Graphical abstract
Keywords: Viruses, Surfaces, Coatings, Antiviral agents, Nanoparticles
Abstract
Viruses pose a serious threat to human health and society in general, as virus infections are one of the main causes of morbidity and mortality. Till May 2022, over 513 million people around the world have been confirmed to be infected and more than 6.2 million have died due to SARS-CoV-2. Although the COVID-19 pandemic will be defeated in the near future, we are likely to face new viral threats in the coming years. One of the important instruments to protect from viruses are antiviral surfaces, which are essentially capable of limiting their spread.
The formulation of the concept of antiviral surfaces is relatively new. In general, five types of mechanism directed against virus spread can be proposed for antiviral surfaces; involving: direct and indirect actions, receptor inactivation, photothermal effect, and antifouling behavior. All antiviral surfaces can be classified into two main types - passive and active. Passive antiviral surfaces are based on superhydrophobic coatings that are able to repel virus contaminated droplets. In turn, viruses can become biologically inert (e.g., blocked or destroyed) upon contact with active antiviral surfaces, as they contain antiviral agents: metal atoms, synthetic or natural polymers, and small molecules. The functionality of antiviral surfaces can be significantly improved with additional properties, such as temperature- or pH-responsivity, multifunctionality, non-specific action on different virus types, long-term application, high antiviral efficiency and self-cleaning.
1. Introduction
Undoubtedly, a pandemic affects the lives of each individual citizen and society as a whole. Being in a turbulent environment for more than a year, we have learned to adapt to new conditions - remote work and online negotiations. The pandemic has left its mark on almost all areas of our activity.
Due to the pandemic, the issue of health and hygiene is in the center of attention. Of course, we all want to be healthy and do everything for it. We wear masks, wash our hands more often, and carry out intensive disinfection. But, unfortunately, the statistics of the pandemic are disappointing. This means that new and more modern methods of fighting infections must be used. The future of hygiene by long-acting disinfectants, just as soap and water were the future of hygiene 100 years ago. The most appealing solution to the problem these days is the design of antiviral coatings of various types, which can be applied to the surfaces encountered daily that serve as the platforms for virus spread. The pandemic has dramatically increased interest and demand for antiviral coatings. Modern biomaterials are one of the human achievements, based on a deeper understanding of how biomaterials interact with biological systems at both the cellular and molecular levels. The ultimate purpose is to create more suitable materials, including antiviral surfaces.
The current situation allows us to assume that the COVID pandemic is not the last one and a new pandemic will appear in the future. Fortunately, previous coronavirus outbreaks of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) passed relatively lightly, providing the opportunity to obtain more information on coronavirus infections [1]. The SARS-CoV-2 pandemic has an enormous influence in the world on the health of people, social behavior, and economic situation. Undoubtedly, great progress has been made in discovering the origin, transmission, pathogenesis, diagnosis, and treatment of SARS-CoV-2 infection. However, more research is required to continue the battle against SARS-CoV-2, and cooperation between governments, academia, and industries is crucial to make this happen [2]. Additional risks of the pandemic can be generated by booming space programs, with hitchhiking Earth’s viruses becoming pathogenic in space [3] or even extraterrestrial viruses infecting humans [4].
Nanomaterials have promising potential to fight against various viruses, either by providing sensitive and economical nanosensors for rapid viral detection, or by developing translatable nanovaccines and broad-spectrum nanomedicine for virus treatment [5]. Great progress in the fabrication of the antiviral nanostructured surfaces is first of all related to the production of antiviral face masks. Although different antiviral mask materials have been patented in this century, we have observed quite recently an accelerated application of both active and passive antiviral surfaces to substantially improve the performance of facial masks [6], [7]. Other applications involve, in addition to personal protection and devices, ubiquitous surfaces of medical and public space. Passive antiviral defense prevents virus contamination without blocking or destroying virus particles. This principal new approach, based on the application of superhydrophobic coatings, protects the surfaces from the adsorption of the virus particles. In contrast to passive antiviral approach, active antiviral surfaces contain agents engaged in antiviral activity, such as metal atoms, synthetic or natural polymers, and small antiviral molecules. These antiviral agents are able to block or destroy virus particles since they have an active impact on virus particles.
As described above, antiviral coatings are very relevant today. Therefore, many research groups are working to create different types of materials that can be used as protective antiviral surfaces. This article attempts to classify, compare, and describe antiviral coatings. Compared to other similar reviews, this article has at least five advantages. First, a modern classification of antiviral surfaces is introduced based on passive and active antiviral surfaces, including different mechanisms of antiviral actions. Second, complex information is presented on the impact of non-specific and specific interactions on virus deposition and infectivity. Third, the problem of the fabrication of the antiviral surfaces is outlined from different viewpoints, including information not only on the synthesis and characterization of the nanostructured antiviral surfaces but also taking into account the physico-chemical and biological properties of the virus particles. Fourth, different types of antiviral coatings are compared, their advantages and disadvantages are shown. And lastly, our attention is not focused on only one species of viruses, but the impact of the antiviral surface on different viruses is compared.
2. Impact of non-specific and specific interactions on virus deposition and infectivity
2.1. Non-specific interactions
The physico-chemical and biological properties of the surface exposed to viruses, such as its chemical composition, charge, hydrophilicity/hydrophobicity and roughness, but also the surface properties of the material before and after colonization by biofilms can affect non-specific virus deposition [8], [9]. Also, the size of the viral particles and their surface properties play an important role in this process. In an aqueous environment, additionally, the characteristics of the water (pH, ionic strength, temperature) are substantial factors [8], [9].
Viruses have different mechanisms of deposition on surfaces depending on the physico-chemical properties of the viral particle. Understanding the mechanisms of viral deposition is important for the development of surfaces with antiviral properties [10].
