Abstract
Objective
Autoimmune limbic encephalitis (ALE) is characterized by memory impairment, psychiatric symptoms, and epileptic seizures. Though, the neuropsychological profile of ALE is not yet well defined. However, there is some evidence that neuropsychological impairments might exceed those related to the limbic system and that different autoantibodies (AABs) are associated with distinguishable pattern of neuropsychological impairments. We provide a comprehensive presentation of neuropsychological performance of ALE in an immune therapy-naïve sample.
Methods
We retrospectively analyzed 69 immunotherapy-naïve ALE-patients (26 seropositive—[8 LGI1-, 4 CASPR2-, 2 GABAB-R-, 3 Hu-, 4 GAD65-, 2 Ma2-, 2 unknown antigen, and 1 Yo-AABs] and 43 seronegative patients, mean age 56.0 years [21.9–78.2], mean disease duration 88 weeks [0–572]). Neuropsychological evaluations comprised of the domains memory, attention, praxis, executive functions, language, social cognition, and psychological symptoms. We compared these functions between seronegative −, seropositive patients with AABs against intracellular neural antigens and seropositive patients with AABs against surface membrane neural antigens.
Results
No effect of AAB group on neuropsychological performance could be detected. Overall, ALE predominantly presents with deficits in long-term memory and memory recognition, autobiographical-episodic memory loss, impairment of emotion recognition, and depressed mood. Furthermore, deficits in praxis of pantomimes and imitations, visuo-construction, and flexibility may occur.
Conclusion
ALE shows a wide spectrum of neuropsychological impairments, which might exceed the limbic system, with no evidence of differences between AAB groups. Neuropsychological assessment for diagnosing ALE should include long-term memory, memory recognition, autobiographical-episodic memory, emotion recognition, and a detailed investigation of depression.
Keywords: Autoimmune limbic encephalitis, Neuropsychological profile, Cognition, Memory, Praxis, Emotion recognition
Introduction
Autoimmune limbic encephalitis (ALE) is clinically characterized by subacute memory deficits, psychiatric symptoms, and seizures (Graus et al., 2016). Mean age at onset is mainly between 50 and 65 years (see e.g., Gadoth et al., 2017; Mueller et al., 2021; Shojima et al., 2019). In T2−/fluid attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI), uni- or bilateral alterations of mesial temporal lobe structures can be detected. Moreover, Electroencephalography (EEG) typically reveals epileptic or slow wave activity involving the mesial temporal lobes, and autoantibodies (AABs) targeting a variety of central nervous system antigens can be detected in serum and cerebrospinal fluid (CSF) together with inflammatory CSF changes (Graus et al., 2016). ALE has first been reported by Brierley, Corsellis, Hierons, and Nevin (1960). They described three cases of severe subacute encephalitis, mainly affecting limbic areas of the brain. All cases presented “progressive dementia,” “severe impairment of memory,” disorientation, and depression. In one case, epileptic seizures occurred (Brierley et al., 1960). Later, Bataller and colleagues reported symptoms of limbic dysfunction such as “confusion, seizures, short-term memory loss, or psychiatric symptoms” (Bataller et al., 2007). Chou and colleagues named retrograde amnesia as a defining cognitive symptom of ALE (Chou et al., 2013). According to the widely recognized clinical approach to diagnose autoimmune encephalitis proposed by Graus and colleagues, the clinical presentation of ALE is characterized by a rapid development of a working memory deficit, confusion, mood changes, and seizures. The authors also state that the subacute development of short-term memory loss is the hallmark of the disorder (Graus et al., 2016). (Alves et al., 2017) report a sample of six patients with ALE in which working memory was preserved. However, these patients suffered from impaired long-term anterograde memory (Alves et al., 2017). Findings of impaired long-term memory were supported by others (Helmstaedter, Winter, Melzer, Lohmann, & Witt, 2019; Holtmann et al., 2018), but not found by Loane et al. (2019). Moreover, whether learning as one step towards memory is already impaired or preserved in ALE is also inconsistent with regard to verbal and nonverbal memory (Butler et al., 2014; Hanert et al., 2019; Helmstaedter et al., 2019; Holtmann et al., 2018; Loane et al., 2019). Others observed loss of autobiographical memories in patients with ALE (Lad, Mullally, Houston, Kelly, & Griffiths, 2019; Witt et al., 2015). Thus, the pattern of memory impairment in ALE is not yet fully understood.
In addition, there is growing evidence that cognitive impairment in ALE may not be restricted to memory functions. There is some evidence that ALE affects also other executive functions, such as word fluency or selective attention (see e.g., Frisch, Malter, Elger, & Helmstaedter, 2013; Hansen et al., 2016; Heine et al., 2018). In addition to the limbic system, working memory is associated with the lateral prefrontal, the supplementary motor, and the parietal cortex (D’Esposito, Postle, Ballard, & Lease, 1999; D’Esposito, Postle, & Rypma, 2000; Müller & Klein, 2014). Word fluency is associated with the thalamus and anterior cingulate cortex as well as a wide bi-hemispherical cortical network (Tucha, Smely, & Lange, 1999). Selective attention is associated with fronto-thalamic connections, the anterior cingulate cortex, and the inferior frontal cortex predominantly of the left hemisphere (Sturm, 2014). Structures of the limbic system comprise of the hypothalamus, amygdala, hippocampus, septum, and the anterior cingulate gyrus, which are directly interconnected by massive fiber pathways (Joseph, 2017). Thus, impairments in these functions might well be due involvement of extra-limbic regions as well.
Heine and colleagues found increased connectivity in ventral and dorsal default mode network regions to be significantly correlated with better memory performance. Moreover, a stronger connectivity of the insula with the salience network and default mode network was linked to impaired memory function (Heine et al., 2018). Unfortunately, Frisch and colleagues found no correlation between structural MRI findings and neuropsychological performance (Frisch et al., 2013), and Hansen and colleagues did not relate cognitive performance to their structural MRI findings (Hansen et al., 2016). In contrast, Bauer and colleagues found a significant alteration of fiber cross-section of the superior longitudinal fascicle and a significant relation between its left/right ratio and verbal memory performance (Bauer et al., 2020). Others found alterations of the basal ganglia, cerebellum and prefrontal associative cortex (Dodich et al., 2016), the motor cortex and the striatum (Navarro et al., 2016), and wide spread affections of white matter (Wagner et al., 2016), but did either not correlate imaging results with cognition or could not find a significant association (Bauer et al., 2020; Dodich et al., 2016; Navarro et al., 2016; Wagner et al., 2016).
Concerning psychological impairments such as depression, pathological tearfulness, altered emotion processing, anxiety, visual hallucinations, delusion, and sleep disorders have been described as well (Argyropoulos et al., 2020; Endres et al., 2020; Holtmann et al., 2018; Sezgin et al., 2018; Somers et al., 2011; Sonderen et al., 2016). However, studies applying standardized assessment of psychological symptoms are sparse.
Several AABs binding to either intracellular or surface membrane neural antigens, have been associated with ALE (Crisp, Kullmann, & Vincent, 2016; Lancaster & Dalmau, 2012; Melzer, Meuth, & Wiendl, 2012). It appears that different ALE associated AAB-types cause distinct structural and functional brain alterations (e.g., Bauer et al., 2020; Wagner et al., 2016), which potentially result in distinct patterns of cognitive impairments. Patients with GAD65 AABs for example, are found to show less impaired memory functions than patients with voltage gated potassium channel-complex (VGKC) AABs (Frisch et al., 2013), AAB-negative patients are reported to show an accelerated long-term forgetting more frequently than AAB positive patients (Helmstaedter et al., 2019) and ALE-patients with LGI1 AABs are reported to show cognitive decline and psychiatric symptoms more frequently than ALE-patients with CASPR2 AABs (Gadoth et al., 2017).
Overall, this raises the question of whether ALE is a single disease entity or a group of diseases predominantly affecting the limbic system or even extra-limbic structures with respect to clinical neuropsychological symptoms.
So far reports on cognitive performance in ALE mainly rely on prevalences of cognitive impairments, on brief cognitive screenings (e.g., Mini Mental State Examination) or on small samples (see e.g., Ahmad, Ramakrishna, Meara, & Doran, 2010; Hansen et al., 2016; Li-hao, Cong-cong, & Hai feng, & Ya-jun, 2018; Yang, Li, Zhao, Liu, & Wang, 2019). Very few studies using a more extensive neuropsychological assessment are mainly based on selected AABs with patients currently undergoing or after immunotherapy (see Finke et al., 2017; Miller et al., 2017) or on mixed samples with and without immunotherapy (see Butler et al., 2014; Helmstaedter et al., 2019). An extensive neuropsychological study on immunotherapy-naive ALE is based on only three patients (see Dodich et al., 2016). Disease duration in these studies ranges from 16 days to 4 years. Furthermore, symptoms of confusion and mood changes in ALE have been poorly addressed in literature so far.
Here, we provide a retrospective analysis of neuropsychological performance in a sizeable immunotherapy-naïve sample of seropositive and seronegative ALE-patients. Analysis of neuropsychological domains includes memory (short-term, long-term, forgetting, recognition, and autobiographical-episodic), attention (intensity and selectivity), praxis (pantomimes, gestures, and visuoconstruction), executive functions (working memory, problem solving, and flexibility), language (fluency and naming), social cognition (recognition of emotions and intensities of emotions), and diverse psychological symptoms. Moreover, we aim to contribute to the question of whether ALE is one single entity or a group of distinguishable diseases from a clinical neuropsychological perspective.
