Abstract
Background:
Alveolar ridge squamous cell carcinoma (ARSCC) is poorly represented in randomized trials.
Methods:
Adults in the National Cancer Database diagnosed with ARSCC between 2010 and 2014 who should be considered for postoperative radiotherapy (PORT) based on National Comprehensive Cancer Network (NCCN)-defined risk factors were identified.
Results:
Eight hundred forty-five (58%) of 1457 patients meeting the inclusion criteria received PORT. PORT was associated with improved overall survival (OS) on unadjusted (hazard ratio [HR] 0.83, 95% confidence interval [CI] 0.70–0.98, P = .02) and multivariable (HR 0.78, 95% CI 0.64–0.94, P = .002) analyses. PORT was associated with significantly improved 5-year OS for patients with 1 (68% vs 58%, P < .001), 2 (52% vs 31%, P < .001), and ≥3 (38% vs 24%, P < .001) NCCN-defined risk factors. Prognostic variables significantly associated with worse OS on multivariable analysis included advanced age, primary tumor size ≥3 cm, high grade, positive margin(s), stage N2-3, level IV/V nodal metastasis, and extranodal extension.
Conclusion:
PORT for resected ARSCC with adverse pathologic features is associated with significantly improved OS.
Keywords: alveolar ridge, oral cancer, radiotherapy, squamous cell carcinoma, surgery
1 |. INTRODUCTION
Alveolar ridge cancer accounts for a considerable proportion of oral cavity cancers worldwide. In Thailand and Nigeria, alveolar ridge is the most common oral cavity site of squamous cell carcinoma (SCC), accounting for 50% and 55% of cases, respecitvely.1,2 In India and Japan, carcinoma of the mandibular alveolar ridge, which is more frequently involved compared to the maxillary alveolar ridge, is the second most common oral cavity cancer site, accounting for 22% and 30% of cases, respectively.3,4 Among 806 Brazilian men and women, alveolar ridge represented the third most common oral cavity site for SCC (behind lip and oral tongue), accounting for 18% of cases.5 In the United States, alveolar ridge cancer is the third most common oral cavity malignancy, accounting for approximately 11% of cases.6
The primary treatment for alveolar ridge cancer is typically surgery. Carcinomas of the alveolar ridge tend to invade bone early, and advanced primary tumors have a relatively high propensity for lymph node metastasis.7 Depending on dental health and the degree of cortical bone invasion, segmental or marginal resection of the mandible for inferior alveolar ridge carcinoma may be needed.8–10 For superior alveolar ridge carcinoma, surgery usually involves resection of bone via alveolectomy, palatectomy, or infrastructure maxillectomy.10 For advanced lesions, selective neck dissection involving at least levels I-III is recommended.11
Postoperative radiotherapy (PORT), with or without systemic therapy, is frequently administered when adverse pathologic features are present. Randomized evidence supports that PORT decreases locoregional recurrence and, in certain instances, improves overall survival (OS); however, only about one-fourth of patients enrolled on European Organisation for Research and Treatment of Cancer 22931 and Radiation Therapy Oncology Group (RTOG) 9501 had oral cavity cancers.12,13 Therefore, there is a lack of prospective data to guide the use of PORT specifically for alveolar ridge carcinoma as this oral cavity subsite is poorly represented in randomized trials. The National Comprehensive Cancer Network (NCCN) guidelines recommend consideration of PORT based on the following adverse risk features: extranodal extension, positive surgical margins, advanced primary T stage (T3-4), advanced nodal disease (N2-3), nodal metastasis in cervical levels IV/V, perineural invasion, vascular embolism, and/or lymphatic invasion.11 The primary objective of this study is to determine the association between PORT and OS for resected alveolar ridge SCC based on the presence and number of adverse pathologic features.
2 |. MATERIALS AND METHODS
2.1 |. Data source
The National Cancer Database (NCDB) is an oncology outcomes database for over 1500 commission-accredited cancer programs in the United States and Puerto Rico, developed jointly by the Commission on Cancer of the American College of Surgeons and the American Cancer Society.14 Approximately 70% of newly diagnosed cancer cases are captured at the institutional level by Certified Cancer Registrars using nationally standardized data item and coding definitions. All NCDB data are de-identified and compliant with Health Insurance Portability and Accountability Act privacy standards. This retrospective cohort study was granted exemption by the Duke University Institutional Review Board.