Virus deposition on surfaces from water or air has probably a similar character as numerous viral particles are deposited on the surfaces from virus-laden respiratory droplets. As is well known, electrostatic interactions between oppositely charged surfaces and viral particles usually attribute to extensive adsorption of viral particles [11], [12]. In general, the behavior of the viral particles is similar to that of colloid particles. The deposition of viruses on surfaces is governed by the total free energy of adsorption [13]. In work [14] it was summarized that knowing the isoelectric point of the virus (pI) enables us to predict its adsorptive interactions with a surface of known charge, as long as the suspending medium conditions are well-defined and controlled. The interactions between virus and surfaces are likely to be governed mainly by long-range electrostatics, while shorter-range van der Waals interactions might be less important [9], [15].
In addition to electrostatic interactions, wettability plays a crucial role in viral particle deposition. The impact of wettability was demonstrated by the successful application of superhydrophilic fabric to separate viruses from virus-containing water droplets and prevent viruses from spreading in water droplets over a long distance [16].
In the excellent work [17], the adsorption of P22 virus-like particles VLPs on surfaces functionalized with silanes with three different terminal groups, i.e. amino- and glycidyloxy-silanes inducing an acid-like and a base-like (polar, hydrophilic) surface tension, and perfluoro-silane inducing a dispersive surface tension (hydrophobic and oleophobic), was studied in detail. The bacteriophage P22 VLP presents an icosahedral structure and can be adsorbed with different orientations, exposing fivefold (S5), threefold (S3), or twofold (S2) symmetry axis. It was observed, that perfluoro-silane strongly enhanced the adsorption of P22 VLPs particles, which exhibited also dominant S5 orientation. In turn, both lower adsorption of P22 bacteriophages and their dominant orientation S2 and S3 characterized the situation on hydrophilic surfaces. It was concluded, that the higher P22 affinity to perfluoro-silanized surface is caused by hydrophobic interactions of this surface with hydrophobic P22 residues at S5 pentons [17].
As outlined above, numerous factors can affect non-specific viral particle deposition, and their majority is related to the physico-chemical properties of both the surfaces and viral particles. They include long-range electrostatics and shorter-range van der Waals components of free energies, as well as their hydrophobic/hydrophilic balances.
We believe, that traditional approaches using non-specific interactions to protect surfaces against virus deposition are not sufficient nowadays. This is mainly due to the fact, that different species of virus have different physico-chemical properties. Hence, ‘ideal’ antiviral surfaces cannot be fabricated without nanotechnological approaches. Therefore, approaches based on active and passive antiviral surfaces have been intensively developed in recent years, widely introducing the concept of antiviral surfaces into bionanotechnology.
2.2. Specific interactions
To develop new methods to inactivate viral particles, it is important to understand their structure as a biological object. In general, an entry of a virus into host cells is governed by specific interactions between viral particles and cellular receptors. Virus–host interactions are complex processes that span from binding to cells, entry, dissemination, and finally lytic or persistent infection. The first step, binding to host molecules that serve as viral receptors, is critical for all subsequent events.
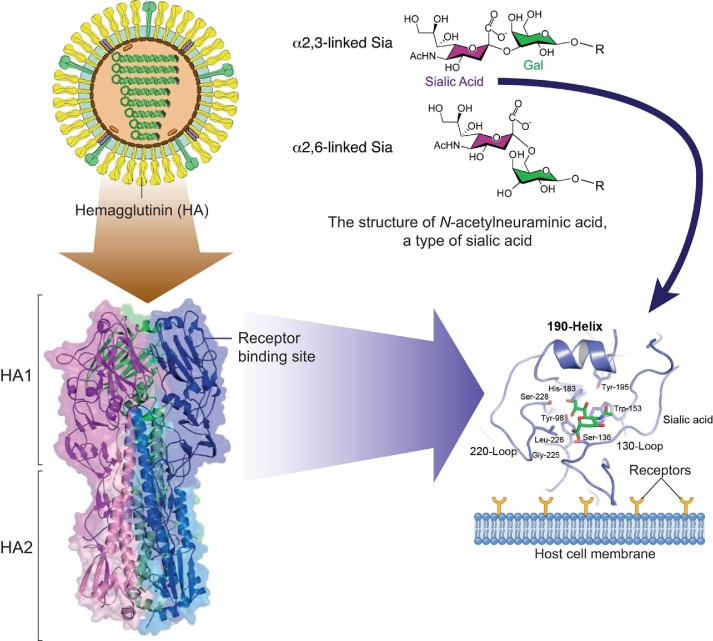
As an example, the influenza virus is an enveloped virus of the Orthomyxoviridae family. The life cycle starts after the attachment of virus to the host cell via viral hemagglutinin. Hemagglutinin is a trimeric surface glycoprotein receptor that recognizes sialic acids on the surface of host cells [18] (Fig. 1 ).
Fig. 1.
Interaction of hemagglutinin (HA) of the influenza A virus with the receptor on the cell surface. Top: left: model of the virion structure of the influenza A virus; right: structure of N-acetylneuraminic acid, a type of sialic acid. Bottom: left: model of structure of hemagglutinin, which is the trimer, all monomers of which consists of two subunits (HA1 and HA2); right: the receptor binding site is magnified and reoriented to depict structural features and conserved residues in the binding pocket, along with the location occupied by bound sialic acid, shown in green. All numbers based on H3 subtype of hemagglutinin [19], [20], [21] (all panels were adapted with permission form Refs. [19], [20], [21]).
