Abstract
It is unknown how providing prospective living donors with information about APOL1, including the benefits and drawbacks of testing, influences their desire for testing. In this study, we surveyed 102 participants with self-reported African ancestry and positive family history of kidney disease, recruited from our nephrology waiting room. We assessed views on APOL1 testing before and after presentation of a set of potential benefits and drawbacks of testing and quantified the self-reported level of influence individual benefits and drawbacks had on participants’ desire for testing in the proposed context of living donation. The majority of participants (92%) were aware of organ donation and more than half (56%) had considered living donation. And though we found no significant change in response following presentation of the potential benefits and the drawbacks of APOL1 testing by study end significance, across all participants, “becoming aware of the potential risk of kidney disease among your immediate family” was the benefit with the highest mean influence (3.3±1.4), while the drawback with the highest mean influence (2.9±1.5) was “some transplant centers may not allow you to donate to a loved one”. This study provides insights into the priorities of prospective living donors and suggests concern for how the information affects family members may strongly influence desires for testing. It also highlights the need for greater community engagement to gain a deeper understanding of the priorities that influence decision making on APOL1 testing.
Keywords: APOL1, genetic testing, kidney transplant, living donor, patient preferences, shared decision-making
1 |. INTRODUCTION
Among individuals of west sub-Saharan ancestry, those with Apolipoprotein L1 (APOL1) high-risk genotypes (i.e., two copies of G1 and/or G2 alleles) are at increased risk of kidney disease and kidney failure.1,2 Deceased donor kidney transplant outcomes appear to be worse for organs with two risk alleles, independent of recipient genotype.3–5 Living donor nephrectomy in individuals with APOL1 high-risk genotypes is associated with an increased incidence of kidney failure.6,7 These data raise questions about the need to incorporate APOL1 testing in the evaluation of prospective living kidney donors.8 The American Society of Transplantation assembled a panel of experts who recommended all potential living donors be informed of the risks associated with APOL1 high-risk genotypes and of the availability of genetic testing.9 As a result, some transplant centers offer APOL1 testing to living donors deemed at-risk for APOL1-mediated kidney disease.10 Implementation of APOL1 testing vary considerably across sites with some centers offering no formal consent procedures or pre-test education prior to APOL1 testing. The variation raises ethical concerns about inadequate consent procedures for prospective living donors who are offered genetic testing.8,9,11
Molecular testing, including APOL1 sequencing, has only recently become more accessible for use in the clinical setting.12–14 Therefore, we do not yet know the burden of APOL1 risk alleles among prospective living donors. Although there is a strong association between APOL1 and kidney disease, only 13% of Black kidney disease patients in the United States are estimated to have a APOL1 high-risk genotype.15 In addition, the lifetime risk for developing kidney disease among individuals with two risk alleles is estimated to only be 10%.16 The low prevalence suggests it follows a two-hit model, where a secondary factor, such as environmental or genomic modifiers, is required to develop kidney disease. However, we still have a limited appreciation of factors that can act as a “second-hit”, and it is unclear whether the hyperfiltration that follows donor-nephrectomy constitutes as a “second-hit” for the disease.
Despite the low prevalence of APOL1-mediated kidney disease, and unanswered questions about the implications of broad implementation of APOL1 testing, some transplant centers prohibit at least some individuals with two risk alleles from donating a kidney.10 Importantly, medical conditions that often preclude donation, like hypertension and diabetes, probably have a higher prevalence among potential Black donors than APOL1-mediated kidney disease. Thus, excluding prospective donors with a high-risk genotype has led to concerns that APOL1 testing creates an additional barrier to living donation, and foster perceptions of bias, for Black patients10,17,18 that may ultimately exacerbate existing health inequities19,20 experienced in Black communities. However, an individual’s APOL1 status may offer valuable prognostic information for prospective living donors. For example, prospective living donors are routinely screened for diabetes, which is widely seen as a contraindication for kidney donation at most, if not all, transplant centers.18 One study found that impaired fasting glucose was associated with a 3-fold higher risk for developing kidney disease among individuals considered otherwise suitable candidates for donation.7 Using a similar estimate model, having two APOL1 risk alleles was associated with a 5-fold higher risk for kidney disease.
Beyond the potential prognostic value of knowing living donors’ APOL1 genotype, there is a growing desire for individuals from Black communities to know about their risk for APOL1-mediated kidney disease.21–25 What remains unclear, however, is whether knowing more about APOL1, including the potential benefits and drawbacks associated with APOL1 testing, influence interest in testing among prospective living donors. In this study, we assessed views on APOL1 testing in the context of living kidney donation among individuals recruited from a nephrology clinic waiting room, before and after presenting them with APOL1 related education, including the potential benefits (e.g., knowledge of personal risk, etc.) and drawbacks (e.g., financial risk, etc.) of testing.
2 |. METHODS
The study was approved by Columbia University’s Institutional Review Board (IRB-AAAR9915). The survey instrument was iteratively developed. The final version was administered electronically using the survey management software Qualtrics (Provo, UT, USA) between October 2018 and June 2019. Prospective participants were in the waiting room accompanying an adult patient who self-identified as Black or African American at registration, and who was scheduled for a nephrology follow-up visit or a kidney transplant evaluation. Eligible participants were at least 18 years of age, able to speak and read in English, had a positive family history of kidney disease but did not have kidney disease themselves, and self-reported having African ancestry.
Individuals deemed eligible who expressed interest in participating in the study used an iPad to view the survey, which was displayed on the Qualtrics platform. The iPad was password protected to ensure confidentiality. Participants were not asked to provide any identifying information such as their name, date of birth, home address, phone number, or email. Only members of Columbia University’s research team had access to the password.
2.1 |. Survey instrument
Before the start of the study, the survey (Appendix A) went through two revisions between July 2018 and September 2018, incorporating feedback offered by the core study team, which was made up of nephrologists, ethicists and living donor coordinators. The instrument’s contents were written at a 5th-grade reading level with a final Flesch Kincaid Grade level of 5.6. The survey included demographic questions, as well as questions on prior experiences with genetic testing, awareness of living kidney donation and if they had considered being a living donor. The survey also included a free text question asking participants how they were related to the individual they accompanied.
