Abstract
Small heterodimer partner (Shp) regulates several metabolic processes, including bile acid levels, but lacks the conserved DNA binding domain. Phylogenetic analysis revealed conserved genetic evolution of SHP, FXR, CYP7A1, and CYP8B1. Shp, although primarily studied as a downstream target of Farnesoid X Receptor (Fxr), has a distinct hepatic role that is poorly understood. Here, we report that liver-specific Shp knockout (LShpKO) mice have impaired negative feedback of Cyp7a1 and Cyp8b1 on bile acid challenge and demonstrate that a single copy of the Shp gene is sufficient to maintain this response. LShpKO mice also exhibit elevated total bile acid pool with ileal bile acid composition mimicking that of cholic acid-fed control mice. Agonistic activation of Fxr (GW4064) in the LShpKO did not alter the elevated basal expression of Cyp8b1 but lowered Cyp7a1 expression. We found that deletion of Shp led to an enrichment of distinct motifs and pathways associated with circadian rhythm, copper ion transport, and DNA synthesis. We confirmed increased expression of metallothionein genes that can regulate copper levels in the absence of SHP. LShpKO livers also displayed a higher basal proliferation that was exacerbated specifically with bile acid challenge either with cholic acid or 3,5-diethoxycarbonyl-1,4-dihydrocollidine but not with another liver mitogen, 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene. Overall, our data indicate that hepatic SHP uniquely regulates certain proliferative and metabolic cues.
Keywords: bile acids, nuclear receptor, small heterodimer partner, enterohepatic recirculation, proliferation
Cholestasis, a condition wherein bile acids (BAs) homeostasis is impaired, leading to excess hepatic accumulation, is commonly observed in several liver diseases (1–4). Sustained exposure to BAs induces injury, dysregulation of the cell cycle, apoptosis, and consequently malignant transformation. Thus, it is essential to understand how bile acid levels are controlled.
Nuclear receptors are ligand-activated transcription factors that coordinate major cellular pathways, including metabolism (5–8). The atypical nuclear receptor, small heterodimer partner (Shp), modulates pathways involved in bile acid homeostasis, fibrolamellar carcinomas, and nonalcoholic fatty liver disease (9–12). Most studies have been performed using global Shp knockout (ShpKO) mice and because BAs are endogenous ligands that bind Farnesoid X Receptor (Fxr) (which in turn can induce Shp), the focus has been laid on elucidating FXR function. SHP is recruited to suppress Cyp7a1 transcription (13–15) to orchestrate a negative feedback loop to prevent BA excess. The severity of the cholestasis in Fxr/Shp double KO mice compared with the individual knockouts revealed cooperative and distinct roles for FXR and SHP (16).
Intriguingly, conditional knockout of Fxr gene in the liver did not increase serum BAs, and the suppression of Cyp7a1 and 8b1 in response to Fxr agonist GW4064 was maintained. Further, tissue-specific roles for -mediated BA homeostasis have been uncovered with conditional knockout of Fxr in the intestine, losing GW4064-mediated suppression of Cyp7a1 but not Cyp8b1 gene expression (17, 18). Therefore, we investigated the liver-specific Shp knockout mice (LShpKO) and examined their response to an acute BA challenge. We examined the expression of genes responsible for hepatic synthesis, transportation, and enterohepatic BA axis in the absence of hepatic Shp.
Mature hepatocytes are quiescent but can be coaxed to proliferate in response to injury. BAs can also act as mitogens, which goes hand in hand with Shp’s postulated role in inhibiting liver proliferation (10, 19–29). We examined if deletion of Shp altered proliferation after treatment with either cholic acid or 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) or ligand-based Car activation. To test the relevance of upon activation, we treated LShpKO mice with GW4064 and examined its response in the liver. At the same time, we mined the publicly available data for ShpKO and FxrKO mice and examined unique and overlapping gene sets (Supplemental Tables 1 and 2) (30) to identify distinct pathways regulated by these 2 receptors. Then using computational tools, we examined if there was a common motif of regulation of genes that were uniquely altered when was deleted.
Materials and Methods
Generation of Floxed Shp and LShpKO Mice
ff Shp mice were obtained from Dr. Kristina Schoonjans’ laboratory. By breeding Albumin-Cre mice with ff Shp mice, we generated heterozygous Shp+/-, and homozygous Shp-/- LShpKO mice. ff Shp mice were used as controls.
Animal Experiments
Adult male mice aged 9 to 21 weeks old were used in this study. Mice were maintained in flow cages at 24°C on the standard 12-hour day/night schedule. Food (including experimental diets) and water were available ad libitum. Control mice were fed standard chow diet, whereas the cholic acid (CA) experimental group were fed 1% CA for 3 days and the DDC experimental group were fed a 0.1% DDC diet for 2 weeks. Mice were sacrificed via cervical dislocation in the fed state at 9 to 10 am. Mice were bled retro-orbitally before sacrifice, and serum was extracted after centrifugation at 7000 rpm for 10 minutes. Serum was transferred to black tubes and stored at -80°C. The liver, gallbladder, and small intestine (ileum) were collected, flash frozen in liquid nitrogen, and stored at -80°C. Mice were gavaged with either vehicle (1% methylcellulose 1% TritonX-100 in PBS) or 50 mg/kg Fxr agonist GW4064. Mice were gavaged twice, first in the evening, second in the morning, and then sacrificed 3 hours after second gavage.
Genotyping Assay
Tail samples were digested in a 200:2 ratio of tail lysate (VIAGEN 102-T) to proteinase K overnight in 55°C water bath overnight and then inactivated at 85°C for 45 minutes. A total of 10 µL of GoTaq buffer, 0.1 µL each of 100µM forward and reverse primers, 7.3 µL of water, and 2.5 µL of digested tail were used in the ff Shp PCR reaction. Alb-Cre reaction used 10 µL of GoTaq buffer, 1 µL of a 10-µM dilution mix of forward and reverse primers, 6.5 µL of water, and 2.5 µL of digested tail. The PCR products were then run on a 1% agarose gel. The wild type (WT) Shp band is seen at 365 bp, whereas floxed Shp band is seen at 415 bp. Positive Alb-Cre was observed at 390 bp. Each PCR product was run alongside a 100-bp DNA ladder (BioLabs) compared with positive and negative controls.
Histological Preparation
Liver and ileum tissues were preserved in 10% neutral buffered formalin for 24 hours at 4°C, transferred to 75% ethanol and stored until processing (up to 4 weeks). Tissue processing occurred in the following steps with each step occurring for 45 minutes: 95% ethanol 2×, 100% ethanol 3×, and xylene 2×. Samples were then embedded in paraffin blocks. Blocks were sectioned at 5 µm and placed on glass slides.
Hematoxylin and Eosin Staining
Tissue sections were deparaffinized and rehydrated through 3 changes of xylene at 5 minutes, 100% ethanol 3× for 3 minutes, 95% ethanol for 3 minutes, 80% ethanol for 3 minutes, 50% ethanol for 3 minutes, and rinsed in tap water. The slides were then placed in Hematoxylin 7211 (Thermo Fisher) for 1 minute and 50 seconds and quickly rinsed in deionized (DI) water until clear. Sections were placed in bluing reagent NaHCO3 for 1 minute, rinsed in DI water, and incubated in Eosin Y (Thermo Scientific) for 25 seconds. Sections were then placed in 100% ethanol 3× for 3 minutes, and xylene 3× for 5 minutes. Sections were left in xylene overnight, and subsequently mounted and coverslipped using Permount (Fisher).
ALT/AST Serum Analysis
Previously collected serum was thawed on ice and alanine transaminase (ALT)/aspartate transaminase (AST) assay was run according to the Thermo Scientific kit protocol. Absorbances were obtained using a Biotek Synergy 2 reader.
