ABSTRACT
Background
The transition from a predominantly milk-based diet to a diverse family diet is a window of opportunity for optimal child growth and development.
Objectives
The study aims to examine the nutritional status and food-consumption patterns of children under 4 y of age in the United Arab Emirates (UAE) and their adherence to nutrient and dietary recommendations.
Methods
A population-based cross-sectional survey of 525 children aged 0–47.9 mo was conducted in 3 major emirates: Abu Dhabi, Dubai, and Sharjah. Anthropometric measurements were obtained, and dietary assessment was conducted using the 24-h dietary recall approach. Usual intakes of energy, macronutrient, and micronutrients, including from supplements, were assessed using PC-SIDE software. Adherence to food-group recommendations was evaluated based on the American Heart Association/American Academy of Pediatrics dietary guidelines.
Results
Among 0–4-y-old children, 10% were stunted, 6% were wasted, 17% were at risk of overweight, 5% were overweight, and 3% were obese. The contribution of sweets and sugar sweetened beverages to energy intake increased from 5% in 6–11.9-mo-old children to 17% in 36–47.9-mo-old children. Compared with dietary guidelines, the lowest adherence was for fruit (13–18%) and vegetables (7–12%), while protein was within the recommendations, and 92% and 89% of children (aged 24 to 35.9 mo and 36 to 47.9 mo, respectively) had high intakes of saturated fat. Almost all toddlers failed to meet the Adequate Intake for fiber. The proportions of children exceeding the free-sugar upper limit increased from 10.6% in infants (0–5.9 mo) to 56.7% in toddlers (12– 23.9 mo). Micronutrient inadequacies were observed, particularly for calcium, zinc, folate, and vitamins A and D.
Conclusions
This study suggests a triple burden of malnutrition among infants and young children in the UAE. Results call for national nutrition intervention strategies aimed at improving dietary quality in the pediatric population.
Keywords: United Arab Emirates, infants and toddlers, young children, malnutrition, stunting, overweight, obesity, dietary intake, food-consumption patterns, dietary adherence
A survey of 525 children aged 0-47.9 months conducted in the UAE suggested a triple burden of malnutrition (overnutrition, undernutrition and micronutrient inadequacies) amongst the study population.
Introduction
The triple burden of malnutrition, characterized by the coexistence of undernutrition, micronutrient deficiencies, and obesity, remains a growing challenge in the Middle East and North Africa (MENA) region (1). The United Arab Emirates (UAE), is one country in the region that has witnessed major economic development, urbanization (90% of the population is urban) (2), and demographic growth, all accompanied by the nutrition transition and increasing rates of obesity and other noncommunicable diseases (NCDs) (3). This nutrition transition is thought to be related to shifts in dietary intakes, whereby traditional dietary practices are eroding and being replaced by westernized food-consumption patterns that are rich in sugar and animal-based (saturated) fats (3). Young children are probably among the most vulnerable population groups to the ongoing nutrition transition and its characteristic changes in dietary habits (4). The modernized food environment typified by creative marketing and increased availability of energy-dense, low-nutrient foods and beverages may affect food-consumption patterns among young children, potentially compromising their growth, development, and nutritional status (5). While little is known on how the nutrition transition is affecting the diets of young children in the UAE, there is evidence for an increasing burden of overweight and obesity in this age group. It was, for instance, estimated that 13% of children under 5 y of age were overweight or obese in the UAE in 2012, a value that exceeded the global prevalence estimate of under-5 overweight/obesity (6.7%), as well as estimates reported from various developing countries (6.1%) (6). In parallel, available evidence suggests that the burden of micronutrient deficiencies remains high in the UAE, with anemia prevalence (hemoglobin <11 g/dL) being estimated at 36.1% among children younger than 5 y (7). These estimates are of concern, given that malnutrition, in any form, can adversely impact growth and development in children, and modulate their susceptibility to disease later in life (8). A significant body of evidence also suggests that unhealthy dietary habits acquired during early childhood may track into adulthood (9–12). Studies have shown that intakes of fruits, vegetables, and discretionary calories such as sugar-sweetened beverages (SSBs) in early childhood tend to predict their consumption levels in later childhood (13, 14). The period of transition from a predominantly milk-based diet in infancy to a diverse family diet may therefore constitute a window of opportunity for nutritional interventions that could instill healthier dietary habits and practices in young children (15).
Despite the growing evidence on the importance of dietary practices and nutrient adequacy in young infants and children, data on food consumption, nutritional status, and dietary intakes remain scarce for this age group (16). This may be particularly true for the UAE, a country that hosts a diverse population of nationals and expatriates (17). It is in this context that this study was conducted, with the aim of investigating the nutritional status and food-consumption patterns among infants and young children (0–4 y) residing in the 3 major emirates of the UAE: Abu Dhabi, Dubai, and Sharjah. For comparison purposes, the sample will include, in addition to nationals, a sample of Arab non-nationals. The specific objectives of the study were as follows: 1) to assess the nutritional status of infants and young children in the UAE, 2) to characterize their food-consumption patterns and shed light on how these consumption patterns may differ between age subgroups, 3) to evaluate their adherence to dietary recommendations, and 4) to assess their macro- and micronutrient intakes in comparison with the US Dietary Reference Intake values, given that local reference values are lacking. The study findings will allow for an appraisal of the nutritional status of infants and young children in the UAE, a better understanding of their food-consumption patterns, and hence a prioritization of policies and interventions aimed at developing healthy dietary habits early in life.
Methods
Study design
This study was designed to assess the anthropometric characteristics, food-consumption patterns, and dietary intakes of a cross-sectional sample of 525 UAE nationals (n = 353) and Arab non-nationals (n = 172). The study sample consisted of children aged 0–47.9 mo residing in the 3 major emirates of the UAE (Abu Dhabi, Dubai, and Sharjah), which host around 85% of the UAE population (18). The study was performed according to the Feeding Infants and Toddler Study (FITS) protocol, the latter being a cross-sectional dietary intake survey of large samples of infants and toddlers aimed at fostering and sharing nutrition knowledge across 10 countries in the world (19). Sample recruitment was performed using a stratified random cluster-sampling framework. Within this framework, the 3 emirates constituted the various strata, and the number of participants recruited from each emirate was proportional to the population size. Each emirate consisted of many districts (regions), and neighborhoods within districts made up the various clusters. Clusters within districts were chosen based on probability proportional to size sampling. In each of the clusters, household selection was based on a systematic sampling approach. For the UAE nationals, sample size calculations were based on an estimated prevalence of 13% of overweight/obesity in under-5 children (6), with a 95% CI and a 5% margin of error. The WHO STEPS Sample Size calculator was used to perform these calculations. In addition to the recruitment of UAE nationals, and for comparison purposes, a sample of Arab non-nationals was recruited with a ratio of 2 nationals to 1 Arab non-national (353 nationals vs. 172 Arab non-nationals). Arab non-nationals consisted of Algerian, Bahraini, Egyptian, Iranian, Iraqi, Jordanian, Lebanese, Libyan, Moroccan, Omani, Palestinian, Saudi Arabian, Somalian, Sudanese, Syrian, Tunisian, and Yemeni nationalities.
The study was performed in accordance with the ethical principles of the Declaration of Helsinki, as revised in 1983. The study protocol along with the screening form, written consent form (for caregivers), and the questionnaires were approved by the Institutional Review Board of the American University of Beirut and by the respective ethical authorities in the UAE, which included the UAE Ministry of Education, the Human Research Ethics Committee of the United Arab Emirates University, the Dubai Scientific Research Ethics Committee of the Dubai Health Authority, the Research Ethics Committee and Subcommittee of the UAE Ministry of Health and Prevention, and the Research Ethics Committee at the University of Sharjah. All study participants were offered a $15 book voucher.
Study population
A total of 525 dyads of young children and their mothers/caregivers were recruited. The study sample included 353 nationals and 172 Arab non-nationals aged 0–4 y, who were recruited from hospitals’ outpatient clinics and primary health care centers between June 2019 and March 2020. Eligibility criteria included the child's age (≤4 y) and absence of chronic diseases, congenital/physical malformations, and inborn errors of metabolism, that may influence anthropometric status or food-consumption patterns. Children of Arab non-nationals having resided in the UAE for less than 3 y, as well as non-Arab children, were excluded from the study.
Administration of the multicomponent questionnaire
Data collection was carried out in the Arabic language by 12 research nutritionists who had received extensive training in the ethical conduct of research, anthropometric assessment (following the WHO and Intergrowth guidelines) (20, 21), dietary assessment, administration of the questionnaire, minimizing interviewer error, and standardization of data-collection procedures across sites. Recruitment of all study subjects occurred in the centers’/clinics’ waiting areas. Indeed, the UAE government stipulates regular health checkups and mandatory vaccinations, specifically in the context of early childhood development and care, with nationals and non-nationals having equal access to these centers/clinics (22). The research nutritionists approached mothers in the center/clinic waiting areas, described the study objectives and protocol, and invited them to participate. Mothers were provided with appropriate information regarding their involvement in the study and the use of the collected anonymous data in published research, hence helping them to make an informed choice. They were made aware that their participation was completely voluntary and were given the opportunity to ask questions. Written informed consent was then obtained, after which the research nutritionists initiated data collection via a face-to-face interview with the mothers, while making sure that the interview was conducted in a private room within the centers/clinics. During the interview, anthropometric measurements were collected from participating children, and mothers were administered an age-specific questionnaire that inquired about demographic and socioeconomic characteristics, early-life feeding practices, and types and frequency of use of micronutrient supplements, in addition to the child's physical activity levels, sedentary behavior, sleeping habits, and food consumption. To preserve confidentiality, data were collected anonymously: each subject was allocated a code, and the link between the code and the subjects’ identifiers was only available to the Principal Investigator (LCI) in the UAE.
The questionnaire development was based on 1) the global FITS protocol (19), 2) a thorough review of relevant literature, and 3) culture-specific considerations of the Arab world, particularly the UAE. Two age-specific questionnaires were used for data collection (1 for children aged 0–23.9 mo and the other for children aged 24–47.9 mo). The content validity of the questionnaires was confirmed by an expert panel consisting of a clinical nutritionist, a community nutritionist, and a nutrition epidemiologist. The questionnaires were translated to Arabic and then back to English to ensure that the translation did not incur any change in meaning.
Anthropometric assessment
Standardized protocols were adopted for the collection of anthropometric measurements. These included length/height, body weight, and midupper arm circumference (MUAC). Anthropometric measurements were interpreted using the WHO Anthro Survey Analyzer (23) and the WHO MUAC criteria for malnutrition (21).
