Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of the coronavirus disease 2019 (COVID-19), and its variants have brought unprecedented impacts to the global public health system, politics, economy, and other fields. Although more than ten COVID-19 specific vaccines have been approved for emergency use, COVID-19 prevention and control still face many challenges. Bacille Calmette–Guérin (BCG) is the only authorized vaccine used to fight against tuberculosis (TB), it has been hypothesized that BCG may prevent and control COVID-19 based on BCG-induced nonspecific immune responses. Herein, we summarized: 1) The nonspecific protection effects of BCG, such as prophylactic protection effects of BCG on nonmycobacterial infections, immunotherapy effects of BCG vaccine, and enhancement effect of BCG vaccine on unrelated vaccines; 2) Recent evidence of BCG's efficacy against SARS-COV-2 infection from ecological studies, analytical analyses, clinical trials, and animal studies; 3) Three possible mechanisms of BCG vaccine and their effects on COVID-19 control including heterologous immunity, trained immunity, and anti-inflammatory effect. We hope that this review will encourage more scientists to investigate further BCG induced non-specific immune responses and explore their mechanisms, which could be a potential tool for addressing the COVID-19 pandemic and COVID-19-like “Black Swan” events to reduce the impacts of infectious disease outbreaks on public health, politics, and economy.
Keywords: COVID-19, Vaccines, SARS-CoV-2, Bacille Calmette-Guérin (BCG), Immunity, Black Swan events
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic is an infectious respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-COV-2). Since the end of 2019, the global public health system, economy, politics, and culture have been profoundly affected by COVID-19 [1]. Scientists generally consider the COVID-19 pandemic to be a “black swan” event, which often has broad and far-reaching effects and can only be recognized and understood with hindsight [2]. According to the World Health Organization (WHO) website (https://covid19.who.int/), as of May 3, 2022, there were 511,965,711 confirmed COVID-19 cases and 6,240,619 deaths worldwide.
Currently, although WHO has approved more than ten vaccines for emergency use (https://www.who.int/teams/regulation-prequalification/eul/COVID-19), and dozens of vaccines are approved by different countries, the spread of the COVID-19 pandemic is still not effectively contained [3], [4], [5], [6]. More notably, SARS-CoV-2 variants keep emerging and may break through the protection of existing vaccines [7], [8], [9]. For instance, researchers found that the SARS-CoV-2 Omicron variant (B.1.1.529) was undetectable in most participants vaccinated with mRNA-1273, BNT162b, or Ad26.COV2.S vaccines [10], [11]. Before the COVID-19 specific vaccines were available, some existing vaccines, such as Bacille Calmette-Guérin (BCG), measles vaccine (MV), oral polio vaccine (OPV), and Measles-Mumps-Rubella (MMR), had started to be repurposed to prevent SARS-CoV-2 infection [12], [13]. BCG is a live attenuated strain derived from Mycobacterium bovis in 1921 and has been widely used for preventing tuberculosis (TB) in children globally [5], [14]. It not only reduces the risk of TB but also protects against many nonmycobacterial infections, especially respiratory virus infections [15], making it a focus as a potential tool against COVID-19 and COVID-19-like “Black Swan” incidents.
Nowadays, numerous studies have been published to evaluate the effects of BCG vaccination on COVID-19 control, either for or against. However, most previous reviews cited these studies without systematically classifying the study types or methods, and actual effects are still elusive. Furthermore, most reviews usually emphasize heterologous immunity and trained immunity involving nonspecific protection mechanisms. However, recent studies have proved only these two mechanisms are not enough to explain the nonspecific protection of BCG on COVID-19 control, and scientists have begun to turn their attention toward the anti-inflammatory effect of BCG on fighting against COVID-19, which was scarcely mentioned in the previous reviews. In addition, as the COVID-19 pandemic continues, different opinions and studies, especially some important clinical trials and animal experiments, continue to be updated. So, an updated review is still needed.
Herein, we summarized BCG-induced nonspecific immune responses and their underlying mechanisms and discussed the potential roles of the BCG vaccine in protective effects against other pathogens, preventing and controlling COVID-19, and dealing with black swan events similar to COVID-19.
2. Nonspecific protection effects of BCG
Vaccine-induced heterologous or nonspecific effects (known as off-target effects) can protect against pathogens other than their intended specific pathogen [16]. Vaccines, including BCG, OPV, MV, smallpox vaccine, and yellow fever vaccine, are believed to have nonspecific effects [13]. Originally, BCG was introduced to prevent TB, leprosy, and Buruli ulcer disease [13]. However, it’s believed that BCG is only moderately efficacious against pulmonary TB, especially in adults, where the efficacy is limited [17]. Interestingly, BCG vaccine was epidemiologically associated with reduced infant mortality independent of its effect on TB [18]. Several observational studies were performed to confirm the protective efficacy of BCG. As a result, a 50% reduction in overall mortality was observed in children in West Africa [19], [20], [21], similarly, a 48% reduction in neonatal mortality was found in infants with low birth weight in Guinea-Bissau [22], [23]. By analyzing the above data, it is not difficult to see that children and infants' mortality decreased by approximately 50% after BCG vaccination. BCG's protection can hardly explain this data against TB alone, which may be related to BCG's protection against unrelated infectious agents (including viruses, bacteria, and protozoa) [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45] (Table 1 ). These beneficial effects have been considered to be related to the nonspecific effects, including prophylactic protection effects, immunotherapy effects, as well as enhancement effects on other vaccines.
Table 1.
Overview of the role of BCG-induced specific cellular immunity against pathogens or diseases in clinical studies.
| Pathogens or diseases | Research subjects | Study type | Effects of BCG | The specific cellular immunity of BCG | Ref |
|---|---|---|---|---|---|
| Yellow fever virus | healthy Dutch male subjects (n = 30) | RCT | Induced epigenetic reprogramming in human monocytes, functional reprogramming, and protection against non-related viral infections | Reduction of viremia was highly correlated with the upregulation of IL-1β | [26] |
| Influenza A (H1N1) | Healthy volunteers (n = 40) | RCT | Resulted in a more pronounced increase and accelerated induction of functional antibody responses against the 2009 pandemic influenza A(H1N1) vaccine strain | Enhanced antibody production and more sustained IFN-γ production | [27] |
| HSV | Patients with genital herpes (n = 155) | RCT | Recurrence over 9 months of follow-up was 0.528 recurrences per month compared with 0.392 in placebo recipients | NA | [28] |
| HPV | Children with common and plane warts (n = 80) | Case series | Improved clearance of viral warts | The activation of CD4 lymphocytes and an increase in IL-1, IL-2, and TNF-α | [29] |
| RRP patients (n = 15) | Case-control | Improved clearance of viral warts | Potentiating Treg differentiation, limiting hyperactivation and virus-specific T cell clones depletion, and restoring a Th1 cytokine background. | [30] | |
| Patients with warts (n = 60) | RCT | Improved clearance of viral warts | NA | [31] | |
| Patients with warts (n = 190) | Non-randomized controlled trial | More effective than topical salicylic acid for treating viral warts | NA | [32] | |
| RSV | Infants (n = 386) | Case-control | A beneficial effect of BCG vaccination for girls | NA | [33] |
| AURTI | Patients with AURTI (n = 34) | RCT | BCG vaccinations in elderly significantly prevent the AURTI | Increase the IFN-γ level as Th1 response and IL-10 as Treg response | [34] |
| RTI | Elderly patients (n = 198) | RCT | Prolonged the time to the new infections, and the incidence of new infections in volunteers vaccinated with BCG or placebo was 25.0% or 42.3% | Production of TNF-α, IL-1β, and IL-10 induced by non-mycobacterial ligands was higher in BCG group than that in placebo group | [35] |
| COVID-19 | Individuals aged 3 months to 75 years (n = 46,889) | RCT | Little evidence of any beneficial effect of BCG revaccination on all-cause mortality | NA | [36] |
| Genital warts | Patients with genital warts (n = 50) | Clinical Trial | BCG had an 80% success rate in treating genital warts with no significant side effects and no recurrence | NA | [37] |
| CIS | Patients with CIS (n = 700) | Meta-Analysis | Intravesical BCG significantly reduces the risk of short and long-term treatment failure compared with intravesical chemotherapy | NA | [38] |
| AD and PD | Patients with BC (n = 12,185) | Multi-center retrospective cohort study | Bladder cancer immunotherapy by BCG is associated with a significantly reduced risk of Alzheimer's Disease and Parkinson's Disease | NA | [39] |
| Cancer | American Indian and Alaska Native schoolchildren | Multi-center retrospective cohort study | The rate of lung cancer was significantly lower in BCG vs placebo recipients | NA | [40] |
| NL and NHL | Subjects (n = 400,611) | Retrospective cohort study | No association was observed between BCG vaccination and either HL or NHL | NA | [41] |
| Cancer | Children aged 1 to 18 years (n = 77,972) | Clinical Trial | BCG vaccination had no protective effect on the subsequent development of cancer | NA | [42] |
| T1D | Participants with or without T1D (n = 282) | Clinical Trial | Long-term and stable blood sugar reduction with BCG vaccinations | BCG-induced demethylation causes increased mRNA expression of the Treg signature genes, such as Foxp3, TNFRSF18, IL2RA, IKZF2, IKZF4, and CTLA4 | [43] |
| T1D | Adults aged 18 to 50 years (n = 85) | RCT | BCG treatment modified the autoimmunity that underlies T1D by stimulating the host innate immune response and has value in the treatment of long-term diabetes | Induction of regulatory T cells, resulting in insulin autoreactive T cell death by stimulating TNF | [44] |
| Typhoid fever | Healthy adult volunteers (n = 30) | Clinical trial | Prior BCG vaccination does not increase adaptive response to TFV | Induced a significant increase in IL-22, IFN-γ, IL-1β, and IL-6 production | [45] |
AD, Alzheimer's disease; AURTI, acute upper respiratory tract infection; BC, bladder cancer; BCG, Bacille Calmette–Guérin; CIS, carcinoma in situ; HL, Hodgkin's lymphoma; HPV, human papillomavirus; HSV, Herpes simplex virus; IFN, interferon; IL, interleukin; NHL, non-Hodgkin's lymphoma; PD, Parkinson's disease; RCT, Randomized Controlled Trial; RRP, Recurrent respiratory papillomatosis; RTI, Respiratory Tract Infections; T1D, type 1 diabetes; TFV, Typhoid fever vaccine.
2.1. Prophylactic protection effects of BCG on nonmycobacterial infections
In animals, BCG vaccination protects against infections of various pathogens, including viruses and bacteria [24]. It protected against influenza virus infection by enhancing efferocytosis and the capacity of macrophages to reduce viral titers of influenza A virus in mice [46], [47]. Muramyl dipeptide (MDP), a peptidoglycan fragment derived from the cell wall component of mycobacteria, could protect against infections of several viruses by triggering the immune responses in mice, such as herpes simplex virus type 2 (HSV-2), vaccinia virus, and Sendai virus [48], [49]. Moreover, previous studies have reported that BCG has a potential ability to induce protection or enhanced resistance against a large number of viruses and bacteria in animal models, including herpes simplex type 1, HSV-2, Japanese encephalitis virus, encephalomyocarditis virus, ectromelia virus, Staphylococcus aureus, M. fortuitum, Salmonella enteritidis, and Klebsiella pneumonia [50], [51], [52], [53], [54], [55], [56], [57], [58], [59].
Clinical studies have been conducted on children or elderly people. BCG vaccination in children improved survival independently of TB prevention as mentioned above. In Spain, the non-TB hospital admissions in infants vaccinated with BCG reduced by 32% for respiratory infections and 53% for sepsis, respectively [60], which could have been due to an effect of BCG on bacterium or virus infection. In Guinea-Bissau, where a significant reduction in neonatal mortality was observed, as mentioned above, BCG vaccination significantly reduced the morbidity of acute lower respiratory tract infection caused by respiratory syncytial virus (RSV) in a case-control study performed in young children [61]. In clinical trials involving older adults, BCG significantly reduced the incidence of acute upper respiratory tract infections and the risk of pneumonia in tuberculin-negative older adults compared with placebo [34], [62]. A recent phase III trial (ACTIVATE, ClinicalTrials.gov NCT03296423) assessed the heterologous effects of BCG vaccination in the elderly (n = 198). Its interim analysis indicated that BCG vaccination dramatically prolonged the time to the new infections, and the incidence of new infections in volunteers vaccinated with BCG or placebo was 25.0% (95% CIs 16.4%-36.1%) or 42.3% (95% CIs 31.9%-53.4%), respectively [35]. Actually, the prophylactic protection effects on acute upper and lower respiratory tract infections and pneumonia in elderly people have encouraged scientists to further validate its role in preventing COVID-19, for elderly people infected by SARS-CoV-2 performed a higher mortality rate due to their underlying diseases [63].
A recent study assessed all-cause mortality following a large BCG revaccination trial between January 1986 and November 1989 in Malawi and didn’t find a beneficial effect of BCG revaccination on all-cause mortality in the 30-year follow-up [36], which may be due to the high proportion of deaths attributable to non-infectious causes as well as the long-time interval since BCG revaccination. In addition, there are sporadic reports that BCG also has a non-specific protective effect on protozoan infections such as Leishmania donovani, Eimeria maxima, Eimeria tenella, and malaria [64], [65], [66], [67]. Still, the relevant data are few, and further studies are needed.
2.2. Immunotherapy effects of BCG vaccine
BCG's nonspecific immunotherapy has effectively treated virus infection, cancer, and autoimmune diseases. For example, in a placebo-controlled trial conducted in Egypt, 65% of children with common warts caused by human papillomavirus (HPV) showed resolution when treated with viable topical BCG [29]. Another placebo-controlled study in Egypt showed that BCG had an 80% success rate in treating genital warts after a maximum of six BCG applications, with no significant side effects and no recurrence [37]. A similar result was observed in another clinical trial in India, in which 28.6% and 48.5% of the patients performed a complete clearance of viral warts after one single BCG vaccination [68] and three BCG vaccinations [31], respectively. In cancer treatment, BCG could reduce the growth of subcutaneously transplanted tumors in mice [69], [70]. As an immunomodulator, BCG has been used to treat human bladder cancer to reduce the risk of recurrence [38]. In addition, BCG vaccination has shown benefits in treating various autoimmune diseases (AIDs), including Alzheimer’s disease, multiple sclerosis (MS), and diabetes [71]. It has been reported that BCG could restore glycemic control in T1D patients by using a repeat vaccination strategy [72]. Moreover, a single BCG vaccination could decrease the characteristic magnetic resonance imaging activity in patients with MS [73], [74].
2.3. Enhancement effects of BCG vaccine on unrelated vaccines
BCG may also improve vaccines' response against viral or bacterial infections. Actually, Freund's Complete Adjuvant, one of the most commonly used adjuvants in immune-related animal experiments, contains the cell wall component of M. tuberculosis and has a potent ability to enhance the immune effects of antigens. Therefore, it’s not unanticipated that the BCG vaccine enhances immune responses of other unrelated vaccines. Furthermore, a previous study reported that single administration of typhoid fever vaccine (TFV) induced a significantly higher level of anti-inflammatory cytokine IL-10, interestingly, this effect can be reversed by using a prime-boost strategy with BCG and TFV, indicating that BCG vaccination could decrease pro-inflammatory responses of TFV vaccination [45]. Similarly, a prime-boost strategy with BCG and hepatitis B vaccine markedly enhanced the levels of humoral and cellular immune responses induced by a single administration of the hepatitis B vaccine [75], [76].