Immunohistochemistry
Liver samples were preserved, sectioned, deparaffinized, and rehydrated as described previously. Tris-EDTA (10 mM Tris, 1 mM EDTA, 0.05% Tween 20, pH 9) buffer was used for antigen retrieval, with samples being microwaved for 35 minutes. Slides were cooled and endogenous peroxidases were quenched by incubating slides in 3% H2O2 in methanol for 15 minutes. Samples were blocked with a buffer of 2% normal goat serum, 1% BSA, 0.25% Tween 20, 0.05% Triton X100 in 1X Tris-buffered saline (TBS) for 1 hour at room temperature. Samples were then incubated in Primary antibody (purified mouse Anti Ki-67, BD Biosciences, catalog no.: 550609) diluted to 1:100 in dilution buffer (1% BSA, 0.05% Triton X100, in 1X TBS) overnight at 4°C. Samples were washed in 1X TBS with Tween 20 and then incubated in secondary antibody (goat anti-mouse IgG (Heavy + Light Chain (H L)) horseradish peroxidase conjugated, BioRad catalog no.: 170-6516) diluted 1:250 in dilution buffer at RT for 1 hour. Samples were washed and DAB reagent (Vector catalog no.: SK-4100) reagent was added according to manufacturer’s protocol. Samples were counterstained in hematoxylin 7211 (Thermo Fisher) for 15 seconds, rinsed in water until it ran clear, placed in bluing reagent NaHCO3 for 1 minutes, and finally rinsed in DI water again. Samples were dehydrated and coverslipped as described previously. Ki-67 cell count analyses were performed by taking 5 random images per sample.
RNA Isolation, cDNA synthesis, and Quantitative RT-PCR
The total RNA was extracted from flash-frozen liver and intestinal tissue according to TRIzol (Ambion) manufacturer’s protocol. RNA quality was checked by measuring the A260/A280 using the Biotek Synergy 2 as well as by 1% bleach agarose TAE gel analysis. A 5-µg volume of RNA was used and treated with DNase I (BioLabs New England) and subsequently deactivated with EDTA and heat. cDNA was made using Maxima reverse transcriptase (Thermo Scientific), random primers (BioLabs New England), dNTP mix 10 mM (Invitrogen), 0.1M DTT(BioLabs New England), and 5× RT buffer (Thermo Scientific). cDNA was then diluted to 12.5 ng/µL using molecular-grade water. Quantitative RT-PCR was performed using SYBR green-based reaction at 50 ng of cDNA per reaction. Relative expression was determined by using 2^ΔCT, with the control gene being 36B4. All primer sequences can be found in Minimum Information for Quantitative Real-Time PCR Experiments guidelines (30).
Histological Analysis
Hematoxylin and eosin-stained liver sections were evaluated microscopically by a pathologist in a blinded manner for various tissue alterations including neutrophil infiltration, apoptosis, mitotic figures, biliary hyperplasia, hepatocellular degeneration/necrosis, megalocytosis, and fibrosis.
Bile Acid Analysis
Total bile acid levels were determined from liver, ileum, gallbladder, and serum using Diazyme’s Total Bile Acid Assay kit. Liver and intestinal tissue was homogenized in 75% ethanol, incubated at 50°C for 2 hours, and centrifuged at 12,000 rpm for 10 minutes. The supernatant was then diluted 4 to 5 time or 35 to 40 times, respectively, before using the assay. Gallbladders were thawed on ice and then pierced to expel its contents. A total of 1 µL of the extract was diluted 20 to 30 times before use in the assay. Serum was thawed on ice and either directly used in assay or diluted 2 times. All samples were diluted in 0.9% saline. Samples were aliquoted in triplicates, and obtained absorbance values were compared to a standard curve composed from 50 µmole/L calibrator dilutions. Total bile acid levels were obtained by calculating bile acid concentration in µM/g of tissue used with the total liver weight used as the tissue weight for serum and gallbladder. All concentrations for each tissue were averaged and then summed together to get an average total bile acid level for each group. The colorimetric analysis was obtained via Biotek Synergy 2 instrument.
BA composition analysis from serum, gallbladder, liver, and intestinal tissue was done with Biocrates bile acids kit and analyzed by ultra-performance liquid chromatography/tandem mass spectrometry using a triple quadrupole mass spectrometer at the DUKE Proteomics and Metabolomics Shared Resource. Briefly, extraction buffer was made by combining 6.8 mL EtOH, 240 µL of 100 mM phosphate buffer, and 960 µL water. Then 3-fold extraction buffer (to 200-mg sample, add 600 µL of extraction buffer) was added to weighed tissue samples and homogenized. Finally, samples were sonicated for 5 minutes in ice water and the stored at -80°C until analysis.
Transcription Factor Analysis
Using publicly available transcriptome data of FxrKO and ShpKO mice, genes that exhibited more than 2-fold change in knockout mice compared with control were examined. Genes unique to each of the 2 genotypes were considered for analysis. We wanted to explore the regulatory sequences of these unique genes that altered only when or only when is deleted. To do this, we collected the first 2000 nucleotides upstream of each of these genes using the Ensembl-BioMart tool and inserted the sequences into MEME-Suite’s Analysis of Motif Enrichment tool, an in silico identifier of motifs enriched in a given list of gene promoters. The database of motifs used by Analysis of Motif Enrichment for this analysis was the HOmo sapiens COmprehensive MOdel COllection (HOCOMOCO), version 11, which derives its mouse motif models from the ChIPMunk motif discovery tool. A motif list for FxrKO and ShpKO was made and compared for transcription factor motifs that were unique to the ShpKO genotype only. This led to a final list of transcription factors that are possibly uniquely regulated by Shp as shown in Supplemental Table 3 (30) as well as a list of uniquely regulated transcription factors by Fxr as shown in Supplemental Table 4 (30). The literature indicates these transcription factors are involved in proliferation and cell-cycle progression. As such, genes involved in the E2f cell-cycle progression pathway and a few genes from the transcriptome data of FxrKO and ShpKO mice that are cell-cycle associated were analyzed using quantitative PCR. The primer sequences used for their analysis are shown in the Minimum Information for Quantitative Real-Time PCR Experiments guidelines (30).
1,4-Bis[2-(3,5-dichloropyridyloxy)]benzene Preparation and Injection
1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TC) was purchased from Sigma-Aldrich in 5-mg quantities. A total of 1 to 1.5 mL of 100% ethanol was added to resuspend the compound, and then 2 mL of corn oil was added to the mixture to achieve a 2.5-mg/mL concentration. This mixture was left to stir overnight to evaporate any residual ethanol in the solution. The ethanol-free TC and corn oil mixture was then injected IP into ff Shp and LShpKO mice. Mice were injected with 5 µL of solution per gram weight and then sacrificed 3 days after injection.
Phylogenetic Analysis
The evolutionary history was inferred using the Neighbor-Joining method. The bootstrap consensus tree inferred from 500 replicates is taken to represent the evolutionary history of the taxa analyzed. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The evolutionary distances were computed using the Poisson correction method and are in the units of the number of amino acid substitutions per site. This analysis involved 77 amino acid sequences. All ambiguous positions were removed for each sequence pair (pairwise deletion option). There were a total of 898 positions in the final dataset. Evolutionary analyses were conducted in MEGA X (31–34).
Copper Quantification
A total of 50 to 100 mg of flash-frozen liver tissue was homogenized in 500 μL of 0.1 mol/L sodium phosphate buffer (pH 7.5), 0.25 mol/L D-sucrose in a loose-fitting Dounce homogenizer. Protein concentration was then determined using ThermoFisher Scientific BCA protein kit. Finally, 35 mg of protein from each of the samples was analyzed with the Sigma-Aldrich Copper Assay Kit (Catalog no. MAK127) using the manufacturer’s protocol.
