Abstract
Background:
Traditional physical frailty (PF) screening tools are resource intensive and unsuitable for remote assessment. In this study, we used five times sit-to-stand test (5×STS) with wearable sensors to determine PF and three key frailty phenotypes (slowness, weakness, and exhaustion) objectively.
Materials and methods:
Older adults (n = 102, age: 76.54 ± 7.72 y, 72% women) performed 5×STS while wearing sensors attached to the trunk and bilateral thigh and shank. Duration of 5×STS was recorded using a stopwatch. Seventeen sensor-derived variables were analyzed to determine the ability of 5×STS to distinguish PF, slowness, weakness, and exhaustion. Binary logistic regression was used, and its area under curve was calculated.
Results:
A strong correlation was observed between sensor-based and manually-recorded 5xSTS durations (r = 0.93, P < 0.0001). Sensor-derived variables indicators of slowness (5×STS duration, hip angular velocity range, and knee angular velocity range), weakness (hip power range and knee power range), and exhaustion (coefficient of variation (CV) of hip angular velocity range, CV of vertical velocity range, and CV of vertical power range) were different between the robust group and prefrail/frail group (P < 0.05) with medium to large effect sizes (Cohen’s d = 0.50–1.09). The results suggested that sensor-derived variables enable identifying PF, slowness, weakness, and exhaustion with an area under curve of 0.861, 0.865, 0.720, and 0.723, respectively.
Conclusions:
Our study suggests that sensor-based 5×STS can provide digital biomarkers of PF, slowness, weakness, and exhaustion. The simplicity, ease of administration in front of a camera, and safety of 5xSTS may facilitate a remote assessment of PF, slowness, weakness, and exhaustion via telemedicine.
Keywords: Physical frailty, Wearable, Sit-to-stand test, Remote patient monitoring, Digital health, Digital biomarker
Introduction
The concept of physical frailty (PF) has increasingly been recognized in preoperative and postoperative evaluation over the last decade.1,2 PF is a state of increased vulnerability in reserve and function across multiple physiological systems.3 While PF can affect any age group, it is more common in older adults.4 In North America, approximately half of all surgical procedures are performed on patients aged 65 y or older.5 Evidence has also documented that the presence of PF in elderly is associated with increased mortality, postoperative complications, prolonged hospitalization, and poor discharge disposition.1,2 Therefore, PF assessment has received considerable attention to evaluate risk of surgical intervention and track recovery postsurgery in older adults.
Although more than 20 different methods for PF assessment identified in a systematic review,6 two of the most popular methods are frailty phenotype and frailty index. The frailty phenotype, proposed by Fried et al.,7 assesses 5 physical components such as unintentional weight loss, slowness, weakness, exhaustion, and low physical activity. Frailty index, proposed by Rockwood et al.,8 considers cumulative health deficits (symptoms, signs, disabilities, diseases, etc.). The frailty index is represented as a ratio between the number of presented deficits and the number of considered deficits. Based on these two popular approaches, a variety of tools have been proposed to identify and screen PF in research and clinical practice (see9 for review). However, current PF assessment tools are resource intensive (e.g., time, equipment, space, etc.)10 and need to be administered by professionals assessing clinical conditions (e.g., gait speed, grip strength, physical activity, etc.).11 Therefore, they are unsuitable for remote assessment. In addition, evidence has documented the importance of routine PF assessment in elderly surgical patients,1,2,12 and there is a need for adequate simple tools to administer PF assessment in primary health care.9,13
Five times sit-to-stand test (5×STS), which represents the sitting and standing movements for 5 times as quickly as possible while being timed,14 could be a practical method for PF assessment in hospital, clinic, home, or other settings. 5×STS is a widely used, well validated, and reliable measure to assess lower extremity strength, balance, and fall risk,15–17 and it has been used for various populations.18 A noticeable advantage of the 5×STS over other functional measures is simple, fast, inexpensive, and reproducible. Although a study has reported that the 30-s chair stand test is correlated with a PF status (i.e., nonfrail, prefrail, and frail) in older adults,19 no study to date has investigated whether the 5xSTS can objectively determine PF and three key frailty phenotypes (slowness, weakness, and exhaustion) in older adults.
Acknowledging this limitation and recent efforts to shift from face-to-face to remote assessments, we investigated the effectiveness of 5×STS with wearable sensors to objectively determine PF and three key frailty phenotypes (slowness, weakness, and exhaustion) inolder adults.In particular, we(1) quantitatively assessed the correlation and agreement between sensor-based 5×STS duration and manually recorded 5xSTS duration, (2) statistically compared the sensor-derived variables between the robust group and the prefrail/frailty group, and (3) used regression modeling with significant sensor-derived variables to identify PF, slowness, weakness, and exhaustion.
Material and methods
Participants and experimental protocols
One hundred two older adults were included in the final analysis. Inclusion criteria included any ambulatory volunteers aged 65 y or older. Participants were excluded if they (1) were unable to walk a distance of 15 feet with or without assistive devices or unable to stand still without moving their feet (i.e., nonambulatory or severe gait or balance problems); (2) had active foot ulcers or infection, major foot deformity, or major amputation; (3) had any clinically significant medical or psychiatric conditions; or (4) were not willing to participate. The initial inclusion and exclusion criteria led to 126 eligible participants. Then we excluded 24 participants who were unable to complete 5×STS (n = 18) or had no sensor data (n = 6) due to a sensor’s technical issue. These additional exclusion criteria led to 102 eligible participants.
The study protocol was approved by the Institutional Review Board at the local institutional review boards including Michael E. DeBakey Veterans Affairs Medical Center, Baylor College of Medicine, and University of Arizona. All participants read and signed the informed consent form before the study.
Demographics included age, gender, height, weight, and body mass index (BMI). The following clinical information was collected such as frailty phenotypes, fall history in last 12 mo, use of walking aid (e.g., canes and walkers), daily number of prescribed medications, Mini-Mental State Examination (MMSE) score, falls self-efficacy scale-International (FES-I) score, and Center for Epidemiologic Studies-Depression (CESD) score. The Fried frailty criteria, well-validated, and the most frequently used method in research and clinical practice, were used to classify all participants into a robust group (RG) or prefrail/frail group (FG). It assesses the presence or absence of 5 phenotypes, including unintentional weight loss, slowness, weakness, exhaustion, and low physical activity.7 Participants with the absence of the 5 phenotypes were assigned to the RG, while participants with the presence of 1 or more of the 5 phenotypes were assigned to the FG. The MMSE assesses cognitive impairment20; the MMSE score of less than 24 indicates cognitive impairment. The FES-I evaluates self-reported concerns about falling21; the FES-I score of 11 or greater indicates a high concern of falling. The CES-D evaluates self-reported depression symptoms22; the CES-D score of 16 or greater suggests a risk for clinical depression.