BCG vaccination prior to other vaccines vaccination has similar effects to co-administration. In 2013, a clinical trial conducted in Australia investigated the non-specific impacts of the BCG vaccine, and results suggested that the geometric mean concentrations of antibodies against pneumococcal, Haemophilus influenzae type B, and tetanus toxoid were significantly higher in the BCG-immunized individuals than that in the non-BCG-immunized individuals [77].
The adjuvant effect of BCG on unrelated vaccines makes it possible to act against these infections [15]. Thus, the enhancement ability of BCG on unrelated vaccines has drawn scientists’ attention to develop a BCG-based vaccination against COVID-19. In a recent animal study, the combination of BCG and spike antigen of SARS-CoV-2 induced high-titer neutralizing antibodies, and Th1 type cellular immune response, resulting in mild symptoms, minimal inflammation, and undetectable viral loads in the lungs of K18-hACE2 mice infected by B.1.1.7 and B.1.351 SARS-CoV-2 variants after a single dose of BCG:CoVac vaccination [78]. These findings demonstrate that BCG-based vaccination may play roles in protecting against SARS-CoV-2 variants infection [78].
3. Is the BCG vaccine effective against SARS-CoV-2 infection?
In the early stages of the COVID-19 pandemic, due to the lack of safe and effective vaccines and effective treatment drugs and protocols, researchers and public health experts recommended using existing licensed vaccines or medicines to treat COVID-19, such as vaccines (BCG, MMR, OPV, and influenza vaccine) [12], [13], [79], antimicrobials (didanosine and hydroxychloroquine didanosine) [80], [81], antibiotics (azithromycin) [82], or antiviral drugs (lopinavir/ritonavir, favipiravir, and remdesivir) [83]. Among them, BCG has received the most attention. A growing number of studies have suggested that BCG plays a protective role in fighting against COVID-19 [84], [85], but the controversy about its true efficacy in COVID-19 control still exists [86]. Therefore, it’s necessary to discuss and distinguish its clear roles in COVID-19 control. This section will highlight recent advances in the nonspecific protection effects of BCG against COVID-19, including ecological studies, analytical studies (mainly case-control and cohort studies), clinical trials, and animal experiments, providing new insights for further clarification and resolution of the controversy. In the literature searches (Fig. 1 ), we used a Boolean Operations “(COVID-19 OR SARS-CoV-2) AND (BCG vaccination OR BCG vaccine) NOT review[pt]” in the PubMed database (http://pubmed.ncbi.nlm.nih.gov) to collect all literature except reviews concerning SARS-CoV-2 or COVID-19 and BCG vaccine. Some unrelated publications with the theme or publication types of meta-analysis, case report, clinical trial protocol, and comments were excluded manually. The remaining publications (n = 54) were divided into ecological studies, analytical studies, clinical trials, and animal experiments for separate review (Table 2 ).
Fig. 1.
Flow chart of the literature search strategy.
Table 2.
A brief summary of the studies involving the BCG vaccination effects on COVID-19 control.
| Study types | Objects | BCG vaccination effect | Conclusions | Ref |
|---|---|---|---|---|
| Ecological study* | Global study | Middle high and high-income countries with a universal BCG policy had significantly lower COVID-19-related mortality and morbidity than those never had a universal BCG policy, with 0.78 ± 0.40 (mean ± s.e.m) vs. 16.39 ± 7.33 deaths per million people (P = 8.64e-04) and 59.54 ± 23.29 vs. 264.90 ± 134.88 cases per million inhabitants (P = 0.0064). | Positive: Countries without universal policies of BCG vaccination were more severely affected by COVID-19 compared to countries with universal and long-standing BCG policies, suggesting broad BCG vaccination along with other measures could slow the spread of COVID-19. | [85] |
| Ecological study | Global study | The morbidity and mortality of COVID-19 in countries with BCG vaccination recommendation were significantly lower than these in countries without BCG vaccination recommendation (P < 0.05 and P < 0.001, respectively). | Positive: The study supports the hypothesis that BCG vaccination is beneficial in reducing the morbidity and mortality of COVID-19, however, many confounders might affect the accuracy. | [14] |
| Ecological study | 135 countries | Linear mixed models revealed both the growth rates of COVID-19 cases and COVID-19–related deaths were significantly slower in countries with mandated BCG vaccinations compared to countries without them (P < 0.001). | Positive: Mandated BCG vaccination can be effective in the fight against COVID-19. | [87] |
| Ecological study | Global study | A strong correlation between the BCG index (degree of universal BCG vaccination deployment in a country) and COVID-19 mortality in different socially similar European countries was observed after mitigating effects of potentially confounding factors. Every 10% increase in the BCG index was associated with a 10.4% reduction in COVID-19 mortality. | Positive: BCG vaccination could have a protective effect against COVID-19. | [88] |
| Ecological study | 186 countries | Countries with universal BCG vaccine had significantly lower total infection and mortality rates, with 0.2979 vs. 3.7445 and 0.0077 vs. 0.0957 per 1000 people, respectively (P < 0.001). | Positive: Countries with universal BCG vaccine had significantly lower total infection and mortality rates caused by COVID-19, suggesting that BCG vaccination could reduce the infection. | [106] |
| Ecological study | Strictly selected countries | BCG vaccination for the preceding 15 years could reduce COVID-19-related deaths per million by 71% (95% CI 53–89%). | Positive: A consistent association between countries with a BCG vaccination for the preceding 15 years and COVID-19 related mortality existed. | [94] |
| Ecological study | 139 countries | The countries having a current BCG policy had a significantly lower mortality rate relative to the countries with a past BCG policy or a BCG policy only for special groups (P < 0.001). | Positive: The presence of current BCG policy rather than the type of strain used in the vaccination program was associated with decreased COVID-19-related disease burden. | [104] |
| Ecological study | Global study | Cases per million inhabitants, deaths per million inhabitants and mortality rates were significantly lower in countries with BCG with vaccination schedule than those without (P < 0.01). | Positive: Countries where BCG vaccination was given at birth showed a lower contagion rate and fewer COVID-19-related deaths, suggesting the BCG vaccine’s protection against COVID-19. | [89] |
| Ecological study | 171 countries | A 30-fold decrease of COVID-19 mortality per population. | Positive: Countries with current universal pediatric BCG policy were associated decrease of COVID-19 mortality compared to countries without the policy. | [109] |
| Ecological study | Countries with at least 500 or 1 000 cases | Both cases/million and deaths/population in countries with a national BCG vaccination program were statistically significantly lower than those that did not have/ceased their national BCG vaccination programs (P < 0.0001 and P < 0.01, respectively). | Positive: The lower than expected number of COVID-19 cases in countries might stem from the BCG immunization induced heterologous protection. | [91] |
| Ecological study | European countries or regions | Using least squares regression and a robust standard error algorithm, the authors found a significant effect exerted by the BCG (P < 0.0005) | Positive: The study confirmed an association between BCG-positive vaccination policy and lower death rates from COVID-19. Implementing BCG vaccination policy might have a significant impact on the control of SARS-CoV-2 epidemic. | [108] |
| Ecological study | Global study | The rate ratio of the cumulative COVID-19 mortality/million was 2.70 per 1 unit decrease in the incidence rate of tuberculosis (per 100,000 people). This association existed even after adjusting for potential confounders. | Positive: An inverse relationship existed between the past epidemic indicators of M. tuberculosis and current COVID-19 impact. | [103] |
| Ecological study | Global study | The occurrence of deaths due to COVID-19 was 21-fold lower in countries with a national BCG vaccination policy than in countries without. | Positive: BCG could have a protective effect by decreasing the occurrence of death due to COVID-19. | [96] |
| meta-regression | 160 countries | The countries that had ≤ 70% and greater than 70% coverage of BCG vaccine reported 6.5 and 10.1 less COVID-19 infections per 10,000 population as compared to countries that reported no coverage, respectively. | Positive: BCG was associated with reduced COVID-19 infections. | [93] |
| Ecological study | Young population in Japan | The prevalence of SARS-CoV-2 infection was significantly negatively correlated with BCG vaccine coverage in 2004 (P < 0.01). Effect size was not calculated. | Positive: The routine infant BCG vaccination coverage in young generation had a significant impact on prevention of local COVID-19 spread in Japan. | [99] |
| Ecological study | 55 countries | There were strong and significant correlations between the number of years of BCG administration and COVID-19-related deaths/million outcomes (P < 0.001) and cases/million outcome (P < 0.05). | Positive: BCG immunization coverage, especially among the most recently vaccinated population, contributed to attenuation of the spread and severity of the COVID-19 pandemic. | [90] |
| Ecological study | 67 countries | Countries with BCG vaccination policy had 58% less mortality as compared with countries without BCG coverage. | Positive: BCG vaccination could have a protective effect against COVID-19 by decreasing mortality. | [105] |
| Ecological study | 140 countries | BCG was marginally associated with fewer reported COVID-19 death rates (P < 0.05). | Positive: There were associations between live vaccine coverage and COVID-19 outcomes. | [111] |
| Ecological study | Global study | TB incidences and deaths due to TB negatively correlated with COVID-19 deaths/million in various time points (P < 0.0001 and P < 0.01, respectively) and correlated with mortality rate at two early time points (both with P < 0.05). | Positive: TB incidences and deaths and BCG vaccination negatively correlated with COVID-19 deaths/million. Countries with high BCG vaccination coverage as well as high TB deaths displayed the lowest COVID-19 deaths/million | [107] |
| Ecological study | 80 malaria-endemic countries | TB prevalence was significantly associated with reduced COVID-19 mortality. Effect size was not calculated. | Positive: TB prevalence was significantly associated with reduced COVID-19 mortality, indicating BCG could have a protective effect against COVID-19. | [101] |
| Ecological study | Organisation for Economic Cooperation and Development countries | The correlation with BCG vaccination policies was statistically significant for both morbidity (P = 0.0186) and mortality (P = 0.00266). Effect size was not calculated. | Positive: Those countries implementing BCG vaccination had a reduced number of COVID-19 morbidity and mortality cases, compared to those who had never implemented a BCG vaccination policy, suggesting the potential protective effect of BCG vaccination against COVID-19. | [98] |
| Ecological study | European countries | There were consistently negative covariations of the cases/million (r(20): −0.5511 to −0.6338; P: 0.0118 to 0.0027) and deaths/million (r(20): −0.2836 to −0.3283; P greater than 0.05) with population’s % latent TB infection at all the time points evaluated. | Positive: The prevalence of tuberculin immunoreactivity was found consistently negatively correlated with COVID-19 infections and mortality. | [100] |
| Ecological study | 173 Countries | A moderately negative association (rho = -0.29) existed between BCG vaccine coverage and COVID-19 mortality rather than COVID-19 morbidity | Positive: The recent BCG vaccine coverage was negatively associated with mortality, but not morbidity of COVID-19. | [92] |
| Ecological study | Global study | There was a significant negative correlation between the year of the establishment of universal BCG vaccination and death/million based on May 15th data (rs = -0.28, P = 0.035). | Positive: COVID-19 deaths/million negatively associated with universal BCG vaccination. | [110] |
| Ecological study | Two European countries | A strong negative correlation of BCG coverage and excess mortality during the early phase of the COVID‐19 pandemic (R2 = 0.7–0.81, P < 0.001) in Europe. | Positive: BCG vaccination could reduce mortality rates of COVID-19 during the first months of the pandemic. | [112] |
| Ecological study | Formerly East and West German federal states | Formerly East and West German federal states where divergent BCG vaccination policies existed performed significant difference in morbidity and mortality (P < 0.05). | Positive: The observations strongly supported the protective effect of BCG vaccination. | [113] |
| Ecological study | 142 Countries | No effect. | Negative: Among the countries with universal BCG vaccination policy, a weak but positive correlation was observed between COVID-19 cases and deaths per million population and BCG vaccination coverage rates. There was no significant correlation between case-fatality rate and BCG coverage at any of the set time points. | [115] |
| Ecological study | All countries from the BCG World Atlas | No effect. | Negative: After correction for confounding variables, most notably testing rates, there was no association between BCG vaccination policy and COVD-19 spread rate or percent mortality. | [118] |
| Ecological study | 97 countries | No effect. | Negative: There was no effect of country-level BCG status on SARS-CoV2 cases or deaths analyzed by a log-linear regression model. There was no statistical evidence for an association between BCG vaccination policy and either SARS-CoV2 morbidity or mortality. | [120] |
| Ecological study | 18 countries | No effect. | Negative: No effect on COVID-19 case fatality rate or number of deaths per population could be demonstrated between countries that had introduced BCG in the 1950 s and those that had not. There was no evidence for a beneficial effect of BCG vaccination on COVID-19 reported cases or fatalities. | [119] |
| Ecological study | Young population in Taiwan, China | No effect. | Negative: BCG immunization might not relate to COVID-19 severity in the young population. | [117] |
| Epidemiological model prediction | Former East and West German states | A 5% heterologous vaccine efficacy of BCG was estimated in the highly vaccinated former East Germany using the COVID-19 International Modeling (CoMo) Consortium model. A comparable BCG vaccination campaign undertaken prior to the pandemic in former West Germany, instituted along with known country-wide transmission reduction measures, was associated with a 37% decrease in projected mortality by mid-summer, 2020. | Positive: A comparable BCG vaccination campaign undertaken prior to the pandemic was associated with decrease in projected mortality. | [130] |
| Cohort study | 5 933 people born before or after the cessation of the universal BCG vaccine program in Israel | No effect. | Negative: BCG vaccination in childhood was associated with a similar rate of positive test results for SARS-CoV-2 compared with no vaccination and this study did not support the idea that BCG vaccination in childhood had a protective effect against COVID-19 in adulthood. | [121] |
| Cohort study | 2 044 848 people born before or after the cessation of the universal BCG vaccine program in Sweden | No effect. | Negative: Regression discontinuity analysis provided strong evidence that receiving the BCG vaccine at birth did not have a protective effect against COVID-19 among middle-aged individuals. | [122] |
| Cohort study | 1 906 Italian physicians | No effect. | Negative: The study did not find possible protective role of BCG vaccination, performed years earlier, against COVID-19. | [123] |
| Cohort study | 200 health care workers in India | No effect. | Negative: The study did not support the beneficial effect of BCG vaccine in protection against the development of COVID-19 disease. | [124] |
| Cohort study | 406 leprosy patients in Brazil | No effect. | Negative: The use of BCG vaccination did not affect the occurrence or severity of COVID-19. | [125] |
| Cohort study | 103 adult patients (18 years or above) with positive SARS-CoV-2 polymerase chain reaction | The patients with prior BCG vaccination had lower mortality (3% vs. 17.9%). | Partial positive: The BCG vaccine had no impact on the severity of COVID-19 but could have a protective role with a low mortality rate in already infected patients. | [136] |
| Cohort study | 2 803 individuals affected with high risk non-muscle-invasive bladder cancer and treated with intra-bladder instillation of BCG in Italy | No effect. | Negative: The study showed no evidence of a protective effect of BCG against COVID-19 in non-muscle-invasive bladder cancer patients. | [139] |
| Cohort study | 167 patients with BCG and 167 without bladder cancer | No effect. | Negative: Intravesical BCG administration did not decrease the frequency of COVID-19 infection. | [138] |
| Cohort study | 102 bladder cancer patients with a history of BCG therapy | No effect. | Negative: No statistically significant association was observed between receiving BCG therapy and developing COVID-19, however, the infection rate in patients who had recently received BCG therapy was lower than those who had received therapy more than a year ago. | [137] |
| Cohort study | 120 COVID-19 patients in Rhode Island, United States | Individuals with BCG vaccination were less likely to require hospital admission during the disease course (3.7% vs. 15.8%, P = 0.019). This association remained unchanged after adjusting for demographics and comorbidities (P = 0.017) using multivariate regression analysis. | Positive: Individuals with BCG vaccination were less likely to require hospital admission during the disease course of COVID-19, suggesting the potential of BCG in preventing more severe COVID-19. | [127] |
