Abstract
A safe and effective vaccine against multidrug-resistant gonorrhea is urgently needed. An experimental peptide vaccine called TMCP2 that mimics an oligosaccharide epitope in gonococcal lipooligosaccharide, when adjuvanted with glucopyranosyl lipid adjuvant–stable emulsion, elicits bactericidal immunoglobulin G and hastens clearance of gonococci in the mouse vaginal colonization model. In this study, we show that efficacy of TMCP2 requires an intact terminal complement pathway, evidenced by loss of activity in C9−/− mice or when C7 function was blocked. In conclusion, TMCP2 vaccine efficacy in the mouse vagina requires membrane attack complex. Serum bactericidal activity may serve as a correlate of protection for TMCP2.
Keywords: Neisseria gonorrhoeae, gonorrhea, vaccine, lipooligosaccharide, complement, terminal complement pathway
An experimental gonococcal peptide vaccine that mimics a gonococcal glycan epitope on lipooligosaccharide requires activation of the terminal complement pathway for its efficacy in the mouse vaginal colonization model of gonorrhea.
The emergence of multidrug-resistant Neisseria gonorrhoeae constitutes a global public health problem. A safe and effective vaccine against gonorrhea is urgently needed. Several gonococcal vaccine candidates are being evaluated in preclinical studies (reviewed in [1]). We previously identified a peptide mimic (mimitope) of the lipooligosaccharide (LOS) epitope recognized by monoclonal antibody (mAb) 2C7, which—when configured as a tetramer (called TMCP2) and adjuvanted with glucopyranosyl lipid adjuvant–stable emulsion (GLA-SE)—attenuated colonization of mice by gonococci [2]. One impediment to the development of gonococcal vaccines is the lack of a correlate of protection. In the current study, we elucidated the mechanism of action of TMCP2 in mice and define a corelate of protection that will facilitate further preclinical development.
METHODS
Bacterial Strain
N. gonorrhoeae strain FA1090 has been described elsewhere [2].
Mouse Strains
C57BL/6 and BALB/c mice were from Jackson Laboratories. C9−/− mice in a C57BL/6 background have been described elsewhere [3].
Antibodies
Function-blocking anti-mouse C7 mAb (immunoglobulin [Ig] G2κ) was produced as described elsewhere [4]. A chimeric human IgG1 derivative of mAb 2C7 with a complement-enhancing Fc mutation (E430G) has also been described elsewhere [5].
Immunization of Mice
Six-week-old C57BL/6 and C9−/− mice were immunized with 3 doses of 50-µg TMCP2 plus 5-µg GLA-SE adjuvant at weeks 0, 3, and 6. BALB/c mice used in experiments with anti-C7 were given a fourth dose of vaccine at 9 weeks. Mice were challenged with N. gonorrhoeae strain FA1090 10–14 days after the last vaccine dose.
LOS Enzyme-Linked Immunosorbent Assay
Antibody elicited against the 2C7 LOS epitope was measured by means of enzyme-linked immunosorbent assay, using LOS purified from 2C7-positive N. gonorrhoeae strain 15253, as described elsewhere [2].
Opsonophagocytosis
Mouse polymorphonuclear neutrophil (PMNs) were elicited by intraperitoneal injection of thioglycolate broth and killing of FA1090 opsonized with normal mouse serum was performed as described elsewhere [6].
Murine Model of Gonococcal Vaginal Colonization
Use of animals was performed in strict accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol (protocol no. A-1717) was approved by the Institutional Animal Care and Use Committee at the University of Massachusetts Medical School. Immunized female mice (10–14 days after the last vaccine dose) in the diestrus phase of the estrous cycle were treated with Premarin (Pfizer) and antibiotics (vancomycin and streptomycin), as described elsewhere [2, 5]. Mice were infected intravaginally with N. gonorrhoeae FA1090 (colony-forming units [CFUs] specified for each experiment). Daily bacterial burdens were measured by enumerating CFUs by rinsing vaginal swab samples in 100 μL of normal saline and then plating serial 10-fold dilutions onto chocolate agar plates containing vancomycin, colistin, nystatin, and trimethoprim sulfate) supplement (Becton Dickinson) plus 100 μg of streptomycin sulfate (Sigma) per milliliter of medium [2].
Statistical Analysis
Clearance of N. gonorrhoeae across groups was compared using 3 characteristics of the data, as described elsewhere [2, 5]: time to clearance, longitudinal trends in mean log10 CFUs, and the cumulative CFUs as area under the curve (AUC). Median time to clearance was estimated using Kaplan-Meier survival curves; the times to clearance were compared between groups using a log-rank test. Mean log10 CFU trends over time were compared between groups using 2-way analysis of variance (ANOVA) and Dunnett multiple comparisons test. The mean AUC (log10 CFUs vs time) was computed for each mouse to estimate the bacterial burden over time (cumulative infection); the means under the curves were compared between groups using 1-way ANOVA (Kruskal-Wallis test) because distributions were skewed or kurtotic; pairwise comparisons between groups was carried out using Dunn post hoc test. Anti-LOS IgG titers were compared across vaccine immunized groups using Mann-Whitney nonparametric test.
