Abstract
Introduction
The increase in drugs available for severe uncontrolled asthma and the lifestyle of these patients make it necessary to implement self-administration programs of these therapies at home. Benralizumab, a monoclonal antibody targeting IL5R, was authorized in Spain for poorly controlled severe eosinophilic asthma. The possibility of administration at home was approved in March 2020 in Spain. The aim of the Auto-Benra study was to evaluate the usability and satisfaction of the benralizumab prefilled syringe and autoinjector and assessing the effectivity of these devices in uncontrolled severe eosinophilic asthma (SEA) in home-self administration.
Methods
This is a retrospective, observational multicenter study uncontrolled SEA patients treated with benralizumab at least with 3 doses self-administered at home before April 30, 2021. Reliability and satisfaction with benralizumab at home were evaluated with subcutaneous administration assessment questionnaire (SQAAQ) and visual analogic scales (VAS). Effectiveness was evaluated in all patients with asthma control test (ACT), Mini Asthma Quality of Life Questionnaire (MiniAQLQ), annual exacerbation rate, oral corticosteroid treatment (OCS) and asthma-related hospitalizations and emergency visits.
Results
Fifty-four patients across 9 hospitals in Spain were included. The mean SQAAQ score was 6.89 (±0.16) points. Patients and their caregivers and doctors report excellent satisfaction by VAS, with no differences between benralizumab devices used (prefilled syringe and autoinjector). Severe exacerbation rate decreased by 65% (p = 0.0007) after benralizumab treatment. ACT score improved on average 6.27 ± 5.35 points (p < 0.0001) and the mean MiniAQLQ increased up to 1.58 ± 1.47 points (p < 0.0001). Twenty-four patients were OCS-dependent and at the end of study 14 patients get complete OCS withdrawal.
Conclusion
AUTO-BENRA study supports the use of benralizumab at home given the excellent results of satisfaction and usability by patients and their caregivers.
Keywords: benralizumab, severe eosinophilic asthma, self-administration, severe asthma, eosinophilic asthma
Introduction
Advances in the knowledge of the physiopathological mechanisms of severe asthma has allowed the development of specific biological therapies for patients with poor control despite optimized maintenance treatment. Benralizumab was recent marketing approval in Spain in January 2019 for uncontrolled severe eosinophilic asthma (SEA). It is a humanized monoclonal antibody that specifically binds to the alpha subunit of the interleukin (IL)-5 receptor, present in the surface of eosinophils and basophils, inducing an antibody-dependent cell-mediated apoptosis by natural-killer (NK) and other cells.1 Its efficacy and safety have been proved in some clinical trials.2–6
Home self-administration of biological therapies has shown to increase health-related quality of life (HRQoL), decreasing costs through a substantial reduction of visits to specialized clinics, and avoiding travelling long distances to the clinic.7–10 Furthermore, the use of autoinjectors positively correlates with medication adherence, which is known to be lower in patients with chronic diseases.11 There are two main types of devices for self-administration therapy: the pre-filled syringe and the autoinjector. In this sense, benralizumab has been evaluated for home self-administration in three studies; GREGALE, to confirm the functionality and correct performance of the pre-filled syringe when used at home in patients with SEA;12 AMES, to check pharmacokinetic parameters between the benralizumab pre-filled syringe and the auto-injector system.13 Finally, the GRECO study to verify that the auto-injector was completely reliable and easy to use self-administered.14 According to these data, Spanish regulatory entities allowed the home-self-use with a pre-filled syringe or single-use autoinjector of benralizumab in March 2020.15 Moreover, self-administration has become vitally important due to the COVID 19 pandemic, in a scenario in which hospital visits could increase the health risk in certain situations. Several national societies, such as the British Thoracic Society (BTS) and the Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC), recommend to promote the home self-administration versus hospital administration during the pandemic.16,17 Furthermore, maintaining the biological therapy in patients with severe asthma is important to keep the disease control and may also be useful to mitigate the clinical course of COVID-19 based on the current and initial evidence.18,19
Likewise, there are scarcely some data regarding the patients and doctors ‘satisfaction with the therapy and particularly, nothing has been reported for benralizumab in SEA in real-life conditions. Patients´ satisfaction has been assessed in some few previous monoclonal antibodies like ixekizumab and galcanezumab.20,21 Both studies used the subcutaneous administration assessment questionnaire (SQAAQ) to explore the satisfaction, the patient’s abilities and confidence in the device. Considering the absence of published evidence, the main objective of this study was to characterize the patient’s and the doctor’s satisfaction with the self-administration of benralizumab, moreover measuring usability of benralizumab devices for SEA in real-world setting. In addition, as secondary objectives, an analysis of the effectiveness of benralizumab in patients treated at home was carried out.
Materials and Methods
Objectives, Study Population and Study Design
AUTO-BENRA study was a retrospective multicenter study conducted at 9 hospitals in Spain with patients with uncontrolled SEA treated with benralizumab at home-use administration. The primary endpoint was to assess patient satisfaction and reliability with the self-administered treatment through the completion of SQAAQ after 3 doses of benralizumab self-administered by the patient of his/her caregiver.