| Cohort study | Individuals recently vaccinated with BCG | BCG-vaccinated individuals had less sickness (an adjusted odds ratio (AOR) of 0.58, P < 0.05) and less incidence of extreme fatigue compared with control group (8.3% vs. 18.9%, AOR 0.37, P < 0.01), when obtained COVID-19. | Positive: BCG vaccination might be associated with a decrease in the incidence of sickness during the COVID-19 pandemic, and lower incidence of extreme fatigue. | [134] |
| Cohort study | 6 201 health care workers in a multisite Los Angeles health care organization | Compared with individuals who were not BCG vaccinated, those with a history of BCG vaccination were less likely to report experiencing COVID-19-related symptoms (75.6% vs. 72.7%; P = 0.017; the percentage of individuals with a serology test positive for SARS-CoV-2 (IgG) was significantly lower for BCG-vaccinated individuals than those who were not BCG vaccinated (2.7% vs 3.8%, P < 0.05) | Positive: A history of BCG vaccination, rather than meningococcal, pneumococcal, or influenza vaccination, was associated with decreased SARS-CoV-2 IgG seroconversion. | [135] |
| Case-control study | 263 039 controls and 167 664 COVID-19 cases of U.S. military veterans | No effect. | Negative: The study did not support the hypothesis that BCG in infancy was protective against COVID-19. | [126] |
| Case-control study | 920 cases and 2 123 controls in Quebec | No effect. | Negative: The vaccinated group was as likely as the unvaccinated group to require hospitalization or to die, indicating BCG did not provide long-term protection against symptomatic COVID-19 or severe forms of the disease. | [133] |
| Case-control study | 175 bladder cancer patients in instillations with BCG | Patients with non-muscle invasive bladder cancer submitted to instillations with BCG have a lower case-fatality rate than the national registry of patients between 70 and 79 years (2.3% vs. 14%, respectively). | Positive: Intravesical BCG could decrease the mortality due to COVID-19. | [140] |
| Cross-sectional study | 123 adults with COVID-19 pneumonia in Istanbul, Turkey | No effect. | Negative: BCG vaccination was not associated with disease severity in COVID-19 pneumonia | [128] |
| Clinical trial | Elderly individuals in India. | Not available. | Positive (indirect evidence): BCG vaccination was associated with enhanced DC subsets and IL-28A/IL-29 in elderly individuals, suggesting its ability to induce non-specific innate immune responses. | [141] |
| Clinical trial* | 301 elderly Greek patients | BCG revaccination resulted in 68% risk reduction for total COVID-19 clinical and microbiological diagnoses. | Positive: BCG revaccination resulted in reduction for total COVID-19 clinical and microbiological diagnoses. | [142] |
| Clinical trial | 280 hospital staffs in United Arab Emirates | The SARS-CoV-2 infection rate in the BCG-unvaccinated group was 8.6% vs. zero in the BCG booster vaccinated group (Fisher's exact test P = 0.004). | Positive: The study demonstrated the potential effectiveness of the booster BCG vaccine, specifically the booster in preventing COVID-19 infections in an elevated-risk healthcare population. | [143] |
| Clinical trial* | 60 COVID-19 patients | Resolution of pneumonia, viremia, ICU admissions, duration thereof, and mortalities were significantly improved in COVID-19 patients receiving BCG and concomitant standard of care compared with those receiving normal saline with concomitant standard of care, with reduction in oxygen requirement decreasing from day 3–4 and improved radiological resolution from day 7–15. | Positive (in therapy): The study showed that BCG could be used in patients with moderate COVID-19 to reduce requirement of oxygen supplemented beds and disease burden in low resource countries. | [144] |
| Clinical trial | 60 participants | The participants from the BCG group and anti-SARS-CoV-2 vaccine group had increased serum cytokine concentrations (i.e., IL-1β, IL-4, IL-6, IL-12p70, IL-13, IL-18, GM-CSF, INF-γ, and TNF-α) and higher neutralizing antibody titers, compared to the group with Placebo-anti-SARS-CoV-2. | Positive: Revaccination with BCG synergized with subsequent vaccination against SARS-CoV-2 in occupationally exposed personnel. | [145] |
| Clinical trial | 695 health care workers in Poland | No effect. | Negative: The statistical analysis did not reveal any significant correlation between the frequency of incidents suspected of COVID-19 and BCG-10 vaccination, the result of the tuberculin test and the number of scars. | [146] |
| Animal experiment | Human-ACE2 transgenic mice | When challenging BCG intravenous vaccinated mice on day 42, mice were largely protected with 85% survival rate vs. 15% in control (P < 0.001), and challenging on day 112, mice were significantly protected with survival rate of 50% compared with controls of 10% (P < 0.01); Intravenous BCG reduced viral loads in the lungs, SARS-CoV-2-associated pulmonary pathology, immune cell infiltration, and chemokine production. | Positive: Prior intravenous, but not subcutaneous, administration of BCG protected human-ACE2 transgenic mice against lethal challenge with SARS-CoV-2 and resulted in reduced viral loads in non-transgenic animals infected with an alpha variant. | [147] |
| Animal experiment | M. tuberculosis infection in two mouse models | Control mice without M. tuberculosis infection lost a significant portion of body weight after challenging by SARS-CoV-2, however, experimental mice infected by M. tuberculosis did not lose significant body weight after challenging with significantly lower virus burden in lungs compared with control mice (P < 0.05) | Positive: M. tuberculosis infection conditioned the lung environment in a manner that was not conducive to SARS-CoV-2 survival. | [148] |
| Animal experiment | Rhesus Macaques | No effect. | Negative: Aerosol BCG vaccination did not enhance the initial clearance of virus or reduce the occurrence of early disease pathology after high dose SARS-CoV-2 challenge. | [149] |
Positive, BCG vaccination or M. tuberculosis infection plays a positive role in COVID-19 control; Negative, BCG vaccination or M. tuberculosis infection doesn’t correlate with COVID-19 control; *, preprints without peer-reviewed.
3.1. Ecological studies
In the early months of the COVID-19 pandemic, there were substantial geographical differences in the speed of the SARS-CoV-2 transmission and the extent of its impact, resulting in significantly higher numbers of confirmed cases and deaths in North America and Western Europe than that in Asia, Africa, and South America. Furthermore, in March 2020, Miller et al. analyzed publicly available data and found that countries with universal BCG vaccination policies had generally lower numbers of COVID-19 infections and deaths [85]. Our previous study observed similar results [14]. The publication of these papers had a huge impact and led to many similar ecological studies, either for or against this theory (Table 2). One hypothesis links the incidence and mortality of COVID-19 to BCG vaccination rates: these substantial differences in morbidity and mortality of COVID-19 may be associated with the universal BCG vaccination policies of developing countries in Asia, Africa, and South America [14]. To confirm this hypothesis, one study compared the number of confirmed cases and deaths from COVID-19 in countries with mandated BCG vaccination or not (until at least 2000). The results revealed that mandatory BCG vaccination may have played a positive role in the fight against COVID-19 [87]. In epidemiological analysis, the interference of potential confounding factors must be considered and excluded as much as possible. Escobar et al. performed an epidemiological study, after eliminating the potential confounding factors such as the COVID-19 epidemic stages, geographical distribution, population density, and age structure, they found a 10% increase in the BCG index resulted in a 10.4% decrease in COVID-19 deaths [88]. Interestingly, a large number of subsequent ecological studies have found a similar phenomenon that BCG vaccination rates and years of implementation of the BCG vaccination policy are significantly negatively correlated with COVID-19 morbidity and mortality [14], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113].
Potential confounding factors must be considered in epidemiological studies to avoid disturbing the results, including differences in health systems, levels of economic development, income, education, demographics, rural and urban environments, timing of COVID-19 outbreaks, number and criteria of diagnostic tests, national control strategies and possible arbitrary selection bias [88], [110], [114]. Besides, there are some inherent uncertainty issues or biases. For example, these studies were based on population-level data, with little investigation and analysis at the individual level (for example, individuals who received BCG vaccination and those who did not), resulting in much information being ignored. Another example is that if studies are carried out during a rapidly progressing pandemic, the number of cases and deaths may be underestimated in many low- and middle-income countries vaccinated with BCG, as these countries have fewer COVID-19 tests and more underreported / misclassified deaths [84], [114], [115]. In addition, although BCG vaccination coverage in South America and South Africa is high, the number of COVID-19 deaths in both of them is still rising rapidly with the global spread of the epidemic. A follow-up analysis from South America and South Africa found a remarkable negative correlation between BCG coverage rate and deaths of COVID-19 reported on April 22, 2020, but this negative correlation was not observed on August 1, 2020 [116], suggesting that the stage of COVID-19 transmission progression is a confounding factor that seriously affects epidemiological statistics. Meanwhile, the association between BCG coverage and COVID-19 mortality varies according to the Healthcare Access and Quality Index (HAQI) in 140 countries, indicating that differences in structural health systems and policies may further confuse linkages at the population level [111]. The early effective epidemic prevention strategies of these countries may also partly explain the low COVID-19 mortality in the early stage of the epidemic, which has been successfully proved by regions or countries such as Taiwan, Hong Kong, New Zealand, and South Korea [117]. Hensel J et al. found that after correction for confounding factors, no association between BCG vaccination policy and COVD-19 spread rate or percent mortality was observed anymore [118], as several other studies reported [119], [120].
In summary, the results from ecological studies, though most of them support the hypothesis, are highly controversial and not very credible or persuasive. Therefore, more plausible clinical trials are needed to determine the nonspecific protective effect of BCG in fighting against COVID-19 infection
3.2. Analytical analysis (case-control and cohort studies)
Except for the ecological studies conducted between countries, some analytical studies, mainly case-control and cohort studies, at the individual level have been undertaken within a country or region, which has fewer differences and potentially confounding factors than the between-countries comparison.
As 2020, a cohort study compared the PCR positive rate of COVID-19 infection among individuals born three years before and after the cessation of the universal BCG vaccination program in Israel, and the results showed that BCG vaccination in childhood did not protect against COVID-19 infection in adulthood [121]. Similarly, evidence from a natural experiment based on a large population (including 1,026,304 people born before 1975 and 1,018,544 born after 1975) in Sweden showed that BCG vaccination at birth didn’t provide adequate protection against COVID-19 infection in middle-aged individuals [122]. Similar results were observed in Italian physicians [123], Indian healthcare workers [124], and Brazilian leprosy patients [125]. Recently, Bates MN et al. conducted a case-control study on COVID-19 disease and BCG vaccination status at birth determined by nationality and year of birth among 430,703 American veterans. They found that early BCG vaccination was not protective against COVID-19 infection [126]. On the contrary, a study during the first months of the COVID-19 pandemic in European countries showed a strong correlation between the years of BCG vaccination programs and age-standardized mortality [112]. Another cohort study in Rhode Island, United States, proved COVID-19 patients with BCG vaccination were less likely to require hospital admission, suggesting the potential of BCG in preventing more severe COVID-19 [127].
The heterogeneity of these findings may be associated with many confounding factors, such as nationality, age, income, and medical care level. In the above studies, the average age of BCG vaccinated people is generally higher than that of non-vaccinated people. In fact, the impact of age on the clinical manifestations and mortality of COVID-19 can not be ignored. Poor immunity and more complications will result in a higher hospitalization rate, severe disease rate, and mortality in the elderly population infected with SARS-CoV-2. Interestingly, a retrospective cross-sectional study in Turkey found significant correlation between age or income rather than BCG vaccination and the seriousness of COVID-19 [128].
In addition, non-pharmaceutical interventions (NPIs) are the potential interferences for evaluating the protective effect of BCG against COVID-19, including handwashing, social distancing, online classes at home, working at home, restrictions on international travel, vaccination policy, and shielding the elderly [129], which can reduce the morbidity and mortality of the COVID-19. In a recent study evaluating the heterologous protective efficacy of BCG vaccination in former East and West Germany using the Oxford-Cornell-founded COVID-19 International Modeling (CoMo) Consortium model revealed that the combination of a comparably efficacious BCG vaccination campaign instituted prior to the onset of the SARS-CoV-2 pandemic, along with an identical suite of NPIs, would decrease the corresponding median infection rate by 40% as well as the median mortality by 37% in former West Germany by mid-summer, 2020 [130].
Furthermore, the duration of non-specific immune response induced by BCG vaccination should be considered. Previous studies indicated that the “trained immunity” stimulated by BCG immunization could be maintained for at least one year [131], and the protective effect caused by BCG vaccination fight against TB infection might be last for 15–20 years [132]. Therefore, we hypothesized that the “trained immunity” induced by BCG vaccination could provide cross-protection against COVID-19. In theory, observational studies should assess the risk of COVID-19 in the first 30 years after BCG vaccination. It is not surprising that the protective effect of BCG on COVID-19 and even TB will decline after 35 years [114]. Therefore, it’s reasonable that no protective effect was found for BCG vaccination during childhood in individuals born in Quebec between 1956 and 1976 [133].
Therefore, exploring whether BCG vaccination provides short-term protection against COVID-19 may be more meaningful. A retrospective study evaluated the safety and COVID-19 symptoms in healthy volunteers who had been vaccinated with and without BCG in recent five years, and the results showed that injection of BCG might be related to the decrease of COVID-19 incidence rate and the occurrence of extreme fatigue [134]. Moreover, a multivariable analysis found that the years of BCG vaccination were negatively correlated with the deaths per million, and the most significant contribution was attributed to the BCG vaccination coverage of young people [90]. These data suggest that BCG may provide short-term protection against COVID-19.
Interestingly, some opposite results of BCG providing short- or long-term protection exist. In a longitudinal, retrospective observational study in 6,201 health care workers (HCWs) with a mean age of above 40 years old, researchers found that the history of BCG vaccination was associated with a decrease in SARS-CoV-2 IgG titers [135]. In addition, a study in young people in Taiwan, China did not find significant correlation between BCG vaccination status and COVID-19 severity [117]. A similar study also found that BCG vaccination did not contribute to reducing the severity of COVID-19 but provided protection to reduce mortality in COVID-19 patients [136]. BCG intravesical treatment is often used in bladder cancer patients, so comparations of SARS-CoV-2 infection rates or mortalities between patients with BCG treated or not were conducted in several studies. Basically, three of the four collected studies reported that no statistically significant association was observed between receiving BCG therapy and developing COVID-19 [137], [138], [139], however, either the small number of recruitment patients or the low prevalence of infection (<1%) might have influenced the results. The remaining paper reported that COVID-19 patients submitted to instillations with BCG have a lower case-fatality rate than the national data (2.3% vs. 14%, respectively) in Chile [140]. In contrast, a comparison with a control group data rather than the national data is believed to be more convincing. COVID-19.
It seems that more ecological studies support the nonspecific protection effect of the BCG vaccine in COVID-19 control (Table 2). In comparison, more analytical studies are against the theory or support the short-term protection against COVID-19 theory. Therefore, these observational and varied findings should be evaluated by large-scale randomized, prospective clinical trials.