2.2 |. Cohort selection
The NCDB was queried for adults age 18 or older diagnosed between January 1, 2010 and December 31, 2014 with alveolar ridge malignancy using International Classification of Diseases for Oncology Classification of Diseases Third Edition (ICD-O-3) topographical codes C030-C039. These years were selected due to incomplete information on pathologic risk factors for patients diagnosed between 2004 and 2009. Patients diagnosed in 2015 were excluded due to missing survival data. Patients with invasive, non-metastatic SCC of the alveolar ridge treated with surgical resection were included. Exclusion criteria included in situ disease, non-squamous histology (ICD-O-3 morphological code other than 8052–8084), unknown surgery status, unknown radiotherapy status, preoperative radiotherapy, radiotherapy dose <50 or >80 Gy, radiotherapy delivered in >68 fractions (exceeding the RTOG 9003 hyper-fractionated regimen), radiotherapy to a non-head/neck site, prior malignancy, stage pT1-2N0 (based on the American Joint Committee on Cancer, Cancer Staging Manual 7th Edition) without identifiable NCCN-defined risk factors, unknown number of resected lymph nodes, and unknown/missing NCCN-defined risk factor data.15 The NCDB does not report information regarding perineural invasion or vascular embolism for alveolar ridge carcinoma, but data for the following six NCCN-defined risk factors are available: extranodal extension, positive surgical margins, pathologic T3-4, clinical or pathologic N2-3, nodal metastasis in cervical levels IV/V, and lymphovascular invasion.16 Radiotherapy dose-fractionation exclusion criteria were utilized due to recently published data demonstrating that anomalous radiotherapy data in the NCDB can affect survival results.17
Patient characteristics collected were age at diagnosis, sex, and Charlson-Deyo comorbidity score (0, 1, or ≥2). Tumor characteristics recorded were primary alveolar ridge site, pathologic T stage, clinical and pathologic N stage, tumor grade, tumor thickness or depth of invasion, bone invasion, surgical margin status, lymphovascular invasion, number of pathologically positive nodes, presence of level IV/V nodal metastasis, and extranodal extension. All patients with coded bone invasion were designated as stage T4. Clinical N stage was only used for patients who did not undergo pathologic nodal assessment. Treatment data collected included number of resected lymph nodes, chemotherapy receipt, days from diagnosis to chemotherapy, radiotherapy receipt, radiotherapy dose, days from diagnosis to radiotherapy, and number of radiotherapy fractions.
2.3 |. Statistical methods
Patients were categorized into a surgery only cohort or a surgery plus PORT cohort. Patients in the latter cohort were coded as receiving radiotherapy after surgery and had a coded radiotherapy start date later than that of surgery relative to the date of diagnosis. Descriptive summary statistics were performed on demographic, tumor, and treatment-related variables. Patient characteristics were compared in the surgery only vs surgery plus PORT cohorts using Chi-square and t tests for categorical and continuous variables, respectively. Patient characteristics were similarly compared in the subgroup of patients who received chemotherapy.
The primary outcome was OS, which was calculated from the date of diagnosis until the date of last contact or death. Unadjusted OS was estimated using the Kaplan-Meier method, and differences in unadjusted OS between study groups were tested using the log-rank test. Kaplan-Meier curves and estimates examined differences between study groups within pre-specified subgroups, including number of NCCN-defined risk factors, percentage of positive lymph nodes, high grade, large primary tumor size, and locally advanced, node-negative tumor (ie, T3-4N0). Hazard ratios from univariate Cox proportional hazards models estimated the unadjusted effects of patients and treatment characteristics on OS.
To estimate the association of PORT with OS after adjustment for covariates, a multivariable Cox proportional hazards model was created to evaluate the impact of NCCN-defined risk factors and treatment characteristics on OS. Prognostic variables with a P-value <.05 on univariate analysis were included in the multivariable model in addition to the following: diagnosis year, gender, comorbidity score, race, income, insurance, education, facility type, facility location, and great circle distance. A robust sandwich covariance estimator was used to account for the correlation of patients treated at the same facility. Patients with missing values for these variables were excluded from the multivariable analysis. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, North Carolina).
3 |. RESULTS
A total of 5852 adults diagnosed with alveolar ridge malignancy between 2010 and 2014 were identified, with 1457 patients meeting inclusion criteria. Figure 1 summarizes the cohort selection process. The most common reasons for exclusion were incomplete pathologic data for all six available NCCN-defined risk factors or stage pT1-2N0 without NCCN-defined risk factors.
FIGURE 1.
