Abstract
Background
Depriving microbes of iron is critical to host defense. Hemeproteins, the largest source of iron within vertebrates, are abundant in infected tissues in aspergillosis due to hemorrhage, but Aspergillus species have been thought to lack heme import mechanisms. We hypothesized that heme provides iron to Aspergillus during invasive pneumonia, thereby worsening the outcomes of the infection.
Methods
We assessed the effect of heme on fungal phenotype in various in vitro conditions and in a neutropenic mouse model of invasive pulmonary aspergillosis.
Results
In mice with neutropenic invasive aspergillosis, we found a progressive and compartmentalized increase in lung heme iron. Fungal cells cultured under low iron conditions took up heme, resulting in increased fungal iron content, resolution of iron starvation, increased conidiation, and enhanced resistance to oxidative stress. Intrapulmonary administration of heme to mice with neutropenic invasive aspergillosis resulted in markedly increased lung fungal burden, lung injury, and mortality, whereas administration of heme analogs or heme with killed Aspergillus did not. Finally, infection caused by fungal germlings cultured in the presence of heme resulted in a more severe infection.
Conclusions
Invasive aspergillosis induces local hemolysis in infected tissues, thereby supplying heme iron to the fungus, leading to lethal infection.
Keywords: fungal infection, hemolysis, immunity, immunocompromised host, invasive pulmonary aspergillosis
We report that invasive aspergillosis results in localized hemorrhage and heme release in infected tissues. The fungus then takes up heme from the environment, thus acquiring the essential element, iron. This renders the organism more virulent during in vivo infection.
The mortality of invasive aspergillosis remains at 30%–50% with the best available therapy [1], underlining the need for better mechanistic understanding of this infection to identify new therapeutic targets. Iron acquisition is integral to microbial pathogenesis and host defense: as part of the innate immune responses, the host suppresses the concentration of extracellular iron ions to exceedingly low levels, thereby withholding this critical element from invading microbes. In contrast, pathogens have evolved sophisticated mechanisms to acquire iron from host tissues. Siderophore-mediated scavenging of extracellular ionic iron is a well defined mechanism of Aspergillus iron acquisition that is essential to its growth and virulence [2]. Host mechanisms of iron sequestration, such as neutrophil lactoferrin, inhibit Aspergillus growth, and therapeutic iron chelation has an additive benefit to antifungal antibiotics [3, 4]. These mechanisms are clinically important, since iron overload is an independent risk for development of invasive aspergillosis in immunocompromised patients [5, 6].
The host hemeproteins, notably hemoglobin, are the largest iron source in vertebrates. Heme iron is covalently bound to a protoporphyrin ring and is thus unavailable for chelation. Importing extracellular heme is a mechanistically distinct form of iron acquisition in many pathogens [7, 8]. However, a prevailing view in the field is that Aspergillus species cannot import heme [9–11]. We found this surprising, because iron acquisition is essential to the growth of Aspergillus species, and heme is a major source of iron in decomposing vegetation [12], the natural habitat of saprophytic fungi. We reasoned that Aspergillus may have evolved a hitherto undiscovered ability to utilize heme as an iron source. Given the association of invasive aspergillosis with tissue hemorrhage, we tested the hypothesis that heme provides iron to Aspergillus during invasive pneumonia, thereby worsening the outcomes of the infection.
MATERIALS AND METHODS
In Vivo Experiments and Tissue Harvest
Animal studies were performed under institutionally approved protocols. Age- and sex-matched 6- to 10-week-old C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) and hepcidin-deficient mice on C57BL/6 background [13] were used in experiments. Aspergillosis was induced as described [14–16], with minor modifications. Neutropenic mice were inoculated with conidia or 6 × 105 germlings. In some experiments, mice were administered 100 µg of hemin (Sigma, St. Louis, MO), equivalent to the heme content of ~1% of mouse blood volume, or a molar equivalent of tin(IV)-protoporphyrin, with conidia and daily thereafter. Intratracheal injection, tissue harvest, bronchoalveolar lavage (BAL), and histology were performed as previously described [17, 18].
In Vitro Studies
Aspergillus fumigatus strain 13073 (American Type Culture Collection, Manassas, VA) or a green fluorescent protein (GFP)-expressing H237 stain (a gift from Dr. David Askew, University of Cincinnati) [19] were cultured at 37°C on Sabouraud’s dextrose agar, in Roswell Park Memorial Institute (RPMI) 1640 medium or in minimal media. Conidia were harvested and germlings generated as previously described [20]. Iron-free minimal media were prepared as previously described [21], omitting the addition of ferrous sulfate to Hutner’s Trace elements and using a final pH of 6.7. Hemin-supplemented or tin(IV)-protoporphyrin-supplemented media were prepared by dissolving in 1 mL 0.1 N NaOH in phosphate-buffered saline (PBS), then dilution in PBS to a final concentration of 500 µM in minimal media. Cultures were incubated at 37°C in 6- or 24-well or 100-mm culture plates. Iron-free media was used for culture without supplementation or supplemented with 50 μM final concentration of heme, tin(IV)-protoporphyrin, or ferric chloride. Tin(IV)-protoporphyrin was handled under low lighting conditions to avoid phototoxicity.
Conidial susceptibility to hydrogen peroxide was measured as described [22]. Fungal dry mass was determined by removing mycelia from liquid culture media, placing them on preweighed filter paper, air drying overnight, and weighing. Conidiation was determined by removing the mycelium from liquid media, rinsing in 4 mL 2 mM PBS 0.1% Tween and enumeration of conidia under a hemocytometer. For measurements of intracellular iron, germlings were washed in 0.1% Tween in PBS, then homogenized in a high-speed bead basher. For microscopic evaluation of Tin(IV)-protoporphyrin fluorescence in hyphae, sterile pipette tips were used to dissociate hyphal mats. Resulting hyphal strands were placed on glass slides, allowed to dry in the dark for 2 hours, and then imaged under a fluorescent microscope (Axio Imager; Carl Zeiss Microscopy, White Plains, NY) using Zeiss no. 15 filter set (excitation BP 546/12, beamsplitter FT 580, emission LP 590). Tin(IV)-protoporphyrin fluorescence was quantified spectroscopically at excitation and emission wavelengths of 425/590 nm.