3.3. Clinical trials
In the absence of direct evidence from clinical trials, the existing epidemiological data are insufficient to support the hypothesis of using the BCG vaccine to prevent and control COVID-19 or other emerging infectious diseases. Therefore, WHO has not recommended BCG for COVID-19 prevention and control through official channels. Our previous study has indicated that more than 51 clinical trials have been conducted worldwide [4]. However, only limited papers were published, including some preprinted papers without peer-reviewed.
As early as July 17, 2020, ICMR-National Institute for Research in Tuberculosis conducted a phase 3 clinical trial to evaluate the effectiveness of the BCG vaccine in decreasing morbidity and mortality of elderly individuals with COVID-19 in India (NCT04475302). The preliminary results observed the proliferation of plasma-like dendritic cells and myeloid dendritic cells and increased levels of IFN-λ1 (IL-29), IFN-λ2 (IL-28a), and IFN-λ3 (IL-28b) and decreased levels of IFN-α and IFN-βin plasma induced by BCG vaccination [141]. These results provide indirect evidence for BCG vaccination to fight SARS-CoV-2 infection by enhancing heterologous immunity.
In a double-blind, randomized phase III ACTIVATE-2 study (NCT04414267), participants were randomized to receive either BCG revaccination or placebo to evaluate whether BCG vaccination can prevent COVID-19 infection in the elderly population [142]. During the 6-month observation, BCG revaccination reduced the risk of COVID-19 clinical and microbiological diagnosis by 68%, suggesting its protective effect in the elderly against COVID-19. Interestingly, three months after BCG vaccination, anti-SARS-CoV-2 antibodies that were induced in mild or asymptomatic infection were detected in more BCG-vaccinated volunteers (4.7%) than the placebo (1.3%), indicating that BCG revaccination may improve the low antibody responses and enhance its protection against COVID-19 infection in mild or asymptomatic patients [142]. This preprinted study provided substantial evidence of the protective efficacy of BCG on COVID-19 prevention. However, one drawback of the study is the small sample size.
Another similar study recruited HCWs who had the BCG vaccine administered at birth in the United Arab Emirates and gave them a booster BCG vaccination or not [143]. There were significant differences in the rate of COVID-19 infection between the booster BCG vaccination group and the BCG unvaccinated group (zero (0/71) vs. 8.6% (18/209), P = 0.004), demonstrating the potential value of a booster BCG vaccination in preventing COVID-19 infectionCOVID-19.
Except for the prophylactic protection effects, the potential therapeutic effect of BCG for COVID-19 control was also evaluated by a Phase II clinical trial. Sixty COVID-19 patients with pneumonia requiring oxygen therapy were recruited and were given an intradermal injection of BCG or normal saline (control group) [144]. Compared with the control group, the BCG group was found to have significantly faster resolution of COVID-19 associated hypoxia, significantly radiological improvement from day 7–15, and a substantially higher percentage of patients with favourable outcomes. These findings suggest that BCG may be a safe and cost-effective treatment that can be used as a standard for treating patients with moderate COVID-19 by reducing the need for oxygen replenishment beds and disease burden in resource-poor countries.
After the vaccines against SARS-CoV-2 were available, the enhancement effect of BCG on the vaccines was also evaluated. A study exploring the effect of revaccination with BCG on the response to a subsequent anti-SARS-CoV-2 vaccine in persons occupationally exposed to COVID-19 patients found that prior BCG vaccination enhanced the increase of serum cytokine concentrations and higher neutralizing antibody titers, indicating the synergistic effects of BCG on subsequent vaccination against SARS-CoV-2 [145].
A most recent multicenter, randomized, double-blind, and placebo-controlled study (Register No. of NCT04648800 at ClinicalTrials.gov) on HCWs in Poland didn’t acquire similar results [146]. The participants in the study with a negative tuberculin test were randomized (1:1), received either the BCG- or placebo vaccine, and then subjected to observation for COVID-19 symptoms occurrence. The BCG vaccination, the tuberculin test result, and the number of scars were not observed to correlate with the suspected COVID-19 incident rate in statistical analysis. However, before the trial, all the participants had previously received at least two doses of the BCG vaccine due to the vaccination policy of Poland, which may decrease the variation between the BCG revaccination group and placebo group and is a significant limitation of this study [146]. In this sense, a control group with never-vaccinated participants may lead to a different result.
All the clinical trials except one with outcomes are for the nonspecific effect of BCG against COVID-19, indicating its promise as a potential tool against COVID-19 and COVID-19-like Black Swan incidents. However, what can not be ignored is that two of the six clinical trials are preprints without peer-reviewed, which may cause some biases in conclusion, and the results need more careful discrimination. The results of clinical trials are still scarce, and we look forward to more results of the over 50 ongoing clinical trials.
3.4. Animal studies
Except for clinical studies, the protective effects of the BCG vaccine against COVID-19 infection were also evaluated in animal models. A recently published study systematically assessed the protective effect of BCG immunization in transgenic mouse models and wild-type mouse models infected by SARS-CoV-2 [147]. It was found that intravenous but not subcutaneous injection of BCG could provide adequate protection on transgenic mice against lethal challenge with SARS-CoV-2, and reduced viral loads were also observed in the lungs obtained from transgenic mice and wild-type mice infected with SARS-CoV-2B.1.1.7 variant [147]. The authors also pointed out that the protective effect of BCG in the mouse model stems from the changes in the composition and function of pulmonary intercellular compartments induced by BCG immunization, which affect the innate response to SARS-CoV-2 infection and subsequent immunopathology. The nonspecific immune response stimulated by the BCG vaccine can promote host resistance to SARS-CoV-2 lethality. This animal study strongly proved the nonspecific protection effects of the BCG vaccine in COVID-19 prevention.
In addition, a study found that M. tuberculosis-infected mice were resistant to secondary SARS-CoV-2 infection [148]. The pathological outcomes and the resistance of mice infected with M. tuberculosis are related to the expansion of T and B lymphocyte subsets during the SARS-CoV-2 challenge. The impact of M. tuberculosis infection on the lung environment is not conducive to the survival of SARS-CoV-2 [148]. The study provides circumstantial evidence for the nonspecific protection effects of the BCG vaccine against COVID-19.
However, a recent study using an aerosol BCG vaccinated rhesus macaques model found that BCG could enhance the production of proinflammatory and immunoregulatory cytokines in serum and increase circulating monocytes and activated γδ T-cell populations by SARS-CoV-2 challenge, but couldn’t enhance the initial clearance of virus after high-dose SARS-CoV-2 challenge [149]. It’s believed the macaque model can’t replicate severe COVID-19 as in susceptible patients, for whom delayed and dysregulated immune responses occur after infection [149]. From this point of view, the study doesn’t indicate aerosol BCG vaccination is helpless to moderate severe COVID-19 disease in susceptible individuals. Also, aerosol delivery may affect the effect of injection, for, as indicated by Hilligan et al. [147], different administration methods can lead to different results. More studies are needed to overcome these limitations to clarify the biases.
In general, animal studies are still too scarce, not only in numbers but also in animal types as well as inoculation methods. Nevertheless, we believe the current animal results provide evidence of the nonspecific effect of BCG against COVID-19.
4. Possible mechanisms and their effects on COVID-19 control
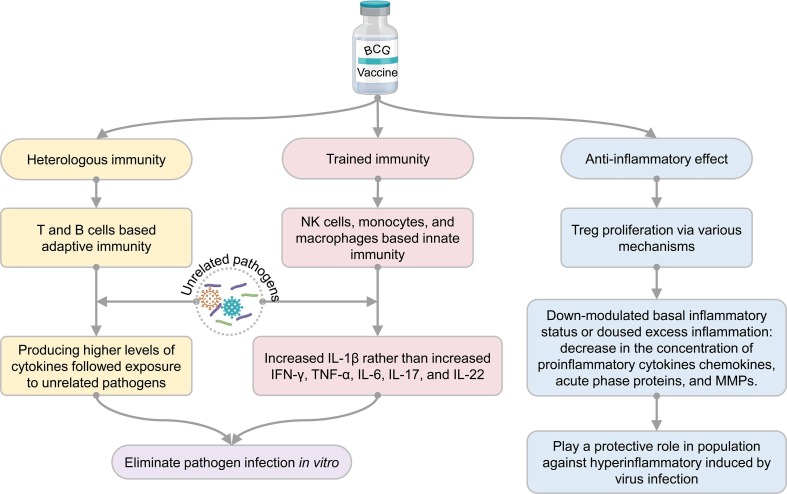
Though controversary of the nonspecific protective effects of BCG vaccine for COVID-19 control exist in ecological or analytical studies, clinical trial and animal experiment results strongly prove the nonspecific protective effects. The nonspecific protective effects of BCG vaccination in human and animal models may be due to three immunological mechanisms: heterologous immunity, training immunity, and anti-inflammatory effect [15], [150] (Fig. 2 ). Furthermore, when SARS-CoV-2 infects the host, BCG vaccination may play a protective role by enhancing the immune response to the virus and help to inhibit the systemic inflammatory immune response of patients caused by SARS-CoV-2 infection [151].
Fig. 2.
The possible mechanisms of immunity induced by BCG vaccination.
4.1. Heterologous immunity
BCG could induce enhanced adaptive immune responses to secondary unrelated infectious agents, called heterologous immunity [152]. Different from natural immunity, adaptive immunity is specific. T lymphocytes and antigen-presenting cells (APCs) recognize each other through MHC molecules to activate non-specific immune responses. At the same time, a part of T lymphocytes becomes memory T lymphocytes, which will realize the homologous antigen in future infections and rapidly induce a more robust immune response. The most significant difference between heterologous immunity and immunity induced by memory T lymphocytes is whether the secondary pathogen is the same. Heterologous immunity means that even if the initial infection is over, the host can generate a strong Th1/Th17 immune response in a short time, releasing high levels of interferon-γ (IFN-γ) and tumor necrosis factor (TNF)-α to combat the invasion of unrelated pathogens [153]. Furthermore, the nonspecific Th1/Th17 response can be enhanced at least one year after BCG vaccination in healthy volunteers [131]. For instance, BCG vaccination protected mice against vaccinia virus and HSV-2 infections by resulting in immunity based on enhanced T cell receptor signal transduction and increased secretion of IFN-γ by memory CD4+ T lymphocytes [154]. A previous study has found that even six months after BCG vaccination, memory CD4+ T lymphocytes are still activated in response to the attack of the vaccinia virus and produce a large amount of IFN-γ to clear the virus [154]. In the treatment of condyloma acuminatum and warts, BCG-activated CD4+ T lymphocytes are able to downregulate HPV gene transcription by secreting high levels of TNF-α [29]. In addition, in treating recurrent respiratory papillomatosis caused by HPV, BCG can fight the virus by stimulating the host to produce Th1-type cytokines [30].
Two mechanisms in these studies were postulated. First, there may be cross-reactive CD4 epitopes between BCG and the vaccinia virus. However, BCG and M. tuberculosis have more than 4000 proteins, and each protein has a large number of CD4+ T lymphocytes epitopes. Moreover, BCG can potentially protect against many pathogen infections, so the probability that all pathogens share BCG epitopes is very low [15]. Therefore, sharing epitopes with other pathogens may be one mechanism of its nonspecific protective effects, but not the only one. For example, a recent study involving SARS-CoV-2 found eight new BCG derived peptides with significant homology with NSP3 or NSP13 derived peptides of SARS-CoV-2 [155], along with several similar studies involving in silico identification of multiple cross-reactive peptides between SARS-CoV-2 and BCG or Mycobacterium sp. antigens, which could drive heterologous immunity induced by BCG vaccination [156], [157], [158], [159], [160]. Furthermore, it was also found that the first stimulation of BCG-derived peptides could significantly enhance the immunoreactivity of CD4+ and CD8+ T cells to corresponding homologous peptides derived from SARS-CoV-2 [155], [161]. Another mechanism is that CD4+ and CD8+ memory T lymphocytes can be triggered in an antigen-independent model. For example, IL-12 and IL-18 induced by heterologous pathogen infection can induce effector and memory CD8+ T lymphocytes to secrete high levels of IFN- γ to eliminate pathogens [154], [162].
4.2. Trained immunity
Traditionally, only B lymphocytes and T lymphocytes are considered to establish memory response through adaptive immunity. However, surprisingly, some studies have shown that BCG vaccination can induce histone modification and genome-wide epigenetic reprogramming of human innate immune cells at the promoter site of the inflammatory cytokine gene, resulting in a more active natural immune response when cells encounter a secondary stimulus [163], [164]. Furthermore, BCG vaccination has been proved to boost nonspecific innate immune responses by inducing higher frequencies of plasmacytoid dendritic cells (pDCs) and myeloid DCs (mDCs) and plasma levels of IL-28A/IL-29 in the elderly population [141]. This nonspecific memory immunity of innate immune cells induced by BCG is called “training immunity” [163], [164]. “Training immunity” is considered to be the critical mechanism for BCG to induce its nonspecific effect. “Training immunity” focuses on immune memory in innate immune cells, especially macrophages, natural killer (NK) cells, and monocytes, rather than an adaptive immune mechanism based on T and B lymphocytes [165], [166], [167] (Fig. 3 ).
Fig. 3.
The immune response of trained immunity induced by BCG.
BCG-induced training immunity has been validated in both animal models and humans. For example, a study showed that BCG vaccination has a protective effect on severe combined immunodeficiency (SCID) mice lacking functional T and B lymphocytes, proving that innate immune cells play a crucial role in mediating nonspecific protection [164]. Here, it is important to note that that BCG vaccination is contraindicated in SCID patients. Similarly, in human volunteers immunized with BCG, it was found that stimulation of peripheral blood mononuclear cells (PBMCs) with unrelated pathogens in vitro could induce significantly high levels of proinflammatory cytokines such as IL-1β and TNF-α, which is related to the increase in activation markers CD11b, CD14, TLR4, and epigenetic reprogramming of human monocytes encoding the promoter site of proinflammatory cytokine gene [164]. These results suggest that BCG-induced increased cytokine secretion of monocytes may improve clinical outcomes in secondary virus infection.
Furthermore, the mechanism behind trained immunity of BCG was also explored. From February to November 2015, Arts RJW and colleagues conducted a randomized placebo-controlled study in 30 healthy Dutch male subjects who received either BCG or placebo [26]. This study analyzed the effect of BCG vaccination on genome-wide histone modification in monocytes, the functional changes induced by BCG in monocytes, and how BCG vaccination affected viral, serological, and immunological parameters. They found that BCG vaccination could induce the whole genome epigenetic reprogramming of monocytes, accompanied by the immune function changes, and mediate the protective effect against the infection of the attenuated yellow fever vaccine (YFV) strain. In addition, this study also found that the reduction of viremia was highly correlated with the upregulation of IL-1β rather than increased IFN-γ, TNF-α, IL-6, IL-17, and IL-22. These data indicated that IL-1β is a xenogeneic cytokine associated with the induction of trained immunity, which is consistent with other findings that IL-1β plays a crucial role in anti-viral immunity [168].
Nevertheless, in the animal study mentioned above, the aerosol BCG vaccination induced trained immunity and primed unconventional T-cell populations in rhesus macaques but didn’t lead to virus clearance after the SARS-CoV-2 challenge [149], indicating the trained immunity may not provide enough protection against COVID-19.
4.3. Anti-inflammatory effect
Both the heterologous immunity and trained immunity mechanisms of BCG enhance its immunity to a nonspecific stimulus. However, in some cases, such as in autoinflammatory or autoimmune responses, a hyperinflammatory reaction induced by a stimulus may lead to further tissue damage [135], [163]. Cytokines play a crucial role in immunopathology during SARS-CoV-2 infection. Compared with healthy donors, significantly higher levels of inflammatory factors, such as IFN-γ, TNF-α, IL-1β, IL-6, IL-10, IL-17, and IL-18, can be seen in patients with COVID-19 [169].