2.2 |. Baseline assessment
A short paragraph that introduced basic background information about APOL1 was developed for this study. This brief section included a simple description of DNA, genes, genetic inheritance, the APOL1 gene, APOL1-mediated risk for kidney disease, and the value of living kidney donation in the management of kidney failure. To determine baseline views on APOL1 testing, participants were asked if they would want APOL1 testing as a potential or hypothetical, living donor and how the presented background information influenced their views on testing.
2.3 |. Educational intervention
Next, participants were provided with educational information on the potential benefits and drawbacks of APOL1 testing. These items were derived from previously published findings and themes relating to views on APOL1 testing identified in participants from Black and African American communities (Table S1 in the Supplementary Appendix).21,23–26 To minimize the influence of the order in which the benefits and drawbacks were presented, we randomized participants into two groups. The randomization was generated by Qualtrics. Individuals in group A were first shown the potential benefits of APOL1 testing followed by the potential drawbacks, while those in group B were presented the drawbacks of testing before the benefits. The participants initial response about APOL1 testing was used as the control, a common study design in the initial development phase of a new exploratory intervention. Using five-point Likert-type items (1-None to 5-Extremely), participants were then asked to rate the level of influence each associated benefit (e.g., knowledge of personal risk) and drawback (e.g., financial risk) had on their desire for APOL1 testing. Immediately after each section, participants were asked if their views on APOL1 testing changed from their original response, in light of the new information. In addition, they were asked to rate to what extent each of the presented benefits and drawbacks influenced their decision on testing.
2.4 |. Statistical analysis
Descriptive statistics are presented as frequencies and percentages for categorical variables and means and standard deviations (SD) for continuous variables. Chi-squared, Fisher’s exact, and two-sample t-tests were used to compare demographics, attitudes, and experiences between survey order groups A and B, and between those who were and were not previously aware of genetic testing. As is the case in prepost design, participants’ baseline response was used as the control. Pre- and post-intervention comparisons were made using McNemar’s test to evaluate potential changes between baseline and presentation of benefits, between baseline and presentation of drawbacks, and between baseline and end of survey. To examine the reported level of influence of each benefit or drawback on participants’ desire for APOL1 testing, the Likert-type item responses were mapped to numeric values ranging from 1 (no influence) to 5 (extremely influential). The mean (SD) level of response was presented per question as well as each individual’s mean level of response to all benefits or all drawbacks. Responses were compared between participants who, at the end of the survey, indicated they would want APOL1 testing and those who would not. Comparisons between participants who wanted testing or not, and participants who changed their initial responses or not, were made using the Wilcoxon rank-sum test.
All analyses were performed using Stata version 15.1 (StataCorp, College Station, TX, USA). We considered P values < .05 as statistically significant
3 |. RESULTS
Information about the recruitment of study participants is presented in Figure 1. In total, 147 individuals were approached for this study. Thirty-six (24%) individuals declined to participate, citing lack of interest. Seven individuals were deemed ineligible: six individuals with kidney disease who would not be able to serve as a living kidney donor, even in a hypothetical scenario, and one individual who denied having African ancestry. Of the 104 individuals who agreed to participate, two participants were unable to complete the survey. Analyses were performed on data from the final cohort of 102 participants who completed the survey.
FIGURE 1.
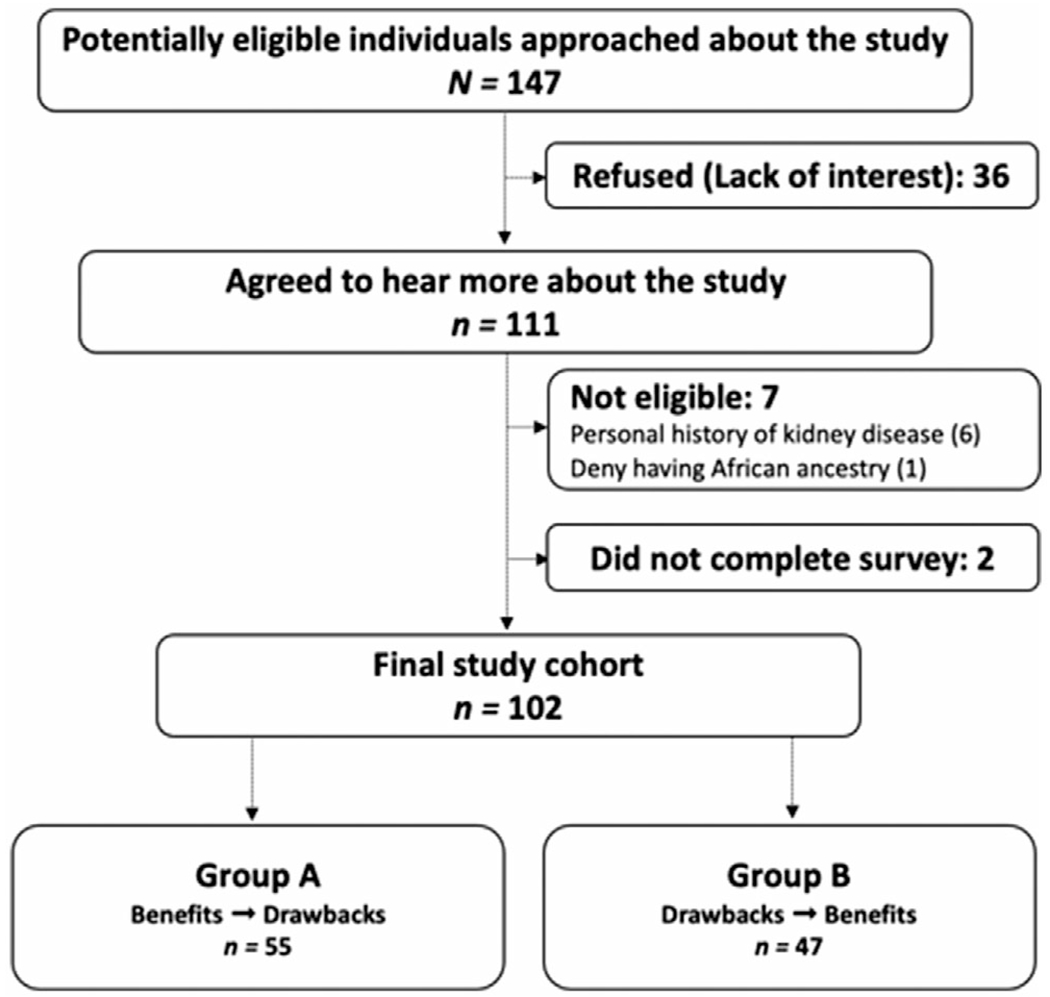
Study enrollment flowchart
Overall, the two randomized participant groups (A; B) did not have any statistically significant differences in demographics, attitudes and experiences, or desires for APOL1 testing at each assessment point in the survey (see Tables 1 and 2)
TABLE 1.