The secondary endpoint was to measure the effectiveness of benralizumab with these devices in self-administration at home.
The study enrolled patients over 18 years of age, with SEA according to the Spanish Guidelines for Asthma Management (GEMA 5.0) criteria, being treated with benralizumab according to its approved indication in Spain, who had received at least three doses of self-administered benralizumab at home before April 30, 2021.22 The recruitment period ended on October 1, 2021. Exclusion criteria was limited just to the incapacity to understand the informed consent, refusal to sign it and/or if the patient or their caregivers had insufficient intellectual capacity to understand the SQAAQ.
Variables Related to Satisfaction a Reliability
SQAAQ is a novel 12-item (see Figure 1), self-administered questionnaire that has not yet been psychometrically validated. The SQAAQ items assess ease of use of the device and patient/caregiver confidence. Each item was answered on a 7-point Likert scale ranging from “strongly disagree” to “strongly agree”. Moreover, satisfaction with the home-use treatment was also measured with a classic visual analog scale (VAS) where it could be answered with a number from 1 to 10 where 0 was not satisfied and 10 was completely satisfied. This VAS was used to assess satisfaction not only with the device but also with the health results (Global VAS) for both the patient and doctor. VAS has not been measured to validate SQAAQ. The concordance of the results between physician/patients VAS and SQAAQ was evaluated.
Figure 1.
Subcutaneous administration assessment questionnaire (SQAAQ).
Notes: Adapted from Callis Duffin K, Bukhalo M, Bobonich MA, et al. Usability of a novel disposable autoinjector device for ixekizumab: results from a qualitative study and an open-label clinical trial, including patient-reported experience. Med Devices (Auckl). 2016;9:361–369.20 Copyright © 2016 Callis Duffin et al. Creative Commons Attribution – Non Commercial (unported, v3.0) License (http://creativecommons.org/licenses/by-nc/3.0/).
SQAAQ scores were registered after the third self-administration of benralizumab. We collected the patient’s VAS for global satisfaction with benralizumab treatment, as well as VAS for the patients´ and doctors´ satisfaction with the self-administration regimen at home. The scale ranged from 0 (completely unsatisfied) to 10 (completely satisfied). Any errors in the self-administration at the different doses, adverse events that might have arisen, and possible mistakes in the manufacture were registered. Patients received a prefilled syringe or autoinjector depending on the availability of each of the devices in each center. Patients fulfilled the questionnaires themselves during each scheduled visit to the specialized centers, according to routine clinical practice.
Clinical-Demographics and Effectiveness Variables
Social-demographic data (race, sex, date of birth, educational level) and clinical outcomes (year of onset of asthma, body mass index, asthma phenotype, concomitant rhinosinusal pathology, comorbidities, tolerance to non-steroidal anti-inflammatory drugs, allergic sensitizations, and previous biological treatment for asthma) were assessed for each patient. We also collected pre- and post-treatment data [counts of eosinophils in absolute value per microliter (µL) in peripheral blood (the closest data after the third self-administration of benralizumab)]; absolute and percentage values concerning the predicted values of the FVC, FEV1 and the FEV1/FVC ratio; self-reported Spanish version of asthma control test (ACT) and Spanish Mini Asthma Quality of Life Questionnaire (MiniAQLQ) scores; number of moderate (add-on or increase up inhaled medications) to severe exacerbations (those that had required systemic corticosteroids at least 3 days or increasing the dose if patients already received them).23–25 Exacerbations that required hospitalization or emergency care attention were also collected. All post-treatment outcomes were collected at least after 6 months of treatment. In prednisone-dependent patients, the dose of prednisone recorded was the closest one to the study enrollment.
Database and Ethics
An encoded computerized database, specifically designed for this purpose, was compiled from patients’ medical records and interviews.The ethics committee for research with medicinal products at Hospital Universitario 12 de Octubre approved the study protocol and assigned the code 21/031 to the project. With this approval, the rest of the participating centers presented at the rest of the ethics committees where they also approved the protocol. This study was performed in accordance with the Declaration of Helsinki.
Statistical Analysis
The characteristics of the patients and all the results were presented using descriptive statistics. Continuous variables were defined by mean, standard deviation, median, interquartile range, minimum and maximum. The categorical variables were described as absolute and relative frequency. In both cases, the number of observations and missing data will be specified. The assumptions of the normal distribution were evaluated using the Shapiro–Wilk test or the Kolmogorov–Smirnov test. The evolution of change from pre to post was calculated using the t-student related sample test, the non-parametric Mann–Whitney-Wilcoxon U-test. McNemar’s exact test or Cochran’s q test, depending on the nature of the variables.
The concordance index will be calculated to obtain the proportion of agreement observed between the global VAS results, per patient, and doctors. The discrepancies of the VAS values were evaluated with the differences of means and statistically significant differences were evaluated through the symmetry test and Pearson’s correlation coefficient. All estimates were accompanied by the 95% confidence interval. All statistical analyses will be performed using Stata version 16 for Windows. The level of significance used was 5% (α = 0.05).