Methods
Ethics Approval
This study was approved by the ethics committee of the Westfälische Wilhelms-University of Münster, Germany (AZ 2013-350-f-S).
Sample
We collected retrospective data of patients admitted under suspicion of ALE between January 1, 2000 and November 30, 2020 at the Department of Neurology with Institute of Translational Neurology of the Westfälische Wilhelms-University of Münster, Germany and selected patients fulfilling criteria for “possible autoimmune encephalitis,” (panel one) “definite autoimmune limbic encephalitis,” (panel two) and “autoantibody-negative but probable autoimmune encephalitis” (panel seven) proposed by Graus et al. (2016). Although Graus and colleagues include multifocal cortical or white matter lesions as indicative of “autoimmune encephalitis” in general, we strictly wanted to select for “autoimmune limbic encephalitis.” Therefore, we limited our analysis to cases in which MRI alterations were restricted to the medial temporal lobes. For panel two, we only considered the criterion of “EEG with epileptic or slow-wave activity involving the temporal lobes” (Graus et al., 2016) to be met if indeed both medial temporal lobes showed abnormalities independent from each other. So, we refer to possible ALE, definite ALE, and autoantibody-negative but probable ALE here. Furthermore, we only included cases that had not yet undergone any causal treatment (immunotherapy or cancer treatment). Thus, our study is based on immunotherapy-naïve patients who only received symptomatic treatment, such as antiseizure, antidepressant, antipsychotic drugs, or a combination thereof.
Neuropsychological Assessment
To obtain a thorough presentation of neuropsychological performance, we collected all neuropsychological test data in the domains of praxis, memory, attention, executive functions, language, social cognition, and psychological symptoms in our cohort. All patients had a comprehensive neuropsychological assessment, with 95.65% of cases being investigated for four or more domains. To date no detailed guidelines for neuropsychological assessment of ALE have been published. Thus, in these retrospective data, the assessed domains and functions vary between patients. Several cognitive functions were assessed with different tests, depending on the patients’ age and severity of impairment, leading to variable numbers of test applications. Thus, results of different tests measuring the same cognitive construct were combined. Furthermore, we included data of all tests, which were applied in at least seven patients, to be able to present the cognitive domains of attention, memory, and executive functions according to neuropsychological diagnostic guidelines (see Müller & Klein, 2014; Sturm, 2014; Thöne-Otto, 2009). An overview of the applied tests and definitions of the neuropsychological constructs can be found in the Appendix. In addition, we report the neuropsychological symptoms first noticed from patients’ history. Moreover, patients were explored for loss of memories of personal experiences, stored in memory before disease (retrograde amnesia of autobiographic-episodes; see e.g., Lad et al., 2019).
Statistical Analysis
We first analyzed by analyses of variance whether ALE-patients with different AAB-types exhibit different neuropsychological performances. We classified ALE groups according to the absence (seronegative) or presence (seropositive) of AAB-types against known intracellular or surface membrane neural antigens. Patients with AABs against an unknown cytoplasmic or nuclear intracellular antigen or an unknown surface membrane “neuropil” antigen on brain tissue were classified as seropositive accordingly.
Across all AAB groups, we calculated mean values and standard deviations (SD) of all neuropsychological functions in standardized z-format to present neuropsychological performance in ALE. In addition, we calculated prevalences of performances below z < −1. Outliers were identified by exceeding the 1.5*inter quartile range (see Laurikkala et al., 2000). They were deleted if they reflected confounded results after review of the medical records (e.g., confounded by vigilance reduction, uncorrected vision etc.) or were corrected if there were typing errors. To facilitate clinical diagnosis of ALE, we report the spectrum of neuropsychological performances with prevalences of performances below cut-off scores (see e.g., Fischer et al., 2014; Renner et al., 2020). Therefore, data from all cognitive tests with validated cut-off scores were used accordingly (see Appendix). For tests without validated cut-off scores, standardized z-values, based on age-related-, and if applicable, education- and gender-related-norms, were used. We regarded a z-value < −1 (that is one SD below mean performance of the normative sample, reflecting that ~15% of the normative sample score this low or lower) as cut-off defining a cognitive deficit, according to common use in clinical (Lezak et al., 2012) and scientific neuropsychological practice (see e.g., Grote et al., 2016; Helmstaedter & Witt, 2012; von Rhein et al., 2017; Wagner et al., 2015). For a statistical analysis with a one-sample z-test see Supplementary material online, Table S2. Mean performances beyond cut-off scores that are present in the majority of patients (i.e., prevalence of 50% or higher) were considered as common for ALE. Unfortunately, in the emotion recognition test, there is just one global cut-off score. No emotional valence specific cut-off scores are available. Thus, we compared the valence ratings among each other in one-sample t-tests (two-tailed) to detect valence specific significant differences. To correct for multiple comparisons, we used the Bonferroni-method.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Sample
We retrospectively gathered data of 69 immunotherapy-naive ALE-patients. For patients characteristics see Table 1. The prevalence of AAB-types is shown in Fig. 1. Hence, 43 patients of our cohort were seronegative, 12 patients had AABs against intracellular neural antigens, and 14 had AABs against surface membrane neural antigens. As symptomatic treatment, 83.6% of the patients received centrally effective drugs.
Table 1.
Sample characteristics. Education years include occupational education (see Thomann et al., 2018). The certainty of the diagnosis refers to diagnostic criteria proposed by Graus et al. (2016)
| Characteristics | Value |
|---|---|
| Sex (number) | |
| Woman | 33 |
| Man | 36 |
| Age (mean, range) | 56.0 years, from 21.9 to 78.2 years |
| Education (mean, range) | 13.3 years, from 8 to 20 years |
| Certainty of diagnosis (number) | |
| Definite ALE | 32 |
| Possible ALE | 34 |
| AAB-negative but probable ALE | 3 |
| Disease duration (mean, range) | 88 weeks (from 0 to 572 weeks) |
Fig. 1.
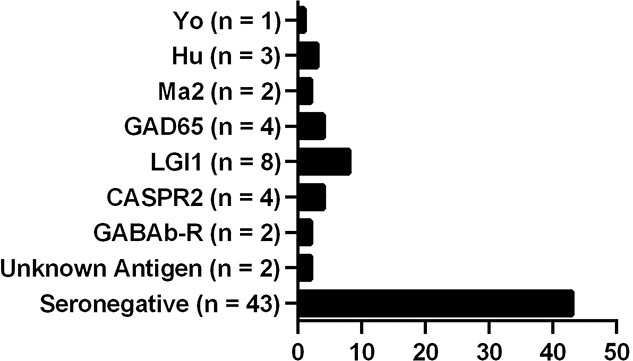
Prevalence of serotypes in the cohort of ALE-patients: 17.4% of patients had AABs against intracellular neural antigens (Hu, Yo, Ma2, GAD65, and unknown antigen), and 20.3% had AABs against surface membrane neural antigens (LGI1, CASPR2, and GABAB-R), and 62.3% of patients were seronegative.
No significant differences between AAB groups (43 seronegative patients, 12 seropositive patients with AABs against intracellular neural antigens, and 14 seropositive patients with AABs against surface membrane neural antigens) were detected regarding neuropsychological (cognitive and psychological) parameters (for details see Supplementary material online, Table S1).
Neuropsychological Performance by Mean Test Scores
For mean scores of neuropsychological performances see Table 2. Note, that variations of individual neuropsychological scores are large regarding both cognitive function and psychological symptoms. The SD here exceed the SD of 1 in a normal population.
Table 2.