Baseline characteristics
| Survey order |
Has heard of genetic testing before this study |
||||||
|---|---|---|---|---|---|---|---|
| Characteristics | All participants | A benefits presented first | B drawbacks presented first | p-value, test statistic (df) | Yes | No | p-value, test statistic (df) |
|
|
|||||||
| n (col %) or mean (± SD) | |||||||
|
|
|||||||
| No. of participants | 102 | 55 | 47 | – | 72 | 30 | – |
| Age (years) | 46 (± 14) | 45 (± 15) | 47 (± 13) | P = .434, t = −.79 (99) | 48 (± 14) | 42 (± 14) | P = .0969, t = −1.68 (99) |
|
| |||||||
| Gender | P = .591, x2 = .29 (1) | P = .817 | |||||
| Male | 32 (31%) | 16 (29%) | 16 (34%) | – | 22 (31%) | 10 (33%) | – |
| Female | 70 (69%) | 39 (71%) | 31 (66%) | 50 (69%) | 20 (67%) | ||
|
| |||||||
| Race | P = .532 | P = .412 | |||||
| Black/African American | 79 (77%) | 45 (82%) | 34 (72%) | – | 58 (81%) | 21 (70%) | – |
| West Indies/Caribbean | 9 (9%) | 4 (7%) | 5 (11%) | 6 (8%) | 3 (10%) | ||
| Mixed/other/no response | 14 (14%) | 6 (11%) | 8 (17%) | 8 (11%) | 6 (20%) | ||
|
| |||||||
| Ethnicity | P = .648 | P = .320 | |||||
| Hispanic | 24 (24%) | 14 (25%) | 10 (21%) | – | 15 (21%) | 9 (30%) | – |
| Non-Hispanic | 78 (76%) | 41 (75%) | 37 (79%) | 57 (79%) | 21 (70%) | ||
|
| |||||||
| Place of birth | P = .733 | P = .027 | |||||
| United States | 65 (64%) | 36 (65%) | 29 (62%) | – | 48 (67%) | 17 (57%) | – |
| West Indies/Caribbean | 31 (30%) | 17 (31%) | 14 (30%) | 23 (32%) | 8 (27%) | ||
| Africa | 5 (5%) | 2 (4%) | 3 (6%) | 1 (1%) | 4 (13%) | ||
| Other | 1 (1%) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (3%) | ||
|
| |||||||
| Relationship to the individual they accompanied | P = .964 | P = .805 | |||||
| First degree (parent/child/sibling) | 56 (55%) | 31 (56%) | 25 (53%) | – | 41 (57%) | 15 (50%) | – |
| Spouse | 25 (25%) | 13 (24%) | 12 (26%) | 17 (24%) | 8 (27%) | ||
| Other/no response | 21 (21%) | 11 (20%) | 10 (21%) | 14 (19%) | 7 (23%) | ||
|
| |||||||
| Highest level of education completed | P = .730 | P = .279 | |||||
| High school/GED or less | 29 (28%) | 15 (27%) | 14 (30%) | – | 16 (22%) | 13 (43%) | – |
| Technical/associate degree | 10 (10%) | 6 (11%) | 4 (9%) | 8 (11%) | 2 (7%) | ||
| Some college | 28 (27%) | 16 (29%) | 12 (26%) | 22 (31%) | 6 (20%) | ||
| Bachelor’s degree | 23 (23%) | 10 (18%) | 13 (28%) | 16 (22%) | 7 (23%) | ||
| Master’s degree or Doctorate | 12 (12%) | 8 (15%) | 4 (9%) | 10 (14%) | 2 (7%) | ||
|
| |||||||
| Current health insurance | P = .911 | P = .048 | |||||
| Private | 45 (44) | 26 (47%) | 19 (40%) | – | 37 (51%) | 8 (27%) | – |
| Public | 32 (31) | 16 (29%) | 16 (34%) | 17 (24%) | 15 (50%) | ||
| Both | 12 (12) | 6 (11%) | 6 (13%) | 8 (11%) | 4 (13%) | ||
| Other/unknown/unsure/no response | 12 (13) | 7 (13%) | 6 (13%) | 10 (14%) | 3 (10%) | ||
|
| |||||||
| Currently employed | P = .306, x2 = 1.05 (1) | P = .375 | |||||
| Yes | 64 (63) | 37 (67%) | 27 (57%) | – | 43 (60%) | 21 (70%) | – |
|
| |||||||
| Attitudes and experiences prior to survey | |||||||
|
| |||||||
| Considers themselves generally healthy | P = .921 | P = .334 | |||||
| Yes | 85 (83%) | 45 (82%) | 40 (85%) | – | 58 (81%) | 27 (90%) | – |
| No | 11 (11%) | 6 (11%) | 5 (11%) | 8 (11%) | 3 (10%) | ||
| Unsure | 6 (6%) | 4 (7%) | 2 (4%) | 6 (8%) | 0 (0%) | ||
|
| |||||||
| Trusts the healthcare system to do the right thing | P = .919 | P = .270 | |||||
| Disagree or neither agree/disagree | 11 (11%) | 6 (11%) | 5 (11%) | – | 10 (14%) | 1 (3%) | – |
| Somewhat agree | 48 (47%) | 27 (49%) | 21 (45%) | 34 (47%) | 14 (47%) | ||
| Strongly agree | 43 (42%) | 22 (40%) | 21 (45%) | 28 (39%) | 15 (50%) | ||
|
| |||||||
| Has heard of organ donation | P = .723 | P = .690 | |||||
| Yes | 94 (92%) | 50 (91%) | 44 (94%) | – | 67 (93%) | 27 (90%) | – |
|
| |||||||
| Has considered organ donation (n = 94) | P = .523, x2 = .41 (1) | P = .625 | |||||
| Yes | 65 (69%) | 36 (72%) | 29 (66%) | – | 45 (67%) | 20 (74% | – |
|
| |||||||
| Has considered becoming a living kidney donor (n = 94) | P = .936 | P = .494 | |||||
| Yes | 53 (56%) | 28 (56%) | 25 (57%) | – | 36 (54%) | 17 (63%) | – |
|
| |||||||
| Registered organ donor (n = 94) | P = .722 | P = .497 | |||||
| Yes | 37 (39%) | 20 (40%) | 17 (39%) | – | 27 (40%) | 10 (37%) | – |
| No | 53 (56%) | 27 (54%) | 26 (59%) | 36 (54%) | 17 (63%) | ||
| Do Not Know/Not Sure | 4 (4%) | 3 (6%) | 1 (2%) | 4 (6%) | 0 (0%) | ||
|
| |||||||
| Has heard of genetic testing | P = .343, x2 = .90 (1) | – | |||||
| Yes | 72 (71%) | 41 (75%) | 31 (66%) | – | – | – | – |
|
| |||||||
| Has considered or been approached for genetic testing (n = 72) | P = .906, x2 = .01 (1) | – | |||||
| Yes | 25 (35%) | 14 (34%) | 11 (35%) | – | – | – | – |
|
| |||||||
| Has participated in genetic testing through a healthcare provider (n = 72) | P = 1.000 | – | |||||
| Yes | 16 (22%) | 9 (22%) | 7 (23%) | – | – | – | – |
|
| |||||||
| Has participated in genetic testing through a commercial service (n = 72) | P = .227 | – | |||||
| Yes | 6 (8%) | 5 (12%) | 1 (3%) | – | – | – | – |
TABLE 2.