Results
Patient Population
Fifty-four patients were recruited in AUTO-BENRA Study. The baseline demographic and clinical characteristics of patients are summarized in Table 1. Median time under benralizumab treatment was 11[7–29] months. Nine patients completed less than 6 months for the evaluation of SQAAQ and VAS Scales, because they switched treatment from other biological drug with similar profile for self-administration or doctors decided to accelerate the training time due the COVID-19 pandemic situation. However, even in these patients, at least the first dose was administered in the hospital to monitor possible adverse events.
Table 1.
Baseline Demographic and Clinical Characteristics of AUTO-BENRA Study
| Demographics Characteristics | N Missed | |
|---|---|---|
| Age y, mean±SD | 57.31±9.97 | |
| Female, n (%) | 34 (62.96%) | |
| Educational level | ||
| High school n (%) | 9 (18.75%) | |
| Vocational training n (%) | 14 (29.17%) | |
| Secondary education n (%) | 17 (35.41%) | |
| Primary education n (%) | 8 (16.67%) | |
| BMI mean±SD | 28.04±4.62 | 1 |
| Current/ex- smoker >10p/year n (%) | 9 (16.7%) | |
| Comorbidities | ||
| GERD n (%) | 32 (59.26%) | |
| OSAS (%) | 5 (9.26%) | |
| Mental disorders n (%) | 13 (24.07%) | |
| Bronchiectasis n (%) | 11 (20.37%) | |
| Nasal polyps n (%) | 25 (26.3%) | |
| AERD n (%) | 16 (30.7%) | |
| Aeroallergen’s sensitization-atopy n (%) | 38 (70.37%) | |
| Biomarkers | ||
| Total IgE KU/L mean ±SD | 554.21±1337.22 | |
| Eosinophils Cells /microliter ±SD | 742.04±441.73 | |
| Clinical Characteristics | ||
| Prednisone-dependent patients n (%) | 24(44.4%) | |
| Severe exacerbation rate Exacerbations/year ±SD | ||
| FEV1 percentage mean ±SD | 68.82±15.92% | 2 |
| ACT points mean±SD | 14.10±4.41 | 2 |
| MiniAQLQ mean ±SD | 3.99±1.30 | 26 |
Abbreviations: BMI, body mass index; GERD, gastroesophageal reflux disease; OSAS, obstructive sleep apnea syndrome; AERD, aspirin-exacerbated respiratory disease; FEV1, forced expiratory volume in first second; ACT, asthma control test; MiniAQLQ, reduced version of asthma quality of life questionnaire.
Mean age of patients was 57.3[±9.97] years, mostly women (62.96%). Thirty-one patients had been previously treated with other biological drugs for severe asthma, suspended due to lack of efficacy, adverse events, or avoiding of intravenous administration of reslizumab due to the COVID-19 pandemic. Four patients had received 3 biologics sequentially (omalizumab, mepolizumab, and reslizumab), another 4 patients had previously received 2 (omalizumab and mepolizumab), 13 patients had exclusively received omalizumab prior to benralizumab, 7 patients only mepolizumab and, finally, 5 patients only reslizumab. The most frequent recorded comorbidity was gastroesophageal reflux (59.3%) and chronic rhinosinusitis with polyposis (46.3%) and 70.4% were atopic. At baseline, mean (SD) blood eosinophil count was 742.04 ±441.73 cells/µL. A 50.9% of the patients used the pre-filled syringe for home self-administration and 49.1% for the autoinjector. Adherence to asthma maintenance treatments was high, with an average score in the inhaler adherence test (TAI) of 49.8 out of 50 points and a 92.6% mean withdrawal at the pharmacy office for inhalers, according to the pharmacy refill data from the National Spanish Health System.
SQAAQ and VAS Results
The mean SQAAQ score was 6.89 (±0.16) points. Patients and their caregivers report excellent satisfaction, safety with the device, and ability to administer medication at home with the devices offered. The question with the worst average score was the one related to the storage conditions of the device (question 6, average score of 6.81 points) and the best-scored question was question 9, related to the opinion of the general ease of use of the device with a score of 6.96 points. The mean VAS score for home administration was 9.40 points by the patient and 9.45 by the doctor. The VAS on global satisfaction with benralizumab treatment was 9.40 points. The concordance between the perception of the doctor and the patient about the treatment was measured with a spearman coefficient of 0.648 indicating a good positive correlation (p < 0.0001). There were no statistical differences between prefilled syringe and autoinjector (see Table 2). There were no errors recorded during the study, nor in the application of the medication by the patient or their caregivers, nor in any manufacturing errors.
Table 2.
