Abstract
Context
Mutations in the NR0B1 gene, also well-known as the DAX1 gene, are known to cause congenital adrenal hypoplasia associated with hypogonadotropic hypogonadism. The abnormal NR0B1 protein fails to suppress the transcription of promoters of steroidogenic enzymes, which are also targets of NR5A1 protein, also well-known as Ad4BP/SF-1 protein. Since NR5A1 and NR0B1 have antagonistic effects on steroidogenesis, the loss of function due to NR0B1 mutations may be compensated by inducing loss of function of NR5A1 protein.
Patient
A middle-aged man was diagnosed with congenital adrenal hypoplasia associated with hypogonadotropic hypogonadism and genetic analysis revealed him to have a novel NR0B1 mutation, c.1222C>T(p.Gln408Ter).
Methods
NR0B1 activity was evaluated in CLK1/4 inhibitor-treated 293T cells via immunoblotting and luciferase assays of the STAR promoter.
Results
TG003 treatment suppressed NR5A1 protein function to compensate for the mutant NR0B1 showing inhibited suppression of transcription. Immunoblotting analyses showed that the phosphorylation status of NR5A1 at Ser203 was attenuated by the CLK1/4 inhibitor.
Conclusion
The specific reduction of NR5A1 phosphorylation by a CLK1/4 inhibitor may alleviate developmental defects in patients with NR0B1 mutations.
Keywords: congenital adrenal hypoplasia, NR0B1 mutation, NR5A1 phosphorylation, CLK1/4 inhibitor
Patients with mutations in the NR0B1 gene, previously called the dosage-sensitive sex reversal, adrenal hypoplasia critical region on chromosome X gene 1 (DAX1) gene, have abnormal development of the adrenal gland and gonadotropic regions of the pituitary gland, resulting in congenital adrenal hypoplasia and hypogonadotropic hypogonadism [1]. Targeted disruption of the Nr0b1 gene in mice results in failure of fetal zone regression in the adrenal cortex, resembling that of patients with a mutant NR0B1 gene [2]. However, these mice show normal adrenocortical function and no abnormalities in the hypothalamus or pituitary, except for defects in spermatogenesis [3]. Further analyses of Nr0b1-knockout mice showed that after aging, these mice show marked adrenal dysplasia resembling the phenotype of patients with a mutant NR0B1 gene [4]. The spectrum of NR0B1 mutations shows that the C-terminal region of the ligand-binding domain is missing in almost all NR0B1 mutations mostly caused by nonsense mutations and deletions [5].
The NR5A1 protein, previously called the Ad4-binding protein/steroidogenic factor-1 (Ad4BP/SF1) protein, transactivates genes encoding steroidogenic enzymes [6], including the steroidogenic acute regulatory protein (STAR) gene [7]. Targeted disruption of Nr5a1 in mice results in impaired organogenesis of the adrenal gland and defects in the gonads [8, 9]. Mutations in the NR5A1 gene have been reported in patients with primary adrenal failure and XY sex reversal [10]. Heterozygous mutations observed include 46XY partial gonadal dysgenesis with normal adrenal function [11]. The differential phenotypes between studies are in part due to the dose-dependent effects of NR0B1 and NR5A1 on their common targets. NR0B1 suppresses NR5A1-transactivated steroidogenic gene promoter activity via its C-terminal domain [12]. Since NR5A1 and NR0B1 have antagonistic effects on steroidogenesis, we hypothesized that NR5A1 loss of function may compensate for NR0B1 mutations, where the transcriptional repressive function of NR0B1 in adrenocortical and reproductive organogenesis is suppressed.
Posttranscriptional-modifying compounds, as well as splicing-modifying compounds, have been studied for their capacity to regulate defects in genetic diseases [13]. Splicing-modifying compounds mainly represent inhibitors of kinases that phosphorylate proteins involved in alternative splicing. The first compound identified, TG003, has been found to inhibit Cdc2-like kinase 1 (CLK1) activity and concomitant SF2/ASF-dependent splicing [14]. Later, it was found to skip mutated exons in Duchenne-type muscular dystrophy in an attempt to convert it to a milder phenotype [15]. More recent studies have shown that one of the derivatives of TG003 can be used orally in mice [16]. Though it has to be tested whether the oral derivative of TG003 can pass through the placenta, future studies are planned to test this in Nr0b1-knockout mice.
Here, we show whether a compound that inhibits NR5A1 phosphorylation can compensate for the transcriptional defects of an NR0B1 mutation.
Methods
Cell Culture, Immunofluorescence, and Reagents
The COS7 (ATCC, CRL1651) and the 293T (ATCC, CRL3216) cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum. Mutant NR0B1 cDNAs encoding p.V126M, p.166R, and p.Q408X were created by gene synthesis (Eurofines Genomics, Tokyo, Japan). COS7 cells were transfected with pcDNA3.1-NR0B1 wild-type or pcDNA3.1-NR0B1 Q408X mutant plasmid. Immunofluorescence assays were performed by fixing the cells with 4% paraformaldehyde/PBS, which were then permeabilized using 0.1% Triton-X100/PBS, and then incubated with an anti-NR0B1 antibody (EP13786) raised against the N-terminal region of NR0B1 (Abcam, Japan) [17]. TG003 was purchased from Alfa Aesar Co., Inc. (Haverhill, MA).