Neuropsychological performance in mean scores and prevalences of deficits. Def. = deficit, min = minimum score, max = maximum score, mean scores, min, max, and standard deviations (SD) are given as standardized z-values (cut-off < −1.0), standardized t-values (T) (test specific cut-off >63) or as raw-scores (R). NA = not available. Deficits in mean performance or with a prevalence of at least 50% are bold
| n | Min | Max | Mean | SDs | n Def. (%) | n No Def. (%) | |
|---|---|---|---|---|---|---|---|
| Praxis | |||||||
| Praxis R | 17 | 51 | 80 | 74.94 | 7.77 | 8 (47.1) | 9 (52.9) |
| Visuo-construction | 37 | −11.04 | 0.98 | −1.33 | 2.73 | 16 (43.2) | 21 (56.8) |
| Memory | |||||||
| Verbal short-term memory | 60 | −2.31 | 2.31 | −0.40 | 1.18 | 18 (30.0) | 42 (70.0) |
| Nonverbal short-term memory | 17 | −3.19 | 1.53 | −0.56 | 1.26 | 5 (29.4) | 12 (70.6) |
| Verbal learning capacity | 68 | −3.29 | 2.49 | −0.45 | 1.26 | 26 (38.2) | 42 (61.8) |
| Nonverbal learning capacity | 36 | −2.33 | 1.08 | −0.86 | 0.99 | 16 (44.4) | 20 (55.6) |
| Verbal immediate recall | 8 | −3.00 | 0.00 | −1.38 | 0.81 | 7 (87.5) | 1 (12.5) |
| Nonverbal immediate recall | 28 | −3.12 | 1.86 | −0.68 | 1.26 | 11 (39.3) | 17 (60.7) |
| Verbal forgetting | 59 | −3.37 | 1.20 | −0.91 | 1.26 | 23 (39.0) | 36 (61.0) |
| Verbal long-term memory | 59 | −3.59 | 1.43 | −1.10 | 1.47 | 30 (50.8) | 29 (49.2) |
| Nonverbal long-term memory | 12 | −3.43 | 1.18 | −1.01 | 1.46 | 6 (50.0) | 6 (50.0) |
| Verbal recognition | 63 | −10.68 | 0.94 | −1.76 | 2.15 | 38 (60.3) | 25 (39.7) |
| Attention | |||||||
| Processing-speed | 60 | −39.64 | 1.79 | −1.67 | 5.92 | 19 (31.7) | 41 (68.3) |
| Divided attention: visual reaction time | 7 | −0.80 | 1.65 | 0.28 | 0.91 | — | 7 (100.0) |
| Divided attention: auditory reaction time | 6 | −1.75 | 0.81 | −0.73 | 0.98 | 3 (50.0) | 3 (50.0) |
| Divided attention: missings | 7 | −5.00 | 0.81 | −0.82 | 1.93 | 1 (14.3) | 6 (85.7) |
| Divided attention: errors | 7 | −1.08 | 1.00 | 0.02 | 0.83 | 1 (14.3) | 6 (85.7) |
| Executive functions | |||||||
| Verbal working memory | 62 | −3.76 | 3.20 | −0.53 | 1.10 | 24 (38.7) | 38 (61.3) |
| Nonverbal working memory | 16 | −3.49 | 1.47 | −0.70 | 1.58 | 6 (37.5) | 10 (62.5) |
| Nonverbal divergent problem solving | 7 | −1.13 | 0.92 | 0.13 | 0.67 | 1 (14.3) | 6 (85.7) |
| Nonverbal convergent problem solving | 7 | −2.33 | 1.00 | 0.09 | 1.12 | 1 (14.3) | 6 (85.7) |
| Flexibility | 57 | −12.69 | 1.61 | −2.03 | 3.36 | 26 (45.6) | 31 (54.4) |
| Language | |||||||
| Semantic word fluency | 21 | −2.05 | 2.05 | −0.47 | 1.16 | 7 (33.3) | 14 (66.7) |
| Lexical word fluency | 12 | −3.35 | 0.33 | −0.90 | 0.96 | 3 (25.0) | 9 (75.0) |
| Naming | 34 | −4.01 | 1.12 | −0.16 | 1.38 | 8 (23.5) | 26 (76.5) |
| Social cognition | |||||||
| Emotion recognition total | 17 | −4.45 | 1.39 | −1.04 | 1.75 | 7 (41.2) | 10 (58.8) |
| Emotion recognition happiness R | 17 | 4.00 | 5.00 | 4.94 | 0.24 | NA | NA |
| Emotion recognition fear R | 17 | 0.00 | 5.00 | 2.29 | 1.40 | NA | NA |
| Emotion recognition disgust R | 17 | 1.00 | 5.00 | 3.18 | 1.24 | NA | NA |
| Emotion recognition anger R | 17 | 3.00 | 5.00 | 3.88 | 0.78 | NA | NA |
| Emotion recognition surprise R | 17 | 2.00 | 5.00 | 4.12 | 1.11 | NA | NA |
| Emotion recognition sadness R | 17 | 1.00 | 5.00 | 3.88 | 1.22 | NA | NA |
| Emotion recognition neutrality R | 17 | 0.00 | 5.00 | 4.18 | 1.51 | NA | NA |
| Intensity of emotions | 12 | −1.03 | 1.29 | 0.3773 | 0.71 | 1 (8.3) | 11 (91.7) |
| Psychological symptoms | |||||||
| Somatization (SCL/BSCL) T | 19 | 32.00 | 80.00 | 51.95 | 13.51 | 4 (21.1) | 15 (78.9) |
| Obsessive–compulsive (SCL/BSCL) T | 19 | 39.00 | 80.00 | 60.26 | 10.02 | 8 (42.1) | 11 (57.9) |
| Interpersonal sensitivity (SCL/BSCL) T | 19 | 39.00 | 80.00 | 51.37 | 11.14 | 3 (15.8) | 16 (84.2) |
| Depression (SCL/BSCL) T | 19 | 39.00 | 76.00 | 57.05 | 9.86 | 7 (36.8) | 12 (63.2) |
| Anxiety (SCL/BSCL) T | 19 | 42.00 | 73.00 | 57.47 | 8.87 | 9 (47.4) | 10 (52.6) |
| Hostility (SCL/BSCL) T | 19 | 41.00 | 80.00 | 52.37 | 11.11 | 3 (15.8) | 16 (84.2) |
| Phobic anxiety (SCL/BSCL) T | 19 | 41.00 | 80.00 | 51.84 | 15.32 | 4 (21.1) | 15 (78.9) |
| Paranoid ideation (SCL/BSCL) T | 19 | 38.00 | 73.00 | 48.84 | 15.36 | 3 (15.8) | 16 (84.2) |
| Psychoticism (SCL/BSCL) T | 19 | 44.00 | 80.00 | 58.05 | 17.14 | 8 (42.1) | 11 (57.9) |
| GSI (SCL/BSCL) T | 19 | 41.00 | 80.00 | 55.83 | 15.86 | 4 (22.2) | 14 (77.8) |
| PSDI (SCL/BSCL) T | 15 | 40.00 | 71.00 | 54.47 | 15.84 | 4 (26.7) | 11 (73.3) |
| PST (SCL/BSCL) T | 18 | 43.00 | 76.00 | 56.60 | 15.60 | 7 (38.9) | 11 (61.1) |
| Depression (BDI-II) R | 23 | 3 | 27 | 14.87 | 7.12 | 14 (60.9) | 9 (39.1) |
| Anxiety (HADS) R | 29 | 0 | 15 | 6.31 | 4.22 | 11 (37.9) | 18 (62.1) |
| Depression (HADS) R | 29 | 0 | 13 | 4.83 | 3.69 | 6 (20.7) | 23 (79.3) |
Cognition
Patients with ALE showed mean scores in deficit range in recalling both verbal and nonverbal information from long-term memory. Moreover, the mean score of verbal recognition was in the deficit range. Unfortunately, nonverbal recognition was not included in our test battery. In contrast, mean scores of short-term memory and working memory, as well as learning ability and nonverbal immediate recall were not in deficit. However, the mean score of verbal immediate recall, a function only assessed in severely impaired patients, was in the defined deficit range. Verbal forgetting just did not exceed the cut-off score. Furthermore, mean scores of visuo-construction, praxis as well as processing speed and flexibility appeared to be in the deficit range in ALE. Concerning social cognition, we found emotion recognition scores to be in the deficit range, with patients showing most pronounced difficulties in recognizing fear in facial expressions (see Tables 2 and 3). Rating of the intensity of given emotions, divided attention, divergent and convergent problem solving, word fluency, and naming were preserved in our cohort (see Table 2).
Table 3.
Differences of recognizing fear and other emotions. Matrix of one sample t-tests (2-tailed, df = 16, Bonferroni-corrected alpha = .008)
| t | p ≤ | |
|---|---|---|
| Fear-happiness | −8.268 | .001 |
| Fear-disgust | −2.151 | .047 |
| Fear-anger | −4.622 | .001 |
| Fear-surprise | −5.122 | .001 |
| Fear-sadness | −5.125 | .001 |
| Fear-neutrality | −4.804 | .001 |
Psychological Symptoms
The mean depression score was elevated in the Beck Depression Inventory-II (BDI-II), whereas it was within normal range in Hospital Anxiety and Depression Scale (HADS). Moreover, there were no elevated scores of anxiety in the HADS nor in psychological strain in Symptom Checklist-90-R (SCL-90-R) and its brief version Brief-Symptom-Checklist (BSCL; Franke, 2002, 2017).
Prevalence of Neuropsychological Impairments
Cognition
Consistent with deficits as defined by mean scores, the majority of patients showed performances of z < − 1 in long-term memory recall (50.8% of verbal long-term memory and 50.0% of nonverbal long-term memory). In ALE-patients with nonverbal long-term memory in the deficit range, 66.7% also showed a deficit in verbal long-term memory. However, 25.0% of all ALE-patients showed neither a deficit in verbal- nor in nonverbal long-term memory. Concerning verbal recognition, 60.3% performed in the deficit range (see Table 2). Moreover, 21.7% of patients reported to have forgotten relevant personal experiences, such as the funeral of the own mother, marriage of a daughter, or own vacations, but have retained the semantic facts about their lives. Furthermore, 50.0% of patients showed a reduced reaction speed to auditory stimuli in divided attention, with one patient actually not being able to respond at all to the auditory subtask in this test (see Table 2). In contrast to cognitive deficits indicated by mean scores, only a minority of patients showed performances below cut-off scores in praxis, visuo-construction, processing speed, flexibility, and emotion recognition (see Table 2). As in the mean scores, only a minority of patients showed performances below cut-off scores in short-term memory, working memory, verbal and nonverbal learning, verbal forgetting, nonverbal immediate recall, nonverbal immediate recall, nonverbal divergent problem solving, semantic and lexical word fluency, convergent problem solving, naming, and in rating intensity of emotions (see Table 2). The neuropsychological complaints patients reported verbally at first consultation are shown in Table 4.
Table 4.