Views about APOL1 testing among participants who had heard of genetic testing prior to enrollment
| Survey order |
Has heard of genetic testing before this study |
||||||
|---|---|---|---|---|---|---|---|
| Characteristic | All participants | A Benefits presented first | B Drawbacks presented first | P-value, test statistic (df) | Yes | No | P-value, test statistic (df) |
|
|
|||||||
| n (col%) | |||||||
|
|
|||||||
| No. of participants | 102 | 55 | 47 | – | 72 | 30 | – |
| Desire for APOL1 testing prior to living kidney donation | |||||||
|
| |||||||
| Initial response | P = .393, x2 = .73 (1) | P = .386 | |||||
| Yes | 61 (60%) | 35 (64%) | 26 (55%) | 41 (57%) | 20 (67%) | ||
|
| |||||||
| How has the basic information about APOL1 provided affected your views on APOL1 testing as a living kidney donor? | P = .451 | P = .018 | |||||
| Less likely to want genetic testing for APOL1 | 6 (6%) | 3 (5%) | 3 (6%) | – | 1 (1%) | 5 (17%) | – |
| No change on desire to get genetic testing | 46 (45%) | 22 (40%) | 24 (51%) | – | 35 (49%) | 11 (37%) | – |
| More likely to want genetic testing for APOL1 | 50 (49%) | 30 (55%) | 20 (43%) | – | 36 (50%) | 14 (47%) | – |
|
| |||||||
| Response after presentation of benefits | P = .135, x2 = 2.23 (1) | P = .979, x2 = .001 (1) | |||||
| Yes | 58 (57%) | 35 (64%) | 23 (49%) | 41 (57%) | 17 (57%) | ||
|
| |||||||
| Response after presentation of drawbacks | P = .101, x2 = 2.69 (1) | P = .210, x2 = 1.57 (1) | |||||
| Yes | 48 (47%) | 30 (55%) | 18 (38%) | 31 (43%) | 17 (57%) | ||
|
| |||||||
| Response after presentation of benefits and drawbacks (i.e., Final response) | P = .572, x2 = .32 (1) | P = .539, x2 = .38 (1) | |||||
| Yes | 53 (52%) | 30 (55%) | 23 (49%) | 36 (50%) | 17 (57%) | ||
|
| |||||||
| Changed their response by the end of the survey | P = .965, x2 = .002 (1) | P = .632 | |||||
| Yes | 28 (27%) | 15 (27%) | 13 (28%) | 21 (29%) | 7 (23%) | ||
3.1 |. Demographics, baseline attitudes and experiences, and initial views about APOL1 testing
The mean age was 46±14 years and approximately two-thirds of participants were female (69%). Eligible participants reported having African ancestry; the majority self-identified as Black/African American (77%), 9% identified as West Indies/Caribbean, and the remaining 14% either did not answer or selected “mixed” or “Other”. In addition, 24% of the cohort also reported being of Hispanic/Latino ethnicity. Sixty-five (64%) individuals reported being born in the United States. Level of education varied widely, with nearly a third of participants (n = 29, 28%) reporting high school or less as their highest level of education. More than half of participants (55%) had both an affected first-degree relative with kidney disease.
Forty-three participants (42%) strongly agreed that they “trust the healthcare system to do the right thing”, 47% somewhat agreed, while only 11% either disagreed or neither agreed or disagreed. The majority of participants (n = 94, 92%) were aware of organ donation prior to their participation in this study. Among them, 53 individuals (56%) had considered becoming a living kidney donor themselves and 37 (39%) reported having registered as organ donors. A majority of participants (n = 72, 71%) were also aware of genetic testing in general. Among them, 35% had considered or been previously approached for genetic testing, including 22% who reported undergoing genetic testing through a healthcare provider, and 8% who had undergone genetic testing through a third-party commercial service (e.g., Ancestry.com, 23andMe). Those who had heard of genetic testing were more likely to have private insurance (51% vs. 27%, Fisher’s exact test P = .048) and to be born in the United States (67% vs. 57%, P = .027) compared to those who had not heard of genetic testing.
Following a brief paragraph about APOL1, 61 (60%) participants responded they would want APOL1 testing if they were considering kidney donation, while 41 (40%) indicated they would not want testing. Among participants aware of genetic testing prior to the survey (n = 72), the basic information about APOL1 either made them more likely to want testing (50%) or did not change their views (49%), except for one participant who reported the information made them less likely to want testing. Responses were significantly different among those who had not heard of genetic testing (n = 30), with 17% reporting that the information about APOL1 made them less likely to want testing, while only 1% of those who had previously heard of genetic testing were less likely to want testing (Fisher’s exact test P = .018).