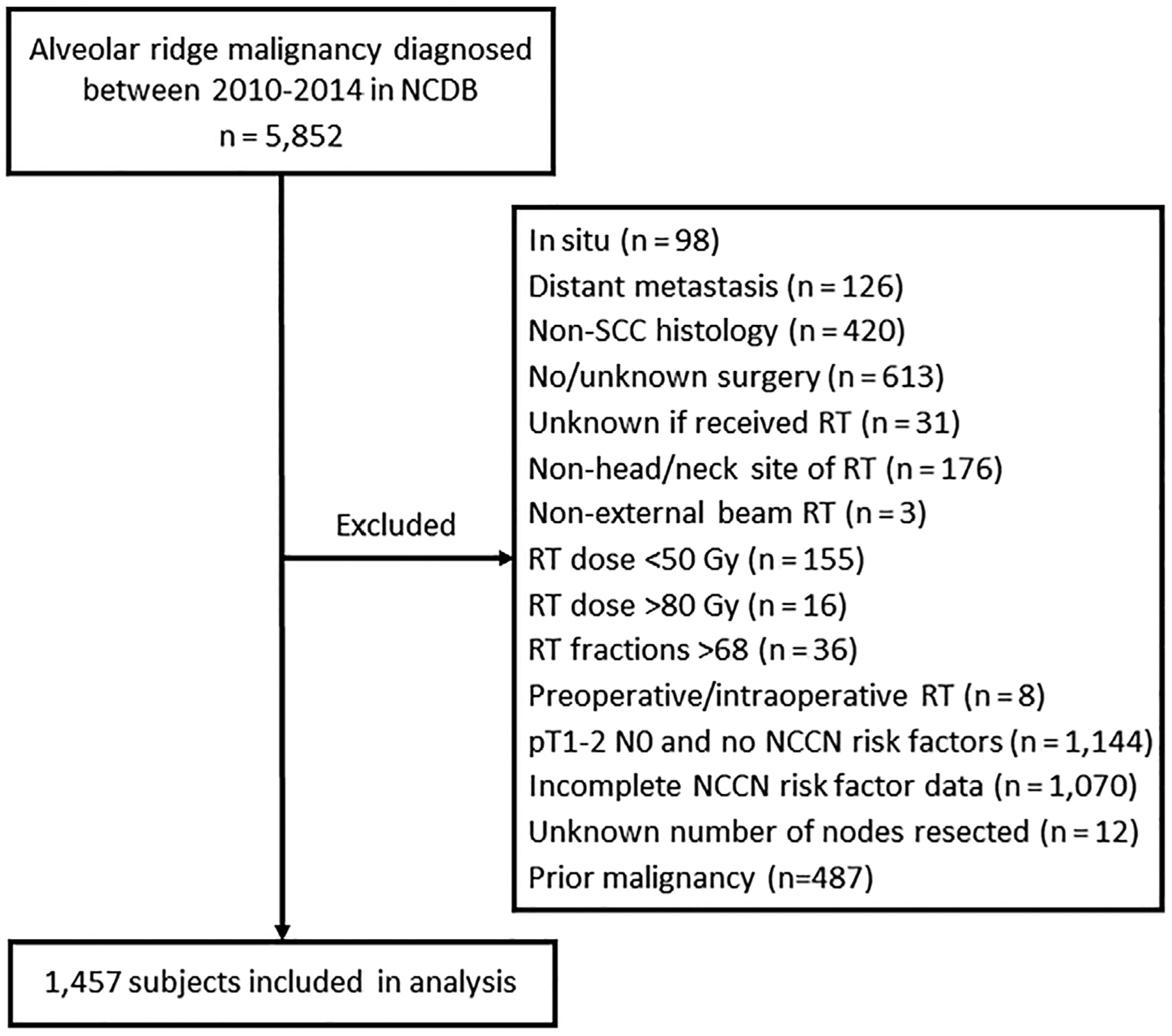
Analytic cohort derivation. NCCN, National Comprehensive Cancer Network; NCDB, National Cancer Database; RT, radiotherapy; SCC, squamous cell carcinoma
Among the 1457 patients meeting inclusion criteria, 845 (58%) received PORT. The patients in the surgery only cohort did not receive PORT for the following coded reasons: not part of the planned first course (75%), declined physician’s recommendation (22%), or deemed contraindicated (4%).
Differences between the surgery only and surgery plus PORT cohorts are summarized in Table 1. Patients who received PORT were significantly more likely to have adverse pathologic risk factors, including larger primary tumor size, stage T3-4, higher tumor grade, lymphovascular invasion, lymph node metastases, stage N2-3, extranodal extension, and multiple NCCN-defined risk factors. Patients in the PORT cohort were also significantly younger, had a lower Charlson-Deyo comorbidity score, and were more likely to have received chemotherapy. The characteristics of the subgroup of patients who received chemotherapy are shown in Supplemental Table 1.
TABLE 1.