Luciferase Assay
The promoter region of human STAR (upstream of 1190 nucleotides from the transcriptional start point) was amplified via genomic polymerase chain reaction and inserted into the pNL3.1 reporter plasmid (pNL3.1-STARprom) (Promega, Madison, WI). pGL4.54 was also co-transfected for internal control using Fugene HD (Promega). After transfection, 293T cells were cultured for the indicated times. Luciferase activity was measured using the Nono-Glo Dual-Luciferase Reporter Assay Kit (Promega). The relative luciferase unit represents the ratio of Nanoluc to firefly luciferase activity.
Western Blot Analysis
Western blotting was performed as previously described [18] in duplicate and quantified, shown as representative of 1 of 3 independent experiments using anti-phospho-NR5A1 (Invitrogen, Waltham, MA) [19]. Antibody for Ad4BP was provided by Prof. Kenichiro Morohashi [20].
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software. Comparative analysis was performed by one-way ANOVA. P value of less than 0.05 was significant.
Results
Case Presentation
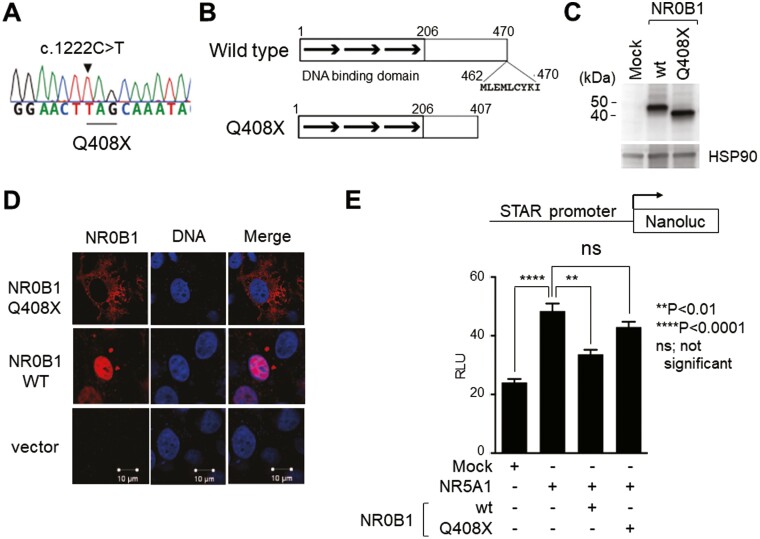
A 55-year-old man was referred to our hospital complaining of fatigue and skin hyperpigmentation. He was diagnosed with Addison’s disease at 7 years of age and took hydrocortisone daily after that. Hypogonadism was diagnosed at 18 years of age. He was examined with gonadotropin-releasing hormone loading test and diagnosed as having hypogonadotropic hypogonadism and has been treated with testosterone enanthate. When he was referred to our hospital, his medication was prednisolone (8 mg/morning) and testosterone (250 mg/3 weeks). He presented pigmentation of his face, whole body, and small testes on physical examinations. Endocrinological tests showed that the morning adrenocorticotropic hormone (ACTH) level was high (116 pg/mL). After we changed prednisolone to hydrocortisone (20 mg/day: 10 mg/morning, 5 mg/afternoon, 5 mg/evening) to measure the cortisol value properly, his morning serum cortisol level was extremely low (< 0.06 µg/dL). Meanwhile, the levels of LH and FSH were low (< 0.10 mIU/mL, 0.25 mIU/mL, respectively). The testosterone level was also low (0.34 ng/mL; measured 3 weeks after administration of testosterone enanthate). These results indicated that the hypogonadism was hypogonadotropic hypogonadism, as he previously diagnosed. The change in treatment to dexamethasone (0.5 mg/morning) and hydrocortisone (5 mg/morning) resulted in a decreased ACTH level (3.4 pg/mL) with considerable improvement in fatigue and pigmentation. At the time of diagnosis of Addison’s disease, the patient seemed to have no signs of tuberculosis, no adrenal metastasis of cancer, and no bleeding into the adrenal glands. The plasma renin activity value was 3.5 ng/mL/h and the plasma aldosterone value was 26.5 pg/mL, which indicated his aldosterone secretion was normal. Besides, the other pituitary hormone secretions were normal. His pituitary was also normal organically on magnetic resonance imaging. He did not have any smelling symptoms and never had been evaluated for genetic abnormalities such as the FGFR1, FGF8, PROKR2, PROK2, KAL1 gene mutations that cause Kallman syndrome. He also never had been evaluated for NR5A1 gene mutations, the cause of adrenal hypoplasia congenita with hypogonadotropic hypogonadism. Genetic evaluation revealed 3 mutations in the NR0B1 gene: c.376G>A, p.Val126Met; c.498G>A, p.166Arg; and c.1222C>T, p.Gln408Ter. Concerning the c.376G>A, p.Val126Met variant (accession:VCV000225425.6), the ClinVar database (ClinVar: last release January 28, 2021) designates this as benign/likely benign by 5 times of clinical testing but without functional evidence. The c.498G>A, p.166Arg variant (accession: VCV000256225.8) has been reported as a silent polymorphism [21-23] and as a possible