Abstract
High follicle-stimulating hormone (FSH) doses during ovarian stimulation are detrimental to ovulatory follicle function and decrease live birth rate in cattle and women. However, the mechanism whereby excessive FSH causes ovarian dysfunction is unknown. This study tested the hypothesis that excessive FSH during ovarian stimulation induces premature luteinization of ovulatory-size follicles. Small ovarian reserve heifers were injected twice daily for 4 days with 70 IU (N = 7 heifers) or 210 IU (N = 6 heifers) Folltropin-V [commercial FSH-enriched preparation of porcine pituitary glands with minor (<1%) luteinizing hormone (LH) contamination, cpFSH]. Ovulatory-size (≥10 mm) follicles were excised from ovaries after the last cpFSH injection and hormone concentrations in follicular fluid (FF) were determined using ELISA. Luteinization was monitored by assessing cumulus cell–oocyte complex (COC) morphology and measuring concentrations of estradiol (E), progesterone (P), and oxytocin (O) in FF. COCs were classified as having compact (cCOC) or expanded (eCOC) cumulus cell layers, and as estrogen-active (E:P in FF ≥1), estrogen-inactive (EI, E:P in FF ≤1 > 0.1), or extreme-estrogen-inactive (EEI, E:P in FF ≤0.1). A high proportion (72%) of ovulatory-size follicles in 210 IU, but not 70 IU, dose heifers displayed eCOCs. The high doses also produced higher proportions of EI or EEI follicles which had lower E:P ratio and/or E but higher P and/or O concentrations compared with the 70 IU dose heifers. In conclusion, excessive cpFSH doses during ovarian stimulation may induce premature luteinization of most ovulatory-size follicles in heifers with small ovarian reserves.
Keywords: ovarian stimulation, small ovarian reserve, ovulatory-size follicles, estradiol, progesterone, oxytocin, cumulus cell expansion, premature luteinization
Excessive doses of Folltropin-V (commercial FSH-enriched preparation from porcine pituitary glands with minor LH contamination) during ovarian stimulation of heifers with small ovarian reserves reduce intrafollicular estradiol while inducing premature luteinization of most ovulatory-size follicles.
Introduction
The use of high gonadotropin doses in women undergoing assisted reproductive technology (ART) causes high oocyte and embryo wastage and decreases live birth rate [1, 2]. The mechanisms whereby high follicle-stimulating hormone (FSH) doses impair ovulatory follicle function are poorly understood.
Ovarian follicle development becomes progressively reliant and ultimately dependent on gonadotropin (FSH and luteinizing hormone, LH) signaling to enhance growth and function. In cattle, the transition of follicles to gonadotropin dependence occurs in 1–3-mm antral follicles and continued follicle growth and maturation are dependent on adequate support from circulating FSH [3, 4]. FSH has a range of functions within the follicle and interacts with many different signaling pathways that contribute to ovulatory follicle function, and oocyte development and quality. These include steroid hormone synthesis, cell proliferation and survival, induction of LH receptor expression, and FSH receptor downregulation [5]. Given the broad, complex intrafollicular effects of FSH on follicular function, the effects of exogenous FSH on ovulatory follicle function likely vary with dosage. For example, estradiol secretion by bovine granulosa cells in response to different FSH doses is curvilinear; estradiol production initially increases in a dose-dependent manner but the highest FSH doses decrease estradiol production [6]. This supports our recent observations of decreased estradiol production by ovulatory-size follicles during ovarian stimulation of cattle with high FSH doses [7]. Taken together, these observations imply that high FSH doses during ovarian stimulation may be detrimental to granulosa cell function in ovulatory follicles, although the mechanism and potential impact of such an effect on ovulatory follicle function and oocyte quality are unclear.
Ovarian stimulation is a method of ART designed to provide continued FSH support by exogenous administration of FSH to promote development of the gonadotropin-dependent follicles to ovulatory-size. Subsequent development and completion of final maturation of ovulatory-size follicles in preparation for ovulation is dependent on the ability of these follicles to respond to LH [8, 9]. Luteinization is the process, regulated by LH, by which the ovulatory follicle is remodeled following ovulation to form the corpus luteum and concomitantly switch from primarily estradiol to progesterone production [10].
Premature luteinization is a term applied to clinical observations of high circulating progesterone concentrations or reduced estradiol:progesterone (E:P) ratio on the day of the ovulatory stimulus in women [11–21]. Premature luteinization impacts 12.3–46.7% of ART cycles in women contributing to poor ART outcomes [22]. There is evidence that premature luteinization can be induced by high doses of recombinant FSH administered during ovarian stimulation in patients undergoing ART [19, 22, 23] and ovarian stimulation in animal models [24]. However, the impact of the altered circulating endocrine profile on ART outcomes is contentious and very few, if any, of the studies in women have directly evaluated the ovulatory-size follicles developing in response to ovarian stimulation, particularly with excessive FSH doses (i.e., doses beyond what is needed to achieve a maximum ovulatory response). Importantly, we observed that high doses of FSH decrease estradiol production in vitro [6] and during superovulation of cattle with a small ovarian reserve [7]. It is unknown to what extent excessive FSH doses during ART cause premature luteinization of ovulatory follicles, thereby compromising oocyte quality and ART outcomes.
The present study, therefore, tests the hypothesis that excessive FSH doses during ovarian stimulation can induce premature luteinization of ovulatory-size follicles. To test this hypothesis, we use the small ovarian reserve heifer (SORH) model validated in our laboratory because it shares numerous phenotypic characteristics of women with small ovarian reserves including a diminished ovarian reserve and low antral follicle count (AFC) [25], low circulating concentrations of AMH [26], heightened FSH secretion [25], and reduced responsiveness to ovarian stimulation [27]. Moreover, the primary reason women seek ART is because they have a small ovarian reserve [28]. However, it is unclear why high FSH doses during ovarian stimulation of individuals with a small ovarian reserve typically result in poor ART outcomes [1, 2].
The present study used the SORH model to measure alterations in well-established intrafollicular markers of luteinization of ovulatory follicles in cattle including cumulus cell expansion [29] and intrafollicular concentrations of estradiol [30–32], progesterone [30–32], and oxytocin [10] in individual ovulatory-size follicles developing in response to ovarian stimulation with either an industry standard (70 IU) or excessive (210 IU) doses of Folltropin-V. Although recombinant hFSH is primarily used during ART in women [33], recombinant bovine FSH is not available for studies in cattle. Nevertheless, Folltropin-V is an FSH-enriched porcine pituitary preparation with minor (<1%) LH contamination [34] used globally for decades to superovulate cattle for embryo transfer [35]. Hereafter, Folltropin-V is referred to as cpFSH.
Even though high cpFSH doses are used in cattle to minimize number of injections during ovarian stimulation [36–39], the highest doses of cpFSH chosen for the present study (3X industry standard 70 IU dose) are unlikely to be used commercially to superovulate cattle. However, very high total FSH doses ranging from <1000 IU to 20 000 IU [1, 2] are used during ART cycles in some women. The cpFSH doses for the present study were selected based on our previous study showing that ovarian stimulation with cpFSH doses only three-fold higher than the industry-standard decreased estradiol production and ovulation rate in the SORH model [7]. We provide evidence in the present study that excessive cpFSH doses induce premature luteinization of a high proportion of the ovulatory-size follicles developing in response to ovarian stimulation. Premature luteinization may also impair oocyte quality, enhance oocyte wastage, and contribute to poor ART outcomes in cattle with small ovarian reserves. These results also indicate a possible need for caution in selecting high gonadotropin doses for human ART.
Methods
Selection of heifers with a low antral follicle count and small ovarian reserve, ovarian stimulation protocol, excision of ovulatory-size follicles from ovaries, and recovery of follicular fluid and cumulus cell–oocyte complexes from ovulatory-size follicles
Selection of 14 heifers aged 11–12 months with a small ovarian reserve and ovarian stimulation procedures were performed as described [7]. Briefly, the number of antral follicles (AFCs) ≥3 mm in diameter in two groups of 50 Holstein heifers (Green Meadow Farms Inc., Ovid-Elsie, MI) was determined using ovarian ultrasonography. Animals were ranked according to AFC and luteolysis was induced in the 25 heifers with the lowest AFC in each group using two prostaglandin F2α (PG; 12.5 mg/mL, Lutylase HighCon, Zoetis) injections administered 10 days apart. AFC for each heifer was determined prior to each PG injection and 4 days after the final PG injection. Individuals determined to consistently have an AFC ≤15 (N = 14 heifers; mean ± SEM AFC = 6.8 ± 0.4) on these days were selected for this study. We have previously shown that age-matched heifers with an AFC ≤15 also have an ovarian reserve (total number of morphologically healthy follicles/oocytes in ovaries) 80% smaller than heifers with an AFC ≥ 25 [26]. Heifers in the present study were housed at the Michigan State University Beef Cattle Teaching and Research Center. All procedures described herein were sanctioned by the Institutional Animal Care and Use Committee at Michigan State University.