Summary of SQAAQ and VAS Score
| Total | Prefilled Syringe | Autoinjector | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| N= 54 | N missed | N=27 | Nmiss | N=26 | N missed | |||
| Device | 0 | |||||||
| Prefilled syringe | 27 (50.94%) | |||||||
| Autoinjector | 26 (49.06%) | |||||||
| Global VAS patient | 9.37 (0.99) | 1 | 9.38 (0.64) | 26 | 1 | 9.35 (1.26) | 0 | 0.89 |
| Patient VAS | 9.38 (1.03) | 1 | 9.50 (0.86) | 26 | 1 | 9.27 (1.19) | 0 | 0.43 |
| Physician VAS | 9.44 (0.92) | 1 | 9.58 (0.58) | 26 | 1 | 9.31 (1.16) | 0 | 0.29 |
| Average 12-SQAAQ | 6.89 (0.16) | 0 | 6.90 (0.16) | 27 | 0 | 6.89 (0.16) | 0 | 0.87 |
Effectiveness Variables
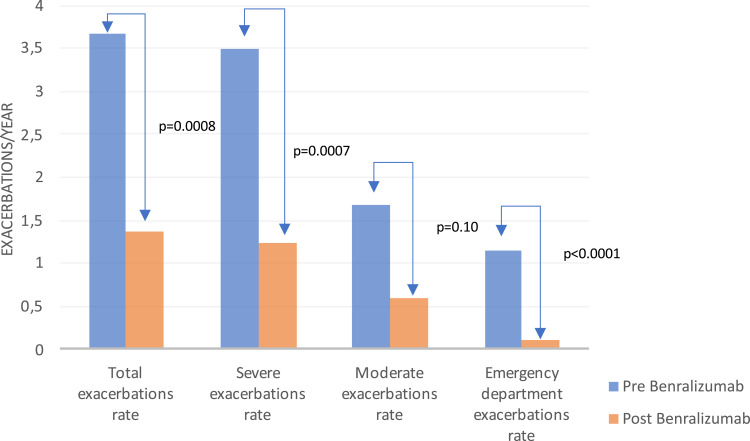
Regarding the effectiveness variables, the annualized rate of exacerbations (see Figure 2) decreased by 63%, reducing from 3.67±3.32 to 1.36±3.19 exacerbations per year (p = 0.0008), severe exacerbations, decreased by 65% (p = 0.0007) exacerbations a year. A 91.2% (p < 0.0001) reduction was observed in exacerbations attended at emergency department. At the beginning of the study, 52 (96.3%) patients had experienced at least one exacerbation, and at the end of the study 38 (73.1%) patients were free of exacerbations. Only 2 patients enrolled in the AUTO-BENRA study had been admitted to the hospital for an asthma attack at baseline, and no one until the study ended. Regarding asthma control, median ACT score improved on average 6.27±5.35 points (p <0.0001, N missed 2 patients) and the median MiniAQLQ raised 1.58±1.47 points (p <0.0001 N missed 17 patients). Only 3 patients had >20 points in ACT (cut-off to consider good asthma control) before benralizumab, and after benralizumab 37 patients had >20 points. Regarding lung function, FEV1 improved by 320.77±458.74 mL on average (p <0.0001, N missed 2 patients), and an improvement of 13.82±15.92% (p < 0.0001) was observed. The FVC improved on average by 239.61±495.62 mL (p = 0.0010) and 9.43±19.45% in percentage value (p = 0.0011). Twenty-four patients (44.4%) were on maintenance systemic corticosteroids with a mean dose of 16.7 mg prednisone per day. At the end of the study, 14 patients (58.3%) achieved complete withdrawal and of the 10 patients undergoing treatment, the average dose downed to 7.38 mg of prednisone daily. Eosinophils decreased a 99.4% (p < 0.0001), drooped to 3.82 eosinophils per microliter to the end of the study. The device complaints or malfunctions in the study were low. No discontinuation due to adverse effects was reported by the investigators.
Figure 2.
Improvement on subtypes of exacerbations rate after benralizumab.
Discussion
Although home self-administration has become a frequent option for most biological treatments, there is an increasing need to verify satisfaction, usability, and reliability with this treatment in patients with SEA to ensure good adherence and good health outcomes.
The AUTO-BENRA study emerged to objectively confirm the good perception of most patients and physicians regarding satisfaction with benralizumab when administered at home. These results demonstrate the great satisfaction, usability, and reliability that patients report through the SQAAQ of the different devices in which benralizumab is presented, wherein a generalized way the average score achieved in each question is close to the maximum, 7 points (totally agree) within 162 administered doses.
Our results are coincident with previous publications, although for different drug and diseases. We have evaluated the SQAAQ scores after 3 doses. We truly believe this evaluation adds robustness to our results. Similar to the ixekizumab trial, the study tried to assess the ease of use and confidence by using an injection device at weeks 0, 4, and 8. For ixekizumab at the first use, 95% of subjects either agreed or strongly agreed that the device was “overall easy to use”, and they felt “confident the dose was complete” according to the SQAAQ.20 Concordantly to our study, continued experience with the self-administration over the course of the trial offered an extremely high proportion of patients or caregivers agreeing or strongly agreeing with each item of the SQAAQ, even in issues in which clinicians are not directly involved, like the storage conditions of the device, the worst average score in our study. For galcanezumab prefilled syringe and autoinjector study, which analyzed patients with at least one administration, most patients had positive responses to the SQAAQ.21 A possible weakness in our study could be that SQAAQ has not been validated in Spanish and it is not a validated measure. However, it helps providing insight into the patient-reported experiences using both devices, syringe and autoinjector. Furthermore, we have confirmed the SQAAQ results, through providing another usability and satisfaction measure, the global VAS for benralizumab treatment and a patients’ VAS for home-us of benralizumab, which has resulted also positively correlated with the opinion of doctors.