RESULTS
Unimpaired Antibody Responses to TMCP2 in C9−/− Mice
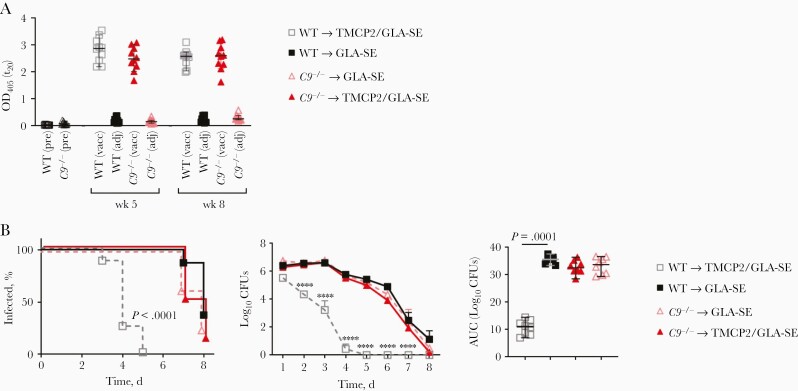
We compared 2C7 epitope-specific anti-LOS IgG responses in C9−/− and wild-type C57BL/6 mice 2 weeks after dose 2 and dose 3 (ie, at weeks 5 and 8, respectively). As shown in Figure 1A, both strains of mice showed similar anti-LOS IgG responses after immunization with TMCP2/GLA-SE.
Figure 1.
An intact terminal complement pathway is required for TMCP2 vaccine efficacy. Wild-type (WT) C57BL/6 or C9−/− mice were immunized with either TMCP2 (50 µg) plus glucopyranosyl lipid adjuvant–stable emulsion (GLA-SE) (5 µg), or GLA-SE alone intramuscularly at 0, 3, and 6 weeks. A, C9−/− mice immunized with TMCP2/GLA-SE mount normal immunoglobulin (Ig) G responses. Anti–lipooligosaccharide (LOS) IgG in sera (n = 10 per group) collected at week 0 (preimmune sera [pre]) and at weeks 5 and 8 (2 weeks after doses 2 and 3, respectively) was measured by means of LOS enzyme-linked immunosorbent assay. Horizontal bars represent medians, and error bars, 95% confidence intervals; differences between the immunized groups were not significant. Abbreviations: adj, GLA-SE adjuvant alone; OD450, optical density at 450 nm; t20, time at 20 minutes; vacc, TMCP2 vaccine plus GLA-SE. B, TMCP2 is ineffective in the absence of C9. WT C57BL/6 mice or C9−/− mice immunized according to the schedule above were challenged with Neisseria gonorrhoeae strain FA1090 (3 × 107 colony-forming units [CFUs]) intravaginally (n = 8 mice per group). Vaginas were swabbed daily to obtain CFUs. Left, Kaplan-Meier curves for time to clearance of infection, analyzed using the Mantel-Cox log-rank test. Middle, Log10 CFU versus time, with groups compared using 2-way analysis of variance (ANOVA); ∗∗∗∗P < .0001. Right, Area under the curve (AUC) analysis; groups were compared using 1-way ANOVA with the Kruskal-Wallis nonparametric test, and pairwise comparisons performed using the Dunn test.
Lack of Efficacy of TMCP2 in Mice Without Functional Terminal Complement
The terminal pathway comprises 5 components: C5b, C6, C7, C8 and C9, plasma proteins that when combined together form the lytic membrane attack complex, penetrating membranes to kill microbes. Complement C9 is the last step in assembly of the terminal complement pathway (membrane attack complex). The role of the early stages of the complement pathway in bacterial killing is well defined; Fc-Fc receptor and C3 fragment-complement receptor 3 interactions, as well as signaling through the C5a receptor, all contribute to opsonophagocytic uptake and killing of neisseriae [7, 8]. We confirmed that opsonophagocytosis remained unaffected in C9−/− mice (Supplementary Figure 1). As shown in Figure 1B, TMCP2 lost all activity in C9−/− mice, suggesting that membrane attack complex formation was essential for TMCP2-mediated protection.