Demographic, tumor, and treatment characteristics
| Variable | Surgery only, n = 612 | Surgery + PORT, n = 845 | P value* |
|---|---|---|---|
| Demographic characteristics | |||
| Age at diagnosis | <.001 | ||
| Median (interquartile range) | 73 (62–82) | 66 (57–74) | |
| <45 | 14 (2) | 21 (3) | |
| 45–54 | 55 (9) | 122 (14) | |
| 55–64 | 115 (19) | 246 (29) | |
| 65–74 | 152 (25) | 249 (30) | |
| 75–84 | 177 (29) | 164 (19) | |
| ≥85 | 99 (16) | 43 (5) | |
| Gender | .08 | ||
| Female | 292 (48) | 364 (43) | |
| Male | 320 (52) | 481 (57) | |
| Charleson-Deyo comorbidity score | .01 | ||
| 0 | 432 (71) | 629 (74) | |
| 1 | 131 (21) | 180 (21) | |
| ≥2 | 49 (8) | 36 (4) | |
| Tumor characteristics | |||
| Primary alveolar ridge site | .61 | ||
| Maxilla | 150 (25) | 192 (23) | |
| Mandible | 413 (68) | 564 (67) | |
| Unknown | 49 (8) | 89 (11) | |
| T stage | <.001 | ||
| 1 | 62 (10) | 35 (4) | |
| 2 | 47 (8) | 42 (5) | |
| 3 | 26 (4) | 16 (2) | |
| 4 | 477 (78) | 752 (89) | |
| N stage | <.001 | ||
| 0 | 412 (67) | 444 (53) | |
| 1 | 95 (16) | 125 (15) | |
| 2 | 104 (17) | 272 (32) | |
| 3 | 1 (<1) | 4 (1) | |
| Size (mm) | .003 | ||
| <10 | 34 (6) | 18 (2) | |
| 10–19 | 105 (17) | 116 (14) | |
| 20–29 | 141 (23) | 218 (26) | |
| 30–39 | 135 (22) | 211 (25) | |
| 40–49 | 87 (14) | 140 (17) | |
| ≥50 | 97 (16) | 129 (15) | |
| Unknown | 13 (2) | 13 (2) | |
| Grade | .08 | ||
| Low | 151 (25) | 171 (20) | |
| Intermediate | 361 (59) | 518 (61) | |
| High | 83 (14) | 137 (16) | |
| Unknown | 17 (3) | 19 (2) | |
| Thickness or depth of invasion (mm) | 3.0 (1.0–9.0) | 2.8 (1.2–13.0) | .11 |
| Bone invasion | .01 | ||
| Yes | 359 (59) | 551 (65) | |
| No | 253 (41) | 294 (35) | |
| Positive surgical margin | .10 | ||
| Yes | 120 (20) | 196 (23) | |
| No | 492 (80) | 649 (77) | |
| Lymphovascular invasion | <.001 | ||
| Yes | 87 (14) | 186 (22) | |
| No | 525 (86) | 659 (78) | |
| Level IV or V nodal metastasis | .97 | ||
| Yes | 33 (5) | 46 (5) | |
| No | 579 (95) | 799 (95) | |
| Extranodal extension | <.001 | ||
| Yes | 63 (10) | 196 (23) | |
| No | 549 (90) | 649 (77) | |
| Number of NCCN risk factors | <.001 | ||
| 0a | 29 (5) | 12 (1) | |
| 1 | 383 (63) | 379 (45) | |
| 2 | 121 (20) | 220 (26) | |
| 3 | 43 (7) | 118 (14) | |
| 4 | 24 (4) | 86 (10) | |
| 5 | 11 (2) | 29 (3) | |
| 6 | 1 (<1) | 1 (<1) | |
| Treatment characteristics | |||
| Chemotherapy | <.001 | ||
| Yes, concurrent with PORT | 0 (0) | 301 (36) | |
| Yes, not concurrent with PORT | 10 (2) | 20 (2) | |
| Yes, sequence unknown | 0 (0) | 30 (4) | |
| No | 569 (93) | 489 (58) | |
| Unknown | 33 (5) | 5 (<1) | |
| Radiotherapy dose (Gy) | N/A | 60 (60–66) | N/A |
| Radiotherapy fractions | N/A | 30 (30–33) | N/A |
Notes: Percentages are out of total population counts unless otherwise indicated, and may not add up to 100 due to rounding or missing values. Continuous variables are presented as median (interquartile range).
Subjects with stage T1-2N1M0.
P-values for categorical variables are from chi-square tests, and P-values from continuous variables are from pooled t tests.
The median follow-up time was 28 months for all patients and 37 months for living patients. Use of PORT was associated with improved OS (56% vs 48% at 5 years, P < .001; Figure 2A). Stratified by number of NCCN-defined risk factors, PORT was associated with significantly improved OS for patients with a single NCCN-defined risk factor (68% vs 58% at 5 years, P < .001; Figure 2B), two NCCN-defined risk factors (52% vs 31% at 5 years, P < .001; Figure 2C), and ≥3 NCCN-defined risk factors (38% vs 24% at 5 years, P < .001; Figure 2D).
FIGURE 2.