Heifers were injected with 70 IU (40 mg, N = 7 heifers) or 210 IU (120 mg, N = 7) doses of cpFSH (Folltropin-V, Lot# 499213, Vetoquinol USA Inc) per injection. As described by the manufacturer, cpFSH contains 700 IU (equivalent to 400 mg of NIH-FSH-P1 with <1 mg NIH-LH-S19) of FSH per 20-mL vial [34]. However, one heifer treated with the 210 IU dose was excluded from the final analysis due to the absence of a response to ovarian stimulation. The 70 IU and 210 IU cpFSH doses were chosen as the industry standard and excessive doses, respectively, based on our previous study [7]. The 210 IU cpFSH dose compared with the 70 IU dose is excessive because it does not increase the number of ovulatory-sized follicles but decreased circulating estradiol concentration and ovulation rate following ovarian stimulation [7].
To synchronize estrous cycles and the day ovarian stimulation began, heifers were administered PG injections 10 days apart followed by a third PG injection 12 h later. On Days 1–2 of the estrous cycle, which is near the time of ovulation and emergence of the first follicular wave (~36 h after last PG injection), animals were randomly assigned to treatment with either 70 IU or 210 IU cpFSH twice daily for 4 days. PG injections were also administered coincident with the seventh and eighth cpFSH injections to regress the newly formed corpus luteum. Antral follicle size and number were monitored by daily ovarian ultrasonography as previously explained [7]. As depicted in Table 1, AFC prior to ovarian stimulation did not differ between cpFSH doses.
Table 1.
Antral follicle count (AFC, follicles ≥3 mm in diameter) prior to ovarian stimulation and number, and diameter of ovulatory-size (≥10 mm) follicles developing in response to ovarian stimulation of heifers with the industry standard, 70 IU, or excessive, 210 IU, cpFSH doseA
| 70 IU, cpFSH | 210 IU, cpFSH | Significance | |
|---|---|---|---|
| AFC prior to cpFSH treatment | 12.9 ± 1.8 | 11.3 ± 1.2 | NSB |
| Number of ovulatory-size follicles | 12.6 ± 3.3 | 19.1 ± 4.4 | NS |
| Diameter (mm) of ovulatory-size follicles | 12.2 ± 0.9 | 11.4 ± 0.3 | NS |
Heifers with a low AFC (≤15 follicles) and small ovarian reserve were subjected to PG injections to synchronize estrous cycles and injected with 70 IU (n = 7 heifers) or 210 IU (n = 6 heifers) cpFSH during ovarian stimulation as explained in Methods. AFC was determined prior to the first cpFSH injection whereas number and diameter of ovulatory-size follicles were determined 12 h after the last cpFSH injection.
NS = P > 0.05.
Ovaries were collected from a single heifer per day alternating between cpFSH dose groups until the study was completed. Heifers were euthanized 12 h after the final cpFSH injection and both ovaries were recovered. Note that in contrast to our previous study using this model [7], heifers were not injected with LH or an LH-like stimulus (for example, human chorionic gonadotropin; hCG) during the ovarian stimulation regimen. Time from the euthanasia of heifers in our study to the collection of ovaries was 33 ± 3 min. In agreement with our previous study [7], the number and diameter of ovulatory-size follicles did not differ between cpFSH doses (Table 1). Ovaries were rinsed with 70% ethanol, washed in Dulbecco’s phosphate Buffered Saline (DPBS), and placed in fresh DPBS for excision of ovulatory-size follicles. Follicular fluid (FF) was aspirated from all ovulatory-size follicles (≥10 mm). For a subset of follicles, the FF was immediately transferred to a separate dish and searched using a dissecting microscope to find the cell–oocyte complex (COC). Between 5 and 15 COCs were recovered from FF of ovulatory-size follicles per heifer. Each COC was classified as having compact or expanded layers of cumulus cells as depicted in the representative images in Figure 1. Hereafter, COCs with compact layers of cumulus cells are referred to as cCOCs whereas COCs with expanded layers of cumulus cells are referred to as eCOCs.
Figure 1.
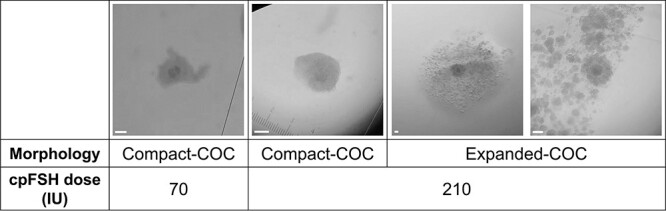
Representative images of cumulus cell–oocyte complexes (COCs) with compact (cCOC) or expanded (eCOC) layers of cumulus cells from heifers treated with the industry-standard, 70 IU, or excessive, 210 IU, cpFSH dose. Holstein heifers with a low AFC and small ovarian reserve were injected with either the 70 IU or 210 IU cpFSH dose during ovarian stimulation and ovulatory-size follicles (≥10 mm) excised from ovaries 12 h after the last cpFSH injection as explained in the Methods section. COCs recovered from FF of each follicle were classified under the dissecting microscope as having a compact-COC (two images on left) or expanded-COC (two images on right). The white scale bar in the bottom right corner of each image represents 130 μm, the diameter of the oocyte for an ovulatory-size follicle [77].
After the COC was removed from FF, the residual FF was recovered, and the volume measured. A protease inhibitor cocktail (cOmplete, EDTA-free Protease Inhibitor Cocktail, Roche, USA) was added to each FF sample at a 1:10 dilution to minimize protein degradation. The FF samples were centrifuged to pellet cellular debris and 200-μL aliquots of the supernatant were stored at −80°C. Thereafter, each remaining ovulatory-size follicle on both ovaries was aspirated and the FF processed as described above but without recovery of the COC. The time elapsed from removal of ovaries, excision of ovulatory-size follicles from ovaries, aspiration of FF, and recovery and classification of COCs for each heifer ranged from 2 to 5 h.
Estradiol (E), progesterone (P), and oxytocin (O) assays
Concentrations of E and P were measured in FF of all ovulatory-size follicles regardless of whether a COC was recovered, whereas O was measured in a subset of ovulatory-size follicles based on COC morphology and the ratio of E:P in FF as explained in the Results section. Estradiol and P concentrations were measured in the same aliquot of FF from each ovulatory-size follicle using commercially available ELISA kits (Cayman Chemical Company, MI, USA). The inter- and intra-assay coefficients of variation for the E assays were 9.3% and 7.0%, respectively, and 11.0% and 9.9%, respectively, for the P assays. Oxytocin concentrations in FF were determined using the Oxytocin ELISA kit (ENZO Life Sciences, Inc). Serial dilutions of bovine serum and FF from small (3–5 mm), medium (8–10 mm), and large (12–15 mm) bovine follicles were evaluated against the standard curve to confirm parallelism. The inter- and intra-assay coefficients of variation were 7.5% and 7.9%, respectively.
Classification of ovulatory-size follicles based on ratio of E:P in FF
Cattle typically have one or two non-ovulatory follicular waves and one ovulatory follicular wave during a ~21-day estrous cycle [40]. We previously established that cattle have both estrogen-active (EA) and estrogen-inactive (EI) follicles during a follicular wave [30–32, 41]. EA follicles are healthy, growing, dominant, non-ovulatory, or ovulatory follicles during follicular waves, whereas EI follicles are either dominant ovulatory follicles undergoing luteinization in response to the preovulatory LH surge or dominant non-ovulatory follicles destined for atresia at the end of a follicular wave [30–32, 41]. In the present study, ovulatory-size follicles were classified based not only on whether the COC had compact or expanded layers of cumulus cells, but also on the ratio of E:P concentrations in FF as follows: EA (E:P in FF ≥1), EI (E:P in FF ≤1 > 0.1), or extreme-estrogen-inactive (EEI, E:P in FF ≤0.1).