3.2 |. Views about APOL1 testing following presentation of the potential benefits and drawbacks
After presentation of the complete educational intervention, only 53 (52%) participants responded that they would want testing (see Table 3 and Table S2 in the Supplementary Results). In total, 28 participants (27%) changed their initial response. Among them, most changed their position indicating they no longer wanted testing (n = 18/28, 64%). Participants who changed their response were younger compared to those who did not (two-sample T test, P = .006). While the change in response following presentation of the drawbacks of APOL1 test was statistically significant (McNemar’s test, P = .0124), there was no significant change after presentation of the benefits (P = .5637) and no significant net change from the beginning of the survey to the end after considering both benefits and drawbacks (P = .1306).
TABLE 3.
Demographics and experiences with living donation and genetic testing among participants who changed their initial response about APOL1 testing by study end
| Changed response from beginning to end |
||||
|---|---|---|---|---|
| Characteristic | All participants | Yes | No | P-value, test statistic (df) |
|
|
||||
| n (col %) or mean (± SD) | ||||
|
|
||||
| No. of participants | 102 | 28 | 74 | – |
| Age (years) | 46 ± 14 | 40 (± 13) | 48 (± 14) | P = .006, t = 2.79 (99) |
|
| ||||
| Gender | P = .289, x2 = 1.12 (1) | |||
| Male | 32 (31%) | 11 (39%) | 21 (28%) | – |
| Female | 70 (69%) | 17 (61%) | 53 (72%) | |
|
| ||||
| Race | P = .391 | |||
| Black/African American | 79 (77%) | 20 (71%) | 59 (80%) | – |
| West Indies/Caribbean | 9 (9%) | 4 (14%) | 5 (7%) | |
| Mixed/other/missing | 14 (14%) | 4 (14%) | 10 (14%) | |
|
| ||||
| Ethnicity | P = .115 | |||
| Hispanic | 24 (24%) | 10 (36%) | 14 (19%) | – |
| Non-Hispanic | 78 (76%) | 18 (64%) | 60 (81%) | |
|
| ||||
| Place of birth | P = .112 | |||
| United States | 65 (64%) | 20 (71%) | 45 (61%) | – |
| West Indies/Caribbean | 31 (30%) | 5 (18%) | 26 (35%) | |
| Africa | 5 (5%) | 3 (11%) | 2 (3%) | |
| Other | 1 (1%) | 0 (0%) | 1 (1%) | |
|
| ||||
| Relationship to the individual with kidney disease | P = .457 | |||
| First degree (parent/child/sibling) | 56 (55%) | 14 (50%) | 42 (57%) | – |
| Spouse | 25 (25%) | 6 (21%) | 19 (26%) | |
| Other/Missing | 21 (21%) | 8 (29%) | 13 (18%) | |
|
| ||||
| Highest level of education completed | P = .258 | |||
| High school/GED or less | 29 (28%) | 7 (25%) | 22 (30%) | – |
| Technical/associate degree | 10 (10%) | 4 (14%) | 6 (8%) | |
| Some college | 28 (27%) | 5 (18%) | 23 (31%) | |
| Bachelor’s degree | 23 (23%) | 6 (21%) | 17 (23%) | |
| Master’s degree or doctorate | 12 (12%) | 6 (21%) | 6 (8%) | |
|
| ||||
| Current health insurance | P = .481 | |||
| Private | 45 (44) | 13 (46%) | 32 (43%) | – |
| Public | 32 (31) | 10 (36%) | 22 (30%) | |
| Both | 12 (12) | 1 (4%) | 11 (15%) | |
| Other/unknown/unsure/missing | 12 (13) | 4 (14%) | 9 (12%) | |
|
| ||||
| Attitudes and experiences prior to survey | ||||
| Considers themselves generally healthy | P = .443 | |||
| Yes | 85 (83%) | 25 (89%) | 60 (81%) | – |
| No | 11 (11%) | 1 (4%) | 10 (14%) | |
| Unsure | 6 (6%) | 2 (7%) | 4 (5%) | |
|
| ||||
| Trusts the healthcare system to do the right thing | P = .203 | |||
| Disagree or Neither Agree/Disagree | 11 (11%) | 4 (14%) | 7 (9%) | – |
| Somewhat agree | 48 (47%) | 16 (57%) | 32 (43%) | |
| Strongly agree | 43 (42%) | 8 (29%) | 35 (47%) | |
|
| ||||
| Has heard of organ donation | P = .681 | |||
| Yes | 94 (92%) | 25 (89%) | 69 (93%) | |
|
| ||||
| Has considered organ donation (n = 94) | P = 1.000 | |||
| Yes | 65 (69%) | 10 (40%) | 27 (39%) | |
|
| ||||
| Has considered becoming a living kidney donor (n = 94) | P = .481 | |||
| Yes | 53 (56%) | 16 (64%) | 37 (54%) | |
|
| ||||
| Registered Organ Donor (n = 94) | P = 1.000 | |||
| Yes | 37 (39%) | 10 (40%) | 27 (39%) | – |
| No | 53 (56%) | 14 (56%) | 39 (57%) | |
| Do Not Know/Not Sure | 4 (4%) | 1 (4%) | 3 (4%) | |
|
| ||||
| Has heard of genetic testing | P = .632 | |||
| Yes | 72 (71%) | 21 (75%) | 51 (69%) | – |
|
| ||||
| Has considered or been approached for genetic testing (n = 72) | P = .280 | |||
| Yes | 25 (35%) | 5 (24%) | 20 (39%) | – |
|
| ||||
| Has participated in genetic testing through a healthcare provider (n = 72) | P = .365 | |||
| Yes | 16 (22%) | 3 (14%) | 13 (25%) | – |
|
| ||||
| Has participated in genetic testing through a commercial service (n = 72) | P = .664 | |||
| Yes | 6 (8%) | 1 (5%) | 5 (10%) | – |
3.3 |. Specific considerations influencing attitudes toward APOL1 testing
The mean individual level of responses across all benefits of testing was 3.1±1.3 and 2.7±1.2 across all drawbacks of testing (see Table 4). Participants who indicated a desire for APOL1 testing by the end of the survey reported higher levels of influence across all benefits (3.7±1.0) and across all drawbacks (3.0±1.1) compared to those who responded that they would not want testing (benefits: 2.4±1.2 and drawbacks: 2.4±1.3). These findings were significant for most factors when comparing the level of influence for each risk. Across all participants, “becoming aware of the potential risk of kidney disease among your immediate family” was the benefit with the highest mean influence (3.3±1.4), while “some transplant centers may not allow you to donate to a loved one” was the drawback with the highest mean influence (2.9±1.5). Overall, there was no significant difference in reported levels of influence of both the benefits and drawbacks between those who changed their response by the end of the survey and those who did not (P = .635 and .619, respectively).