Statistical Analysis
All data are represented as mean ± SD. A 2-tailed unpaired t test was used to determine significance between 2 groups. Two-way ANOVA analysis of variance Bonferroni test was used to determine significance between 2 groups under 2 conditions. Statistics were performed using the GraphPad Prism 6 software. Outliers were determined using GraphPad and removed from the analysis. Statistical significance was considered as *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001.
Supplemental Data
Supplemental figures and tables can be found in an online repository (30).
Results
A Single Copy of the Hepatic Shp Allele Is Sufficient to Maintain BA Homeostasis
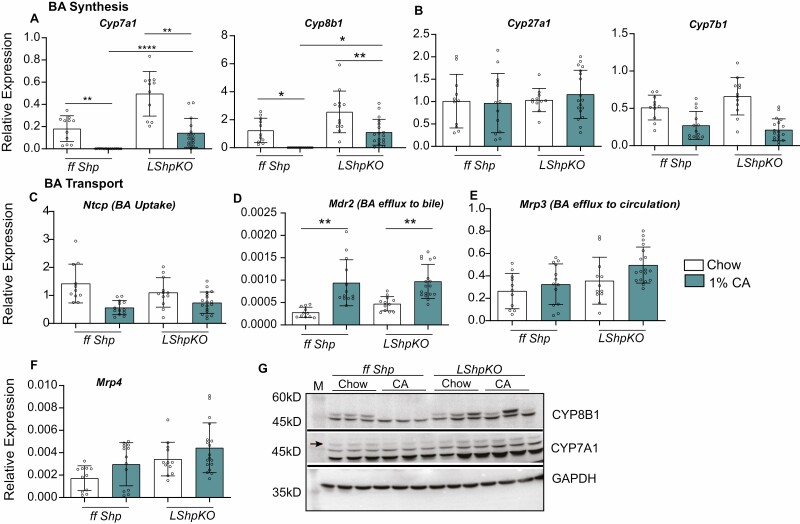
We generated liver-specific Shp heterozygous (Shp+/-) and homozygous knockout (LShpKO) mice using albumin-specific Cre-recombinase. Both of these genotypes maintained intact expression of the Fxr transcript (Supplemental Fig. 1) (30). Intriguingly, Shp expression was comparable between the control mice expressing both functional alleles to that of the heterozygous Shp knockout possessing only 1 functional allele. Next, we challenged ff Shp, Shp+/-, and LShpKO mice with either chow or 1% CA diet and analyzed the expression of genes involved in the BA synthesis and transportation pathways. We found that basal Cyp7a1 and Cyp8b1 mRNA levels were significantly higher when Shp was deleted in the liver, even under chow conditions (Fig. 1A). The expression of alternative BA synthesis genes, Cyp27a1 and Cyp7b1, remained unchanged irrespective of Shp expression (Fig. 1B). We found that none of the canalicular and peripheral hepatic export pumps were altered except for the increase in Mdr2 transcripts in LShpKO livers (Fig. 1C-F). On challenging with a 1% CA diet, ff Shp mice showed the expected suppression of BA synthesis pathways Cyp7a1 and Cyp8b1, which was blunted in the absence of hepatic Shp (Fig. 1A). However, Shp+/- mice maintained this repression of BA synthesis and had comparable expression patterns of the BA transporters as that of ff Shp mice, indicating a single copy of the allele is enough to maintain the repressive function (Supplemental Fig. 3 and 4) (30).
Figure 1.
Relative expression of bile acid synthesis and transport genes in ff Shp and LShpKO mice on chow and 1% CA diets. (A-B) Expression of key bile acid synthesis genes, and (C-F) Expression of hepatic bile acid uptake and canalicular bile acid export pumps, (G) Western blot analysis of proteins responsible for classical bile acid synthesis. One-way ANOVA analysis of variance Bonferroni test was used to determine significance between groups. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
We also investigated the evolution of SHP-mediated BA regulation by performing phylogenetic analysis using the protein sequences of SHP, CYP7A1, and CYP8B1. When examining the dendrograms of these proteins, we noted that the number of speciation events and diversity of the leaves of SHP and CYP8B1 more closely resembled each other than SHP and CYP7A1 (Supplemental Fig. 2) (30). Because of the relatively simple 2-exon structure of SHP, we hypothesized that SHP was an ancestral gene. Evidence from the dendrogram suggests that this is not the case. In fact, the 2 major branches present in the FXR phylogenetic tree are drastically reduced in size in the tree constructed for SHP. Further, FXR and CYP7A1 contain more speciation events based on the 60 different sequences analyzed.
Loss of Hepatic Shp Leads to Elevated BA Pool and Alters its Tissue Distribution
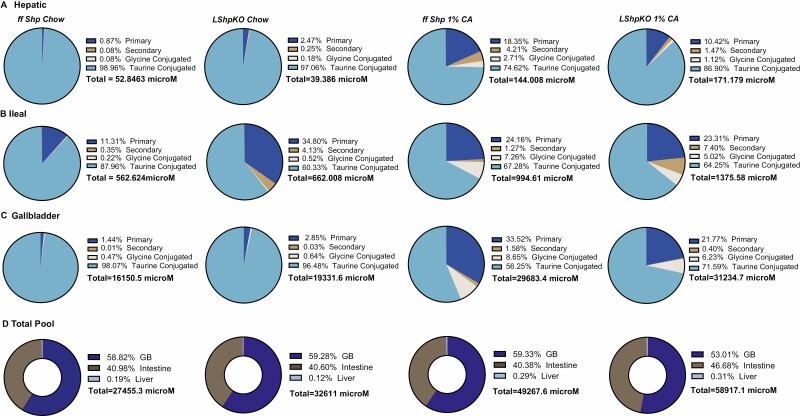
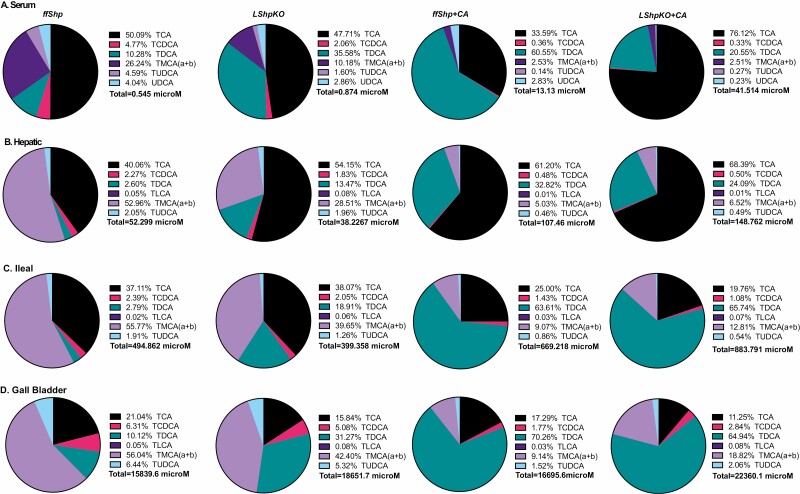
To characterize BA homeostasis in LShpKO animals, we measured BA levels from chow and CA-fed control and knockout mice. We found that hepatic Shp deficiency shifted the distribution of BAs in the enterohepatic tissues (liver, intestine, and gallbladder, Fig. 2A-C). Under basal chow, LShpKO displayed altered BA composition with a higher ileal and gallbladder BA content. Also, we found an increase in unconjugated primary and secondary BAs, whereas taurine-conjugated species were proportionally reduced in the ileum and liver. Upon CA feeding, we observed a decrease in taurine-conjugated BAs and a corresponding rise in unconjugated BA ratios in the liver (Fig. 2A). In the ileum, higher taurine-conjugated BAs with a particular increase in the secondary BA, Tauro deoxycholic acid (TDCA) was seen in both the cohorts of mice (Fig. 2B). Gallbladder exhibited a pronounced reduction in taurine-conjugated BA proportion (Fig. 2C). The total BA concentrations and their tissue-specific distribution are shown in Fig. 2D. Although we see changes in the proportions of the unconjugated and conjugated BAs, it is important to note that CA diet does increase the total concentrations of taurine-conjugated BA in both the genotypes (Fig. 3). Intriguingly, we find a robust decrease in Tauro muricholic acid (TMCA) accompanied by a dramatic increase TDCA in the serum, liver, ileum, and gallbladder of LShpKO mice even under chow conditions (Fig. 3), indicating changes in the primary to secondary BA ratios. Overall, LShpKO mice displayed higher BA pool and varied composition than the ff Shp mice under chow and CA diet (Fig. 2D and Fig. 3).