Frequency of patients’ complaints at first consultation.
| Cognitive complaints | n/65 | % |
|---|---|---|
| Memory: Anterograde amnesia | 25 | 39.1 |
| More than one listed | 18 | 28.1 |
| None | 16 | 25.0 |
| Executive functions | 1 | 1.6 |
| Attention | 3 | 4.7 |
| Word fluency | 1 | 1.6 |
| Memory: Amnesia of autobiographic-episodes | n/69 | % |
| Yes | 15 | 21.7 |
| No | 54 | 78.3 |
| Psychological complaints | n/63 | % |
| Depressed mood/ rumination/ psychomotor retardation |
16 | 25.4 |
| More than one listed | 8 | 12.7 |
| Irritability/aggression | 5 | 7.9 |
| Sleep disturbance/tiredness | 2 | 3.2 |
| Tearfulness | 1 | 1.6 |
| No affect | 1 | 1.6 |
| Fear of future | 1 | 1.6 |
| None | 29 | 46.0 |
Discussion
This study presents the neuropsychological performance of immunotherapy-naïve patients with ALE. No significant differences in neuropsychological performance between AAB groups could be detected. Thus, absence or presence of AAB-types against known intracellular or surface membrane neural antigens does not seem to be a relevant factor in neuropsychological presentation of ALE.
The performances of ALE-patients show large variations. Thus, deficits as defined by a mean performance with a z-value < −1 in the ALE sample have to be interpreted carefully. Therefore, we give the prevalence of deficits within the ALE patient sample to illustrate how likely patients present specific symptoms and consider only those deficits as common for ALE that appear in 50% or more of all patients. Such an approach is frequently used to describe (relatively variable) cognitive functioning in neuroimmunological disorders (see e.g., Massman et al., 1996; Tran, Milanovic, Holshausen, & Bowie, 2021; Weissenborn, Ennen, Schomerus, Rückert, & Hecker, 2001). However, it should be taken into account that ~15% of the normal population show a performance below a z-value of −1 due to premorbid limited functioning.
Given such large variations in neuropsychological performances in our relatively small ALE sample, this approach appears more appropriate compared with statistical testing using for example, z-tests averaging the performances of individual patients by comparing the mean scores and SDs of the patient sample with data of the normative sample of the respective test. Moreover, data of the normative sample are not publicly available for all neuropsychological tests performed in our ALE, especially as we combined tests. The neuropsychological functions are discussed in detail in the following section. Several neuropsychological impairments in this cohort indicate a possible extra-limbic involvement in ALE.
Praxis
Apraxia has not been reported in ALE before. Here, 8 of 17 patients showed symptoms of apraxia. The concept of apraxia traditionally comprises the domains of (i) imitation of gestures, (ii) communicative gestures, (iii) simple use of tools and objects, and (iv) complex use of tools and objects (Goldenberg, 2009). Goldenberg suggests to specify the impaired domain in apraxia (Goldenberg, 2009). However, the Kölner Apraxie Screening (KAS, Cologne Apraxia Screening, see Appendix) used in our study is restricted to imitations of gestures and pantomimes of object use. It must be noted that patients in our cohort with a mean score of 74.9 only slightly underscored the cut-off score of KAS of 76 on a scale from 80 to 0. Second, this cut-off score is based on patients suffering from stroke of the left hemisphere. The authors point out that apraxia related to the right hemisphere shows different characteristics. Because the KAS does not adequately represent this, its cut-off score might underestimate apraxia in ALE. Taken together, we state that patients in ALE show mild apraxia of pantomimes and imitation of gestures.
The ability to imitate gestures is associated with left parietal cortical function and the ability to pantomime the use of objects is associated with left hemispheric function (Goldenberg, 2009). This indicates an affection of the left parietal cortex in ALE or even wider left hemispheric networks.
In our cohort visuo-construction was also in deficit with regard to the mean score, but not with regard to prevalence. An impaired visuo-construction was also found in a smaller cohort of 12 patients with autoimmune epilepsy (see Holtmann et al., 2018). In contrast, Hanert and colleagues did not find a difference in visuo-construction compared with controls (Hanert et al., 2019). Butler and colleagues report that none of their patients scored below z ≤ −1.67 (Butler et al., 2014). That does not contradict our findings, because in our cohort the mean score was just −1.33. The majority of patients did not show a conspicuous z-score below −1.
Visuo-construction is known to involve parietal and frontal regions of both hemispheres (De Renzi, 1982). However, Heine and colleagues could not show alterations of frontoparietal networks in ALE (Heine et al., 2018). This is possibly because of the relatively low prevalence of pathological impairments in visuo-construction as we also observed in our cohort. Unfortunately, Heine and colleagues do not report the results of visuo-constructive performance in their cohort (Heine et al., 2018). They only described decreased functional connectivity between the salience network, which is commonly coactivated in a wide range of cognitive tasks (Menon, 2015) and left parietal cortical areas (Heine et al., 2018). The latter finding is at least consistent with findings on praxis in our cohort.
Memory
We observed below average short-term memory performance as well as working memory performance for both verbal and nonverbal information, but not in deficit range with regard to the mean performance with only a minority of patients performing below cut-off values. We state that short-term memory and working memory can be impaired in ALE and even be in deficit as defined by z-values below −1 but only in a minority of patients. This is plausible as hippocampal function was found to be relevant for maintenance of information in working memory (Leszczyński, 2015). On the other hand, this may also point towards a possible involvement of extra-limbic regions in ALE-patients with respective deficits, because working memory is mainly performed by the dorsolateral prefrontal cortex (Curtis & D’Esposito, 2003; D’Esposito et al., 2000).
In contrast, we found patients with ALE to be in deficit in verbal and nonverbal long-term memory and in verbal recognition memory (nonverbal recognition memory was not included in our test battery) with regard to the mean performance and prevalence (50% or more) in our cohort. One might assume that a unimodal (verbal or nonverbal) memory deficit would be inconsistent with the diagnosis of ALE given the observed bimodal long-term memory deficits. However, note that only 66.7% in this cohort showed a bimodal long-term memory deficit. In 25% of our ALE-patients no long-term memory deficit was observed since their memory impairment remained subclinical. Nonverbal immediate recall with regard to the mean performance and prevalence of deficit was not affected in a clinically relevant manner. However, verbal immediate recall was in deficit concerning mean performance and deficit prevalence. This finding can be attributed to the small number of eight patients selected for this test by reasons of an overall strong impairment, reflected by the fact that none of them reached a z-value >0.
The fact that we found recognition of verbal memory to be in the deficit range indicates that the impairment is not due to a mere impairment of recall but mainly to an impairment of information consolidation. If it were due to an impaired recall only, properly stored information could be passively recognized despite an impaired process of active recall.
Another striking feature in ALE is an impaired memory for personal experiences that is, autobiographic-episodes (see Lad et al., 2019; Witt et al., 2015). Since this type of impairment was not explicitly reported by default in the medical records of our retrospective study, our cohort’s true prevalence is unknown. However, in our experience, impaired memories for personal experiences often cannot be recognized, illustrating that they are lost indeed. Autobiographic episodic memory not only engages limbic structures, such as the left anterior medial prefrontal cortex, the medial temporal and posterior cingulate cortex and diencephalic regions, but also requires the right temporo-parietal cortex for reconstruction of spatial context (Levine, Svoboda, Hay, Winocur, & Moscovitch, 2002). Given that the impairment of autobiographical-episodic memory causes a severe burden in the affected patients (Levine et al., 2002), more research is clearly needed.
The findings of previous studies of memory functions in ALE are inconsistent (see Introduction). First, there appears to be some confusion in the literature due to a lack of clear definition of these cognitive constructs, since for example, Graus and colleagues in some passages refer to short-term memory and in others to working memory deficits (see Graus et al., 2016). Second, sample sizes are small, ranging from 7 to 23 patients (see Helmstaedter et al., 2019; Holtmann et al., 2018; Loane et al., 2019). Because we found high SD in our large sample, varying results in small samples are to be expected. Third, samples are not comparable, as some contain different AAB-types (see Holtmann et al., 2018), whereas others are restricted to specific AABs (see Butler et al., 2014; Hanert et al., 2019; Loane et al., 2019). We here compare groups of AAB-types. Forth, there are different definitions of neuropsychological impairments: Butler and colleagues used a z-score of −1 as cut-off, whereas Loane and colleagues use one of −1.67 (see Butler et al., 2014; Loane et al., 2019). Moreover, others define impairments as significant difference from control groups (see Hanert et al., 2019; Helmstaedter et al., 2019).
Attention
The mean processing speed—but none of the other attention parameters—in our cohort was in deficit. Only a minority of patients scored below the respective cut-off score, consistent with an earlier study on 15 patients with LGI1 AABs (Hanert et al., 2019). Others found reduced processing speed in comparison to a healthy control group (Butler et al., 2014; Day, Babulal, Rajasekar, Stout, & Roe, 2020; Sola-Valls et al., 2020). However, it is debatable, how valid processing speed assessed by the Trail Making Test (TMT-A, see e.g., Reitan et al., 1956) is a measure of attention. It appears to be used for this purpose for example, in the EpiTrack battery (Helmstaedter, 2005), but it is also described as measure of visuo-spatial scanning (Lezak et al., 2012; Thompson et al., 1999). Whether an impaired reaction time on auditory stimuli in a divided attention task is representative for ALE cannot be stated based on the small number of test applications in our cohort. In addition, the symptomatic treatment might have influenced the outcome. The major neuropsychological side-effects of centrally effective drugs are tiredness and impaired attention. Thus, the impaired attention functioning may be affected by centrally effective symptomatic treatment, taken by the ALE-patients despite being immunotherapy-naïve. Correspondingly, patients rarely complained about attention deficits at first consultation before symptomatic treatment was initiated.