TNF-α and IFN-γ are mainly driving inflammatory macrophage phenotype in the lung of severe COVID-19 patients [170], and IL-6, IL-1β, and high serum levels of IL-6, IL-8, and TNF-α in hospitalized patients were strong and independent predictors for survival [169]. The severity of the COVID-19 disease has been reported to be significantly associated with excessive inflammation and high levels of circulating cytokines [171]. In order to investigate whether the immune response induced by BCG vaccination is also harmful to COVID-19 patients, Kumar et al. detected the levels of cytokines, chemokines, acute phase proteins (APPs), matrix metalloproteinases (MMPs), and growth factors in 82 older adults after BCG vaccination [172]. Surprisingly, the results showed that BCG vaccination reduced the concentrations of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6), chemokines (CCL2 and CXCL10), APPs, and MMPs, suggesting that BCG vaccination may protect the elderly from severe COVID-19 by modulating systemic inflammation [172]. Therefore, it has been proposed that the immune response induced by BCG vaccination is not associated with hyperinflammation but with downregulation of the underlying inflammatory status, which may be protective against inflammatory disease in the elderly population [172]. Another large cohort study in healthy volunteers showed that BCG vaccination could increase the antimicrobial response of innate immune cells and reduce systemic inflammation by decreasing the level of pro-inflammatory cytokines in a gender-dependent model [173]. In animal studies, immune regulatory cells were also found in the cervical lymph nodes draining BCG vaccinated site on the dorsum of the ear in guinea pigs [174].
In a condition of high inflammation, BCG can trigger anti-inflammatory or tolerogenic effects to reduce the damage caused by excessive inflammatory responses, but the mechanism behind it is currently unclear [150], [173]. Considering that BCG plays a role in generating beneficial outcomes for several autoimmune diseases mentioned above [43], [71], [175], it’s not inconceivable that BCG can trigger the anti-inflammatory response. Furthermore, it has been reported that BCG can trigger Treg proliferation via various mechanisms [150]. For example, preliminary studies proved that neonatal BCG vaccination could induce CD4+ CD25+ T cells to produce IL-10 [176] and stimulate CD8+ T cells to express classical markers of Treg such as Foxp3 and CD25, which was shown to suppress the Th1 effector response [177]. In addition, BCG vaccination can activate human dendritic cells to upregulate sonic hedgehog (SHH) signaling, resulting in the expression of programmed death-ligand 1 (PD-L1) and prostaglandin E2 (PGE2), which can promote Treg proliferation [178].
In treating autoimmune diseases, BCG can enhance glycolysis, and Tregs are highly dependent on glycolysis to develop and maintain tolerance, thereby reducing inflammation in autoimmune diseases [71], [179], [180]. In addition, BCG can also induce demethylation of multiple CpG methylation sites of major Treg signature genes such as foxp3, il2ra, and ctla4, and upregulate their expression in lymphocytes [43]. Although BCG-induced Tregs have not been reported in acute inflammation caused by viral infection, in severe COVID-19, BCG-induced immune tolerance can suppress excessive inflammatory responses and benefit patients.
5. Discussion and prospect
“Black Swan” is an event with a small probability but a significant impact, while “Gray Rhino” is a metaphor for a potential crisis with a large probability and great effects. The “Black Swan” is unpredictable and uncontrollable, while the “Gray Rhinos” can't be prevented because of neglect, disregard, and contempt of contradictions. It is not hard to see that the “Black Swans” and the “Gray Rhinos” often go hand in hand. The sudden outbreak of COVID-19 in 2020, for example, is a classic “Black Swan” event. However, this outbreak also has the characteristics of “Gray Rhinos.” The precursor to the COVID-19 pandemic can be traced back to the SARS pandemic in 2003, after which many coronaviruses have been discovered, including SARS-CoV-1, MERS-CoV, and SARS-CoV-2 [181]. The suspected animal-to-human transmission indicates their significant pandemic potential. Therefore, we should not ignore the possibility of hypothetical SARS-CoV-X or other new viruses from animals but should be vigilant and prepared for emerging infectious diseases that may appear worldwide. In a COVID-19 International Modeling Consortium mathematical model, a study has proved that even with low (5 to 15%) effectiveness, heterologous vaccination interventions could reduce COVID-19 cases, hospitalization, and mortality in endemics [182]. In this sense, nonspecific vaccines for multiple pathogens may be effective emergency tools and need more study.
Considering that a number of effective COVID-19 vaccines are currently available, the clinical trials confirming BCG’s protective effects against COVID-19 or the discussion on its actual effects might seem trivial. However, there are several important reasons why these studies remain vital. Firstly, developing countries have a natural disadvantage in COVID-19 vaccine availability, and BCG vaccination can be used as a temporary solution. As our previous studies reported, BCG vaccination programmes are available in most developing countries [4], [5], [14]. Therefore, before obtaining enough COVID-19 vaccine, the BCG vaccination strategy can play an essential role in reducing the COVID-19 incidence rate, hospitalization rate, and mortality. Secondly, the continuous appearance of new variants of SARS-CoV-2 is continuously increasing escape rates and challenging the existing vaccines. The employment of vaccines with nonspecific protective immunity could thus prove very important when such viral variants become most prevailing. Thirdly, evidence that BCG prevents respiratory viral infections can provide lessons to address future infectious disease challenges like COVID-19. Also, before specific vaccines against COVID-19-like infectious diseases are available, a strategy employing such vaccines can be used as a “bridge vaccination” to protect the population partially.
In conclusion, though previous ecological studies and analytical analysis lead to opposite results, clinical trials and animal experiments provide strong evidence supporting the nonspecific effect of BCG. Furthermore, the mechanisms of heterologous immunity, trained immunity, and anti-inflammatory effect are believed to have worked together in this process. This review hopes to encourage more researchers to further confirm the nonspecific protective effect induced by BCG and the mechanism behind it, explore the possibility of using BCG as a potential tool to combat COVID-19 and COVID-19-like “black swan” events, and provide suggestions for reducing public health impacts and avoiding high societal and economic toll.
6. Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Funding
This study was funded by the Beijing Municipal Science & Technology Commission (Grant No. 19L2065) and the Chinese PLA General Hospital (Grant No. QNC19047) to WPG as well as Medical Science and Technology Projects (19SWAQ04, BWS20J021, A3705011904-06, and JJ2020A01) and Jiangsu Province Social Development Projects (BE2020631 and ZDXKB2016024) to YXL.
CRediT authorship contribution statement
Wenping Gong: Data curation, Formal analysis, Funding acquisition, Methodology, Software, Writing – original draft. Yingqing Mao: Data curation, Formal analysis, Funding acquisition, Writing – original draft. Yuexi Li: Conceptualization, Writing – review & editing. Yong Qi: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Jia Z., Gong W. Will Mutations in the Spike Protein of SARS-CoV-2 Lead to the Failure of COVID-19 Vaccines? J. Korean Med. Sci. 2021;36 doi: 10.3346/jkms.2021.36.e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valeras A.S. COVID-19: Complexity and the Black Swan. Families Syst. Health. 2020;38(2):221–223. doi: 10.1037/fsh0000486. [DOI] [PubMed] [Google Scholar]
- 3.Liang J., Liu G., Yu S., Yang Y., Li Y., Tian H., Chen Z., Gong W. Dynamic Changes in Chest CT Images Over 167 Days in 11 Patients with COVID-19. A Case Ser. Literature Rev. Zoonoses. 2021;1(1) doi: 10.15212/ZOONOSES-2021-1001. [DOI] [Google Scholar]
- 4.Gong W., Aspatwar A., Wang S., Parkkila S., Wu X. COVID-19 pandemic: SARS-CoV-2 specific vaccines and challenges, protection via BCG trained immunity, and clinical trials. Expert Rev. Vaccines. 2021;20(7):857–880. doi: 10.1080/14760584.2021.1938550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aspatwar A., Gong W., Wang S., Wu X., Parkkila S. Tuberculosis vaccine BCG: the magical effect of the old vaccine in the fight against the COVID-19 pandemic. Int. Rev. Immunol. 2022;41(2):283–296. doi: 10.1080/08830185.2021.1922685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C., Shi Q.i., Dong X.-P. SARS-CoV-2 Lambda Variant: Spatiotemporal Distribution and Potential Public Health Impact. Zoonoses. 2021;1(1) doi: 10.15212/ZOONOSES-2021-0009. [DOI] [Google Scholar]
- 7.Li X., To K.-W., Yuen K.-Y. Variants of SARS Coronavirus-2 and Their Potential Impact on the Future of the COVID-19 Pandemic. Zoonoses. 2021;1(1) doi: 10.15212/ZOONOSES-2021-1003. [DOI] [Google Scholar]
- 8.Shi Q.i., Dong X.-P. Rapid Global Spread of the SARS-CoV-2 Delta (B.1.617.2) Variant: Spatiotemporal Variation and Public Health Impact. Zoonoses. 2021;1(1) doi: 10.15212/ZOONOSES-2021-0005. [DOI] [Google Scholar]
- 9.Liu Y., Liu J., Shi P.-Y. SARS-CoV-2 Variants and Vaccination. Zoonoses. 2021;2(1) doi: 10.15212/ZOONOSES-2022-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Beltran W.F., Denis K.J.S., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2021 doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin S.i., Cui M., Sun S., Zhou J., Du Z., Cui Y., Fan H. Genome Characterization and Potential Risk Assessment of the Novel SARS-CoV-2 Variant Omicron (B.1.1.529) Zoonoses. 2021;1(1) doi: 10.15212/ZOONOSES-2021-0024. [DOI] [Google Scholar]
- 12.Mosaddeghi P., Shahabinezhad F., Dorvash M., Goodarzi M., Negahdaripour M. Harnessing the non-specific immunogenic effects of available vaccines to combat COVID-19. Hum Vaccin Immunother. 2021;17(6):1650–1661. doi: 10.1080/21645515.2020.1833577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma D. Repurposing of the childhood vaccines: could we train the immune system against the SARS-CoV-2. Expert Rev. Vaccines. 2021;20(9):1051–1057. doi: 10.1080/14760584.2021.1960161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong W., Wu X. Is the tuberculosis vaccine BCG an alternative weapon for developing countries to defeat COVID-19? Indian J Tuberc. 2021;68(3):401–404. doi: 10.1016/j.ijtb.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parmar K., Siddiqui A., Nugent K. Bacillus Calmette-Guerin Vaccine and Nonspecific Immunity. Am. J. Medical Sci. 2021;361(6):683–689. doi: 10.1016/j.amjms.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saadatian-Elahi M., Aaby P., Shann F., Netea M.G., Levy O., Louis J., Picot V., Greenberg M., Warren W. Heterologous vaccine effects. Vaccine. 2016;34(34):3923–3930. doi: 10.1016/j.vaccine.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Mangtani P., Abubakar I., Ariti C., Beynon R., Pimpin L., Fine P.E., et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin. Infectious Dis.: An Official Publication Infect. Diseases Soc. Am. 2014;58:470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 18.Shann F. Nonspecific effects of vaccines and the reduction of mortality in children. Clin. Ther. 2013;35(2):109–114. doi: 10.1016/j.clinthera.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Garly M.-L., Martins C.L., Balé C., Baldé M.A., Hedegaard K.L., Gustafson P., Lisse I.M., Whittle H.C., Aaby P. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine. 2003;21(21-22):2782–2790. doi: 10.1016/s0264-410x(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 20.Roth A., Gustafson P., Nhaga A., Djana Q., Poulsen A., Garly M.L., et al. BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int. J. Epidemiol. 2005;34:540–547. doi: 10.1093/ije/dyh392. [DOI] [PubMed] [Google Scholar]
- 21.Kristensen I., Aaby P., Jensen H. Routine vaccinations and child survival: follow up study in Guinea-Bissau. West Africa. Bmj. 2000;321:1435–1438. doi: 10.1136/bmj.321.7274.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aaby P., Roth A., Ravn H., Napirna B.M., Rodrigues A., Lisse I.M., et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J. Infectious Dis. 2011;204:245–252. doi: 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 23.Biering-Sørensen S., Aaby P., Napirna B.M., Roth A., Ravn H., Rodrigues A., Whittle H., Benn C.S. Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guérin vaccination at first health center contact. Pediatr. Infect. Dis. J. 2012;31(3):306–308. doi: 10.1097/INF.0b013e3182458289. [DOI] [PubMed] [Google Scholar]
- 24.Moorlag S.J.C.F.M., Arts R.J.W., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infection: The Official Publication Eur. Soc. Clin. Microbiol. Infectious Dis. 2019;25(12):1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Higgins J.P., Soares-Weiser K., López-López J.A., Kakourou A., Chaplin K., Christensen H., et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. Bmj. 2016;355 doi: 10.1136/bmj.i5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arts R.J.W., Moorlag S.J.C.F.M., Novakovic B., Li Y., Wang S.-Y., Oosting M., Kumar V., Xavier R.J., Wijmenga C., Joosten L.A.B., Reusken C.B.E.M., Benn C.S., Aaby P., Koopmans M.P., Stunnenberg H.G., van Crevel R., Netea M.G. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe. 2018;23(1):89–100.e5. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Leentjens J., Kox M., Stokman R., Gerretsen J., Diavatopoulos D.A., van Crevel R., et al. BCG vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: a randomized, placebo-controlled pilot study. J. Infectious Dis. 2015;212:1930–1938. doi: 10.1093/infdis/jiv332. [DOI] [PubMed] [Google Scholar]
- 28.Douglas J.M., Vontver L.A., Stamm W.E., Reeves W.C., Critchlow C., Remington M.L., Holmes K.K., Corey L. Ineffectiveness and toxicity of BCG vaccine for the prevention of recurrent genital herpes. Antimicrob. Agents Chemother. 1985;27(2):203–206. doi: 10.1128/aac.27.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salem A., Nofal A., Hosny D. Treatment of common and plane warts in children with topical viable Bacillus Calmette-Guerin. Pediatr. Dermatol. 2013;30(1):60–63. doi: 10.1111/j.1525-1470.2012.01848.x. [DOI] [PubMed] [Google Scholar]
- 30.Vetskova E.K., Muhtarova M.N., Avramov T.I., Stefanova T.R., Chalakov I.J., Nikolova M.H. Immunomodulatory effects of BCG in patients with recurrent respiratory papillomatosis. Folia Med (Plovdiv). 2013;55:49–54. doi: 10.2478/folmed-2013-0005. [DOI] [PubMed] [Google Scholar]
- 31.Podder I., Bhattacharya S., Mishra V., Sarkar TusharKanti, Chandra S., Sil A., Pal S., Kumar D., Saha A., Shome K., Bandyopadhyay D., Das NilayKanti. Immunotherapy in viral warts with intradermal Bacillus Calmette-Guerin vaccine versus intradermal tuberculin purified protein derivative: A double-blind, randomized controlled trial comparing effectiveness and safety in a tertiary care center in Eastern India. Indian J. Dermatol. Venereol. leprol. 2017;83(3):411. doi: 10.4103/0378-6323.193623. [DOI] [PubMed] [Google Scholar]
