. Functional outcome in people at high risk for psychosis predicted by thalamic glutamate levels and prefronto-striatal activation. Schizophr Bull. 41(2):429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . 1994. Diagnostic and statistical manual of mental disorders. 4th ed (DSM-IV). Washington DC: APA. [Google Scholar]
- Barbey AK, Koenigs M, Grafman J. 2013. Dorsolateral prefrontal contributions to human working memory. Cortex. 49(5):1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidet-Caulet A, Buchanan KG, Viswanath H, Black J, Scabini D, Bonnet-Brilhault F, Knight RT. 2015. Impaired facilitatory mechanisms of auditory attention after damage of the lateral prefrontal cortex. Cereb Cortex. 25(11):4126–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodatsch M, Brockhaus-Dumke A, Klosterkotter J, Ruhrmann S. 2015. Forecasting psychosis by event-related potentials-systematic review and specific meta-analysis. Biol Psychiatry. 77(11):951–958. [DOI] [PubMed] [Google Scholar]
- Bois C, Whalley HC, McIntosh AM, Lawrie SM. 2015. Structural magnetic resonance imaging markers of susceptibility and transition to schizophrenia: a review of familial and clinical high risk population studies. J Psychopharmacol. 29(2):144–154. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Yucel M, Pantelis C. 2012. The effects of gender on grey matter abnormalities in major psychoses: a comparative voxelwise meta-analysis of schizophrenia and bipolar disorder. Psychol Med. 42(2):295–307. [DOI] [PubMed] [Google Scholar]
- Bora E, Lin A, Wood SJ, Yung AR, McGorry PD, Pantelis C. 2014. Cognitive deficits in youth with familial and clinical high risk to psychosis: a systematic review and meta-analysis. Acta Psychiatr Scand. 130(1):1–15. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Riecher-Rossler A, Dazzan P, Chitnis X, Aston J, Drewe M, Gschwandtner U, Haller S, Pfluger M, Rechsteiner E, et al. 2007. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 61(10):1148–1156. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TG, McEwen S, Addington J, Bearden CE, Cadenhead K, et al. 2015. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 77(2):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceyhan E, Hosakere M, Nishino T, Alexopoulos J, Todd RD, Botteron KN, Miller MI, Ratnanather JT. 2011. Statistical analysis of cortical morphometrics using pooled distances based on labeled cortical distance maps. J Math Imaging Vis. 40(1):20–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, Asai I, Detraux J, Gautam S, Moller HJ, Ndetei DM, et al. 2011. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 10(1):52–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 31(3):968–980. [DOI] [PubMed] [Google Scholar]
- Fischl B. 2012. FreeSurfer. Neuroimage. 62(2):774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Yung AR, Wood SJ, Phillips LJ, Nelson B, Cotton S, Velakoulis D, McGorry PD, Pantelis C, Yucel M. 2008. Anatomic abnormalities of the anterior cingulate cortex before psychosis onset: an MRI study of ultra-high-risk individuals. Biol Psychiatry. 64(9):758–765. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, Barale F, Caverzasi E, McGuire P. 2012. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 69(3):220–229. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Cappucciati M, Rutigliano G, Lee TY, Beverly Q, Bonoldi I, Lelli J, Kaar SJ, Gago E, Rocchetti M, et al. 2016. Towards a standard psychometric diagnostic interview for subjects at ultra high risk of psychosis: CAARMS versus SIPS. Psychiatry J. 2016:7146341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Eskin E. 2011. Random-effects model aimed at discovering associations in meta-analysis of genome-wide association studies. Am J Hum Genet. 88(5):586–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser M, Zhang JP, Sheridan EM, Burdick KE, Mogil R, Kane JM, Auther A, Carrion RE, Cornblatt BA, Correll CU. 2017. Neuropsychological test performance to enhance identification of subjects at clinical high risk for psychosis and to be most promising for predictive algorithms for conversion to psychosis: a meta-analysis. J Clin Psychiatry. 78(1):e28–e40. [DOI] [PubMed] [Google Scholar]