Regarding the effectiveness of benralizumab, its efficacy has been widely demonstrated in pivotal clinical trials. Benralizumab reduced exacerbations in CALIMA (56 weeks) and SIROCCO (48 weeks) studies by 28% (RR = 0.72; 95% CI 0.54–0.95; p = 0.019) and 51%, respectively (RR = 0.49; 95% CI: 0.37–0.64; p < 0.001) vs placebo. In the SIROCCO study, the rate of exacerbations that led to the hospitalization (secondary variable) was reduced in a 63% (RR = 0.37; 95% CI: 0.20–0.67; p = 0.0010).2,3 In the ZONDA trial (steroid-dependent patients) the 70% reduction in exacerbations was obtained (RR = 0.30; 95% CI: 0.17–0.53; p < 0.001).4 AUTO-BENRA study was not placebo controlled. In real-world evidence (RWE), the evidence about effectiveness of benralizumab is limited. There are several case series of patients, but most of them included less than half a hundred patients and only one with 130 (Kavanagh et al).26–38 In most RWE studies, results in reducing the rate of exacerbations exceed those reported in clinical trials. Similarly, in AUTO-BENRA, data on the reduction in the rate of exacerbations outstrip the reality of clinical trials, achieving a 73.4% reduction in patients with zero exacerbation/year, a rate not communicated in other RWE studies.
In other clinical outcomes, the improvement with benralizumab was again evident. In the RWE groups, where the effect of benralizumab on asthma control measured by ACT has been assessed, improvements of up to 7.1 points (Padilla-Galo et al) and 6 points (Bagnasco et al) have been achieved.27,29 In our study, we observed an important effect on asthma control with an improvement of almost 7 points in our population, ranging from 14.1± 4.23 points to 20.37 points± 4.41. This implied a significant improvement in asthma-controlled patients in this group. Also, few groups have assessed the effect on quality of life of benralizumab in RWE. Interestingly, the improvement in quality of life, measured by the MiniAQLQ in the AUTO-BENRA study, is the highest recorded in RWE – studies to date and much higher than the 0.5 points considered as clinically relevant.39 It has been even higher than the 0.89 points reported in Kavanagh et al.26 Improvement in lung function has been well reported with benralizumab. In tSIROCCO and CALIMA trials, FEV1 improvement was 398 mL vs 239 mL at 48 weeks (p = 0.001) and 330 mL vs 215 mL placebo at 56 weeks (p = 0.01), respectively.2,3 RWE studies report greater improvements in lung function, measured with spirometry and other techniques like oscillometry.40 In our study, improvements exceed 320 mL, with improvement considered clinically relevant, as occurs in most RWE studies, where improvements have been observed even above 680 mL.35 Exacerbations and lung function were evaluated in studies in SEA population with or without CRSwNP, obtaining better results in the subpopulation with CRSwNP.33,41 CRSwNP population is likely to become a clinical marker of eosinophilic endotype in the Caucasian population, and therefore the response to benralizumab will be better. In our case, the population did not show significant differences in the results with the general population, possibly because the number of patients with CRSwNP was low (24 patients).
One important issue is oral corticosteroids (OCS) sparing rate due to benralizumab. According to clinical trials, benralizumab reduced the median dose reduction of OCS by 75% compared to the basal dose at 28 weeks vs 25%, with placebo (OR 4.12 (95% CI 2.22–7.63) p <0.0017).4 Withdrawal of OCS with benralizumab was 52% compared to 19% with placebo (OR 4.19 (1.58–11.12) p=0.002) in patients with a basal prednisone dose ≤12.5 mg/day.4 In most series of RWE, more than half of the steroid-dependent patients achieve withdrawal, and in some up to 80% of the steroid-dependent population.33 In our study, we achieved withdrawal in just over half of the patients who were on OCS, and those who could not withdraw them reduced the dose of prednisone by half.
The first limitation of our study could be that SQAAQ has not been validated. Other limitations of our study are the lack of a control group. Furthermore, we only have one recorded measure of FEV1, corticosteroid doses, questionnaires such as the ACT and MiniAQLQ corresponding to the closest date to enrollment.
In conclusion, we report the first study that objectively evaluates satisfaction with home-use of biologic therapy in SEA. We observed how benralizumab home-use represents an important change in their lives, reflected in enormous satisfaction and confidence with the treatment, without losing effectiveness and offering high safety. In times like the current COVID-19 pandemic and patients with specific risks such as infectious or related to hospital displacement, results from this study support that this treatment regimen could be the best choice. However, more studies, probably with a prospective design, could add validation and more data on the satisfaction and usability even cost saving of these devices while maintaining the effectiveness of the drug.