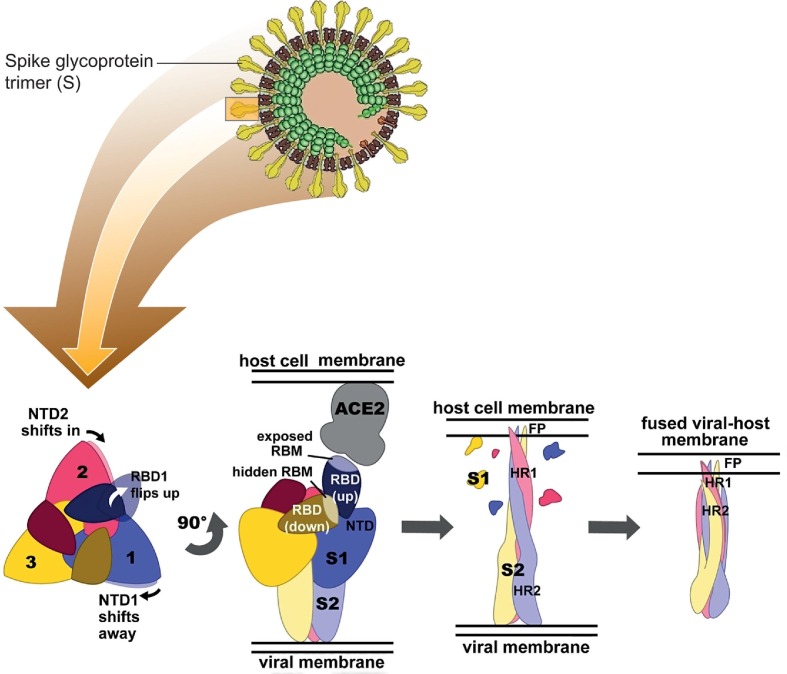
In turn, the coronavirus spike (S) glycoprotein is the main antigen presented at the viral surface of coronaviruses. The CoV S protein is responsible for host cell attachment and mediates host cell membrane and viral membrane fusion during infection. The S protein includes the receptor binding domain (RBD), with the receptor binding motif (RBM) that directly interacts with the receptors of angiotensin converting enzyme 2 (ACE2) in host cells. The application of substances for effective blocking of the virus receptor binding motifs is a promising method for the fabrication of the antiviral surfaces (Fig. 2 ).
Fig. 2.
Model of the virion structure of SARS-CoV-2 (top) and conformational changes in the spike ectodomain during membrane fusion (bottom). Bottom: left: top view of prefusion SARS-CoV-2 S. The conformational changes in adjacent N-terminal domains NTDs (NTD1 and NTD2) as the receptor-binding domain (RBD) of protomer shifts to the upward position are indicated with arrows; middle-left: side view of prefusion SARS-CoV-2 S with two RBDs in the down conformation (RBMs (receptor-binding motif) hidden) and one RBD in the upward conformation (RBM exposed), bound to the ACE2 receptor; middle-right: side view of postfusion SARS-CoV-2 S with S1 (receptor-binding subunit of spike glycoptotein) shed and S2 (membrane-anchored subunit of spike glycoptotein) subunits elongated towards the host cell membrane with FP (fusion peptide) inserted; right: side view of postfusion SARS-CoV-2 S after a collapse that allowed HR2 (heptad repeats 2) to form a six-helix bundle with HR1 (heptad repeats 1), resulting in fusion of the viral membrane with the host cell membrane [22], [23] (all panels were adapted with permission form Refs. [22], [23]).
3. Approaches based on active and passive antiviral surfaces
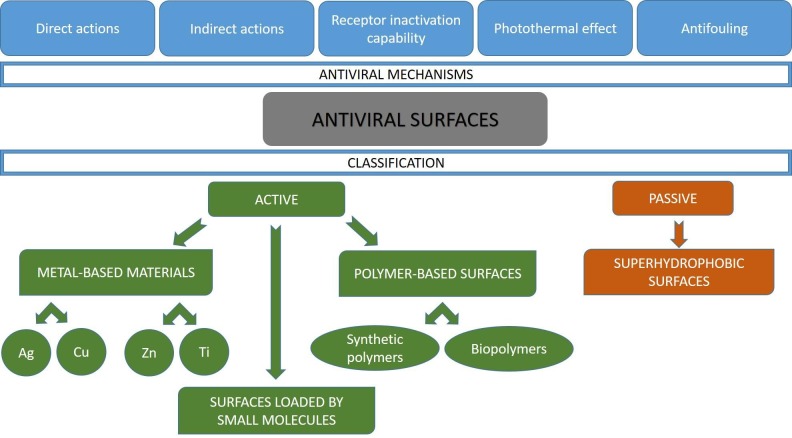
The term ‘antiviral surfaces’ has appeared first as a title in 2020 [24], implying the novelty of the formulation of this concept. The strategy to prevent virus transmission through disruption of virus survival or deposition on ubiquitous surfaces is based on different mechanisms ‘added’ to the surfaces with antiviral agents or surface modifications [24]. In general, five main antiviral mechanisms can be proposed, as shown in Fig. 3 ; involving: direct and indirect actions, virus receptor inactivation, photothermal effect and antifouling behavior (for superhydrophobic surfaces). The direct action of the antiviral agents is based on the inactivation of the viral particles at contact. The indirect action of antiviral agents leads to the stimulated formation of highly reactive species, such as reactive oxygen species, which are able to effectively act on viral particles. Sometimes, the photothermal mechanism is also considered as an indirect antiviral action, but it is so specific that it should be assigned to a separate group. The photothermal effect consists of energy absorption by a nanostructured material, and the resulting energy dissipation as a heat in the vicinity of the nanostructure. Receptor inactivation relies on blocking virus receptors, which are then unable to interact with host receptors. Finally, the antifouling behavior of the superhydrophobic coatings repels virus contaminated droplets.
Fig. 3.
Types of antiviral mechanisms and classification of active and passive antiviral surfaces.
In addition, it is convenient to divide all antiviral surfaces into two main types – passive and active ones. Passive antiviral surfaces prevent the deposition of viruses through the application of superhydrophobic coatings. In turn, active antiviral surfaces contain agents engaged in antiviral activity, such as metal atoms, synthetic or natural polymers, and small molecules. They include metal-based materials, coatings loaded by small molecules, and polymer-based coatings. An appropriate discussion on different types of surfaces and related mechanisms of antiviral actions is given below.