Statistical analyses
All statistical analyses were performed using the Statistical Analysis System (SAS 9.4) software [42]. Data were transformed before analyses if not normally distributed. Specifically, data for E, P, and O concentrations were log transformed, while data for E:P ratios were arcsine transformed. When overall treatment effects were significant (P ≤ 0.05), Fisher LSD [42] was used to determine whether means differed (P ≤ 0.05). Note that degrees of freedom during statistical analyses were based on the number of heifers (N = 7 heifers treated with 70 IU cpFSH dose or 6 heifers treated with 210 IU cpFSH dose), not the number of ovulatory-size follicles.
To assess whether the excessive, 210 IU, versus industry-standard, 70 IU, cpFSH doses during ovarian stimulation induced premature luteinization of the ovulatory-size follicles, five separate analyses were performed on the present data set. First, Fisher’s exact test was used to determine whether the proportion of ovulatory-size follicles with cCOCs or eCOCs differed (P ≤ 0.05) between heifers undergoing ovarian stimulation with the 70 IU versus 210 IU cpFSH doses. Second, given that the industry standard dose did not yield any eCOC, two t-tests were performed. The first t-test evaluated the effect of dose by comparing the concentrations of E, P, O, and E:P in compact follicles from the 70 IU versus 210 IU cpFSH dose. The second t-test evaluated the impact of COC expansion, unique to the 210 IU dose group, by comparing the concentrations of E, P, O, and E:P in the cCOCs 210 IU follicles to those in the eCOCs 210 IU follicles. Third, analysis of variance (ANOVA) was used to determine whether concentrations of E, P, O, and E:P in FF of the ovulatory-size follicles differed (P ≤ 0.05) between heifers treated with the 70 IU versus 210 IU cpFSH dose. Fourth, chi-square analysis was used to determine whether proportion of ovulatory-size follicles classified as EA, EI, and EEI differed (P ≤ 0.05) between heifers undergoing ovarian stimulation with the 70 IU versus 210 IU cpFSH dose. Fifth, ANOVA was used to determine whether concentrations of E, P, O, and E:P in FF of ovulatory-size follicles with cCOCs or eCOCs differed (P ≤ 0.05) between follicles classified as EA, EI, or EEI for the heifers undergoing ovarian stimulation with 70 IU versus 210 IU cpFSH dose.
Results
Analysis 1: Effect of cpFSH dose during ovarian stimulation on proportion of ovulatory-size follicles in heifers with cCOC or eCOC
Only cCOCs were observed in ovulatory-size follicles in heifers undergoing ovarian stimulation with the 70 IU cpFSH dose whereas ovulatory-size follicles in heifers treated with the 210 IU cpFSH dose had both cCOCs and eCOCs. As shown in Table 2, the heifers undergoing ovarian stimulation with 210 IU versus 70 IU cpFSH dose had a lower (P < 0.001) proportion of ovulatory-size follicles with cCOCs (28 ± 3% vs. 100 ± 0%, mean ± SEM) but a higher (P < 0.001) proportion of ovulatory-size follicles with eCOCs (72 ± 3% vs. 0%). Representative images of the cCOC and eCOC phenotype are depicted in Figure 1. Although the eCOCs are indeed expanded, the clumped appearance of the cumulus cell mass (most right photo in Figure 1) is indicative of poor oocyte quality [43].
Table 2.
Summary of follicle group characteristics (including proportion per heifer, follicle diameter, estradiol (E), progesterone (P), and oxytocin (O) concentrations), when follicles were categorized by cpFSH dose (70 or 210 IU), COC morphology (compact, expanded), and E:P in follicular fluid (estrogen-active (EA); estrogen-inactive (EI); or extreme-estrogen-inactive (EEI)A
| cpFSH (IU) | COC morphology | E:P ratio in FF | Proportion (%) per heifer | Follicle diameter (mm) | Concentration (ng/mL) | ||
|---|---|---|---|---|---|---|---|
| Estradiol | Progesterone | Oxytocin | |||||
| 70 | Compact | EA [4.11 ± 0.83] | 79 ± 6 | 12.0 ± 0.8 | 191 ± 28 (N = 7, 48)B | 49 ± 10 (N = 7, 48) | 0.28 ± 0.03 (N = 7, 12) |
| 70 | Compact | EI** [0.68 ± 0.04] | 21 ± 6 | 11.4 ± 1.5 | 59 ± 10** (N = 5, 11) | 114 ± 41 (N = 5, 11) | 0.25 ± 0.04 (N = 5, 10) |
| 210 | Compact | EA [2.61 ± 0.45] | 28 ± 3 | 11.5 ± 0.5 | 207 ± 33 (N = 6, 20) | 104 ± 23 (N = 6, 20) | 0.40 ± 0.04 (N = 6, 12) |
| 210 | Expanded | EA [2.17 ± 0.41] | 26 ± 5 | 12.3 ± 0.7 | 203 ± 31 (N = 6, 21) | 110 ± 10 (N = 6, 21) | 0.34 ± 0.05 (N = 6, 12) |
| 210 | Expanded | EI*** [0.59 ± 0.12] | 24 ± 8 | 11.7 ± 0.5 | 116 ± 27 (N = 6, 16) | 268 ± 75* (N = 6, 16) | 0.76 ± 0.15 (N = 6, 12) |
| 210 | Expanded | EEI*** [0.03 ± 0.01] | 22 ± 3 | 11.6 ± 0.5 | 33 ± 10*** (N = 6, 15) | 2643 ± 1027*** (N = 6, 15) | 6.80 ± 2.43*** (N = 6, 12) |
Asterisks (*P < 0.05, **P < 0.01, ***P < 0.001) denote differences between the means (±SEM) for control heifers versus other heifer groups (not follicles).
Holstein heifers with a low AFC and small ovarian reserve were injected with either the 70 IU or 210 IU cpFSH dose and ovulatory-size follicles were excised from ovaries as explained in Methods. The COCs recovered from the FF of 5–15 ovulatory-size follicles per heifer were classified as either having compact or expanded layers of cumulus cells and each ovulatory-size follicles was also classified based on E:P in FF as EA (E:P in FF ≥1), EI (E:P in FF <1 > 0.1), or EEI in FF = ≤0.1) as explained in Methods. This table summarizes the follicular characteristics in this subset of follicles in which we had information on COC morphology as well as intrafollicular ratio of E:P. The hormone concentrations in the EA ovulatory-size follicles with a compact COC in heifers undergoing ovarian stimulation with the 70 IU cpFSH dose (depicted in the first row of this table) were considered representative of preovulatory follicles in cattle [28, 31, 33] and thus used as control values.
N = number of heifers, or number of ovulatory-size follicles.
Analysis 2: Relationship between COC morphology (cCOC vs. eCOC) and concentrations of E, P, O, and E:P in FF of ovulatory-size follicles for heifers undergoing ovarian stimulation with the 70 IU or 210 IU cpFSH dose
This analysis was designed to determine whether concentrations of E, P, O, or E:P in FF differed between the ovulatory-size follicles with cCOCs versus eCOCs for the heifers undergoing ovarian stimulation with the 70 IU or 210 IU cpFSH dose. Ovulatory-size follicles with cCOCs had similar E, P, and O concentrations and ratio of E:P, regardless of cpFSH dose (Figure 2). However, for heifers undergoing ovarian stimulation with the 210 IU cpFSH dose, the ovulatory-size follicles with eCOCs not only had a decreased E and E:P ratio but also increased P and O concentrations in FF compared with the ovulatory-size follicles with cCOCs (Figure 2).
Figure 2.