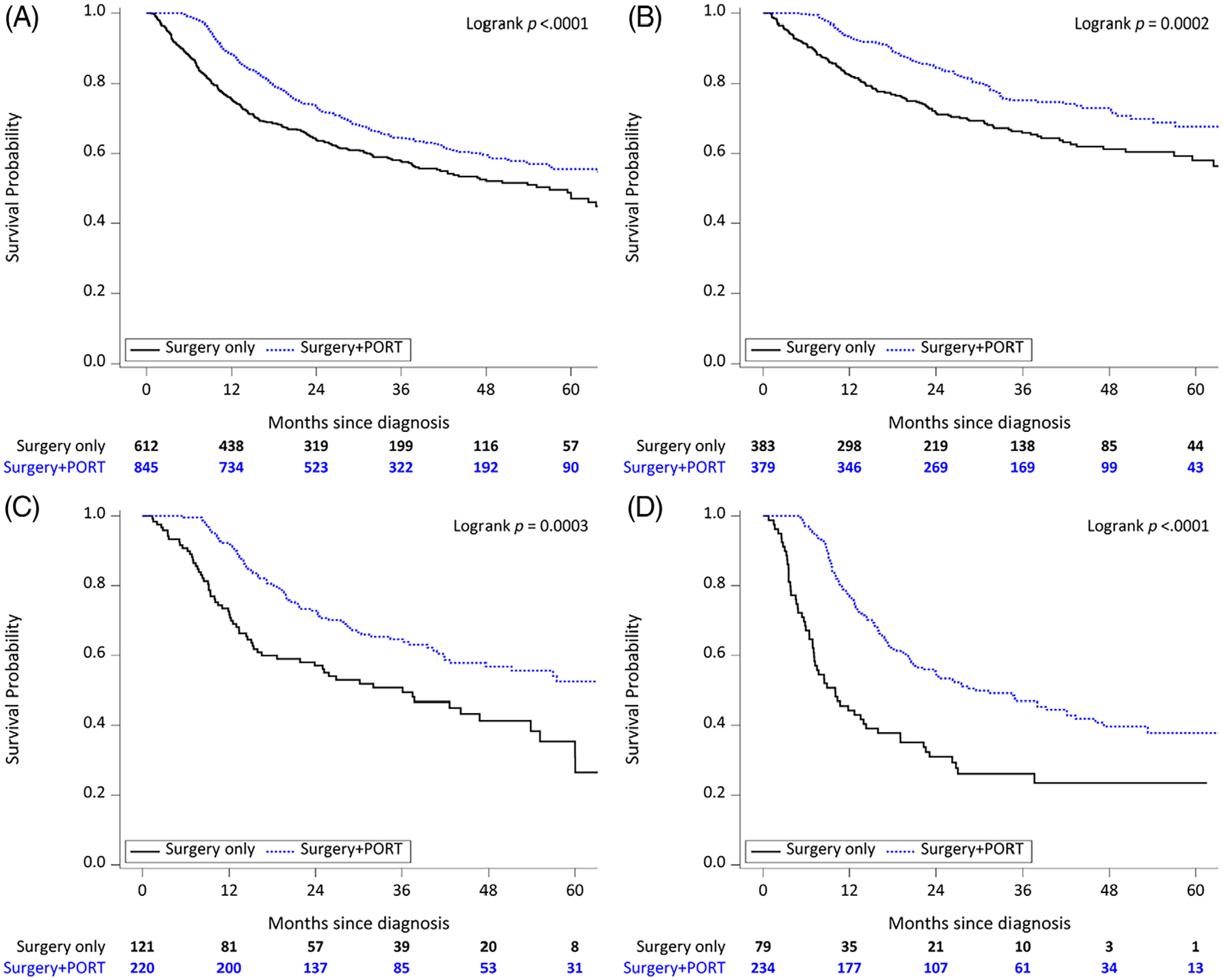
Kaplan-Meier overall survival curves for surgery only versus surgery plus postoperative radiotherapy (PORT) among (A) all patients, (B) patients with 1 National Comprehensive Cancer Network (NCCN)-defined risk factor, (C) patients with 2 NCCN-defined risk factors, and (D) patients with ≥3 NCCN-defined risk factors
Table 2 summarizes the univariate analysis results with respect to OS. Receipt of PORT was significantly associated with improved OS (hazard ratio [HR] 0.83, 95% confidence interval [CI] 0.70–0.98). Each of the assessable NCCN-defined risk factors was significantly associated with OS except for stage T3-4 (HR 1.07, 95% CI 0.84–1.37). Other prognostic variables significantly associated with worse OS included advanced age at diagnosis (HR 1.03, 95% CI 1.02–1.04), high tumor grade (HR 1.61, 95% CI 1.23–2.09), all tumor size categories ≥3 cm, and increasing number of NCCN-defined risk factors (HR 1.43, 95% CI 1.33–1.54). These results did not appreciably change even after adjusting for PORT (Supplemental Table 2).
TABLE 2.
Univariate overall survival (N = 1457)
| Variable | HR (95% CI) | P value |
|---|---|---|
| Age at diagnosis | 1.03 (1.02–1.04) | <.001 |
| Charleson-Deyo comorbidity score (ref: 0) | ||
| 1 | 1.18 (0.94–1.47) | .08 |
| ≥2 | 1.44 (1.00–2.09) | |
| Insurance (ref: private) | ||
| Government | 1.55 (1.29–1.86) | <.001 |
| Uninsured | 1.29 (0.82–2.03) | |
| Mandible primary | 0.93 (0.77–1.13) | .48 |
| Tumor size in mm (ref: <10) | <.001 | |
| 10–19 | 1.22 (0.74–2.01) | |
| 20–29 | 1.26 (0.76–2.09) | |
| 30–39 | 1.96 (1.22–3.17) | |
| 40–49 | 1.84 (1.09–3.08) | |
| ≥50 | 2.55 (1.48–4.40) | |
| Grade (ref: low) | .001 | |
| Intermediate | 1.13 (0.93–1.37) | |
| High | 1.61 (1.23–2.09) | |
| Thickness or depth of invasion in mm | 1.00 (1.00–1.01) | .29 |
| Bone invasion | 1.02 (0.86–1.21) | .84 |
| T-stage 3 or 4 | 1.07 (0.84–1.37) | .56 |
| Positive surgical margin | 1.40 (1.18–1.66) | <.001 |
| Lymphovascular invasion | 1.47 (1.20–1.80) | <.001 |
| N-stage 2 or 3 | 2.32 (1.96–2.73) | <.001 |
| Level IV or V nodal metastasis | 2.80 (2.03–3.84) | <.001 |
| Extranodal extension | 2.10 (1.74–2.54) | <.001 |
| Number of NCCN risk factors | 1.43 (1.33–1.55) | <.001 |
| PORT | 0.83 (0.70–0.98) | .02 |
| Chemotherapy | 1.24 (1.00–1.53) | .05 |
Notes: Hazard ratios (HR), confidence intervals (CI), and P-values are from Cox proportional hazards models. Postoperative radiotherapy (PORT) and chemotherapy were assessed as time-varying. Other insignificant prognostic factors included gender (P = .63), diagnosis year (P = .67), race (P = .79), income (P = .29), education (P = .37), facility type (P = .45), and facility location (P = .35).
