Abstract
Background
HIV Prevention Trials Network 084 demonstrated that long-acting injectable cabotegravir (CAB) was superior to daily oral tenofovir (TFV) disoproxil fumarate (TDF)/emtricitabine (FTC) for preventing human immunodeficiency virus (HIV) infection in sub-Saharan African women. This report describes HIV infections that occurred in the trial before unblinding.
Methods
Testing was performed using HIV diagnostic assays, viral load testing, a single-copy RNA assay, and HIV genotyping. Plasma CAB, plasma TFV, and intraerythrocytic TFV-diphosphate concentrations were determined by liquid chromatography–tandem mass spectrometry.
Results
Forty HIV infections were identified (CAB arm, 1 baseline infection, 3 incident infections; TDF/FTC arm, 36 incident infections). The incident infections in the CAB arm included 2 with no recent drug exposure and no CAB injections and 1 with delayed injections; in 35 of 36 cases in the TDF/FTC arm, drug concentrations indicated low or no adherence. None of the cases had CAB resistance. Nine women in the TDF/FTC arm had nonnucleoside reverse-transcriptase inhibitor resistance; 1 had the nucleoside reverse-transcriptase inhibitor resistance mutation, M184V.
Conclusions
Almost all incident HIV infections occurred in the setting of unquantifiable or low drug concentrations. CAB resistance was not detected. Transmitted nonnucleoside reverse-transcriptase inhibitor resistance was common; 1 woman may have acquired nucleoside reverse-transcriptase inhibitor resistance from study drug exposure.
Keywords: Africa, cabotegravir, HIV, HPTN 084, injectable, long-acting, preexposure prophylaxis, prevention, TDF/FTC, women
Human immunodeficiency virus (HIV) infections were characterized in women receiving long-acting cabotegravir or oral tenofovir disoproxil fumarate/emtricitabine for HIV prevention. Almost all incident HIV infections occurred in the setting of unquantifiable or low drug concentrations. Cabotegravir resistance was not detected.
Daily oral tenofovir (TFV) disoproxil fumarate/emtricitabine (TDF/FTC) is approved by the US Food and Drug Administration (FDA) and recommended by the World Health Organization for human immunodeficiency virus (HIV) preexposure prophylaxis (PrEP). The efficacy of this regimen is highly dependent on adherence, particularly in women [1, 2]. Use of long-acting drugs for PrEP may reduce HIV risk and provide alternative PrEP options that overcome barriers to adherence [3]. Cabotegravir (CAB) is an HIV integrase strand transfer inhibitor (INSTI). Long-acting injectable CAB (CAB-LA) was recently approved by the US FDA for maintenance of viral suppression as part of a 2-drug regimen for HIV treatment [4].
The HIV Prevention Trials Network (HPTN) 083 and 084 trials demonstrated that CAB-LA is superior to daily oral TDF/FTC for HIV PrEP [5, 6]. Both trials included a double-blind, double-dummy comparison of 2 regimens: active CAB plus TDF/FTC placebo versus active TDF/FTC plus CAB placebo. HPTN 083 enrolled cisgender men and transgender women who had sex with men at 43 sites in the United States, Latin America, Asia, and Africa. HPTN 084 enrolled cisgender women at 20 sites in sub-Saharan Africa. Both trials were unblinded in 2020 after Data Safety Monitoring Board meetings concluding that CAB-LA was superior to oral TDF/FTC, with a 66% reduction in HIV incidence in HPTN 083 [5] and an 88% reduction in incidence in HPTN 084 [6]. TDF/FTC adherence was assessed in randomly selected participants; 74.2% of samples tested in HPTN 083 and 46.4% of those tested in HPTN 084 had TFV concentrations consistent with good adherence to the daily oral TDF/FTC regimen (>40 ng/mL) [5, 6].
In HPTN 083, CAB exposure was associated with prolonged viral suppression and diminished/delayed HIV antibody expression among infected participants [7]. This resulted in a delay in HIV diagnosis at study sites in most incident HIV infections; in many cases, participants received study drug after they were infected. Delays in HIV diagnosis contributed to emergence of INSTI resistance in some cases. Low or unquantifiable concentrations of study drugs near the time of the first HIV-positive visit accounted for almost half of the incident infections in the CAB arm and the majority of them in the TDF/FTC arm [7]. This report includes analysis of HIV infections observed in the blinded phase of HPTN 084, including analysis of drug concentrations, the impact of CAB and TDF/FTC on detection of HIV infection, and HIV drug resistance.
METHODS
Study Cohort
HPTN 084 (NCT03164564) enrolled 3224 women in Botswana, Eswatini, Kenya, Malawi, South Africa, Uganda, and Zimbabwe. Participants in the TDF/FTC arm received daily oral TDF/FTC plus placebo. Participants in the CAB arm received 5 weeks of daily oral CAB followed by CAB injections (2 injections 4 weeks apart, then every 8 weeks) plus placebo (Figure 1). Participants were screened for HIV infection at each visit. Plasma samples were stored at every visit. Dried blood spot (DBS) samples were stored at selected visits (Figure 1). This report includes data from the blinded phase of the trial (6 December 2016 through 5 November 2020).
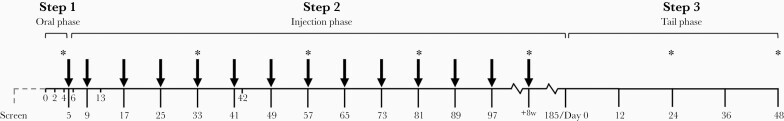
Figure 1.