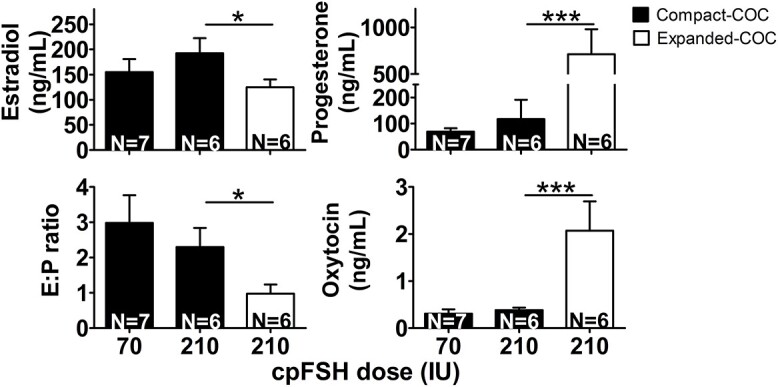
Effect of cpFSH dose (IU) and COC morphology (cCOC, eCOC) on concentrations of estradiol (E), progesterone (P), oxytocin (O), and ratio of E:P in follicular fluid (FF). Holstein heifers with a low AFC and small ovarian reserve were injected with either the industry-standard, 70 IU, or the excessive, 210 IU, cpFSH dose during ovarian stimulation and ovulatory-size follicles were excised from ovaries 12 h after the last cpFSH injection as explained in the Methods section. COC morphology was assessed in 5–15 ovulatory-size follicles per heifer and the concentration of E, P, and O was measured in the FF of these follicles. Total number of ovulatory-size follicles in each cpFSH dose-COC morphology group of heifers for the E, P, and E:P measurements follows: 70 IU-cCOC = 59; 210 IU-cCOC = 20; 210 IU-eCOC = 51. For O, a random subset of ovulatory-size follicles was selected for analysis in each cpFSH dose-COC morphology group of heifers as follows: 70 IU-cCOC = 22; 210 IU-cCOC = 12; 210 IU-eCOC = 36. Bars represent the mean ± SEM for heifers (not follicles). Lines above bars indicate which means were compared whereas asterisks (*P < 0.05, ***P < 0.001) denote differences between means for heifers. N = number of heifers.
Analysis 3: Overall effect of cpFSH dose during ovarian stimulation of heifers on concentrations of E, P, and O and E:P in FF
Estradiol concentrations and E:P in FF did not differ between heifers treated with the 70 or 210 IU cpFSH dose (Figure 3). In contrast, P and O concentrations were seven- and six-fold higher, respectively, in heifers undergoing ovarian stimulation with the 210 IU versus 70 IU cpFSH dose (Figure 3).
Figure 3.
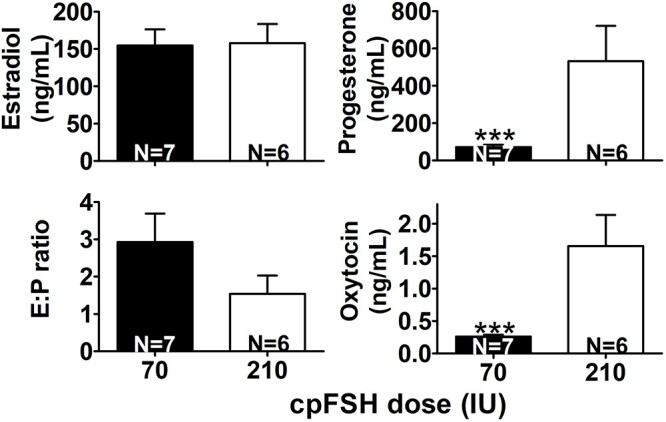
Effect of cpFSH dose (IU) on concentrations of estradiol (E), progesterone (P), oxytocin (O), and ratio of E:P in follicular fluid (FF). Holstein heifers with a low AFC and small ovarian reserve were injected with either the industry-standard, 70 IU, or the excessive, 210 IU, cpFSH dose during ovarian stimulation and ovulatory-size follicles were excised from ovaries and FF aspirated 12 h after the last cpFSH injection as explained in Methods. Concentrations of E and P in FF were measured in 5–24 follicles per heifer (n = a total of 93 ovulatory-size follicles from seven heifers) treated with 70 IU cpFSH and in 15–23 follicles per heifer (n = 114 ovulatory-size follicles from six heifers) treated with 210 IU cpFSH dose. Oxytocin was measured in two to five follicles per heifer (n = 22 of the 93 ovulatory-size follicles from the seven heifers) treated with 70 IU cpFSH and in seven to nine follicles per heifer (n = 48 of the 114 ovulatory-size follicles from the six heifers) treated with 210 IU cpFSH. N within bars indicates number of heifers treated with each FSH dose and the bars represent the mean (±SEM) concentration of E, P, E:P ratio, and O for heifers (not follicles). Asterisks indicate differences between means were significant (***P < 0.001).
Analysis 4: Effect of cpFSH dose during ovarian stimulation on diameter and proportion of ovulatory-size follicles in heifers classified as EA, EI, and EEI
We examined whether cpFSH dose impacted the diameter and proportion of ovulatory-size follicles classified as EA versus EI or EEI. As depicted in Table 2, the ovulatory-size follicles for heifers stimulated with the 70 IU cpFSH dose were classified as EA (79 ± 6%) or EI (21 ± 6%), whereas the ovulatory-size follicles for heifers stimulated with the 210 IU cpFSH dose were classified as EA (54 ± 9%), EI (24 ± 8%), and EEI (22 ± 3%). Diameters were similar among the EA, EI, and EEI ovulatory-size follicles regardless of cpFSH dose or COC morphology (Table 2). However, the heifers stimulated with 210 IU versus 70 IU cpFSH dose had a lower (P < 0.001) proportion of ovulatory-size follicles classified as EA (54 ± 7% vs. 79 ± 6%), a similar proportion classified as EI (24 ± 8% vs. 21 ± 6%) and a higher (P < 0.001) proportion classified as EEI (22 ± 3% vs. 0%).
Analysis 5: Relationship between COC morphology (cCOCs vs. eCOCs) and concentrations of E, P, O, and E:P in FF of ovulatory-size follicles classified as EA, EI, or EEI in the heifers undergoing ovarian stimulation with different cpFSH doses
Dominant follicles classified as EA [30, 32] and containing cCOCs [44] are representative of ovulatory follicles in cattle in the period preceding the pre-ovulatory LH surge or ovulatory stimulus. Thus, the E, P, and O concentrations and E:P in FF in the EA ovulatory-size follicles with cCOCs in the heifers stimulated with the industry-standard, 70 IU, cpFSH dose were considered the control values to use to determine whether cpFSH dose, COC morphology, and the E:P follicle classification scheme were associated with alterations in the E, P, or O concentrations or E:P in FF.
In comparison with the control follicle group (70 IU, cCOC and EA), the E:P ratio and E concentrations were lower in the EI ovulatory-size follicles with cCOCs for heifers stimulated with the 70 IU cpFSH dose and in the EI and EEI ovulatory-size follicles which contained eCOCs in the heifers stimulated with the 210 IU cpFSH dose (Table 2). In addition, compared with the control follicle group, P in FF was higher in the EI and EEI ovulatory-size follicles, while O was only higher in the EEI ovulatory-size follicles, both of which were from heifers stimulated with the 210 IU cpFSH dose and contained eCOCs (Table 2).
Discussion
The present results provide direct evidence in the SORH model that in the absence of a preovulatory LH or LH-like (e.g., hCG) stimulus, most ovulatory-size follicles developing in response to ovarian stimulation with an excessive cpFSH dose (three-fold greater than industry-standard) have (i) expanded layers of cumulus cells, (ii) decreased capacity to produce estradiol but enhanced capacity to produce progesterone resulting in a decreased ratio of estradiol:progesterone in FF, and/or (iii) enhanced capacity to produce oxytocin. Because in vivo, these phenotypic changes occur in ovulatory follicles in response to the preovulatory gonadotropin surge and in dominant non-ovulatory follicles destined for atresia at the end of a follicular wave in cattle [10, 29–32, 45], they are hereafter collectively referred to as premature luteinization of ovulatory-size follicles.