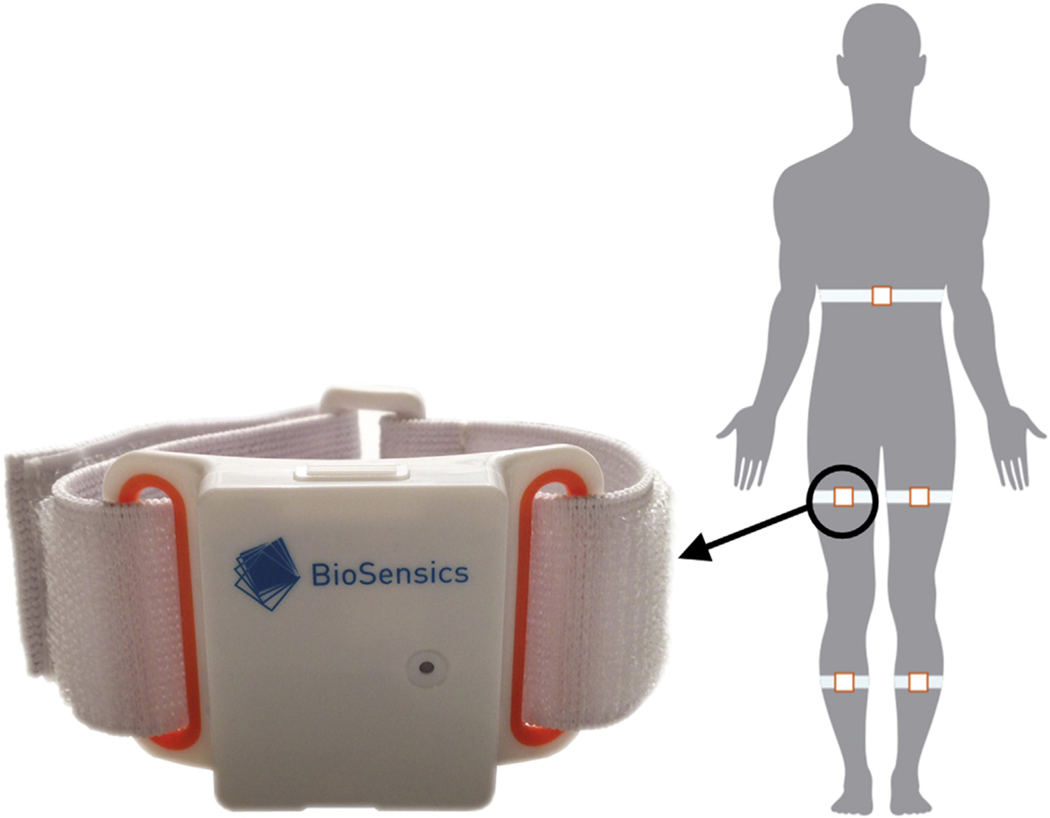
At the beginning of the experimental session, all participants were instrumented with wearable sensors (LegSys+, BioSensics, Watertown, MA, USA).23 The wearable sensor consists of a triaxial accelerometer and gyroscope. Custom software installed on a standard laptop receives the accelerations, angular velocities, and quaternions wirelessly via Bluetooth. A research staff attached 5 wearable sensors with an elastic belt and velcro closure to a participant’s trunk and bilateral thigh and shank, as shown in Figure 1. After instrumentation, all participants were instructed to sit on a chair (no wheel)by resting their back and folding their arms across their chest. All participants were then asked to perform 5×STS as quickly as possible at the count of go and without their back or leg resting on the chair between the interval of repetition. A clinician manually recorded 5×STS duration using a stopwatch. Sensor data (i.e., accelerations, angular velocities, and quaternions) were saved on the laptop at a rate of 100 Hz. All participants completed the 5×STS, and there were no system malfunctions during all experimental trials.
Fig. 1 –
Wearable sensor and sensor attachment.
Sensor data processing and variable extraction
MATLAB (The MathWorks, Natick, MA) was used to process recorded sensor data (accelerations, angular velocities, and quaternions). From sensor data, sensor-based 5×STS duration and 8 primary variables were defined. Eight primary variables include hip angle range, hip angular velocity range, hip power range, knee angle range, knee angular velocity range, knee power range, vertical velocity range, and vertical power range. These 8 primary variables were computed for each STS cycle, and their mean and coefficient of variance (CV: standard deviation divided by the mean) were computed across 5 STS cycles. Thus, 17 sensor variables (i.e., sensor-based 5xSTS duration + 8 primary variables × 2 variable types (mean and CV)) were extracted from sensor data. Slowness was determined by sensor-based 5xSTS duration, mean of hip angular velocity range, mean of knee angular velocity range, and mean of vertical velocity range.24,25 Weakness was determined by mean of hip angle range, mean of hip power range, mean of knee angle range, mean of knee power range, and mean of vertical power range.24,25 Exhaustion was determined by CV of each key feature.25
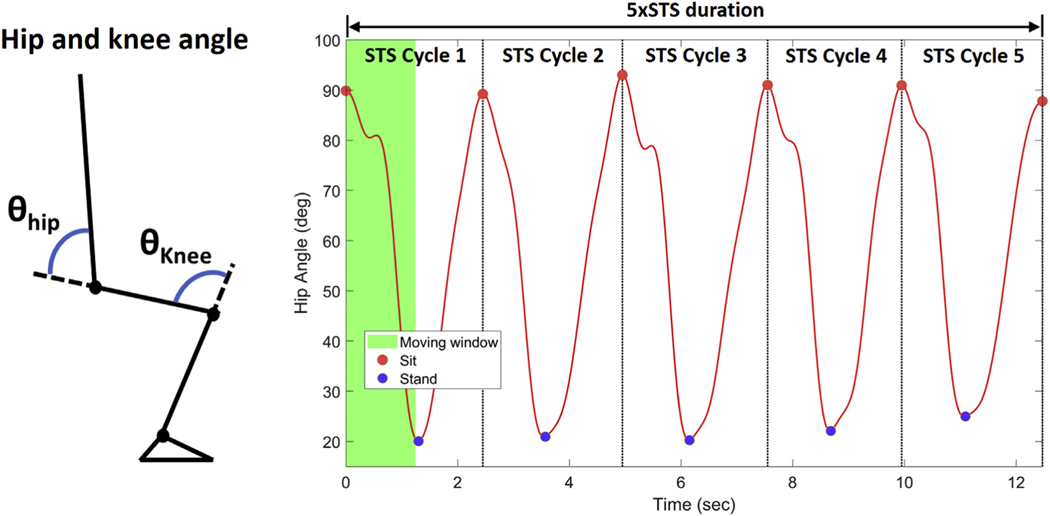
Figure 2A shows a definition of hip and knee angle. Hip angle was computed using quaternions of sensors attached to the trunk and both thighs. Similarly, knee angle was computed using quaternions of sensors attached to both thighs and shanks. Hip and knee angle data were low pass filtered with a fourth order Butterworth filter(cutoff frequency of 2 Hz) to remove high frequency. The cutoff frequency of 2 Hz was determined by the power spectral density analysis of hip and knee angle data and based on the results of the previous study.26 Hip and knee angular velocity was computed as the time derivative of hip and knee angle, respectively. Power was estimated as a product of angular velocity and angular acceleration,24,25 as shown in Equation 1:
| (1) |
Fig. 2 –
Hip and knee angle definition and a representative hip angle with the definition of each STS cycle.