HIV Prevention Trials Network (HPTN) 084 study schema. The study included 3 steps: an oral lead-in phase (step 1), an injection phase (step 2), and an open-label tail phase (step 3). In the tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) arm, participants received daily TDF/FTC in steps 1 and 2 (coformulated tablets; 300-mg TDF plus 200-mg FTC), along with placebo tablets (in step 1) and placebo injections (in step 2). In the cabotegravir (CAB) arm, participants received oral daily CAB in step 1 (30-mg tablets) and long-acting CAB (CAB-LA) injections in step 2 (600 mg; 3 mL), with placebo tablets in steps 1 and 2. All participants received daily open-label TDF/FTC in step 3. The first and second injections were given 4 weeks apart at weeks 5 and 9; participants then received injections every 8 weeks (arrows). Plasma samples were collected at every visit. Dried blood spot (DBS) samples were prepared and stored at weeks 4 and week 33, followed by every third injection visit (asterisks); DBS samples were also stored at weeks 24 and 48 in step 3. Study drugs were discontinued if participants had a reactive or positive human immunodeficiency virus (HIV) test result, declined drug administration, or had significant adverse events. Study drugs were also stopped for other reasons at the discretion of the site principal investigator.
Laboratory Methods
The methods used to identify and characterize HIV infections were the same in HPTN 083 and HPTN 084, except for the approach used to select HIV screening tests at study sites [7].
HIV Testing at Study Sites
Study sites performed HIV testing using locally available assays. All participants had a negative HIV RNA test result within 14 days prior to enrollment. HIV testing at enrollment and follow-up visits was performed using 1 or 2 HIV rapid tests (with ≥1 test cleared by the US FDA) and an instrumented, laboratory-based antigen-antibody (Ag/Ab) test. A centralized committee reviewed HIV test results if a reactive or positive result was obtained and provided guidance on further testing. HIV DNA testing using an ultrasensitive assay was performed in real time at Johns Hopkins University for cases with inconclusive test results, at the direction of the committee. The first visit near the time of HIV diagnosis where a reactive or positive HIV test was obtained at a study site was defined as the first site-positive visit.
Retrospective Testing
Additional testing was performed at the HPTN Laboratory Center and other laboratories in the United States to confirm and characterize HIV infections.
Identification of HIV infections
Additional testing with the following assays was performed in all cases where a reactive or positive test result was obtained at a study site: Architect HIV Ag/Ab Combo Test (Ag/Ab test; Abbott Diagnostics), Geenius HIV 1/2 Supplemental Assay (confirmatory antibody test; Bio-Rad Laboratories), or Aptima HIV-1 RNA Qualitative Assay (qualitative RNA test; limit of quantification, 30 copies/mL; Hologic). An independent Endpoint Adjudication Committee reviewed test results from study sites and the HPTN Laboratory Center. This committee made a final determination of HIV status and identified the first HIV-positive visit in each case.
Viral Load Testing
HIV viral load was quantified using the RealTime HIV-1 Viral Load Assay (Abbott Molecular). A validated dilution method was used for testing; if HIV RNA was not detected, the standard viral load assay was performed (limit of quantification, 40 copies/mL). Selected samples were tested at the University of Pittsburgh using a single-copy HIV RNA assay [8].
HIV Drug Resistance Testing
HIV resistance testing was performed at Monogram Biosciences using the GenoSure PRIme and PhenoSense INSTI assays. The GenoSure PRIme assay provides data for nucleoside/nucleotide reverse-transcriptase inhibitors (NRTIs), nonnucleoside reverse-transcriptase inhibitors (NNRTIs), protease inhibitors, and INSTIs. Drug resistance mutations were identified using the 2019 IAS Drug Resistance Mutations Update [9].
Antiretroviral Drug Testing
Concentrations of TFV and CAB in plasma and TFV-diphosphate (TFV-DP) in DBS were quantified using liquid chromatography–tandem mass spectrometry (lower limits of quantification: CAB, 0.025 mcg/mL; TFV, 0.31 ng/mL; and TFV-DP, 31.3 fmol per punch). CAB concentrations were measured in the CAB arm at all study visits before infection. The CAB protein-adjusted in vitro 90% inhibitory concentration (PA-IC90) is 0.166 mcg/mL [10, 11]. CAB concentrations were stratified into 4 groups: <1× PA-IC90, 1–4× PA-IC90 (0.166–0.664 mcg/mL), 4–8× PA-IC90 (0.664–1.33 mcg/mL), and ≥8× PA-IC90 (≥1.33 mcg/mL) [7]. In the TDF/FTC arm, TFV and TFV-DP concentrations were measured at selected study visits. Adherence to daily oral TDF/FTC was determined based on results of plasma and DBS testing, comparing results to established adherence benchmarks (Supplementary File 1).
Ethical Considerations
The HPTN 084 protocol was reviewed and approved by institutional review boards and/or ethics committees for all participating sites and at ministries of health, as appropriate. Written informed consent was obtained for all participants.
RESULTS
Classification of HIV Infections
Forty HIV infections were identified (4 in the CAB arm, 36 in the TDF/FTC arm). Case numbers were assigned using a classification system similar to that used in HPTN 083 (Table 1). At the time of study unblinding, all 4 infections in the CAB arm were classified as incident infections. Subsequent testing at the HPTN Laboratory Center revealed that 1 of 4 participants had HIV infection at enrollment; this case was reclassified as a baseline infection (case A1). In the CAB arm, 2 participants with incident infection received no CAB injections and had no recent CAB exposure (cases B1 and B2). The third incident infection occurred during the injection phase of the study in a participant with delayed injection visits (case DX). All 36 infections in the TDF/FTC arm were incident infections.