To identify candidate A fumigatus heme metabolism proteins, we queried the Aspergillus (http://www.aspgd.org) and Candida (http://www.candidagenome.org) genome databases for the following ontology terms: heme transmembrane transporter activity (GO:0015232), heme transmembrane transport (GO:0035351), heme import into cell (GO:0140420), heme export from vacuole to cytoplasm (GO:0140357), endocytic heme import into cell (GO:0140421), heme import across plasma membrane (GO:1904334), heme transport (GO:0015886), iron acquisition from host (GO:0044847), and hemoglobin binding (GO:0030492). We then searched for annotated genes in Candida or non-fumigatus Aspergillus species that were orthologous or a best hit to a gene in A fumigatus using the Aspergillus genome database, and we added orthologous or best hits to PUG1 in Saccharomyces cerevisiae due to its role in heme uptake [23]. We prioritized the list using validated polymerase chain reaction primers from the literature, yielding the following final list of possible genes targets: CfmA/Af6g14090, CfmB/Af6g10580, CfmC/Af6g06690, GpdA [24], aspHS/Afu3G00590 [25], MirD/Afu3g03440 [26], FlcA/Afu4g13340, and Afu2g17650, Afu2g06100 [27].
Other Measurements
Fungal colony-forming units (CFUs) were measured by homogenizing freshly excised lungs in 1 mL distilled water at 50 Hz for 10 minutes (TissueLyser LT; QIAGEN, Venlo, Netherlands). Serial 2-fold dilutions of the samples in water were then cultured in duplicate at 37°C on Sabourad’s dextrose agar containing 0.05% Triton X-100. After 24 hours, plates were photographed and CFUs were counted. Commercial enzyme-linked immunosorbent assays were used to measure the concentrations of albumin (Abcam, Cambridge, United Kingdom); 1,3 β-glucan (Associates of Cape Cod, East Falmouth, MA); hemoglobin (Cayman Chemical, Ann Arbor, MI); and hepcidin (Intrinsic Life Sciences, La Jolla, CA). Total protein was measured using the Bradford assay (Bio-Rad, Hercules, CA). Fungal messenger ribonucleic acid (RNA) was isolated using a commercial kit (QIAGEN) after grinding in liquid nitrogen, then transcribed into complementary deoxyribonucleic acid (Bio-Rad) and amplified with SYBR Green, using A fumigatus-specific primers gathered from the literature (Table 1). Murine tissue RNA was extracted and hepcidin expression quantified as previously described [28]. Plasma iron concentrations were measured with a commercial kit (Sekisui Diagnostics). Tissue and cell culture iron were measured using the ferrozine assay, as previously described [14, 29], with the following modification: 2 aliquots from each sample was run in parallel, 1 treated with an equal volumes 20% trichloroacetic acid 2 N HCl in distilled water, and 4.5% KMnO4 (mixed immediately before use) and 1 with 2 N HCl only. Since KMnO4 is required to liberate iron from heme, the aliquots processed without KMnO4 measured nonheme iron, and the difference between the 2 measurements from each sample represented heme iron.
Table 1.
Aspergillus fumigatus Primer Sequences Used in the Study
| Gene | Forward | Reverse |
|---|---|---|
| β-actin | 5’ TCA TCA TGC GCG ACA GCT TA | 5’ CGT GCT TGG GGT AGC TG |
| Afu2g06100 | 5’ TGG TAA CCA GGG ATC TTT CG | 5’ TAG GCC TCA ATG CCA GAA AC |
| Afu2g17650 | 5’ TGC TGA TGA AAG TGG TCT GC | 5’ AAG CCG ATA AAG ACC AGA CG |
| aspHS | 5’ TGG TAC AAG GAC GGT GAC AA | 5’ GTC CCA CTG GAC TCT TCC AA |
| CfmA | 5’ ATG TCT CTG CTC CCA AGC CAA CTA | 5’ CTA AAG GAT AAT CAA GGC AGC GAG |
| CfmB | 5’ ATG CAC TTC TCT CGT ACT TCC CT | 5’ CAA CAA CAC CTG CCA CAT TT |
| CfmC | 5’ CCT GCT CTT CGC GCA TGC CT | 5’ GCG CAA TGA GAC CAA TGT CCT TGT |
| FlcA | 5’ GAG GAT GTG GCC AAA CAG AT | 5’ GTA ACG GGT ATC GGG GTT TT |
| GpdA | 5’ TCC ACT ATG CTG CCT ACA TGC TCA | 5’ TGC CGT TGA GAG AAG GAA TGA CCT |
| HapX | 5’ CCC ATC AGC CAG GCT ACA AA | 5’ AGG CGT CGG CAC AAG ATA AA |
| MirD | 5’ ATC GGC ACA AGG CAA AGA AGA G | 5’ ATG GAA AAC GGC AGG AGA AAA A |
Statistical Analyses
Data were analyzed in Prism software (version 9; GraphPad Software, San Diego, CA). At a single time point, the Mann-Whitney test was used to compare 2 groups, and one-way analysis of variance (ANOVA) with a Tukey multiple comparison posttest was used to analyze more than 2 groups. Change over time was assessed using the Friedman test, and changes between 2 groups over time or over a range of concentrations were compared using 2-way ANOVA with Tukey multiple comparison posttest. Survival data were expressed using Kaplan-Meier curves and compared by log-rank test. In cases where Tukey multiple comparison posttest was used, multiplicity-adjusted P values are reported. Two-sided probability values <.05 were considered statistically significant.