responsible mutation in an infant with a double variant of c.192C>A and c.498G>A [24]. In that case, c.192C>A is a nonsense mutation thus c.498G>A is a silent polymorphism in this case, too. Lastly, a very similar NR0B1 triple mutation with our case was reported as c.376G>A, p.Val126Met; c.498G>A, p.166Arg; and c.1225C>T, p.Gln409Ter [25], with the difference underlined but not specified as the mutation responsible. It is well-known that of the approximate 200 mutations found in NR0B1 patients, all result in alteration of the C-terminus of the NR0B1 protein [26] which is associated with the main transcriptional suppressive activity per se [27]. Thus, among the mutations in our case, we were prompted to test c.1222C>T, p.Gln408Ter (Q408X) (Fig. 1A), a nonsense mutation resulting in truncation of the C-terminus of the protein which is associated with the main transcriptional suppressive activity (Fig. 1B and 1C) [27]. The termination codon of this mutation is located downstream of the region where the resulting mRNA is destined to nonsense-mediated decay which is, but not always, 50 nucleotides upstream from the last exon junction [28] and thus the resulting truncated protein was detected (Fig. 1C). Immunofluorescence analyses showed that this mutation (Q408X) impaired nuclear localization, as previously reported for NR0B1 mutations (Fig. 1D) [29]. Accordingly, transient expression of the NR0B1 Q408X mutant failed to suppress the NR5A1-induced STAR promoter activity in COS7 cells (Fig. 1E). The results in Fig. 1 follow the characteristics of almost all other known NR0B1 mutations [30, 31].
Figure 1.
Functional features of the novel NR0B1 mutation. (A) Chromatogram of the c.1222C>T, p.Gln408Ter (Q408X) mutation in the case presented. (B) Scheme of the NR0B1 gene structure (upper) and resulting truncated protein of the Q408X mutation. (C) Immunoblot of wild-type and Q408X-mutant NR0B1 using anti-NR0B1 antibody. (D) Immunofluorescence of COS7 cells transfected with wild-type and Q408X-mutant NR0B1 using anti-NR0B1 antibody and merged via DAPI staining. (E) STAR promoter analyses of 293T cells transfected with NR5A1 along with wild-type and Q408X-mutant NR0B1 as indicated. One-way ANOVA, **P < 0.01, ****P < 0.0001, ns; not significant. Abbreviations/gene designations: NR0B1, dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1; NR5A1, Ad4-binding protein/steroidogenic factor-1; DAPI, 4′,6-diamidino-2-phenylindole; STAR, steroidogenic acute regulatory protein.
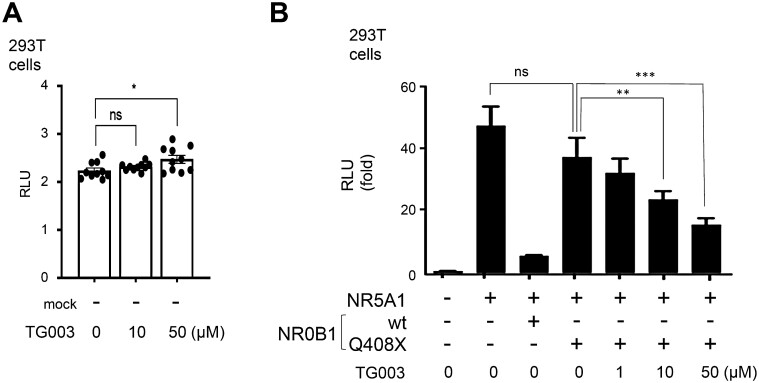
A straightforward strategy to correct this failure in suppressing NR5A1-induced STAR promoter by NR0B1 due to its mutation would be to correct the NR0B1 mutation. However, an alternative would be to suppress NR5A1 function. During experiments of treating steroidogenic cells with splice-modifying compounds, we found a CLK1/4 inhibitor, TG003, as a candidate to suppress NR5A1 function. First, we tested whether the mock (promoter-less) construct was influenced by TG003 treatment. As shown in Fig. 2A, the mock construct had almost null activity, with no difference between 0 and 10 µM TG003. 50 µM TG003 showed a significant increase but at very low level and at least there was no suppressive activity in 293T cells. This means TG003 had no suppressive activity on the basic transcription apparatus. TG003 was added to 293T cells co-transfected with the human STAR promoter luciferase reporter, NR5A1, NR0B1, and NR0B1 Q408X expression vectors. After transfection, the culture medium was changed to DMEM containing 10% charcoal dextran–treated fetal bovine serum (FBS) with TG003 (0, 10, or 50 μM) [15] or solvent, was added the following day. We found that TG003 significantly alleviated the impaired transcriptional suppressive activity of the NR0B1 Q408X mutation at 24 hours after transfection (Fig. 2B).