Table 1.
Classification of Human Immunodeficiency Virus Infectionsa
| Infections, No. | ||
|---|---|---|
| Infection Type and Classification Group | CAB Arm (n= 4) | TDF/FTC Arm (n = 36) |
| Baseline | 1 | 0 |
| HIV positive at study enrollment | Case A1 | |
| Incident | 3 | 36 |
| No recent CAB exposure | Cases B1 and B2 | |
| Infected during the CAB injection phase | Case DX | |
| Inconsistent/poor TDF/FTC adherence | Cases E1-E35 | |
| Partial TDF/FTC adherence | Case E36 | |
Abbreviations: CAB, cabotegravir; FTC, emtricitabine; HIV, human immunodeficiency virus; TDF, tenofovir disoproxil fumarate.
This table shows the system used to classify cases in the CAB and TDF/FTC arms. Groups were assigned based on study arm and timing of HIV infection/exposure to study drug. Each case was assigned a group letter and a case number (eg, case A1). Cases in the TDF/FTC arm are numbered based on the number of days between study enrollment and the first HIV-positive visit, from the longest (846 days [case E1] ) to the shortest (12 days [case E36]) interval.
Study Drug Exposure and Drug Concentrations
CAB Arm
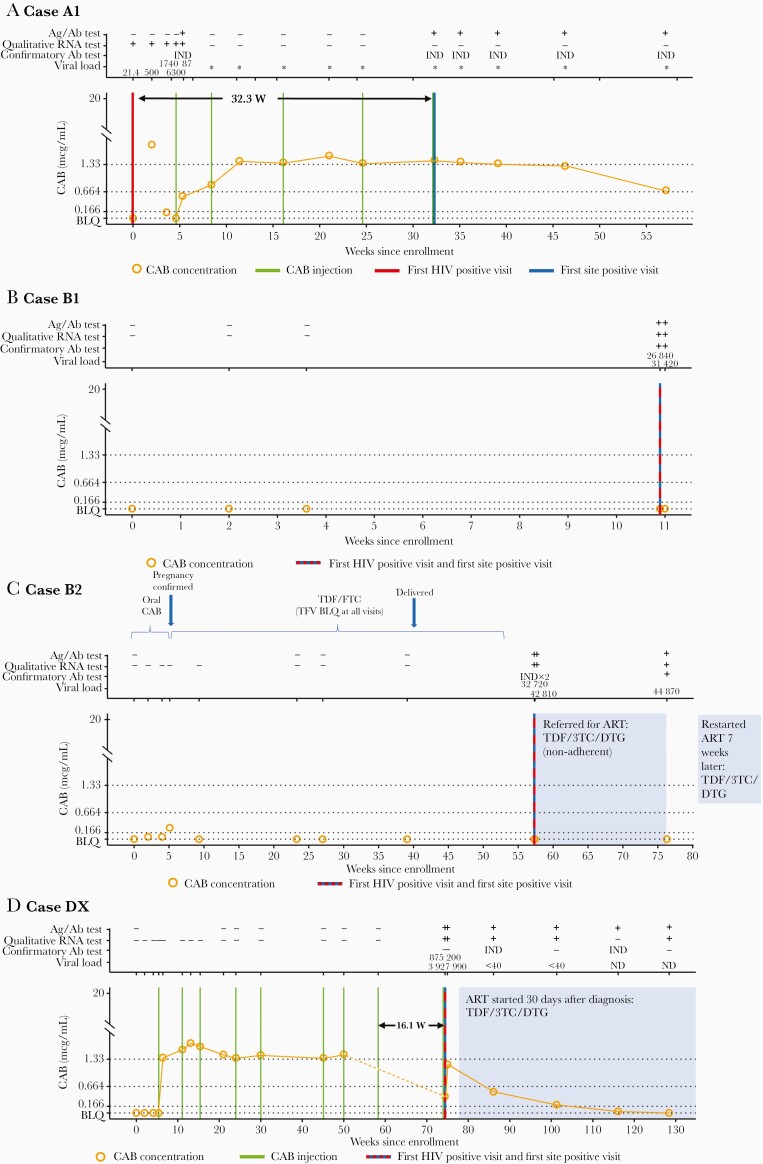
Laboratory results and key events for the 4 CAB cases are shown in Figure 2. Additional information is provided in Supplementary File 2. In case A1, the participant had HIV infection at study enrollment, before CAB administration (Figure 2A). The site first detected evidence of HIV infection 32.3 weeks later. This participant received oral CAB and 5 CAB injections before the first site-positive visit. CAB concentrations indicate that the participant was not taking CAB consistently during the oral lead-in period. During the injection phase of the study, CAB concentrations did not exceed 8× PA-IC90 until after the second injection and remained above this level through the first site-positive visit. At that time, the CAB concentration was 2.58 mcg/mL.
Figure 2.