RESULTS
Pulmonary Hemorrhage During Invasive Aspergillosis Makes Tissue Iron Available
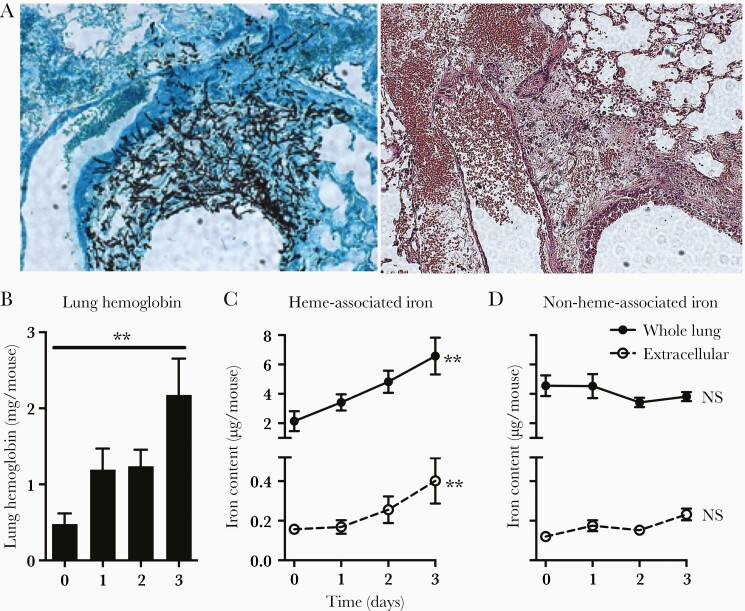
Pulmonary hemorrhage is a feature of invasive aspergillosis in humans and mouse models. Consistent with this, histologic assessment of lungs of neutropenic mice with invasive aspergillosis showed hyphal penetration of blood vessel walls and extensive hemorrhage into the lung parenchyma (Figure 1A). We quantified the lung hemorrhage and found lung hemoglobin content to progressively increase in the first 3 days of infection (Figure 1B), which correlated with the increase in heme-associated iron in the lungs (including both intra- and extracellular iron pools), and extracellular iron in the bronchoalveolar lavage fluid supernatant (Figure 1C), indicating that infection was associated with hemolysis in the lung. In contrast, lung nonheme-associated iron did not change significantly during the infection (Figure 1D).
Figure 1.
Lung hemorrhage during invasive pulmonary aspergillosis. (A) Lung histology on day 2 of infection. Serial sections of the lung show invasion of a pulmonary venule by fungal hyphae from an adjacent airway and alveoli (left, Gomori's methenamine silver stain), and hemorrhage into the lung parenchyma (right, hematoxylin and eosin stain). Original magnification ×100. (B) Hemoglobin concentration in whole lung homogenates. (C and D) Heme- and nonheme-associated iron in whole lungs (intra- and extracellular) and supernatant of bronchoalveolar lavage fluid (extracellular). Data represent mean ± standard error of the mean of n = 5–10 animals per time point in each panel. **, P < .01; time 0 represents neutropenic but uninfected animals. NS, no significant difference.
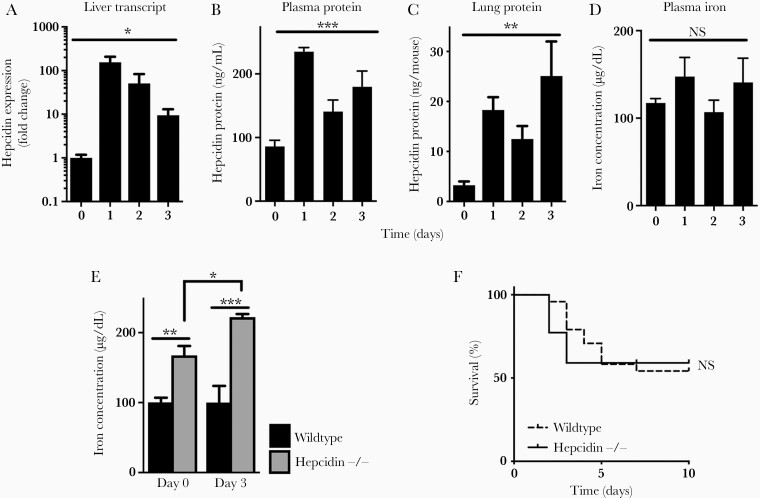
Hepcidin is a hormone produced by the liver during infection, which potently reduces plasma iron concentration, limiting the availability of this essential element to invading pathogens [30]. Similar to other infections, hepcidin transcript in the liver was robustly induced during aspergillosis (Figure 2A), associated with progressive increase in plasma and lung hepcidin protein concentration (Figure 2B and C). We were surprised to find, however, that this increase in hepcidin had no detectable effect on plasma iron concentration (Figure 2D). To probe this unexpected result, we examined the effect of hepcidin deficiency on plasma iron levels during the infection. Compared with wild-type animals, hepcidin-deficient mice had elevated plasma iron at baseline, which became further elevated during invasive aspergillosis (Figure 2E), similar to other infections [30]. Despite this, hepcidin deficiency had no effect on the outcome of aspergillosis (Figure 2F). Because iron acquisition is critical to Aspergillus pathogenicity [10], we hypothesized that, during infection, Aspergillus had access to heme-bound iron within the lung.
Figure 2.
Hepcidin induction and plasma iron during invasive pulmonary aspergillosis. (A) Liver hepcidin messenger ribonucleic acid in the liver normalized to glyceraldehyde 3-phosphate dehydrogenase expression and then to day 0. (B and C) Hepcidin protein concentration in plasma and whole lung homogenate after infection. (D) Plasma iron concentration. (E) Comparison of plasma iron concentration in wild-type and hepcidin-deficient mice before and during invasive aspergillosis. (F) Survival of neutropenic wild-type and hepcidin-deficient mice during invasive aspergillosis. Time 0 represents neutropenic but uninfected animals. Data in panels A–E represent mean ± standard error of the mean of n = 3–10 animals per time point; for panel F, n = 22–24 per group, combined results of 2 experiments. *, **, and *** denote P < .05, P < .01, and P < .001, respectively. NS, no significant difference.