Figure 2.
Compensation of the transcriptional suppression defect of the NR0B1 mutant via treatment with TG003. STAR promoter analyses of 293T cells transfected with NR5A1 and NR0B1 wild-type or NR0B1 Q408X mutant treated with TG003 (0, 1, 10, or 50 μM) for 24 hours. One-way ANOVA, **P < 0.01, ***P < 0.001, ****P < 0.0001. Gene designations: NR0B1, dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1; NR5A1, Ad4-binding protein/steroidogenic factor-1; STAR, steroidogenic acute regulatory protein.
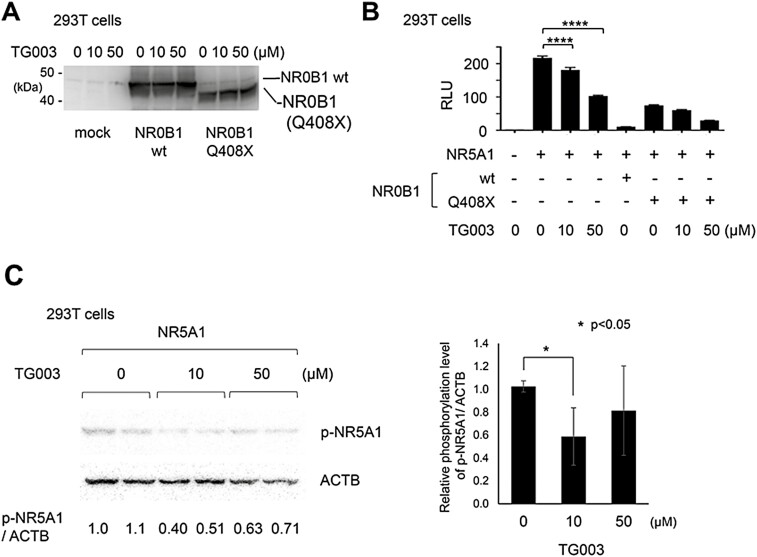
To determine whether this recovery effect of TG003 on the inhibited transcriptional suppressive activity of the NR0B1 Q408X mutation was due to the induced regulation of alternative splicing by TG003, we performed immunoblotting using an NR0B1 antibody. NR0B1 Q408X was expressed at the expected size but accompanied by a slight vague band corresponding to the full-length NR0B1 (Fig. 3A). There was no accessory band that corresponds to a splicing variant such as intron retention of intron 1. We further investigated whether NR5A1 was involved in this TG003 effect. Addition of TG003 to 293T cells transfected with NR5A1 showed a significant dose-dependent suppressive effect on NR5A1-induced STAR promoter activity (Fig. 3B). This was also observed for NR5A1 + NR0B1 Q408X protein, where TG003 showed a significant dose-dependent suppressive effect on the inhibited suppressive effect of NR0B1 Q408X (Fig. 3B). However, when we performed a western blot analysis of NR5A1 there was no change of expression of the full-length NR5A1 or of the short variant detected in this condition (Fig. 3C). Next, we sought whether TG003 inhibited phosphorylation of NR5A1 which would mean that CLK1/4 phosphorylates NR5A1. Using a phospho-NR5A1 (p-NR5A1) antibody, we found that TG003 significantly suppressed phospho-NR5A1 expression at a concentration of 10 µM, whereas 50 µM had a lower effect due to its slightly harmful effect on 293T cells as seen in the relatively low expression of actin (ACTB) (Fig. 3C). This supported the possibility of compensating for the defect of transcriptional suppression in NR0B1 mutants via TG003 treatment at 10 µM (Fig. 2B) by inhibiting the phosphorylation of NR5A1 at the same concentration of TG003 (Fig. 3C).
Figure 3.
(A, B) TG003-mediated suppression of STAR promoter activity in an NR0B1-independent manner. TG003 (0, 10, or 50 μM) did not alter the expression of NR0B1 wild-type or NR0B1 Q408X transiently expressed in 293T cells (A). TG003 (0, 10, or 50 μM) suppressed STAR promoter activity in the absence of NR0B1 and also where the NR0B1 Q408X mutant had a defect in transcriptional suppression of STAR promoter activity. One-way ANOVA, ****P < 0.0001 (B). NR0B1, dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1; STAR, steroidogenic acute regulatory protein. (C) Decreased phosphorylation of NR5A1 via TG003 treatment. Left panel shows representative western blot analysis of 3 independent assays in duplicate of NR5A1-transiently-transfected 293T cells treated with TG003 (0, 10, or 50 μM). Right graph shows the relative phosphorylation rate of NR5A1 (p-NR5A1) in TG003-treated cells compared to that in nontreated cells. One-way ANOVA, *P < 0.05. Gene designations: NR0B1, dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1; NR5A1, Ad4-binding protein/steroidogenic factor-1; STAR, steroidogenic acute regulatory protein.