Among children younger than 24 mo, the measurement of length was performed using the Seca 333 WLAN measuring rod to the last completed millimeter, while body weight was measured using an electronic pan-type pediatric scale that has a 0.1-kg accuracy (Seca 331 EMR Ready Baby Scale). In children older than 24 mo, the measurement of height was obtained using a stadiometer (Seca 217; with shoes removed), while body weight was measured to the nearest 0.1 kg using the standard clinical balance (Seca model 11770), with the child wearing light clothing and barefoot. MUAC was measured to the nearest 0.1 cm using a calibrated plastic strip at the midpoint between the elbow and shoulder of the child's left arm with the arm hanging relaxed down the side of the body. All anthropometric measurements were obtained twice, and a maximal allowable difference was set. The average of both measurements was retained. BMI was calculated as weight in kilograms divided by height in meter squared (24), and its evaluation was performed using the WHO-2006 criteria based on gender and age-specific z scores (20). Accordingly, wasting was defined as BMI-for-age z score (BAZ) < −2, normal as −2 ≤ BAZ ≤ +1, possible risk of overweight as +1 ≤ BAZ ≤ +2, overweight as +2 ≤ BAZ ≤ +3, and obese as BAZ > +3.
Stunting was identified based on the height-for-age z score (HAZ) WHO classification (HAZ <2 indicates not stunted; HAZ ≥ −2 indicates stunted) (21). MUAC measurements were also interpreted based on the WHO criteria, whereby values below 110 mm indicated severe acute undernutrition and values between 110 mm and 120 mm indicated moderate malnutrition (21). There were no available MUAC cutoff points for infants less than 6 mo of age.
Dietary intake assessment
Dietary intake was evaluated using a paper-based 24-h recall (24-HR), according to the 5-step multiple pass approach of the USDA (25), an approach that was used in several previous studies conducted by our group in other Arab countries (26–28). In order to assist in portion-size estimation, the study participants were given the choice of reporting portion size in terms of standard household measures (such as cups, tablespoons, teaspoons), or using the 2-dimensional visual chart for food portions (29). The research nutritionists collected information related to the occasion and time of each meal, location of food consumption, type of food consumed, portion size, preparation method, and the brand when applicable. Information on water intake was also collected. A second 24-HR was obtained from a randomly selected subsample (47%) of consenting participants to take into consideration within-person variance when estimating usual dietary intakes (n = 245; 71.4% nationals and 28.6% non-nationals).
Food items, as consumed, were categorized into 10 food groups based on their nutrient profile. The food groups were grains and grain products, fruits, vegetables, milk and milk products, meats and other protein sources, mixed dishes, savory snacks, sweets and sweetened beverages, fats and oils, condiments, and sauces.
To further compare intakes with the recommendations of the American Heart Association/American Academy of Pediatrics (AHA/AAP) for healthy eating patterns, recipes were disaggregated into separate ingredients, then grouped into 5 main groups: milk and dairy, lean meat and beans, fruits, vegetables, and grains (30, 31). Intakes of these food groups were then compared with the AHA/AAP-recommended number of servings by age and gender. The aforementioned recommendations were not established for children below 12 mo of age.
Dietary information was analyzed using the Nutritionist Pro software (version 5.1.0, 2014, First Data Bank, Nutritionist Pro; Axxya Systems). Based on culture-specific recipes, mixed and traditional UAE dishes were entered into the Nutritionist Pro software as single food items. The food-composition databases that were used included a combination of the USDA single food items, the food-composition tables for the Middle East (32), product packaging and related websites when applicable, as well as those from published studies reporting on specific UAE dishes (33, 34). Daily intakes of energy and macro- and micronutrients were determined for each participating infant/child. Energy and nutrient intakes from breast milk were also determined using the Nutritionist Pro software. On the interview days, 161 children (58.3%) had consumed breast milk on either day 1, day 2, or both (86.7% of 0–5.9-mo-old infants; 56.2% of 6–11.9-mo-old infants, and 24.4% of toddlers aged 12–23.9 mo). During the 24-HR, mothers were asked to report the number of times as well as the duration of breastfeeding. To estimate the amount of human milk consumed and hence the corresponding nutrient intakes, we adopted the methodology previously described in FITS (35). Accordingly, infants who were exclusively breastfed were assigned a standard reference value of 780 mL of human milk per day if aged 0–5.9 mo and 600 mL/d if aged 6–11.9 mo. In partially breastfed infants, human-milk intake was calculated as 780 mL/d minus the amount of “formula milk (mL/d)” ingested on the day of the recall if aged 0–5.9 mo or 600 mL/d minus the amount of “formula milk (mL/d)” ingested if aged 6–11.9 mo. In older children aged 12–17.9 mo and 18–23.9 mo, the daily amount of human milk was calculated as 89 mL or 59 mL, respectively, for every feeding occasion reported by the mother.
The participants’ usual dietary energy intakes (EIs) were compared with their estimated energy requirements (EERs), which were calculated using the 2006 Institute of Medicine of the National Academies published guide (36), using sex- and age-specific equations. For children younger than 3 y, EER equations do not account for physical activity level (PAL), while for children older than 3 y, EER equations take into consideration 4 PALs: sedentary (PAL: 1.0–1.39), low activity (PAL: 1.4–1.59), active (PAL: 1.6–1.89), and very active (PAL: 1.9–2.5). In our study, we did not obtain information on physical activity or sedentary behavior, and hence, for 3–4-y-old children, low physical activity (physical activity coefficient = 1.13 for boys and 1.16 for girls) was adopted in all EER calculations.
Data analysis
All analyses were stratified by age, based on the FITS protocol, and hence the sample was divided into 5 age groups: 0–5.9 mo (infants), 6–11.9 mo (infants), 12–23.9 mo (toddlers), 24–35.9 mo (toddlers), and 36–47.9 mo (preschoolers). Data analysis was performed using STATA (version 12.0; StataCorp) and the significance level was set at P < 0.05.
Frequencies and proportions were used for all categorical variables and means ± SEs for continuous variables. To test for statistical significance, chi-square (χ2) test was used for categorical variables.
Socioeconomic status was assessed based on the crowding index (calculated as the number of people living in a household per number of rooms in the house) and household monthly income. The crowding index is an internationally recognized proxy measure of socioeconomic status that considers an index >1 as an overcrowded household with few economic resources (37). Anthropometric measurements were assessed according to WHO recommendations for children under 5 y (21).
Food sources of energy (kilocalories/capita/day) and their percentage contribution to EI (%EI) were computed (based on the first 24-HR). The proportions of children adhering to the AHA/AAP dietary recommendations were calculated and differences between age groups were examined using the chi-square test. The usual macro- and micronutrient intakes from food sources and dietary supplements were estimated using PC-SIDE software, produced by Iowa State University (2001) using the combined method (38, 39). To assess the adequacy of macro- and micronutrient intakes, values were evaluated using DRIs as published by the Institute of Medicine. For micronutrients, intakes were compared with the Estimated Average Requirements (EARs), Tolerable Upper Intake Level (UL), and Adequate Intake (AI). AI was only used when there were no established EAR values, in which case a group mean intake at or above the AI suggests that the prevalence of inadequacy is probably low (40); if alternatively a group's mean intake is inferior to the AI, then intakes may need to increase, but it is not possible to accurately quantify the prevalence of inadequacy (40). The proportions of children with intakes outside the recommended ranges of the Acceptable Macronutrient Distribution Range (AMDR) for fat, protein, and carbohydrate were assessed for children older than 1 y of age.
Results
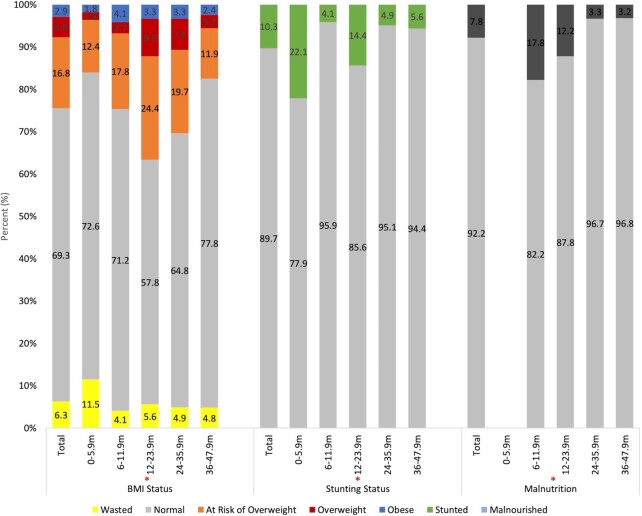
Table 1 presents the sociodemographic and household characteristics of the study population. The sample consisted of 525 infants and children aged between 0 and 47.9 mo, including 353 nationals and 172 Arab non-nationals, with equal distribution of girls and boys. A higher socioeconomic status was observed among national families compared with the Arab non-nationals, with a significant difference in household monthly income. Using the crowding index as a proxy measure of socioeconomic status, 97% of Arab non-national households had a crowding index above 1 as compared to 87% of nationals. Assessing family education, approximately 47% of children's mothers and 45% of fathers had a university education. A significant difference in educational level was observed between nationals and Arab non-nationals, whereby a significantly higher proportion of Arab non-nationals had completed their higher education. At the time of the interview, the employment rate was higher for fathers as compared to mothers (97% vs. 28%), with no significant difference between nationals and Arab non-nationals. Table 1 also shows the anthropometric characteristics of the study population by nationality status. Approximately 10% of all infants and children were stunted, 6% were wasted, 17% were at risk of overweight, 5% were overweight, and 3% were obese. The prevalence of stunting was significantly higher in nationals compared with Arab non-nationals (12.5% vs. 5.8%, respectively; P < 0.05), whereas no significant differences were observed for BMI status. Figure 1 shows the anthropometric characteristics of the study population by age. The highest proportions of stunting (22%) and wasting (12%) were observed in the 0–5.9-mo age group. The risk of overweight was lowest among preschoolers aged 36–47.9 mo (11.9%) as compared with toddlers aged 12–23.9 mo and 24–35.9 mo (24.4% and 19.7%, respectively). Similarly, the highest prevalence of overweight was found among children aged 12–23.9 mo (8.9%), whereas only 7.4% and 3.2% were overweight among toddlers aged 24–35.9 mo and 36–47.9 mo, respectively (P ≤ 0.001). Significant differences in the proportion of malnourished children (based on MUAC) were also observed among the 4 age groups, with the highest prevalence among those aged 6–11.9 mo (P < 0.001).
TABLE 1.