Aspergillus fumigatus Can Take Up and Utilize Heme
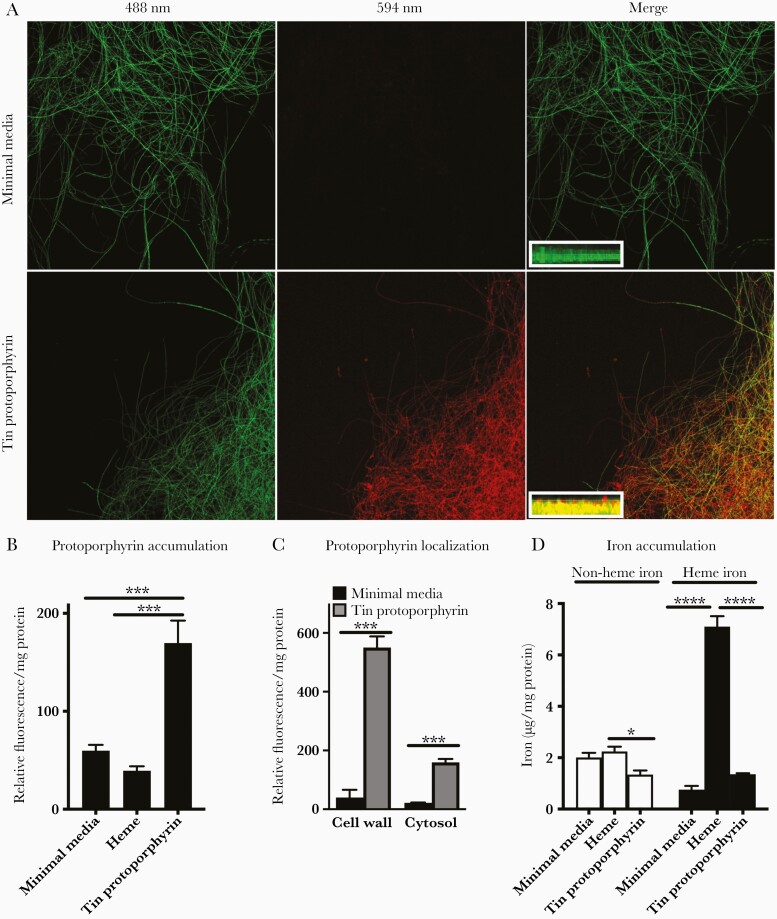
We began by in vitro examination of Aspergillus in iron-deficient media supplemented with various iron sources. We used tin(IV)-protoporphyrin as a heme analog because this molecule is composed of a protoporphyrin-IX ring identical to heme, but it contains a tin, rather than iron, atom that is covalently bound to the center of the ring. Tin(IV)-protoporphyrin is fluorescent, allowing for its identification in cells. We first incubated a transgenic strain of Aspergillus expressing GFP in hyphal cytoplasm [19], and we found that fungal hyphae cultured in minimal media with tin(IV)-protoporphyrin acquired the combined fluorescence of tin(IV)-protoporphyrin and GFP (Figure 3A and B). Cell wall and cytoplasmic fluorescence were 8- and 10-fold higher, respectively, in A fumigatus incubated with tin(IV)-protoporphyrin compared with minimal media (Figure 3C). Consistent with this, Aspergillus hyphae cultured with heme-containing media contained markedly higher levels of heme iron compared with cells cultured in minimal media or in tin protoporphyrin, but only marginally increased nonheme iron compared with tin protoporphyrin (Figure 3D).
Figure 3.
Aspergillus uptake of heme and the heme analog, tin-protoporphyrin. (A) Representative confocal images of a green fluorescent protein (GFP)-expressing Aspergillus fumigatus hyphae cultured in minimal media or minimal media supplemented with tin(IV) protoporphyrin, obtained with indicated laser emission wavelengths, and 2D Z-stack images of hyphal fragments (insets, 0.5-μm steps) showing colocalization of GFP (green) and tin-protoporphyrin fluorescence (red) in the fungal cytoplasm. Original magnifications ×200 for main panels and ×630 for insets. (B and C) Fluorescence at excitation/emission of 425/590 nm normalized to total protein of A fumigatus cultured for 2 days in indicated media. (D) Heme- and nonheme iron recovered from the cytosolic fraction normalized to cytosolic protein content of A fumigatus cultured for 2 days in indicated media. Data represent mean ± standard error of the mean of n = 3–6 replicates in each panel. *, ***, and **** denote P < .05, P < .001, and P < .0001, respectively.
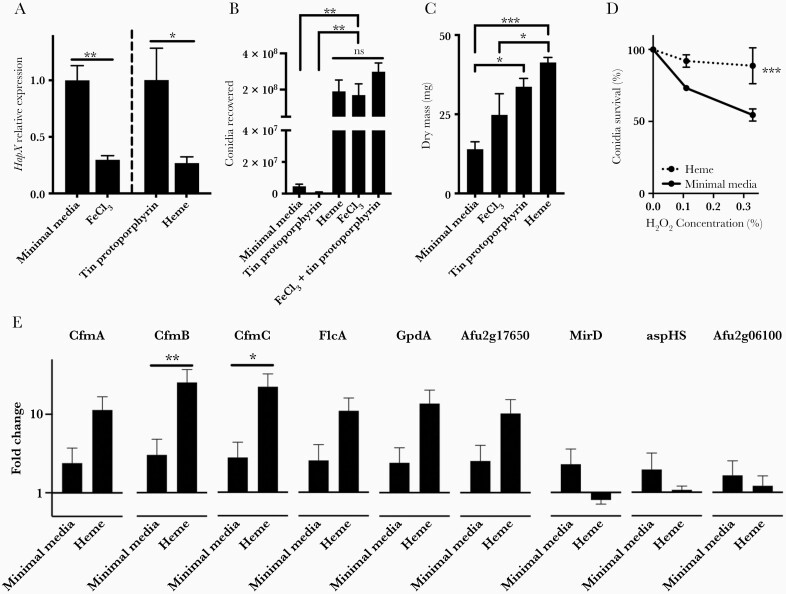
To quantify the effect of heme uptake by Aspergillus, we began by assessing the expression of the fungal expression of HapX, an Aspergillus transcription factor expressed during iron starvation [31], after exposure of the fungus to equimolar concentrations of heme, tin-protoporphyrin, or ferric chloride. As expected, the addition of ferric chloride to minimal media resulted in down-regulation of HapX. Fungal cells cultured with heme displayed a similar suppression of HapX expression compared with cells cultured with tin(IV)-protoporphyrin, indicating that exogenous heme provides bioavailable iron to A fumigatus and relieves iron starvation (Figure 4A). We further noted that Aspergillus grown in heme-containing media produced visibly larger colonies. We quantified this, and we found that fungi cultured in minimal media supplemented with heme or ferric chloride generated markedly more conidia than those cultured in minimal media alone or with tin(IV)-protoporphyrin. In addition, there was no difference between conidia generated from fungal colonies cultured with ferric chloride, heme, and the combination of ferric chloride and tin(IV)-protoporphyrin—the latter indicating that tin(IV)-protoporphyrin does not interfere with conidiation (Figure 4B). The dry mass of the mycelium cultured with heme was greater than both the mycelium cultured in minimal media and with ferric chloride. It is interesting to note that the dry mass of the mycelium cultured with heme was comparable to the dry mass of the mycelium cultured with tin(IV)-protoporphyrin (Figure 4C). We interpret this as evidence that fungal cells under starvation conditions can use the porphyrin ring as an energy source. Conidia from colonies cultured in the presence of heme were also found to display resistance to oxidative killing (Figure 4D).