Table 3 summarizes the results of the multivariable Cox regression analysis with respect to OS. Receipt of PORT remained significantly associated with improved OS (HR 0.73, 95% CI 0.60–0.89, P = .002). Prognostic variables significantly associated with worse OS on multivariable analysis included advanced age at diagnosis, tumor size ≥3 cm, high tumor grade, positive surgical margin, stage N2-3, level IV/V nodal metastasis, and extranodal extension.
TABLE 3.
Multivariable overall survival model (N = 1335)
| Variable | HR (95% CI) | P value |
|---|---|---|
| Age at diagnosis | 1.04 (1.03–1.05) | <.001 |
| Tumor size in mm (ref: <10) | <.001 | |
| 10–19 | 1.46 (0.85–2.49) | |
| 20–29 | 1.34 (0.78–2.33) | |
| 30–39 | 2.25 (1.32–3.84) | |
| 40–49 | 1.96 (1.13–3.39) | |
| ≥50 | 2.92 (1.67–5.13) | |
| Grade (ref: low) | .04 | |
| Intermediate | 0.97 (0.79–1.20) | |
| High | 1.31 (0.97–1.77) | |
| T-stage 3 or 4 | 1.01 (0.75–1.35) | .94 |
| Positive surgical margin | 1.56 (1.29–1.88) | <.001 |
| Lymphovascular invasion | 1.05 (0.84–1.31) | .65 |
| N-stage 2 or 3 | 1.96 (1.54–2.50) | <.001 |
| Level IV or V nodal metastasis | 1.80 (1.25–2.57) | .001 |
| Extranodal extension | 1.44 (1.08–1.92) | .01 |
| PORT | 0.78 (0.64–0.94) | .01 |
| Chemotherapy | 1.11 (0.84–1.46) | .47 |
Notes: Hazard ratios (HR), confidence intervals (CI), and P values are from Cox proportional hazards models. A robust sandwich covariance estimator was used to account for correlation of patients treated at the same facility. The multivariable model was adjusted for diagnosis year (P = .27), gender (P = .21), comorbidity score (P = .10), race (P = .90), income (P = .68), insurance (P = .46), education (P = .68), facility type (P = .63), facility location (P = .75), and great circle distance (P = .02). Postoperative radiotherapy (PORT) and chemotherapy were assessed as time-varying.
In a subgroup analysis of 779 patients with stage T3-4N0 disease, PORT was associated with improved OS on unadjusted analysis (66% vs 54% at 5 years; P < .001). Univariate analysis in this subgroup revealed that PORT was associated with improved OS (HR 0.71, 95% CI 0.55–0.92; P = .009). However, PORT was no longer significantly associated with OS in the T3-4N0 subgroup on multivariable analysis (HR 0.85, 95% CI 0.63–1.13; P = .26).
4 |. DISCUSSION
Although alveolar ridge SCC accounts for a considerable proportion of oral cavity cancers worldwide, prospective data are lacking to guide the use of PORT given that these patients have been poorly represented in randomized trials. The observation that one-third of patients in the present analysis were ≥75 years old at diagnosis may contribute to their poor representation on randomized trials. Among patients with NCCN-defined risk factors in the present NCDB analysis (45% of whom had multiple NCCN-defined risk factors), completing a course of PORT was associated with a clinically meaningful survival benefit. With the exception of stage T3-4 disease, all assessable NCCN-defined risk factors were significantly associated with worse OS on univariate and multivariable analyses. Because small tumors with minimal bone invasion are T4 by definition, the majority of patients in this study had stage T4 disease. Notably, primary tumor size was more prognostic than pathologic T stage, and primary tumor size ≥3 cm was consistently associated with worse OS on unadjusted subgroup analysis, univariate analysis, and multivariable analysis.
The NCCN guidelines recommend consideration of PORT with or without systemic therapy for resected oral cavity cancers with extranodal extension, positive surgical margins, advanced T stage (T3-4), advanced N stage (N2-3), nodal metastasis in cervical levels IV/V, lymphatic invasion, perineural invasion, and/or vascular embolism. Unfortunately, the NCDB does not report information regarding the latter two risk factors. The present analysis identified two other adverse pathologic features that warrant consideration of PORT, especially when clustered with other risk factors: primary tumor size ≥3 cm and high tumor grade.