Case summaries for the cabotegravir (CAB) arm), including laboratory results and key events for participants (cases A1, B1, B2, and DX). The full set of laboratory data for these cases, including results from testing performed at study sites and results from human immunodeficiency virus (HIV) resistance testing, is provided in Supplementary File 2. Annotations above each graph show results obtained from testing performed at the HIV Prevention Trials Network (HPTN) Laboratory Center, displayed as reactive/positive (plus sign [+]), negative/nonreactive (minus sign [−]), or indeterminate (IND). Viral load values represent HIV RNA copies per milliliter; a value <40 copies/mL indicates that HIV RNA was detected but was below the limit of quantification (BLQ). Asterisks signify negative single-copy RNA test results (no RNA detected). The graphs show plasma CAB concentrations and key events by weeks since study enrollment. The arrow in A shows the weeks between the first HIV-positive visit and the first site-positive visit (visit at which the site had a reactive or positive HIV test result); the arrow in D, the weeks between the eighth injection and the first HIV-positive visit. Horizontal lines indicate the following CAB concentration cutoffs: 1.33 mcg/mL, 8× the protein-adjusted in vitro 90% inhibitory concentration (PA-IC90); 0.664 mcg/mL, 4× PA-IC90, and 0.166 mcg/mL, 1× PA-IC90. BLQ indicates that the CAB concentration was <0.025 mcg/mL, shaded areas indicate periods of antiretroviral treatment (ART). Abbreviations: 3TC: lamivudine; Ab, antibody; Ag/Ab, antigen-antibody test; DTG, dolutegravir; FTC, emtricitabine. ND, not detected; TDF, tenofovir disoproxil fumarate; TFV, tenofovir.
In case B1, HIV infection was detected by HIV rapid tests 10.9 weeks after enrollment; this participant did not receive any CAB injections. CAB concentrations were below the limit of quantification at all visits (Figure 2B).
In case B2, the participant discontinued oral CAB 5.1 weeks after enrollment due to pregnancy (Figure 2C). CAB concentrations were <4× PA-IC90 during the oral lead-in phase. The participant was provided open-label TDF/FTC during pregnancy; however, the concentration of TFV was unquantifiable at all visits, indicating poor adherence or nonadherence to the daily oral regimen. The first HIV-positive visit occurred 1 year after oral CAB was stopped, 3.1 weeks after TDF/FTC provision was interrupted because the participant was not able to refill the medication, and 17.3 weeks after delivery of a healthy infant. The participant was referred for antiretroviral treatment after diagnosis but did not achieve viral suppression owing to nonadherence. The infant tested negative for HIV at birth and 1 year later.
In case DX, the participant acquired HIV while receiving CAB injections (Figure 2D). CAB concentrations in the oral phase were all below the limit of quantification, suggesting nonadherence to study drug. The participant received 9 CAB injections; the ninth injection was administered at the first site-positive visit, before the site received the reactive Ag/Ab test result. Three injections occurred outside protocol-specified allowable windows, including the eighth and ninth injections, which occurred 16.1 weeks apart. After the initiation of CAB injections, the CAB concentration was ≥8× PA-IC90 in all plasma samples collected before the first HIV-positive visit but <4× PA-IC90 (0.416 mcg/mL) at the first HIV-positive visit. It was not possible to determine the CAB concentration at the time of the eighth injection owing to a sample processing error.
TDF/FTC Arm
Laboratory results and interpretation of TDF/FTC adherence data for TDF cases are shown in Supplementary File 3. In HPTN 084, DBS samples were available only at the first HIV-positive visit in 9 of 36 incident cases. Based on results from plasma and DBS samples, none of the 36 TDF/FTC cases had drug concentrations consistent with good adherence (7 doses per week). Only 1 case had drug concentrations consistent with partial adherence (case E36; 4–6 doses weekly). In this case, results for all HIV assays performed at the HPTN Laboratory Center were nonreactive or negative (Supplementary File 3). The possibility of a sample mix-up should be considered when interpreting laboratory results for this case. In 2 cases, drug concentrations indicated inconsistent adherence (cases E6 and E31; 2–4 doses weekly). The remaining 33 cases were classified as having poor adherence (<2 doses weekly). Four cases classified as having poor adherence based on low TFV-DP concentrations had high plasma TFV concentrations (>100 ng/mL; cases E4, E9, E21, and E30) (Supplementary File 3); these participants likely had inconsistent or poor adherence to TDF/FTC but may have taken the product shortly before attending study visits.
Delayed Detection of HIV Infection
In 1 CAB and 8 TDF/FTC cases, there was a delay between the first HIV-positive visit and the first site-positive visit. In the CAB arm, retrospective testing revealed that the participant in case A1 was HIV positive at study enrollment (Figure 2A and Supplementary File 2). This participant had a positive qualitative RNA test at enrollment; the viral load test was positive with RNA detected below the limit of quantification (<40 copies/mL). A viral load of 21.4 copies/mL was obtained using the single-copy RNA assay. During the oral lead–in phase, the viral load ranged from 500 to 6300 copies/mL. HIV RNA was not detected after the first CAB injection using the qualitative RNA assay or the single-copy RNA assay (>48 weeks total; 23.9 weeks while receiving CAB injections and 24.9 weeks after the last injection). The Ag/Ab test performed at the HPTN Laboratory Center was reactive at the visit after the first CAB injection (week 5) and then not until week 33, when the site first detected the infection. The confirmatory antibody test result was indeterminate at all visits tested (>57 weeks after enrollment).
