Abstract
Background
Minimizing right ventricular (RV) pacing to reduce the progression of heart failure is an established practice. Proprietary algorithms to reduce unnecessary RV pacing have been incorporated into both simple and complex cardiac pacemaker devices, for reducing the possibility of heart failure and arrhythmias.
Case summary
We present a case of a 43-year-old male implanted with a dual-chamber primary prevention implantable cardioverter-defibrillator (AUTOGEN EL, Boston Scientific) for sudden cardiac death. At the time of implant, the patient had hypertrophic cardiomyopathy with mild left ventricular (LV) systolic impairment, and sinus rhythm with intact atrioventricular (AV) conduction. The patient developed progression of his disease with symptoms (dyspnoea) and LV impairment. This led to a decision to activate the minimal RV pacing algorithm (RYTHMIQ™). A deterioration in AV conduction caused intrinsic ventricular beats to fall in the atrial blanking period, and subsequent VVI backup pacing resulted in R on T pacing. This induced ventricular arrhythmia. RYTHMIQ™ was subsequently deactivated, and the patient has had no further device-induced arrhythmias.
Discussion
Numerous studies have demonstrated the adverse effect of RV pacing on LV function. Minimizing RV pacing is, therefore, encouraged in individuals with intact AV conduction. However, underlying conduction abnormalities must be assessed prior to activating algorithms designed to minimize RV pacing. This case demonstrates the importance of careful intracardiac electrogram interpretation and individual case-based device programming, to avoid device-induced complications.
Keywords: Case report, Implantable cardioverter-defibrillator, Ventricular arrhythmia, RYTHMIQ
Learning points.
RYTHMIQ™ should be used with caution in patients with known intermittent atrioventricular (AV) dissociation.
In patients with no ventricular pacing indication, a Wenckebach pacing test or pacing at sensor indicated rate may be performed to assess AV conduction abnormalities.
Introduction
Widespread research has demonstrated that although dual-chamber pacing restores atrioventricular (AV) synchrony, right ventricular (RV) pacing-induced mechanical dyssynchrony may lead to progressive left ventricular (LV) dysfunction, promoting heart failure.1 This encouraged the design of programmable device algorithms to reduce unnecessary RV pacing. Here, we present an iatrogenic case of device-induced ventricular arrhythmia.
Timeline
| Date | Events |
|---|---|
| 2009 | Presented with Dyspnoea. Diagnosis of hypertrophic cardiomyopathy with mild left ventricular (LV) systolic impairment and family history of sudden cardiac death. No other past medical history. Implanted with primary prevention implantable cardioverter-defibrillator (ICD). |
| 2015 | Elective ICD generator replacement |
| 2016 | Presented with worsening dyspnoea. Echocardiogram demonstrated moderate LV systolic impairment. RYTHMIQ™ was programmed on. |
| 17 May 2017 | Device check demonstrated normal function and parameters |
| 18 May 2017 | Device-induced ventricular arrhythmia. Physical examination demonstrated no abnormalities. |
Case presentation
A 43-year-old male was implanted with a dual-chamber implantable cardioverter-defibrillator (ICD) for primary prevention. The patient presented with dyspnoea and had a family history of sudden cardiac death (SCD), hypertrophic cardiomyopathy, and mild LV systolic impairment (Class IIB recommendation for an ICD with a 5% risk of SCD at 5 years). No other abnormalities were detected. The underlying rhythm was sinus with first-degree AV Block (PR interval 340 ms) and a QRS duration of 90 ms. The ICD (AUTOGEN EL, Boston Scientific) was programmed to DDDR mode with rates of 70–120 b.p.m., and sensed and paced AV delays of 80 ms and 110 ms, respectively. Subsequent device follow-ups demonstrated normal device function and measurements, >95% atrial and ventricular pacing, and patient was symptomatically improving.
Seven years after the initial presentation, patient presented with symptoms of progressive dyspnoea and a follow-up echocardiogram demonstrated moderate LV systolic impairment (Table 1). As a result, RYTHMIQ™ and AV Search+ device features were enabled to minimize RV pacing. A device check 7 months later verified normal device and lead parameters (Figure 1). Atrial and ventricular pacing were 100% and 1%, respectively. As patient’s symptoms were improving, no changes to the device settings were made. The following day, the patient experienced a single episode of appropriate device (shock) therapy for ventricular fibrillation. Interrogation of device electrograms demonstrated initiation of ventricular arrhythmia due to R on T pacing.
Table 1.