Acknowledgments
Acknowledgments to the rest of the members of AIRE Group: David Gonzalez-de-Olano, Aythami Henriquez, Remedios Cárdenas Contreras, María Victoria Mugica Garcia, Beatriz Rodriguez Jiménez, Maria Rubio Pérez, Maria Vázquez de la Torre Gaspar, and Armando Bueso Fernández.
Funding Statement
The study has not received direct funding from any company. The AIRE Group meetings have been sponsored by AstraZeneca.
Disclosure
Ismael Garcia-Moguel reports grants and personal fees from AstraZeneca, GSK, TEVA, SANOFI, NOVARTIS, CHIESI, ORION PHARMA, LETI, ALLERGY THERAPEUTICS, and STALLERGENES during the conduct of the study and has been on advisory boards and/or been a speaker or investigator for: Novartis, AstraZeneca, Teva, GSK, Sanofi Genzyme, Chiesi, Allergy therapeutics, Leti, Stallergenes, ALK-Abelló, Mundipharma, Pfizer and Orion Pharma. Ana Rosado reports personal fees from GSK, Astra-Zeneca, and Novartis, outside the submitted work, has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing and/or educational events from AstraZeneca, GSK, Novartis, and support for attending meetings and/or travel from GSK and Chiesi. Aida Gomez Cardeñosa reports personal fees from ASTRAZENECA, GSK, Allergopharma, allergy therapeutics, Stallergenes, LetiPharma, Roxall, Mundipharma, Chiesi, and alk abelló, outside the submitted work, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing and/or educational events from Leti Pharma, Alk Abello, Stallergenes, Allergopharma AstraZeneca, Gsk, Mundipharma, Chiesi, and Bial, and support for attending meetings and/or travel from Allergy Therapeutics, Roxall Letipharma, Sanofi, Gsk Stallergenes, Chiesi, and Allergopharma. Mar Gandolfo-Cano has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing and/or educational events from AstraZeneca, Sanofi, GlaxoSmithKline, Novartis, Leti, Allergy Therapeutics, and Chiesi and support for attending meetings and/or travel from Leti, Inmunotek, Allergy Therapeutics, and Chiesi. Teresa Robledo Echarren has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing and/or educational events from AstraZeneca, GSK, Sanofi, Bial, and Stallergenes, support for attending meetings and/or travel from Diater, Immunotek, and Stallergenes, and participated on a Data Safety Monitoring Board or Advisory Board for Allergy therapeutics and stallergenes. Maria del Mar Moro Moro reports personal fees from SANOFI, ASTRA ZENECA, and GSK, outside the submitted work, has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing and/or educational events from GSK, Astra-Zeneca, Sanofi, Novartis, ALK, and Chiesi, and support for attending meetings and/or travel from Allergy therapeutics, LETI, and ALK. Mª del Mar Reaño Martos has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing and/or educational events from AstraZeneca, GSK, and ALK, support for attending meetings and travel from Allergy therapeutics and payment for expert testimony from Leti and Stallergenes. Rafael Pineda-Pineda reports personal fees from Astra Zeneca, Sanofi, GLAXOSMITHKLINE, and INMUNOTEK, non-financial support from NOVARTIS, LETI, ALLERGY THERAPEUTICS, and DIATER outside the submitted work and has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing and/or educational events, support for attending meetings and/or travel from AstraZeneca, Sanofi, GlaxoSmithKline, Novartis, Leti, Inmunotek, Diater, Allergy Therapeutics, and Roxall. Marcela Valverde-Monge reports personal fees from GSK and Organon outside the submitted work, has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing and educational events from GSK, and support for attending meetings and travel from Allergy therapeutics. Javier Domínguez-Ortega reports personal fees from ASTRAZENECA, GSK, SANOFI, NOVARTIS, and TEVA, outside the submitted work, has received funding for research and honoraria for consultancy and conferences from AstraZeneca, Chiesi, and GSK, Bial, Novartis, Sanofi, and Teva, and and speaker fees from ALK, LETI Pharma, and Mundipharma. The authors report no other potential conflicts of interest in relation to this work.