- Higuchi Y, Seo T, Miyanishi T, Kawasaki Y, Suzuki M, Sumiyoshi T. 2014. Mismatch negativity and p3a/reorienting complex in subjects with schizophrenia or at-risk mental state. Front Behav Neurosci. 8:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaskelainen E, Juola P, Hirvonen N, McGrath JJ, Saha S, Isohanni M, Veijola J, Miettunen J. 2013. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. 39(6):1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda Y, Sumiyoshi T, Keefe R, Ishimoto Y, Numata S, Ohmori T. 2007. Brief assessment of cognition in schizophrenia: validation of the japanese version. Psychiatry Clin Neurosci. 61(6):602–609. [DOI] [PubMed] [Google Scholar]
- Kasparek T, Prikryl R, Mikl M, Schwarz D, Ceskova E, Krupa P. 2007. Prefrontal but not temporal grey matter changes in males with first-episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 31(1):151–157. [DOI] [PubMed] [Google Scholar]
- Kikinis Z, Fallon JH, Niznikiewicz M, Nestor P, Davidson C, Bobrow L, Pelavin PE, Fischl B, Yendiki A, McCarley RW, et al. 2010. Gray matter volume reduction in rostral middle frontal gyrus in patients with chronic schizophrenia. Schizophr Res. 123(2–3):153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N, Schmitt GJ, Gaser C, Bottlender R, Scheuerecker J, McGuire P, Burgermeister B, Born C, Reiser M, Moller HJ, et al. 2009. Neuroanatomical correlates of different vulnerability states for psychosis and their clinical outcomes. Br J Psychiatry. 195(3):218–226. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, et al. 2003. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 60(9):878–888. [DOI] [PubMed] [Google Scholar]
- Lee NA, Priebe CE, Miller MI, Ratnanather JT. 2008. Validation of alternating kernel mixture method: application to tissue segmentation of cortical and subcortical structures. J Biomed Biotechnol. 2008:346129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Riecher-Rossler A, Meisenzahl EM, Tognin S, Wood SJ, Borgwardt SJ, Koutsouleris N, Yung AR, Stone JM, Phillips LJ, et al. 2011. Neuroanatomical abnormalities that predate the onset of psychosis: a multicenter study. Arch Gen Psychiatry. 68(5):489–495. [DOI] [PubMed] [Google Scholar]
- Miller MI, Massie AB, Ratnanather JT, Botteron KN, Csernansky JG. 2000. Bayesian construction of geometrically based cortical thickness metrics. Neuroimage. 12(6):676–687. [DOI] [PubMed] [Google Scholar]
- Miller MI, Hosakere M, Barker AR, Priebe CE, Lee N, Ratnanather JT, Wang L, Gado M, Morris JC, Csernansky JG. 2003. Labeled cortical mantle distance maps of the cingulate quantify differences between dementia of the Alzheimer type and healthy aging. Proc Natl Acad Sci U S A. 100(25):15172–15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. 2003. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 29(4):703–715. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Suzuki M, Matsumoto K, Murakami M, Takeshi K, Miyakoshi T, Ito F, Yamazawa R, Kobayashi H, Nemoto T, et al. 2009. Clinical practice and research activities for early psychiatric intervention at japanese leading centres. Early Interv Psychiatry. 3(1):5–9. [DOI] [PubMed] [Google Scholar]
- Nesvag R, Lawyer G, Varnas K, Fjell AM, Walhovd KB, Frigessi A, Jonsson EG, Agartz I. 2008. Regional thinning of the cerebral cortex in schizophrenia: effects of diagnosis, age and antipsychotic medication. Schizophr Res. 98(1–3):16–28. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, et al. 2003. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 361(9354):281–288. [DOI] [PubMed] [Google Scholar]
- Pereira JB, Ibarretxe-Bilbao N, Marti MJ, Compta Y, Junque C, Bargallo N, Tolosa E. 2012. Assessment of cortical degeneration in patients with parkinson's disease by voxel-based morphometry, cortical folding, and cortical thickness. Hum Brain Mapp. 33(11):2521–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. 1995. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the talairach coordinate system. Cereb Cortex. 5(4):323–337. [DOI] [PubMed] [Google Scholar]
- Ratnanather JT, Wang L, Nebel MB, Hosakere M, Han X, Csernansky JG, Miller MI. 2004. Validation of semiautomated methods for quantifying cingulate cortical metrics in schizophrenia. Psychiatry Res. 132(1):53–68. [DOI] [PubMed] [Google Scholar]