Discussion
NR0B1 has been shown to suppress transcription [32], which is deeply involved in organogenesis [3, 4] and steroidogenesis [33] of the adrenal gland in relation to NR5A1, a master regulator of steroidogenesis [6, 34-36]. While disruption of NR5A1 leads to a complete lack of adrenal and gonadal tissue development in both sexes [8], disruption of NR0B1 in mice shows possible adrenal hyperfunction at birth for both sexes, and impaired spermatogenesis without affecting female gonads [3]. This is different from human NR0B1 mutations, where adrenal hypoplasia is mostly observed in early infancy [37]. However, subsequent studies have shown that these mice containing disrupted NR0B1 show marked adrenal dysplasia at old age, and a defect in adrenocortical subcapsular progenitor cells is observed [4], where NR5A1 is not expressed [38]. CRISPR/Cas9-mediated NR0B1 knockout studies on monkeys show phenotypes closely resembling human NR0B1 abnormalities [39]. Although the major function of NR0B1 is its transcriptional repression activity, it has been reported that NR0B1 upregulated Oct4 expression in mouse embryonic stem (ES) and embryonic carcinoma F9 cells by interacting with liver receptor homolog 1 (LRH-1) and the steroid receptor RNA activator (SRA) [40]. Moreover, the adrenocortical function increases prior to the adrenocortical hypofunction in NR0B1 mutated patients as well as model mice [41]. Thus, the findings of the present report, before therapeutic application, should be used as a tool to seek whether the possible transient hyperfunction prior to adrenocortical hypofunction is due to positive regulation by NR0B1 or NR5A1 phosphorylation. An orally available compound of TG003 [16] can be used in NR0B1 mutant mouse model [4] for this purpose.
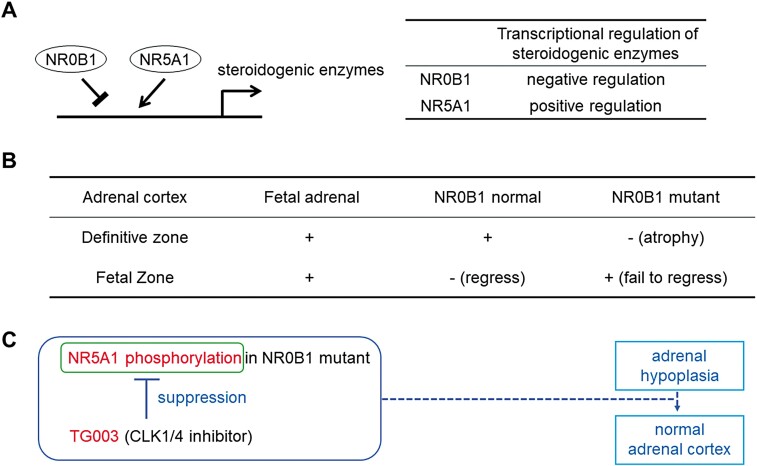
We showed here that suppressing the phosphorylation of NR5A1 could compensate for the defect in transcriptional suppressive activities of NR0B1 mutations. However, this study has a few limitations. First, among the posttranscriptional modifications of NR5A1, sumoylation is known to be involved in synergistic [42] and antagonistic transcriptional regulation of promoters with multiple NR5A1-binding sites (Ad4 sites) [43]. It has been reported that sumoylation is not involved in TG003 action, at least for RanBP2 [44]; however, we need to exclude the possibility of sumoylation of NR5A1 by TG003. Second, since NR5A1 is not expressed in adrenocortical subcapsular progenitor cells [38], this NR5A1-targeting strategy for NR0B1 mutations shown here may not be effective. However, an opportunity may arise when these progenitor cells differentiate into adrenocortical cells where NR5A1 is expressed. In fact, the adrenal fetal zone fails to regress in patients with NR0B1 mutations [37], and in Nr0b1-knockout mice [4] or monkeys [39], and that NR5A1 and NR0B1 antagonistically act on a fetal adrenal enhancer FAdE in the NR5A1 gene, possibly targeting cells of the adrenal fetal zone [45]. Fourth, there were slight variables between each STAR promoter analysis. The number of cells was always constant but other reasons such as different passages of 293T cells might have influenced the absolute but not relative values of the promoter analyses. Moreover, the concentration of TG003 at 50 µM slightly influenced cell viability, evidenced by the significant increase of STAR-luc reporter activity at 50 µM TG003 (Fig. 2A) and drop off in ACTB signal in Fig. 2B. However, a clear dose-dependency was shown in Fig. 2B where 1 µM TG003 was added. We did not assay the effect of TG003 on STAR-luc reporter activity because it is known as a specific inhibitor of splicing-related CLK1/4 activity [14] that has no influence on the general transcriptional machinery. To check whether TG003 has an effect on NR5A1 in the absence of NR0B1 Q408X is an important control but we doubt NR0B1 Q408X would have an enhancing effect on the TG003 inhibiting effect on NR5A1 phosphorylation. Furthermore, the consequent therapeutic target is NR0B1 Q408X not NR5A1. TG003 inhibition of NR5A1 phosphorylation is a proposed alternative to alleviate the defect of the NR0B1 mutation (Fig. 4A-4C).