- 32.Al-Yassen A.Q., Al-Maliki S.K., Al-Asadi J.N. The Bacillus Calmette-Guérin (BCG) Vaccine: Is it a better choice for the treatment of viral warts? Sultan Qaboos Univ. Medical J. 2020;20(3):330. doi: 10.18295/squmj.2020.20.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stensballe L.G., Nante E., Jensen I.P., Kofoed P.-E., Poulsen A., Jensen H., Newport M., Marchant A., Aaby P. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: a beneficial effect of BCG vaccination for girls: Community based case–control study. Vaccine. 2005;23(10):1251–1257. doi: 10.1016/j.vaccine.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Wardhana D.EA., Sultana A., Mandang V.V., Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta medica Indonesiana. 2011;43:185–190. [PubMed] [Google Scholar]
- 35.Giamarellos-Bourboulis E.J., Tsilika M., Moorlag S., Antonakos N., Kotsaki A., Domínguez-Andrés J., Kyriazopoulou E., Gkavogianni T., Adami M.-E., Damoraki G., Koufargyris P., Karageorgos A., Bolanou A., Koenen H., van Crevel R., Droggiti D.-I., Renieris G., Papadopoulos A., Netea M.G. Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly. Cell. 2020;183(2):315–323.e9. doi: 10.1016/j.cell.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glynn J.R., Dube A., Fielding K., Crampin A.C., Kanjala C., Fine P.E.M. The effect of BCG revaccination on all-cause mortality beyond infancy: 30-year follow-up of a population-based, double-blind, randomised placebo-controlled trial in Malawi. Lancet. Infect. Dis. 2021;21(11):1590–1597. doi: 10.1016/S1473-3099(20)30994-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metawea B., El-Nashar A.-R., Kamel I., Kassem W., Shamloul R. Application of viable bacille Calmette-Guérin topically as a potential therapeutic modality in condylomata acuminata: a placebo-controlled study. Urology. 2005;65(2):247–250. doi: 10.1016/j.urology.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 38.Sylvester R.J., van der MEIJDEN A.P.M., Lamm D.L. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J. Urol. 2002;168(5):1964–1970. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 39.Klinger D., Hill B.L., Barda N., Halperin E., Gofrit O.N., Greenblatt C.L., et al. Bladder Cancer Immunotherapy by BCG Is Associated with a Significantly Reduced Risk of Alzheimer's Disease and Parkinson's Disease. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Usher N.T., Chang S., Howard R.S., Martinez A., Harrison L.H., Santosham M., Aronson N.E. Association of BCG Vaccination in Childhood With Subsequent Cancer Diagnoses: A 60-Year Follow-up of a Clinical Trial. JAMA network open. 2019;2(9):e1912014. doi: 10.1001/jamanetworkopen.2019.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salmon C., Conus F., Parent M.-É., Benedetti A., Rousseau M.-C. Association between Bacillus Calmette-Guérin vaccination and lymphoma: a population-based birth cohort study. J. Intern. Med. 2019;286(5):583–595. doi: 10.1111/joim.12965. [DOI] [PubMed] [Google Scholar]
- 42.Snider D.E., Comstock G.W., Martinez I., Caras G.J. Efficacy of BCG vaccination in prevention of cancer: an update. J. Natl Cancer Inst. 1978;60:785–788. doi: 10.1093/jnci/60.4.785. [DOI] [PubMed] [Google Scholar]
- 43.Kühtreiber W.M., Tran L., Kim T., Dybala M., Nguyen B., Plager S., Huang D., Janes S., Defusco A., Baum D., Zheng H., Faustman D.L. Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations. npj Vaccines. 2018;3(1) doi: 10.1038/s41541-018-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faustman D.L., Wang L., Okubo Y., Burger D., Ban L., Man G., Zheng H., Schoenfeld D., Pompei R., Avruch J., Nathan D.M., Doherty T.M. Proof-of-concept, randomized, controlled clinical trial of Bacillus-Calmette-Guerin for treatment of long-term type 1 diabetes. PLoS ONE. 2012;7(8):e41756. doi: 10.1371/journal.pone.0041756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blok B.A., Arts R.J.W., van Crevel R., Aaby P., Joosten L.A.B., Benn C.S., Netea M.G. Differential effects of BCG vaccine on immune responses induced by vi polysaccharide typhoid fever vaccination: an explorative randomized trial. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39(6):1177–1184. doi: 10.1007/s10096-020-03813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spencer J.C., Ganguly R., Waldman R.H. Nonspecific protection of mice against influenza virus infection by local or systemic immunization with Bacille Calmette-Guérin. J. Infect. Dis. 1977;136:171–175. doi: 10.1093/infdis/136.2.171. [DOI] [PubMed] [Google Scholar]
- 47.Mukherjee S., Subramaniam R., Chen H., Smith A., Keshava S., Shams H., Vij N. Boosting efferocytosis in alveolar space using BCG vaccine to protect host against influenza pneumonia. PLoS ONE. 2017;12(7):e0180143. doi: 10.1371/journal.pone.0180143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ikeda S., Negishi T., Nishimura C. Enhancement of non-specific resistance to viral infection by muramyldipeptide and its analogs. Antiviral Res. 1985;5(4):207–215. doi: 10.1016/0166-3542(85)90025-7. [DOI] [PubMed] [Google Scholar]
- 49.Ishihara C., Mizukoshi N., Iida J., Kato K., Yamamoto K., Azuma I. Suppression of Sendai virus growth by treatment with N alpha-acetylmuramyl-L-alanyl-D-isoglutaminyl-N epsilon-stearoyl-L-lysine in mice. Vaccine. 1987;5:295–301. doi: 10.1016/0264-410x(87)90155-1. [DOI] [PubMed] [Google Scholar]
- 50.Floc'h F., Werner G.H. Increased resistance to virus infections of mice inoculated with BCG (Bacillus calmette-guérin) Annales d'immunologie. 1976;127:173–186. [PubMed] [Google Scholar]
- 51.S.E. Starr, A.M. Visintine, M.O. Tomeh, A.J. Nahmias, Effects of immunostimulants on resistance of newborn mice to herpes simplex type 2 infection. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY). 1976;152:57-60. [DOI] [PubMed]
- 52.Kulkarni S., Mukherjee S., Pandey A., Dahake R., Padmanabhan U., Chowdhary A.S. Bacillus Calmette-Guérin Confers Neuroprotection in a Murine Model of Japanese Encephalitis. NeuroImmunoModulation. 2016;23(5-6):278–286. doi: 10.1159/000452171. [DOI] [PubMed] [Google Scholar]
- 53.Lodmell D.L., Ewalt L.C. Enhanced resistance against encephalomyocarditis virus infection in mice, induced by a nonviable Mycobacterium tuberculosis oil-droplet vaccine. Infect. Immun. 1978;19(1):225–230. doi: 10.1128/iai.19.1.225-230.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lodmell D.L., Ewalt L.C. Induction of enhanced resistance against encephalomyocarditis virus infection of mice by nonviable Mycobacterium tuberculosis: mechanisms of protection. Infect. Immun. 1978;22(3):740–745. doi: 10.1128/iai.22.3.740-745.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suenaga T., Okuyama T., Yoshida I., Azuma M. Effect of Mycobacterium tuberculosis BCG infection on the resistance of mice to ectromelia virus infection: participation of interferon in enhanced resistance. Infect. Immun. 1978;20(1):312–314. doi: 10.1128/iai.20.1.312-314.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakuma T., Suenaga T., Yoshida I., Azuma M. Mechanisms of enhanced resistance of Mycobacterium bovis BCG-treated mice to ectromelia virus infection. Infect. Immun. 1983;42(2):567–573. doi: 10.1128/iai.42.2.567-573.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fox A.E., Evans G.L., Turner F.J., Schwartz B.S., Blaustein A. Stimulation of nonspecific resistance to infection by a crude cell wall preparation from Mycobacterium phlei. J. Bacteriol. 1966;92(1):1–5. doi: 10.1128/jb.92.1.1-5.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiss D.W., Bonhag R.S., Parks J.A. Studies on the heterologous immunogenicity of a menthanol-insoluble fraction of attenuated tubercle bacilli (BCG) I. Antimicrobial Protection. J. Exp Med. 1964;119:53–70. doi: 10.1084/jem.119.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ujiie A., Watanabe M., Ushiba D. Non-specific resistance of the BCG vaccinated mice to the infection with Salmonella enteritidis (I) Nihon Saikingaku Zasshi. 1966;21:675–682. [PubMed] [Google Scholar]
- 60.de Castro M.J., Pardo-Seco J., Martinón-Torres F. Nonspecific (heterologous) protection of neonatal bcg vaccination against hospitalization due to respiratory infection and sepsis. Clin. Infectious Dis.: An Official Publication Infectious Dis. Soc. Am. 2015;60:1611–1619. doi: 10.1093/cid/civ144. [DOI] [PubMed] [Google Scholar]
- 61.Stensballe L.G., Nante E., Jensen I.P., Kofoed P.-E., Poulsen A., Jensen H., Newport M., Marchant A., Aaby P. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: a beneficial effect of BCG vaccination for girls community based case-control study. Vaccine. 2005;23(10):1251–1257. doi: 10.1016/j.vaccine.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Ohrui T., Nakayama K., Fukushima T., Chiba H., Sasaki H. Sasaki H. Nihon Ronen Igakkai zasshi Japanese J. Geriatrics. 2005;42(1):34–36. doi: 10.3143/geriatrics.42.34. [DOI] [PubMed] [Google Scholar]
- 63.Levin A.T., Hanage W.P., Owusu-Boaitey N., Cochran K.B., Walsh S.P., Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur. J. Epidemiol. 2020;35(12):1123–1138. doi: 10.1007/s10654-020-00698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.dos Santos J.C., Vilela Teodoro Silva M., Ribeiro-Dias F., Joosten L.A.B. Non-specific effects of BCG in protozoal infections: tegumentary leishmaniasis and malaria. Clin. Microbiol. Infect. 2019;25(12):1479–1483. doi: 10.1016/j.cmi.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 65.Smrkovski L.L., Larson C.L. Effect of treatment with BCG on the course of visceral leishmaniasis in BALB/c mice. Infect. Immun. 1977;16(1):249–257. doi: 10.1128/iai.16.1.249-257.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li W.-C., Zhang X.-k., Du L., Pan L.e., Gong P.-T., Li J.-H., Yang J.u., Li H.e., Zhang X.-C. Eimeria maxima: efficacy of recombinant Mycobacterium bovis BCG expressing apical membrane antigen1 against homologous infection. Parasitol. Res. 2013;112(11):3825–3833. doi: 10.1007/s00436-013-3570-5. [DOI] [PubMed] [Google Scholar]
- 67.Wang Q., Chen L., Li J., Zheng J., Cai N., Gong P., Li S., Li H.e., Zhang X. A novel recombinant BCG vaccine encoding eimeria tenella rhomboid and chicken IL-2 induces protective immunity against coccidiosis. Korean J. Parasitol. 2014;52(3):251–256. doi: 10.3347/kjp.2014.52.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daulatabad D., Pandhi D., Singal A. BCG vaccine for immunotherapy in warts: is it really safe in a tuberculosis endemic area? Dermatol. Ther. 2016;29(3):168–172. doi: 10.1111/dth.12336. [DOI] [PubMed] [Google Scholar]
- 69.Jaisinghani A.K., Dey V.K., Suresh M.S., Saxena A. Bacillus Calmette-Guerin Immunotherapy for Recurrent Multiple Warts: An Open-Label Uncontrolled Study. Indian J. Dermatol. 2019;64(2):164. doi: 10.4103/ijd.IJD_558_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weiss D.W., Bonhag R.S., Deome K.B. Protective activity of fractions of tubercle bacilli against isologous tumours in mice. Nature. 1961;190(4779):889–891. doi: 10.1038/190889a0. [DOI] [PubMed] [Google Scholar]
- 71.Ristori G., Faustman D., Matarese G., Romano S., Salvetti M. Bridging the gap between vaccination with Bacille Calmette-Guérin (BCG) and immunological tolerance: the cases of type 1 diabetes and multiple sclerosis. Curr. Opin. Immunol. 2018;55:89–96. doi: 10.1016/j.coi.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 72.Faustman D.L. Benefits of BCG-induced metabolic switch from oxidative phosphorylation to aerobic glycolysis in autoimmune and nervous system diseases. J. Intern. Med. 2020;288(6):641–650. doi: 10.1111/joim.13050. [DOI] [PubMed] [Google Scholar]
- 73.Tea F., Lopez J.A., Ramanathan S., Merheb V., Lee F.X.Z., Zou A., Pilli D., Patrick E., van der Walt A., Monif M., Tantsis E.M., Yiu E.M., Vucic S., Henderson A.P.D., Fok A., Fraser C.L., Lechner-Scott J., Reddel S.W., Broadley S., Barnett M.H., Brown D.A., Lunemann J.D., Dale R.C., Brilot F. Characterization of the human myelin oligodendrocyte glycoprotein antibody response in demyelination. Acta Neuropathol Commun. 2019;7(1) doi: 10.1186/s40478-019-0786-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ristori G., Buzzi M.G., Sabatini U., Giugni E., Bastianello S., Viselli F., et al. Use of Bacille Calmette-Guèrin (BCG) in multiple sclerosis. Neurology. 1999;53:1588–1589. doi: 10.1212/wnl.53.7.1588. [DOI] [PubMed] [Google Scholar]
- 75.Ota M.O.C., Vekemans J., Schlegel-Haueter S.E., Fielding K., Sanneh M., Kidd M., Newport M.J., Aaby P., Whittle H., Lambert P.-H., McAdam K.P.W.J., Siegrist C.-A., Marchant A. Influence of Mycobacterium bovis bacillus Calmette-Guérin on antibody and cytokine responses to human neonatal vaccination. J. Immunol. (Baltimore, Md: 1950) 2002;168(2):919–925. doi: 10.4049/jimmunol.168.2.919. [DOI] [PubMed] [Google Scholar]
- 76.Scheid A., Borriello F., Pietrasanta C., Christou H., Diray-Arce J., Pettengill M.A., Joshi S., Li N., Bergelson I., Kollmann T., Dowling D.J., Levy O. Adjuvant Effect of Bacille Calmette-Guérin on Hepatitis B Vaccine Immunogenicity in the Preterm and Term Newborn. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.0002910.3389/fimmu.2018.00029.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ritz N., Mui M., Balloch A., Curtis N. Non-specific effect of Bacille Calmette-Guérin vaccine on the immune response to routine immunisations. Vaccine. 2013;31(30):3098–3103. doi: 10.1016/j.vaccine.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 78.Counoupas C., Johansen M.D., Stella A.O., Nguyen D.H., Ferguson A.L., Aggarwal A., Bhattacharyya N.D., Grey A., Hutchings O., Patel K., Siddiquee R., Stewart E.L., Feng C.G., Hansbro N.G., Palendira U., Steain M.C., Saunders B.M., Low J.K.K., Mackay J.P., Kelleher A.D., Britton W.J., Turville S.G., Hansbro P.M., Triccas J.A. A single dose, BCG-adjuvanted COVID-19 vaccine provides sterilising immunity against SARS-CoV-2 infection. npj Vaccines. 2021;6(1) doi: 10.1038/s41541-021-00406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ayoub B.M. COVID-19 vaccination clinical trials should consider multiple doses of BCG. Pharmazie. 2020;75:159. doi: 10.1691/ph.2020.0444. [DOI] [PubMed] [Google Scholar]
- 80.Alakwaa F.M., Gilbert J.A. Repurposing Didanosine as a Potential Treatment for COVID-19 Using Single-Cell RNA Sequencing Data. mSystems. 2020;5(2) doi: 10.1128/mSystems.00297-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang T.Y., Zhong B. Meeting the potential emergency global drug supply challenge of hydroxychloroquine for COVID-19. Med Drug Discov. 2020;5:100036. doi: 10.1016/j.medidd.2020.100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Damle B., Vourvahis M., Wang E., Leaney J., Corrigan B. Clinical Pharmacology Perspectives on the Antiviral Activity of Azithromycin and Use in COVID-19. Clin. Pharmacol. Ther. 2020;108(2):201–211. doi: 10.1002/cpt.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ford N., Vitoria M., Rangaraj A., Norris S.L., Calmy A., Doherty M. Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS or COVID-19: initial assessment. J. Int. AIDS Soc. 2020;23(4) doi: 10.1002/jia2.v23.410.1002/jia2.25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vashishtha V.M. Are BCG-induced non-specific effects adequate to provide protection against COVID-19? Hum Vaccin Immunother. 2021;17(1):88–91. doi: 10.1080/21645515.2020.1794219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.A. Miller, M.J. Reandelar, K. Fasciglione, V. Roumenova, Y. Li, G.H. Otazu, Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. medRxiv. 2020:2020.03.24.20042937.