- Sasabayashi D, Takayanagi Y, Takahashi T, Katagiri N, Sakuma A, Obara C, Katsura M, Okada N, Koike S, Yamasue H, et al. 2020. Subcortical brain volume abnormalities in individuals with an at-risk mental state. Schizophr Bull. 46(4):834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz CC, Koch K, Wagner G, Roebel M, Schachtzabel C, Gaser C, Nenadic I, Reichenbach JR, Sauer H, Schlosser RG. 2010. Reduced cortical thickness in first episode schizophrenia. Schizophr Res. 116(2–3):204–209. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Shapiro DI, Stone WS, Woodberry KA, Ronzio A, Cornblatt BA, Addington J, Bearden CE, Cadenhead KS, Cannon TD, et al. 2016. Association of neurocognition with transition to psychosis: baseline functioning in the second phase of the north american prodrome longitudinal study. JAMA Psychiatry. 73(12):1239–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford MR, Jackson H, Mayo-Wilson E, Morrison AP, Kendall T. 2013. Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ. 346:f185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Zhou SY, Takahashi T, Hagino H, Kawasaki Y, Niu L, Matsui M, Seto H, Kurachi M. 2005. Differential contributions of prefrontal and temporolimbic pathology to mechanisms of psychosis. Brain. 128(Pt 9):2109–2122. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M. 2018. Brain morphologic changes in early stages of psychosis: Implications for clinical application and early intervention. Psychiatry Clin Neurosci. 72(8):556–571. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Yung AR, Phillips LJ, Soulsby B, McGorry PD, Tanino R, Zhou SY, Suzuki M, Velakoulis D, et al. 2009. Insular cortex gray matter changes in individuals at ultra-high-risk of developing psychosis. Schizophr Res. 111(1–3):94–102. [DOI] [PubMed] [Google Scholar]
- Takayanagi M, Wentz J, Takayanagi Y, Schretlen DJ, Ceyhan E, Wang L, Suzuki M, Sawa A, Barta PE, Ratnanather JT, et al. 2013. Reduced anterior cingulate gray matter volume and thickness in subjects with deficit schizophrenia. Schizophr Res. 150(2–3):484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Sasabayashi D, Takahashi T, Furuichi A, Kido M, Nishikawa Y, Nakamura M, Noguchi K, Suzuki M. 2019. Reduced cortical thickness in schizophrenia and schizotypal disorder. Schizophr Bull. 46(2):387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Takahashi T, Orikabe L, Mozue Y, Kawasaki Y, Nakamura K, Sato Y, Itokawa M, Yamasue H, Kasai K, et al. 2011. Classification of first-episode schizophrenia patients and healthy subjects by automated MRI measures of regional brain volume and cortical thickness. PLoS One. 6(6):e21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Kulason S, Sasabayashi D, Takahashi T, Katagiri N, Sakuma A, Obara C, Nakamura M, Kido M, Furuichi A, et al. 2017. Reduced thickness of the anterior cingulate cortex in individuals with an at-risk mental state who later develop psychosis. Schizophr Bull. 43(4):907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Kulason S, Sasabayashi D, Takahashi T, Katagiri N, Sakuma A, Ohmuro N, Katsura M, Nishiyama S, Nakamura M, et al. 2020. Structural MRI study of the planum temporale in individuals with an at-risk mental state using labeled cortical distance mapping. Front Psychiatry. 11:593952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognin S, Riecher-Rossler A, Meisenzahl EM, Wood SJ, Hutton C, Borgwardt SJ, Koutsouleris N, Yung AR, Allen P, Phillips LJ, et al. 2014. Reduced parahippocampal cortical thickness in subjects at ultra-high risk for psychosis. Psychol Med. 44(3):489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, Agartz I, Westlye LT, Haukvik UK, Dale AM, et al. 2016. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 21(4):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita A, De Peri L, Deste G, Barlati S, Sacchetti E. 2015. The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: Does the class matter? A meta-analysis and meta-regression of longitudinal magnetic resonance imaging studies. Biol Psychiatry. 78(6):403–412. [DOI] [PubMed] [Google Scholar]
- Vossel S, Weidner R, Driver J, Friston KJ, Fink GR. 2012. Deconstructing the architecture of dorsal and ventral attention systems with dynamic causal modeling. J Neurosci. 32(31):10637–10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. 1986. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 43(2):114–124. [DOI] [PubMed] [Google Scholar]
- Wentz J. 2012. A pipeline for cortical analysis of regional changes in MCI and autism. MSE thesis. Baltimore, MD: Johns Hopkins University.
- Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell'Olio M, Francey SM, Cosgrave EM, Killackey E, Stanford C, et al. 2005. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust N Z J Psychiatry. 39(11–12):964–971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