Sociodemographic and anthropometric characteristics of UAE infants and young children aged 0 to 47.9 mo, by nationality: FITS 20201
| Total, n (%) | Nationals, n (%) | Arab non-nationals, n (%) | χ2, P | |
|---|---|---|---|---|
| n (%) | 525 (100) | 353 (67.2) | 172 (32.8) | |
| Sociodemographic characteristics | ||||
| Age distribution | χ2 = 7.66, P = 0.105 |
|||
| 0 to 5.9 mo | 113 (21.5) | 83 (23.5) | 30 (17.4) | |
| 6 to 11.9 mo | 73 (13.9) | 41 (11.6) | 32 (18.6) | |
| 12 to 23.9 mo | 90 (17.1) | 56 (15.9) | 34 (19.8) | |
| 24 to 35.9 mo | 123 (23.4) | 86 (24.4) | 37 (21.5) | |
| 36 to 48 mo | 126 (24.0) | 87 (24.6) | 39 (22.7) | |
| Gender | χ2 = 8.32, P = 0.004* |
|||
| Male | 264 (50.3) | 162 (45.9) | 102 (59.3) | |
| Female | 261 (49.7) | 191 (54.1) | 70 (40.7) | |
| Household monthly income2 | χ2 = 25.94, P = 0.000* |
|||
| <30,000 DHS | 163 (31.0) | 101 (28.6) | 62 (36.0) | |
| 30,000 to 50,000 DHS | 47 (9.0) | 44 (12.5) | 3 (1.7) | |
| 50,000 to 100,000 DHS | 12 (2.3) | 12 (3.4) | 0 (0.0) | |
| >100,000 DHS | 4 (0.8) | 4 (1.1) | 0 (0.0) | |
| Don't know/refuse | 299 (57.0) | 192 (54.4) | 107 (62.2) | |
| Crowding index | χ2 = 11.16, P = 0.001* |
|||
| <1 person/room | 50 (9.6) | 44 (2.7) | 6 (3.5) | |
| ≥1 persons/room | 469 (90.4) | 303 (87.3) | 166 (96.5) | |
| Education of mother | χ2 = 24.45, P = 0.000* |
|||
| Less than elementary3 | 4 (0.8) | 2 (0.6) | 2 (1.2) | |
| Elementary to secondary4 | 252 (48.0) | 195 (55.2) | 57 (33.1) | |
| University degree | 247 (47.0) | 146 (41.4) | 101 (58.7) | |
| Graduate/professional degree | 22 (4.2) | 10 (2.8) | 12 (7.0) | |
| Education of father | χ2 = 29.61, P = 0.000* |
|||
| Elementary to secondary4 | 243 (46.4) | 192 (54.4) | 51 (29.8) | |
| University degree | 238 (45.4) | 140 (39.7) | 98 (57.3) | |
| Graduate/professional degree | 43 (8.2) | 21 (5.9) | 22 (12.9) | |
| Mother employed | χ2 = 0.06, P = 0.807 |
|||
| Yes | 149 (28.4) | 99 (28.0) | 50 (29.1) | |
| No | 376 (71.6) | 254 (72.0) | 122 (70.9) | |
| Father employed | χ2 = 0.82, P = 0.365 |
|||
| Yes | 510 (97.3) | 342 (96.9) | 168 (98.2) | |
| No | 14 (2.7) | 11 (3.1) | 3 (1.8) | |
| Anthropometric characteristics | ||||
| Height for age | χ2 = 5.59, P = 0.018* |
|||
| Stunted | 54 (10.3) | 44 (12.5) | 10 (5.8) | |
| Not stunted | 470 (89.7) | 308 (87.5) | 162 (94.2) | |
| BMI status | χ2 = 3.11, P = 0.539 |
|||
| Wasted | 33 (6.3) | 22 (6.3) | 11 (6.4) | |
| Normal | 363 (69.3) | 252 (71.6) | 111 (64.5) | |
| At risk of overweight | 88 (16.8) | 54 (15.3) | 34 (19.8) | |
| Overweight | 25 (4.8) | 15 (4.3) | 10 (5.8) | |
| Obese | 15 (2.9) | 9 (2.6) | 6 (3.5) | |
| Dietary supplement use | χ2 = 0.64, P = 0.425 |
|||
| Yes | 210 (40.0) | 137 (38.8) | 73 (42.4) | |
| No | 315 (60.0) | 216 (61.2) | 99 (57.6) | |
n = 525. *Indicates significance at P < 0.05. DHS, Dirhams; FITS, Feeding Infants and Toddler Study; UAE, United Arab Emirates.
1 US dollars = 3.67 UAE Dirhams.
Less than elementary includes being illiterate, not attending school, or being able to read and write only.
Elementary to secondary includes primary school, intermediate school, high school, or technical diploma.
FIGURE 1.
Anthropometric characteristics of UAE infants and young children aged 0 to 47.9 mo (%), FITS 2020. Stunted if HAZ < −2; not stunted if HAZ ≥ −2. Interpretation of anthropometric measurements was based on the WHO classification. Note: MUAC reference values have not been established for infants 0–5.9 mo. Only toddlers and young children >6 mo were considered. Total n for BMI status and stunting status is 524 (1 child had missing weight); total n for malnutrition is 411 (excluding 0–5.9-mo children). *Indicates significant difference between the age groups in the proportion of children with corresponding anthropometric characteristics. FITS, Feeding Infants and Toddler Study; HAZ, height-for-age z score; m, months; MUAC, midupper arm circumference; UAE, United Arab Emirates.
Table 2 shows EI estimates for the study population. As expected, mean EI was lowest in the youngest age group of 0 to 5.9 mo (667.7 kcal/d) and highest among older children aged 36 to 47.9 mo (1208.5 kcal/d). The proportion of children consuming below EERs significantly increased from 7% at age 0–5.9 mo to almost 68% at 36–47.9 mo. In parallel, the proportion of children exceeding EERs significantly decreased from 93% in those 0–5.9 mo to 33% in those 36–47.9 mo of age. There was a significant difference between nationals and Arab non-nationals regarding energy consumption. More specifically, Arab non-nationals had a significantly higher proportion (47%) of children consuming below the EER, in comparison with nationals (37%) (data not shown).
TABLE 2.
Estimated dietary energy intake and % contribution to estimated energy needs among UAE infants and young children by age in months: FITS 20201
| Age group | |||||
|---|---|---|---|---|---|
| 0–5.9 mo | 6–11.9 mo | 12–23.9 mo | 24–35.9 mo | 36–47.9 mo | |
| Energy intake, kcal | 667.7 ± 17.5 | 840.5 ± 31.5 | 1001.3 ± 37.1 | 1135.2 ± 22.6 | 1208.5 ± 21.9 |
| EER | 429.2 ± 12.6 | 703.1 ± 12.6 | 890.0 ± 17.3 | 1125.6 ± 20.5 | 1326.2 ± 8.5 |
| %EER2 | 167.7 ± 5.3 | 122.9 ± 5.4 | 116.9 ± 5.3 | 103.4 ± 2.4 | 91.3 ± 1.6 |
| Energy inadequacy, n (%) | |||||
| % below EER | 7.1 | 30.1 | 41.1 | 46.3 | 67.5 |
| % above EER | 92.9 | 69.9 | 58.9 | 53.7 | 32.5 |
Values are means ± SEs unless otherwise indicated; n = 525. EER, estimated energy requirement; FITS, Feeding Infants and Toddler Study; UAE, United Arab Emirates.
%EER = energy intake/EER × 100.
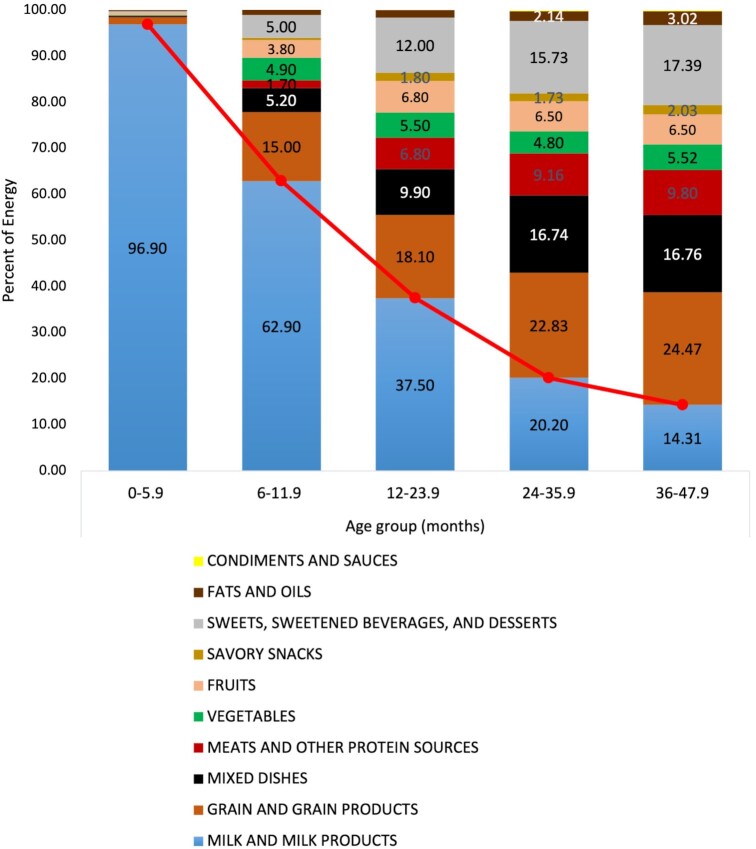
Food items, as consumed, were categorized into 10 food groups. Figure 2 shows the contribution of these food groups to daily EI in the study population. During infancy (0–5.9 mo), the predominant source of energy was milk (breast milk or infant formula), contributing 97% of EI. The contribution of milk to EI decreased with age, whereby it was estimated at 63% of EI among infants aged 6–11.9 mo, 38% among toddlers aged 12–23.9 mo, 20% among toddlers aged 24–35.9 mo, and reached 14% among preschoolers aged 36–47.9 mo. Gradually, grains and grain products became the main source of energy in the children's diet, starting from 15% (6–11.9 mo) and increasing to 18% (12–23.9 mo), 23% (24–35.9 mo), and 24% among children aged 36–47.9 mo. Similarly, the energy contributed by sweets, sweetened beverages, and desserts increased from approximately 5% at 6–11.9 mo, to 12% at 12–23.9 mo, to 16% at 24–35.9 mo, reaching 17% at 36–47.9 mo of age. Vegetables accounted for approximately 5% of EI across all age groups, while the contribution of fruits to EI increased from 4% among infants aged 6–11.9 mo to approximately 7% among older toddlers and preschoolers. The contribution of meats and mixed dishes to EI increased from 2% and 5% among children aged 6–11.9 mo, respectively, to 7% and 10% in those aged 12–23.9 mo. These 2 food groups contributed approximately 10% and 17% in the older 2 age groups, respectively. No significant differences were found between nationals and Arab non-nationals in terms of percentage contribution of any of the food groups to total EI.
FIGURE 2.
Percentage of energy from major food groups, as consumed by 0- to 47.9-mo-old infants and children in the UAE, FITS 2020 (n = 525). The red line displays the trend in the contribution of milk to energy intake among the different age groups. FITS, Feeding Infants and Toddler Study; UAE, United Arab Emirates.