Detection of HIV infection was also delayed at the study site in 8 TDF/FTC cases; all 8 cases had acute (RNA only) infection at the first HIV-positive visit. Six of these cases occurred within the first 4 months after study enrollment. In 4 of them (cases E23, E26, E30, and E33), the confirmatory antibody test was positive at the next visit (Supplementary File 3). In these cases, the median viral load was 2265 copies/mL at the first HIV-positive visit (range, 601–25 340 copies/mL). All 4 participants had poor adherence to blinded oral TDF/FTC. The time between the first HIV-positive visit and the first site-positive visit in these cases ranged from 5 to 8.1 weeks.
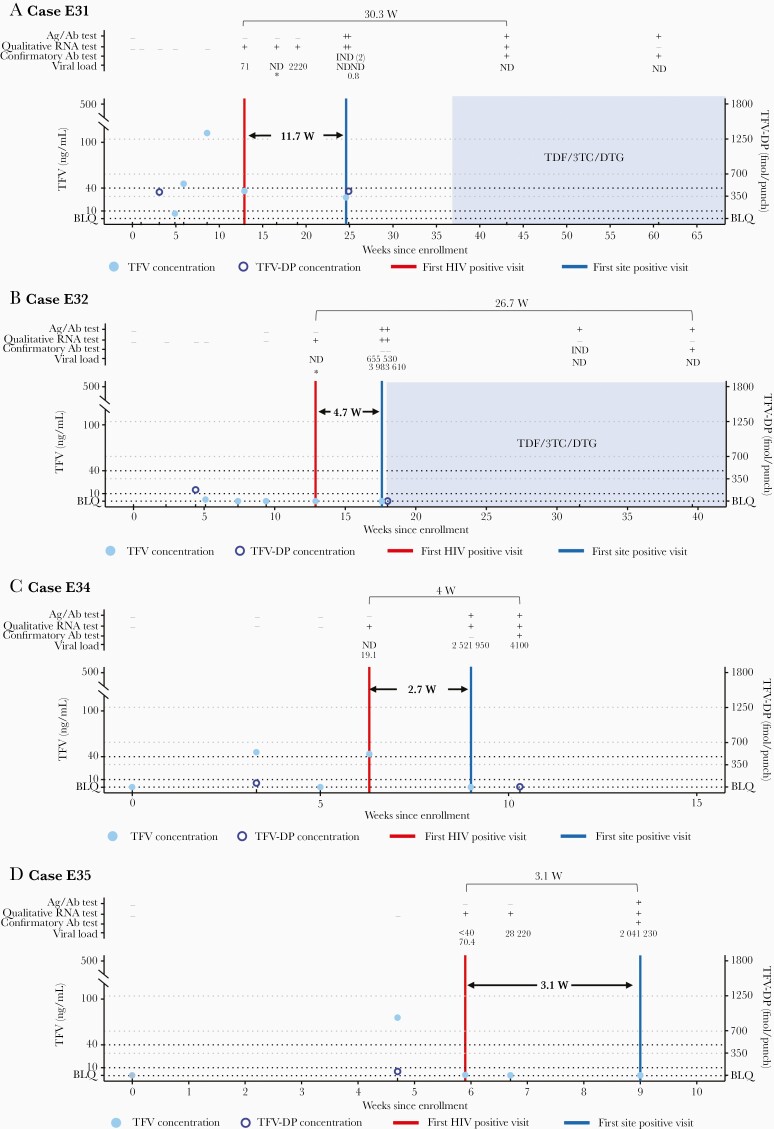
In the other 4 cases with delayed detection (cases E31, E32, E34, and E35), all HIV testing at the study site had nonreactive/negative results at the first HIV-positive study visit; the confirmatory antibody result at the next visit was negative or indeterminate (Figure 3 and Supplementary File 4). Three of these participants had poor adherence to blinded oral TDF/FTC (cases E32, E34, and E35), and 1 had inconsistent adherence (case E31). The time between the first HIV-positive visit and the first site-positive visit ranged from 2.7 to 11.7 weeks. In 2 of these cases, the standard viral load assay was negative at the first HIV-positive visit (cases E32 and E34); the single-copy RNA assay result was negative in case E32 and 19.1 copies/mL in case E34. In the other 2 cases, HIV RNA was detected at the first HIV-positive visit using the standard viral load assay (71 copies/mL in case E31; below the limit of quantification in case E35); the single-copy RNA assay result was 70.4 copies/mL in case E35. In all 4 cases, the Ag/Ab test was nonreactive at the first HIV-positive visit; in 1 case, the following visit also had a nonreactive Ag/Ab result. The time between the first HIV-positive visit and the visit with a positive confirmatory antibody test ranged from 3.1 to 30.3 weeks.
Figure 3.
Selected case summaries for the tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) arm, including laboratory results and key events for the 4 participants for whom antibody detection at the study sites was delayed >1 study visit (cases E31, E32, E34, and E35). The full set of laboratory data for these cases, including results from testing performed at study sites and results from human immunodeficiency virus (HIV) resistance testing, is provided in Supplementary File 4. Annotations above each graph show results obtained from testing performed at the HIV Prevention Trials Network (HPTN) Laboratory Center, displayed as reactive/positive (plus sign [+]), negative/nonreactive (minus sign [−]), or indeterminate (IND). Viral load values represent the HIV RNA copies per milliliter; a value <40 copies/mL indicates that HIV RNA was detected but was below the limit of quantification (BLQ). Asterisks signify negative single-copy RNA test results (no RNA detected). The graphs show tenofovir (TFV) and TFV-diphosphate (TFV-DP) concentrations and key events by weeks since study enrollment. Arrows show the weeks between the first HIV-positive visit (red vertical line) and the first site-positive visit (visit at which the site had a reactive or positive HIV test result (blue vertical line); dashed black lines, TFV concentration cutoffs used for adherence assessments (10 and 40 ng/mL, which correspond to 4 and 7 doses per week, respectively); dashed gray lines, TFV-DP concentrations cutoffs used for adherence assessments (350, 700, and 1250 fmol per punch, correspond to 2, 4, and 7 doses per week, respectively). The dashed line labeled BLQ indicates drug concentrations <0.31 ng/mL for TFV and <31.3 fmol per punch for TFV-DP, and shaded areas indicate periods of antiretroviral treatment (ART). Abbreviations: 3TC, lamivudine; Ab, antibody; Ag/Ab: antigen-antibody; DTG, dolutegravir; ND, not detected.