Hip angular acceleration (αhip) was computed as the time derivative of hip angular velocity (ωhip), and knee angular acceleration (αknee) was computed as the time derivative of hip angular velocity (ωknee). Vertical velocity was computed by the integration of measured vertical acceleration from a sensor attached to the trunk. Vertical power was computed as a product of vertical velocity and vertical acceleration.
Each STS cycle was determined by the following steps: (1) find a minimum and maximum hip angle within a moving window (bin width and step = 5xSTS duration/(5×2); estimated sit-to-stand time), (2) check whether the minimum and maximum hip angle is a zero-crossing point of hip angular velocity, (3) select a maximum hip angle if multiple maximum hip angles (due to oscillatory motion while sitting) are detected within a moving window (bin width = 5xSTS duration/(5×2), step = 100 ms (sampling period)), (4) select a minimum hip angle if multiple minimum hip angles (due to oscillatory motion while standing) are detected within a moving window (bin width = 5×STS duration/(5×2), step = 100 ms [sampling period]), (5) determine 6 maximum hip angles corresponding to sitting and 5 minimum hip angles corresponding to standing, and (6) determine each STS cycle between two consecutive maximum hip angles, as shown in Figure 2B. A sensor-based 5xSTS duration shown in Figure 2B indicates the time between the first and last maximum hip angle.
Correlation and agreement analysis
Correlation and agreement between sensor-based and manually recorded 5×STS durations were analyzed quantitatively. Pearson’s correlation analysis was used to determine linear relationship between two measurements and measure the strength and direction of linear relationship. Pearson’s correlation coefficient (r) ranging from −1 to 1 was computed to measure the strength of linear relationship; the closer the coefficients are to +1.0 or −1.0, the greater the strength of linear relationship is. Linear regression was performed to assess the direction of linear relationship. While correlation analysis describes linear relationship between two sets of data, it does not describe an agreement between two sets of data.27 Therefore, the Bland-Altman analysis was used to assess the agreement between sensor-based and manually recorded 5×STS durations.
The Bland-Altman analysis is a widely used technique in clinical and medical research to investigate the agreement between two methods of the same measurement by constructing limits of agreement.27 The Bland-Altman analysis determines the bias (mean difference ), as a measure of accuracy, and the limits of agreement, as a measure of precision, between two methods of the same measurement. The bias allows the identification of any systematic difference between two methods of the same measurement, and the limits of agreement estimate interval within which a proportion of the differences between two methods of the same measurement lie. The bias (mean difference ) was computed between sensor-based and manually recorded 5×STS durations. The limits of agreement were computed by using the mean difference and the standard deviation (s) of the differences between sensor-based and manually recorded 5×STS durations,27 as shown in Equation 2:
| (2) |
In addition, accuracy (standard deviation of differences between sensor-based and manually recorded 5×STS durations) and dispersion (range of limits of agreement) was computed.
Statistical analysis
All statistical analysis was performed by SPSS (IBM Corp, Armonk, NY). Outcome measures are participants’ demographics, clinical information, and 17 sensor-derived variables.
The Shapiro-Wilk test for normality was performed for all outcome measures. To assess differences of continuous demographics and clinical information between the RG and FG, a one-way analysis of variance was conducted for normally distributed variables, and a Mann-Whitney U test was conducted for non-normally distributed variables. For categorical variables, a chi-square test was conducted. Linear regression analysis was conducted for sensor-based and manually recorded 5×STS durations measured from all participants. To assess systematic bias between sensor-based and manually recorded 5×STS durations, a one-sample t-test (a paired t-test of 5×STS durations from each measurement method) was28 conducted, whereby the hypothesis that the true mean of the differences is zero, corresponding to no bias between the methods, was tested. For 17 sensor-derived variables, one-way analysis of covariance (ANCOVA) for normally distributed variables and Quade’s ANCOVA for non-normally distributed variables were conducted to assess the main effect of the group, considering BMI as adjusting variables because BMI was statistically different between the RG and FG. Using Cohen’s d, an effect size was calculated for 18 variables. Values were defined as small (0.20–0.49), medium (0.50–0.79), large (0.80–1.29), and very large (above 1.30).29 Values of less than 0.20 were classified as having no noticeable effect.29 For categorical variables, an effect size was calculated using an odds ratio with 95% confidence interval. Binary logistic regression was used, and its area under curve (AUC) was calculated to determine the ability of 17 sensor-derived variables to identify PF, slowness, weakness, and exhaustion. Four models were built, such as a PF, slowness, weakness, and exhaustion model. The presence and absence of PF, slowness, weakness, and exhaustion were used as a dependent variable for the PF, slowness, weakness, and exhaustion model. For each model, significant sensor-derived variables determined by ANCOVA were used as independent variables. An AUC of 0.8 to 0.9 is considered excellent, an AUC of 0.7 to 0.8 is considered acceptable, and an AUC below 0.7 is considered unacceptable.30 BMI was used as adjusting variables for all models. Significance was defined at the 2-sided P < 0.05 level.