Executive Functions and Language
Studies reporting in detail executive functions and language in ALE are scarce. We found flexibility to be in deficit with regard to mean performance. Prevalence was inconspicuous in our cohort. However, variance is high. Some ALE-patients scored very far below average, whereas others performed within the average range. Although earlier studies found flexibility to be severely impaired in some patients (Hanert et al., 2019), other studies found flexibility in ALE to be significantly worse than in controls or rather in the lower average range (Holtmann et al., 2018; Sola-Valls et al., 2020). Some researchers barely reported patients with a z-score below −1.67 (Loane et al., 2019). In this respect, the large range of flexibility performance we observed is consistent with the literature. Flexibility involves both parietal lobes, premotor and supplementary motor cortex and primary visual cortex and visual association cortex (Karimpoor et al., 2017).
Nonverbal problem solving appeared normal in our ALE cohort, whereas word fluency was not in deficit, but below average with a minor prevalence of impairments. Other researchers also found word fluency to be impaired or worse than in control patients (Hanert et al., 2019; Hansen et al., 2016; Holtmann et al., 2018; Sola-Valls et al., 2020). Word fluency requires recall of words from memory. The fact that no deficit could be detected here appears to support the notion that in ALE memory consolidation seems to be more severely impaired than recall. Intact naming performance in this cohort appears to be in line with this.
Social Cognition
Emotion recognition was in deficit with regard to mean performance in our cohort but not concerning prevalence. Notably, patients had most pronounced difficulties in recognizing fear. Emotion recognition is mainly associated with the amygdala and the ventromesial frontal lobe (Adolphs, 2002; Heberlein, Padon, Gillihan, Farah, & Fellows, 2008), so our finding is consistent with lesions of the limbic system in ALE. This could be relevant in the differential diagnosis to late onset temporal lobe epilepsy associated with hippocampal sclerosis occurring independently from ALE (see Hlobil et al., 2007). In contrast, rating of arousal in facial expression was unimpaired for all emotions. This finding is in agreement with a study in a small sample of three ALE-patients using pictures of faces (Dodich et al., 2016). Other researchers could show impairments in autonomic and cognitive discrimination of stimulus arousal in ALE and herpes encephalitis (HE), respectively, but could not find impairments in the evaluation of emotion valence using various stimuli (Gläscher & Adolphs, 2003; Holtmann et al., 2018). It is well known that the amygdala reacts stronger to faces than to scenes (Hariri, Tessitore, Mattay, Fera, & Weinberger, 2002). Therefore, one can assume that a functional impairment of the amygdala in emotion processing might only become apparent under high activity demands in ALE.
Psychological Symptoms
ALE-patients are often reported to show symptoms of depressed mood, but also other psychological symptoms such as anxiety, visual hallucinations, delusion, and sleep disorders (see Endres et al., 2020; Sezgin et al., 2018; Somers et al., 2011; Van Sonderen et al., 2016). Using the BDI-II (containing 21 items), we could confirm that the majority, ~60%, of ALE-patients report elevated symptoms of depression. In contrast, Klein and colleagues found symptoms of depression and anxiety in a large cohort of 316 ALE-patients with VGKC antibodies in only 16.5% (Klein et al., 2013) of their patients. We were unable to detect depression in ALE with other screening instruments for depression assessing fewer symptoms of depression than the BDI-II such as the HADS (using seven items to assess symptoms of depression), the BSCL (containing six items of depression), and the SCL-90R (containing 13 items).
In our experience, symptoms of depression in ALE often precede physical and cognitive symptoms. Patients are commonly clueless about the cause of their tearfulness and depressed mood. In assessing neuropsychological symptoms in ALE, we suggest to be careful not to erroneously attribute an affective reaction on psychosocial burden as evidence for ALE. It is assumable that at least in part, depression in ALE is endogenous. Becker, Berg, Lesch, and Becker (2001)) summarized evidence pointing towards an involvement of the limbic system in primary depression and in secondary-to-Parkinson disease depression (Becker et al., 2001). The authors found a reduced echogenicity in transcranial sonography and an increase signal intensity in T2-weighted MRI in the brain stem midline indicating structural alterations (Becker et al., 2001). Because the basal limbic system encompasses noradrenergic nuclei (nucleus coeruleus), serotonergic nuclei (raphe complex), and dopaminergic nuclei (ventral tegmental nucleus) in the midbrain, an impairment of the limbic system would inevitably result in a reduction of these monoaminergic transmitters, which is associated with depression (Becker et al., 2001). Becker and colleagues point out that the main fiber complex constituting this area is the middle forebrain bundle. Other fiber tracts such as the longitudinal fasciculus (Becker et al., 2001) were also found to be altered in ALE (Wagner et al., 2016). This could support our assumption of an endogenous contribution of symptoms of depression in ALE. Though this needs to be clarified in further research.
Neuropsychological Complaints
Clinicians contacted for first consultation should be aware that in patients with suspected ALE most frequent psychological complaints are symptoms of depressed mood occurring in 25%. Moreover, ~33% of patients already indicate at first consultation that cognitive impairments might exceed memory problems. These cognitive and psychological symptoms typically develop in a subacute manner. Loss of memory of autobiographical episodes and impaired emotion recognition seem to be rather specific complaints.
Limitations
Due to retrospective data collection, the number of patients on which the evaluation of distinct neuropsychological functions is based, varies considerably. However, we have only included data from tests applied in at least seven patients to be able to present the cognitive domains of attention, memory, and executive functions with its respective functions according to neuropsychological diagnostic guidelines (see Müller & Klein, 2014; Sturm, 2014; Thöne-Otto, 2009). Nevertheless, it would have been desirable to include nonverbal recognition memory in our test battery and to obtain more data on problem solving and divided attention. The partially small number of patients on which the data of separate cognitive functions are based limits the power to detect significant differences between AAB groups. Besides, this study cannot rule out possible differences between various AAB-types.
Because the data originate from a monocentric cohort, the generalizability of the results to the population of ALE is limited. The strict selection of patients with ALE prohibits generalization to other forms of autoimmune encephalitis especially to those with multifocal cortical and subcortical grey or white matter lesions.
The neuropsychological performance of ALE presented here is likely confounded by symptomatic medications, which 83.6% of the patients took at the time of investigation. However, this fact should not compromise the clinical value of this presentation as it reflects the common clinical situation when establishing the diagnosis of ALE.
For a definite proof that assessed cognitive impairments are attributed to extra-limbic structural alterations, sophisticated structural and functional imaging studies in relation to cognitive performance are required.
Conclusion
This study provides a detailed clinical neuropsychological presentation of ALE in immunotherapy-naïve patients. It confirms that memory impairments and symptoms of depression form hallmarks of ALE. It also provides further and new insights: (i) Memory impairment predominantly pertains to a consolidation impairment for new information resulting in long-term memory and recognition deficits. (ii) Short-term and working memory can be impaired, but usually only at a subclinical level. (iii) Recall of retrograde autobiographical episodes can be affected and (iv) ALE-patients suffer from deficits in emotion recognition especially regarding fear. In addition, we found (v) apraxia in pantomimes and imitation of gestures, which has not yet been described in ALE-patients. Further deficits may include impaired visuo-construction, processing speed, and flexibility. However, the performances between patients vary considerably.
Taken together, neuropsychological deficits presented in our cohort indicate possible involvement of brain networks outside the limbic system in ALE.
Neuropsychological assessment for diagnosing ALE should include long-term memory, memory recognition, autobiographical-episodic memory, emotion recognition, and a detailed investigation of symptoms of depression.
Supplementary Material
Contributor Information
Christoph Mueller, Department of Neurology with Institute of Translational Neurology, Westfälische Wilhelms-University of Münster, Münster, Germany.
Lisa Langenbruch, Department of Neurology with Institute of Translational Neurology, Westfälische Wilhelms-University of Münster, Münster, Germany.
Johanna M H Rau, Department of Neurology with Institute of Translational Neurology, Westfälische Wilhelms-University of Münster, Münster, Germany.
Tobias Brix, Institute of Medical Informatics, Westfälische Wilhelms-University of Münster, Münster, Germany.
Christine Strippel, Department of Neurology with Institute of Translational Neurology, Westfälische Wilhelms-University of Münster, Münster, Germany.
Andre Dik, Department of Neurology with Institute of Translational Neurology, Westfälische Wilhelms-University of Münster, Münster, Germany.
Kristin S Golombeck, Department of Neurology with Institute of Translational Neurology, Westfälische Wilhelms-University of Münster, Münster, Germany.
Constanze Mönig, Department of Neurology with Institute of Translational Neurology, Westfälische Wilhelms-University of Münster, Münster, Germany.
Andreas Johnen, Department of Neurology with Institute of Translational Neurology, Westfälische Wilhelms-University of Münster, Münster, Germany.
Saskia Räuber, Department of Neurology with Institute of Translational Neurology, Westfälische Wilhelms-University of Münster, Münster, Germany; Department of Neurology, Medical Faculty, Heinrich-Heine University of Düsseldorf, Düsseldorf, Germany.
Heinz Wiendl, Department of Neurology with Institute of Translational Neurology, Westfälische Wilhelms-University of Münster, Münster, Germany.
Sven G Meuth, Department of Neurology with Institute of Translational Neurology, Westfälische Wilhelms-University of Münster, Münster, Germany; Department of Neurology, Medical Faculty, Heinrich-Heine University of Düsseldorf, Düsseldorf, Germany.
Jens Bölte, Institute of Psychology, Westfälische Wilhelms-University of Münster, Münster, Germany.
Stjepana Kovac, Department of Neurology with Institute of Translational Neurology, Westfälische Wilhelms-University of Münster, Münster, Germany.