Figure 4.
Effect of heme uptake on Aspergillus. (A) Expression of fungal HapX normalized to β-actin in Aspergillus fumigatus cultured for 2 days in indicated media. (B) Conidia recovered after 4 days of culture in indicated media. (C) Dry mass of mycelia after 2 days of culture in indicated media. (D) Survival of conidia after exposure to hydrogen peroxide. (E) Relative expression of candidate fungal heme transport genes normalized to β-actin in A fumigatus cultured for 2 days in indicated media. Data represent mean ± standard error of the mean of n = 3–8 replicates in each panel. *, **, and *** denote P < .05, P < .01, and P < .001, respectively. NS, no significant difference.
Next, we sought further evidence that Aspergillus heme uptake alters its transcriptional profile. In addition to the iron-sensing transcription factor HapX (Figure 4A), we quantified the Aspergillus siderophore transporter MirD and the hemolysin aspHS [26, 32–34]. We also reasoned that any proteins involved in heme uptake and metabolism in Aspergillus may share homology with proteins with these functions in other fungi. CfmA, CfmB, and CfmC are Aspergillus proteins homologous to Candida heme transporters. Likewise, Aspergillus FlcA and its paralogs Afu2g17650 and Afu2g06100 were tested because their Candida albicans homolog, CaFLC1, mediates heme uptake [27, 35], and glyceraldehyde-3-phosphate dehydrogenase (encoded by Aspergillus gene GpdA) acts as a heme chaperone in yeast [36]. We thus quantified the transcription of these 9 genes in fungal cultures in minimal media alone or with minimal media supplemented with heme (Figure 4E). The transcription of 2 genes, CfmB and CfmC, was 20-fold greater in the presence of heme, indicating a transcriptional response of the fungus to the presence of heme in its environment. Although some of these changes to fungal phenotype (such as conidiation) do not occur during in vivo infection, taken together, these data indicate that Aspergillus heme acquisition is sufficient to alter its biologic behavior.
Heme Worsens the Outcome of Invasive Aspergillosis
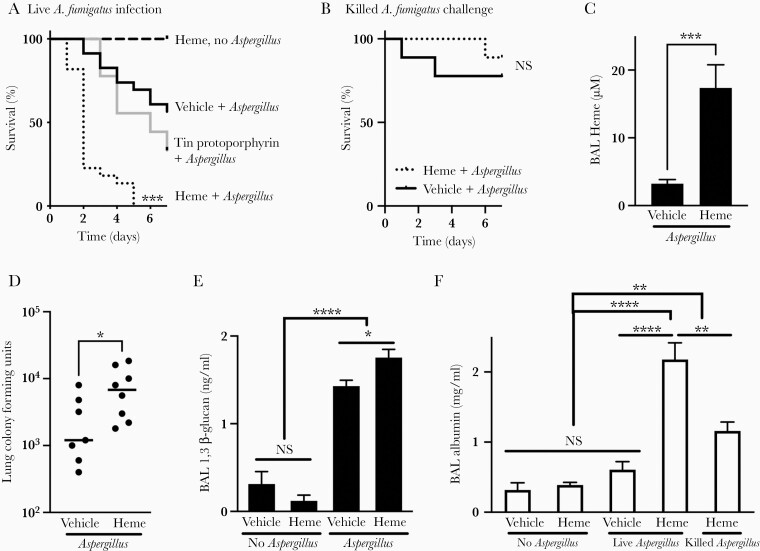
To investigate whether heme is relevant to the pathogenesis of invasive pulmonary aspergillosis in vivo, neutropenic mice were intratracheally inoculated with A fumigatus alone, heme alone, or conidia together with heme or a molar equivalent of tin(IV)-protoporphyrin. Heme alone had no detectable effect in neutropenic mice, but coadministration of heme with Aspergillus resulted in markedly worsened infection outcome compared with vehicle or tin(IV)-protoporphyrin (Figure 5A). To assess whether the detrimental effect of heme was a consequence of exacerbating passive lung injury in response to Aspergillus antigens rather than response to an active infection, we also assessed the outcome when animals were inoculated with heme or vehicle together with ethanol-killed Aspergillus germlings, and we found that animals inoculated with dead germlings had low mortality, regardless of administration of heme (Figure 5B).
Figure 5.
The effect of heme on the outcome of invasive pulmonary aspergillosis. (A and B) Survival of neutropenic mice, intratracheally challenged with indicated inocula. n = 6–23 per group, combined from 2 experiments. (C) Bronchoalveolar lavage (BAL) supernatant heme content in neutropenic mice on day 3 of infection. (D and E) Lung fungal content measured as lung colony-forming units and BAL fluid β-glucan content in neutropenic mice on day 3 of infection. (F) Extent of lung injury, as measured by BAL fluid albumin content, in neutropenic mice on day 3 of infection. Data in C–F represent mean ± standard error of the mean of n = 4–15 animals in each panel, combined from 3 experiments. *, **, and **** denote P < .05, P < .01, and P < .0001, respectively. NS, no significant difference.
Administration of exogenous heme in these experiments resulted in elevated extracellular heme in the lungs (Figure 5C) and increased lung fungal content, as assessed by CFUs, and BAL (1,3)-β-glucan, a major fungal cell wall component synthesized during exponential hyphal growth [37]. Coadministration of Aspergillus with heme resulted in 6-fold higher lung fungal CFUs and 23-fold higher BAL β-glucan (Figure 5D and E). We also quantified the extent of lung injury induced by the infection by comparing the albumin content of bronchoalveolar lavage fluid, a measure of loss of alveolar epithelial integrity. Heme alone did not cause measurable lung injury, and challenge with live Aspergillus alone, or ethanol-killed germlings together with heme, resulted in minor lung injury. In contrast, coadministration of heme with live fungi resulted in markedly increased lung injury (Figure 5F). Heme therefore appeared to contribute to both increased fungal growth and increased lung injury during invasive aspergillosis.