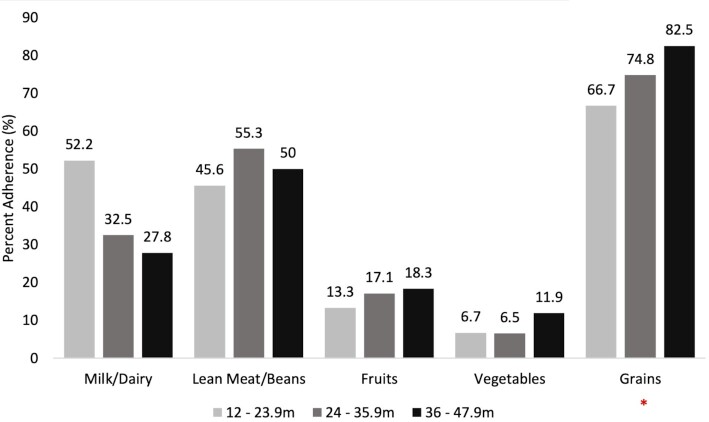
In children older than 1 y, the consumption level of the different food groups was compared with the dietary recommendations of AHA/AAP (31, 41), after the disaggregation of mixed dishes. As shown in Figure 3, there was high adherence to the recommended servings of grains (67% to 83%), with the highest proportion of adherence observed in the older age groups (P = 0.03). A significant difference was observed between nationals and Arab non-nationals, whereby nationals had a significantly higher proportion of children adhering to grain recommendations (79% vs. 68%; P = 0.03) (data not shown). Almost half of the children were adhering to the meat-and-bean intake recommendations (46–55%), whereas adherence to milk and dairy recommendations significantly decreased from 52% in 12–23.9-mo-old toddlers to 33% in 24–35.9-mo-old toddlers, reaching 28% in preschoolers. The lowest adherence levels were observed for fruits and vegetables, with the proportions of adherence being the lowest in the younger age groups.
FIGURE 3.
Adherence to food-group recommendations among UAE toddlers and preschoolers aged 12–47.9 mo (%), FITS 2020 compared with the AHA/AAP 2005 guidelines. Adherence assessment was based on the recommended servings for the various food groups by age and gender based on the AHA/AAP dietary recommendations for children. Recipes of composite foods were disaggregated prior to the assessment of adherence to dietary recommendations. *Indicates significant difference between the age groups in the proportion of children adhering to food-group recommendations. AAP, American Academy of Pediatrics; AHA, American Heart Association; FITS, Feeding Infants and Toddler Study; m, months; UAE, United Arab Emirates.
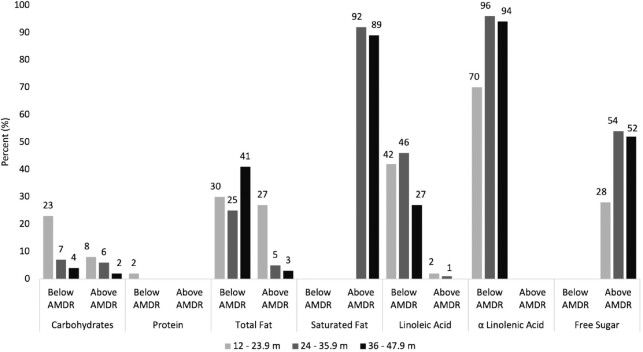
Table 3 and Figure 4 summarize the usual macro- and micronutrient intakes (from food and dietary supplements) of the study population. Macro- and micronutrient intakes and their percentage compliance with DRIs are shown in Supplemental Table 1. In total, 40% of all children consumed dietary supplements. Average intake of total fat was 52.4% of EI and 39.2% of EI among infants aged 0–5.9 mo and 6–11.9 mo, respectively, with the majority of infants (95% and 69%, respectively) exceeding the AI for total fat. As expected, fat intake was lower among older age groups (31.7–32.1% of EI), with only 5% of toddlers aged 24–35.9 mo and 3% of preschoolers exceeding the AMDR for fat. While fat consumption was within the ADMR, almost 90% of children aged 12 to 47.9 mo exceeded the WHO limit of 8% of energy from saturated fat per day. Additionally, intake of linoleic acid (18:2n–6) was below the AMDR in 42% of toddlers (12–23.9 mo), 46% of toddlers aged 24–35.9 mo, and 27% of preschoolers. As for alpha-linolenic acid (18:3n–3) , intake was below the AMDR in 70% of toddlers (12–23.9 mo), 96% of those aged 24–35.9 mo, and 94% of preschool-aged children. A significant difference was noted between nationals and Arab non-national toddlers (n = 339) in percentage adherence to total fat consumption as %EI. Nationals had a significantly higher proportion of children (39.7%) consuming below the AMDR for total fat as compared with Arab non-nationals (16.4%; P < 0.001), whereas the ercentage of children exceeding the total fat AMDR was significantly higher among Arab non-nationals (15.5% vs. 7.4%) (data not shown).
TABLE 3.
Usual macro- and micronutrient intakes (from food and supplements) in infants and children aged 0 to 47.9 mo: FITS 20201
| Nutrient intake2 | 0–5.9 mo (n = 113) | 6–11.9 mo (n = 73) | 12–23.9 mo (n = 90) | 24–35.9 mo (n = 123) | 36–47.9 mo (n = 126) |
|---|---|---|---|---|---|
| Energy, kcal/d | 667.8 ± 17.5 | 840.5 ± 31.5 | 1001.7 ± 37.1 | 1135.7 ± 22.6 | 1209.2 ± 21.9 |
| Fat, g/d | 38.4 ± 0.7 | 36.6 ± 1.6 | 39.2 ± 1.7 | 40.0 ± 0.8 | 42.4 ± 0.8 |
| Saturated fat, g/d | 15.5 ± 0.4 | 12.5 ± 0.8 | 13.7 ± 0.7 | 13.7 ± 0.4 | 14.8 ± 0.4 |
| Monounsaturated fat, g/d | 12.6 ± 0.4 | 10.3 ± 0.8 | 11.6 ± 0.6 | 12.9 ± 0.3 | 14.1 ± 0.3 |
| Polyunsaturated fat, g/d | 4.7 ± 0.2 | 4.7 ± 0.3 | 6.2 ± 0.4 | 7.8 ± 0.2 | 8.2 ± 0.2 |
| Linoleic acid, g/d | 4.6 ± 0.2 | 5.3 ± 0.3 | 6.2 ± 0.4 | 6.7 ± 0.2 | 7.6 ± 0.2 |
| ɑ-Linolenic acid, g/d | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.0 |
| DHA, g/d | 0.02 ± 0.00 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.078 ± 0.003 | 0.072 ± 0.003 |
| Carbohydrates, g/d | 66.1 ± 2.4 | 103.5 ± 4.1 | 129.3 ± 5.3 | 152.9 ± 3.4 | 163.4 ± 3.1 |
| Total sugar, g/d | 54.5 ± 1.8 | 49.9 ± 3.4 | 54.5 ± 3.0 | 56.4 ± 1.7 | 60.1 ± 1.7 |
| Free sugar, g/d | 4.7 ± 1.3 | 9.8 ± 2.1 | 18.1 ± 1.5 | 29.5 ± 1.0 | 31.2 ± 0.9 |
| Added sugars, g/d | 0.6 ± 0.2 | 5.1 ± 0.8 | 13.6 ± 1.4 | 22.8 ± 0.9 | 25.4 ± 0.9 |
| Protein, g/d | 13.2 ± 0.6 | 21.8 ± 1.0 | 32.9 ± 1.5 | 42.9 ± 0.8 | 44.7 ± 0.8 |
| Dietary fiber, g/d | 0.2 ± 0.1 | 3.5 ± 0.3 | 6.5 ± 0.5 | 8.5 ± 0.3 | 9.7 ± 0.3 |
| Total fat, % | 52.4 ± 0.5 | 39.2 ± 0.9 | 35.3 ± 0.8 | 32.1 ± 0.4 | 31.7 ± 0.4 |
| Saturated fat, % | 21.5 ± 0.5 | 13.4 ± 0.7 | 12.5 ± 0.5 | 10.9 ± 0.2 | 11.0 ± 0.2 |
| Linoleic acid, % | — | — | 5.4 ± 0.2 | 5.3 ± 0.1 | 5.7 ± 0.1 |
| ɑ-Linolenic acid, % | — | — | 0.5 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 |
| Carbohydrate, % | 39.6 ± 0.8 | 49.6 ± 1.1 | 51.6 ± 1.0 | 53.9 ± 0.6 | 54.2 ± 0.5 |
| Total sugar, % | 33.5 ± 0.9 | 24.1 ± 0.4 | 22.3 ± 1.0 | 20.1 ± 0.6 | 20.0 ± 0.5 |
| Free sugar, % | 2.3 ± 0.5 | 4.4 ± 0.7 | 7.4 ± 0.5 | 10.6 ± 0.3 | 10.5 ± 0.3 |
| Added sugars, % | 0.3 ± 0.1 | 2.6 ± 0.4 | 5.5 ± 0.7 | 8.2 ± 0.3 | 8.5 ± 0.3 |
| Protein, % | 7.6 ± 0.2 | 10.2 ± 0.3 | 13.1 ± 0.4 | 15.3 ± 0.2 | 14.9 ± 0.2 |
| Vitamin C, mg/d | 60.1 ± 2.8 | 71.1 ± 3.8 | 66.1 ± 3.5 | 72.3 ± 2.8 | 71.9 ± 2.8 |
| Thiamin, mg/d | 0.4 ± 0.0 | 0.7 ± 0.0 | 0.8 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| Riboflavin, mg/d | 0.7 ± 0.0 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.0 | 1.2 ± 0.0 |
| Niacin, mg/d | 3.5 ± 0.2 | 6.0 ± 0.5 | 10.4 ± 0.8 | 12.2 ± 0.4 | 13.1 ± 0.4 |
| Vitamin B-6, mg/d | 0.2 ± 0.0 | 0.6 ± 0.0 | 0.8 ± 0.0 | 1.1 ± 0.0 | 1.2 ± 0.0 |
| Folate, μ/d | 94.5 ± 7.0 | 176.0 ± 10.6 | 224.1 ± 3.0 | 293.4 ± 9.1 | 318.0 ± 8.7 |
| Vitamin B-12, μ/d | 0.9 ± 0.1 | 1.2 ± 0.1 | 2.0 ± 0.2 | 3.0 ± 0.2 | 3.0 ± 0.2 |
| Vitamin D, μ/d | 10.8 ± 0.7 | 12.2 ± 0.9 | 10.2 ± 0.8 | 5.8 ± 0.9 | 4.6 ± 0.4 |
| Vitamin A, RE | 584.6 ± 14.6 | 596.8 ± 32.3 | 505.5 ± 27.7 | 403.2 ± 30.7 | 411.6 ± 23.3 |
| Vitamin K, μ/d | 11.8 ± 2.1 | 25.2 ± 3.2 | 32.3 ± 3.3 | 45.2 ± 1.8 | 50.9 ± 1.7 |
| Calcium, mg/d | 427.9 ± 22.6 | 571.1 ± 32.9 | 649.4 ± 33.2 | 649.3 ± 14.7 | 655.7 ± 14.5 |
| Phosphorus, mg/d | 214.1 ± 11.0 | 384.1 ± 23.5 | 703.7 ± 39.4 | 737.3 ± 10.3 | 755.5 ± 10.8 |
| Iron, mg/d | 4.0 ± 0.5 | 10.3 ± 1.0 | 8.9 ± 0.7 | 9.1 ± 0.5 | 9.4 ± 0.4 |
| Zinc, mg/d | 2.2 ± 0.2 | 3.7 ± 0.3 | 4.4 ± 0.3 | 4.9 ± 0.1 | 5.3 ± 0.1 |
| Sodium, mg/d | 240.9 ± 16.6 | 445.7 ± 31.2 | 920.5 ± 56.9 | 1240.9 ± 37.6 | 1350.1 ± 32.8 |
| Potassium, mg/d | 620.1 ± 26.7 | 1047.3 ± 47.5 | 1364.3 ± 61.1 | 1548.3 ± 20.3 | 1593.3 ± 22.9 |
Values are means ± SEs; n = 525. RE, retinol equivalents.