Figure 2.
Pie charts depicting (A) hepatic, (B) ileal, and (C) gallbladder bile acid composition in ff Shp and LShpKO mice on chow and 1% CA diets. Individual bile acid values were totalled and then categorized based on whether they were unconjugated primary, unconjugated secondary, glycine conjugated, or taurine conjugated. The sum of all the individual bile acid analytes in each tissue were then used to calculate the tissue-specific bile acid concentrations (D). Total concentrations and distribution of the bile acid pool shown in micromoles.
Figure 3.
Pie charts specifically depicting the taurine conjugated bile acid composition in the (A) serum, (B) liver, (C) ileum, and (D) gallbladder in ff Shp and LShpKO mice on chow and 1% CA diets. Concentrations are shown in micromoles.
To gain insight into why ileal BA contributions were elevated in the absence of hepatic Shp, we investigated the major enterokine Fgf15 as well as several intestinal transport genes. We observed increased expression of Fgf15 in LShpKO animals under chow consistent with high ileal BAs (Supplemental Figure 5) (30). However, the transcript levels of ileal import and efflux BA transporters remained similar between LShpKO and control mice. Thus, we do not know why ileal BAs are elevated when SHP is deleted in the liver.
Loss of Hepatic Shp Induces a Mild Proliferative Response in the Liver
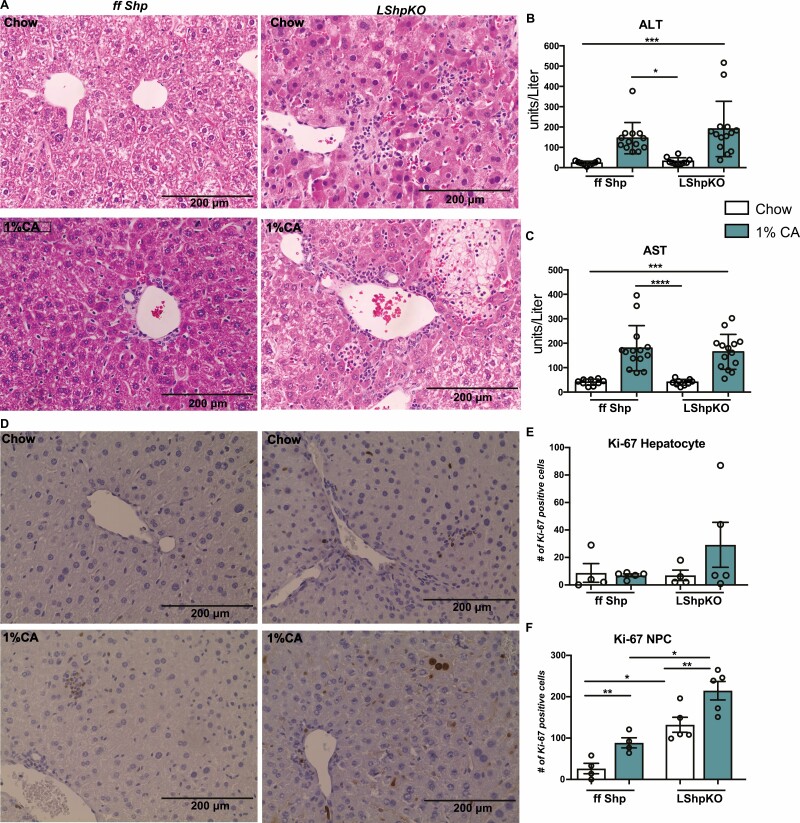
We examined hematoxylin and eosin-stained liver sections microscopically for inflammatory and morphological changes. Liver from LShpKO mice displayed no overt morphological differences from control mice; however, we saw a higher incidence of hepatocellular degeneration/necrosis under 1% CA with increased infiltration of neutrophils in LShpKO mice (Fig. 4A). Hepatic serum injury markers ALT and AST were comparable between ff Shp and LShpKO animals (Fig. 4B and 4C). To test if the injury secondary to CA-diet induced proliferation in mice that lack hepatic Shp, we performed Ki-67 immunostaining. We then quantified the number of hepatocytes and nonparenchymal cells (NPCs) that were undergoing cell division. Compared with ffShp control, we found more Ki-67-positive nuclei in LShpKO mice that is further enhanced upon 1% CA feeding (Fig. 4E and 4F).
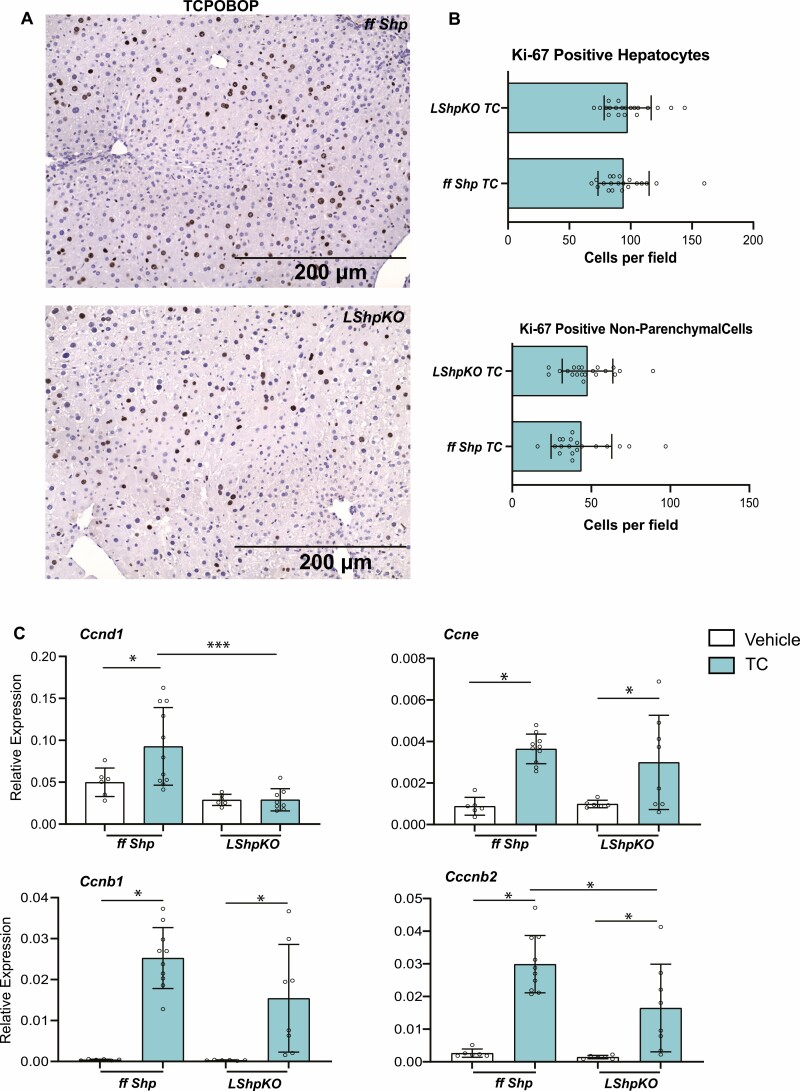
Figure 4.