The most compelling data in the present study supporting the overall impact of excessive cpFSH on ovulatory follicle function were the overt observation that most (72 ± 3%) ovulatory-size follicles in excessive cpFSH dose-treated heifers contained COCs with an expanded layer of cumulus cells. In contrast, all the ovulatory-size follicles in heifers undergoing ovarian stimulation with the industry-standard cpFSH dose contained COCs with a compact layer of cumulus cells. This was an unexpected finding because cumulus cell expansion is one of the key events initiated by the preovulatory gonadotropin surge in cattle [29, 45] and other species including women [46, 47]. However, the ovaries in the present study were recovered 12 h after the last cpFSH injection and even though cpFSH contains minor LH contamination [34, 35] an LH or LH-like (hCG) ovulatory stimulus was not provided, early ovulations were not detected in the SORH model in the present study. Previous studies report that increased ratio of LH in various FSH preparations impairs the superovulatory response in cattle perhaps by causing early ovulation, premature luteinization, or early resumption of meiosis [35, 48]. However, the microenvironments for individual ovulatory-size follicles in these studies [35, 48] were not examined. The results of the present study indicate that ovarian stimulation of the SORH model with high doses of cpFSH causes cumulus expansion in COCs from most ovulatory-size follicles. Whether the cumulus expansion observed in the present study was caused by excessive FSH action, or alternatively by an interaction of the excessive FSH with endogenous secretion of LH or other unknown hormones, or with the minor LH contamination in cpFSH was not established in the present study. Nevertheless, although potential LH effects on premature cumulus expansion of COCs in ovulatory-size follicles cannot be completely ruled out in the present study, several studies show that ovarian stimulation protocols utilizing FSH (including the cpFSH preparation used herein) reduce LH pulse frequency and mean peripheral LH concentrations [49–51]. In addition, the half-life of LH in the bovine [52] and other species [53] is only 20–30 min compared with the 4–5 h half-life for FSH in cattle [54, 55] which likely explains why others using LH assays that cross react with both endogenous LH in cattle and porcine LH in cpFSH report that cpFSH injections do not enhance overall circulating LH concentrations compared with untreated controls [49–51]. In support of relatively low endogenous LH concentrations during ovarian stimulation with cpFSH, we confirmed in our previous study [7] that even with eight injections at 12-h intervals of cpFSH doses three-fold higher than the industry-standard dose during ovarian stimulation, luteal production of progesterone is not enhanced nor did early ovulation occur in the SORH model in the present or previous study [7] as would be expected if the preparation contained significant amounts of biologically active LH. In our previous study [7], we also observed that the high cpFSH doses did not increase circulating estradiol concentrations compared with the industry-standard dose indicating that LH-induced thecal production of androgens (estradiol substrate) was also very likely not enhanced. These combined observations indicate that cumulus cell expansion observed in ovulatory-size follicles during ovarian stimulation of the SORH model with high cpFSH doses in the present study is likely caused by excessive FSH stimulation and unlikely to be a primary response to significant endogenous or exogenous LH signaling.
The ovarian stimulation protocol used here, coupled with hormonal measurements and classification of individual ovulatory-size follicles based on COC morphology and ratio of E:P in FF, provides new insights into the impact of excessive cpFSH doses on the development of ovulatory-size follicles. For instance, the cpFSH injections in heifers were initiated at the emergence of the first follicular wave (Day 1 of the estrous cycle) when about a dozen 3–4-mm antral follicles were present. These cpFSH injections were continued for three additional days resulting in the development of ~13–19 ovulatory-size follicles (≥10 mm) during the ovarian stimulation period. However, the excessive, compared with the industry-standard, cpFSH dose did not increase the number of ovulatory-size follicles, as observed previously using the same heifer model [7]. Instead, it increased the heterogeneity of the ovulatory-size follicles, which were separated into four distinct phenotypes. The ovulatory-size follicles from heifers undergoing ovarian stimulation with the industry-standard cpFSH dose had mostly (79 ± 6%) EA ovulatory-size follicles with cCOCs. These are the hormonal and morphological characteristics of normal ovulatory follicles in cattle [30, 32, 41]. In contrast, the heifers undergoing ovarian stimulation with excessive cpFSH doses had ovulatory-size follicles with relatively equal proportions of four distinct phenotypes. One follicular phenotype (210 IU with cCOCs and EA) mimicked the control phenotype while the remaining three follicular phenotypes exhibited one (expanded cumulus; EA with eCOCs), two (expanded cumulus and high P in FF; EI with eCOCs), or three (expanded cumulus and high P and O in FF; EEI with eCOCs) phenotypic characteristics of prematurely luteinized ovulatory-size follicles. Whether these four types of ovulatory-size follicles represented sequential changes (for example, from an EA ovulatory-size follicle with cCOCs into the EEI ovulatory-size follicle with an eCOCs) or distinct trajectories that ovulatory-size follicles follow in response to ovarian stimulation with excessive cpFSH doses, is yet to be determined. Nevertheless, this heterogeneity very likely contributes to the inverse relationship we have reported between FSH doses during ovarian stimulation with oocyte recovery and live birth rate during ART in women [1, 2] and reduced estradiol production and decreased ovulation rate in cattle [7].
However, the excessive cpFSH dose did not cause premature luteinization of all the ovulatory-size follicles developing in response to ovarian stimulation in the present study. In fact, the control phenotype for ovulatory-size follicles was also observed in 28 ± 3% of ovulatory-size follicles in the heifers that were stimulated with the excessive cpFSH dose. The reason for this is unclear but may be explained by differences in the capacity of the granulosa and cumulus cells in individual ovulatory-size follicles to respond to the excessive cpFSH dose or differences in exposure to serum FSH. In sheep, granulosa cells from different follicles from the same individual have varying capacities to respond to stimulation with FSH and hCG as measured by in vitro cAMP production [56]. Furthermore, follicular development is hierarchical during follicular waves in cattle [57]. Differences in ovarian vascular supply to individual follicles [58, 59] may cause different distributions of exogenous FSH administered during ovarian stimulation to individual follicles. This unequal distribution of FSH to individual follicles could explain why some ovulatory-size follicles in heifers undergoing ovarian stimulation with the excessive cpFSH dose in the present study did not undergo premature luteinization and the observed heterogeneity of follicles.
Expansion of the cumulus cell layers of the COCs is not only the predominate symptom of premature luteinization, but it can also occur independent of concomitant changes in intrafollicular hormone concentrations in the ovulatory-size follicles in heifers undergoing ovarian stimulation with the excessive cpFSH doses in the present study. Nearly 30% of the ovulatory-size follicles in heifers undergoing ovarian stimulation with excessive cpFSH doses had COCs with an expanded cumulus layer but no alterations in intrafollicular hormone concentrations compared with not only the control follicle group (70 IU, cCOCs and EA), but also the EA, ovulatory-size follicles with COCs with a compact layer of cumulus cells in heifers undergoing ovarian stimulation with the excessive cpFSH dose. Although the implications of the premature cumulus expansion phenotype are unknown, this suggests that other maturational processes linked to cumulus cell expansion, including for example, increased glucose utilization [60], ovulation [47], oocyte maturation [61], and prevention of luteinization and downregulation of FSHR and modulation of FSH-induced steroidogenesis [62–64], and the developmental potential of oocytes in cattle [45, 65] and other species [47, 66], may also be altered during ovarian stimulation of heifers with excessive cpFSH doses. Clinical methods to distinguish the EA ovulatory-size follicles with COCs with a compact layer of cumulus cells from prematurely luteinized ones, have not been developed, but may be necessary to improve ART outcomes.
Our previous study using the same SORH model demonstrated that excessive cpFSH doses decreased capacity of ovulatory-size follicles to ovulate in response to an ovulatory stimulus [7]. However, it is unknown if premature expansion of the COCs in ovulatory-size follicles developing in response to the excessive cpFSH dose observed in the present study also impairs responsiveness of these follicles to an ovulatory stimulus like hCG or GnRH. If so, this could explain why we observed a reduced ovulation rate in heifers undergoing ovarian stimulation with excessive cpFSH doses in our previous study [7].
Ovarian stimulation of heifers with the excessive cpFSH dose in the present study altered intrafollicular production of hormonal markers of luteinization. These include established markers seen in cattle and other species including humans, such as changes in estradiol [30, 32, 41], progesterone [30, 32, 41], and oxytocin [6, 67–69]. This observation provides direct endocrine evidence, linked to the differences in COC morphology, that excessive cpFSH doses induce premature luteinization in a large proportion of the ovulatory-size follicles developing in response to ovarian stimulation. For example, a higher proportion (46%) of the ovulatory-size follicles in heifers undergoing ovarian stimulation with the excessive, compared with the industry-standard, cpFSH dose in the present study were classified as EI and EEI with COCs with expanded layers of cumulus cells. These same ovulatory-size follicles also had E:P ratio that were lower while progesterone concentrations in FF were higher compared with the EA ovulatory-size follicles in heifers with COCs with compact layers of cumulus cells undergoing ovarian stimulation with the industry-standard cpFSH dose (control). However, whether this intrafollicular shift from estradiol to progesterone production by ovulatory-size follicles developing during ovarian stimulation of heifers with excessive cpFSH doses also resulted in enhanced circulating concentrations of progesterone, similar to that observed during ART in some women [17, 19, 21], was not determined in the present study.