Echocardiogram parameters of hypertrophic cardiomyopathy2
| Left ventricular dimensions and volumes | Initial echocardiogram | Echocardiogram in 2016 | Normal range (male) | |
|---|---|---|---|---|
| IVS diastole (mm) | 17 | 18 | 6–12 | |
| LVPW diastole (mm) | 9.5 | 8.8 | 6–12 | |
| LVID diastole (mm) | 40 | 42 | 37–56 | |
| LVEDV indexed ml/m2 | 71.4 | 78.1 | 30–79 | |
| LVEF % | Mildly impaired (visually) | Moderately impaired (visually) | ≥55% | |
| Eccentric | Concentric | |||
| Relative wall thickness in left ventricular hypertrophy | 0.66 | 0.64 | ≤0.42 | >0.42 |
IVS, interventricular septum; LVPW, left ventricular posterior wall; LVID, left ventricular internal diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction.
Figure 1.
Device measurements taken 1 day prior to shock.
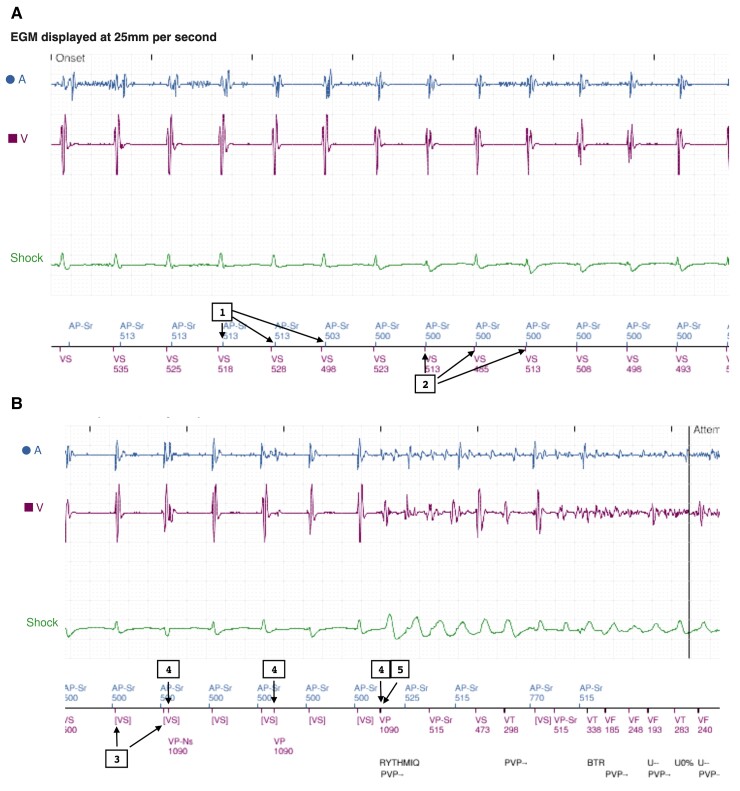
The alert demonstrated an episode of shock delivered for ventricular arrhythmia. The trace (Figure 2A) begins with atrial pacing and ventricular sensing (VS) at the sensor indicated rate (SIR) (120 ppm) (1,2). As the RYTHMIQ™’s criteria were met (VS within A-A + 150 ms), the device remained in AAIR mode. In the presence of AV block and subsequent ventricular beats falling in the atrial blanking period (Figure 2B), the device failed to detect the intrinsic ventricular events: [VS] (3). This initiated mode switch to backup VVI pacing [lower rate limit (LRL) set at 70 ppm, therefore, VVI backup is 55 ppm (1090 ms)], with ventricular paced beats on the 3rd, 6th, and 9th beats (4). We believe the closely coupled V-V timing resulted in R on T pacing (5), initiating ventricular fibrillation.
Figure 2.
Example of ventricular arrhythmia in the context of RYTHMIQ. (A) Device functioning in AAIR mode at sensor indicated rate and (B) back up VVI pacing followed by R on T pacing-induced ventricular arrhythmia.
Discussion
RYTHMIQ™ and AV Search+
RYTHMIQ™ is a Boston Scientific feature designed to encourage intrinsic conduction. It is a programmable feature and is nominally set to off. RYTHMIQ™ operates in AAI(R) pacing mode with asynchronous VVI backup pacing during normal AV conduction. A loss of AV synchrony will automatically switch the device to DDD(R) mode, and a return in normal AV conduction will switch back to AAI mode with VVI backup.
RYTHMIQ™:
AAI(R) pacing is delivered at the LRL and/or SIR.