3.1. Approaches based on passive antiviral surfaces
The first step to defend the surface from virus invasion is to prevent them from adsorption of virus particles. In recent years, a new trend of surface design has been intensively developed to protect them from the accumulation of proteins, bacteria, and marine organisms [25]. In case of viruses, an analogous approach is completely new and only a limited number of papers describe passive antiviral surfaces able to prevent virus contamination without blocking or destroying virus particles.
In the case of SARS-CoV-2, virus-laden respiratory droplets (from less than 1 to 2000 μm in diameter [26]) are released from infectious people. The virus laden airborne droplets can be deposited on surfaces of various objects to form fomites, which may cause indirect infection. Recently, a principally new approach was developed to protect the surfaces from virus-laden droplets, based on the application of superhydrophobic coatings. Superhydrophobic coatings are defined as coatings with water contact angles greater than 150° and sliding angles less than 10°, attributed mainly to two parameters: low surface energy and micro- and nano-hierarchical surface roughness [27], [28]. Theoretical reflections on the application of superhydrophobic surfaces as effective antiviral surfaces were presented by Meguid [29]. Based on these considerations, the surface should repel contaminated droplets or body fluids to prevent adhesion, keeping the virus encapsulated and suppressing surface contaminants [29].
Theoretical assumptions presented in [29] were confirmed in excellent experimental works [10], [30]. In the first work [10], two superhydrophobic coatings, i.e., commercially available Glaco (SOFT99, GLACO) and hydrophobic aerosil coating, were fabricated on six commonly used substrates (copper, glass, glove, face mask, plastic, and steel) with different wettability. Both coatings are composed of silanized silica nanoparticles with a comparable average diameter of nearly 30 nm. The general scheme of the fabrication of passive antiviral surfaces is presented in Fig. 4 a. The adhesion of SARS-CoV-2 viruses was determined not by static contact angles, but by dynamic contact angles that characterize solid–liquid adhesion. The amount of attached viruses was determined to be proportional to the cube of solid–liquid adhesion [10]. Furthermore, practically no virus attachment was concluded on superhydrophobic surfaces with adhesion reduced below 1 mN [10]. Compared to bare surfaces, all superhydrophobic surfaces exhibited a significant reduction in SARS-CoV-2 attachment, at least 99.997% and 23.6% on Aerosil and Glaco coatings, respectively. This strategy was continued in work [30], where materials encountered daily, such as glass, fabric (polyester), steel, copper, mask, nitrile glove, and paper, were used as substrates. Repellent superhydrophobic coatings were fabricated by functionalizing various nanostructures, including SiO2, TiO2 nanoparticles, CuO nanoflakes, and Ag nanocrystals. The results obtained confirmed the results presented in a previous publication [10]. Fig. 4b illustrates the formation of fomite on typical surfaces and the prevention of fomite formation on superhydrophobic surfaces.
Fig. 4.
General scheme of fabrication of the passive antiviral surfaces (a); fomite formation for typical surfaces, and prevention of fomite formation for superhydrophobic surfaces (b).
3.2. Approaches based on active antiviral surfaces
3.2.1. Metal-based antiviral surfaces
In general, metal-based antiviral surfaces can be grouped in two classes. The first class includes ‘pure’ metal surfaces, including entirely metallic products, whereas the second class includes products with metalized surfaces or coatings composed of metallic nanoparticles, often embedded in polymer matrix [31], [32], [33], [34], [35].
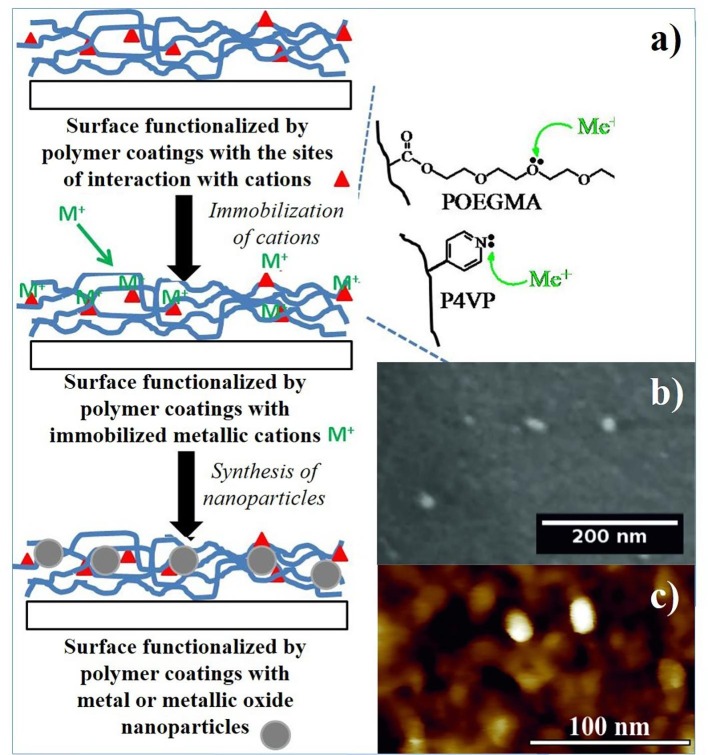
A simple fabrication of nanocomposite coatings based on polymers and metallic nanoparticles is presented in Fig. 5 a. In this approach, interactions between specific motifs of coated polymers and metallic cations are employed. For example, the strong affinity between metallic cations and nitrogen in the pyridyl group of poly(4-vinylpyridine) (P4VP) or with oxygen in the ether groups of poly(oligo(ethylene glycol)methacrylates (POEGMA) leads to immobilization of metallic cations in polymer coatings. Finally, metallic nanoparticles can be synthesized using different chemical approaches [31], [32], [33], [34]. Typical micrographs of silver nanoparticles incorporated in P4VP coatings, provided by scanning electron microscopy and atomic force microscopy, are presented in Fig. 5b and 5c, respectively.