- 86.Fekrvand S., Yazdani R., Olbrich P., Gennery A., Rosenzweig S.D., Condino-Neto A., Azizi G., Rafiemanesh H., Hassanpour G., Rezaei N., Abolhassani H., Aghamohammadi A. Primary Immunodeficiency Diseases and Bacillus Calmette-Guérin (BCG)-Vaccine-Derived Complications: A Systematic Review. The Journal of Allergy and Clinical Immunology. In Practice. 2020;8(4):1371–1386. doi: 10.1016/j.jaip.2020.01.038. [DOI] [PubMed] [Google Scholar]
- 87.Berg M.K., Yu Q., Salvador C.E., Melani I., Kitayama S. Mandated Bacillus Calmette-Guérin (BCG) vaccination predicts flattened curves for the spread of COVID-19. Sci. Adv. 2020;6(32) doi: 10.1126/sciadv.abc1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Escobar L.E., Molina-Cruz A., Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19) PNAS. 2020;117(30):17720–17726. doi: 10.1073/pnas.2008410117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Covián C., Retamal-Díaz A., Bueno S.M., Kalergis A.M. Could BCG Vaccination Induce Protective Trained Immunity for SARS-CoV-2? Front. Immunol. 2020;11:970. doi: 10.3389/fimmu.2020.00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klinger D., Blass I., Rappoport N., Linial M. Significantly Improved COVID-19 outcomes in countries with higher BCG vaccination coverage: a multivariable analysis. Vaccines (Basel) 2020;8 doi: 10.3390/vaccines8030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gursel M., Gursel I. Is global BCG vaccination-induced trained immunity relevant to the progression of SARS-CoV-2 pandemic? Allergy. 2020;75(7):1815–1819. doi: 10.1111/all.14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Urashima M., Otani K., Hasegawa Y., Akutsu T. BCG Vaccination and Mortality of COVID-19 across 173 Countries: An Ecological Study. Int. J. Environ. Res. Public Health. 2020;17(15):5589. doi: 10.3390/ijerph17155589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joy M., Malavika B., Asirvatham E.S., Sudarsanam T.D., Jeyaseelan L. Is BCG associated with reduced incidence of COVID-19? A meta-regression of global data from 160 countries. Clinical epidemiology and global health. 2021;9:202–203. doi: 10.1016/j.cegh.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brooks N.A., Puri A., Garg S., Nag S., Corbo J., Turabi A.E., Kaka N., Zemmel R.W., Hegarty P.K., Kamat A.M. The association of Coronavirus Disease-19 mortality and prior bacille Calmette-Guerin vaccination: a robust ecological analysis using unsupervised machine learning. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-020-80787-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li W.X. Worldwide inverse correlation between Bacille Calmette-Guérin (BCG) immunization and COVID-19 mortality. Infection. 2021;49(3):463–473. doi: 10.1007/s15010-020-01566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jirjees F.J., Dallal Bashi Y.H., Al-Obaidi H.J. COVID-19 Death and BCG Vaccination Programs Worldwide. Tuberc Respir Dis (Seoul). 2021;84(1):13–21. doi: 10.4046/trd.2020.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Islam M.Z., Zahan M.-E., Al‐Bari M.A.A. Convergence between global BCG vaccination and COVID-19 pandemic. J. Med. Virol. 2021;93(3):1496–1505. doi: 10.1002/jmv.26450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Senoo Y., Suzuki Y., Tsuda K., Tanimoto T., Takahashi K. Association between COVID-19 morbidity and mortality rates and BCG vaccination policies in OECD countries. J Infect Prev. 2021;22(2):91–93. doi: 10.1177/1757177420976812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kinoshita M., Tanaka M. Impact of routine infant BCG vaccination on COVID-19. J. infection. 2020;81(4):625–633. doi: 10.1016/j.jinf.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Singh S., Maurya R.P., Singh R.K. “Trained immunity” from Mycobacterium spp. exposure or BCG vaccination and COVID-19 outcomes. PLoS pathogens. 2020;16:e1008969. doi: 10.1371/journal.ppat.1008969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Raham T.F. Influence of malaria endemicity and tuberculosis prevalence on COVID-19 mortality. Public Health. 2021;194:33–35. doi: 10.1016/j.puhe.2021.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sayed A., Challa K.T., Arja S. Epidemiological Differences of COVID-19 Over the World. Cureus. 2020;12 doi: 10.7759/cureus.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Inoue K., Kashima S., Izzo A.A. Association of the past epidemic of Mycobacterium tuberculosis with mortality and incidence of COVID-19. PLoS ONE. 2021;16(6):e0253169. doi: 10.1371/journal.pone.0253169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chaudhari V.L., Godbole C.J., Gandhe P.P., Gogtay N.J., Thatte U.M. Association of Bacillus Calmette Guerin Vaccine Strains with COVID-19 Morbidity and Mortality - Evaluation of Global Data. Indian J. Community Med. 2021;46(4):727. doi: 10.4103/ijcm.IJCM_103_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kumar A., Misra S., Verma V., Vishwakarma R.K., Kamal V.K., Nath M., Prakash K., Upadhyay A.D., Sahu J.K., Lo Iacono G. Global impact of environmental temperature and BCG vaccination coverage on the transmissibility and fatality rate of COVID-19. PLoS ONE. 2020;15(10):e0240710. doi: 10.1371/journal.pone.0240710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abdulah D.M., Hassan A.B. Exploration of Association Between Respiratory Vaccinations With Infection and Mortality Rates of COVID-19. Disaster Med Public Health Prep. 2021;1–16 doi: 10.1017/dmp.2021.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pathak S., Jolly M.K., Nandi D. Countries with high deaths due to flu and tuberculosis demonstrate lower COVID-19 mortality: roles of vaccinations. Hum. Vaccin. Immunother. 2021;17(9):2851–2862. doi: 10.1080/21645515.2021.1908058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hidvégi M., Nichelatti M. Bacillus Calmette-Guerin vaccination Policy and Consumption of Ammonium Chloride-Enriched Confectioneries May Be Factors Reducing COVID-19 Death Rates in Europe. Isr Med. Assoc. J. 2020;22:501–504. [PubMed] [Google Scholar]
- 109.Ebina-Shibuya R., Horita N., Namkoong H.o., Kaneko T. Current national policies for infant universal bacille Calmette-Guérin vaccination were associated with lower mortality from coronavirus disease 2019. Clin. Exp. Vaccine Res. 2020;9(2):179. doi: 10.7774/cevr.2020.9.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Szigeti R., Kellermayer D., Trakimas G., Kellermayer R., Roques P. BCG epidemiology supports its protection against COVID-19? A word of caution. PloS one. 2020;15(10):e0240203. doi: 10.1371/journal.pone.0240203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ogimi C., Qu P., Boeckh M., Bender Ignacio R.A., Zangeneh S.Z. Association between live childhood vaccines and COVID-19 outcomes: a national-level analysis. Epidemiol. Infect. 2021;149 doi: 10.1017/S0950268821000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lerm M. On the relationship between BCG coverage and national COVID-19 outcome: could 'heterologous' herd immunity explain why some countries are better off? J. Intern. Med. 2020;288(6):682–688. doi: 10.1111/joim.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hauer J., Fischer U., Auer F., Borkhardt A. Regional BCG vaccination policy in former East- and West Germany may impact on both severity of SARS-CoV-2 and incidence of childhood leukemia. Leukemia. 2020;34(8):2217–2219. doi: 10.1038/s41375-020-0871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sarinho E., Goudouris E., Solé D. BCG vaccine: Worrying proposal for COVID-19. Vaccine. 2021;39(3):460–462. doi: 10.1016/j.vaccine.2020.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meena J, Yadav A, Kumar J. BCG Vaccination Policy and Protection Against COVID-19. Indian J. Pediatrics 2020;87:749. [DOI] [PMC free article] [PubMed]
- 116.Lindestam Arlehamn C.S., Sette A., Peters B. Lack of evidence for BCG vaccine protection from severe COVID-19. PNAS. 2020;117(41):25203–25204. doi: 10.1073/pnas.2016733117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Su W.-J., Chang C.-H., Wang J.-L., Chen S.-F., Yang C.-H. COVID-19 Severity and Neonatal BCG Vaccination among Young Population in Taiwan. Int. J. Environ. Res. Public Health. 2021;18(8):4303. doi: 10.3390/ijerph18084303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hensel J., McAndrews K.M., McGrail D.J., Dowlatshahi D.P., LeBleu V.S., Kalluri R. Protection against SARS-CoV-2 by BCG vaccination is not supported by epidemiological analyses. Sci. Rep. 2020;10:18377. doi: 10.1038/s41598-020-75491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wassenaar T.M., Buzard G.S., Newman D.J. BCG vaccination early in life does not improve COVID-19 outcome of elderly populations, based on nationally reported data. Lett. Appl. Microbiol. 2020;71(5):498–505. doi: 10.1111/lam.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chimoyi L., Velen K., Churchyard G.J., Wallis R., Lewis J.J., Charalambous S., Izzo A.A. An ecological study to evaluate the association of Bacillus Calmette-Guerin (BCG) vaccination on cases of SARS-CoV2 infection and mortality from COVID-19. PLoS ONE. 2020;15(12):e0243707. doi: 10.1371/journal.pone.0243707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hamiel U., Kozer E., Youngster I. SARS-CoV-2 Rates in BCG-Vaccinated and Unvaccinated Young Adults. JAMA. 2020;323(22):2340. doi: 10.1001/jama.2020.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.de Chaisemartin C, de Chaisemartin L. Bacille Calmette-Guérin Vaccination in Infancy Does Not Protect Against Coronavirus Disease 2019 (COVID-19): Evidence From a Natural Experiment in Sweden. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2021;72:e501-e5. [DOI] [PMC free article] [PubMed]
- 123.Patella V., Sanduzzi A., Bruzzese D., Florio G., Brancaccio R., Fabbrocini G., et al. A Survey Among Italian Physicians During COVID-19 Outbreak Could Bacillus Calmette-Guérin Vaccine Be Effective Against SARS-CoV2? Front Pharmacol. 2021;12:646570. doi: 10.3389/fphar.2021.646570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chauhan A., Singh M., Agarwal A., Jaiswal N., M Lakshmi P.V., Singh M. Exploring the role of bacillus calmette-guerin vaccination in protection against COVID-19. Int. J. Mycobacteriol. 2021;10(4):433. doi: 10.4103/ijmy.ijmy_179_21. [DOI] [PubMed] [Google Scholar]
- 125.Cerqueira S.R.P.S., Deps P.D., Cunha D.V., Bezerra N.V.F., Barroso D.H., Pinheiro A.B.S., Pillegi G.S., Repsold T.A.R., Kurizky P.S., Collin S.M., Gomes C.M., Fava V.M. The influence of leprosy-related clinical and epidemiological variables in the occurrence and severity of COVID-19: A prospective real-world cohort study. PLoS Negl Trop Dis. 2021;15(7):e0009635. doi: 10.1371/journal.pntd.0009635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bates M.N., Herron T.J., Lwi S.J., Baldo J.V. BCG vaccination at birth and COVID-19: a case-control study among U.S. military Veterans. Hum. Vaccin. Immunother. 2021:1–8. doi: 10.1080/21645515.2021.1981084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Weng C.-H., Saal A., Butt W.- W.-W., Bica N., Fisher J.Q., Tao J., Chan P.A. Bacillus Calmette-Guérin vaccination and clinical characteristics and outcomes of COVID-19 in Rhode Island, United States: a cohort study. Epidemiol. Infect. 2020;148 doi: 10.1017/S0950268820001569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aksu K, Lu TBN, Zkan P. Factors determining COVID-19 pneumonia severity in a country with routine BCG vaccination. 2020. [DOI] [PMC free article] [PubMed]
- 129.School O.M., editor. Oxford Martin Programme on Global Development O. Policy Responses to the Coronavirus Pandemic. Oxford Martin School; Internet: 2022. [Google Scholar]
- 130.Marín-Hernández D., Nixon D.F., Hupert N. Anticipated reduction in COVID-19 mortality due to population-wide BCG vaccination: evidence from Germany. Hum Vaccin Immunother. 2021;17(8):2451–2453. doi: 10.1080/21645515.2021.1872344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kleinnijenhuis J., Quintin J., Preijers F., Benn C.S., Joosten L.A.B., Jacobs C., van Loenhout J., Xavier R.J., Aaby P., van der Meer J.W.M., van Crevel R., Netea M.G. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014;6(2):152–158. doi: 10.1159/000355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nguipdop-Djomo P., Heldal E., Rodrigues L.C., Abubakar I., Mangtani P. Duration of BCG protection against tuberculosis and change in effectiveness with time since vaccination in Norway: a retrospective population-based cohort study. Lancet. Infect. Dis. 2016;16(2):219–226. doi: 10.1016/S1473-3099(15)00400-4. [DOI] [PubMed] [Google Scholar]
- 133.Pépin J., Labbé A.-C., Carignan A., Parent M.-E., Yu J., Grenier C., Beauchemin S., De Wals P., Valiquette L., Rousseau M.-C. Does BCG provide long-term protection against SARS-CoV-2 infection? A case-control study in Quebec. Canada. Vaccine. 2021;39(50):7300–7307. doi: 10.1016/j.vaccine.2021.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moorlag S.J.C.F.M., van Deuren R.C., van Werkhoven C.H., Jaeger M., Debisarun P., Taks E., Mourits V.P., Koeken V.A.C.M., de Bree L.C.J., ten Doesschate T., Cleophas M.C., Smeekens S., Oosting M., van de Veerdonk F.L., Joosten L.A.B., ten Oever J., van der Meer J.W.M., Curtis N., Aaby P., Stabell-Benn C., Giamarellos-Bourboulis E.J., Bonten M., van Crevel R., Netea M.G. Safety and COVID-19 Symptoms in Individuals Recently Vaccinated with BCG: a Retrospective Cohort Study. Cell reports Medicine. 2020;1(5):100073. doi: 10.1016/j.xcrm.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rivas M.N., Ebinger J.E., Wu M., Sun N., Braun J., Sobhani K., et al. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J. Clin. Investigation. 2021;131 doi: 10.1172/JCI145157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Khanum I., Kumar L., Awan S., Jamil B. Severity of COVID-19 in bacillus Calmette-Guérin vaccinated population. Clin. Exp. Vaccine Res. 2021;10(3):276. doi: 10.7774/cevr.2021.10.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ohadian Moghadam S., Abbasi B., Nowroozi A., Amini E., Nowroozi M.R., Momeni S.A., Niroomand H. A possible protective role for Bacillus Calmette-Guérin therapy in urinary bladder cancer in the era of COVID-19: a brief report. Clin. Exp. Vaccine Res. 2021;10(2):191. doi: 10.7774/cevr.2021.10.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Karabay O., Köse O., Tocoglu A., Uysal B., Dheir H., Yaylaci S., Guclu E. Investigation of the frequency of COVID-19 in patients treated with intravesical BCG. Rev. Assoc. Med. Bras. 1992;66(suppl 2):91–95. doi: 10.1590/1806-9282.66.S2.91. [DOI] [PubMed] [Google Scholar]
- 139.Fedeli U., Porreca A., Colicchia M., Schievano E., Artibani W., Biasio L.R., Palù G. Intravescical instillation of Calmette-Guérin bacillus and COVID-19 risk. Hum Vaccin Immunother. 2021;17(2):416–417. doi: 10.1080/21645515.2020.1805994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gallegos H., Rojas P.A., Sepúlveda F., Zúñiga Á., San Francisco I.F. Protective role of intravesical BCG in COVID-19 severity. BMC Urol. 2021;21:50. doi: 10.1186/s12894-021-00823-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kumar N.P., Padmapriyadarsini C., Rajamanickam A., Bhavani P.K., Nancy A., Jeyadeepa B., Selvaraj N., Ashokan D., Renji R.M., Venkataramani V., Tripathy S., Babu S. BCG vaccination induces enhanced frequencies of dendritic cells and altered plasma levels of type I and type III interferons in elderly individuals. Int. J. Infectious Dis.: IJID: Official Publication Int. Soc. Infect. Dis. 2021;110:98–104. doi: 10.1016/j.ijid.2021.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.M. Tsilika, E. Taks, K. Dolianitis, A. Kotsaki, K. Leventogiannis, C. Damoulari et al. ACTIVATE-2: a double-blind randomized trial of BCG vaccination against COVID-19 in individuals at risk. medRxiv. 2021:2021.05.20.21257520. [DOI] [PMC free article] [PubMed]
- 143.Amirlak L., Haddad R., Hardy J.D., Khaled N.S., Chung M.H., Amirlak B. Effectiveness of booster BCG vaccination in preventing Covid-19 infection. Hum. Vaccin Immunother. 2021;17(11):3913–3915. doi: 10.1080/21645515.2021.1956228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.U. Padmanabhan, S. Mukherjee, R. Borse, S. Joshi, R. Deshmukh, Phase II Clinical trial for Evaluation of BCG as potential therapy for COVID-19. medRxiv. 2020:2020.10.28.20221630.