VVI backup pacing is delivered at a rate of 15 ppm below the programmed LRL, i.e. LRL programmed at 60 ppm, VVI backup will be 45 ppm. The backup VVI pacing rate is limited to a minimum of 30 ppm and maximum of 60 ppm.
The device switches from AAI(R) to DDD(R) mode when three slow ventricular beats are detected in a window of 11 beats. A slow ventricular event is defined as: (i) ventricular paced beat, (ii) V-V interval greater than A-A interval + 150 ms.
In DDD(R) mode, the device uses AV Search + algorithm to assess the return of normal AV conduction.
AV Search+:
AV Search+ promotes intrinsic conduction by periodically extending the AV delay. This occurs every 32–1024 ventricular cycles with AV Search delay of 30–400 ms (both programmable search parameters).
If <2 out of the last 10 ventricular events are paced or sustained conduction is detected for ≥25 beats, the device will switch to AAI(R) mode with VVI backup.
Note: If AV Search+ is programmed off, switch from AAI(R) with VVI backup to DDD(R) mode will only occur once, until the device is reprogrammed.
Atrioventricular conduction disease is a common indication for device implantation. As per the European Society of Cardiology guideline for cardiac pacing and cardiac resynchronization therapy, AV synchronous pacing is recommended in advanced AV block, however, ventricular pacing for prolonged first-degree AV delay is an ongoing debate.3
A prolonged AV delay impairs LV filling, and reducing ventricular pacing may not be desirable in view of the haemodynamic response.4 On the contrary, several studies have demonstrated RV pacing to be associated with an increased risk of death and heart failure hospitalization, ventricular dyssynchrony and propensity to atrial fibrillation, questioning the optimal device setting in patients with prolonged AV delay.5,6
To provide an appropriate level of AV synchronous pacing, many pacemaker manufacturers (St Jude Medical/Abbott, Boston Scientific, Sorin, Biotronik, and Medtronic) have incorporated a minimal RV pacing feature into both simple and complex devices. With the exception of RYTHMIQ™, other algorithms are designed to provide a backup synchronous ventricular response to A-A intervals that are missing ventricular sensed events (number of allowed non-conducted atrial events and programmable AV interval vary between manufacturers). Thus, reducing the likelihood of R on T pacing (Table 2).
Table 2.
Device algorithms designed to reduce ventricular pacing
| Boston Scientific |
RYTHMIQ™
|
| Medtronic |
Managed ventricular pacing (MVP)
|
| St. Jude Medical™ |
Ventricular intrinsic preference (VIP)
|
| Sorin |
SafeR
|
| Biotronik |
AV hysteresis
|
The RYTHMIQ™ feature, which is present in simple pacemaker devices, may have proven fatal for our patient if he did not have a defibrillator. Similar incidences with RYTHMIQ™ were previously reported to be proarrhythmic, though these were as a result of short-long-short coupling intervals.7,8
Our case highlights the importance of regular follow-up and individual case-based device programming to prevent potential device issues. RYTHMIQ™ stores a 20 s electrogram data in the Arrhythmia logbook which must be carefully interpreted to distinguish between pathological and algorithm-induced arrhythmias. In addition, a simple Wenckebach pacing test or pacing at SIR may be performed to assess AV conduction abnormalities before activating RYTHMIQ™. A Wenckebach test involves pacing a patient’s heart at a faster rate than their AV node can handle (most often at the programmed max sensor/track rate). In some cases, this can result in 1st degree AV block or Wenckebach, which can be viewed on the paced electrocardiogram. This will help determine appropriate settings to avoid possible complications.9 Patients with heart failure who require ventricular pacing due to AV conduction disease may additionally benefit from cardiac resynchronization therapy (Table 3).10 This will help restore ventricular synchrony and may improve LV systolic function.11 Our patient did not meet the criteria for a cardiac resynchronization therapy (QRS duration; 90 ms, mild LV function), however, this patient may benefit from the emerging conduction system pacing, which hopes to preserve or normalize biventricular activation with pacing.12 In light of this arrhythmia episode, RYTHMIQ™ was deactivated in the patient’s ICD. A subsequent 3 year follow-up demonstrated no further device-induced arrhythmias.
Table 3.