Results
Table 1 summarizes the participants’ demographics and clinical information. All continuous data were presented as mean ± standard deviation. Categorical data were expressed as count (percentage). Among 5 demographics reported in Table 1, weight and BMI were significantly different between the RG and FG (P = 0.001 and P < 0.0001, respectively). Fifty two percentages, 33%, 38%, 13%, and 32% of participants in the FG presented slowness, weakness, exhaustion, weight loss, and low physical activity, respectively. In addition, the use of walking aid (P = 0.001), daily prescribed medications (P = 0.019), overall FES-I scores (P < 0.0001), and FES-I scores of participants who has a high concern of falling (P < 0.0001) were significantly different between the RG and FG.
Table 1 –
Demographics and clinical information for the robust group (RG) and prefrail/frail group (FG).
| No./total no. (%), by group |
P-value | OR (95% CI) | ||
|---|---|---|---|---|
| RG (n = 42) | FG (n = 60) | |||
| Demographics | ||||
| Age, y | 74.79 ± 6.64 | 76.57 ± 8.00 | 0.085 | - |
| Female, n (%) | 34/42 (81.0) | 39/60 (65.0) | 0.079 | 2.288 (0.898–5.830) |
| Height, m | 162.09 ± 7.34 | 164.90 ± 10.77 | 0.230 | - |
| Weight, kg | 66.77 ± 12.21 | 78.61 ± 19.95 | 0.001* | - |
| BMI, kg/m2 | 25.40 ± 4.23 | 28.70 ± 5.79 | <0.0001* | - |
| Clinical information | ||||
| Frailty phenotype, units on a scale | 0 | 1.68 ± 0.79 | - | - |
| Presence of slowness, n (%) | 0(0) | 31/60 (51.67) | - | - |
| Presence of weakness, n (%) | 0(0) | 20/60 (33.33) | - | - |
| Presence of exhaustion, n (%) | 0(0) | 23/60 (38.33) | - | - |
| Presence of weight loss, n (%) | 0(0) | 8/60 (13.33) | - | - |
| Presence of low physical activity, n (%) | 0(0) | 19/60 (31.67) | - | - |
| Fall incidence in last 12 mo, % | 12/34 (35.3) | 22/43 (51.2) | 0.164 | 0.521 (0.207–1.311) |
| Use of walking aida | 3/35 (8.6) | 18/43 (41.9) | 0.001* | 0.130 (0.034–0.492) |
| Daily prescribed medications, n | 2.63 ± 1.72 | 4.81 ± 4.10 | 0.019* | - |
| Cognitive function (MMSE), score | 29.15 ± 1.18 | 28.81 ± 1.36 | 0.144 | - |
| Concern for fall (FES-I), score | 20.54 ± 3.77 | 29.45 ± 9.64 | <0.0001* | - |
| High concern (≥28) | 2/41 (4.9) | 30/56 (53.6) | <0.0001* | 0.044 (0.010–0.202) |
| Depression (CES-D), score | 6.45 ± 5.82 | 8.91 ± 7.18 | 0.098 | - |
| Depressed (≥16) | 4/42 (9.5) | 12/56 (21.4) | 0.115 | 0.386 (0.115–1.297) |