Estimated usual intakes calculated from the PC-side software (Iowa State University).
FIGURE 4.
Proportion of UAE infants and children ages 0–47.9 mo consuming below or above the AMDR for macronutrients, FITS 2020 (n = 525). AMDR, Acceptable Macronutrient Distribution Range; FITS, Feeding Infants and Toddler Study; m, months; UAE, United Arab Emirates.
Approximately half of the 0–5.9-mo-old (51%) and 6–11.9-mo-old (59%) infants had intakes above the AI for carbohydrates. In children aged 1 y and above, the proportions of those consuming above the AMDR for carbohydrates ranged from only 2% to 8%. The proportions of children consuming below the AMDR for carbohydrates were 4% and 7% in preschoolers and toddlers aged 24–35.9 mo, respectively, reaching 23% in younger toddlers aged 12–23.9 mo. A significant difference was noted between nationals and Arab non-nationals in percentage adherence to carbohydrate consumption, whereby a higher proportion of Arab non-nationals had intakes below the AMDR (9.3%) as compared with nationals (5.1%) (data not shown). Almost all toddlers and preschoolers aged 24–47.9 mo had dietary fiber intakes below the AI. A high proportion of children were consuming free sugars above the upper limits of 5% and 10% of energy set by the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) and WHO for under 2- and above 2-y-old children, respectively (12, 42). Specifically, around one-fifth of infants aged 6–11.9 mo and more than half of toddlers and preschoolers exceeded the free-sugar intake limits, respectively. Adequate protein intake was observed across all age groups in the study population, whereby 89% of infants aged 0–5.9 mo consumed above the AI, and the majority of older children were consuming within the AMDR.
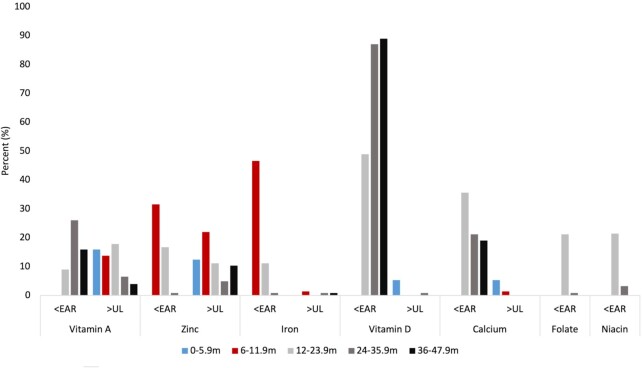
Analysis of adherence to micronutrient intake recommendations in the sample is shown in Supplemental Table 1 and Figure 5. The data revealed a low intake of vitamin D among all age groups, whereby most toddlers and preschoolers aged 24–47.9 mo were consuming below the vitamin D EAR value. With regard to vitamin A, intake was below the EAR in 9%, 26%, and 16% of children aged 12–23.9 mo, 24–35.9 mo, and 36–47.9 mo, respectively. In contrast, approximately 15% of infants aged 0–11.9 mo were consuming above the UL for vitamin A.
FIGURE 5.
Proportion of UAE infants and children aged 0–47.9 mo not adhering to DRIs for micronutrients, FITS 2020 (n = 525). EAR, Estimated Average Requirement; FITS, Feeding Infants and Toddler Study; m, months; UAE, United Arab Emirates; UL, Tolerable Upper Intake Level.
Inadequate intake of zinc was noted among infants aged 6–11.9 mo, whereby 32% were consuming below the EAR value, with the proportion decreasing with age, to 17% among toddlers aged 12–23.9 mo. Simultaneously, 22% of infants (6–11.9 mo), 11% of toddlers (12–23.9 mo), 5% of toddlers aged 24–35.9 mo, and 10% of preschoolers were consuming above the UL for zinc. Inadequate intakes of iron and folate were only noted in specific age groups. For iron, inadequate intake was found among 46% of infants aged 6–11.9 mo. A significant difference was noted between nationals and Arab non-nationals, whereby 5.7% of nationals had an iron intake below the EAR compared with 15% of Arab non-nationals. With regard to folate, inadequate intake was found among 21% of toddlers aged 12–23.9 mo.
Inadequate intake of calcium (below the EAR) was also noted among 36% of toddlers (12–23.9 mo), with the prevalence decreasing with age. In addition, a small percentage of children adhered to potassium intake recommendation, whereby only 0.8% and 4% of children aged 24–35.9 mo and 36–47.9 mo reached the AI, respectively. Conversely, a high intake of sodium was noted across all age groups with around 48% of toddlers and preschoolers (12–47.9 mo) exceeding the sodium Chronic Disease Risk Reduction (CDRR) intake level of 1200 mg for 1–3.9-y-old children (43).
Discussion
To our knowledge, this is the first study to provide a comprehensive overview of the nutritional status and adequacy of dietary and nutrient intakes among children under 4 y of age in the UAE. The study findings suggest the presence of a triple burden of malnutrition, including undernutrition, overweight, and micronutrient inadequacies in this age group, while describing the transition of dietary intake from a predominantly milk-based diet in infancy to a more diverse family diet in older age groups. The study findings highlight suboptimal food-consumption practices characterized by low consumption of fruits and vegetables accompanied by high intakes of sweets and SSBs. A high percentage of children also had a high consumption of saturated fat and free sugars coupled with a very low intake of dietary fiber and essential fatty acids.
Anthropometric measurements of the study population showed a relatively high proportion of stunted and wasted children, exceeding those reported from neighboring countries such as Saudi Arabia and Kuwait (44). These findings are of concern given that adequate nutritional status in early childhood is essential to ensure proper growth and development, and to prevent chronic diseases later in life (45). A poor nutritional status among children has been also shown to impact cognition and school performance, leading to poor productivity later in life and a higher risk of poverty (45). There is therefore a need to better elucidate the determinants of child undernutrition in the UAE, which could include socioeconomic factors, maternal educational level, food availability, access to health services, exposure to infectious diseases, inadequate exclusive breastfeeding and complementary feeding practices, insufficient energy and micronutrient intakes, or low birth weight (LBW) (46). Our study did not aim to examine the predictors of child undernutrition in the UAE, but our results showed that, except for infants aged below 6 mo, the proportions of children who had caloric intake below the EER were relatively high, ranging between 30% and 67%, an observation that may at least partially explain the prevalent acute malnutrition (wasting). In addition, stunting, which reflects a more chronic type of undernutrition, may result from failure to meet micronutrient requirements (47), a fact that was also observed in our study. In addition, the prevalence of LBW, a significant predictor of child stunting and wasting (48), was previously shown to be high in the UAE and to exceed the prevalence observed in neighboring countries. According to the WHO, the prevalence of LBW in the UAE was 12.7% in 2015, compared to 9.9% in Kuwait and 7.3% in Qatar (49). Further studies are needed to better understand the determinants of stunting in the UAE, especially that, in our study, nationals (who had a higher socioeconomic status) had a significantly higher prevalence of stunting compared with non-nationals.
In parallel with undernutrition, this study showed a high prevalence of risk of overweight, overweight, and obesity. The coexistence of under- and overnutrition, as highlighted in our study, is not unique to the UAE and may possibly be driven by an accelerated catch-up growth in LBW children, as well as by environmental and socioeconomic drivers (50, 51). It is important to note that UAE is ranked as 15th worldwide for age-adjusted comparative prevalence of diabetes mellitus, which has obesity as a strong predisposing factor (52).
Conversely, the reported mean EIs of the study participants exceeded the EERs by a considerable margin in all age groups, which may either reflect actual overconsumption among study participants or alternatively suggest a potential overreporting of intakes by caregivers (51). In fact, overreporting may occur given the subconscious inclination among parents to portray their child as eating well (53) and given the potential difficulties for caregivers to estimate the amount of food that was actually consumed by the child rather than the amount that was offered: food losses associated with spillage or spitting up, may be important, particularly in younger children (53). In our study, the proportion of children consuming above EERs in the UAE (32.5% to 92.9%) was higher than those reported from developed countries such as the United States, where low percentages of children consumed above EERs (8%) (51). This finding is significant as overconsumption of energy is a principal cause of excessive weight gain among children and may thus be contributing to the growing epidemic of childhood and adult obesity in the UAE (54).
Assessment of the contribution of food groups to EI from infancy to preschool age showed a transition in food-consumption patterns with age, similar to that observed in other studies conducted in Europe and the United States (55, 56). Our study showed that milk and milk products remained the highest contributor to EI up to 24 mo of age, which is in alignment with the WHO recommendations (57). However, the intake of this food group rapidly decreased in older age groups. A study by Wiley (58) assessed the relation between milk consumption and height among children in the United States, based on data stemming from the NHANES 1999–2002. Results revealed that child milk consumption was a significant predictor of height at 12–18 y and was associated with a lower risk of stunting. In our sample of UAE children, grains and grain products were one of the highest contributors to EI across all age groups; however, this was accompanied by a very low consumption of dietary fiber, indicating that grains are consumed in their refined state. A diet rich in dietary fiber during childhood is important as it may promote a healthy bowel function and decrease the risk of developing diabetes and obesity later in life (59). The study also revealed a high contribution of sweets and SSBs to EI, which was higher in older age groups, especially in preschoolers aged 35 to 47.9 mo. This is worrying, as research has shown that early consumption of sweets and SSBs displaces nutrient-dense food (such as fruits, vegetables, and whole grains) and compromises the nutritional status of children (60). More importantly, early consumption of sweets and SSBs increases the likelihood of their consumption in later childhood and is correlated with overweight and increased adiposity among infants, toddlers, and preschool children (14, 60, 61). A meta-analysis by the WHO showed that decreased intake of SSBs significantly reduced body weight (0.80 kg), whereas weight gain was associated with increased consumption of SSBs (62). Similar data were reported from the United States where SSB consumption during infancy was associated with higher odds of obesity at 6 y (63).