Figure 4.
The molecular pathways and potential targets of TG003. (A) Transcriptional regulation of steroidogenic enzymes by NR0B1 and NR5A1. (B) Adrenal cortex zones on fetal adrenal, adrenal of NR0B1 normal, and adrenal of NR0B1 mutant. (C) The diagram of TG003 effects. Gene designations: NR0B1, dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1; NR5A1, Ad4-binding protein/steroidogenic factor-1.
Lastly, TG003 is a well-known splice-modifying compound with a specific inhibitory effect on CLK1/4 [14, 46]. Here, we report that TG003 inhibited the phosphorylation of NR5A1. However, previous studies have not reported whether TG003 or CLK1/4 targets NR5A1. NR5A1 is phosphorylated by CDK7 [47] and ERK2 [48]. It is not known whether TG003 or CLK1/4 is related to CDK7 or ERK2. Future studies should clarify the mechanism of TG003-inhibited NR5A1 phosphorylation in its signal transduction pathway. We believe the effect of TG003 does not necessarily have to be specific to NR5A1 or NR0B1. TG003 surely might have an effect on background activation since it was not screened for its specificity on NR5A1 phosphorylation. This can be overcome by structure-function screening using TG003 as a leading compound and NR5A1 phosphorylation as the target. We believe we will find a more specific TG003-related compound that will specifically inhibit NR5A1 phosphorylation. The concept of this report is not to immediately apply TG003 to therapy. If clinicians come across a patient with a NR0B1 mutation as well as other patients with adrenal failure, adequate and suitable steroid replacement with suppression of ACTH level should be applied to monitor adequate replacement and repress skin complications, as in the present case.
In conclusion, we propose that the suppression of NR5A1 phosphorylation by TG003 can compensate for transcriptional defects in NR0B1 mutants by regulating steroidogenic enzymes in adrenocortical development and steroidogenesis.
Glossary
Abbreviations
- ACTH
adrenocorticotropic hormone
- DMEM
Dulbecco’s Modified Eagle Medium
- FSH
follicle-stimulating hormone
- LH
luteinizing hormone
- NR0B1
dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1
- NR5A1
Ad4-binding protein/steroidogenic factor-1
- STAR
steroidogenic acute regulatory protein
Contributor Information
Ichiro Abe, Email: abe1ro@fukuoka-u.ac.jp.
Kenji Ohe, Email: ohekenji@fukuoka-u.ac.jp.
Financial Support
I.A. was funded by a Grant of The Clinical Research Promotion Foundation (grant no. 180477), K.O. was funded by a Grant-in-Aid for Scientific Research (C) (grant no. 18K09212 and 15H05721) and T.T. was funded by a Grant-in-Aid for Scientific Research (C) (grant no. 16K09815 and 20K08922) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. K.O., T.T., and T.Y. were funded by the Suisyo Kenkyu Project, Fukuoka University (167005 and 197010), and the Center for Clinical and Translational Research of Kyushu University (A147).
Disclosures
We have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Achermann JC, Meeks JJ, Jameson JL. Phenotypic spectrum of mutations in DAX-1 and SF-1. Mol Cell Endocrinol. 2001;185(1-2):17-25. doi: 10.1016/s0303-7207(01)00619-0 [DOI] [PubMed] [Google Scholar]
- 2. Kopp P. Targeted disruption of the Ahch (Dax-1) gene: knockout of old concepts. Eur J Endocrinol. 1999;140(4):291-292. doi: 10.1530/eje.0.1400291 [DOI] [PubMed] [Google Scholar]
- 3. Yu RN, Ito M, Saunders TL, et al. Role of Ahch in gonadal development and gametogenesis. Nat Genet. 1998;20(4):353-357. doi: 10.1038/3822 [DOI] [PubMed] [Google Scholar]
- 4. Scheys JO, Heaton JH, Hammer GD. Evidence of adrenal failure in aging DAX1-deficient mice. Endocrinology. 2011;152(9):3430-3439. doi: 10.1210/en.2010-0986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCabe ER. DAX1: increasing complexity in the roles of this novel nuclear receptor. Mol Cell Endocrinol. 2007;26(5-266):179-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lala DS, Rice DA, Parker KL. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992;6(8):1249-1258. doi: 10.1210/mend.6.8.1406703 [DOI] [PubMed] [Google Scholar]
- 7. Sugawara T, Holt JA, Kiriakidou M, Strauss JF 3rd. Steroidogenic factor 1-dependent promoter activity of the human steroidogenic acute regulatory protein (StAR) gene. Biochemistry. 1996;35(28):9052-9059. doi: 10.1021/bi960057r [DOI] [PubMed] [Google Scholar]