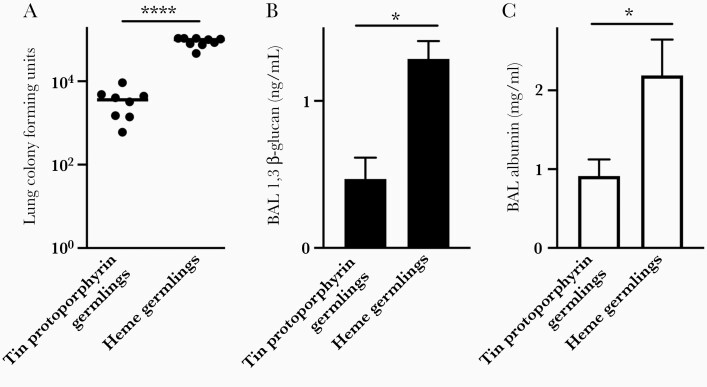
We recognize that, during in vivo coadministration of heme with Aspergillus, it is not possible to differentiate between the effect of heme on the host and its effect on the fungus. To address this problem, we cultured Aspergillus germlings in minimal media supplemented with tin(IV)-protoporphyrin or heme, conditions that result in generation of a similar mycelial biomass (Figure 4C). We then washed the resulting fungal cells extensively, and we inoculated them intratracheally into neutropenic mice. Mice challenged with germlings cultured in the presence of heme had a markedly higher lung fungal burden as measured by colony-forming units and β-glucan content, and 2-fold greater lung injury, compared with mice inoculated with germlings cultured with tin(IV)-protoporphyrin (Figure 6). We thus conclude that, independent of the effect of heme on the host, availability of heme to Aspergillus is sufficient to result in a more severe infection.
Figure 6.
The effect of fungal heme exposure on severity of invasive aspergillosis. Lung fungal content measured as lung colony-forming units (A) and bronchoalveolar lavage (BAL) fluid β-glucan content (B), and extent of lung injury, as measured by BAL fluid albumin content (C) in neutropenic mice on day 2 after intratracheal challenge with Aspergillus germlings that were culture in minimal media supplemented with tin(IV)-protoporphyrin or heme. Data represent mean ± standard error of the mean of n = 5–9 animals per group, combined from 2 experiments. * and **** denote P < .05 and P < .0001, respectively.
DISCUSSION
We report a time-dependent release of heme in the lungs during murine invasive aspergillosis, consistent with histologic evidence of alveolar hemorrhage in human infection [38]. In contrast to a prevailing notion in the field, we found (1) that A fumigatus can import and utilize exogenous heme and (2) that extracellular heme availability to the fungus enhances its virulence.
Aspergillus heme utilization as an iron source adds to the literature on the importance of iron acquisition in invasive aspergillosis: Aspergillus strains deficient in siderophores are avirulent in immunocompromised mice [2]. In a model of Aspergillus keratitis, providing iron to the organism in vivo resulted in increased fungal burden, whereas administration of the iron chelator lactoferrin improved outcomes [39]. In an orthotopic tracheal transplant model, which mimics airway-invasive aspergillosis in lung transplant recipients, infection was associated with increased iron availability to the fungus and microhemorrhage in the allograft [40]. In a computational model of invasive aspergillosis, iron availability to the fungus dramatically influenced predicted growth of the fungus [14]. Our data add to this literature by showing heme acquisition to be a nonredundant mode of iron acquisition by the fungus, and that the tissue hemorrhage provides a rich source of iron to the organism, rendering it resistant to systemic hypoferremia orchestrated by the host.
Mechanisms of heme acquisition have been extensively documented as contributing to bacterial virulence [8]. Although less well studied in fungi, heme acquisition has been discovered in Candida, Cryptococcus neoformans, Paracoccidioides species, and Schizosaccharomyces pombe [7]. Our findings are consistent with improved protein expression of Aspergillus oryzae in heme-containing media [41] and evidence that Aspergillus niger is capable of heme utilization, rescuing the lethal phenotype of mutants incapable of endogenous heme synthesis [42, 43]. It is notable that although our observations contradict a prevailing notion in the field, they do not formally conflict with previously published data: the contention that Aspergillus species cannot import heme arose from observations that mutant strains with disrupted siderophore synthesis have impaired growth and conidiation that was rescued by culture in iron-supplement media or with exogenous siderophores, but not by culture with blood or heme, and were avirulent in neutropenic mice [21, 44]. This work implicated the fungal siderophore system as indispensable to in vitro growth and in vivo virulence regardless of heme presence, but it did not address heme accessibility to Aspergillus strains with intact siderophore systems. In this context, the current report provides evidence that, under conditions of iron starvation, (1) heme is imported and utilized by wild-type A fumigatus, providing it with an iron source and enhancing its in vivo pathogenicity and growth, and (2) that siderophore-mediated iron acquisition and heme uptake thus play crucial and nonredundant roles in fungal virulence.
We assessed the transcription of several Aspergillus proteins with potential relevance to heme metabolism to document the response of A fumigatus to heme, among which HapX, CfmB, and CfmC were found to be differentially regulated (Figure 4). HapX is essential to Aspergillus siderophore production, and its downregulation in the presence of heme indicates relief from iron starvation [45]. The Common in Fungal Extracellular Membrane (CFEM) domain identifies a family of heme-import proteins in several fungal species. The Aspergillus CfmA, CfmB, and CfmC are CFEM-containing proteins with homology to Candida counterparts involved in heme handling [34, 46, 47]. We interpret the upregulation of CfmB and CfmC in the presence of heme as evidence that the fungus can detect and respond to heme, but we recognize this as hypothesis-generating regarding their role in mediating heme import in Aspergillus.
Systemic hemolysis has long been recognized to predispose to numerous infections [48]. Our data suggest that invasive aspergillosis represents a special case of local hemolysis, wherein angioinvasion promotes tissue hemorrhage, release of hemoglobin from lysis of red blood cells, and its degradation to heme—resulting in increasing extracellular heme iron as the infection progresses (Figure 1C). Aspergillus conidia and germling are phagocytosed, and they are either rapidly killed or kill the phagocytosing cell within hours [19, 49]; Aspergillus thus does not persist in an intracellular niche. We therefore speculate that fungal heme uptake occurs only extracellularly during infection, but our data leave open the possibility that phagocytosed fungal elements exposed to heme also kill host cells more rapidly during their brief intracellular phase.