The AHA/AAP food recommendations were used to assess the adequacy of food-group intakes among UAE toddlers and preschool children aged between 12 and 47.9 mo. Findings highlight an alarmingly low consumption of fruits and vegetables among UAE toddlers and preschoolers (only 13% to 18% of children aged 12–23.9 mo and 36–47.9 mo were adherent to fruit intake recommendations, respectively and 7% to 12% of children aged 12–23.9 mo and 36–47.9 were adherent to vegetable intake recommendations, respectively)). A low intake of fruits and vegetables may increase the odds of developing adult NCDs and micronutrient deficiencies (64). Additionally, early intake of a variety of fruits and vegetables has been reported as a factor predicting the likelihood of their consumption in late childhood (65). Our study also showed a significant decrease in the proportion of children adhering to the dietary recommendations for milk/dairy, from 52% in younger children to 28% in older ones. Adequate consumption of milk and milk products is essential for optimal child growth and development as milk contains high levels of energy, proteins, and micro- and macronutrients that are crucial for child bone growth and development (55, 56).
Similar to the US FITS, macronutrient intake data in our study showed that a high proportion of children over 12 mo of age were consuming below the AMDR for fat. However, this low intake was coupled with a high proportion of children (89–92%) exceeding the recommendations for saturated fat (set at <8% of EI by the WHO) (66). The observed proportions of children exceeding the saturated-fat WHO limit are considerably higher than those reported by the 2016 US FITS. This is of concern as a large body of evidence has shown that a high intake of saturated fat during childhood is positively associated with an increased risk of developing cardiovascular disease, hypertension, and elevated blood cholesterol concentrations later in life (67, 68). Increased intake of saturated fat was also correlated with weight gain and obesity during late childhood, which may persist into adulthood (69). In addition to the aforementioned health consequences, a low intake of fat, coupled with high intakes of saturated fat, puts children at risk of suboptimal intakes of essential fatty acids, linolenic and linoleic fatty acids. Indeed, low intakes of essential fatty acids were observed among children participating in our study, when optimal intakes of such nutrients are pivotal during childhood for proper cognitive and nervous system development (70).
With regard to adherence to protein and carbohydrate intake recommendations, the majority of toddlers and children in the UAE were consuming within the AMDR recommendations, except for fiber, where only 0.8–2.4% of children (aged 12 –47.9 mo) achieved the AI for this nutrient. This may be the result of the observed low intake of fruits, vegetables, and whole grains. Our study also showed a high contribution of free sugars to total EI, whereby infants and preschoolers exceeded the recommendation of 5% to 10% of energy from free sugars as recommended by ESPGHAN and the WHO (12, 42). This high intake of free sugars during childhood was suggested to displace the intake of vital nutrients and result in micronutrient deficiencies, obesity, and NCDs in adulthood (60, 71).
Concerning micronutrient intakes, the study revealed a low intake of iron among infants aged 6–11.9 mo, a finding that is in line with that reported by the 2016 FITS in the United States (18% and 7% of infants and toddlers aged 6–11.9 and 12–23.9 mo, respectively) (72). Considering that dietary iron inadequacy is the most common cause of iron deficiency anemia among children (73), particularly in the MENA region (74), the observed low intakes of iron among children in the UAE are of concern. Anemia and iron deficiency during infancy increase the risk of mortality, and are associated with impaired cognitive development, inadequate behavioral development, poor school performance, and decreased productivity later in life (75). Additionally, very low vitamin D intakes were observed among children participating in our study, which is in line with findings described by other countries in the region such as Saudi Arabia (KSA) and Lebanon (74), as well as other parts of the world such as Canada and the United Kingdom (76, 77). Ensuring an adequate vitamin D intake among children is pivotal since vitamin D has many important biological and physiological functions, and contributes to the prevention of rickets, growth failure, and respiratory infections (78, 79). Vitamin D deficiency is widespread in the MENA region and hence oral vitamin D supplementation for children, including breastfed infants, those with inadequate sun exposure, inadequate time spent outdoors, or obesity, is highly recommended by pediatric authorities (80). On the other hand, a high intake of vitamin A was observed, whereby intakes exceeded the UL in 4–18% of children in the study sample. In the UAE, foods such as milk and oil have been fortified with vitamin A to prevent vitamin A deficiency; hence, the observed high intake in some children may be the result of the consumption of both dietary supplements and vitamin A–fortified foods (81). Although an intake above the UL was observed, a nonnegligible proportion of children (7–16%) were still consuming below the EAR for this vitamin. To prevent micronutrient deficiencies among children, the AAP has recommended the use of vitamin D and iron supplements among infants (72, 82) and the WHO has proposed the supplementation of infants with vitamin A (83).
The study findings have also shown that a high proportion of older children had intakes of zinc below the EAR. This is concerning as suboptimal intakes of zinc may lead to deficiencies, which are associated with impaired child growth, stunting, increased morbidity and mortality, and low weight gain in children less than 5 y of age (84). Conversely, and similar to the US FITS study (72), the current study also revealed intakes of zinc above the UL. It is important to note that the UL for zinc has been repeatedly criticized as it is based on only 1 study and it is only slightly higher than the RDA (85). However, given that high intakes of zinc may result in an impaired immune function among children and may cause anemia (86, 87), it is important to further understand the factors contributing to excessive zinc intake in this population. Low intakes of folate were also observed in our study, putting children at risk for developing megaloblastic anemia, which is one of the hallmarks of folate deficiency (88). The observed results on sodium intake showed that a considerable proportion of children had high intakes compared with the recommendations. This is similar to what was reported by the US FITS (72) and highlights potential health risks since excessive exposure to sodium in childhood may facilitate the development of a preference for salt that persists later in life (89), and may lead to increased blood pressure in salt-sensitive individuals, even in childhood (90).
The strengths of this study include the fact that we have assessed total usual nutrient intakes from both dietary supplements and foods to assess dietary adequacy among children. We have also obtained measurements of anthropometric characteristics using standardized protocols rather than relying on self-reporting. For comparison purposes, our study included a sample of Arab non-nationals in addition to nationals, at a ratio of 1:2. The results suggest that there may be significant differences in nutritional status and food-consumption practices between these groups, and these differences may be linked to socioeconomic disparities between the groups. Thus, although the study sample was small and may not allow for the generalizability of these differences, the study findings call for more targeted research investigating the diet and nutritional status of various population groups living in the UAE.
The findings of our study should also be considered in light of the following limitations. First, a potential limitation is the use of the 24-HR for dietary assessment, which may be associated with reporting bias by caregivers (91). However, despite the well-known limitations of the 24-HR approach, such as its reliance on memory and potential day-to-day variation, the 24-HR may give accurate estimates of EI at the population level (92). In addition, in our study, dietary information was collected using the multiple pass approach, which helps in reducing the limitations of the 24-HR (93). All 24-HRs were administered by research nutritionists who had participated in extensive training prior to data collection in order to decrease interviewer errors. Second, in our study, we did not obtain information on physical activity or sedentary behavior, which may have impacted EER estimations in 3–4-y-old children. Third, although this study covers 3 major emirates in the UAE, which are home to 85% of the country's total population, the exclusion of the remaining 15% in 4 emirates would constitute a limitation.
In conclusion, the study provided a comprehensive overview of the nutritional status, dietary intakes, dietary adequacy, and the transition in diets from infancy to preschool age in the UAE. The study findings suggest the presence of a triple burden of malnutrition including undernutrition, overweight, and micronutrient inadequacies in this age group. The study showed poor adherence to the recommendations for nutrient-dense foods such as fruits and vegetables and milk/dairy. It also highlighted high intakes of sweets and SSBs, saturated fat, and free sugars, coupled with low intakes of dietary fiber, essential fatty acids, and suboptimal intakes of micronutrients (vitamin D, zinc, iron). Our findings provide the necessary evidence for revisiting policies and strategies to improve the quality of diets among infants and young children in the UAE, thus mitigating the burden of malnutrition, obesity, and NCDs.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Damian Mehers for the data entry software development. We also acknowledge Alicia L Carriquiry for her assistance with the PC-Side software. The authors’ responsibilities were as follows—NCH: provided project administration and conceptualization and assisted in data interpretation; LMN and FAN: contributed to the writing of the manuscript and interpretation of the results and provided essential feedback on different drafts; LNC: contributed to the writing of the manuscript; LICI, ASAD, HIA, WHA, and MNM: conducted the research, data collection, data entry, data cleaning, and interpretation of results; FSC: statistically analyzed and reported the data; SAK: provided significant contribution to sampling of study population and statistical analysis of results; LMO and ANK: contributed to the work's conception and provided essential feedback on different drafts; and all authors read and approved the final manuscript.
Notes
Supported by Nestlé Research (Societé des Produits Nestlé S.A.), Lausanne, Switzerland, through a written contract with the American University of Beirut in Lebanon, and written subcontracts with the United Arab Emirates University, the University of Sharjah, and Tathqeef Health Treatment Undertakings Services in the United Arab Emirates.
Author disclosures: LMO is an employee of Société des Produits Nestlé, S.A., Nestlé Research. ANK is a consultant for Nestlé Research. All the other authors report no conflicts of interest. The funding agency had no involvement in the training, implementation of the study, or the collection, analysis, and interpretation of the data.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: AAP, American Academy of Pediatrics; AHA, American Heart Association; AI, Adequate Intake; AMDR, Acceptable Macronutrient Distribution Range; BAZ, BMI-for-age z score; EAR, Estimated Average Requirement; EER, estimated energy requirement; EI, energy intake; ESPGHAN, European Society for Paediatric Gastroenterology Hepatology and Nutrition; FITS, Feeding Infants and Toddler Study; HAZ, height-for-age z score; LBW, low birth weight; MENA, Middle East and North Africa; MUAC, midupper arm circumference; NCD, noncommunicable disease; PAL, physical activity level; SSB, sugar-sweetened beverage; UAE, United Arab Emirates; UL, Tolerable Upper Intake Level; 24-HR, 24-h dietary recall.
Contributor Information
Lara M Nasreddine, Department of Nutrition and Food Sciences, Faculty of Agricultural and Food Sciences, American University of Beirut, Riad El Solh, Beirut, Lebanon.
Farah A Naja, Department of Clinical Nutrition and Dietetics, College of Health Sciences, University of Sharjah, Sharjah, United Arab Emirates.
Nahla C Hwalla, Department of Nutrition and Food Sciences, Faculty of Agricultural and Food Sciences, American University of Beirut, Riad El Solh, Beirut, Lebanon.
Habiba I Ali, Department of Nutrition and Health, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, United Arab Emirates.
Maysm N Mohamad, Department of Nutrition and Health, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, United Arab Emirates.
Fatima Al Zahraa S Chokor, Department of Nutrition and Food Sciences, Faculty of Agricultural and Food Sciences, American University of Beirut, Riad El Solh, Beirut, Lebanon.
Lara N Chehade, Department of Nutrition and Food Sciences, Faculty of Agricultural and Food Sciences, American University of Beirut, Riad El Solh, Beirut, Lebanon.
Lynda M O'Neill, Nestlé Institute of Health Sciences, Nestlé Research Center, Société des Produits Nestlé S.A., Vers-chez-les-Blanc, Lausanne, Switzerland.
Samer A Kharroubi, Department of Nutrition and Food Sciences, Faculty of Agricultural and Food Sciences, American University of Beirut, Riad El Solh, Beirut, Lebanon.