- 8. Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77(4):481-490. doi: 10.1016/0092-8674(94)90211-9 [DOI] [PubMed] [Google Scholar]
- 9. Sadovsky Y, Crawford PA, Woodson KG, et al. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci U S A. 1995;92(24):10939-10943. doi: 10.1073/pnas.92.24.10939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet. 1999;22(2):125-126. doi: 10.1038/9629 [DOI] [PubMed] [Google Scholar]
- 11. Kohler B, Achermann JC. Update--steroidogenic factor 1 (SF-1, NR5A1). Minerva Endocrinol. 2010;35(2):73-86. [PubMed] [Google Scholar]
- 12. Achermann JC, Ito M, Silverman BL, et al. Missense mutations cluster within the carboxyl-terminal region of DAX-1 and impair transcriptional repression. J Clin Endocrinol Metab. 2001;86(7):3171-3175. doi: 10.1210/jcem.86.7.7660 [DOI] [PubMed] [Google Scholar]
- 13. Ohe K, Hagiwara M. Modulation of alternative splicing with chemical compounds in new therapeutics for human diseases. ACS Chem Biol. 2015;10(4):914-924. doi: 10.1021/cb500697f [DOI] [PubMed] [Google Scholar]
- 14. Muraki M, Ohkawara B, Hosoya T, et al. Manipulation of alternative splicing by a newly developed inhibitor of clks. J Biol Chem. 2004;279(23):24246-24254. doi: 10.1074/jbc.M314298200 [DOI] [PubMed] [Google Scholar]
- 15. Nishida A, Kataoka N, Takeshima Y, et al. Chemical treatment enhances skipping of a mutated exon in the dystrophin gene. Nat Commun. 2011;2:308. 10.1038/ncomms1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sako Y, Ninomiya K, Okuno Y, et al. Development of an orally available inhibitor of CLK1 for skipping a mutated dystrophin exon in duchenne muscular dystrophy. Sci Rep. 2017;7:46126. doi: 10.1038/srep46126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. RRID: AB_2857966. https://antibodyregistry.org/search.php?q=AB_2857966
- 18. Nomiyama T, Kawanami T, Irie S, et al. Exendin-4, a GLP-1 receptor agonist, attenuates prostate cancer growth. Diabetes. 2014;63(11):3891-905. doi: 10.2337/db13-1169 [DOI] [PubMed] [Google Scholar]
- 19. RRID: AB_2662789. https://antibodyregistry.org/search.php?q= AB_2662789
- 20. RRID: AB_2622228. https://antibodyregistry.org/search.php?q= AB_2622228
- 21. Mou L, Xie N, Yang L, et al. A novel mutation of DAX-1 associated with secretory azoospermia. PLoS One. 2015;10(7):e0133997. doi: 10.1371/journal.pone.0133997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Achermann JC, Gu WX, Kotlar TJ, et al. Mutational analysis of DAX1 in patients with hypogonadotropic hypogonadism or pubertal delay. J Clin Endocrinol Metab. 1999;84(12):4497-500. doi: 10.1210/jcem.84.12.6269 [DOI] [PubMed] [Google Scholar]
- 23. Reutens AT, Achermann JC, Ito M, et al. Clinical and functional effects of mutations in the DAX-1 gene in patients with adrenal hypoplasia congenita. J Clin Endocrinol Metab. 1999;84(2):504-511. doi: 10.1210/jcem.84.2.5468 [DOI] [PubMed] [Google Scholar]
- 24. Wheeler B, George PM, Mackenzie K, Hunt P, Potter HC, Florkowski CM. Three cases of congenital adrenal hypoplasia with novel mutations in the (NROB1) DAX-1 gene. Ann Clin Biochem. 2008;45(Pt 6):606-609. doi: 10.1258/acb.2008.008038 [DOI] [PubMed] [Google Scholar]
- 25. Xu XQ, Feng YY, Yuan WX, Huang K, Liang L, Fu JF. Novel mutations in DAX1 of X-linked adrenal hypoplasia congenita over several generations in one family. Endocr Pract. 2013;19(4):e105-e111. doi: 10.4158/EP12368.CR [DOI] [PubMed] [Google Scholar]
- 26. Fan DB, Li L, Zhang HH. Nonsense variant of NR0B1 causes hormone disorders associated with congenital adrenal hyperplasia. Sci Rep. 2021;11(1):16066. doi: 10.1038/s41598-021-95642-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lalli E, Bardoni B, Zazopoulos E, et al. A transcriptional silencing domain in DAX-1 whose mutation causes adrenal hypoplasia congenita. Mol Endocrinol. 1997;11(13):1950-1960. doi: 10.1210/mend.11.13.0038 [DOI] [PubMed] [Google Scholar]
- 28. Supek F, Lehner B, Lindeboom RGH. To NMD or not to NMD: nonsense-mediated mRNA decay in cancer and other genetic diseases. Trends Genet. 2021;37(7):657-668. doi: 10.1016/j.tig.2020.11.002 [DOI] [PubMed] [Google Scholar]
- 29. Lehmann SG, Lalli E, Sassone-Corsi P. X-linked adrenal hypoplasia congenita is caused by abnormal nuclear localization of the DAX-1 protein. Proc Natl Acad Sci USA. 2002;99(12):8225-8230. doi: 10.1073/pnas.122044099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lalli E, Sassone-Corsi P. DAX-1, an unusual orphan receptor at the crossroads of steroidogenic function and sexual differentiation. Mol Endocrinol. 2003;17(8):1445-1453. doi: 10.1210/me.2003-0159 [DOI] [PubMed] [Google Scholar]