Values are presented as mean ± standard deviation (SD) or n (%).
The asterisk symbol represents a significant difference between the groups.
OR = odds ratio; CI = confidence interval; MMSE = Mini-Mental State Examination; FES-I = Fall Efficacy Scale-International; CES-D = Center for Epidemiological Studies Depression.
Canes and walkers.
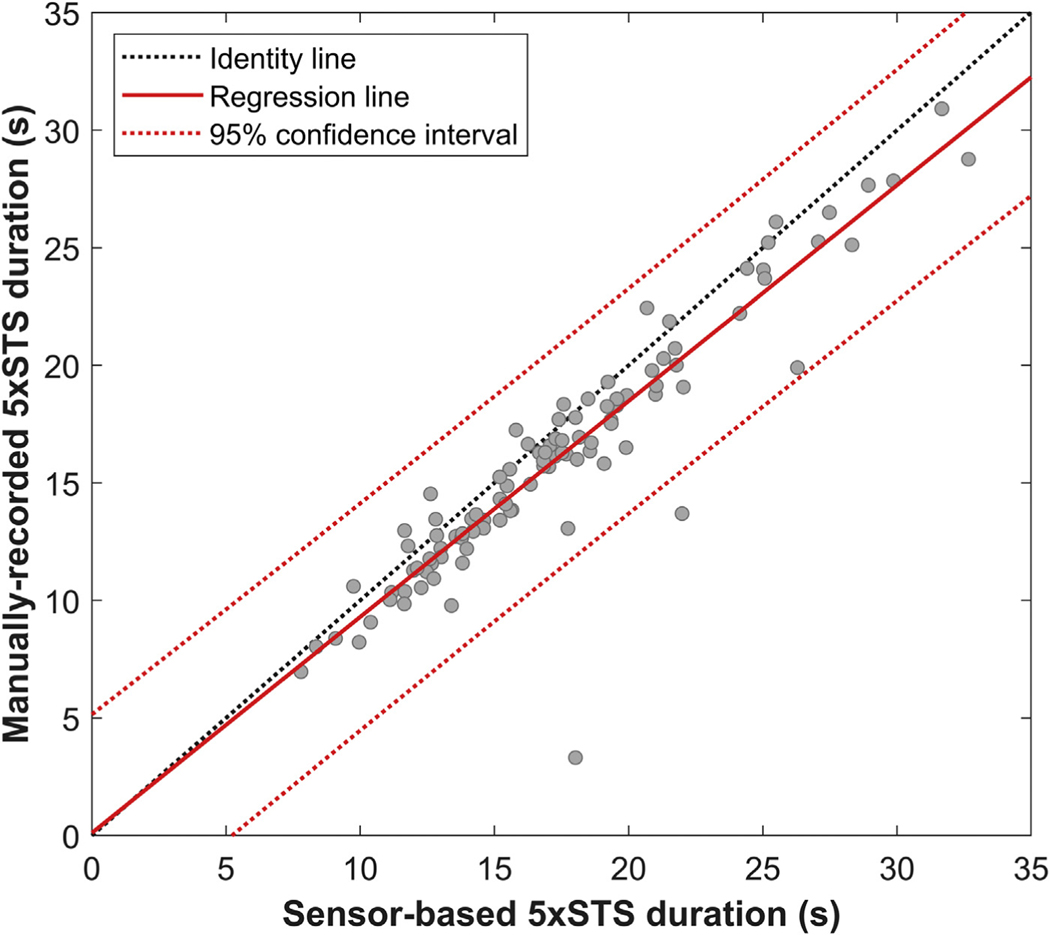
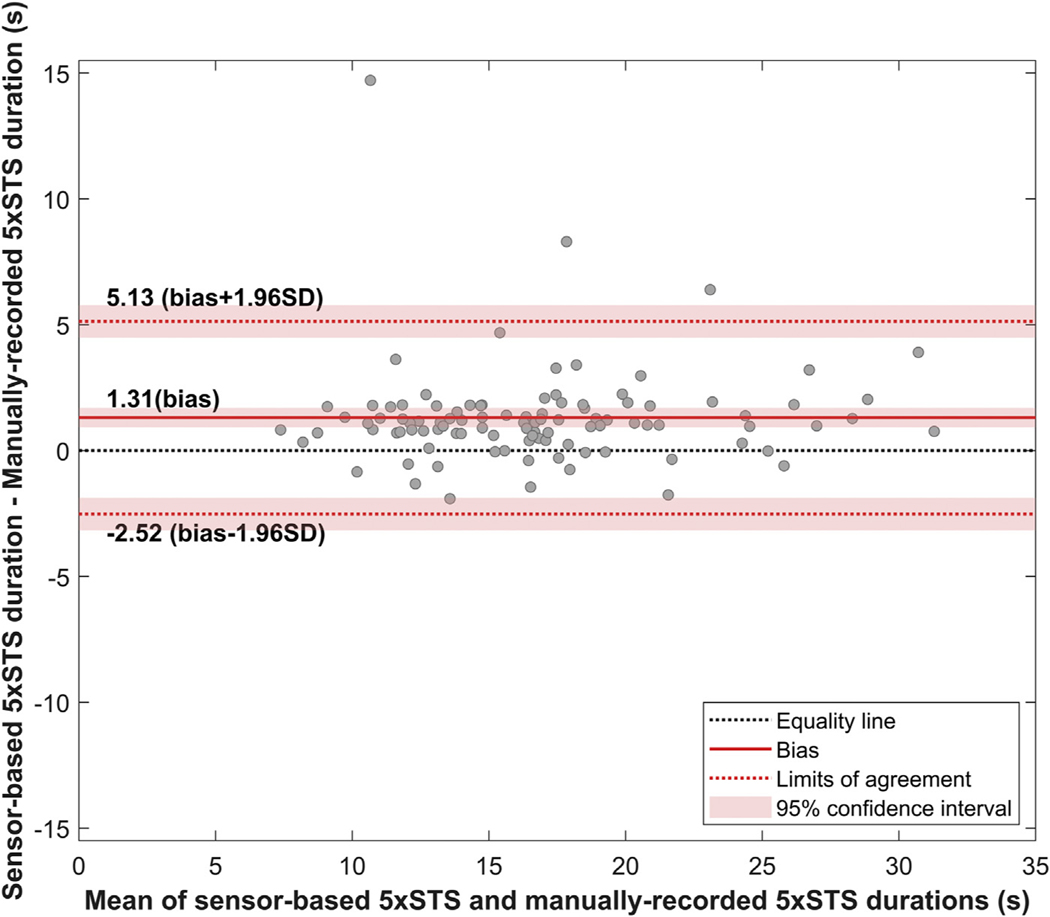
Figure 3 shows the results of linear regression, which indicate a significant correlation between sensor-based and manually recorded 5xSTS durations (Pearson’s correlation coefficient r = 0.93, P < 0.0001). Figure 4 shows the Bland-Altman plot, which visualizes the difference in two continuous measurements (i.e., sensor-based and manually recorded 5×STS durations). The Bland-Altman analysis showed a bias of 1.31 s, with limits of agreement between −2.52 s and 5.13. The accuracy and dispersion were 1.95 s and 7.65 s. The paired t-test indicated that the bias is significantly higher than the equality (P < 0.0001). This result was also confirmed by that the line of equality is not in the 95% confidence interval of the bias, as shown in Figure 4.
Fig. 3 –
The regression line between sensor-based and manually recorded 5×STS.
Fig. 4 –
Bland-Altman plot for sensor-based and manually recorded 5×STS.
The statistical analyses of the ability of 17 variables to distinguish PF are reported in Table 2. One-way ANCOVA showed the significant main effect of the group for 8 variables. Compared with the RG, sensor-based 5xSTS duration, mean of hip angular velocity range, and mean of knee angular velocity range were significantly slower for the FG (P < 0.0001), which are indicators of slowness. Mean of hip power range and mean of knee power range were significantly lower for the FG than for the RG (P < 0.001), which are indicators of weakness. Compared with the RG, CV of hip angular velocity range, CV of vertical velocity range, and CV of vertical power range were significantly higher for the FG (P < 0.05), which are indicators of exhaustion. The Cohen’s d effect size was observed as large (0.80–1.29) for the 5 variables (sensor-based 5×STS duration, mean of hip angular velocity range, mean of knee angular velocity range, mean of hip power range, and mean of knee power range) and medium (0.50–0.79) for the 3 variables (CV of hip angular velocity range, CV of vertical velocity range, and CV of vertical power range).