Cardiac defibrillator options for patients with left ventricular dysfunction and a left ventricular ejection fraction of ≤35%
| QRS duration | New York Heart Association | |||
|---|---|---|---|---|
| I | II | III | IV | |
| <120 ms | ICD if at high risk of SCD | ICD and CRT not clinically indicated | ||
| 120–149 ms without left bundle branch block | ICD | CRT-P | ||
| 120–149 ms with left bundle branch block | ICD | CRT-D | CRT-P or CRT-D | CRT-P |
| ≥150 ms with or without left bundle branch block | CRT-D | CRT-P or CRT-D | CRT-P | |
Adapted from National Institute for Health and Care Excellence [TA314].10
ICD, implantable cardioverter-defibrillator; CRT-P, cardiac resynchronisation therapy-pacemaker; CRT-D, cardiac resynchronization therapy-defibrillator.
RYTHMIQ™ has been designed to promote intrinsic conduction with the safety of backup pacing. We highlight the need for careful individual assessment prior to programming this feature on, to avoid pacing-induced arrhythmias.
Supplementary Material
Lead author biography
Vivetha is a PhD candidate at the University of Leicester. Research interests include Ventricular Arrhythmia and Sudden Cardiac Death.
Supplementary material
Supplementary material is available at European Heart Journal—Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for the submission and publication of this case, including images, has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: V.P. is supported by the NIHR Leicester Biomedical Research Centre with a Research Fellowship. G.A.N. is supported by a British Heart Foundation Programme Grant (RG/17/3/32774). W.N. and G.A.N. are supported by a Medical Research Council Biomedical Catalyst Developmental Pathway Funding Scheme (MR/S037306/1).
References
- 1. O'Keefe JH Jr, Abuissa H, Jones PG, Thompson RC, Bateman TM, McGhie AI, Ramza BM, Steinhaus DM. Effect of chronic right ventricular apical pacing on left ventricular function. Am J Cardiol 2005;95:771–773. [DOI] [PubMed] [Google Scholar]
- 2. Harkness A, Ring L, Augustine DX, Oxborough D, Robinson S, Sharma V. Normal reference intervals for cardiac dimensions and function for use in echocardiographic practice: a guideline from the British Society of Echocardiography. Echo Res Pract 2020;7:G1–G18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Authors/Task Force Members, Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt O-A, Cleland J, Deharo J-C, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013;34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 4. Keene D, Arnold A, Shun-Shin MJ, Howard JP, Sohaib SA, Moore P, Tanner M, Quereshi N, Muthumala A, Chandresekeran B, Foley P, Leyva F, Adhya S, Falaschetti E, Tsang H, Vijayaraman P, Cleland JGF, Stegemann B, Francis DP, Whinnett ZI. Rationale and design of the randomized multicentre His Optimized Pacing Evaluated for Heart Failure (HOPE-HF) trial. ESC Heart Fail 2018;5:965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, Sharma A. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA 2002;288:3115–3123. [DOI] [PubMed] [Google Scholar]
- 6. Sweeney MO, Bank AJ, Nsah E, Koullick M, Zeng QC, Hettrick D, Sheldon T, Lamas GA. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med 2007;357:1000–1008. [DOI] [PubMed] [Google Scholar]
- 7. Monkhouse C, Dillon T, Chow AW, Behar JM. AV hysteresis causing initiation of recurrent atrial arrhythmias. Pacing Clin Electrophysiol 2018;41:1552–1554. [DOI] [PubMed] [Google Scholar]
- 8. Nguyen T, Sieira J, Casado-Arroyo R. Increased risk of ventricular fibrillation associated with RYTHMIQ™: lessons learned. J Interv Card Electrophysiol 2017;48:111–112. [DOI] [PubMed] [Google Scholar]
- 9. Adachi M, Igawa O, Yano A, Miake J, Inoue Y, Ogura K, Kato M, Iitsuka K, Hisatome I. Long-term reliability of AAI mode pacing in patients with sinus node dysfunction and low Wenckebach block rate. Europace 2008;10:134–137. [DOI] [PubMed] [Google Scholar]
- 10. National Institute of Health and Care Excellence . NICE technology appraisal [TA 314]: Implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure (review of TA95 and TA120). 2014.
- 11. St John Sutton MG, Plappert T, Abraham WT, Smith AL, DeLurgio DB, Leon AR, Loh E, Kocovic DZ, Fisher WG, Ellestad M, Messenger J, Kruger K, Hilpisch KE, Hill MRS. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation 2003;107:1985–1990. [DOI] [PubMed] [Google Scholar]
- 12. Sharma PS, Dandamudi G, Herweg B, Wilson D, Singh R, Naperkowski A, Koneru JN, Ellenbogen KA, Vijayaraman P. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: a multicenter experience. Heart Rhythm 2018;15:413–420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.