HIV Drug Resistance
HIV genotyping was performed at visits before antiretroviral treatment initiation where the viral load was >500 copies/mL (viremic visits; Table 2). Results were obtained at the first viremic visit for all 4 cases in the CAB arm and at subsequent visits in 3 of these cases. Three cases were subtype C and 1 was subtype A1 infection. There was no evidence of drug resistance in any of these cases. The INSTI polymorphism, L74I, was detected in 1 case of subtype C HIV infection. Phenotyping was successful for 3 of the 4 cases; none had reduced INSTI susceptibility.
Table 2.
Human Immunodeficiency Virus Drug Resistance (Tenofovir Disoproxil Fumarate/Emtricitabine Arm)
| Case | Week of Visit | DRMsa | Drug Resistanceb |
|---|---|---|---|
| E7 | 89 | NNRTI: K103N, P225H; PI: K20R, M36I, L89M | EFV, NVP, (DOR) |
| E9 | 81 | NNRTI: K103N, P225H; PI: K20R, M36I, I62V, I85V | EFV, NVP, (DOR) |
| E11 | 65 | NNRTI: E138A; PI: K20R, M36I, I62I/V, L89M | RPV |
| E12 | 65 | NNRTI: V90I, K103N, E138A; PI: K20R, M36I, L89M | EFV, NVP, RPV |
| E17 | 49 | NNRTI: K101E; PI: M36I, D60E, L89M | RPV, (EFV), (NVP) |
| E18 | 49 | NNRTI: K103K/N; PI: M36I | EFV, NVP |
| E24 | 33 | NNRTI: K103N; PI: M36I, L89M | EFV, NVP |
| E32 | 17 | NRTI: M184V; NNRTI: K103N; PI: M36I, L89M | EFV, NVP, FTC, 3TC, (ddI) |
| E33 | 9 | NNRTI: K103N; PI: M36I, I62V, L89M | EFV, NVP |
Abbreviations: 3TC, lamivudine; ddI, didanosine; DOR, doravirine; DRMs, drug resistance mutations; EFV, efavirenz; FTC, emtricitabine; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse-transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; RPV, rilpivirine.
Results from human immunodeficiency virus (HIV) genotyping for cases in the tenofovir disoproxil fumarate/FTC arm with ≥1 major DRM noted in the 2019 IAS drug resistance mutations update [8]. HIV genotyping was performed at the first study visit with a viral load >500 copies/mL; this was the first HIV-positive visit for 8 of the 9 cases (in case E32, the first sample with a viral load >500 copies/mL was collected 33 days after the first HIV-positive visit). All 9 cases had subtype C infection. Major protease inhibitor mutations were not detected. Major DRMs associated with resistance to integrase strand transfer inhibitors were not detected. Bold text indicates major DRMs and italics, drugs that affect susceptibility to study drugs (M184V only); underlined mutations are recommended for surveillance of transmitted drug resistance [12].
Drugs noted as having reduced susceptibility (resistant) on reports from Monogram Biosciences; those in parentheses are noted as having partial susceptibility (resistance possible). HIV genotyping results were obtained at follow-up visits for cases E7 and E33 (7 and 39 days later, respectively); no change in mutations was detected in these cases. Three additional cases that had no drug resistance at the first viremic visit had genotyping results at follow-up visits (cases E23, E26, and E35; 16–57 days later); no change in mutations was detected in these case.
Genotyping results were obtained at the first viremic visit for 33 of 36 TDF/FTC cases (1 had no viremic sample; 2 failed testing); genotyping results were also obtained at subsequent visits in 5 of the 33 cases (Table 1). Twenty-nine cases had subtype C infection, 2 had subtype A1 infection, and 2 had intersubtype recombinant HIV (C/K and A/C). Nine of the cases had NNRTI resistance at the first viremic visit; 1 also had the M184V mutation at this visit (case E32). INSTI polymorphisms were detected in 10 of the 33 cases.
DISCUSSION
In HPTN 084, CAB-LA and oral TDF/FTC were both highly effective for HIV prevention. Detailed characterization of HIV infections helps explain why some women acquired HIV and provides new insights into the impact of CAB-LA PrEP on detection of HIV infection.
In all 3 incident cases in the CAB arm of HPTN 084, the CAB concentration at the first HIV-positive visit was below the level predicted to be protective against HIV infection based on nonhuman primate simian-human immunodeficiency virus challenge studies [10, 13]. Two of these cases were in women who had no recent CAB exposure and received no CAB injections. The third occurred in a woman who had delayed CAB injections; this woman had a low CAB concentration at the first HIV-positive visit which was >16 weeks after the previous injection. The low CAB concentration at the first HIV-positive visit in this case is not unexpected based on the apparent terminal phase half-life of CAB-LA in cisgender women (8.6 weeks) [14]. In HPTN 084, none of the cisgender women in the CAB arm acquired HIV infection while receiving on-time injections. In contrast, in the HPTN 083 trial of cisgender men and transgender women, 4 participants with incident infection in the CAB arm were infected despite on-time injections with expected drug concentrations at the first HIV-positive visit [7].