Table 2 –
Comparison of sensor-driven variables between the robust group (RG) and prefrail/frail group (FG).
| Phynotype | Sensor-driven variables | Unit | RG (n = 42) | FG (n = 60) | P-value | Effect size |
|---|---|---|---|---|---|---|
| Slowness | Sensor-based 5xSTS duration | s | 14.44 ± 2.99 | 19.44 ± 5.44 | <0.0001* | 1.09 |
| Mean of hip angular velocity range | deg/s | 307.40 ± 67.80 | 244.04 ± 59.40 | <0.0001* | 1.01 | |
| Mean of knee angular velocity range | deg/s | 203.27 ± 42.54 | 166.73 ± 34.17 | <0.0001* | 0.97 | |
| Mean of vertical velocity range | m/s | 0.15 ± 0.04 | 0.18 ± 0.07 | 0.292 | 0.44 | |
| Weakness | Mean of hip angle range | deg | 77.30 ± 12.60 | 77.36 ± 13.15 | 0.668 | 0.01 |
| Mean of hip power range | deg2/s3 × 103 | 147.22 ± 77.54 | 86.72 ± 46.42 | <0.0001* | 0.99 | |
| Mean of knee angle range | deg | 61.36 ± 10.36 | 61.66 ± 11.04 | 0.491 | 0.03 | |
| Mean of knee power range | deg2/s3 × 103 | 60.51 ± 31.61 | 39.35 ± 18.78 | <0.0001* | 0.85 | |
| Mean of vertical power range | m2/s3 | 0.15 ± 0.04 | 0.18 ± 0.07 | 0.261 | 0.44 | |
| Exhaustion | CV of hip angle range | % | 5.39 ± 5.05 | 5.55 ± 3.69 | 0.228 | 0.04 |
| CV of hip angular velocity range | % | 7.22 ± 3.67 | 8.97 ± 3.29 | 0.032* | 0.50 | |
| CV of hip power range | % | 19.24 ± 8.17 | 23.19 ± 12.14 | 0.056 | 0.37 | |
| CV of knee angle range | % | 5.00 ± 3.76 | 5.90 ± 4.62 | 0.143 | 0.21 | |
| CV of knee angular velocity range | % | 7.28 ± 3.74 | 8.44 ± 4.30 | 0.178 | 0.28 | |
| CV of knee power range | % | 18.87 ± 7.86 | 21.59 ± 12.38 | 0.557 | 0.25 | |
| CV of vertical velocity range | % | 14.89 ± 8.03 | 22.42 ± 13.70 | 0.002* | 0.64 | |
| CV of vertical power range | % | 15.39 ± 7.95 | 22.69 ± 12.61 | 0.002* | 0.67 |
All analyses were adjusted with body mass index.
The asterisk symbol represents a significant difference between the groups.
CV = coefficient of variation
Table 3 reports the results of binary logistic regression and its AUC for PF, slowness, weakness, and exhaustion. An AUC of the PF, slowness, weakness, and exhaustion model was 0.86, 0.88, 0.72, and 0.72, respectively. The AUC of the PF and slowness model was within an excellent range (0.8 to 0.9), and the AUC of theweakness and exhaustion model was within an acceptable range (0.7 to 0.8).
Table 3 –
Results of binary logistic regression and its area under curve (AUC) for physical frailty, slowness, weakness, and exhaustion.
| Model (dependent variable) | Independent variables | AUC |
|---|---|---|
| Physical frailty | Sensor-based 5xSTS duration | 0.861 |
| Mean of hip angular velocity range | ||
| Mean of knee angular velocity range | ||
| Mean of hip power range | ||
| Mean of knee power range | ||
| CV of hip angular velocity range | ||
| CV of vertical velocity range | ||
| CV of vertical power range | ||
| Slowness | Sensor-based 5xSTS duration | 0.865 |
| Mean of hip angular velocity range | ||
| Mean of knee angular velocity range | ||
| Weakness | Mean of hip power range | 0.720 |
| Mean of knee power range | ||
| Exhaustion | CV of hip angular velocity range | 0.723 |
| CV of vertical velocity range | ||
| CV of vertical power range |
All analyses were adjusted with body mass index.
CV = coefficient of variation.
Discussion
The primary findings are that (1) a strong correlation was observed between sensor-based and manually recorded 5×STS durations, (2) 8 sensor-derived variables indicating slowness, weakness, and exhaustion were significantly different between the RG and FG, and (3) sensor-derived variables were able to identify PF, slowness, weakness, and exhaustion objectively.
Consistent with the previous study,31 the result of Pearson’s correlation coefficient and linear regression showed that the sensor-based 5×STS duration strongly correlates with the and manually recorded 5×STS duration. However, the result of the Bland-Altman analysis showed a significant systematic difference between sensor-based and manually-recorded 5×STS durations, which indicates the lack of agreement. We attribute this result to a difference between the start signal given by a clinician and the movement’s start. We also infer that the observed systematic difference can be related to the accuracy of a clinician, who marked the start and stop of 5×STS by simultaneously supervising the participants. Our inference is supported by previous findings that sensor-based sit-to-stand measures showed consistent test-retest reliability in older adults.32,33
The results of ANCOVA indicated 8 significant sensor-derived variables between the RG and FG, with an effect size of a medium to large (0.50–1.09). Consistent with the previous study,19 our results showed that the sensor-based 5xSTS duration distinguishes PF. Each significant sensor-derived variable was associated with weakness, slowness, or exhaustion. Compared with the RG, the FG had (1) a slow sensor-based 5×STS duration, mean of hip angular velocity range, and mean of knee angular velocity range, which are indicators of slowness, (2) a lower mean of hip power range and mean of knee power range, which are indicators of weakness, and (3) a higher CV of hip angular velocity range, CV of vertical velocity range, and CV of vertical power range, which are indicators of exhaustion. Previous studies have demonstrated that frailty is associated with kinematics (e.g., velocity and acceleration) and kinetics (e.g., power and moment) of hip and ankle joints while performing sit-to-stand tasks.19,34,35 Frail older adults show slower sit-to-stand motion than robust older adults, which is linked to the cautious motion described by slower hip and knee joint movements.34 Frail older adults also show reduced power in hip and knee joints compared with robust older adults,19 which is related to muscle strength.35 Other previous studies have demonstrated that frail older adults show a larger CV of joint movements than robust older adults.24,25,36 Therefore, our results confirm and extend previous studies that the 8 significant sensor-derived variables can be indicators of slowness, weakness, and exhaustion. Our results of binary logistic regression and its AUC indicated that the four models with significant sensor-derived variables have an ability to identify PF, slowness, weakness, and exhaustion, with an acceptable to excellent AUC (0.72–0.86).30 Therefore, we suggest that sensor-based 5xSTS could provide digital biomarkers of PF, slowness, weakness, and exhaustion in older adults.