Fig. 5.
Fabrication of nanocomposite coating based on polymers and metallic nanoparticles (a). Typical images of Ag nanoparticles incorporated in P4VP coatings, provided by scanning electron microscopy (b) and atomic force microscopy (c).
More than 30 metals and their salts or oxides have antiviral potential, including silver (Ag), gold (Au), bismuth (Bi), cobalt (Co), copper (Cu), iron (Fe), mercury (Hg), manganese (Mn), nickel (Ni), lead (Pb), platinum (Pt), antimony (Sb), tin (Sn), zinc (Zn) and titanium (Ti) [36]. Some of these metallic antiviral agents are extremely toxic to humans, highly polluting the environment, or too expensive for application at the industrial scale. Therefore, today, great practical applications may be proposed only for silver, copper, zinc, titanium, and their intermetallic-based antiviral coatings.
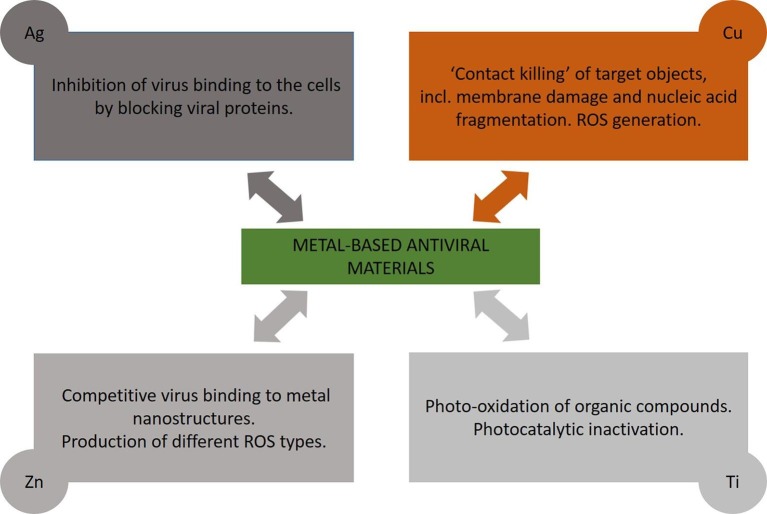
We review in the following the reports on metal-based antiviral surfaces using Ag, Cu, Zn and Ti, with representative studies outlined in Table 1 , with respect to coating material and virus type. In turn, the main concluded mechanisms of the antiviral actions of metal-based coatings are summarized in Fig. 6 .
Table 1.
Metal-based antiviral surfaces.
| Coating Materials | Virus | References |
|---|---|---|
| Cu and Cu2O | HCoV-229E | [38] |
| AgNPs with an aqueous extract | influenza A virus (strain A/PR/8) | [39] |
| Silver nanoparticles (AgNPs) | Chikungunya virus | [40] |
| Ag2O/AgO NPs | Herpes Simplex virus (HSV-1) | [41] |
| AgNPs | HIV-1 | [42] |
| AgNPs (10–80 nm, with or without polysaccharide coating | Monkeypox virus Zaire strain (MPOX-Z | [44] |
| Ag-NPs coated polyurethane condom | HIV-1 and herpes simplex virus (HSV) | [45] |
| Silver nanoparticles (AgNP) | SARS-CoV-2 | [47] |
| Ag/SiO2 nanoparticle film | SARS-CoV-2 | [50] |
| Copper or stainless steel | Influenza A virus | [58] |
| Alloys, containing over 60% copper | Murine norovirus (MNV-1) | [59] |
| Cu2O | bacteriophages T4 and Qβ | [60] |
| Copper iodide (CuI) particles | A/California/07/09 (H1N1) pdm (A/H1N1pdm) | [61] |
| Copper oxide particles | Human immunodeficiency viruses (HIV-1), human influenza A virus (H1N1) and avian influenza virus (H9N2) | [62] |
| Cuprous oxide Nanoparticles CuO-NPs | Hepatitis C Virus (HCV) | [64] |
| Copper surface | Human coronavirus 229E (HuCoV-229E) | [65] |
| CuO nanoparticle | SARS-CoV-2 | [50] |
| ZnO nanostructures | Herpes simplex virus (HSV-1) | [79] |
| TiO2- and TiO2–Ag (Ti:Ag atomic ratio 1:0.04)-coated ceramic tiles | SARS-CoV-2 | [84] |
| TiO2 nano-colloids | Newcastle virus (NDV) | [85] |
| TiO2 particles | MS-2 phage and Escherichia coli | [86] |
| TiO2 | Murine norovirus, HSV-1, bacteriophage MS2, Hepatitis B, human rotavirus strain Odelia, a simian rotavirus strain SA11, human astrovirus serotype 1 (HAstV-1), and FCV strain F4 | [87], [88], [89], [90], [91] |
Fig. 6.
Main mechanisms of the antiviral actions of metal-based coatings.
3.2.1.1. Silver-based antiviral surfaces
The biological activity of silver (Ag) has been recognized for thousands of years. Since ancient times, silver has been widely used in containers to prevent putrefaction of liquids and foods [37]. Nowadays, silver is one of the best known antiviral materials with promising advantages. However, extensive production and application of the silver coatings are strongly limited due to relatively high costs.
Recent research showed that 50 nm thick Ag film coated on glass substrates by thermal evaporation has a viral titer of coronavirus 229E essentially lower than for native glass [38], but silver nanoparticle coatings demonstrated significantly better antiviral results. It is likely that silver-based materials are more effective in the nanoparticle form, where the nanoparticles can directly bind to viral proteins.
