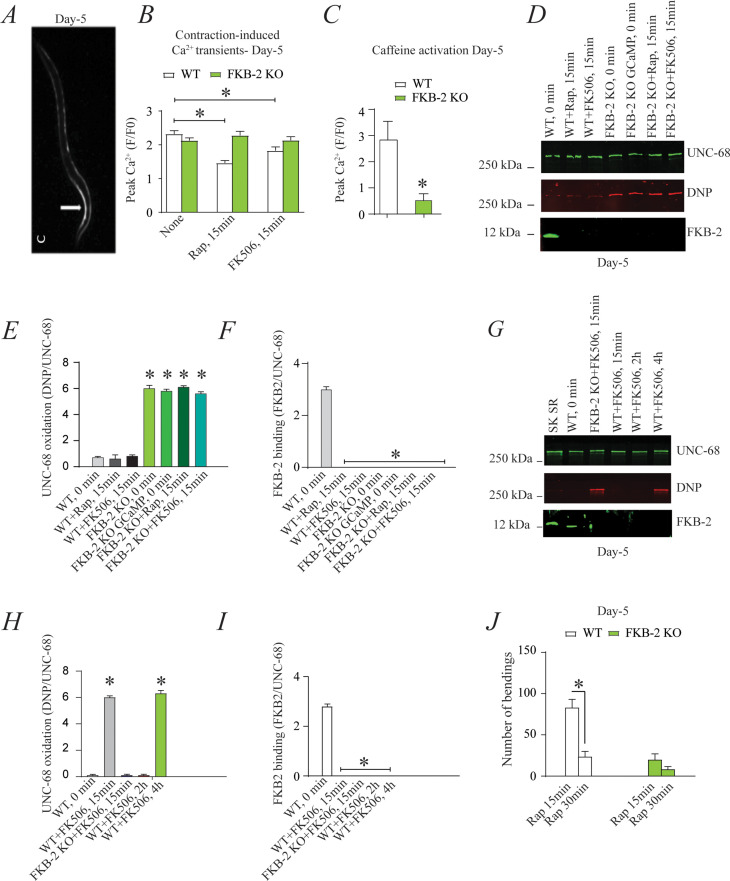
Figure 3. Depleting FKB-2 from UNC-68 causes UNC-68 oxidation (A) Representative image of caffeine activated calcium transient in GCaMP2 wild type (WT) at day 5; arrow denotes peak fluorescence in body wall muscle.
(B) Intracellular calcium (Ca2+) transients in day 5 age-synchronized populations of WT and FKB-2 (ok3007) nematodes treated with 15 μM and 50 μM rapamycin and FK506, respectively (treatment was applied for 15 min). (C) Fluorescence intensity following caffeine activation in age-matched GCaMP2: WT vs. GCaMP2: FKB-2 KO worms at day 5. (D) UNC-68 was immunoprecipitated and immunoblotted using anti-ryanodine receptor, anti-calstabin, and dinitrophenyl (DNP; marker of oxidation) antibodies in nematodes (at day 5) acutely treated with 15 μM and 50 μM rapamycin and FK506, respectively (treatment was applied for 15 min). (E–F) Quantification of the band intensity shown in (D): band intensity was defined as the ratio of either DNP (marker of UNC-68 oxidation) or FKB-2 binding over its corresponding /UNC-68’s expression. (G) UNC-68 was immunoprecipitated after 0, 15 min, 2 hr, and 4 hr FK506 exposure of the nematodes (at day 5). Representative immunoblots from triplicate experiments. (H–I) Quantification of the band intensity shown in (G): band intensity was defined as the ratio of either DNP (marker of UNC-68 oxidation) or FKB-2 binding over its corresponding /UNC-68’s expression. (J) Graph showing number of bends recorded for WT vs. FKB-2 KO worms (Day 5) treated for 20 and 30 min with 15 μM and 50 μM rapamycin and FK506, respectively. N ≥ 15 per group. Data are means ± SEM from triplicate experiments. One-way ANOVA shows * p<0.05 vs. WT for results shown in panel E, F, H, and I. Two-way ANOVA was used for results comparison in panel B, and t-test was used for results shown in C and J. SK SR; sarcoplasmic reticulum fraction from mouse skeletal muscle used as external control reference and was not quantified in the bar graphs. The time 0 min refers to untreated worms. Figure 3—source data 1.