Nico Melzer, Department of Neurology with Institute of Translational Neurology, Westfälische Wilhelms-University of Münster, Münster, Germany; Department of Neurology, Medical Faculty, Heinrich-Heine University of Düsseldorf, Düsseldorf, Germany.
Funding
None declared.
Conflicts of Interest
None.
Appendix: Aggregation of neuropsychological symptoms in ALE
| Domains and functions | Definition | Tests and Description | Cut-off | Reference |
|---|---|---|---|---|
| Praxia | ||||
| Praxia | to perform movements purposefully | Kölner Apraxie Screening (KAS): pantomimes of object use and imitations of gestures shall be executed. | Mean score ≤ 76 | (Weiss et al., 2013) |
| Visuo-construction (constructive praxia) | Constructing a whole out of elements | Rey Complex Figure Test (RCFT): copy a complex figure consisting of 18 geometrical elements. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) figure: Copy four, more simple figures. We combined these two tests referring to “visuo-construction”. | z < −1 | (Meyers & Meyers, 1995, Welsh et al., 1994) |
| Memory | ||||
| Verbal short-term memory | Verbal information that can be kept in mind for a few seconds | Digit Span test of the Wechsler Memory Scale-Revised (WMS-R): Reproduction of increasingly longer sequences of numbers. | z < −1 | (Wechsler, 1987) |
| Nonverbal short-term memory | Nonverbal information that can be kept in mind for a few seconds | Wechsler Memory Scale-Revised (WMS-R), Spatial Span: Reproduction of increasingly longer spatially separated sequences of taps on blocks. | z < −1 | (Wechsler, 1987) |
| Verbal learning capacity | Ability to acquire new verbal information | A list of words is to be learned over repeated learning trials in the Verbaler Lern- und Merkfähigkeitstest (VLMT) and in CERAD. We comprised them as “verbal learning capacity”. | z < −1 | (Helmstaedter et al., 2001, Welsh et al., 1994) |
| Nonverbal learning capacity | Ability to acquire new nonverbal information | Diagnosticum für Zerebralschädigung (DCS-II). Learn images of figures and reproduce them with stics over learning trials (DCS_Rcum) | z < −1 | (Weidlich et al., 2011) |
| Verbal immediate recall | Retrieval of verbal information stored in memory immediately after learning | CERAD: recall of learned words a few moments after learning | z < −1 | (Welsh et al., 1994) |
| Nonverbal immediate recall | Retrieval of nonverbal information stored in memory immediately after learning | CERAD: figures are to be drawn from memory, after a few moments. Likewise, in the RCFT. We combined these two to “nonverbal immediate recall”. | z < −1 | (Welsh et al., 1994, Meyers & Meyers, 1995) |
| Verbal forgetting | Loss of stored verbal information | The difference between amount of words reproduced after the last learning trial of the VLMT and the recall | z < −1 | (Helmstaedter et al., 2001) |
| Verbal long-term memory | Recall of verbal information with a delay longer than short-term memory interval. | VLMT: learned word list is asked to recall after a 20–30 minute delay | z < −1 | (Helmstaedter et al., 2001) |
| Nonverbal long-term memory | Recall of nonverbal information with a delay longer than short-term memory interval. | RCFT: Reproduction 30 minutes after copying. | z < −1 | (Meyers & Meyers, 1995) |
| Verbal recognition | To remember that certain verbal information had been learned when it is presented again | In VLMT and CERAD, after recall trial, learned words are presented again together with distractors. The task is to recognize which word had been learned earlier and which not. In the CERAD the accuracy of word recognition is presented in the discriminability index. We comprised the accuracy of word recognition in VLMT and CERAD in “verbal recognition” inspite of different time delays in VLMT and CERAD. | z < −1 | (Helmstaedter et al., 2001, Welsh et al., 1994) |
| Nonverbal recognition | remember that certain nonverbal information had been learned when it is presented again | Not reported in the main text, because of too few cases. | ||
| Attention | ||||
| Processing-speed | Speed of processing information is a common operationalization of attention intensity. | Trail Making Test (TMT) –A: numbers spread on a page shall be connected with a pencil in ascending order as fast as possible. We applied the TMT-A without time-constraints. | z < −1 | (Tombaugh, 2004) |
| Divided attention | Pay attention on two or more tasks simultaneously. | Testbatterie zur Aufmerksamkeitsprüfung (TAP), Divided Attention: patients are presented visual stimuli on a screen and simultaneously auditory stimuli, to react on. Reaction speed on both stimuli as well as accuracy are recorded. | z < −1 | (Zimmermann & Fimm, 2014) |
| Executive Functions | ||||
| Verbal working memory | Maintenance and manipulation of verbal information in mind. | WMS-R: Reproduction of digit spans backwards | z < −1 | (Wechsler, 1987) |
| Nonverbal working memory | Maintenance and manipulation of nonverbal information in mind. | WMS-R: Reproduction of spatial spans backwards | z < −1 | (Wechsler, 1987) |
| Verbal divergent problem solving | Verbal fluency | See language: semantic and lexical fluency | z < −1 | (Aschenbrenner et al., 2000) |
| Nonverbal divergent problem solving | Production of as many different solutions as possible. | Hamasch 5 Punkte Test (H5PT): fields with each five dots in the same orientation on a paper. Patients are asked to draw as many different pattern as possible by connecting at least two dots per field. | z < −1 | (Haid et al., 2004) |
| Nonverbal convergent problem solving | Find the one solution of a problem | D-KEFS, Tower Test: wooden slices have to be moved and placed on stics, following specific rules to build given towers with a minimum amount of movements. | z < −1 | (Delis et al. 2001) |
| Flexibility | Ability to switch between tasks | TMT-B: numbers and letters shall be connected alternating in ascending order, as fast as possible. We applied no time constraints | z < −1 | (Tombaugh, 2004) |
| Language | ||||
| Semantic word fluency (verbal convergent problem solving) | Enumerating words of a distinct category | Regensburger Wortflüssigkeitstest (RWT): name as many animals as possible within one minute. | z < −1 | (Aschenbrenner et al., 2000) |
| Lexical word fluency (verbal convergent problem solving) | Enumerating words of a distinct first letter | RWT: name as many words starting with “S”, as possible within one minute. Word fluency can also be classified as verbal divergent problem solving (see above). | z < −1 | (Aschenbrenner et al., 2000) |
| Naming | Naming of presented objects | Name drawings of presented objects in the Boston Naming Test (BNT) or Wortproduktionsprüfung (WPP) | z < −1 | (Merten, 2004, Blanken et al., 1999) |
| Social Cognition | ||||
| Emotion recognition | Identifying emotions | Social Cognition and Emotional Assessment (Mini-Sea), Facial Emotional Recognition” test: emotions expressed in faces shall be identified on pictures | z < −1 | (Bertoux et al., 2012) |
| Intensity of emotions | Identifying the arousal of emotions | Facial Expressions of Emotions – Stimuli and Test (FEEST): intensity of emotions has to be rated in facial expressions on photographs | z < −1 | (Young et al., 2002) |
| Psychological Symptoms | ||||
| diverse | psychological strain | The Symptom Checklist-90-R (SCL-90-R) and Brief-Symptom-Checklist (BSCL): self-report questionnaires to evaluate psychological problems and symptoms on 9 subscales and 3 resulting global scales. We combined SCL-90-R and BSCL by referring to “SCL_BSCL”. | T > 60 used | (Franke, 2002, Franke, 2017) |
| Depressed mood | symptom of depression | Beck Depression Inventory-II (BDI-II): self-report questionnaires to evaluate symptoms of depression. | score ≥ 14 for mild or more severe symptomatology. | (Hautzinger et al., 2006)) |
| Anxiety and depressed mood | Symptoms of anxiety disorder and depression | Hospital Anxiety and Depression Scale (HADS): self-report questionnaires to evaluate symptoms of anxiety and depression on separate subscales. | Subscale score ≥ 8 for possible presence of anxiety or depressive disorder | (Herrmann-Lingen, 2011) |
References
- Adolphs, R. (2002). Neural systems for recognizing emotion. Current Opinion in Neurobiology, 12(2), 169–177. [DOI] [PubMed] [Google Scholar]
- Alves P, Maruta C, Albuquerque L, Martins IP. (2017). Dissociation findings between short-term and long-term memory in autoimmune limbic encephalitis. Journal of the Neurological Sciences [Internet] 381:126–127. Available from: 10.1016/j.jns.2017.08.016Adolphs, R. (2002). Neural systems for recognizing emotion. Current Opinion in Neurobiology, 12(2), 169–177. [DOI] [PubMed] [Google Scholar]