The depth of bone invasion by the primary tumor (eg, cortical vs medullary) is not captured within the NCDB. A meta-analysis of oral cavity SCC demonstrated no association between mandibular cortical invasion and OS on both unadjusted and adjusted analyses, which is in contrast to deeper medullary mandibular invasion.18 Alveolar ridge SCC tends to invade bone early and small tumors may have minimal cortical erosion, which technically warrants consideration of PORT based on NCCN guidelines. To determine whether concomitant nodal disease was potentially driving the survival benefit associated with PORT for patients with T3-4 disease, we performed a subgroup analysis of >700 patients with T3-4N0 alveolar ridge SCC. For these node-negative patients with locally advanced primary tumors, multivariable analysis showed no significant association between PORT and OS. A retrospective study of 41 patients with cortical invasion identified no difference in locoregional control rates compared to 396 patients without bone invasion; however, the authors were hesitant to recommend omission of PORT because more than double the proportion of patients with cortical invasion received PORT (73% vs 34%).19 Another retrospective study of 32 patients with cortical erosion reported local recurrence outcomes of patients treated with PORT (2/3), chemoradiotherapy (2/4), and no adjuvant therapy (4/25). Due to the small patient numbers in these groups, the authors were unable to determine the effect of adjuvant therapy on local control.20 A retrospective analysis of 302 patients with oral SCC demonstrated that bone invasion was not significantly associated with recurrence-free survival on multivariable analysis, although all patients received PORT.21
In contrast to other oral cavity sites, tumor thickness/depth of invasion of alveolar ridge SCC was not significantly associated with OS in the present study. However, there were two notable shortcomings with the coding of tumor thickness/depth of invasion in this NCDB analysis. First, these two distinct pathologic features were coded as a single variable, which is problematic because of their differing numerical thresholds for predicting regional nodal metastasis.22 Second, nearly half (48%) had missing data. A literature review and meta-analysis identified tumor thickness and depth of invasion as important prognostic factors for oral cavity cancers, although primary tumors involving the thin mucosal layer of the alveolar ridge were not included in these publications.23,24
This study is subject to the inherent shortcomings of the NCDB, including coding errors, potentially incomplete or incorrect radiotherapy doses, and missing data for certain variables leading to exclusion of some patients from analysis. A major limitation given the retrospective study design is selection bias leading to imbalance between the study groups. For example, patients in the PORT cohort tended to be younger with fewer comorbidities, and thus sufficiently healthy to receive adjuvant treatment. Therefore, older patients with more comorbidities in the surgery only cohort can account for some of the survival differences noted. Nevertheless, the PORT cohort also had significantly increased proportions of all adverse pathologic features except for positive surgical margin and lymph node metastases in cervical levels IV/V. Furthermore, PORT remained associated with improved OS on multivariable analysis. The dose of PORT most commonly used in this analysis (60–66 Gy in 2 Gy daily fractions) is similar to doses used in randomized studies that have demonstrated a benefit of adjuvant radiotherapy or concurrent chemoradiotherapy for high-risk head and neck cancer.12,13,25,26 The present analysis cannot address appropriate PORT treatment volumes due to limitations in radiotherapy data available in the NCDB. Additionally, because locoregional recurrence data are not captured in the NCDB, we cannot rule out the possibility of a locoregional control benefit even in the absence of a survival benefit in the subgroup of patients with stage T3-4N0.
In conclusion, PORT for resected alveolar ridge SCC with adverse pathologic features is associated with significantly improved OS. PORT with or without systemic therapy should be considered for patients with primary tumors ≥3 cm (especially in the presence of other adverse pathologic features such as lymphovascular invasion, perineural invasion, or high tumor grade), positive surgical margin(s), or advanced nodal disease (ie, stage N2-3, metastasis in cervical levels IV/V, or extranodal extension).
Supplementary Material
Abbreviations:
- NCDB
National Cancer Database
- NCCN
National Comprehensive Cancer Network
- OS
overall survival
- PORT
postoperative radiotherapy
- RTOG
Radiation Therapy Oncology Group
- SCC
squamous cell carcinoma
Footnotes
Abstract previously presented at the National Comprehensive Cancer Network (NCCN) Annual Conference on March 22, 2019 in Orlando, FL.