Histological analysis of liver tissue and Injury markers after BA challenge. (A) Hematoxylin and eosin staining of liver tissue depicting hepatic inflammation and necrotic tissue in ff Shp and LShpKO mice on chow and 1% CA diets. We find increased numbers of infiltrating neutrophils in liver of LShpKO mice (indicated with arrows) and the discrete focus of hepatocellular degeneration/necrosis (LShpKO 1% CA) indicated by an asterisk. (B-C) Liver injury markers ALT/AST levels. (D) Ki-67 immunohistochemistry of liver tissue samples showing proliferation of hepatocytes and nonparenchymal cells (NPCs). (E-F) quantification of proliferating hepatocytes and NPCs. One-way ANOVA analysis of variance Bonferroni test was used to determine significance between groups. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. N = 5-14 mice per group with 5 random images taken per mouse. Values shown as mean ± SD.
To test if the excessive proliferative response was unique to BA overload, we challenged LShpKO mice with a potent mitogen, TC, an agonist of nuclear receptor, constitutive androstane receptor (CAR) (9, 35–37). Upon activation, CAR translocates to the nucleus to induce proliferation and detoxification networks (38). LShpKO and ff Shp mice were injected with TC or corn oil as a vehicle and gene expression profile of several cyclins was examined and Ki-67 immunostaining was performed. We did not observe increased proliferation in the absence of hepatic Shp. Instead, Ki-67-positive nuclei in the liver were comparable between ffShp mice and LShpKO. However, TC-mediated Ccnd1 increase was lost, whereas Ccnb2 induction was blunted in LShpKO mice (Fig. 5C), suggesting that Shp may play a role in the S to the G2 transition. On the other hand, increases in Ccnb1 and Ccne transcripts were maintained in the absence of Shp (Fig. 5C) (30), indicating the presence of compensatory signals in LShpKO mice.
Figure 5.
Characterization of Car-mediated proliferation in LShpKO mice. (A) Ki-67 immunohistochemistry of liver tissue samples showing proliferation of hepatocytes and nonparenchymal cells (NPCs) treated with TC. (B) Quantification of proliferating hepatocytes. Quantification of proliferating NPCs. (C) Relative expression of several cyclins involved in the progression of the cell cycle. One-way ANOVA analysis of variance Bonferroni test was used to determine significance between 2 groups under 2 conditions. Two-tailed unpaired t test was used to determine significance between 2 groups. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Activation of Fxr Does Not Alleviate Transcriptional Changes Caused by Shp Deficiency
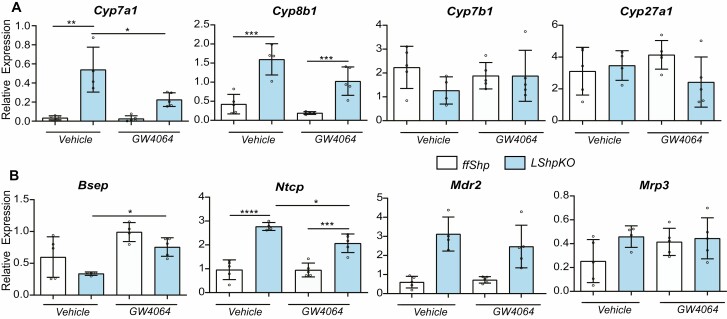
Shp is primarily understood as a downstream target of Fxr in the liver and intestine (14, 39, 40). However, Shp can act coordinately with Fxr to maintain bile acid homeostasis (41–44). To examine the response to activation in the absence of Shp, we treated control and LShpKO animals with vehicle (1% methylcellulose with 1% Triton X-100) or synthetic agonist for Fxr, GW4064 (50 mg/kg). We observed no significant microscopic differences in livers from LShpKO mice administered vehicle or GW4064. The alternative BA synthesis genes Cyp27a1 and Cyp7b1 remained unaltered between all 4 groups (Fig. 6A). The canalicular export (Mdr2) and hepatic bile acid uptake (Ntcp) were increased in the absence of Shp, but this increase was noted regardless of FXR activation (Fig. 6B). Intriguingly, the increase in Cyp7a1 gene was significantly reduced and Cyp8b1 was also lowered in LShpKO livers on GW4064 treatment (Fig. 6A).
Figure 6.
(A) Expression of key bile acid synthesis genes in WT and LShpKO animals treated with either vehicle or GW4064. (B) Relative expression of hepatic uptake and canalicular bile acid pumps in WT and LShpKO animals treated with either vehicle or GW4064. One-way ANOVA analysis of variance Bonferroni test was used to determine significance between 2 groups under 3 conditions. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
Finally, to determine genes that uniquely regulates, we mined the previously published data (16) and identified several unique genes whose expressions are explicitly altered in the absence of and compared them with those that are changed in the absence of (Supplemental Table 1 and 2) (30). Gene ontology analysis of these unique genes revealed enrichment of circadian, amino, and carboxylic acid metabolism, copper ion transport, and DNA synthesis pathways in ShpKO livers. In FxrKO livers, we noted changes in lipid, triglyceride, and nucleotide-sugar metabolism, iron ion, acetylation, chemotaxis, cholesterol, and glucose metabolism. Next, we examined if these unique genes exhibit common or overlapping motifs near their transcription start site to identify whether there is enrichment of specific TFs in the absence of SHP. Using in silico MEME-suite (45), we found potential motifs for THAP11, ZNF143, HOXA9, STAT6, and the Ets family including SPI1 and ELF2 enriched in the promoters of genes dysregulated in ShpKO livers. Because THAP11 and ZNF143 are transcription factors known to assist E2F target genes (46, 47) and HOXA9 is known to influence CDK6 and ccnd1 we examined the cell-cycle genes in LShpKO mice. To determine if FXR activation also influences the expression of these genes, we compared LShpKO and ff Shp mice treated with vehicle or GW4064 or a 1% CA diet. Overall, we found that proliferative pathways are distinctly regulated by GW4064 than by a CA diet. For example, we were surprised to find GW4064-specific suppression of E2f1, and Cdk6, which is not seen in the CA-treated samples (Supplemental Fig. 6) (30). Importantly, these suppressive effects were Shp independent. Intriguingly, Ezh2 expression is significantly decreased in the LShpKO mice when treated with CA, indicating a positive regulatory role for SHP.
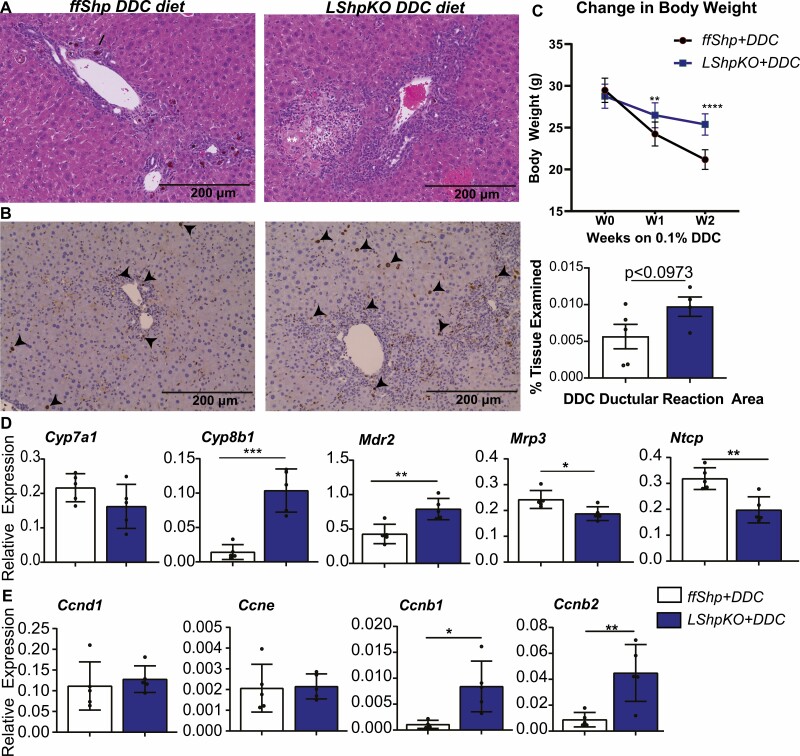
DDC Diet Promotes Inflammation and Proliferation in the Absence of Hepatic SHP
To probe the pro-proliferative role in LShpKO mice, we challenged them with DDC for 2 weeks. Intriguingly, the loss of body weight during the DDC feeding was considerably lower in the LShpKO than the ffShp mice (Fig. 7). Inhibiting ferrochelatase with DDC is typically associated with increase in bile acids, porphyrin plug formation, and ductular reaction surrounding the bile ducts. The ductular reaction is predominantly characterized by neutrophil infiltration and bile duct proliferation. However, microscopic evaluation revealed an increased incidence and severity of ductular reaction and hepatocellular degeneration/necrosis in sections of liver from LShpKO mice (Fig. 7A). We also observed a significant increase in the expression of Cyp8b1 and Mdr2 indicating higher 12 hydroxy BA synthesis and BA efflux to bile along with a decrease in the expression of BA uptake transporter, Ntcp, and systemic efflux transporter, Mrp3 (Fig. 7D). These data suggest that misregulation in BA homeostasis pathway may contribute to the tissue necrosis and increased inflammation observed in LShpKO mice. We also examined Ki-67 immunohistochemistry and observed a robust increase in positively stained hepatocytes and nonparenchymal cell when hepatic Shp is deleted (Fig. 7B). This proliferative phenotype is supported by increased expression of cyclinB1 and B2 but not cyclinD1 in LShpKO livers after the DDC diet (Fig. 7E).