Although beyond the scope of the present study, it is critical to understand the mechanism whereby excessive FSH doses during ovarian stimulation may induce premature luteinization of the developing ovulatory-size follicles. High concentrations of purified FSH trigger ovulation in rodents and primates [46]. In addition, in vitro maturation (IVM) experiments demonstrate that high FSH alone induces cumulus cell expansion [46], and this is particularly important, given the absence of evidence for functional LH receptor expression in the cumulus cells [70–72]. Even in vivo, cumulus cells rely on a paracrine signaling loop to respond to the preovulatory LH surge. Specifically, LH stimulates production of EGF-like factors in the granulosa cells which then signal via the EGF receptor, expressed on the cumulus cells, mediating the effects of LH on the cumulus cells [73]. However, FSH can also induce the expression of EGF-like factors including AREG and EREG in the granulosa and cumulus cells, and supplementation of IVM media with AREG and EREG resulted in cumulus cell expansion and oocyte maturation [74–76]. It is worth noting in these and other in vitro embryo production experiments, the proportions of COCs that underwent cumulus cell expansion following IVM varied considerably, a further indication of the heterogeneity in cumulus cell responsiveness to FSH [71, 74]. Based on the similarities in cumulus cell gene expression profiles, others have suggested that in vitro, FSH signaling mimics some of the downstream pathways induced by LH in vivo [29]. Thus, the variable capacities of COCs to respond to supraphysiological concentrations of FSH may explain why most, but not all, of the ovulatory-size follicles in the heifers undergoing ovarian stimulation with the excessive cpFSH doses had COCs with an expanded layer of cumulus cells in the present study. The expanded cumulus cell phenotype observed herein provides compelling evidence that excessive cpFSH doses during ovarian stimulation of heifers with a low AFC and small ovarian reserve induces premature luteinization of a large proportion of the ovulatory-sized follicles.
In summary, the heifers undergoing ovarian stimulation with the excessive, 210 IU, compared with the industry-standard, 70 IU, cpFSH dose had ovulatory-size follicles containing primarily COCs with expanded layers of cumulus cells and classified as EI or EEI. The EI and EEI ovulatory-size follicles with COCs with expanded layers of cumulus cells in the excessive dose heifers had a lower estradiol or E:P ratio but higher progesterone or oxytocin concentrations compared with ovulatory-size follicles in the 70 IU FSH dose heifers. The excessive FSH doses used during ovarian stimulation are concluded to induce premature luteinization of most ovulatory-size follicles in heifers with small ovarian reserves. Whether premature luteinization of ovulatory-size follicles also impairs their capacity to ovulate in response to an LH stimulus, initiates atresia or alters oocyte maturation and quality thereby contributing to oocyte wastage, is unknown. Moreover, it is also unknown whether premature luteinization of ovulatory-size follicles during ovarian stimulation with excessive cpFSH doses is unique to heifers with a small ovarian reserve. The answers to these questions are, however, critical to improving the efficiency of ovarian stimulation protocols and ART outcomes not only in cattle, but perhaps in other mono-ovulatory mammals, like women. Finally, we acknowledge a limitation of the current study was the LH contamination of the cpFSH preparation utilized during ovarian stimulation. Although, we provide evidence that this contaminating LH is unlikely to contribute to the observed differences in COC morphology and intrafollicular hormone concentrations, future studies utilizing recombinant bovine FSH are required to confirm our findings.
Data availability
Data available upon request.
Authors’ contributions
ZC was involved in experimental design, performed tissue collection and follicular fluid analyses, prepared the first draft of the manuscript, and all subsequent revisions. KK contributed to experimental design, selected small ovarian reserve animals, performed ovarian stimulation, ovarian ultrasonography, and tissue collection, and contributed to revising the manuscript. MR was involved in experimental design, tissue collection, and manuscript revision. KL was involved in experimental design, interpretation of data, and contributed to all revisions of the manuscript. JI oversaw this project, including experimental design, analysis and interpretation of data and the planning and preparation of all drafts of this manuscript.
Acknowledgment
The authors would like to acknowledge Green Meadow Farms Inc. for continuing to allow us to identify and purchase small ovarian reserve heifers and the Beef Cattle Research and Teaching Center at Michigan State University and its staff for maintenance and care of animals used for this project.
Footnotes
† Grant support: This study was supported by the Agriculture and Food Research Initiative Competitive USDA-NIH Dual Purpose Program (Grant no. 2017-67015-26084), the USDA National Institute of Food and Agriculture, the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number T32HD087166, and AgBioResearch at Michigan State University.
Contributor Information
Zaramasina L Clark, Reproductive and Developmental Sciences Program, Department of Animal Science, Michigan State University, East Lansing, MI, USA.
Kaitlin R Karl, Reproductive and Developmental Sciences Program, Department of Animal Science, Michigan State University, East Lansing, MI, USA.
Meghan L Ruebel, Reproductive and Developmental Sciences Program, Department of Animal Science, Michigan State University, East Lansing, MI, USA.
Keith E Latham, Reproductive and Developmental Sciences Program, Department of Animal Science, Michigan State University, East Lansing, MI, USA.
James J Ireland, Reproductive and Developmental Sciences Program, Department of Animal Science, Michigan State University, East Lansing, MI, USA.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1. Baker VL, Brown MB, Luke B, Smith GW, Ireland JJ. Gonadotropin dose is negatively correlated with live birth rate: analysis of more than 650,000 assisted reproductive technology cycles. Fertil Steril 2015; 104:1145–1152.e1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clark ZL, Thakur M, Leach RE, Ireland JJ. FSH dose is negatively correlated with number of oocytes retrieved: analysis of a data set with ~650,000 ART cycles that previously identified an inverse relationship between FSH dose and live birth rate. J Assist Reprod Genet 2021; 38:1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McNatty KP, Juengel JL, Pitman JL. Oocyte-somatic cell interactions and ovulation rate: effects on oocyte quality and embryo yield. Reprod Biol Insights 2014; 7:1–8. [Google Scholar]
- 4. Jaiswal RS, Singh J, Adams GP. Developmental pattern of small antral follicles in the bovine ovary. Biol Reprod 2004; 71:1244–1251. [DOI] [PubMed] [Google Scholar]
- 5. Gloaguen P, Crépieux P, Heitzler D, Poupon A, Reiter E. Mapping the follicle-stimulating hormone-induced signaling networks. Front Endocrinol 2011; 2:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scheetz D, Folger JK, Smith GW, Ireland JJ. Granulosa cells are refractory to FSH action in individuals with a low antral follicle count. Reprod Fertil Dev 2012; 24:327–336. [DOI] [PubMed] [Google Scholar]
- 7. Karl KR, Jimenez-Krassel F, Gibbings E, Ireland JLH, Clark ZL, Tempelman RJ, Latham KE, Ireland JJ. Negative impact of high doses of follicle-stimulating hormone during superovulation on the ovulatory follicle function in small ovarian reserve dairy heifers. Biol Reprod 2020; 104:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction 2005; 130:791–799. [DOI] [PubMed] [Google Scholar]