Wafaa H Ayesh, Public Health Protection Department, Dubai Health Authority, Dubai, United Arab Emirates.
Amira N Kassis, Whiteboard Nutrition Science, Beaconsfield, Quebec, Canada.
Leila I Cheikh Ismail, Email: lcheikhismail@sharjah.ac.ae, Department of Clinical Nutrition and Dietetics, College of Health Sciences, University of Sharjah, Sharjah, United Arab Emirates; Nuffield Department of Women's and Reproductive Health, University of Oxford, Oxford, United Kingdom.
Ayesha S Al Dhaheri, Email: ayesha_aldhaheri@uaeu.ac.ae, Department of Nutrition and Health, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, United Arab Emirates.
Data Availability
Data described in the manuscript, codebook, and analytic code will be made available upon request pending application and approval by the investigators.
References
- 1. Shenggen F. Overcoming the triple burden of malnutrition in the MENA region. [Internet]. International Food Policy Research Institute; 2015; [cited 2021 Jan 5]. Available from: https://www.ifpri.org/blog/overcoming-triple-burden-malnutrition-mena-region%E2%80%A8. [Google Scholar]
- 2. The World Bank . World urbanization prospects—population division. [Internet]. The World Bank Group; 2018; [cited 2022 Mar 28]. Available from: https://data.worldbank.org/indicator/SP.URB.TOTL.IN.ZS?locations=AE. [Google Scholar]
- 3. Itani L, Radwan H, Hashim M, Hasan H, Obaid RS, Ghazal HAet al. Dietary patterns and their associations with gestational weight gain in the United Arab Emirates: results from the MISC cohort. Nutr J. 2020;19(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moghames P, Hammami N, Hwalla N, Yazbeck N, Shoaib H, Nasreddine Let al. Validity and reliability of a food frequency questionnaire to estimate dietary intake among Lebanese children. Nutr J. 2015;15(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . Marketing of foods high in fat, salt and sugar to children: update 2012–2013. [Internet]. Copenhagen (Denmark): WHO Regional Office for Europe; 2013; [cited 2021 Jan 5]. Available from: . [Google Scholar]
- 6. De Onis M, Blössner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92(5):1257–64. [DOI] [PubMed] [Google Scholar]
- 7. Miller CJ, Dunn EV, Abdouni SF, Shaheen HM, Ullah MS. Factors associated with iron depletion and iron deficiency anemia among Arabic preschool children of the United Arab Emirates. Saudi Med J. 2004;25(7):843–7. [PubMed] [Google Scholar]
- 8. Bhutta ZA, Berkley JA, Bandsma RH, Kerac M, Trehan I, Briend A. Severe childhood malnutrition. Nat Rev Dis Primers. 2017;3(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123(4):1177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Birch LL, Doub AE. Learning to eat: birth to age 2y. Am J Clin Nutr. 2014;99(3):723S–8S. [DOI] [PubMed] [Google Scholar]
- 11. Luque V, Escribano J, Closa-Monasterolo R, Zaragoza-Jordana M, Ferré N, Grote Vet al. Unhealthy dietary patterns established in infancy track to mid-childhood: the EU Childhood Obesity Project. J Nutr. 2018;148(5):752–9. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization . Diet, nutrition and the prevention of chronic diseases: report of a joint WHO/FAO expert consultation. [Internet]. Geneva (Switzerland): WHO; 2003; [cited 2021 Sep 8]. Available from:http://apps.who.int/iris/bitstream/handle/10665/42665/WHO_TRS_916.pdf?sequence=1. [Google Scholar]
- 13. Grimm KA, Kim SA, Yaroch AL, Scanlon KS. Fruit and vegetable intake during infancy and early childhood. Pediatrics. 2014;134(Suppl 1):S63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park S, Pan L, Sherry B, Li R. The association of sugar-sweetened beverage intake during infancy with sugar-sweetened beverage intake at 6 years of age. Pediatrics. 2014;134(Suppl 1):S56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwartz C, Scholtens PA, Lalanne A, Weenen H, Nicklaus S. Development of healthy eating habits early in life. Review of recent evidence and selected guidelines. Appetite. 2011;57(3):796–807. [DOI] [PubMed] [Google Scholar]
- 16. Hauck FR, Thompson JM, Tanabe KO, Moon RY, Vennemann MM. Breastfeeding and reduced risk of sudden infant death syndrome: a meta-analysis. Pediatrics. 2011;128(1):103–10. [DOI] [PubMed] [Google Scholar]
- 17. The United Arab Emirates' Government portal . UAE fact sheet: the official portal of the UAE government. [Internet]. United Arab Emirates; 2022; [cited 2022 Mar 26]. Available from: https://u.ae/en/about-the-uae/fact-sheet. [Google Scholar]
- 18. US Central Intelligence Agency . The world factbook. [Internet]. Washington (DC): Central Intelligence Agency; [cited 2021 Sep 8]. Available from: https://www.cia.gov/the-world-factbook/countries/united-arab-emirates/. [Google Scholar]
- 19. Anater AS, Catellier DJ, Levine BA, Krotki KP, Jacquier EF, Eldridge ALet al. The Feeding Infants and Toddlers Study (FITS) 2016: study design and methods. J Nutr. 2018;148(Suppl 3):1516S–24S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization . WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. [Internet]. Geneva (Switzerland): WHO; 2006; [cited 2021 Jun 5]. Available from: https://apps.who.int/iris/handle/10665/43413. [Google Scholar]
- 21. World Health Organization . Guideline: assessing and managing children at primary health-care facilities to prevent overweight and obesity in the context of the double burden of malnutrition: updates for the Integrated Management of Childhood Illness (IMCI). [Internet]. Geneva (Switzerland): WHO; 2017; [cited 2021 Jun 5]. Available from: https://www.who.int/publications/i/item/9789241550123. [PubMed] [Google Scholar]
- 22. The United Arab Emirates' Government portal . Children's health. [Internet]. United Arab Emirates; 2022; [cited 2022 Mar 26]. Available from: https://u.ae/en/information-and-services/health-and-fitness/health-of-vulnerable-groups/childrenshealth#:∼:text=The%20UAE%20Government%20mandates%20vaccinations,DPT%3A%20Diphtheria%2C%20Pertussis%20and%20Tetanus. [Google Scholar]
- 23. World Health Organization . The WHO Anthro survey analyser. [Internet]. Geneva (Switzerland): WHO; 2018; [cited 2019 Jun 10]. Available from: https://worldhealthorg.shinyapps.io/anthro/. [Google Scholar]
- 24. NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults (US) . Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Bethesda (MD): National Heart, Lung, and Blood Institute; 1998. [Google Scholar]
- 25. Steinfeldt L, Anand J, Murayi T. Food reporting patterns in the USDA automated multiple-pass method. Procedia Food Sci. 2013;2:145–56. [Google Scholar]
- 26. Nasreddine L, Chamieh MC, Ayoub J, Hwalla N, Sibai A-M, Naja F. Sex disparities in dietary intake across the lifespan: the case of Lebanon. Nutr J. 2020;19(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abou-Rizk J, Jeremias T, Cocuz G, Nasreddine L, Jomaa L, Hwalla Net al. Food insecurity, low dietary diversity and poor mental health among Syrian refugee mothers living in vulnerable areas of Greater Beirut, Lebanon. Br J Nutr. 2021:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jomaa LH, Naja FA, Kharroubi SA, Diab-El-Harake MH, Hwalla NC. Food insecurity is associated with compromised dietary intake and quality among Lebanese mothers: findings from a national cross-sectional study. Public Health Nutr. 2020;23(15):2687–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Posner BM, Smigelski C, Duggal A, Morgan JL, Cobb J, Cupples LA. Validation of two-dimensional models for estimation of portion size in nutrition research. J Am Diet Assoc. 1992;92(6):738–41. [PubMed] [Google Scholar]
- 30. Wilson AC, Forsyth JS, Greene SA, Irvine L, Hau C, Howie PW. Relation of infant diet to childhood health: seven year follow up of cohort of children in Dundee infant feeding study. BMJ. 1998;316(7124):21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gidding SS, Dennison BA, Birch LL, Daniels SR, Gillman MW, Lichtenstein AHet al. Dietary recommendations for children and adolescents: a guide for practitioners. Pediatrics. 2006;117(2):544–59. [DOI] [PubMed] [Google Scholar]
- 32. Pellet P, Shadarevian S. Food composition. Food composition tables for use in the Middle East. 3rd ed.Beirut (Lebanon): AUB Press; 2013. [Google Scholar]
- 33. Al Dhaheri AS, Al Ma'awali AK, Laleye LC, Washi SA, Jarrar AH, Al Meqbaal FTet al. The effect of nutritional composition on the glycemic index and glycemic load values of selected Emirati foods. BMC Nutr. 2015;1(1):1–8. [Google Scholar]
- 34. Al Dhaheri AS, Henry CJK, Mohamad MN, Ohuma EO, Ismail LC, Al Meqbaali FTet al. Glycaemic index and glycaemic load values of commonly consumed foods in the United Arab Emirates. Br J Nutr. 2017;117(8):1110–7. [DOI] [PubMed] [Google Scholar]
- 35. Briefel RR, Kalb LM, Condon E, Deming DM, Clusen NA, Fox MKet al. The Feeding Infants and Toddlers Study 2008: study design and methods. J Am Diet Assoc. 2010;110(12):S16–26. [DOI] [PubMed] [Google Scholar]
- 36. Otten JJ, Hellwig JP, Meyers LD. Dietary Reference Intakes: the essential guide to nutrient requirements. Washington (DC): National Academies Press; 2006. [Google Scholar]
- 37. Galobardes B, Shaw M, Lawlor DA, Lynch JW. Indicators of socioeconomic position (part 2). J Epidemiol Comm Health. 2006;60(2):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Taha Z, Garemo M, Nanda J. Patterns of breastfeeding practices among infants and young children in Abu Dhabi, United Arab Emirates. Int Breastfeed J. 2018;13(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bailey RL, Dodd KW, Gahche JJ, Dwyer JT, Cowan AE, Jun Set al. Best practices for dietary supplement assessment and estimation of total usual nutrient intakes in population-level research and monitoring. J Nutr. 2019;149(2):181–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Murphy SP, Guenther PM, Kretsch MJ. Using the Dietary Reference Intakes to assess intakes of groups: pitfalls to avoid. J Am Diet Assoc. 2006;106(10):1550–3. [DOI] [PubMed] [Google Scholar]
- 41. American Heart Association . Dietary recommendations for healthy children. [Internet]. Dallas (TX): American Heart Association; 2018; [cited 2020 Mar 17]. Available from: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/dietary-recommendations-for-healthy-children. [Google Scholar]
- 42. Fidler Mis N, Braegger C, Bronsky J, Campoy C, Domellöf M, Embleton NDet al. Sugar in infants, children and adolescents: a position paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2017;65(6):681–96. [DOI] [PubMed] [Google Scholar]