- Ahmad, A., Ramakrishna, S., Meara, J., & Doran, M. (2010). Autoimmune limbic encephalitis: A reversible form of rapidly progressive amnesia and seizures. Journal of the Royal College of Physicians of Edinburgh, 40(2), 123–125. 10.4997/JRCPE.2010.208. [DOI] [PubMed] [Google Scholar]
- Argyropoulos, G. P. D., Moore, L., Loane, C., Roca-Fernandez, A., Lage-Martinez, C., Gurau, O., et al. (2020). Pathologic tearfulness after limbic encephalitis: A novel disorder and its neural basis. Neurology, 94(12), e1320–e1335. 10.1212/WNL.0000000000008934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller, L., Kleopa, K. A., Wu, G. F., Rossi, J. E., Rosenfeld, M. R., & Dalmau, J. (2007). Autoimmune limbic encephalitis in 39 patients: Immunophenotypes and outcomes. Journal of Neurology, Neurosurgery and Psychiatry, 78(4), 381–385. 10.1136/jnnp.2006.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, T., Ernst, L., David, B., Becker, A. J., Wagner, J., Witt, J. A., et al. (2020). Fixel-based analysis links white matter characteristics, serostatus and clinical features in limbic encephalitis. NeuroImage: Clinical, 27(May), 102289. 10.1016/j.nicl.2020.102289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, G., Berg, D., Lesch, K. P., & Becker, T. (2001). Basal limbic system alteration in major depression: A hypothesis supported by transcranial sonography and MRI findings. International Journal of Neuropsychopharmacology, 4(1), 21–31. 10.1017/S1461145701002164. [DOI] [PubMed] [Google Scholar]
- Brierley, J. B., Corsellis, J. A. N., Hierons, R., & Nevin, S. (1960). Subacute encephalitis of later adult life. Mainly affecting the limbic areas. Brain, 83(3), 357–368. 10.1093/brain/83.3.357. [DOI] [Google Scholar]
- Butler, C. R., Miller, T. D., Kaur, M. S., Baker, I. W., Boothroyd, G. D., Illman, N. A., et al. (2014). Persistent anterograde amnesia following limbic encephalitis associated with antibodies to the voltage-gated potassium channel complex. Journal of Neurology, Neurosurgery and Psychiatry, 85(4), 387–391. 10.1136/jnnp-2013-306724. [DOI] [PubMed] [Google Scholar]
- Chou, I. J., Wang, H. S., Lin, J. J., Kuo, C. F., Lin, K. L., Chou, M. L., et al. (2013). Limbic encephalitis in taiwanese children and adolescence: A single center study. Pediatrics and Neonatology, 54(4), 246–253. 10.1016/j.pedneo.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Crisp, S. J., Kullmann, D. M., & Vincent, A. (2016). Autoimmune synaptopathies. Nature Reviews Neuroscience, 17(2), 103–117. 10.1038/nrn.2015.27. [DOI] [PubMed] [Google Scholar]
- Curtis, C. E., & D’Esposito, M. (2003). Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences, 7(9), 415–423. 10.1016/S1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- D’Esposito, M., Postle, B. R., Ballard, D., & Lease, J. (1999). Maintenance versus manipulation of information held in working memory: An event-related fMRI study. Brain and Cognition, 41(1), 66–86. 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- D’Esposito, M., Postle, B. R., & Rypma, B. (2000). Prefrontal cortical contributions to working memory: Evidence from event-related fMRI studies. Experimental Brain Research, 133(1), 3–11. 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Day, G. S., Babulal, G. M., Rajasekar, G., Stout, S., & Roe, C. M. (2020). The road to recovery: A pilot study of driving Behaviors following antibody-mediated encephalitis. Frontiers in Neurology, 11(July), 1–8. 10.3389/fneur.2020.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodich, A., Cerami, C., Iannaccone, S., Marcone, A., Alongi, P., Crespi, C., et al. (2016). Neuropsychological and FDG-PET profiles in VGKC autoimmune limbic encephalitis. Brain and Cognition, 108, 81–87. 10.1016/j.bandc.2016.07.010. [DOI] [PubMed] [Google Scholar]
- Endres, D., Prüss, H., Dressing, A., Schneider, J., Feige, B., Schweizer, T., et al. (2020). Psychiatric manifestation of anti-LGI1 encephalitis. Brain Sciences, 10(6), 1–12. 10.3390/brainsci10060375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke, C., Prüss, H., Heine, J., Reuter, S., Kopp, U. A., Wegner, F., et al. (2017). Evaluation of cognitive deficits and structural hippocampal damage in encephalitis with leucine-rich, glioma-inactivated 1 antibodies. JAMA Neurology, 74(1), 50–59. 10.1001/jamaneurol.2016.4226. [DOI] [PubMed] [Google Scholar]
- Fischer, M., Kunkel, A., Bublak, P., Faiss, J. H., Hoffmann, F., Sailer, M., et al. (2014). How reliable is the classification of cognitive impairment across different criteria in early and late stages of multiple sclerosis? Journal of the Neurological Sciences, 343(1–2), 91–99. 10.1016/j.jns.2014.05.042. [DOI] [PubMed] [Google Scholar]
- Frisch, C., Malter, M. P., Elger, C. E., & Helmstaedter, C. (2013). Neuropsychological course of voltage-gated potassium channel and glutamic acid decarboxylase antibody related limbic encephalitis. European Journal of Neurology, 20(9), 1297–1304. 10.1111/ene.12186. [DOI] [PubMed] [Google Scholar]
- Gadoth, A., Pittock, S. J., Dubey, D., McKeon, A., Britton, J. W., Schmeling, J. E., et al. (2017). Expanded phenotypes and outcomes among 256 LGI1/CASPR2-IgG–positive patients. Annals of Neurology, 82(1), 79–92. 10.1002/ana.24979. [DOI] [PubMed] [Google Scholar]
- Gläscher, J., & Adolphs, R. (2003). Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. Journal of Neuroscience, 23(32), 10274–10282. 10.1523/jneurosci.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus, F., Titulaer, M. J., Balu, R., Benseler, S., Bien, C. G., Cellucci, T., et al. (2016). A clinical approach to diagnosis of autoimmune encephalitis. The Lancet Neurology, 15(4), 391–404. 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote, A., Witt, J. A., Surges, R., Von Lehe, M., Pieper, M., Elger, C. E., et al. (2016). A second chance-reoperation in patients with failed surgery for intractable epilepsy: Long-term outcome, neuropsychology and complications. Journal of Neurology, Neurosurgery and Psychiatry, 87(4), 379–385. 10.1136/jnnp-2015-310322. [DOI] [PubMed] [Google Scholar]
- Hanert, A., Rave, J., Granert, O., Ziegler, M., Pedersen, A., Born, J., et al. (2019). Hippocampal dentate gyrus atrophy predicts pattern separation impairment in patients with LGI1 encephalitis. Neuroscience, 400, 120–131. 10.1016/j.neuroscience.2018.12.046. [DOI] [PubMed] [Google Scholar]
- Hansen, N., Widman, G., Witt, J. A., Wagner, J., Becker, A. J., Elger, C. E., et al. (2016). Seizure control and cognitive improvement via immunotherapy in late onset epilepsy patients with paraneoplastic versus GAD65 autoantibody-associated limbic encephalitis. Epilepsy and Behavior, 65, 18–24. 10.1016/j.yebeh.2016.10.016. [DOI] [PubMed] [Google Scholar]
- Hariri, A. R., Tessitore, A., Mattay, V. S., Fera, F., & Weinberger, D. R. (2002). The amygdala response to emotional stimuli: A comparison of faces and scenes. NeuroImage, 17(1), 317–323. 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Heberlein, A. S., Padon, A. A., Gillihan, S. J., Farah, M. J., & Fellows, L. K. (2008). Ventromedial frontal lobe plays a critical role in facial emotion recognition. Journal of Cognitive Neuroscience, 20(4), 721–733. 10.1162/jocn.2008.20049. [DOI] [PubMed] [Google Scholar]
- Heine, J., Prüss, H., Kopp, U. A., Wegner, F., Then Bergh, F., Münte, T., et al. (2018). Beyond the limbic system: Disruption and functional compensation of large-scale brain networks in patients with anti-LGI1 encephalitis. Journal of Neurology, Neurosurgery and Psychiatry, 89, 1191–1199. 10.1136/jnnp-2017-317780. [DOI] [PubMed] [Google Scholar]
- Helmstaedter, C., Winter, B., Melzer, N., Lohmann, H., & Witt, J. A. (2019). Accelerated long-term forgetting in focal epilepsies with special consideration given to patients with diagnosed and suspected limbic encephalitis. Cortex, 110(February), 58–68. 10.1016/j.cortex.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Helmstaedter, C., & Witt, J. A. (2012). Multifactorial etiology of interictal behavior in frontal and temporal lobe epilepsy. Epilepsia, 53(10), 1765–1773. 10.1111/j.1528-1167.2012.03602.x. [DOI] [PubMed] [Google Scholar]
- Holtmann, O., Schlossmacher, I., Moenig, C., Johnen, A., Rutter, L. M., Tenberge, J. G., et al. (2018). Amygdala enlargement and emotional responses in (autoimmune) temporal lobe epilepsy. Scientific Reports, 8(1), 1–11. 10.1038/s41598-018-27914-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph, R. G. (2017). Limbic system – Hypothalamus, amygdala, hippocampus, septal nuclei, cingulate (4th ed.). Cambridge: Cosmology Science Publishers. [Google Scholar]