- 9. Webb R, England BG. Identification of the ovulatory follicle in the ewe: associated changes in follicular size, thecal and granulosa cell luteinizing hormone receptors, antral fluid steroids, and circulating hormones during the preovulatory period. Endocrinology 1982; 110:873–881. [DOI] [PubMed] [Google Scholar]
- 10. Murphy BD. Models of luteinization. Biol Reprod 2000; 63:2–11. [DOI] [PubMed] [Google Scholar]
- 11. Fanchin R, de Ziegler D, Taieb J, Hazout A, Frydman R. Premature elevation of plasma progesterone alters pregnancy rates of in vitro fertilization and embryo transfer. Fertil Steril 1993; 59:1090–1094. [DOI] [PubMed] [Google Scholar]
- 12. Hofmann GE, Bentzien F, Bergh PA, John Garrisi G, Williams MC, Guzman I, Navot D. Premature luteinization in controlled ovarian hyperstimulation has no adverse effect on oocyte and embryo quality. Fertil Steril 1993; 60:675–679. [DOI] [PubMed] [Google Scholar]
- 13. Hofmann GE, Khoury J, Johnson CA, Thie J, Scott RT. Premature luteinization during controlled ovarian hyperstimulation for in vitro fertilization-embryo transfer has no impact on pregnancy outcome. Fertil Steril 1996; 66:980–986. [DOI] [PubMed] [Google Scholar]
- 14. Howles CM, Macnamee MC, Edwards RG. Follicular development and early luteal function of conception and non-conceptional cycles after human in-vitro fertilization: endocrine correlates. Hum Reprod 1987; 2:17–21. [DOI] [PubMed] [Google Scholar]
- 15. Lejeune B, Degueldre M, Camus M, Vekemans M, Opsomer L, Leroy F. In vitro fertilization and embryo transfer as related to endogenous luteinizing hormone rise or human chorionic gonadotropin administration *. Fertil Steril 1986; 45:377–383. [DOI] [PubMed] [Google Scholar]
- 16. Mascarenhas M, Kamath MS, Chandy A, Kunjummen AT. Progesterone/Estradiol ratio as a predictor in the ART cycles with premature progesterone elevation on the day of hCG trigger. J Reprod Infertil 2015; 16:155–161. [PMC free article] [PubMed] [Google Scholar]
- 17. Schoolcraft W, Sinton E, Schlenker T, Huynh D, Hamilton F, Meldrum DR. Lower pregnancy rate with premature luteinization during pituitary suppression with leuprolide acetate *. Fertil Steril 1991; 55:563–566. [PubMed] [Google Scholar]
- 18. Stanger JD, Yovich JL. Reduced in-vitro fertilization of human oocytes from patients with raised basal luteinizing hormone levels during the follicular phase. Br J Obstet Gynaecol 1985; 92:385–393. [DOI] [PubMed] [Google Scholar]
- 19. Ubaldi F, Camus M, Smitz J, Bennink HC, Van Steirteghem A, Devroey P. Premature luteinization in in vitro fertilization cycles using gonadotropin-releasing hormone agonist (GnRH-a) and recombinant follicle-stimulating hormone (FSH) and GnRH-a and urinary FSH. Fertil Steril 1996; 66:275–280. [DOI] [PubMed] [Google Scholar]
- 20. Younis JS, Haddad S, Matilsky M, Ben-Ami M. Premature luteinization: Could it be an early manifestation of low ovarian reserve? Fertil Steril 1998; 69:461–465. [DOI] [PubMed] [Google Scholar]
- 21. Lawrenz B, Melado L, Fatemi H. Premature progesterone rise in ART-cycles. Reprod Biol 2018; 18:1–4. [DOI] [PubMed] [Google Scholar]
- 22. Kaponis A, Chronopoulou E, Decavalas G. The curious case of premature luteinization. J Asst Reprod Gen 2018; 35:1723–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bosch E, Valencia I, Escudero E, Crespo J, Simón C, Remohí J, Pellicer A. Premature luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil Steril 2003; 80:1444–1449. [DOI] [PubMed] [Google Scholar]
- 24. Tapanainen JS, Lapolt PS, Perlas E, Hsueh AJ. Induction of ovarian follicle luteinization by recombinant follicle-stimulating hormone. Endocrinology 1993; 133:2875–2880. [DOI] [PubMed] [Google Scholar]
- 25. Burns DS, Jimenez-Krassel F, Ireland JLH, Knight PG, Ireland JJ. Numbers of antral follicles during follicular waves in cattle: evidence for high variation among animals, very high repeatability in individuals, and an inverse association with serum follicle-stimulating hormone concentrations. Biol Reprod 2005; 73:54–62. [DOI] [PubMed] [Google Scholar]
- 26. Ireland JLH, Scheetz D, Jimenez-Krassel F, Themmen APN, Ward F, Lonergan P, Smith GW, Perez GI, Evans ACO, Ireland JJ. Antral follicle count reliably predicts number of morphologically healthy oocytes and follicles in ovaries of young adult cattle. Biol Reprod 2008; 79:1219–1225. [DOI] [PubMed] [Google Scholar]
- 27. Ireland JJ, Ward F, Jimenez-Krassel F, Ireland JLH, Smith GW, Lonergan P, Evans ACO. Follicle numbers are highly repeatable within individual animals but are inversely correlated with FSH concentrations and the proportion of good-quality embryos after ovarian stimulation in cattle. Hum Reprod 2007; 22:1687–1695. [DOI] [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention ASfRM, Society for Assisted Reproductive Technology . Assisted Reproductive Technology National Summary Report. Atlanta (GA): US Dept of Health and Human Services; 2015: 2013. [Google Scholar]
- 29. Assidi M, Richard FJ, Sirard M-A. FSH in vitro versus LH in vivo: similar genomic effects on the cumulus. J Ovarian Res 2013; 6:68–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ireland JJ, Roche JF. Development of antral follicles in cattle after prostaglandin-induced luteolysis: changes in serum hormones, steroids in follicular fluid, and gonadotropin receptors. Endocrinology 1982; 111:2077–2086. [DOI] [PubMed] [Google Scholar]
- 31. Ireland JJ, Roche JF. Development of nonovulatory antral follicles in heifers: changes in steroids in follicular fluid and receptors for Gonadotr opins*. Endocrinology 1983; 112:150–156. [DOI] [PubMed] [Google Scholar]
- 32. Ireland JJ, Roche JF. Growth and differentiation of large antral follicles after spontaneous luteolysis in heifers: changes in concentration of hormones in follicular fluid and specific binding of gonadotropins to follicles. J Anim Sci 1983; 57:157–167. [DOI] [PubMed] [Google Scholar]
- 33. Lunenfeld B, Bilger W, Longobardi S, Alam V, D'Hooghe T, Sunkara SK. The development of gonadotropins for clinical use in the treatment of infertility. Front Endocrinol 2019; 10:429–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Folltropin-V . In: vol. 2021. VETOQUINOL N.-A. INC., Commerical Division, 2000, CHEMIN GEORGES, LAVALTRIE, QC, J57 3S5. [Google Scholar]
- 35. Mapletoft RJ, Steward KB, Adams GP. Recent advances in the superovulation in cattle. Reprod Nutr Dev 2002; 42:601–611. [DOI] [PubMed] [Google Scholar]
- 36. Bo GA, Hockley DK, Nasser LF, Mapletoft RJ. Superovulatory response to a single subcutaneous injection of Folltropin-V in beef cattle. Theriogenology 1994; 42:963–975. [DOI] [PubMed] [Google Scholar]
- 37. Tríbulo A, Rogan D, Tribulo H, Tribulo R, Alasino RV, Beltramo D, Bianco I, Mapletoft RJ, Bó GA. Superstimulation of ovarian follicular development in beef cattle with a single intramuscular injection of Folltropin-V. Anim Reprod Sci 2011; 129:7–13. [DOI] [PubMed] [Google Scholar]
- 38. Ongaratto FL, Tríbulo A, Ramos M, Rodriguez P, Bó GA. Oocyte recovery rates and in vitro blastocyst production in cattle treated with a single injection of Folltropin-V diluted in a slow-release formulation. Reprod Fertil Dev 2010; 23:202–203. [Google Scholar]
- 39. Carvalho PD, Hackbart KS, Bender RW, Baez GM, Dresch AR, Guenther JN, Souza AH, Fricke PM. Use of a single injection of long-acting recombinant bovine FSH to superovulate Holstein heifers: a preliminary study. Theriogenology 2014; 82:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ireland JJ, Mihm M, Austin E, Diskin MG, Roche JF. Historical perspective of turnover of dominant follicles during the bovine estrous cycle: key concepts, studies, advancements, and terms. J Dairy Sci 2000; 83:1648–1658. [DOI] [PubMed] [Google Scholar]
- 41. Sunderland SJ, Crowe MA, Boland MP, Roche JF, Ireland JJ. Selection, dominance and atresia of follicles during the oestrous cycle of heifers. J Reprod Fertil 1994; 101:547–555. [DOI] [PubMed] [Google Scholar]