TABLE 4.
Specific considerations influencing views toward APOL1 testing
| Final response-would want genetic testing for APOL1 before kidney donation |
Changed response from beginning to end |
||||||
|---|---|---|---|---|---|---|---|
| Level of influence of each presented benefit/drawback | All Participants | Yes | No | P-value, test statistic | Yes | No | P-value, test statistic |
|
|
|||||||
| mean (± SD) on a scale of 1-5 (none to extremely) | |||||||
|
|
|||||||
| No. of participants | 102 | 53 | 49 | – | 28 | 74 | – |
| Benefits | |||||||
| Becoming aware of your risk for kidney disease related to APOL1 | 2.88 (± 1.42) | 3.47 (± 1.24) | 2.27 (± 1.35) | P < .001, z = −4.27 | 2.75 (± 1.40) | 2.93 (± 1.44) | P = .575, z = .56 |
| Becoming more educated about potential kidney disease related to APOL1 | 3.16 (± 1.38) | 3.72 (± 1.17) | 2.55 (± 1.34) | P < .001, z = −4.20 | 3.07 (± 1.30) | 3.19 (± 1.41) | P = .662, z = .44 |
| Becoming aware of the potential risk of kidney disease among your immediate family | 3.29 (± 1.44) | 3.96 (± 1.11) | 2.57 (± 1.41) | P < .001, z = −4.82 | 3.29 (± 1.33) | 3.30 (± 1.49) | P = .847, z = .19 |
| Contributing to your current knowledge of APOL1 | (3.05 ± 1.41) | 3.72 (± 1.10) | 2.33 (± 1.36) | P < .001, z = −4.90 | 3.04 (± 1.32) | 3.05 (± 1.45) | P = .924, z = .10 |
|
| |||||||
| Drawbacks | |||||||
| You and your family members’ perception of the level of risk for kidney disease may change | 2.51 (± 1.37) | 2.74 (± 1.31) | 2.27 (± 1.41) | P = .064, z = −1.86 | 2.68 (± 1.33) | 2.44 (± 1.39) | P = .375, z = −.89 |
| You may need to share this information with the recipient who may no longer want your kidney | 2.65 (± 1.40) | 3.04 (± 1.37) | 2.22 (± 1.31) | P = .003, z = −2.96 | 2.50 (± 1.20) | 2.70 (± 1.47) | P = .577, z = .56 |
| You may have long-term financial risk with respect to your health and life insurance | 2.77 (± 1.55) | 3.02 (± 1.46) | 2.51 (1.62) | P = .071, z = −1.81 | 3.11 (± 1.50) | 2.65 (± 1.57) | P = .196, z = −1.29 |
| Some transplant centers may not allowyou to donate if you have two copies of the higher-risk forms | 2.85 (± 1.47) | 3.15 (± 1.35) | 2.53 (± 1.54) | P = .028, z = −2.20 | 2.82 (± 1.36) | 2.86 (± 1.52) | P = .869, z = .17 |
| You may feel stressed and anxious if you learned you had two higher-risk forms of the gene | 2.74 (± 1.43) | 3.00 (± 1.30) | 2.45 (± 1.53) | P = .033, z = −2.13 | 2.79 (± 1.42) | 2.75 (± 1.45) | P = .809, z = −.24 |
|
| |||||||
| Average individual level of response | |||||||
| Across all benefits | 3.10 (± 1.30) | 3.72 (± 1.27) | 2.43 (± 1.24) | P < .001, z = −5.02 | 3.04 (± 1.16) | 3.12 (± 1.36) | P = .635, z = .48 |
| Across all drawbacks | 2.71 (± 1.24) | 3.00 (± 1.08) | 2.40 (± 1.34) | P = .016, z = −2.41 | 2.78 (± 1.18) | 2.68 (± 1.27) | P = .619, z = −.50 |
Notes: “Final response” corresponds to participants response after presentation of both the benefits and drawbacks; Comparisons between participants who wanted testing or not, and participants who changed their initial responses or not, were made using the Wilcoxon rank-sum test.
4 |. DISCUSSION
In this study, we examined how providing written education influenced views on APOL1 testing among potential, or hypothetical living kidney donors with a positive family history of kidney disease and self-reported African ancestry. We also asked participants to rate the level of influence each of the presented benefits and drawbacks had on their desire for APOL1 testing.
The majority of participants were aware of organ donation, with more than half having considered living donation, and more than two-thirds of participants were familiar with genetic testing prior to enrollment. At the start of our study, more than half of participants were interested in APOL1 testing. However, there was no significant change in response following presentation of the potential benefits and the drawbacks of APOL1 testing by study end. Overall, there did not appear to be meaningful associations between interest in APOL1 testing and participants’ educational level, awareness of living organ donation, prior experience with genetic testing, level of trust in the healthcare system, and the order in which the benefits and drawbacks of APOL1 testing were presented to them.