References
- 1.Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125(6):1344–1353.e2. doi: 10.1016/j.jaci.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 2.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115–2127. doi: 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 3.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–2214. doi: 10.1016/S0140-6736(16)31322-8 [DOI] [PubMed] [Google Scholar]
- 4.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–2458. doi: 10.1056/NEJMoa1703501 [DOI] [PubMed] [Google Scholar]
- 5.Chipps BE, Newbold P, Hirsch I, Trudo F, Goldman M. Benralizumab efficacy by atopy status and serum immunoglobulin E for patients with severe, uncontrolled asthma. Ann Allergy Asthma Immunol. 2018;120(5):504–511.e4. doi: 10.1016/j.anai.2018.01.030 [DOI] [PubMed] [Google Scholar]
- 6.Liu T, Wang F, Wang G, Mao H. Efficacy and safety of benralizumab in patients with eosinophilic asthma: a meta-analysis of randomized placebo-controlled trials. Front Med. 2018;12(3):340–349. doi: 10.1007/s11684-017-0565-0 [DOI] [PubMed] [Google Scholar]
- 7.Tetteh EK, Morris S, Titcheneker-Hooker N. Discrete-choice modelling of patient preferences for modes of drug administration. Health Econ Rev. 2017;7(1):26. doi: 10.1186/s13561-017-0162-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoner KL, Harder H, Fallowfield LJ, Jenkins VA. Intravenous versus subcutaneous drug administration. Which do patients prefer? A systematic review. Patient. 2014. doi: 10.1007/s40271-014-0075-y [DOI] [PubMed] [Google Scholar]
- 9.Scalone L, Sarzi-Puttini P, Sinigaglia L, et al. Patients’, physicians’, nurses’, and pharmacists’ preferences on the characteristics of biologic agents used in the treatment of rheumatic diseases. Patient Prefer Adherence. 2018;12:2153–2168. doi: 10.2147/PPA.S168458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huynh TK, Ostergaard A, Egsmose C, Madsen OR. Preferences of patients and health professionals for route and frequency of administration of biologic agents in the treatment of rheumatoid arthritis. Patient Prefer Adherence. 2014;8:93–99. doi: 10.2147/PPA.S55156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pozzilli C, Schweikert B, Ecari U, Oentrich W. Supportive strategies to improve adherence to IFN beta-1b in Multiple Sclerosis — results of the BetaPlus observational cohort study. J Neurol Sci. 2011;307(1–2):120–126. doi: 10.1016/j.jns.2011.04.026 [DOI] [PubMed] [Google Scholar]
- 12.Ferguson GT, Mansur AH, Jacobs JS, et al. Assessment of an accessorized pre-filled syringe for home-administered benralizumab in severe asthma. J Asthma Allergy. 2018;11:63–72. doi: 10.2147/JAA.S157762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin UJ, Fuhr R, Forte P, et al. Comparison of autoinjector with accessorized prefilled syringe for benralizumab pharmacokinetic exposure: AMES trial results [published online ahead of print, 2019 Sep 20]. J Asthma. 2019;2019:1–9. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson GT, Cole J, Aurivillius M, et al. Single-use autoinjector functionality and reliability for at-home administration of benralizumab for patients with severe asthma: GRECO trial results. J Asthma Allergy. 2019;12:363–373. doi: 10.2147/JAA.S224266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benralizumab Spanish official prescribing information. Available from: https://cima.aemps.es/cima/pdfs/es/ft/1171252001/FT_1171252001.pdf. Accessed May 2, 2022.
- 16.The British Thoracic Society. COVID-19: information for the respiratory community; 2022. Available from: https://www.brit-thoracic.org.uk/about-us/covid-19-information-for-the-respiratory-community/. Accessed May 2, 2022.
- 17.Societa' Italiana di Allergologia, Asma ed Immunologia Clinica. Documento di Indirizzo SIAAIC per Pazienti Allergici Respiratori ed i Centri di Allergologia, Asma e Immunologia Clinica [SIAAIC recommendations for respiratory allergy patients and centers for allergy, asthma and immunology clinic]; 2020. Available from: http://www.siaaic.org/wp-content/uploads/2020/04/Documento-di-Indirizzo-SIAAIC.pdf. Accessed May 2, 2022. Italian.
- 18.Busse WW. Biological treatments for severe asthma: a major advance in asthma care. Allergol Int. 2019;68(2):158–166. doi: 10.1016/j.alit.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 19.Poddighe D, Kovzel E. Impact of anti-type 2 inflammation biologic therapy on COVID-19 clinical course and outcome. J Inflamm Res. 2021;14:6845–6853. doi: 10.2147/JIR.S345665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callis Duffin K, Bukhalo M, Bobonich MA, et al. Usability of a novel disposable autoinjector device for ixekizumab: results from a qualitative study and an open-label clinical trial, including patient-reported experience. Med Devices. 2016;9:361–369. doi: 10.2147/MDER.S113752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stauffer VL, Sides R, Lanteri-Minet M, et al. Comparison between prefilled syringe and autoinjector devices on patient-reported experiences and pharmacokinetics in galcanezumab studies. Patient Prefer Adherence. 2018;12:1785–1795. doi: 10.2147/PPA.S170636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guía Española para el Manejo del Asma [Spanish guidelines for the management of asthma]. GEMA5.0 [homepage]; 2022. Available from: www.gemasma.com. Accessed May 2, 2022. Spanish.