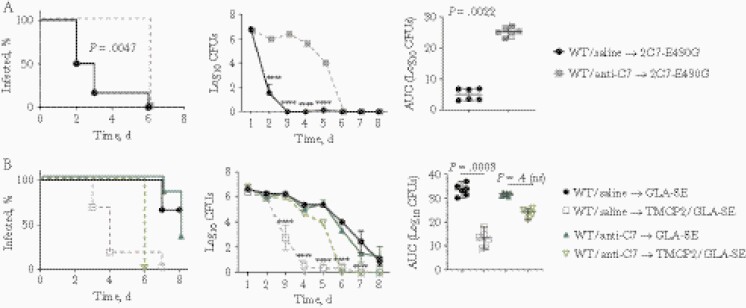
A second independent line of evidence for the role of the terminal pathway in the efficacy of TMCP2 was provided by a function-blocking anti-C7 mAb [4]. This mAb, at the dose used in the current study, completely blocks terminal pathway in mice for ≥48 hours after administration [4]. To confirm that C7 function was blocked in our study, we tested the efficacy of a human IgG1 chimeric mAb 2C7 with the E430G Fc mutation that promotes the formation of Fc hexamers on the bacterial surface, increases C1 complex engagement, and enhances classical complement pathway activation; activity of mAb 2C7-E430G requires the terminal pathway for its efficacy [5]. Administering anti-C7 rendered the chimeric mAb 2C7 ineffective (Figure 2A). Similarly, TMCP2 immunization also failed to hasten the clearance of gonococcal colonization in mice given anti-C7 (Figure 2B), confirming results obtained with C9−/− mice.
Figure 2.
C7 is required for activity of the TMCP2 vaccine. A, Verification of the function of anti-mouse C7 function blocking monoclonal antibody (mAb). Wild-type (WT) BALB/c mice (n = 6 per group) were treated intravenously with either saline or with anti-mouse C7 mAb (1 mg) on day −1 and then infected with Neisseria gonorrhoeae FA1090 (2.6 × 107 colony-forming units [CFUs]) on day 0. Anti-C7 or saline (control) was administered again on days 2 and 5. Vaginas were swabbed daily to enumerate CFUs. Left, Kaplan-Meier curves for time to clearance of infection, analyzed using the Mantel-Cox log-rank test. Middle, Log10 CFU count versus time, with groups compared using 2-way analysis of variance (ANOVA); ∗∗∗∗P < .0001. Right, Area under the curve (AUC) analysis with groups compared using the Mann-Whitney test. B, Blocking C7 function decreases the efficacy of TMCP2. WT BALB/c mice were infected with FA1090 and treated with anti-C7 (or saline), as indicated in B, with CFU counts monitored daily. Left, Kaplan-Meier curves for time to clearance of infection. Middle, Log10 CFU counts versus time, with groups compared using 2-way ANOVA; ∗∗∗∗P < .0001. Right, AUC analysis, with groups compared using 1-way ANOVA with the Kruskal-Wallis nonparametric test, and pairwise comparisons performed using the Dunn test.
DISCUSSION
Serum bactericidal activity is widely accepted as a correlate of protection against meningococcal disease. A major obstacle in the development of gonococcal vaccines is the lack of an established correlate of protection. A group B meningococcal vaccine (4CMenB) showed 31% efficacy in retrospective epidemiologic study [9]; however, its mechanism of action remains unclear. In the current study, we showed that activity of a candidate gonococcal vaccine in the mouse vaginal colonization model relies on a functional terminal complement pathway (Figures 1 and 2). These results mirror prior data with passively administered chimeric mAb 2C7 [5], which targets the same LOS epitope that is mimicked by TMCP2 [10]. We also show that antibody responses to TMCP2 in C9−/− mice are intact, consistent with normal antibody responses in humans with terminal complement deficiencies given meningococcal vaccines [11, 12].
C9-deficient human serum can also kill N. gonorrhoeae strains that are susceptible to killing by complement-sufficient human serum, but at rates far slower than seen in normal serum [13]. By contrast, C8-depleted human serum did not kill gonococci even at later time points [13]. Our data demonstrate that membrane attack complex formation is essential for activity of anti-gonococcal LOS antibodies in mice; either absence of C9 or inhibition of C7 ablates activity. Delayed killing reported in vitro with C9-depleted/C9-deficient serum [13] may not suffice for vaccine efficacy in vivo, although we acknowledge that differences in gonococcal strains and sources of complement may preclude extrapolation of our data to humans.
The presence of active complement in the female mouse genital tract that can support gonococcal killing is consistent with previous findings showing that human cervical secretions contain hemolytically active complement [14]. These data and our group’s previous results with mAb 2C7 [5] suggest that serum bactericidal assay may serve as a correlate of protection for the TMCP2 vaccine. N. gonorrhoeae have evolved numerous strategies to evade killing by neutrophils [7]; it is therefore not surprising that opsonophagocytosis may not contribute significantly to clearance of gonococci. Accordingly, depletion of PMNs did not negatively impact the efficacy of mAb 2C7 [5].