Genetic testing is historically underutilized by minority populations, reflecting inequities in health-care access and concerns about its applications.27,28 Efforts to operationalize APOL1 testing directly impacts individuals from Black communities, who have endured abuses done to them in the name of research and medicine.29,30 Therefore, it is paramount that transplant centers that offer APOL1 testing to their prospective living donors can ensure their informed consent.31–33 And, because clinicians often lack the time and resources to provide comprehensive pre-test counseling,13 there is a need for novel ways of delivering the requisite information in order to promote shared decision-making between prospective living donors and their providers.11,34
Similar to prior community-based studies, including one which asked participants hypothetical questions about APOL1 testing in transplantation,22 we also found broad general interest for APOL1 testing among our participants.21,24,25 And, like other studies, participants in our cohort also had varying levels of trust in the healthcare system.35–38 Studies on attitudes toward genetic testing among African Americans have shown that the perceived benefits of knowing the genetic results outweigh higher levels of mistrust and contributes to greater interest in genetic testing.39–41 However, assessing the personal benefits of APOL1 testing is complex. It relies on an appreciation of seemingly counterintuitive degrees of risk associated with APOL1, along with the unknown risk to a person with two risk alleles who undergoes donor nephrectomy, as well as an awareness for the potential negative consequences (i.e., drawbacks) of undergoing testing, and/or, learning the results- which can extend beyond the individual who undergoes the testing (e.g., preclude donation, lead to loss of privacy, discrimination, stigmatization and psychological harm, etc.). Though prior community-based qualitative studies have identified some of the factors that influence views on APOL1 testing among African American communities,21,24,25 little is known about how providing information about the benefits and drawbacks of APOL1 testing influences the desire for testing among prospective living donors.
Unlike these earlier studies, we set out to quantify the extent each of the presented benefits and drawbacks influenced participants decisions on testing. Across all participants, the benefit with the highest influence was “becoming aware of the potential risk of disease among your immediate family”, while “becoming aware of your risk for kidney disease that is related to this gene” was the lowest. In addition, we also found that the drawback with the greatest influence was “some transplant centers may not allow you to donate to a loved one”. Together, this suggests that although awareness of one’s own hereditary predisposition to kidney disease (i.e., the personal benefit of undergoing APOL1 testing) may be an important factor, we found that concern for how the information may affect family members was potentially more influential in the desire for testing across all participants. In addition to highlighting the limitations of using written, one-size-fits-all, educational content, our findings support the need for further research into whether customized educational approaches focused on addressing concerns about the implications of testing on family members, are able to address the needs of potential living donors and ensure their informed decision on APOL1 testing. Ultimately, our study’s findings are a reminder of the importance of providing culturally-sensitive, comprehensive pre-test education to all individuals who are offered genetic testing.13,42–44
There are many strengths in our study. We evaluated views about APOL1 testing in the context of living donation among a cohort of individuals with a positive family history of kidney disease and self-reported African ancestry. Using an easy-to-read educational intervention, derived from common themes in the literature, we also assessed how the presented content changed perceptions about testing. Limitations of this study include that the survey was conducted at one academic center, and the survey did not undergo independent validation although we employed an iterative process in developing it. In addition, the questions posed with regards to living donation were hypothetical in nature, similar to a recent study about APOL1 testing in transplantation by Berrigan et al.22 Although, more than half of participants in our study both had an affected first-degree relative with kidney disease and had considered living donation, making them more likely to face APOL1 testing as part of a clinical evaluation, compared to community dwelling participants in their study. Finally, while some would suggest the use of a correction for multiple hypothesis testing, we have chosen not to do so in our analysis given the limited number of hypotheses being tested and to avoid inflating the risk of a type II error among other considerations.45,46
Ultimately, further empirical work is needed to more fully understand the interplay between attitudes toward APOL1 testing among prospective living donors from Black communities and varying degrees of trust in healthcare systems, as well as on the potential impact of genomic literacy and numeracy when evaluating the benefits and drawbacks of APOL1 testing. This requires greater engagement and collaboration with Black communities, along with efforts to gain a deeper understanding of the priorities and informational needs of individuals from at-risk communities, and the additional factors that influence decision-making about organ donation and APOL1 testing. Together, these efforts will inform the design and development of dynamic, customizable, educational approaches that can ensure individuals offered genomic testing have the requisite knowledge to provide informed consent, and facilitate broader implementation of APOL1 testing in prospective living donors.
Supplementary Material
ACKNOWLEDGMENTS
The project was supported by grants from the National Institutes of Health through grants R01-MD014161 (JMD, SM, ASI, DS), U01-DK116041 (ASI), U01-DK116042 (JMD), U01-DK116066 (SM, DS), TL1TR001875 (JGN), KL2TR001874 (JGN), the National Kidney Foundation’s Young Investigator Award (JGN) and the Mid-America Transplant Foundation (TJM).
Footnotes
CONFLICT OF INTEREST
None.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Freedman BI, Kopp JB, Langefeld CD, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol. 2010;21:1422–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nadkarni GN, Gignoux CR, Sorokin EP, et al. Worldwide frequencies of APOL1 renal risk variants. N Engl J Med. 2018;379:2571–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman BI, Julian BA, Pastan SO, et al. Apolipoprotein L1 gene variants in deceased organ donors are associated with renal allograft failure. Am J Transplant. 2015;15:1615–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reeves-Daniel AM, Depalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11:1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman BI, Pastan SO, Israni AK, et al. APOL1 genotype and kidney transplantation outcomes from deceased african american donors. Transplantation. 2016;100:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doshi MD, Ortigosa-Goggins M, Garg AX, et al. APOL1 genotype and renal function of black living donors. J Am Soc Nephrol. 2018;29:1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locke JE, Sawinski D, Reed RD, et al. Apolipoprotein L1 and chronic kidney disease risk in young potential living kidney donors. Ann Surg. 2018;267:1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mcintosh T, Mohan S, Sawinski D, Iltis A, Dubois JM. Variation of ApoL1 testing practices for living kidney donors. Prog Transplant. 2020;30:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newell KA, Formica RN, Gill JS, et al. Integrating APOL1 gene variants into renal transplantation: considerations arising from the american society of transplantation expert conference. Am J Transplant. 2017;17:901–911. [DOI] [PubMed] [Google Scholar]