Evidence has documented the importance of routine PF assessments for predicting the risk of adverse health outcomes in older adults during and after surgical intervention.1,2,12 Health professionals must be trained to administer them and interpret the results. The most common assessments, the frailty phenotype method, and the frailty index method, however, are proving inadequate for remote assessments via telemedicine.
The frailty phenotype method, which uses a phenotypic or biologic-driven frailty model, assesses 5 components (unintentional weight loss, slowness, weakness, exhaustion, and low physical activity) in the physical domain, and requires approximately 15 to 20 min to administer.7 The method, which uses equipment for weakness assessments of handgrip strength and enough physical space (4.57 m) for slowness assessments of walking, also requires patients to fill out questionnaires about weight loss, exhaustion, and low physical activity. The 4.57 m walking test performed in front of a telemedicine camera may be unsafe because many older adults have gait and balance instability.37
The frailty index method, which uses a deficit-driven frailty model, assesses more than 70 deficits considering health deficits (e.g., symptoms, signs, disabilities, diseases, etc.) based on patient-reported outcomes using questionnaires. A study has demonstrated that patients prefer performing a functional test over patient-reported outcomes as a functional test provides objective results.38 Although several modified frailty index methods including lesser deficits are available (e.g.,39,40), the results of the frailty index method mainly serve as a red flag for potential problems because the method consists of a high number of general signs or symptoms. Moreover, the results cannot be used to determine an immediate effect of preventive or therapeutic interventions because they are insensitive to change over short time intervals.
Considering these factors and our findings, we assert that sensor-based 5xSTS has advantages over the two common PF assessment methods for telemedicine application in older adults because: (1) it is a simple, fast, inexpensive, and objective PF assessment protocol; (2) it can be used for routine PF assessments; (3) it can provide insights into three key frailty phenotypes (slowness, weakness, and exhaustion); and (4) it can be used in hospital, clinic, home, or other settings. It can remotely monitor changes in physical fitness in response to an intervention or preventive care as well as tracking health deterioration over time. Our sensor-based 5xSTS should complement the use of two common PF assessment methods which convey other types of information.
Of note, the main purpose of the present study was to examine the feasibility of sensor-based 5×STS to identify PF, slowness, weakness, and exhaustion in older adults. However, the limitations of this study are its small sample size and imbalanced gender within and between the groups. In our future studies in larger samples with balanced gender, the relationship between preoperative PF identified sensor-based 5×STS and postoperative adverse outcomes will be addressed. Within future studies, we will explore the validity of sensor-based 5×STS to identify PF and risk of adverse outcomes postsurgery remotely. In addition, we will use the machine learning technique to determine the least number of sensors and variables for identifying PF, slowness, weakness, and exhaustion, which will potentially reduce system cost and complexity.
Conclusion
To our knowledge, this is the first study to demonstrate the ability of sensor-based 5×STS performed by older adults to identify PF and three key frailty phenotypes (slowness, weakness, and exhaustion). Recall that PF is associated with adverse health outcomes postsurgery in older adults, prompt and accurate identification of PF could help clinicians plan tailored treatments and predict the risk of adverse outcomes during preoperative and postoperative care. Objectively identifying three key frailty phenotypes by sensor-based 5×STS may be used to optimize operation planning and risk reduction protocols. We believe that our findings can inform the future design of technologies aiming at assessing PF by performing a simple, safe, fast, inexpensive, and reproducible 5×STS.
Telemedicine is expected to increase in the post-coronavirus disease era. Already, health professionals are using wearable sensors, mobile technology, the Internet of things, artificial intelligence, and cloud computing in telemedicine applications.41–43 Our sensor-based 5×STS will make it easier than ever for the medical community to assess and monitor patients remotely as they perform 5×STS at home in front of a standard telemedicine camera. The addition of telemedicine could address the limitation of attachment of wearable sensors and their management as some wearables have already been deployed in telemedicine applications for remote monitoring of blood pressure, heart rate/electrocardiogram, blood oxygen level, etc. Our camera-based 5xSTS, which we are currently developing, is an alternative when patients have limited (or no) access to sensors. In the very near future, wearable sensor-based or camera-based 5×STS could facilitate remote assessments of PF, slowness, weakness, and exhaustion via telemedicine.
Acknowledgment
The authors thank Ms. Ana Enriquez, Mr. Manuel Gardea, Mr. Ivan Maria, Ms. Luciana Narvaez, and Ms. Maria Noun for their help with data collection, IRB, and analysis. In addition, the authors thank all participants as well as other research coordinators, student helpers, and research interns who have contributed in participants’ recruitment.
Funding: This work was supported in part by the Department of Veterans Affairs, Veterans Health Administration: VA ACCESS Program (Sharafkhaneh, A.), and grants from the National Institute of Health/National Institute on Aging [grant numbers 1R42AG060853-01, R42AG032748, and 3SB1AG032748-06S1, Najafi, B.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors.
Footnotes
Disclosure
Because this paper is from Baylor College of Medicine, Dr. LeMaire recused himself from the entire editorial decisionmaking process.