Figure 7.
Characterization of 0.1% DDC diet in LShpKO mice. (A) Hematoxylin and eosin staining of ff Shp and LShpKO mice after being fed 0.1 % DDC for 2 weeks. Arrows point toward areas of ductular reaction, neutrophil infiltration, and necrotic patches of tissue. (B) Ki-67 immunostaining is seen in both hepatocytes and NPCs (arrowheads point to positive stain). Body weight change observed in ff Shp and LShpKO fed 0.1% DDC monitored over the course of 2 weeks. (D) Expression of bile acid homeostasis genes involved in bile acid synthesis and transport and key cyclins expressed during the cell cycle in ff Shp and LShpKO fed 0.1% DDC for 2 weeks. Significance calculated using an unpaired 2-tailed t-test. *P ≤ 0.05, **P ≤ 0.01.
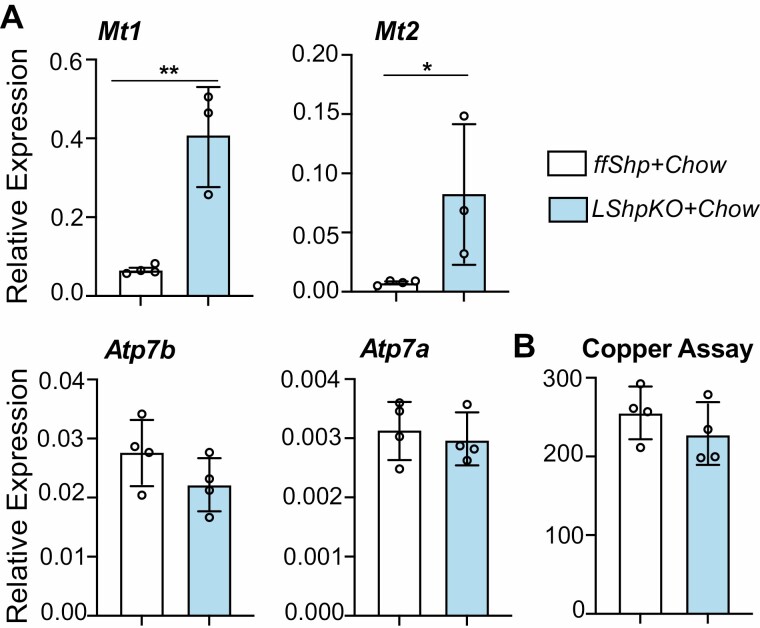
Shp Deficiency Increases the Expression of Genes Related to Copper Regulation
To further explore one of the unique functional aspects of SHP based on our analysis, we investigated copper transporters and the hepatic copper levels in control and LShpKO mice. Under basal chow conditions, the 2 key copper transporters, Atp7a and Atp7b (Fig. 8A), showed no significant difference in their transcript levels, albeit a modest 20% decrease in Atp7b in the absence of SHP. However, we did observe a significant increase in the expression of metallothionein genes Mt1 and Mt2, but hepatic copper levels remain unchanged (Fig. 8B).
Figure 8.
(A) Relative expression of genes involved copper ion binding and transport in ff Shp and LShpKO mice on a chow diet. (B) Quantification of hepatic copper content obtained by using the Sigma Aldrich Copper Assay Kit. Significance calculated using an unpaired 2-tailed t test. *P ≤ 0.05, **P ≤ 0.01.
Discussion
Several studies based on the global knockout of nuclear receptor Shp have been published over the past decades (5, 6, 8, 14, 17). Still, there is a lack of tissue-specific characterization of Shp. Here, we found that deletion of hepatic Shp leads to increased ileal bile acid concentrations and induced proliferation under chow and cholestatic conditions. A single copy of Shp allele in the heterozygous mice displayed comparable Shp transcript levels, indicating that possibly increased transcription of the wild-type allele may compensate for the loss. For instance, the protein levels of Errα were comparable between control and Errα heterozygous mice (48). We also identified several genes that are uniquely regulated by independently of activation. To determine if the evolution of the SHP, FXR, and BA synthesis was convergent, we conducted a phylogenetic analysis across 80 different species. The dendrogram results suggest that the 2 major branches present in the FXR phylogenetic tree are drastically reduced in size in the SHP tree. Branching between FXR and CYP7A1, as well as SHP and CYP8B1, seem more similar (Supplemental Fig, 2) (30).
We examined BA homeostasis in LShpKO mice and found that the negative feedback loop initiated by excessive amounts of bile acids is impaired. Cyp7a1 and Cyp8b1 genes were expressed at increased levels without Shp (Fig. 1). In line with these data, LShpKO animals displayed higher bile acid content when compared with control animals. BA composition analysis revealed higher ratios of unconjugated primary and secondary bile acids in the liver, ileum, and gallbladder of the LShpKO mice (Fig. 2). Upon CA diet, primary bile acid percentage decreases in serum and gallbladder, whereas secondary BAs in the ileum increase when hepatic Shp is deleted. Consistent with increased Cyp8b1 expression, LShpKO animals have higher hepatic taurocholate. Intriguingly, we find a robust increase in TDCA concentrations in the BA pool (liver, gall bladder and intestine as shown in Fig. 3), suggesting that in the absence of hepatic Shp, ileal microbial production of secondary BAs is elevated. Changes in hepatic composition and conjugation have been associated with liver injury and likely explain the increased inflammation, proliferation, and necrotic patches observed in LShpKO mice. To understand the alterations in ileal BA composition and increase in BA levels upon CA diet in LShpKO mice, we analyzed ileal and hepatic transporter gene expression. Although the transcript levels of these transporters were comparable between control and LShpKO mice (Supplemental Fig, 4) (30), it is possible that their protein expression may be altered. In addition, the availability of more primary BAs in LShpKO mice may lead to higher secondary BA synthesis by the gut microbiota, changing BA composition. Overall, our findings indicate that enterohepatic recirculation and BA homeostasis is defective in the absence of hepatic SHP.