We recognize that, independent of its effects on the fungus, heme affects host cells, resulting in complex pro-oxidant, inflammatory, and immunoregulatory effects [50]. During aspergillosis, extracellular heme thus likely affects both the host and Aspergillus concurrently. Our data do not preclude these direct host effects but provide evidence that heme alters the fungal transcriptional profile, increases its growth, and enhances its resistance to oxidative killing. More importantly, fungal exposure to heme before inoculation into neutropenic mice causes a more severe infection, indicating that exposure heme is sufficient to render Aspergillus more virulent.
CONCLUSIONS
This work has several implications for future research. First, the mechanisms by which Aspergillus takes up heme, such as heme receptors, heme endocytosis, and other extracellular heme binding proteins, are of substantial interest. Second, the direct effects of heme on the host during invasive aspergillosis may be important in dictating the local immune responses during invasive aspergillosis. Finally, strategies aimed at limiting the availability of heme to Aspergillus may be relevant therapeutically.
Notes
Presented in part: American Thoracic Society International Conference, August 2020, Philadelphia, PA.
Financial support. This work was funded by National Institutes of Health Grants EB024501, AI135128, and AI117397.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Cadena J, Thompson GR III, Patterson TF.. Invasive aspergillosis: current strategies for diagnosis and management. Infect Dis Clin North Am 2016; 30:125–42. [DOI] [PubMed] [Google Scholar]
- 2. Haas H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat Prod Rep 2014; 31:1266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ibrahim AS, Gebremariam T, French SW, Edwards JE Jr, Spellberg B.. The iron chelator deferasirox enhances liposomal amphotericin B efficacy in treating murine invasive pulmonary aspergillosis. J Antimicrob Chemother 2010; 65:289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zarember KA, Sugui JA, Chang YC, Kwon-Chung KJ, Gallin JI.. Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion. J Immunol 2007; 178:6367–73. [DOI] [PubMed] [Google Scholar]
- 5. Garcia-Vidal C, Upton A, Kirby KA, Marr KA.. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis 2008; 47:1041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kontoyiannis DP, Chamilos G, Lewis RE, et al. Increased bone marrow iron stores is an independent risk factor for invasive aspergillosis in patients with high-risk hematologic malignancies and recipients of allogeneic hematopoietic stem cell transplantation. Cancer 2007; 110:1303–6. [DOI] [PubMed] [Google Scholar]
- 7. Roy U, Kornitzer D.. Heme-iron acquisition in fungi. Curr Opin Microbiol 2019; 52:77–83. [DOI] [PubMed] [Google Scholar]
- 8. Huang W, Wilks A.. Extracellular heme uptake and the challenge of bacterial cell membranes. Annu Rev Biochem 2017; 86:799–823. [DOI] [PubMed] [Google Scholar]
- 9. Haas H. Iron—a key nexus in the virulence of aspergillus fumigatus. Front Microbiol 2012; 3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blatzer M, Latgé J-P.. Metal-homeostasis in the pathobiology of the opportunistic human fungal pathogen Aspergillus fumigatus. Curr Opin Microbiol 2017; 40:152–9. [DOI] [PubMed] [Google Scholar]
- 11. Schrettl M, Ibrahim-Granet O, Droin S, Huerre M, Latgé J-P, Haas H.. The crucial role of the Aspergillus fumigatus siderophore system in interaction with alveolar macrophages. Microbes Infect 2010; 12:1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ancuceanu R, Dinu M, Hovaneţ MV, Anghel AI, Popescu CV, Negreş SA.. Survey of plant iron content-A semi-systematic review. Nutrients 2015; 7:10320–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lesbordes-Brion J-C, Viatte L, Bennoun M, et al. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood 2006; 108:1402–5. [DOI] [PubMed] [Google Scholar]
- 14. Oremland M, Michels KR, Bettina AM, Lawrence C, Mehrad B, Laubenbacher R.. A computational model of invasive aspergillosis in the lung and the role of iron. BMC Syst Biol 2016; 10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park SJ, Burdick MD, Brix WK, et al. Neutropenia enhances lung dendritic cell recruitment in response to Aspergillus via a cytokine-to-chemokine amplification loop. J Immunol 2010; 185:6190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cagnina RE, Michels KR, Bettina AM, et al. Neutrophil-derived tumor necrosis factor drives fungal acute lung injury in chronic granulomatous disease. J Infect Dis 2021; 224:1225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barletta KE, Cagnina RE, Burdick MD, Linden J, Mehrad B.. Adenosine A(2B) receptor deficiency promotes host defenses against Gram-negative bacterial pneumonia. Am J Respir Crit Care Med 2012; 186:1044–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen L, Zhang Z, Barletta KE, Burdick MD, Mehrad B.. Heterogeneity of lung mononuclear phagocytes during pneumonia: contribution of chemokine receptors. Am J Physiol Lung Cell Mol Physiol 2013; 305:L702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhabhra R, Miley MD, Mylonakis E, et al. Disruption of the Aspergillus fumigatus gene encoding nucleolar protein CgrA impairs thermotolerant growth and reduces virulence. Infect Immun 2004; 72:4731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park SJ, Burdick MD, Mehrad B.. Neutrophils mediate maturation and efflux of lung dendritic cells in response to Aspergillus fumigatus germ tubes. Infect Immun 2012; 80:1759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schrettl M, Bignell E, Kragl C, et al. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J Exp Med 2004; 200:1213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eisendle M, Schrettl M, Kragl C, Müller D, Illmer P, Haas H.. The intracellular siderophore ferricrocin is involved in iron storage, oxidative-stress resistance, germination, and sexual development in Aspergillus nidulans. Eukaryot Cell 2006; 5:1596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Protchenko O, Shakoury-Elizeh M, Keane P, Storey J, Androphy R, Philpott CC.. Role of PUG1 in inducible porphyrin and heme transport in Saccharomyces cerevisiae. Eukaryot Cell 2008; 7:859–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vaknin Y, Shadkchan Y, Levdansky E, Morozov M, Romano J, Osherov N.. The three Aspergillus fumigatus CFEM-domain GPI-anchored proteins (CfmA-C) affect cell-wall stability but do not play a role in fungal virulence. Fungal Genet Biol 2014; 63:55–64. [DOI] [PubMed] [Google Scholar]