REFERENCES
- 1.Buigues C, Juarros-Folgado P, Fernández-Garrido J, NavarroMartínez R, Cauli O. Frailty syndrome and pre-operative risk evaluation: a systematic review. Arch Gerontol Geriatr. 2015;61:309–321. [DOI] [PubMed] [Google Scholar]
- 2.Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and postoperative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xue Q-L. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surgeons. 2010;210:901–908. [DOI] [PubMed] [Google Scholar]
- 6.de Vries NM, Staal JB, van Ravensberg CD, et al. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10:104–114. [DOI] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Ser A Biol Sci Med Sci. 2001;56:M146–156. [DOI] [PubMed] [Google Scholar]
- 8.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faller JW, Pereira DDN, de Souza S, et al. Instruments for the detection of frailty syndrome in older adults: a systematic review. PLoS One. 2019;14:e0216166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3–10. [DOI] [PubMed] [Google Scholar]
- 11.Bruyère O, Buckinx F, Beaudart C, et al. How clinical practitioners assess frailty in their daily practice: an international survey. Aging Clin Exp Res. 2017;29:905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birkelbach O, Mörgeli R, Spies C, et al. Routine frailty assessment predicts postoperative complications in elderly patients across surgical disciplines - a retrospective observational study. BMC Anesthesiol. 2019;19:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Lepeleire J, Degryse J, Illiffe S, Mann E, Buntinx F. Family physicians need easy instruments for frailty. Age Ageing. 2008;37:484. author reply 484–485. [DOI] [PubMed] [Google Scholar]
- 14.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. [DOI] [PubMed] [Google Scholar]
- 15.Bohannon RW. Test-retest reliability of the five-repetition sitto-stand test: a systematic review of the literature involving adults. J Strength Cond Res. 2011;25:3205–3207. [DOI] [PubMed] [Google Scholar]
- 16.Teo TWL, Mong Y. Ng SSM the repetitive five-times-sit-tostand test: its reliability in older adults. Int J Ther Rehabil. 2014;20:122–130. [Google Scholar]
- 17.Wallmann HW, Evans NS, Day C, Neellym KR. Interrater reliability of the five-times-sit-to-stand test. Home Health Care Mgt Pract. 2013;25:13–17. [Google Scholar]
- 18.Whitney SL, Wrisley DM, Marchetti GF, et al. Clinical measurement of sit-to-stand performance in people with balance disorders: validity of data for the five-times-sit-tostand test. Phys Ther. 2005;85:1034–1045. [PubMed] [Google Scholar]
- 19.Millor N, Lecumberri P, Gomez M, Martinez-Ramirez A, Izquierdo M. An evaluation of the 30-s chair stand test in older adults: frailty detection based on kinematic parameters from a single inertial unit. J Neuroeng Rehabil. 2013;10:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurlowicz L, Wallace M. The mini-mental state examination (MMSE). J Gerontol Nurs. 1999;25:8–9. [DOI] [PubMed] [Google Scholar]
- 21.Yardley L, Beyer N, Hauer K, et al. Development and initial validation of the falls efficacy scale-international (FES-I). Age Ageing. 2005;34:614–619. [DOI] [PubMed] [Google Scholar]
- 22.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 23.Thiede R, Toosizadeh N, Mills JL, et al. Gait and balance assessments as early indicators of frailty in patients with known peripheral artery disease. Clin Biomech (Bristol Avon). 2016;32:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H, Joseph B, Enriquez A, Najafi B. Toward using a smartwatch to monitor frailty in a hospital setting: using a single wrist-wearable sensor to assess frailty in bedbound inpatients. Gerontology. 2018;64:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toosizadeh N, Mohler J, Najafi B. Assessing upper extremity motion: an innovative method to identify frailty. J Am Geriatr Soc. 2015;63:1181–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bohannon RW, Bubela DJ, Magasi SR, Wang Y-C, Gershon RC. Sit-to-stand test: performance and determinants across the age-span. Isokinet Exerc Sci. 2010;18:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giavarina D. Understanding bland altman analysis. Biochem Med (Zagreb). 2015;25:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bunting KV, Steeds RP, Slater LT, et al. A practical guide to assess the reproducibility of echocardiographic measurements. J Am Soc Echocardiogr. 2019;32:1505–1515. [DOI] [PubMed] [Google Scholar]
- 29.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Burlington: Taylor & Francis; Elsevier Science; 2013. [Google Scholar]
- 30.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5:1315–1316. [DOI] [PubMed] [Google Scholar]
- 31.van Lummel RC, Walgaard S, Maier AB, et al. The instrumented sit-to-stand test (iSTS) has greater clinical relevance than the manually recorded sit-to-stand test in older adults. PLoS One. 2016;11:e0157968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Regterschot GR, Zhang W, Baldus H, Stevens M, Zijlstra W. Test-retest reliability of sensor-based sit-to-stand measures in young and older adults. Gait Posture. 2014;40:220–224. [DOI] [PubMed] [Google Scholar]
- 33.Regterschot GRH, Folkersma M, Zhang W, et al. Sensitivity of sensor-based sit-to-stand peak power to the effects of training leg strength, leg power and balance in older adults. Gait Posture. 2014;39:303–307. [DOI] [PubMed] [Google Scholar]
- 34.Hassani A, Kubicki A, Brost V, Mourey F, Yang F. Kinematic analysis of motor strategies in frail aged adults during the timed up and go: how to spot the motor frailty? Clin Interv Aging. 2015;10:505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gross MM, Stevenson PJ, Charette SL, Pyka G, Marcus R. Effect of muscle strength and movement speed on the biomechanics of rising from a chair in healthy elderly and young women. Gait Posture. 1998;8:175–185. [DOI] [PubMed] [Google Scholar]
- 36.Yanquez FJ, Peterson A, Weinkauf C, et al. Sensor-based upper-extremity frailty assessment for the vascular surgery risk stratification. J Surg Res. 2020;246:403–410. [DOI] [PubMed] [Google Scholar]
- 37.Salzman B. Gait and balance disorders in older adults. Am Fam Phys. 2010;82:61–68. [PubMed] [Google Scholar]
- 38.Joswig H, Stienen MN, Smoll NR, et al. Patients’ preference of the timed up and go test or patient-reported outcome measures before and after surgery for lumbar degenerative disk disease. World Neurosurg. 2017;99:26–30. [DOI] [PubMed] [Google Scholar]
- 39.Lansbury LN, Roberts HC, Clift E, et al. Use of the electronic Frailty Index to identify vulnerable patients: a pilot study in primary care. Br J Gen Pract. 2017;67:e751–e756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khalafallah AM, Huq S, Jimenez AE, Brem H, Mukherjee D. The 5-factor modified frailty index: an effective predictor of mortality in brain tumor patients. J Neurosurg. 2020:1–9. [DOI] [PubMed]
- 41.Wu M, Luo J. Wearable technology applications in healthcare: a literature review. Online J Nurs Inform. 2019;23. [Google Scholar]
- 42.Haghi M, Thurow K, Stoll R. Wearable devices in medical internet of things: scientific research and commercially available devices. Healthc Inform Res. 2017;23:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shoultz TH, Evans HL. Telemedicine and mobile technology. In: Itani KMF, Reda DJ, eds. Clinical Trials Design in Operative and Non Operative Invasive Procedures. Cham: Springer International Publishing; 2017:427–431. [Google Scholar]