SHP is known to function as a transcriptional repressor and can generally be induced through various factors such as xenobiotics, BAs, genetic defects, and CpG methylation (5, 19). LShpKO animals display increased proliferation under basal conditions (Fig. 4), which was further enhanced upon CA or DDC feeding, suggesting that Shp could be playing a role in BA-mediated proliferation in hepatocytes and other nonparenchymal cells. The observed increases in proliferation in the absence of SHP are consistent with its previously postulated role as a tumor suppressor gene. We further explored the proliferative aspects of Shp. Because SHP is known to act as a corepressor, we examined the activation of another pro-proliferative nuclear receptor, CAR by its agonist TC. LShpKO mice maintained the TC-mediated induction of cyclin B1, B2, and E expression. Surprisingly, though we noted a complete loss of cyclin D1 upregulation in LShpKO livers, the increase in Ki-67-positive nuclei was maintained indicative of other redundant mechanisms.
GW4064 treatment in WT and LShpKO mice led us to examine the consequence of FXR activation in the presence and absence of SHP. GW4064 treatment did not rescue expression of genes involved in BA metabolism in LShpKO animals. Of the several different pro-proliferative genes we examined, intriguingly, E2f1, Grn, and Cdk6 expression was distinctly regulated by GW4064 compared with CA in ff Shp livers. Such differences between the synthetic and the biological ligands of FXR have been previously reported in metabolic outcomes (49).
Because we were interested in examining -specific regulation, we then mined the previously published transcriptomic data from the livers of the whole body Shp KO and identified several distinct genes represented in different signaling pathways, including copper ion transport, circadian regulation, and metabolism. Upon further investigation, we found increased expression of Mt1 and Mt2 genes that are known to buffer copper levels (50–53). However, under basal conditions, hepatic copper content was unaltered in LShpKO mice. It is possible that SHP-mediated copper regulation may be relevant under a certain pathological context, which needs to be explored. Taken together, our findings show that hepatic Shp is important to maintain tissue-specific BA content and composition, modulate liver proliferation, and possibly metal ion homeostasis.
Acknowledgments
Dr. Kristina Schoonajans provided the floxed SHP mice at the Ecolé Polytechnique Féderalé de Lausanne, Lausanne, Switzerland. We thank the DUKE proteomics and metabolomics shared resources for performing the bile acid analysis.
Glossary
Abbreviations
- ALT
alanine transaminase
- AST
aspartate transaminase
- BA
bile acid
- CA
cholic acid
- CAR
constitutive androstane receptor
- DDC
3,5-diethoxycarbonyl-1,4-dihydrocollidine
- DI
deionized water
- Fxr
Farnesoid X Receptor
- LShpKO
liver-specific ShpKO
- NPC
nonparenchymal cell
- RT
room temperature
- Shp
small heterodimer partner
- ShpKO
Shp knockout
- TBS
Shp knockout
- TC
1,4-bis[2-(3,5-dichloropyridyloxy)]benzene
- TDCA
; WT,
Financial Support
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases, R01 and minority supplement DK113080, American Cancer Society RSG132315, and Cancer Center at Illinois seed grant at Urbana-Champaign to S.A.
Author Contributions
R.P.H.S., P.K., N.D., and A.H. performed the experiments. A.H. performed all the studies with the Shp+/- mice. N.D. did the motif analysis and quantitative PCR analysis of the DDC experiment. R.P.H.S. and P.K. did the other experiments. Histology was analyzed by K.B. and data analysis was done by R.P.H.S., P.K., N.D., A.H., and S.A. All the authors did the interpretation of data, and R.P.H.S., P.K., and S.A. wrote the manuscript; S.A. was responsible for acquiring the funds, study design, and study supervision.
Disclosures
The authors have nothing to disclose
Data Availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Marcellin P, Kutala BK. Liver diseases: a major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018;38(Suppl. 1):2-6. doi: 10.1111/liv.13682. [DOI] [PubMed] [Google Scholar]
- 2. Byass P. The global burden of liver disease: a challenge for methods and for public health. BMC Med. 2014;12:159. Published 2014 Sep 18. doi: 10.1186/s12916-014-0159-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N Engl J Med. 1998;339(17):1217-1227. [DOI] [PubMed] [Google Scholar]
- 4. Yokoda RT, Rodriguez EA. Review: pathogenesis of cholestatic liver diseases. World J Hepatol. 2020;12(8):423-435. doi: 10.4254/wjh.v12.i8.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilczek E, Szparecki G, Lukasik D, et al. Loss of the orphan nuclear receptor Shp is more pronounced in fibrolamellar carcinoma than in typical hepatocellular carcinoma. PLoS One. 2012;7(1):e30944e30944. doi: 10.1371/journal.pone.0030944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang L, Han Y, Kim CS, Lee YK, Moore DD. Resistance of Shp-null mice to bile acid-induced liver damage. J Biol Chem. 2003;278(45):44475-44481. doi: 10.1074/jbc.M305258200. [DOI] [PubMed] [Google Scholar]
- 7. Claudel T, Zollner G, Wagner M, Trauner M. Role of nuclear receptors for bile acid metabolism, bile secretion, cholestasis, and gallstone disease. Biochim Biophys Acta. 2011;1812(8):867-878. doi: 10.1016/j.bbadis.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 8. Kerr TA, Saeki S, Schneider M, et al. Loss of nuclear receptor Shp impairs but does not eliminate negative feedback regulation of bile acid synthesis. Dev Cell. 2002;2(6):713-720. doi: 10.1016/s1534-5807(02)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zou A, Lehn S, Magee N, Zhang Y. New insights into orphan nuclear receptor Shp in liver cancer. Nucl Receptor Res. 2015;2:101162. doi: 10.11131/2015/101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bae Y, Kemper JK, Kemper B. Repression of Car-mediated transactivation of CYP2B genes by the orphan nuclear receptor, short heterodimer partner (Shp). DNA Cell Biol. 2004;23:81-91. doi: 10.1089/104454904322759894. [DOI] [PubMed] [Google Scholar]
- 11. Kininis M, Kraus WL. A global view of transcriptional regulation by nuclear receptors: gene expression, factor localization, and DNA sequence analysis. Nucl Receptor Signal. 2008;6:e005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kir S, Zhang Y, Gerard RD, Kliewer SA, Mangelsdorf DJ. Nuclear receptors HNF4α and LRH-1 cooperate in regulating Cyp7a1 in vivo. J Biol Chem. 2012;287(49):41334-41341. doi: 10.1074/jbc.M112.421834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goodwin B, et al. A regulatory cascade of the nuclear receptors Fxr, Shp-1, and LRH-1 represses bile acid biosynthesis Mol. Cell. 2000;6(3):517-526. [DOI] [PubMed] [Google Scholar]
- 15. Wang L, et al. Redundant pathways for negative feedback regulation of bile acid production Dev. Cell. 2002;2:721-773. [DOI] [PubMed] [Google Scholar]
- 16. Anakk S, Watanabe M, Ochsner SA, McKenna NJ, Finegold MJ, Moore DD. Combined deletion of Fxr and Shp in mice induces Cyp17a1 and results in juvenile onset cholestasis. J Clin Invest. 2011;121(1):86-95. doi: 10.1172/JCI42846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim I, Ahn SH, Inagaki T, et al. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007;48(12):2664-2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 18. Cariello M, Peres C, Zerlotin R, et al. Long-term administration of nuclear bile acid receptor FXR agonist prevents spontaneous hepatocarcinogenesis in Abcb4-/- Mice. Sci Rep. 2017;7(1):11203. doi: 10.1038/s41598-017-11549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He N, Park K, Zhang Y, Huang J, Lu S, Wang L. Epigenetic inhibition of nuclear receptor small heterodimer partner is associated with and regulates hepatocellular carcinoma growth. Gastroenterology. 2008;134(3):793-802. doi: 10.1053/j.gastro.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 20. Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115(24):5651-5661. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589-604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Y, Xu P, Park K, Choi Y, Moore DD, Wang L. Orphan receptor small heterodimer partner suppresses tumorigenesis by modulating cyclin D1 expression and cellular proliferation. Hepatology. 2008;48(1):289-298. doi: 10.1002/hep.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Columbano A, Ledda Columbano GM, Pibiri M, Concas D, Reddy JK, Rao MS. Peroxisome proliferator-activated receptor-alpha mice show enhanced hepatocyte proliferation in response to the hepatomitogen 1,4-bis [2-(3,5-dichloropyridyloxy)] benzene, a ligand of constitutive androstane receptor. Hepatology. 2001;34(2):262-266. [DOI] [PubMed] [Google Scholar]