- Klein, C. J., Lennon, V., Aston, P. A., McKeon, A., O’Toole, O., Quek, A., et al. (2013). Insights from LGI1 and CASPR2 potassium channel complex autoantibody subtyping. JAMA Neurology, 70(2), 229–234. 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad, M., Mullally, S. L., Houston, A. L., Kelly, T., & Griffiths, T. D. (2019). Characterizing memory loss in patients with autoimmune limbic encephalitis hippocampal lesions. Hippocampus, 29(11), 1114–1120. 10.1002/hipo.23150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster, E., & Dalmau, J. (2012). Neuronal autoantigens-pathogenesis, associated disorders and antibody testing. Nature Reviews Neurology, 8(7), 380–390. 10.1038/nrneurol.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszczyński, M. (2015). Rhythmic working memory activation in the human hippocampus. Cell reports, 13(6), 1272–1282. [DOI] [PubMed] [Google Scholar]
- Levine, B., Svoboda, E., Hay, J. F., Winocur, G., & Moscovitch, M. (2002). Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychology and Aging, 17(4), 677–689. 10.1037/0882-7974.17.4.677. [DOI] [PubMed] [Google Scholar]
- Li-hao, L., Cong-cong, M., & Hai feng, Z., & Ya-jun, L. (2018). Clinical and electrographic characteristics of seizures in LGI1-antibody encephalitis. Epilepsy and Behavior, 88, 277–282. 10.1016/j.yebeh.2018.08.019. [DOI] [PubMed] [Google Scholar]
- Loane, C., Argyropoulos, G. P. D., Roca-Fernández, A., Lage, C., Sheerin, F., Ahmed, S., et al. (2019). Hippocampal network abnormalities explain amnesia after VGKCC-Ab related autoimmune limbic encephalitis. Journal of Neurology, Neurosurgery and Psychiatry, 90(9), 965–974. 10.1136/jnnp-2018-320168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massman, P. J., Sims, J., Cooke, N., Haverkamp, L. J., Appel, V., & Appel, S. H. (1996). Prevalence and correlates of neuropsychological deficits in amyotrophic lateral sclerosis. Journal of Neurology Neurosurgery and Psychiatry, 61(5), 450–455. 10.1136/jnnp.61.5.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer, N., Meuth, S. G., & Wiendl, H. (2012). Neuron-directed autoimmunity in the central nervous system: Entities, mechanisms, diagnostic clues, and therapeutic options. Current Opinion in Neurology, 25(3), 341–348. 10.1097/WCO.0b013e3283531efb. [DOI] [PubMed] [Google Scholar]
- Menon, V. (2015). Salience Network. In: Arthur W. Toga, editor. Brain Mapping: An Encyclopedic Reference, vol. 2, pp. 597–611. Academic Press: Elsevier, Stanford, CA, USA. [Google Scholar]
- Miller, T. D., Chong, T. T. J., Aimola Davies, A. M., Ng, T. W. C., Johnson, M. R., Irani, S. R., et al. (2017). Focal CA3 hippocampal subfield atrophy following LGI1 VGKC-complex antibody limbic encephalitis. Brain, 140(5), 1212–1219. 10.1093/brain/awx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, C., Langenbruch, L. M., Rau, J. M. H., Brix, T., Strippel, C., Dik, A., et al. (2021). Determinants of cognition in autoimmune limbic encephalitis—A retrospective cohort study. Hippocampus July, 1–12. 10.1002/hipo.23375. [DOI] [PubMed] [Google Scholar]
- Müller, S. V., & Klein, T. (2014). 95 Diagnostik und Therapie von exekutiven Dysfunktionen bei neurologischen Erkrankungen. Leitlinien Für Diagnostik Und Therapie in Der Neurologie.. 10.1055/b-0034-37878. [DOI] [Google Scholar]
- Nascimento Alves, P., Maruta, C., Albuquerque, L., & Martins, I. P. (2017). Dissociation findings between short-term and long-term memory in autoimmune limbic encephalitis. Journal of the Neurological Sciences, 381, 126–127. 10.1016/j.jns.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Navarro, V., Kas, A., Apartis, E., Chami, L., Rogemond, V., Levy, P., et al. (2016). Motor cortex and hippocampus are the two main cortical targets in LGI1-antibody encephalitis. Brain, 139(4), 1079–1093. 10.1093/brain/aww012. [DOI] [PubMed] [Google Scholar]
- Renner, A., Baetge, S. J., Filser, M., Ullrich, S., Lassek, C., & Penner, I. K. (2020). Characterizing cognitive deficits and potential predictors in multiple sclerosis: A large nationwide study applying brief international cognitive assessment for multiple sclerosis in standard clinical care. Journal of Neuropsychology, 14(3), 347–369. 10.1111/jnp.12202. [DOI] [PubMed] [Google Scholar]
- Sezgin, M., Çıkrıkçılı, U., Kulaksızoğlu, I. B., Vanlı-Yavuz, E. N., Bebek, N., Tüzün, E., et al. (2018). The psychiatric profiles of patients with temporal lobe epilepsy associated with neuronal auto-antibodies. Neurological Sciences and Neurophysiology, 35(2), 84–90. 10.5152/NSN.2018.10681. [DOI] [Google Scholar]
- Shojima, Y., Nishioka, K., Watanabe, M., Jo, T., Tanaka, K., Takashima, H., et al. (2019). Clinical characterization of definite autoimmune limbic encephalitis: A 30-case series. Internal Medicine, 58(23), 3369–3378. 10.2169/internalmedicine.3029-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola-Valls, N., Ariño, H., Escudero, D., Solana, E., Lladó, A., Sánchez-Valle, R., et al. (2020). Telemedicine assessment of long-term cognitive and functional status in anti-leucine-rich, glioma-inactivated 1 encephalitis. Neurology(R) Neuroimmunology & Neuroinflammation, 7(2). 10.1212/NXI.0000000000000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers, K. J., Lennon, V. A., Rundell, J. R., Pittock, S. J., Drubach, D. A., Trenerry, M. R., et al. (2011). Psychiatric manifestations of voltage-gated potassium-channel complex autoimmunity. Journal of Neuropsychiatry and Clinical Neurosciences, 23(4), 425–433. 10.1176/jnp.23.4.jnp425. [DOI] [PubMed] [Google Scholar]
- Sturm, W. (2014). 93 Diagnostik und Therapie von Aufmerksamkeitsstörungen bei neurologischen Erkrankungen. Leitlinien Für Diagnostik Und Therapie in Der Neurologie, 1–18. 10.1055/b-0034-37876. [DOI] [Google Scholar]
- Thompson, M. D., Scott, J. G., Dickson, S. W., Schoenfeld, J. D., Ruwe, W. D., & Adams, R. L. (1999). Clinical utility of the trail making test practice time. Clinical Neuropsychologist, 13(4), 450–455. 10.1076/1385-4046(199911)13:04;1-Y;FT450. [DOI] [PubMed] [Google Scholar]
- Thöne-Otto, A. I. T. (2009). Gedächtnisstörungen. In Sturm, W., Herrmann, M., & Münte, T. (Eds.), Lehrbuch der Klinischen Neuropsychologie. Grundagen, Methoden, Diagnostik, Therapie (2nd ed., pp. 453–479). Spektrum Akademischer Verlag. [Google Scholar]
- Tran, T., Milanovic, M., Holshausen, K., & Bowie, C. R. (2021). What is normal cognition in depression? Prevalence and functional correlates of normative versus idiographic cognitive impairment. In Neuropsychology, 35(1), 33–41. 10.1037/neu0000717. [DOI] [PubMed] [Google Scholar]
- Tucha, O., Smely, C., & Lange, K. W. (1999). Verbal and figural fluency in patients with mass lesions of the left or right frontal lobes. Journal of Clinical and Experimental Neuropsychology, 21(2), 229–236. 10.1076/jcen.21.2.229.928. [DOI] [PubMed] [Google Scholar]
- Van Sonderen, A., Coenders, E. C., Sanchez, E., De, M. A. A. M., Van, M. H., Wirtz, P. W., et al. (2016). Anti-LGI1 encephalitis – Clinical syndrome and long-term follow-up. Neurology, 87(14), 1–8. 10.1212/WNL.0000000000003173. [DOI] [PubMed] [Google Scholar]
- von Rhein, B., Wagner, J., Widman, G., Malter, M. P., Elger, C. E., & Helmstaedter, C. (2017). Suspected antibody negative autoimmune limbic encephalitis: Outcome of immunotherapy. Acta Neurologica Scandinavica, 135(1), 134–141. 10.1111/ane.12575. [DOI] [PubMed] [Google Scholar]
- Wagner, J., Schoene-Bake, J. C., Witt, J. A., Helmstaedter, C., Malter, M. P., Stoecker, W., et al. (2016). Distinct white matter integrity in glutamic acid decarboxylase and voltage-gated potassium channel-complex antibody-associated limbic encephalitis. Epilepsia, 57(3), 475–483. 10.1111/epi.13297. [DOI] [PubMed] [Google Scholar]
- Wagner, J., Witt, J. A., Helmstaedter, C., Malter, M. P., Weber, B., & Elger, C. E. (2015). Automated volumetry of the mesiotemporal structures in antibody-associated limbic encephalitis. Journal of Neurology, Neurosurgery and Psychiatry, 86(7), 735–742. 10.1136/jnnp-2014-307875. [DOI] [PubMed] [Google Scholar]
- Weissenborn, K., Ennen, J. C., Schomerus, H., Rückert, N., & Hecker, H. (2001). Neuropsychological characterization of hepatic encephalopathy. Journal of Hepatology, 34(5), 768–773. 10.1016/S0168-8278(01)00026-5. [DOI] [PubMed] [Google Scholar]
- Witt, J. A., Vogt, V. L., Widman, G., Langen, K. J., Elger, C. E., & Helmstaedter, C. (2015). Loss of autonoetic consciousness of recent autobiographical episodes and accelerated long-term forgetting in a patient with previously unrecognized glutamic acid decarboxylase antibody related limbic encephalitis. Frontiers in Neurology, 6(MAY), 1–8. 10.3389/fneur.2015.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X., Li, A. N., Zhao, X. H., Liu, X. W., & Wang, S. J. (2019). Clinical features of patients with anti-leucine-rich glioma inactivated-1 protein associated encephalitis: A Chinese case series. International Journal of Neuroscience, 129(8), 754–761. 10.1080/00207454.2019.1567507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