- 31. Hasegawa Y, Takahashi Y, Kezuka Y, et al. Identification and analysis of a novel NR0B1 mutation in late-onset adrenal hypoplasia congenita and hypogonadism. J Endocr Soc. 2021;5(2):bvaa176. doi: 10.1210/jendso/bvaa176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zanaria E, Muscatelli F, Bardoni B, et al. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature. 1994;372(6507):635-641. doi: 10.1038/372635a0 [DOI] [PubMed] [Google Scholar]
- 33. Lalli E, Melner MH, Stocco DM, Sassone-Corsi P. DAX-1 blocks steroid production at multiple levels. Endocrinology. 1998;139(10):4237-4243. doi: 10.1210/endo.139.10.6217 [DOI] [PubMed] [Google Scholar]
- 34. Crawford PA, Dorn C, Sadovsky Y, Milbrandt J. Nuclear receptor DAX-1 recruits nuclear receptor corepressor N-CoR to steroidogenic factor 1. Mol Cell Biol. 1998;18(5):2949-2956. doi: 10.1128/MCB.18.5.2949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ito M, Yu R, Jameson JL. DAX-1 inhibits SF-1-mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol Cell Biol. 1997;17(3):1476-1483. doi: 10.1128/MCB.17.3.1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kawabe K, Shikayama T, Tsuboi H, et al. Dax-1 as one of the target genes of Ad4BP/SF-1. Mol Endocrinol. 1999;13(8):1267-1284. doi: 10.1210/mend.13.8.0325 [DOI] [PubMed] [Google Scholar]
- 37. Muscatelli F, Strom TM, Walker AP, et al. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature. 1994;372(6507):672-676. doi: 10.1038/372672a0 [DOI] [PubMed] [Google Scholar]
- 38. Huang CC, Miyagawa S, Matsumaru D, Parker KL, Yao HH. Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology. 2010;151(3):1119-1128. doi: 10.1210/en.2009-0814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kang Y, Zheng B, Shen B, et al. CRISPR/Cas9-mediated DAX1 knockout in the monkey recapitulates human AHC-HH. Hum Mol Genet. 2015;24(25):7255-7264. doi: 10.1093/hmg/ddv425 [DOI] [PubMed] [Google Scholar]
- 40. Kelly VR, Xu B, Kuick R, Koenig RJ, Hammer GD. DAX1 up-regulates Oct4 expression in mouse embryonic stem cells via LRH-1 and SRA. Mol Endocrinol. 2010;24(12):2281-2291. doi: 10.1210/me.2010-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Walczak EM, Hammer GD. Regulation of the adrenocortical stem cell niche: implications for disease. Nat Rev Endocrinol. 2015;11(1):14-28. doi: 10.1038/nrendo.2014.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Komatsu T, Mizusaki H, Mukai T, et al. Small ubiquitin-like modifier 1 (SUMO-1) modification of the synergy control motif of Ad4 binding protein/steroidogenic factor 1 (Ad4BP/SF-1) regulates synergistic transcription between Ad4BP/SF-1 and Sox9. Mol Endocrinol. 2004;18(10):2451-2462. doi: 10.1210/me.2004-0173 [DOI] [PubMed] [Google Scholar]
- 43. Morohashi K, Honda S, Inomata Y, Handa H, Omura T. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem. 1992;267(25):17913-17919. [PubMed] [Google Scholar]
- 44. Saitoh N, Sakamoto C, Hagiwara M, Agredano-Moreno LT, Jimenez-Garcia LF, Nakao M. The distribution of phosphorylated SR proteins and alternative splicing are regulated by RANBP2. Mol Biol Cell. 2012;23(6):1115-1128. doi: 10.1091/mbc.E11-09-0783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xing Y, Morohashi KI, Ingraham HA, Hammer GD. Timing of adrenal regression controlled by synergistic interaction between Sf1 sumoylation and DAX1. Development. 2017;144(20):3798-3807. doi: 10.1242/dev.150516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosenthal AS, Tanega C, Shen M, et al. An Inhibitor of the Cdc2-like Kinase 4 (Clk4). Probe Reports from the NIH Molecular Libraries Program. Bethesda, MD; 2010. [Google Scholar]
- 47. Lewis AE, Rusten M, Hoivik EA, et al. Phosphorylation of steroidogenic factor 1 is mediated by cyclin-dependent kinase 7. Mol Endocrinol. 2008;22(1):91-104. doi: 10.1210/me.2006-0478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hoivik EA, Aumo L, Aesoy R, et al. Deoxyribonucleic acid methylation controls cell type-specific expression of steroidogenic factor 1. Endocrinology. 2008;149(11):5599-609. doi: 10.1210/en.2008-0104 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.