- 25. Abad-Diaz-De-Cerio A, Fernandez-Molina JV, Ramirez-Garcia A, et al. The aspHS gene as a new target for detecting Aspergillus fumigatus during infections by quantitative real-time PCR. Med Mycol 2013; 51:545–54. [DOI] [PubMed] [Google Scholar]
- 26. Irmer H, Tarazona S, Sasse C, et al. RNAseq analysis of Aspergillus fumigatus in blood reveals a just wait and see resting stage behavior. BMC Genomics 2015; 16:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Castro PA de, Chiaratto J, Morais ER, et al. . The putative flavin carrier family FlcA-C is important for Aspergillus fumigatus virulence. Virulence. 2017; 8:797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Michels KR, Zhang Z, Bettina AM, et al. Hepcidin-mediated iron sequestration protects against bacterial dissemination during pneumonia. JCI Insight 2017; 2:e92002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Riemer J, Hoepken HH, Czerwinska H, Robinson SR, Dringen R.. Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal Biochem 2004; 331:370–5. [DOI] [PubMed] [Google Scholar]
- 30. Michels K, Nemeth E, Ganz T, Mehrad B.. Hepcidin and host defense against infectious diseases. PLoS Pathog 2015; 11:e1004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schrettl M, Beckmann N, Varga J, et al. HapX-mediated adaption to iron starvation is crucial for virulence of Aspergillus fumigatus. PLoS Pathog 2010; 6:e1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wartenberg D, Lapp K, Jacobsen ID, et al. Secretome analysis of Aspergillus fumigatus reveals Asp-hemolysin as a major secreted protein. Int J Med Microbiol 2011; 301:602–11. [DOI] [PubMed] [Google Scholar]
- 33. Yokota K, Shimada H, Kamaguchi A, Sakaguchi O.. Studies on the toxin of Aspergillus fumigatus. VII. Purification and some properities of hemolytic toxin (asp-hemolysin) from culture filtrates and mycelia. Microbiol Immunol 1977; 21:11–22. [DOI] [PubMed] [Google Scholar]
- 34. Mulvihill ED, Moloney NM, Owens RA, Dolan SK, Russell L, Doyle S.. Functional investigation of iron-responsive microsomal proteins, including MirC, in Aspergillus fumigates. Front Microbiol 2017; 8:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Protchenko O, Rodriguez-Suarez R, Androphy R, Bussey H, Philpott CC.. A screen for genes of heme uptake identifies the FLC family required for import of FAD into the endoplasmic reticulum. J Biol Chem. 2006; 281:21445–57. [DOI] [PubMed] [Google Scholar]
- 36. Sweeny EA, Singh AB, Chakravarti R, et al. Glyceraldehyde-3-phosphate dehydrogenase is a chaperone that allocates labile heme in cells. J Biol Chem 2018; 293:14557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beauvais A, Drake R, Ng K, Diaquin M, Latgé JP.. Characterization of the 1,3-beta-glucan synthase of Aspergillus fumigatus. J Gen Microbiol 1993; 139:3071–8. [DOI] [PubMed] [Google Scholar]
- 38. Stergiopoulou T, Meletiadis J, Roilides E, et al. Host-dependent patterns of tissue injury in invasive pulmonary aspergillosis. Am J Clin Pathol 2007; 127:349–55. [DOI] [PubMed] [Google Scholar]
- 39. Leal SM Jr, Roy S, Vareechon C, et al. Targeting iron acquisition blocks infection with the fungal pathogens Aspergillus fumigatus and Fusarium oxysporum. PLoS Pathog 2013; 9:e1003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hsu JL, Manouvakhova OV, Clemons KV, et al. Microhemorrhage-associated tissue iron enhances the risk for invasion in a mouse model of airway transplantation. Sci Transl Med 2018; 10:eaag2616. doi: 10.1126/scitranslmed.aag2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stewart P, Whitwam RE, Kersten PJ, Cullen D, Tien M.. Efficient expression of a Phanerochaete chrysosporium manganese peroxidase gene in Aspergillus oryzae. Appl Environ Microbiol 1996; 62:860–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Franken ACW, Werner ER, Haas H, et al. The role of coproporphyrinogen III oxidase and ferrochelatase genes in heme biosynthesis and regulation in Aspergillus niger. Appl Microbiol Biotechnol 2013; 97:9773–85. [DOI] [PubMed] [Google Scholar]
- 43. Franken ACW, Lokman BC, Ram AFJ, van den Hondel CAMJJ, de Weert S, Punt PJ.. Analysis of the role of the Aspergillus niger aminolevulinic acid synthase (hemA) gene illustrates the difference between regulation of yeast and fungal haem- and sirohaem-dependent pathways. FEMS Microbiol Lett 2012; 335:104–12. [DOI] [PubMed] [Google Scholar]
- 44. Eisendle M, Oberegger H, Zadra I, Haas H.. The siderophore system is essential for viability of Aspergillus nidulans: functional analysis of two genes encoding l-ornithine N 5-monooxygenase (sidA) and a non-ribosomal peptide synthetase (sidC). Mol Microbiol 2003; 49:359–75. [DOI] [PubMed] [Google Scholar]
- 45. Misslinger M, Hortschansky P, Brakhage AA, Haas H.. Fungal iron homeostasis with a focus on Aspergillus fumigatus. Biochim Biophys Acta Mol Cell Res 2021; 1868:118885. [DOI] [PubMed] [Google Scholar]
- 46. Weissman Z, Kornitzer DA.. family of Candida cell surface haem-binding proteins involved in haemin and haemoglobin-iron utilization. Mol Microbiol 2004; 53:1209–20. [DOI] [PubMed] [Google Scholar]
- 47. Ding C, Vidanes GM, Maguire SL, et al. Conserved and divergent roles of Bcr1 and CFEM proteins in Candida parapsilosis and Candida albicans. PLoS One 2011; 6:e28151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Berkowitz FE. Hemolysis and infection: categories and mechanisms of their interrelationship. Rev Infect Dis 1991; 13:1151–62. [DOI] [PubMed] [Google Scholar]
- 49. Wasylnka JA, Moore MM.. Aspergillus fumigatus conidia survive and germinate in acidic organelles of A549 epithelial cells. J Cell Sci 2003; 116:1579–87. [DOI] [PubMed] [Google Scholar]
- 50. Martins R, Knapp S.. Heme and hemolysis in innate immunity: adding insult to injury. Curr Opin Immunol 2018; 50:14–20. [DOI] [PubMed] [Google Scholar]