- 42. Inc. SI. SAS/SHARE® 9.4: User’s Guide, 2nd ed. Cary, NC: SAS Institute Inc.; 2021; 104:695–705. [Google Scholar]
- 43. Boni R. Origin and effects of oocyte quality in cattle. Anim Reprod 2012; 9:333–320. [Google Scholar]
- 44. Assidi M, Dieleman SJ, Sirard MA. Cumulus cell gene expression following the LH surge in bovine preovulatory follicles: potential early markers of oocyte competence. Reproduction 2010; 140:835–852. [DOI] [PubMed] [Google Scholar]
- 45. Aardema H, Roelen BAJ, van Tol HTA, Oei CHY, Gadella BM, Vos PLAM. Follicular 17β-estradiol and progesterone concentrations and degree of cumulus cell expansion as predictors of in vivo-matured oocyte developmental competence in superstimulated heifers. Theriogenology 2013; 80:576–583. [DOI] [PubMed] [Google Scholar]
- 46. Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update 2007; 13:289–312. [DOI] [PubMed] [Google Scholar]
- 47. Robker RL, Hennebold JD, Russell DL. Coordination of ovulation and oocyte maturation: a good egg at the right time. Endocrinology 2018; 159:3209–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kanitz W, Becker F, Schneider F, Kanitz E, Leiding C, Nohner H-P, Pöhland R. Superovulation in cattle: practical aspects of gonadotropin treatment and insemination. Reprod Nutr Dev 2002; 42:587–599. [DOI] [PubMed] [Google Scholar]
- 49. Ben Jebara MK, Carrière PD, Price CA. Decreased pulsatile LH secretion in heifers superovulated with eCG or FSH. Theriogenology 1994; 42:685–694. [DOI] [PubMed] [Google Scholar]
- 50. Gosselin N, Price CA, Roy R, Carrière PD. Decreased lh pulsatility during initiation of gonadotropin superovulation treatment in the cow: evidence for negative feedback other than estradiol and progesterone. Theriogenology 2000; 54:507–521. [DOI] [PubMed] [Google Scholar]
- 51. Price CA, Carrière PD, Gosselin N, Kohram H, Guilbault LA. Effects of superovulation on endogenous LH secretion in cattle, and consequences for embryo production. Theriogenology 1999; 51:37–46. [DOI] [PubMed] [Google Scholar]
- 52. Baenziger JU, Kumar S, Brodbeck RM, Smith PL, Beranek MC. Circulatory half-life but not interaction with the lutropin/chorionic gonadotropin receptor is modulated by sulfation of bovine lutropin oligosaccharides. P N A S 1992; 89:334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yen SSC, Llerena O, Little B, Pearson OH. Disappearance rates of endogenous luteinizing hormone and chorionic gonadotropin in man. J Clin Endocrinol Metab 1968; 28:1763–1767. [DOI] [PubMed] [Google Scholar]
- 54. Walsh JH, Mantovani R, Duby RT, Overstrom EW, Dobrinsky JR, Enright WJ, Roche JF, Boland MP. The effects of once or twice daily injections of pFSH on superovulatory response in heifers. Theriogenology 1993; 40:313–321. [DOI] [PubMed] [Google Scholar]
- 55. Laster DB. Disappearance and uptake of [125I]FSH in the rat, rabbit, ewe and cow. Reproduction 1972; 30:407–415. [DOI] [PubMed] [Google Scholar]
- 56. McNatty KP, Heath DA, Hudson NL, Reader KL, Quirke L, Lun S, Juengel JL. The conflict between hierarchical ovarian follicular development and superovulation treatment. Reproduction 2010; 140:287–294. [DOI] [PubMed] [Google Scholar]
- 57. Ginther OJ, Bergfelt DR, Beg MA, Kot K. Follicle selection in cattle: relationships among growth rate, diameter ranking, and capacity for dominance. Biol Reprod 2001; 65:645–350. [DOI] [PubMed] [Google Scholar]
- 58. Acosta TJ, Hayashi KG, Matsui M, Miyamoto A. Changes in follicular vascularity during the first follicular wave in lactating cows. J Reprod Dev 2005; 51:273–280. [DOI] [PubMed] [Google Scholar]
- 59. Fraser HM. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol 2006; 4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sutton-McDowall ML, Gilchrist RB, Thompson JG. Cumulus expansion and glucose utilisation by bovine cumulus–oocyte complexes during in vitro maturation: the influence of glucosamine and follicle-stimulating hormone. Reproduction 2004; 128:313–319. [DOI] [PubMed] [Google Scholar]
- 61. Nevoral J, Orsák M, Klein P, Petr J, Dvořáková M, Weingartová I, Vyskočilová A, Zámostná K, Krejčová T, Jílek F. Cumulus cell expansion, its role in oocyte biology and perspectives of measurement: a review. Sci Agric Bohem 2015; 45:212–225. [Google Scholar]
- 62. El-Fouly MA, Cook B, Nekola M, Nalbandov AV. Role of the ovum in follicular luteinization. Endocrinology 1970; 87:288–293. [PubMed] [Google Scholar]
- 63. Otsuka F, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic Protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J Biol Chem 2001; 276:11387–11392. [DOI] [PubMed] [Google Scholar]
- 64. Otsuka F, Yao Z, Lee T-h, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15: identification of target cells and biological functions*. J Biol Chem 2000; 275:39523–39528. [DOI] [PubMed] [Google Scholar]
- 65. Furnus CC, de Matos DG, Moses DF. Cumulus expansion during in vitro maturation of bovine oocytes: relationship with intracellular glutathione level and its role on subsequent embryo development. Mol Reprod Dev 1998; 51:76–83. [DOI] [PubMed] [Google Scholar]
- 66. Chen L, Russell PT, Larsen WJ. Functional significance of cumulus expansion in the mouse: roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass. Mol Reprod Dev 1993; 34:87–93. [DOI] [PubMed] [Google Scholar]
- 67. Voss AK, Fortune JE. Oxytocin secretion by bovine granulosa cells: effects of stage of follicular development, gonadotropins, and coculture with theca interna. Endocrinology 1991; 128:1991–1999. [DOI] [PubMed] [Google Scholar]
- 68. Voss AK, Fortune JE. Oxytocin/neurophysin-I messenger ribonucleic acid in bovine granulosa cells increases after the luteinizing hormone (LH) surge and is stimulated by LH in vitro. Endocrinology 1992; 131:2755–2762. [DOI] [PubMed] [Google Scholar]
- 69. Meidan R, Wolfenson D, Thatcher WW, Gilad E, Aflalo L, Greber Y, Shoshani E, Girsh E. Oxytocin and estradiol concentrations in follicular fluid as a means for the classification of large bovine follicles. Theriogenology 1993; 39:421–432. [DOI] [PubMed] [Google Scholar]
- 70. Amsterdam A, Koch Y, Lieberman ME, Lindner HR. Distribution of binding sites for human chorionic gonadotropin in the preovulatory follicle of the rat. J Cell Biol 1975; 67:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Calder MD, Caveney AN, Smith LC, Watson AJ. Responsiveness of bovine cumulus-oocyte-complexes (COC) to porcine and recombinant human FSH, and the effect of COC quality on gonadotropin receptor and Cx43 marker gene mRNAs during maturation in vitro. Reprod Biol Endocrinol 2003; 1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. van Tol HT, van Eijk MJ, Mummery CL, van den Hurk R, Bevers MM. Influence of FSH and hCG on the resumption of meiosis of bovine oocytes surrounded by cumulus cells connected to membrana granulosa. Mol Reprod Dev 1996; 45:218–224. [DOI] [PubMed] [Google Scholar]
- 73. Richani D, Gilchrist RB. The epidermal growth factor network: role in oocyte growth, maturation and developmental competence. Hum Reprod Update 2017; 24:1–14. [DOI] [PubMed] [Google Scholar]
- 74. Caixeta ES, Machado MF, Ripamonte P, Price C, Buratini J. Effects of FSH on the expression of receptors for oocyte-secreted factors and members of the EGF-like family during in vitro maturation in cattle. Reprod Fertil Dev 2013; 25:890–899. [DOI] [PubMed] [Google Scholar]
- 75. Procházka R, Petlach M, Nagyová E, Němcová L. Effect of epidermal growth factor-like peptides on pig cumulus cell expansion, oocyte maturation, and acquisition of developmental competence in vitro: comparison with gonadotropins. Reproduction 2011; 141:425–435. [DOI] [PubMed] [Google Scholar]
- 76. Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol 2006; 20:1352–1365. [DOI] [PubMed] [Google Scholar]
- 77. Webb R, Gosden RG, Telfer EE, Moor RM. Factors affecting folliculogenesis in ruminants. Animal Science 1999; 68:257–284. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available upon request.