A recent study showed that C6−/− mice (derived from the Peru-Coppock strain) had impaired innate immune responses, including defective expression of surface adhesion molecules, generation of superoxide anion, and appearance of reactive oxygen species and histone release after activation of PMNs, along with defective phagocytosis [15]. Loss of C6, C7, or C8 activity does not impair opsonophagocytic killing of meningococci by neutrophils derived from normal healthy individuals [8, 11]. To minimize the possibility of impaired neutrophil function in mice genetically deficient in C9, we confirmed the role of terminal complement using a function-blocking anti-C7 mAb in wild-type mice.
In conclusion, terminal complement is necessary for efficacy of a peptide vaccine that targets gonococcal LOS. The serum bactericidal assay may serve as a correlate of protection for the TMCP2 vaccine.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by the National Institutes of Health (grants R01 AI114790, AI132296, AI141181 and R21 AI146621) and by Health and Care Research Wales (fellowship award to W. M. Z.).
Potential conflicts of interest. B. P. M. serves as a consultant for Jansen and Kyra Pharmaceuticals, and B. P. M. and W. M. Z. are inventors on patents for anti-C7 antibodies. J. S. and F. B. have financial interests in Genmab (stocks and/or warrants) and are inventors on patents relating to bispecific and avidity-engineered molecules. S. R. serves as a consultant for Apellis Pharmaceuticals and Ionis Pharmaceuticals. P. A. R. is listed as an inventor on patents related to the TMCP2 vaccine (assignee: University of Massachusetts Medical School). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rice PA, Shafer WM, Ram S, Jerse AE.. Neisseria gonorrhoeae: drug resistance, mouse models, and vaccine development. Annu Rev Microbiol 2017; 71:665–86. [DOI] [PubMed] [Google Scholar]
- 2. Gulati S, Pennington MW, Czerwinski A, et al. Preclinical efficacy of a lipooligosaccharide peptide mimic candidate gonococcal vaccine. Mbio 2019; 10:e02552-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ueda Y, Miwa T, Ito D, et al. Differential contribution of C5aR and C5b-9 pathways to renal thrombic microangiopathy and macrovascular thrombosis in mice carrying an atypical hemolytic syndrome-related factor H mutation. Kidney Int 2019; 96:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zelek WM, Morgan BP.. Monoclonal antibodies capable of inhibiting complement downstream of C5 in multiple species. Front Immunol 2020; 11:612402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gulati S, Beurskens FJ, de Kreuk BJ, et al. Complement alone drives efficacy of a chimeric antigonococcal monoclonal antibody. PloS Biol 2019; 17:e3000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu H, Jerse AE.. Alpha-2,3-sialyltransferase enhances Neisseria gonorrhoeae survival during experimental murine genital tract infection. Infect Immun 2006; 74:4094–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palmer A, Criss AK.. Gonococcal defenses against antimicrobial activities of neutrophils. Trends Microbiol 2018; 26:1022–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Konar M, Granoff DM.. Eculizumab treatment and impaired opsonophagocytic killing of meningococci by whole blood from immunized adults. Blood 2017; 130:891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petousis-Harris H, Paynter J, Morgan J, et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet 2017; 390:1603–10. [DOI] [PubMed] [Google Scholar]
- 10. Ngampasutadol J, Rice PA, Walsh MT, Gulati S.. Characterization of a peptide vaccine candidate mimicking an oligosaccharide epitope of Neisseria gonorrhoeae and resultant immune responses and function. Vaccine 2006; 24:157–70. [DOI] [PubMed] [Google Scholar]
- 11. Van den Broek B, van Els C, Kuipers B, et al. Multi-component meningococcal serogroup B (MenB)-4C vaccine induces effective opsonophagocytic killing in children with a complement deficiency. Clin Exp Immunol 2019; 198:381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fijen CA, Kuijper EJ, Drogari-Apiranthitou M, Van Leeuwen Y, Daha MR, Dankert J.. Protection against meningococcal serogroup ACYW disease in complement-deficient individuals vaccinated with the tetravalent meningococcal capsular polysaccharide vaccine [see comments]. Clin Exp Immunol 1998; 114:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harriman GR, Esser AF, Podack ER, et al. The role of C9 in complement-mediated killing of Neisseria. J Immunol 1981; 127:2386–90. [PubMed] [Google Scholar]
- 14. Price RJ, Boettcher B.. The presence of complement in human cervical mucus and its possible relevance to infertility in women with complement-dependent sperm-immobilizing antibodies. Fertil Steril 1979; 32:61–6. [DOI] [PubMed] [Google Scholar]
- 15. Fattahi F, Grailer JJ, Parlett M, et al. Requirement of complement C6 for intact innate immune responses in mice. J Immunol 2020; 205:251–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.