- 23.Vega JM, Badia X, Badiola C, et al. Validation of the Spanish version of the Asthma Control Test (ACT). J Asthma. 2007;44(10):867–872. doi: 10.1080/02770900701752615 [DOI] [PubMed] [Google Scholar]
- 24.Sanjuàs C, Alonso J, Sanchís J, et al. Cuestinario de calidad de vida en pacientes con asma: la versión española del Asthma Quality of Life Questionnaire [The quality-of-life questionnaire with asthma patients: the Spanish version of the Asthma Quality of Life Questionnaire]. Arch Bronconeumol. 1995;31(5):219–226. Spanish. doi: 10.1016/S0300-2896(15)30927-3 [DOI] [PubMed] [Google Scholar]
- 25.Crimi C, Campisi R, Noto A, et al. Comparability of asthma control test scores between self and physician-administered test. Respir Med. 2020;170:106015. doi: 10.1016/j.rmed.2020.106015 [DOI] [PubMed] [Google Scholar]
- 26.Kavanagh JE, Hearn AP, Dhariwal J, et al. Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest. 2021;159(2):496–506. doi: 10.1016/j.chest.2020.08.2083 [DOI] [PubMed] [Google Scholar]
- 27.Bagnasco D, Brussino L, Bonavia M, et al. Efficacy of Benralizumab in severe asthma in real life and focus on nasal polyposis. Respir Med. 2020;171:106080. doi: 10.1016/j.rmed.2020.106080 [DOI] [PubMed] [Google Scholar]
- 28.Padilla-Galo A, García-Ruiz AJ, Levy Abitbol RC, et al. Real-life cost-effectiveness of benralizumab in patients with severe asthma. Respir Res. 2021;22(1):163. doi: 10.1186/s12931-021-01758-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Padilla-Galo A, Levy-Abitbol R, Olveira C, et al. Real-life experience with benralizumab during 6 months. BMC Pulm Med. 2020;20(1):184. doi: 10.1186/s12890-020-01220-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miralles López JC, Escudero Pastor AI, Carbonell Martínez A, Navarro Garrido C, Pacheco B, Petryk Petryk Y. Quality of life in severe asthmatic patients treated with benralizumab. Eur Ann Allergy Clin Immunol. 2021. doi: 10.23822/EurAnnACI.1764-1489.218 [DOI] [PubMed] [Google Scholar]
- 31.Yamada H, Nakajima M, Matsuyama M, et al. Identification of distinct phenotypes related to benralizumab responsiveness in patients with severe eosinophilic asthma. PLoS One. 2021;16(3):e0248305. doi: 10.1371/journal.pone.0248305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelaia C, Crimi C, Benfante A, et al. Therapeutic effects of benralizumab assessed in patients with severe eosinophilic asthma: real-life evaluation correlated with allergic and non-allergic phenotype expression. J Asthma Allerg. 2021;Volume 14:163–173. doi: 10.2147/JAA.S297273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe S, Suzukawa M, Tashimo H, et al. High serum cytokine levels may predict the responsiveness of patients with severe asthma to benralizumab. J Asthma. 2021;1–9. doi: 10.1080/02770903.2021.1942039 [DOI] [PubMed] [Google Scholar]
- 34.Watanabe H, Shirai T, Hirai K, et al. Blood eosinophil count and FeNO to predict benralizumab effectiveness in real-life severe asthma patients. J Asthma. 2021;1–9. doi: 10.1080/02770903.2021.1963769 [DOI] [PubMed] [Google Scholar]
- 35.Di Bona D, Crimi C, D’Uggento AM, et al. Effectiveness of benralizumab in severe eosinophilic asthma: distinct sub-phenotypes of response identified by cluster analysis. Clin Exp Allergy. 2021;52:312–323. doi: 10.1111/cea.14026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nolasco S, Crimi C, Pelaia C, et al. Benralizumab effectiveness in severe eosinophilic asthma with and without chronic rhinosinusitis with nasal polyps: a real-world multicenter study. J Allergy Clin Immunol Pract. 2021;9(12):4371–4380.e4. doi: 10.1016/j.jaip.2021.08.004 [DOI] [PubMed] [Google Scholar]
- 37.Menzella F, Fontana M, Galeone C, et al. Real world effectiveness of benralizumab on respiratory function and asthma control. Multidiscip Respir Med. 2021;16(1):785. doi: 10.4081/mrm.2021.785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menzella F, Ruggiero P, Galeone C, Scelfo C, Bagnasco D, Facciolongo N. Significant improvement in lung function and asthma control after benralizumab treatment for severe refractory eosinophilic asthma. Pulm Pharmacol Ther. 2020;64:101966. doi: 10.1016/j.pupt.2020.101966 [DOI] [PubMed] [Google Scholar]
- 39.Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55(1):1900588. doi: 10.1183/13993003.00588-2019 [DOI] [PubMed] [Google Scholar]
- 40.Shirai T, Akamatsu T, Hirai K, et al. Oscillometry improves earlier than spirometry after benralizumab initiation in severe asthma. Allergy. 2020;75(10):2678–2680. doi: 10.1111/all.14339 [DOI] [PubMed] [Google Scholar]
- 41.Canonica GW, Harrison TW, Chanez P, et al. Benralizumab improves symptoms of patients with severe, eosinophilic asthma with a diagnosis of nasal polyposis. Allergy. 2021;77:150–161. [DOI] [PubMed] [Google Scholar]