- 43. National Academies of Sciences Engineering Medicine . Dietary Reference Intakes for sodium and potassium. Washington (DC): National Academies Press; 2019. [PubMed] [Google Scholar]
- 44. United Nations International Children's Emergency Fund. The state of the world's children 2019: children, food and nutrition: growing well in a changing world. [Internet]. New York: UNICEF; 2019; [cited 2021 Jul 27]. Available from:https://www.unicef.org/media/60806/file/SOWC-2019.pdf. [Google Scholar]
- 45. Farrag NS, Cheskin LJ, Farag MK. A systematic review of childhood obesity in the Middle East and North Africa (MENA) region: prevalence and risk factors meta-analysis. Adv Pediatr Nutr. 2017;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Motbainor A, Taye A. Wasting in under five children is significantly varied between rice producing and non-producing households of Libokemkem district, Amhara region, Ethiopia. BMC Pediatr. 2019;19(1):300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Branca F, Ferrari M. Impact of micronutrient deficiencies on growth: the stunting syndrome. Ann Nutr Metab. 2002;46(1):8–17. [DOI] [PubMed] [Google Scholar]
- 48. Abbas F, Kumar R, Mahmood T, Somrongthong R. Impact of children born with low birth weight on stunting and wasting in Sindh province of Pakistan: a propensity score matching approach. Sci Rep. 2021;11(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. United Nations International Children's Emergency Fund; World Health Organization . Low birthweight estimates: levels and trends 2000–2015. [Internet]. Geneva (Switzerland): WHO; 2019; [cited 2021 Aug 8]. Available from: https://www.unicef.org/media/53711/file/UNICEF-WHO%20Low%20birthweight%20estimates%202019%20.pdf. [Google Scholar]
- 50. Jain V, Singhal A. Catch up growth in low birth weight infants: striking a healthy balance. Rev Endocr Metab Disord. 2012;13(2):141–7. [DOI] [PubMed] [Google Scholar]
- 51. Butte NF, Fox MK, Briefel RR, Siega-Riz AM, Dwyer JT, Deming DMet al. Nutrient intakes of US infants, toddlers, and preschoolers meet or exceed Dietary Reference Intakes. J Am Diet Assoc. 2010;110(12):S27–37. [DOI] [PubMed] [Google Scholar]
- 52. International Diabetes Federation . Diabetes atlas. 7th ed.Brussels (Belgium): International Diabetes Federation; 2015. [Google Scholar]
- 53. Devaney B, Ziegler P, Pac S, Karwe V, Barr SI. Nutrient intakes of infants and toddlers. J Am Diet Assoc. 2004;104(Suppl 1):14–21. [DOI] [PubMed] [Google Scholar]
- 54. Lee EY, Yoon K-H. Epidemic obesity in children and adolescents: risk factors and prevention. Front Med. 2018;12(6):658–66. [DOI] [PubMed] [Google Scholar]
- 55. Huysentruyt K, Laire D, Van Avondt T, De Schepper J, Vandenplas Y. Energy and macronutrient intakes and adherence to dietary guidelines of infants and toddlers in Belgium. Eur J Nutr. 2016;55(4):1595–604. [DOI] [PubMed] [Google Scholar]
- 56. Reidy KC, Deming DM, Briefel RR, Fox MK, Saavedra JM, Eldridge AL. Early development of dietary patterns: transitions in the contribution of food groups to total energy—Feeding Infants and Toddlers Study, 2008. BMC Nutr. 2017;3(1):5. [Google Scholar]
- 57. World Health Organization . Infant and young child feeding: model chapter for textbooks for medical students and allied health professionals. [Internet]. Geneva (Switzerland): WHO; 2009; [cited 2021 Aug 20]. Available from: https://apps.who.int/iris/bitstream/handle/10665/44117/9789241597494_eng.pdf?sequence=1&isAllowed=y. [PubMed] [Google Scholar]
- 58. Wiley AS. Does milk make children grow? Relationships between milk consumption and height in NHANES 1999–2002. Am J Hum Biol. 2005;17(4):425–41. [DOI] [PubMed] [Google Scholar]
- 59. Kranz S, Brauchla M, Slavin JL, Miller KB. What do we know about dietary fiber intake in children and health? The effects of fiber intake on constipation, obesity, and diabetes in children. Adv Nutr. 2012;3(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Young BE, Krebs NF. Complementary feeding: critical considerations to optimize growth, nutrition, and feeding behavior. Curr Pediatr Rep. 2013;1(4):247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Malik VS, Willett WC, Hu FB. Sugar-sweetened beverages and BMI in children and adolescents: reanalyses of a meta-analysis. Am J Clin Nutr. 2009;89(1):438–9. [DOI] [PubMed] [Google Scholar]
- 62. Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98(4):1084–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pan L, Li R, Park S, Galuska DA, Sherry B, Freedman DS. A longitudinal analysis of sugar-sweetened beverage intake in infancy and obesity at 6 years. Pediatrics. 2014;134(Suppl 1):S29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Albani V, Butler LT, Traill WB, Kennedy OB. Understanding fruit and vegetable consumption in children and adolescents. The contributions of affect, self-concept and habit strength. Appetite. 2018;120:398–408. [DOI] [PubMed] [Google Scholar]
- 65. Skinner JD, Carruth BR, Bounds W, Ziegler PJ. Children's food preferences: a longitudinal analysis. J Am Diet Assoc. 2002;102(11):1638–47. [DOI] [PubMed] [Google Scholar]
- 66. World Health Organization; Food and Agriculture Organization . Interim summary of conclusions and dietary recommendations on total fat & fatty acids. From the Joint FAO/WHO Expert Consultation on Fats and Fatty Acids in Human Nutrition. Ann Nutr Metab. 2009;55(1-3):1–308.20104000 [Google Scholar]
- 67. Doucet E, Almeras N, White M, Despres J, Bouchard C, Tremblay A. Dietary fat composition and human adiposity. Eur J Clin Nutr. 1998;52(1):2–6. [DOI] [PubMed] [Google Scholar]
- 68. Hooper L, Martin N, Jimoh OF, Kirk C, Foster E, Abdelhamid AS. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 2020(8):CD011737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Maffeis C, Tatò L. Long-term effects of childhood obesity on morbidity and mortality. Horm Res Paediatr. 2001;55(1):42–5. [DOI] [PubMed] [Google Scholar]
- 70. Uauy R, Peirano P, Hoffman D, Mena P, Birch D, Birch E. Role of essential fatty acids in the function of the developing nervous system. Lipids. 1996;31(1):S167–76. [DOI] [PubMed] [Google Scholar]
- 71. Warner ML, Harley K, Bradman A, Vargas G, Eskenazi B. Soda consumption and overweight status of 2-year-old Mexican-American children in California. Obesity (Silver Spring). 2006;14(11):1966–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bailey RL, Catellier DJ, Jun S, Dwyer JT, Jacquier EF, Anater ASet al. Total usual nutrient intakes of US children (under 48 months): findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148(Suppl 3):1557S–66S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pettit K, Rowley J, Brown N. Iron deficiency. Paediatr Child Health. 2011;21:8, 339–43. [Google Scholar]
- 74. Hwalla N, Al Dhaheri AS, Radwan H, Alfawaz HA, Fouda MA, Al-Daghri NMet al. The prevalence of micronutrient deficiencies and inadequacies in the Middle East and approaches to interventions. Nutrients. 2017;9(3):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Burke RM, Leon JS, Suchdev PS. Identification, prevention and treatment of iron deficiency during the first 1000 days. Nutrients. 2014;6(10):4093–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Friel JK, Hanning RM, Isaak CA, Prowse D, Miller AC. Canadian infants' nutrient intakes from complementary foods during the first year of life. BMC Pediatr. 2010;10(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gibson S, Sidnell A. Nutrient adequacy and imbalance among young children aged 1–3 years in the UK. Nutr Bull. 2014;39(2):172–80. [Google Scholar]
- 78. Hatun S, Ozkan B, Orbak Z, Doneray H, Cizmecioglu F, Toprak Det al. Vitamin D deficiency in early infancy. J Nutr. 2005;135(2):279–82. [DOI] [PubMed] [Google Scholar]
- 79. Ladhani S, Srinivasan L, Buchanan C, Allgrove J. Presentation of vitamin D deficiency. Arch Dis Child. 2004;89(8):781–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Braegger C, Campoy C, Colomb V, Decsi T, Domellof M, Fewtrell Met al. Vitamin D in the healthy European paediatric population. J Pediatr Gastroenterol Nutr. 2013;56(6):692–701. [DOI] [PubMed] [Google Scholar]
- 81. Laleye LC, Kerkadi AH, Wasesa AA, Rao MV, Aboubacar A. Assessment of vitamin D and vitamin A intake by female students at the United Arab Emirates University based on self-reported dietary and selected fortified food consumption. Int J Food Sci Nutr. 2011;62(4):370–6. [DOI] [PubMed] [Google Scholar]
- 82. Baker RD, Greer FR. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics. 2010;126(5):1040–50. [DOI] [PubMed] [Google Scholar]
- 83. World Health Organization . Guideline: vitamin A supplementation in infants and children 6–59 months of age. [Internet]. Geneva (Switzerland): WHO; 2011; [cited 2021 Nov 3]. Available from: https://www.who.int/publications/i/item/9789241501767. [PubMed] [Google Scholar]
- 84. Black R, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition 1: maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–60. [DOI] [PubMed] [Google Scholar]
- 85. Zlotkin S. A critical assessment of the upper intake levels for infants and children. J Nutr. 2006;136(2):502S–6S. [DOI] [PubMed] [Google Scholar]
- 86. Dardenne M. Zinc and immune function. Eur J Clin Nutr. 2002;56(S3):S20–3. [DOI] [PubMed] [Google Scholar]
- 87. Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. 2010;7(4):1342–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Warzyszynska J, Kim Y-I. Folate in human health and disease. New York: John Wiley & Sons, Ltd; 2014. [Google Scholar]
- 89. Stein LJ, Cowart BJ, Beauchamp GK. The development of salty taste acceptance is related to dietary experience in human infants: a prospective study. Am J Clin Nutr. 2012;95(1):123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lava SA, Bianchetti MG, Simonetti GD. Salt intake in children and its consequences on blood pressure. Pediatr Nephrol. 2015;30(9):1389–96. [DOI] [PubMed] [Google Scholar]
- 91. Westerterp KR, Goris AH. Validity of the assessment of dietary intake: problems of misreporting. Curr Opin Clin Nutr Metab Care. 2002;5(5):489–93. [DOI] [PubMed] [Google Scholar]
- 92. Livingstone M, Robson P. Measurement of dietary intake in children. Proc Nutr Soc. 2000;59(2):279–93. [DOI] [PubMed] [Google Scholar]
- 93. Burrows TL, Martin RJ, Collins CE. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J Am Diet Assoc. 2010;110(10):1501–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, codebook, and analytic code will be made available upon request pending application and approval by the investigators.