- 145.Ramos-Martinez E., Falfán-Valencia R., Pérez-Rubio G., Andrade W.A., Rojas-Serrano J., Ambrocio-Ortiz E., Galicia-Álvarez D.S., Bárcenas-Montiel I., Velasco-Medina A., Velázquez-Sámano G. Effect of BCG Revaccination on Occupationally Exposed Medical Personnel Vaccinated against SARS-CoV-2. Cells. 2021;10(11):3179. doi: 10.3390/cells10113179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Czajka H., Zapolnik P., Krzych Ł., Kmiecik W., Stopyra L., Nowakowska A., Jackowska T., Darmochwał-Kolarz D., Szymański H., Radziewicz-Winnicki I., Mazur A. A Multi-Center, Randomised, Double-Blind, Placebo-Controlled Phase III Clinical Trial Evaluating the Impact of BCG Re-Vaccination on the Incidence and Severity of SARS-CoV-2 Infections among Symptomatic Healthcare Professionals during the COVID-19 Pandemic in Poland-First Results. Vaccines (Basel) 2022;10(2):314. doi: 10.3390/vaccines10020314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hilligan K.L., Namasivayam S., Clancy C.S., O’Mard D., Oland S.D., Robertson S.J., Baker P.J., Castro E., Garza N.L., Lafont B.A.P., Johnson R., Ronchese F., Mayer-Barber K.D., Best S.M., Sher A. Intravenous administration of BCG protects mice against lethal SARS-CoV-2 challenge. J. Exp. Med. 2022;219(2) doi: 10.1084/jem.20211862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rosas Mejia O., Gloag E.S., Li J., Ruane-Foster M., Claeys T.A., Farkas D., Wang S.-H., Farkas L., Xin G., Robinson R.T., Salgame P. Mice infected with Mycobacterium tuberculosis are resistant to acute disease caused by secondary infection with SARS-CoV-2. PLoS Pathog. 2022;18(3):e1010093. doi: 10.1371/journal.ppat.1010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.White A.D., Sibley L., Sarfas C., Morrison A.L., Bewley K., Churchward C., Fotheringham S., Gkolfinos K., Gooch K., Handley A., Humphries H.E., Hunter L., Kennard C., Longet S., Mabbutt A., Moffatt M., Rayner E., Tipton T., Watson R., Hall Y., Bodman-Smith M., Gleeson F., Dennis M., Salguero F.J., Carroll M., McShane H., Cookson W., Hopkin J., Sharpe S. Influence of Aerosol Delivered BCG Vaccination on Immunological and Disease Parameters Following SARS-CoV-2 Challenge in Rhesus Macaques. Front. Immunol. 2021;12:801799. doi: 10.3389/fimmu.2021.801799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Basak P., Sachdeva N., Dayal D. Can BCG vaccine protect against COVID-19 via trained immunity and tolerogenesis? BioEssays. 2021;43(3):e2000200. doi: 10.1002/bies.202000200. [DOI] [PubMed] [Google Scholar]
- 151.Prentice S., Dockrell H.M. Antituberculosis BCG vaccination: more reasons for varying innate and adaptive immune responses. J. Clin. Invest. 2020;130:5121–5123. doi: 10.1172/JCI141317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Goodridge H.S., Ahmed S.S., Curtis N., Kollmann T.R., Levy O., Netea M.G., Pollard A.J., van Crevel R., Wilson C.B. Harnessing the beneficial heterologous effects of vaccination. Nat. Rev. Immunol. 2016;16(6):392–400. doi: 10.1038/nri.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Benn C.S., Netea M.G., Selin L.K., Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34(9):431–439. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 154.Mathurin K.S., Martens G.W., Kornfeld H., Welsh R.M. CD4 T-cell-mediated heterologous immunity between mycobacteria and poxviruses. J. Virol. 2009;83(8):3528–3539. doi: 10.1128/JVI.02393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Eggenhuizen P.J., Ng B.H., Chang J., Fell A.L., Cheong R.M.Y., Wong W.Y., Gan P.-Y., Holdsworth S.R., Ooi J.D. BCG Vaccine Derived Peptides Induce SARS-CoV-2 T Cell Cross-Reactivity. Front. Immunol. 2021;12:692729. doi: 10.3389/fimmu.2021.692729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tarabini R.F., Rigo M.M., Faustino Fonseca A., Rubin F., Bellé R., Kavraki L.E., Ferreto T.C., Amaral Antunes D., de Souza A.P.D. Large-Scale Structure-Based Screening of Potential T Cell Cross-Reactivities Involving Peptide-Targets From BCG Vaccine and SARS-CoV-2. Front. Immunol. 2021;12:812176. doi: 10.3389/fimmu.2021.812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tomita Y., Sato R., Ikeda T., Sakagami T. BCG vaccine may generate cross-reactive T cells against SARS-CoV-2: In silico analyses and a hypothesis. Vaccine. 2020;38(41):6352–6356. doi: 10.1016/j.vaccine.2020.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Finotti P. Sequence similarity of HSP65 of Mycobacterium bovis BCG with SARS-CoV-2 spike and nuclear proteins: may it predict an antigen-dependent immune protection of BCG against COVID-19? Cell Stress Chaperones. 2022;27(1):37–43. doi: 10.1007/s12192-021-01244-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nuovo G., Tili E., Suster D., Matys E., Hupp L., Magro C. Strong homology between SARS-CoV-2 envelope protein and a Mycobacterium sp. antigen allows rapid diagnosis of Mycobacterial infections and may provide specific anti-SARS-CoV-2 immunity via the BCG vaccine. Ann. Diagnostic Pathol. 2020;48:151600. doi: 10.1016/j.anndiagpath.2020.151600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Haddad-Boubaker S., Othman H., Touati R., Ayouni K., Lakhal M., Ben Mustapha I., Ghedira K., Kharrat M., Triki H. In silico comparative study of SARS-CoV-2 proteins and antigenic proteins in BCG, OPV, MMR and other vaccines: evidence of a possible putative protective effect. BMC Bioinf. 2021;22(1) doi: 10.1186/s12859-021-04045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Urbán S., Paragi G., Burián K., McLean G.R., Virok D.P. Identification of similar epitopes between severe acute respiratory syndrome coronavirus-2 and Bacillus Calmette-Guérin: potential for cross-reactive adaptive immunity. Clin. Transl. Immunol. 2020;9(12) doi: 10.1002/cti2.v9.1210.1002/cti2.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Berg R.E., Crossley E., Murray S., Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J. Exp. Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Netea M.G., Joosten L.A.B., Latz E., Mills K.H.G., Natoli G., Stunnenberg H.G., O’Neill L.A.J., Xavier R.J. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352(6284) doi: 10.1126/science:aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kleinnijenhuis J., Quintin J., Preijers F., Joosten L.A., Ifrim D.C., Saeed S., et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. PNAS. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Butkeviciute E., Jones C.E., Smith S.G. Heterologous effects of infant BCG vaccination: potential mechanisms of immunity. Future Microbiol. 2018;13(10):1193–1208. doi: 10.2217/fmb-2018-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.de Bree L.C.J., Koeken V.A.C.M., Joosten L.A.B., Aaby P., Benn C.S., van Crevel R., Netea M.G. Non-specific effects of vaccines: Current evidence and potential implications. Semin. Immunol. 2018;39:35–43. doi: 10.1016/j.smim.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 167.Malik Y.S., Ansari M.I., Ganesh B., Sircar S., Bhat S., Pande T., Vinodhkumar O.R., Kumar P., Iqbal Yatoo M., Tiwari R., Touil N., Patel S.K., Pathak M., Sharun K., Dhama K. BCG vaccine: a hope to control COVID-19 pandemic amid crisis. Hum. Vaccin Immunother. 2020;16(12):2954–2962. doi: 10.1080/21645515.2020.1818522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rathinam V.A.K., Fitzgerald K.A. Inflammasomes and anti-viral immunity. J. Clin. Immunol. 2010;30(5):632–637. doi: 10.1007/s10875-010-9431-4. [DOI] [PubMed] [Google Scholar]
- 169.Del Valle D.M., Kim-Schulze S., Huang H.-H., Beckmann N.D., Nirenberg S., Wang B.o., Lavin Y., Swartz T.H., Madduri D., Stock A., Marron T.U., Xie H., Patel M., Tuballes K., Van Oekelen O., Rahman A., Kovatch P., Aberg J.A., Schadt E., Jagannath S., Mazumdar M., Charney A.W., Firpo-Betancourt A., Mendu D.R., Jhang J., Reich D., Sigel K., Cordon-Cardo C., Feldmann M., Parekh S., Merad M., Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang F., Mears J.R., Shakib L., Beynor J.I., Shanaj S., Korsunsky I., Nathan A., Donlin L.T., Raychaudhuri S. IFN-γ and TNF-α drive a CXCL10+ CCL2+ macrophage phenotype expanded in severe COVID-19 lungs and inflammatory diseases with tissue inflammation. Genome Med. 2021;13(1) doi: 10.1186/s13073-021-00881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schultze J.L., Aschenbrenner A.C. COVID-19 and the human innate immune system. Cell. 2021;184(7):1671–1692. doi: 10.1016/j.cell.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pavan Kumar N., Padmapriyadarsini C., Rajamanickam A., Marinaik S.B., Nancy A., Padmanaban S., Akbar N., Murhekar M., Babu S. Effect of BCG vaccination on proinflammatory responses in elderly individuals. Sci. Adv. 2021;7(32) doi: 10.1126/sciadv.abg7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Koeken V.A., de Bree L.C.J., Mourits V.P., Moorlag S.J., Walk J., Cirovic B., et al. BCG vaccination in humans inhibits systemic inflammation in a sex-dependent manner. J. Clin Invest. 2020;130:5591–5602. doi: 10.1172/JCI133935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vergkizi S., Nikolakakis I. Bacillus Calmette-Guérin (BCG) vaccine generates immunoregulatory cells in the cervical lymph nodes in guinea pigs injected intra dermally. Vaccine. 2020;38(48):7629–7637. doi: 10.1016/j.vaccine.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kowalewicz-Kulbat M., Locht C. BCG and protection against inflammatory and auto-immune diseases. Expert Rev Vaccines. 2017;16(7):699–708. doi: 10.1080/14760584.2017.1333906. [DOI] [PubMed] [Google Scholar]
- 176.Akkoc T., Aydogan M., Yildiz A., Karakoc-Aydiner E., Eifan A., Keles S., et al. Neonatal BCG vaccination induces IL-10 production by CD4+ CD25+ T cells. Pediatr. Allergy Immunol. 2010;21:1059–1063. doi: 10.1111/j.1399-3038.2010.01051.x. [DOI] [PubMed] [Google Scholar]
- 177.Boer M.C., van Meijgaarden K.E., Bastid J., Ottenhoff T.H.M., Joosten S.A. CD39 is involved in mediating suppression by Mycobacterium bovis BCG-activated human CD8(+) CD39(+) regulatory T cells. Eur. J. Immunol. 2013;43(7):1925–1932. doi: 10.1002/eji.201243286. [DOI] [PubMed] [Google Scholar]
- 178.Holla S., Stephen-Victor E., Prakhar P., Sharma M., Saha C., Udupa V., Kaveri S.V., Bayry J., Balaji K.N. Mycobacteria-responsive sonic hedgehog signaling mediates programmed death-ligand 1- and prostaglandin E2-induced regulatory T cell expansion. Sci. Rep. 2016;6(1) doi: 10.1038/srep24193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.De Rosa V., Galgani M., Porcellini A., Colamatteo A., Santopaolo M., Zuchegna C., Romano A., De Simone S., Procaccini C., La Rocca C., Carrieri P.B., Maniscalco G.T., Salvetti M., Buscarinu M.C., Franzese A., Mozzillo E., La Cava A., Matarese G. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat. Immunol. 2015;16(11):1174–1184. doi: 10.1038/ni.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Procaccini C., Carbone F., Di Silvestre D., Brambilla F., De Rosa V., Galgani M., Faicchia D., Marone G., Tramontano D., Corona M., Alviggi C., Porcellini A., La Cava A., Mauri P., Matarese G. The Proteomic Landscape of Human Ex Vivo Regulatory and Conventional T Cells Reveals Specific Metabolic Requirements. Immunity. 2016;44(2):406–421. doi: 10.1016/j.immuni.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.To K.-W., Sridhar S., Chiu K.-Y., Hung D.-L., Li X., Hung I.-N., Tam A.R., Chung T.-H., Chan J.-W., Zhang A.-X., Cheng V.-C., Yuen K.-Y. Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic. Emerg Microbes Infect. 2021;10(1):507–535. doi: 10.1080/22221751.2021.1898291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.N. Hupert, D. Marín-Hernández, B. Gao, R. Águas, D.F. Nixon, Heterologous vaccination interventions to reduce pandemic morbidity and mortality: Modeling the US winter 2020 COVID-19 wave, in: Proceedings of the National Academy of Sciences of the United States of America. 2022;119. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.