In HPTN 084, retrospective testing revealed that 1 woman in the CAB arm had HIV infection at enrollment; retrospective testing also identified 7 baseline infections in HPTN 083 (4 in the CAB arm, 3 in the TDF/FTC arm). In both trials, all baseline infections would have been detected at enrollment, and almost all incident infections would have been detected at the first HIV-positive visit using a viral load assay with a limit of quantification of 40 copies/mL.
HPTN 083 and 084 are now transitioning to open-label extension studies in which all participants will be offered CAB-LA. In extension studies, HIV RNA testing will be performed at every study visit to screen for HIV infection. Even with this approach, it is still possible that some participants with HIV infection will receive a single CAB-LA injection before the RNA result is available, and that some will have HIV RNA levels below the limit of detection of most viral load assays. Further analyses are needed to evaluate the usefulness and cost of using HIV RNA testing to screen for HIV infection in persons receiving CAB-LA for PrEP outside a clinical trial setting.
None of the women acquired HIV infection during the CAB oral lead-in period. It is notable that all 3 women with incident infection in the CAB arm had low or unquantifiable CAB concentrations during this phase of the study, indicating low or nonadherence to blinded oral CAB. Participants in the open-label extension studies will have the option of starting CAB injections without an oral lead-in period. Suboptimal adherence to daily pill taking was observed in the TDF/FTC arm of HPTN 084. Adherence to daily TDF/FTC is especially important for women; a prior study suggests that 6–7 doses per week are required to achieve vaginal TFV concentrations associated with HIV protection [15]. Thirty-five of the 36 incident infections in the TDF/FTC arm of HPTN 084 occurred in women who had suboptimal drug concentrations near the time of the first HIV-positive visit. Discordant plasma TFV and DBS TFV-DP concentrations were observed in some women, suggesting that dosing may have occurred directly before a study visit without consistent study product use. TDF/FTC adherence was also assessed in both trials in a random subset of participants; the portion of participants with good adherence was much lower in HPTN 084 than in HPTN 083 [5, 6]. These findings highlight the need for PrEP products for women that do not rely on daily oral pill taking.
Detection of HIV infection was also delayed in both trials for some cases in the TDF/FTC arm. These delays were usually short, with HIV infection confirmed at the next study visit. Many of these cases occurred because the participant had acute HIV infection at the first HIV-positive visit, reflecting the frequency of study visits. These delays may not affect detection of HIV infection in clinical practice, since all of these infections were detected within 3 months of the first HIV-positive visit, the maximum recommended interval for HIV testing in persons receiving oral TDF/FTC PrEP [16].
We did not identify any cases with INSTI resistance in HPTN 084. The high frequency of transmitted NNRTI drug resistance observed in this cohort is concerning and emphasizes the importance of drug resistance testing and use of protease inhibitor– or INSTI-based treatment regimens where appropriate. Emergence of NRTI resistance in participants in the TDF/FTC arms of both trials was rare, most likely because most participants who acquired HIV infection in the TDF/FTC arms had poor adherence to the TDF/FTC regimen.
HPTN 084 demonstrated that CAB-LA and oral TDF/FTC are both highly effective for HIV prevention in women. All but 1 of the 39 incident infections occurred in women with delayed or discontinued CAB use or inconsistent or poor adherence to TDF/FTC. Drug concentrations were below predicted protective target concentrations in all of these cases. Detection of HIV infection was delayed in some participants in both study arms. Data from HPTN 084 and HPTN 083 show that these delays were more frequent and longer in persons receiving CAB than in those receiving TDF/FTC. These delays resulted in drug administration to persons with undetected HIV infection in almost all cases; INSTI resistance emerged in some of these cases in HPTN 083 [7]. HPTN 084 and 083 are transitioning to extension studies in which all participants will be offered open-label CAB-LA. Ongoing analysis of samples and data from the 2 trials will provide more information about the correlates of HIV protection with CAB-LA PrEP and the impact of CAB-LA on viral replication, antibody expression, and drug resistance.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the HIV Prevention Trials Network (HPTN) 084 study team and participants; the HPTN 084 site principal investigators; the laboratory staff at the study sites and the HPTN Laboratory Center; laboratory staff at the University of Pittsburgh; Michael Seisa, Yolanda Lie, and laboratory staff at Monogram Biosciences; the members of the HPTN 084 Endpoint Adjudication Committee and HIV Alias Committee (Carole Wallis, Aida Asmelah, Nittaya Phanuphak, Adrian Puren, Francesca Conradie, Halima Dawood, and Michelle Moorhouse); investigators at ViiV Healthcare; Richard Clark and other investigators at Gilead Sciences; Sheryl Zwerski; and Raphael Landovitz.