- 10.Garg N, Lentine KL, Inker LA, et al. The kidney evaluation of living kidney donor candidates: uS practices in 2017. Am J Transplant. 2020;20:3379–3389. [DOI] [PubMed] [Google Scholar]
- 11.Ross LF, Thistlethwaite JR. Introducing genetic tests with uncertain implications in living donor kidney transplantation: apoL1 as a case study. Prog Transplant. 2016;26:203–206. [DOI] [PubMed] [Google Scholar]
- 12.Groopman EE, Marasa M, Cameron-Christie S, et al. Diagnostic utility of exome sequencing for kidney disease. N Engl J Med. 2019;380:142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nestor JG, Marasa M, Milo-Rasouly H, et al. Pilot study of return of genetic results to patients in adult nephrology. Clin J Am Soc Nephrol. 2020;15:651–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cocchi E, Nestor JG, Gharavi AG. Clinical genetic screening in adult patients with kidney disease. Clin J Am Soc Nephrol. 2020;15:1497–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grams ME, Rebholz CM, Chen Y, et al. APOL1 risk, and eGFR decline in the general population. J Am Soc Nephrol. 2016;27:2842–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parsa A, Kao WHL, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lunsford SL, Simpson KS, Chavin KD, et al. Racial disparities in living kidney donation: is there a lack of willing donors or an excess of medically unsuitable candidates?. Transplantation. 2006;82:876–881. [DOI] [PubMed] [Google Scholar]
- 18.Garg N, Lentine KL, Inker LA, et al. Metabolic, cardiovascular, and substance use evaluation of living kidney donor candidates: US practices in 2017. Am J Transplant. 2020;20:3390–3400. [DOI] [PubMed] [Google Scholar]
- 19.Kumar K, Tonascia JM, Muzaale AD, et al. Racial differences in completion of the living kidney donor evaluation process. Clin Transplant. 2018;32:e13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lentine KL, Mandelbrot D. Addressing disparities in living donor kidney transplantation: a call to action. Clin J Am Soc Nephrol. 2018;13:1909–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umeukeje EM, Young BA, Fullerton SM, et al. You are just now telling us about this? African American perspectives of testing for genetic susceptibility to kidney disease. J Am Soc Nephrol. 2019;30:526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berrigan M, Austrie J, Fleishman A, et al. Opinions of African American adults about the use of apolipoprotein L1 (ApoL1) genetic testing in living kidney donation and transplantation. Am J Transplant. 2021;21:1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young BA, Fullerton SM, Wilson JG, et al. Clinical genetic testing for APOL1: are we there yet?. Semin Nephrol. 2017;37:552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon EJ, Amórtegui D, Blancas I, Wicklund C, Friedewald J, Sharp RR. African American living donors’ attitudes about APOL1 genetic testing: a mixed methods study. Am J Kidney Dis. 2018;72:819–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon EJ, Amórtegui D, Blancas I, Wicklund C, Friedewald J, Sharp RR. A focus group study on african american living donors’ treatment preferences, sociocultural factors, and health beliefs about apolipoprotein L1 genetic testing. Prog Transplant. 2019;29:239–247. [DOI] [PubMed] [Google Scholar]
- 26.Mohan S, Iltis AS, Sawinski D, Dubois JM.APOL1 genetic testing in living kidney transplant donors. Am J Kidney Dis. 2019:538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hann KEJ, Freeman M, Fraser L, et al. Awareness, knowledge, perceptions, and attitudes towards genetic testing for cancer risk among ethnic minority groups: a systematic review. BMC Public Health. 2017;17:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halbert CH, Mcdonald JA, Magwood G, Jefferson M. Beliefs about genetically targeted care in African Americans. J Natl Med Assoc. 2017;109:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lea DH, Kaphingst KA, Bowen D, Lipkus I, Hadley DW. Communicating genetic and genomic information: health literacy and numeracy considerations. Public Health Genomics. 2011;14:279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaphingst KA, Stafford JD, Mcgowan LD, Seo J, Lachance CR, Goodman MS. Effects of racial and ethnic group and health literacy on responses to genomic risk information in a medically underserved population. Health Psychol. 2015;34:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Directors ABO. Points to consider in the clinical application of genomic sequencing. Genet Med. 2012;14:759–761. [DOI] [PubMed] [Google Scholar]
- 32.Kaphingst K, Facio F, Cheng M-R, et al. Effects of informed consent for individual genome sequencing on relevant knowledge. Clin Genet. 2012;82:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lerman C, Kerner J, Gomez-Caminero A, et al. Controlled trial of pretest education approaches to enhance informed decision-making for BRCA1 gene testing. J Natl Cancer Inst. 1997;89:148–157. [DOI] [PubMed] [Google Scholar]
- 34.Delikurt T, Williamson GR, Anastasiadou V, Skirton H. A systematic review of factors that act as barriers to patient referral to genetic services. Eur J Hum Genet. 2015;23:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armstrong K Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. Jama. 2005;293:1729–1736. [DOI] [PubMed] [Google Scholar]
- 36.Thompson HS, Valdimarsdottir HB, Jandorf L, Redd W. Perceived disadvantages and concerns about abuses of genetic testing for cancer risk: differences across African American, Latina and Caucasian women. Patient Educ Couns. 2003;51:217–227. [DOI] [PubMed] [Google Scholar]
- 37.Suther S, Kiros G-E. Barriers to the use of genetic testing: a study of racial and ethnic disparities. Genet Med. 2009;11:655–662. [DOI] [PubMed] [Google Scholar]
- 38.Sheppard VB, Mays D, Tercyak KP, Laveist T. Medical mistrust influences black women’s level of engagement in BRCA 1/2 genetic counseling and testing. J Natl Med Assoc. 2013;105:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hensley Alford S, Mcbride CM, Reid RJ, Larson EB, Baxevanis AD, Brody LC. Participation in genetic testing research varies by social group. Public Health Genom. 2011;14:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams I,Christopher J, Williams KP, Sheppard VB. What black women know and want to know about counseling and testing for BRCA1/2. J Cancer Educ. 2015;30:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lerman C, Hughes C, Benkendorf JL, et al. Racial differences in testing motivation and psychological distress following pretest education for BRCA1 gene testing. Cancer Epidemiol Biomarkers Prev. 1999;8:361–367. [PubMed] [Google Scholar]
- 43.Rodriguez EM, Saad-Harfouche FG, Miller A, et al. Engaging diverse populations in biospecimen donation: results from the Hoy y Manana study. J Community Genet. 2016;7:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gustafson SL, Gettig EA, Watt-Morse M, Krishnamurti L. Health beliefs among African American women regarding genetic testing and counseling for sickle cell disease. Genet Med. 2007;9:303–310. [DOI] [PubMed] [Google Scholar]
- 45.Althouse AD. Adjust for multiple comparisons? It’s not that simple. Ann Thorac Surg. 2016;101:1644–1645. [DOI] [PubMed] [Google Scholar]
- 46.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