In work [39], a simple and inexpensive ultra-sonication method was used to synthesize quasi-silver nanoparticles (AgNPs) with an aqueous extract from Panax ginseng roots. The biomolecules present in these plant extracts are often used to reduce metal ions to nanoparticles in a single-step green synthesis route. The AgNPs were spherical with sizes ranging from approximately 5–15 nm. They were also shown to be virucidal against the influenza A virus (strain A/PR/8) [39]. In a similar work [40], AgNPs were synthesized using Andrographis paniculata, Phyllanthus niruri, and Tinospora cordifolia and their antiviral properties against the chikungunya virus were demonstrated. An in vitro antiviral assay of AgNPs showed that AgNPs from A. paniculata were the most effective, followed by AgNPs from T. cordifolia and P. niruri. In turn, two blue-green algal strains, Oscillatoria sp. and Spirulina platensis, were used to biosynthesize Ag2O/AgO NPs 41]. The obtained nanoparticles showed a high reduction rate (reaching 49%) of the initial HSV-1 particles.
Research on AgNP interaction with HIV-1 demonstrated the inhibited infectivity of the virus in vitro, caused by binding of NPs to the disulfide bond regions of the CD4 binding domain within the gp120 glycoprotein subunit [42], [43]. In the work [43], the binding of AgNP to the gp120 subunit was size dependent as particles greater than 10 nm were not observed to be attached to the viral envelope.
AgNPs (10 to80 nm, with or without polysaccharide coating) were evaluated for efficacy against the monkeypox virus Zaire strain (MPOX-Z, CDC isolate V79-I-005). All types of Ag-NPs showed a significant dose-dependent effect of test compound concentration on the mean number of plaque-forming units [44]. In turn, in the work [45] an Ag-NP coated polyurethane condom was developed, presenting the ability to efficiently inactivate HIV-1 and herpes simplex virus (HSV) infectiousness.
The excellent review prepared by Galdiero et al. summarized the application of AgNP against a wide range of viruses, including retroviridae viruses (i.e. HIV virus), herpesvirus, paramyxoviridae viruses (such as respiratory syncytial virus), Hepatitis B virus, influenza virus, by interfering with the binding of cellular receptors or inhibiting viral replication [46].
Recently, numerous papers have been devoted to the impact of silver coatings and nanoparticles on SARS-CoV-2 [47], [48], [49], [50]. In the works [47], [48], [49], [50], where AgNPs of different sizes and concentrations were tested, it was shown that nanoparticles of diameter around 10 nm are effective in inhibiting extracellular SARS-CoV-2 at concentrations ranging between 1 and 10 ppm, while cytotoxic effect for cells was observed at concentrations of 20 ppm and above. Antiviral tests for cotton fabric surface with photodeposited 10 nm AgNPs exhibited a specific viral reduction of 97% for SARS-CoV-2 [48]. Sputtered coatings of silver nanocluster/ silica composite that were deposited on FFP3 masks showed a reduction in virus infectivity of one order of magnitude for coatings containing a lower silver concentration, and the complete removal of the infectivity at a higher silver concentration [49]. The Ag/SiO2 nanoparticle film exhibited a drastic 75% reduction in viral load after 5 min of contact and up to 98% reduction after 120 min [50].
As shown above, silver coatings based on nanoparticles or capped nanoparticles as well as composition materials are effective against a number of viruses such as human immunodeficiency virus (HIV), respiratory syncytial virus, hepatitis B virus, herpesvirus, paramyxoviridae viruses (such as respiratory syncytial virus), influenza virus and SARS-CoV-2.
The main mechanism used by silver structures to act against viruses involves the proteins found on the surface of the virus. In general, silver structures inhibit the binding of the virus to cells (Fig. 6) [51], [52]. Infection with influenza viruses H1N1 and H7N3, HPIV3, Ad3, RSV, RVF, and SARS-CoV-2 begins by binding the S protein to the specific host receptor on the cell membrane [51], [53], [54]. This protein allows the virus to bind to the ACE2 receptor conversion enzyme of the host cells [51], [55]. The disulfide bonds in the S protein, blocked by silver, cannot interact with the ACE2 receptor conversion enzyme [51], [56], [57].
3.2.1.2. Copper-based antiviral surfaces
Copper-based antiviral materials belong to the promising ones because they are relatively inexpensive and easy to fabricate. The first attempt to study antiviral properties of copper-based coatings was presented in 2007 in work [58], where influenza A virus particles were incubated on copper or stainless steel. After incubation for 24 h on the stainless surface, nearly 500,000 virus particles were still infective, in contrast incubation for 6 h on the copper surface, where only 500 particles were active. In another similar work [59] rapid inactivation of murine norovirus (MNV-1) on alloys, containing more than 60% copper, at room temperature, was reported. The creation of reactive oxygen species (ROS) was found to be not important for the inactivation mechanism. In turn, in work [60] cuprous oxide (Cu2O), cupric oxide (CuO), cuprous sulfide (Cu2S) and cupric sulfide (CuS) were tested as antiviral surfaces against bacteriophages T4 and Qβ. The results obtained demonstrated excellent antiviral properties only for Cu2O surfaces, explained by the fact that neither ROS nor copper ions but rather direct contact with the surface of cuprous compounds cause the denaturation or degradation of viral biomolecules, resulting in virus inactivation. Very good antiviral activity of nanosized copper iodide (CuI) particles, which generate hydroxyl radicals, was shown for influenza virus A/California/07/09 (H1N1) pdm (A/H1N1pdm) [61]. Borkow et al. [62], [63] tested the impact of copper oxide introduced into polymer materials, especially in medical masks, where mask layers contained nearly 2% w/w copper oxide particles, against human immunodeficiency viruses (HIV-1), human influenza A virus (H1N1) and avian influenza virus (H9N2). The materials obtained demonstrated excellent antiviral properties. Importantly, no infectious human influenza A viral titer was recovered from the copper oxide containing masks in 30 min in contrast to the control masks [63].