- 24. Ledda-Columbano GM, Pibiri M, Loi R, Perra A, Shinozuka H, Columbano A. Early increase in cyclin D1 expression and accelerated entry of mouse hepatocytes into S phase after administration of the mitogen 1,4-bis[2-(3,5-dichloropyridyloxy)] benzene. Am J Pathol. 2000;156(1):91-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kowalik MA, Saliba C, Pibiri M, et al. Yes-associated protein regulation of adaptive liver enlargement and hepatocellular carcinoma development in mice. Hepatology. 2011;53(6):2086-2096. doi: 10.1002/hep.24289. [DOI] [PubMed] [Google Scholar]
- 26. Abe T, Amaike Y, Shizu R. Role of YAP activation in nuclear receptor Car-mediated proliferation of mouse hepatocytes. Toxicol Sci. 2018;165(2):408-419. doi: 10.1093/toxsci/kfy149. [DOI] [PubMed] [Google Scholar]
- 27. Cipriani S, Carino A, Masullo D, et al. Decoding the role of the nuclear receptor Shp in regulating hepatic stellate cells and liver fibrogenesis. Sci Rep. 2017;7:41055. doi: 10.1038/srep41055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baek SH, Kim KI. Emerging roles of orphan nuclear receptors in cancer. Annu Rev Physiol. 2014;76:177-195. doi: 10.1146/annurev-physiol-030212-183758. [DOI] [PubMed] [Google Scholar]
- 29. Park YY, Choi HS, Lee JS. Systems-level analysis of gene expression data revealed NR0B2/Shp as potential tumor suppressor in human liver cancer. Mol Cells. 2010;30(5):485-491. doi: 10.1007/s10059-010-0136-6. [DOI] [PubMed] [Google Scholar]
- 30. Ryan S, Peter K, Nathanlown D, Angela H, Sayeepriyadarshini A, Keith B. “Loss of hepatic small heterodimer partner elevates ileal bile acids and alters cell cycle related genes in male mice.” Mendeley Data. 2022;V4. doi: 10.17632/zb6mmp3zm3.4. Link: https://data.mendeley.com/datasets/zb6mmp3zm3/draft?a=506bd922-69a3-4cfb-8b30-4166a1e4c57b. [DOI] [Google Scholar]
- 31. Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406-425. [DOI] [PubMed] [Google Scholar]
- 32. Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39(4):783-791. [DOI] [PubMed] [Google Scholar]
- 33. Zuckerkandl E, Pauling L. Evolutionary divergence and convergence in proteins. Edited in Evolving Genes and Proteins by Bryson V. and Vogel H.J., 1965; 97-166. New York: Academic Press. [Google Scholar]
- 34. Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Akinrotimi O, Riessen R, VanDuyne P, et al. Small heterodimer partner deletion prevents hepatic steatosis and when combined with farnesoid X receptor loss protects against type 2 diabetes in mice. Hepatology. 2017;66(6):1854-1865. doi: 10.1002/hep.29305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Costa RH, Kalinichenko VV, Tan Y, Wang IC. The Car nuclear receptor and hepatocyte proliferation. Hepatology. 2005 Nov;42(5):1004-8. doi: 10.1002/hep.20953. Erratum in: Hepatology. 2005 Dec;42(6):1471. Kalinchenko, Vladimir V [corrected to Kalinichenko, Vladimir V]. PMID: 16250031. [DOI] [PubMed] [Google Scholar]
- 37. Wang YM, Ong SS, Chai SC, Chen T. Role of Car and PXR in xenobiotic sensing and metabolism. Expert Opin Drug Metab Toxicol. 2012;8(7):803-817. doi: 10.1517/17425255.2012.685237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seol W, Choi HS, Moore DD. “An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors.” Science 272.5266:1336–1339. [DOI] [PubMed] [Google Scholar]
- 39. Tzameli I, Pissios P, Schuetz EG, Moore DD. The xenobiotic compound 1,4- bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor Car. Mol Cell Biol. 2000;20:2951-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chong HK, Infante AM, Seo YK, Jeon TI, Zhang Y, et al. Genome-wide interrogation of hepatic Fxr reveals an asymmetric IR-1 motif and synergy with LRH-1. Nucleic Acids Res. 2010;38:6007-6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang YD, Chen WD, Moore DD, Huang W. Fxr: a metabolic regulator and cell protector. Cell Res. 2008;18(11):1087-1095. [DOI] [PubMed] [Google Scholar]
- 42. Johansson L, Båvner A, Thomsen JS, Färnegårdh M, Gustafsson JA, Treuter E. The orphan nuclear receptor Shp utilizes conserved LXXLL-related motifs for interactions with ligand-activated estrogen receptors. Mol Cell Biol. 2000;20(4):1124-1133. doi: 10.1128/mcb.20.4.1124-1133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Båvner A, Sanyal S, Gustafsson JA, Treuter E. Transcriptional corepression by Shp: molecular mechanisms and physiological consequences. Trends Endocrinol Metab. 2005;16(10):478-488. doi: 10.1016/j.tem.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 44. Zhang Y, Hagedorn CH, Wang L. Role of nuclear receptor Shp in metabolism and cancer. Biochim Biophys Acta. 2011;1812(8):893-908. doi: 10.1016/j.bbadis.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bailey TL, Johnson J, Grant CE, Noble WS. “The MEME Suite.” Nucleic Acids Res. 2015;43(W1):W39-W49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parker JB, Yin H, Vinckevicius A, Chakravarti D. Host cell factor-1 recruitment to E2F-bound and cell-cycle-control genes is mediated by THAP11 and ZNF143. Cell Rep. 2014;9(3):967-982. doi: 10.1016/j.celrep.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Seth A, Watson DK. ETS transcription factors and their emerging roles in human cancer. Eur J Cancer. 2005;41(16):2462-2478. doi: 10.1016/j.ejca.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 48. Luo J, Sladek R, Carrier J, Bader JA, Richard D, Giguère V. Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor alpha. Mol Cell Biol. 2003;23(22):7947-7956. doi: 10.1128/MCB.23.22.7947-7956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Watanabe M, Horai Y, Houten SM, et al. Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. J Biol Chem. 2011;286(30):26913-26920. doi: 10.1074/jbc.M111.248203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Davis SR, McMahon RJ, Cousins RJ. Metallothionein knockout and transgenic mice exhibit altered intestinal processing of zinc with uniform zinc-dependent zinc transporter-1 expression. J Nutr. 1998;128(5):825-831. doi: 10.1093/jn/128.5.825. [DOI] [PubMed] [Google Scholar]
- 51. Tokuda E, Okawa E, Watanabe S, Ono S-I. Overexpression of metallothionein-I, a copper-regulating protein, attenuates intracellular copper dyshomeostasis and extends lifespan in a mouse model of amyotrophic lateral sclerosis caused by mutant superoxide dismutase-1. Hum Mol Genet. 2014;23(5):1271-1285. doi: 10.1093/hmg/ddt517. [DOI] [PubMed] [Google Scholar]
- 52. Gudekar N, Shanbhag V, Wang Y, et al. Metallothioneins regulate ATP7A trafficking and control cell viability during copper deficiency and excess. Sci Rep. 2020;10:7856. doi: 10.1038/s41598-020-64521-3\. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tapia L, González-Agüero M, Cisternas MF, et al. Metallothionein is crucial for safe intracellular copper storage and cell survival at normal and supra-physiological exposure levels. Biochem J. 2004;378(Pt 2):617-624. doi: 10.1042/BJ20031174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.