Publisher's Disclaimer: DISCLAIMER
Publisher's Disclaimer: The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Jainkittivong A, Swasdison S, Thangpisityotin M, Langlais RP. Oral squamous cell carcinoma: a clinicopathological study of 342 Thai cases. J Contemp Dent Pract. 2009;10:E033–E040. [PubMed] [Google Scholar]
- 2.Effiom OA, Adeyemo WL, Omitola OG, Ajayi OF, Emmanuel MM, Gbotolorun OM. Oral squamous cell carcinoma: a clinicopathologic review of 233 cases in Lagos, Nigeria. J Oral Maxillofac Surg. 2008;66:1595–1599. [DOI] [PubMed] [Google Scholar]
- 3.Tandon P, Dadhich A, Saluja H, Bawane S, Sachdeva S. The prevalence of squamous cell carcinoma in different sites of oral cavity at our Rural Health Care Centre in Loni, Maharashtra – a retrospective 10-year study. Contemp Oncol. 2017; 21:178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shingaki S, Nomura T, Takada M, Kobayashi T, Suzuki I, Nakajima T. Squamous cell carcinomas of the mandibular alveolus: analysis of prognostic factors. Oncology. 2002;62:17–24. [DOI] [PubMed] [Google Scholar]
- 5.Alves AM, Correa MB, Silva KDD, et al. Demographic and clinical profile of oral squamous cell carcinoma from a service-based population. Braz Dent J. 2017;28:301–306. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs CD, Barbour AB, Mowery YM. The relative distribution of oral cancer in the United States by subsite. Oral Oncol. 2019; 89:56–58. [DOI] [PubMed] [Google Scholar]
- 7.Siriwardena B, Rambukewela IK, Pitakotuwage TN, Udagama M, Kumarasiri PVR, Tilakaratne WM. A predictive model to determine the pattern of nodal metastasis in oral squamous cell carcinoma. Biomed Res Int. 2018;2018: 8925818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel RS, Dirven R, Clark JR, Swinson BD, Gao K, O’Brien CJ. The prognostic impact of extent of bone invasion and extent of bone resection in oral carcinoma. Laryngoscope. 2008;118: 780–785. [DOI] [PubMed] [Google Scholar]
- 9.Genden EM, Rinaldo A, Jacobson A, et al. Management of mandibular invasion: when is a marginal mandibulectomy appropriate? Oral Oncol. 2005;41:776–782. [DOI] [PubMed] [Google Scholar]
- 10.Truitt TO, Gleich LL, Huntress GP, Gluckman JL. Surgical management of hard palate malignancies. Otolaryngology–Head and Neck Surgery. 1999;121(5):548–552. 10.1016/s0194-5998(99)70084-7. [DOI] [PubMed] [Google Scholar]
- 11.Cronin KA, Lake AJ, Scott S, et al. Annual report to the nation on the status of cancer, part I: National Cancer Statistics. Cancer. 2018;124:2785–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. [DOI] [PubMed] [Google Scholar]
- 13.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. [DOI] [PubMed] [Google Scholar]
- 14.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15: 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2014;89:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–5067. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs CD, Carpenter DJ, Hong JC, Havrilesky LJ, Sosa JA, Chino JP. Radiation records in the National Cancer Database: variations in coding and/or practice can significantly alter survival results. JCO Clin Cancer Inform. 2019;3:1–9. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Lin J, Men Y, Yang W, Mi F, Li L. Does medullary versus cortical invasion of the mandible affect prognosis in patients with oral squamous cell carcinoma? J Oral Maxillofac Surg. 2017;75:403–415. [DOI] [PubMed] [Google Scholar]
- 19.Ebrahimi A, Murali R, Gao K, Elliott MS, Clark JR. The prognostic and staging implications of bone invasion in oral squamous cell carcinoma. Cancer. 2011;117:4460–4467. [DOI] [PubMed] [Google Scholar]
- 20.Petrovic I, Montero PH, Migliacci JC, et al. Influence of bone invasion on outcomes after marginal mandibulectomy in squamous cell carcinoma of the oral cavity. J Craniomaxillofac Surg. 2017;45:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan K-H, Wang H-M, Kang C-J, et al. Treatment results of postoperative radiotherapy on squamous cell carcinoma of the oral cavity: coexistence of multiple minor risk factors results in higher recurrence rates. Int J Radiat Oncol Biol Phys. 2010;77: 1024–1029. [DOI] [PubMed] [Google Scholar]
- 22.Liu B, Amaratunga R, Veness M, et al. Tumor depth of invasion vs tumor thickness in determining risk of nodal disease in early oral tongue squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2018;102:e331–e332. [Google Scholar]
- 23.Pentenero M, Gandolfo S, Carrozzo M. Importance of tumor thickness and depth of invasion in nodal involvement and prognosis of oral squamous cell carcinoma: a review of the literature. Head Neck. 2005;27:1080–1091. [DOI] [PubMed] [Google Scholar]
- 24.Huang SH, Hwang D, Lockwood G, Goldstein DP, O’Sullivan B. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity. Cancer. 2009;115:1489–1497. [DOI] [PubMed] [Google Scholar]
- 25.Bachaud JM, Cohen-Jonathan E, Alzieu C, David JM, Serrano E, Daly-Schveitzer N. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: final report of a randomized trial. Int J Radiat Oncol Biol Phys. 1996;36:999–1004. [DOI] [PubMed] [Google Scholar]
- 26.Fietkau R, Lautenschläger C, Sauer R, et al. Postoperative concurrent radiochemotherapy versus radiotherapy in high-risk SCCA of the head and neck: results of the German phase III trial ARO 96–3. J Clin Oncol. 2006;24:5507. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