Hang and coworkers [64] showed the ability of the cuprous oxide nanoparticles to significantly inhibit the infectivity of Hepatitis C virus (HCV) infection. In addition, CuO-NPs blocked HCV infection both at the attachment and entry stages. Warnes et al. declared that human coronavirus 229E (HuCoV-229E) was inactivated on a copper surface in less than 30 min if the copper alloy included more than 90% of the copper [65]. A similar observation was presented in work [38] where copper-containing thin films demonstrated strong virucidal effects in contrast to non-copper-containing metals and metal oxides coatings, namely, ZnO, ZTO, Ag, and TiO2, which do not show antiviral activity against HuCoV-229E.
Intensive application of copper-based antiviral materials began two years ago, when different research groups almost simultaneously demonstrated the very high efficiency of the copper materials against severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) [66]. In the work presented by van Doremalen and colleagues [66], SARS-CoV-2 was not detected after 4 h of contact with copper. Similarly to the copper surface, the CuO nanoparticle film significantly reduced the viral load already after 30 min (54% reduction), and after 120 min it reached a reduction up to 76% [50]. The cupric oxide (CuO) coating was deposited onto the glass from a dispersion of cuprous oxide (Cu2O) in ethanol and then thermally treated at 700 °C, oxidizing the cuprous oxide to cupric oxide and sintered the particles into a robust film. The SARS-CoV-2 infectivity of the CuO film was reduced by 99.8% in 30 min [67]. In the work [68] two transparent surface coatings were prepared using polydopamine (PDA) as the adhesive element. The first coating consisted of PDA and a very thin layer of copper that was deposited onto PDA by electroless deposition. The second coating was designed by PDA/Cu2O that was sprayed in a single step. Both coatings inactivated nearly 100% of the SARS-CoV-2 virus in 1 h.
In work [69] a copper-coated KF94 mask was fabricated by vacuum surface treatments, including irradiation with an oxygen ion beam and sputtering deposition of copper. The modified mask demonstrated a high filtration efficiency reducing the amount of virus particles by more than 75%. In another work [70] three-layer medical masks were produced, containing a total of 4.4% CuS (w/w) (2.2% CuS coated & 2.2% CuS impregnated) nylon fibers in the outer layer and 17.6% CuS (w/w) impregnated nylon in the middle layer. The three-layered mask with CuS almost completely blocked virus-containing droplet passage for short exposure periods of 1 to 2 min and showed 80% efficacy for longer exposure times of 5 to10 min.
The luminore (cold spray liquid polymetal alloy consisting of copper) coating effectively inactivated SARS-CoV-2, the ebola virus, and the marburg virus in vitro [71]. The prepared surfaces inactivated 99% of SARS-CoV-2 during 2 h. In a similar work [72] a cold spray system (Lightspee3D) was used to fabricate a textured copper coating that had high viricidal activity with 96% inactivated viral particles in 2 h.
As seen above, the antiviral properties of copper-based materials are well known, but the mechanisms of their action are not undoubtedly clarified yet. Probably, different mechanisms are included in virus inactivation simultaneously (Fig. 6). One of the most important is the ‘contact killing’ of target objects, including very rapid accumulation of copper ions, followed by membrane damage and nucleic acid fragmentation [73], [74], [75]. Another possible mechanism of the antivirus action is the generation of ROS. In work [76] five viruses of different biochemical and structural compositions were successfully inactivated by copper (II). At this time, the addition of peroxide synergistically increased the virucidal activity of copper (II) ions, allowing more intense generating of reactive hydroxyl radicals in a Fenton-type reaction: Cu+ + H2O2 → Cu2+ + OH− + •OH.
Reactive radicals are capable of participating in the oxidation of proteins and lipids.
3.2.1.3. Zinc-based antiviral surfaces
The antibacterial properties of the zinc oxide have been well known since the times of Pharaohs, and historical records show that zinc oxide was used in many ointments for the treatment of injuries and boils even in 2000 BCE [77], [78]. First work on antiviral properties of ZnO was published in 2011 where different zinc oxide (ZnO) micro-nano structures capped with multiple nanoscopic spikes mimicking cell-induced filopodia were synthesized by a simple flame transport synthesis approach using a sacrificial polymer (polyvinyl butyrol) as the local host. Zn and polyvinyl butyrol powders were mixed in particular proportion with the help of ethanol and then heated in a simple furnace at 900 °C for 1 h. The negatively charged ZnO structures obtained efficiently trap the virions of Herpes simplex virus type-1 (HSV-1), preventing them from entering human corneal fibroblasts, a natural target cell for HSV-1 infection [79]. The novel virostatic mechanism concluded involves the binding of viral to ZnO nanoscopic spikes, due to the electrostatic interaction between positively charged viral envelope glycoproteins and negatively charged oxygen vacancies, which competes with the binding of the virus to the negatively charged cell surface receptor of the heparan sulfate proteoglycan [79]. In a similar work [80] three types of ZnO nanoparticles, including hydroxyl group rich ZnO-NPs (H-ZnONPs), oleic acid modified ZnO-NPs (OA-Zn-ONPs) and chitosan-zinc oxide nanoparticles (C-ZnO-NPs), were chemically synthesized by the co-precipitation method. For the preparation of H-ZnO-NPs or C-ZnO-NPs, polyethylene glycol 6000 or chitosan was dissolved in ethanol and mixed with ZnCl2 solution followed by the addition of NaOH. OA-ZnONPs were synthesized with H-ZnONPs coating oleic acid on the surface of the nanoparticles. H-ZnONPs and OA-ZnONPs had a rod shape with an average width of 7 nm, in contrast to C-ZnONPs where a spherical shape was observed. HSV-1 incubation with H-ZnONPs and C-ZnONPs showed greater time-dependent inhibition and complete inactivation of the virus within a period of 24 h, while OA-ZnONPs exhibited a lower (15%) reduction in viral titer [80].