Author contributions. All authors participated in the study, contributed to manuscript preparation, and reviewed the manuscript. S. H. E. (protocol virologist, HPTN 084) designed the study, analyzed data, and drafted the manuscript. J. M. F. (virologist, HPTN Laboratory Center) analyzed data and drafted the manuscript. E. P. M. (deputy director, HPTN Laboratory Center) coordinated laboratory testing. G. C. (data analyst, HPTN Statistical and Data Management Center) performed data management and analysis. V. C. coordinated and performed virology testing. Y. A., international quality assurance/quality control coordinator for the HPTN Laboratory Center. P. R. and P. S. assisted with sample management and virology testing. C. D. H. coordinated and performed cabotegravir and tenofovir (TFV) testing. L. R. B. coordinated and performed TFV-diphosphate (DP) testing. C. P. provided expertise on human immunodeficiency virus (HIV) drug resistance testing. D .P. was responsible for HIV DNA testing. R. K. provided graphic arts support. C. W. H., protocol pharmacologist for HPTN 084. P. L. A. was responsible for TFV-DP testing. J. F., HPTN 084 study coordinator. J. M. was responsible for single-copy RNA testing. A. A., the HPTN 084 National Institutes of Health medical officer. A.R., M. S. C., S. F., and J. F. R. provided pharmaceutical support. C. A. M., P. H., E. S., J. M., and G. N., respectively, were site investigators for Johannesburg, South Africa; Zimbabwe; KwaZulu-Natal, South Africa; Uganda; and Cape Town, South Africa. M. S. C., principal investigator for the HPTN Leadership and Operations Center. J. P. H., HPTN 084 protocol statistician; M. H., HPTN 084 protocol cochair; B. H., HPTN 084 statistician; and S. D. M., HPTN 084 protocol chair. M. A. M. (lead laboratory pharmacologist) designed the study, analyzed data, and drafted the manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, Office of the Director, National Institutes of Health, the National Institute on Drug Abuse, and the National Institute of Mental Health (grants UM1AI068619, UM1AI068617, and UM1AI068613).
Potential conflicts of interest. C. P. is an employee, officer and shareholder of Laboratory Corporation of America. C. W. H. has received research funding and honoraria and has served on scientific advisory boards for ViiV/GlaxoSmithKline, Gilead, and Merck; he is a founder and fiduciary of Prionde Biopharma and holds US patents related to HIV prevention. P. L. A. has received research funding from Gilead and honoraria from Gilead, Merck, and ViiV. A. R., S. F., and M. S. C. are employees of ViiV Healthcare. J. F. R. is an employee and stockholder of Gilead Sciences. S. D. M. received a donation of Truvada from Gilead Sciences for research studies. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 11th International AIDS Society Conference on HIV Science, 18–21 July 2021.
References
- 1. Riddell J, Amico KR, Mayer KH.. HIV preexposure prophylaxis: a review. JAMA 2018; 319:1261–8. [DOI] [PubMed] [Google Scholar]
- 2. Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gulick RM, Flexner C.. Long-acting HIV drugs for treatment and prevention. Annu Rev Med 2019; 70:137–50. [DOI] [PubMed] [Google Scholar]
- 4. US Food and Drug Administration. FDA approves first extended-release, injectable drug regimen for adults living with HIV. https://www.fda.gov/news-events/press-announcements/fda-approves-first-extended-release-injectable-drug-regimen-adults-living-hiv. Accessed 7 May 2021.
- 5. Landovitz RJ, Donnell D, Clement ME, et al. . Long-acting cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med 2021; 385:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delany-Moretlwe S, Hughes JP, Bock P, et al. ; for the HPTN 084 Study Team. . Long acting injectable cabotegravir is safe and effective in preventing HIV infection in cisgender women: results from HPTN 084. Presented at HIV Research for Prevention (R4P) Virtual; 27 January 2021. Abstract LB1479. https://programme.hivr4p.org/abstract/abstract/1479. [Google Scholar]
- 7. Marzinke MA, Grinsztejn B, Fogel JM, et al. . Characterization of human immunodeficiency virus (HIV) infection in cisgender men and transgender women who have sex with men receiving injectable cabotegravir for HIV prevention: HPTN 083. J Infect Dis 2021; 224:1581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tosiano MA, Jacobs JL, Shutt KA, Cyktor JC, Mellors JW.. A simpler and more sensitive single-copy HIV-1 RNA Assay for quantification of persistent HIV-1 viremia in individuals on suppressive antiretroviral therapy. J Clin Microbiol 2019; 57:e01714–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wensing AM, Calvez V, Ceccherini-Silberstein F, et al. 2019 update of the drug resistance mutations in HIV-1. Top Antivir Med 2019; 27:111–21. [PMC free article] [PubMed] [Google Scholar]
- 10. Andrews CD, Spreen WR, Mohri H, et al. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science 2014; 343:1151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spreen W, Min S, Ford SL, et al. Pharmacokinetics, safety, and monotherapy antiviral activity of GSK1265744, an HIV integrase strand transfer inhibitor. HIV Clin Trials 2013; 14:192–203. [DOI] [PubMed] [Google Scholar]
- 12. Bennett DE, Camacho RJ, Otelea D, et al. . Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One 2009; 4:e4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andrews CD, Yueh YL, Spreen WR, et al. A long-acting integrase inhibitor protects female macaques from repeated high-dose intravaginal SHIV challenge. Sci Transl Med 2015; 7:270ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Landovitz RJ, Li S, EronJJ, Jr., et al. Tail-phase safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected adults: a secondary analysis of the HPTN 077 trial. Lancet HIV 2020; 7:e472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cottrell ML, Yang KH, Prince HM, et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis 2016; 214:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Food and Drug Administration. Prescribing information for TRUVADA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021752s061lbl.pdf. Accessed 30 May 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.