In work [81] polyethylene films were covered with a coating consisting of methyl-hydroxy-propyl cellulose with ZnO-NPs (70 nm). Packaging demonstrated moderate activity against phi 6 phages only for ZnONPs supplemented with geraniol and carvacrol. In work [82] virucidal activity against H1N1 influenza virus was not observed at any concentrations of ZnO-NPs or polyethylene glycol modified ZnONPs after incubation of the viral suspensions and nanoparticles suspensions in non-toxic concentration during 4 h, suggesting that nanoparticles could not act directly against the H1N1 particle resulting in viral inactivation.
ZnO films about 135 nm thick, deposited by atmospheric pressure spatial chemical vapor deposition, did not show viral titers for human coronavirus 229E (HCoV-229E) in contrast to unmodified glass at any stage of the desiccation [38]. In similar work [50] ZnO-NPs deposited on glass substrates did not appear to significantly affect the SARS-CoV-2 stability.
The impact of zinc-based coatings on virus stability (Fig. 6) depends strongly on species, from high antiviral activity against herpes simplex virus type1 to low against human coronavirus. ZnO materials are able to release Zn2+ ions and also to absorb UV–Vis light and split the elements of water, producing different types of reactive oxygen species (ROS) such as superoxides, hydroxyl radicals, and hydrogen peroxide, which apparently damage lipids, proteins, carbohydrates and DNA [78].
3.2.1.4. Titanium-based antiviral surfaces
Titanium-based structures are one of the most popular antiviral coatings due to their great photo-oxidation of organic compounds and relatively low toxicity [83]. The combination of TiO2 with transition metal ions or anions essentially improves photocatalytic activity and antiviral properties, respectively.
TiO2 films ∼40 nm thick synthesized by spin coating on glass substrates did not inactivate HCoV-229E under ambient laboratory conditions [38]. Contrary to a previous study, work [84] describes an excellent inactivation of SARS-CoV-2 using TiO2- and TiO2–Ag (Ti:Ag atomic ratio 1:0.04) coated ceramic tiles after illumination with ordinary interior light.
TiO2 nano-colloids synthesized by the sonochemical method demonstrated antiviral activity against Newcastle virus (NDV). Possible antiviral mechanism includes damage to the lipids found in viral envelope. The glycoproteins spikes were damaged and their attachment was blocked and therefore could not initiate infection [85].
Very interesting results were presented in work [86], where the photocatalytic inactivation of MS-2 phage and Escherichia coli on TiO2 particles during illumination by lamp with wavelengths in the range of 300 to 420 nm was compared. Obtained results imply that the MS-2 phage was inactivated mainly by the free hydroxyl radical in the solution bulk but E. coli was inactivated by both, the free and the surface-bound hydroxyl radicals. In particular, MS-2 phage surface has both hydrophobic and negatively charged hydrophilic regions in contrast to the surface of TiO2 that is predominantly negatively charged at pH 7.1, and the electrostatic repulsion between the TiO2 particles and MS-2 phage should be present. Therefore, the adsorption of MS-2 phage onto the surfaces of the TiO2 particles is not favored, and the direct contact between the phage cells and the illuminated TiO2 surface should be minimal. Hence, inactivation of MS-2 phage is concluded to occur through free hydroxyl radical [86].
In similar works [87], [88], [89], [90], [91], successful photocatalytic inactivation using ambient, visible or UV-lights was described for murine norovirus, HSV-1, bacteriophage MS2, Hepatitis B, human rotavirus strain Odelia, a simian rotavirus strain SA11, human astrovirus serotype 1 (HAstV-1), and FCV strain F4. However, TiO2 based virus inactivation using ambient lights is a relatively slow process [89].
Photocatalytic inactivation of virus with titanium based surfaces considers the role of complex photooxidants, such as the hydroxyl radical (•OH), the superoxide radical (O2 •-), and hydrogen peroxide (H2O2), etc. (Fig. 6). Two kinds of hydroxyl radicals, one in the bulk solution phase and the other on the surfaces of TiO2 particles, were reported to act in the heterogeneous TiO2 photocatalytic reaction. Probably both of them are able to inactivate viruses inducing denaturation of the protein in the capsids.
3.2.2. Photothermal effect of the nanofunctionalized surfaces against viruses
A fundamentally new approach for virus disinfection on surfaces is stimulated by the ability of some nanomaterials to initiate photothermal effect by converting into heat the energy gained from absorbed light [92], [93], [94], [95], [96], [97]. Different photothermal mechanisms are related with various materials [98], [99]: Metal nanoparticles can exhibit plasmonic localized rise in temperature with light-excited oscillations of delocalized surface electrons resulting in resistive heating [99]. Wavelength of absorbed light depends on the size and shape of plasmonic nanoparticles [98]. For semiconductors, light with energy hν higher that bandgap produces electron-hole pairs that relax non-radiatively to band edges with energy difference converted into heat [99] (Fig. 6). Element doping of semiconductor can broaden absorption wavelength [98]. In turn, in carbon and polymeric materials electrons can be excited with small light energy hν from π HOMO to π* LUMO state, followed by electronic relaxation with heat converted through thermal molecular vibrations [98], [99] (Fig. 7 ). Photothermal sterilization is a fast, contactless, low-cost, and widely available method, capable of decontaminating the masks, respirators and contact surfaces. The energy gained from photothermal mechanisms is dissipated to the surroundings of the nanostructure as local heat that destroys virus.
Fig. 7.
Photothermal effect (for semiconductors and polymers) of the surfaces nanofunctionalized against viruses.
Only a limited amount of papers presented the applications of photothermal effect against viral infections in vitro. In work [100], shellac/copper nanoparticles (CuNPs) were applied as a nanocoating hybrid to a non-woven surgical mask, increasing the hydrophobicity of the surface and repelling aqueous droplets. The functionalized surfaces showed outstanding photoactivity (combined photocatalytic and photothermal properties) even under solar illumination, demonstrating self-sterilization of the masks. Under solar illumination, the temperature obtained by the photoactive antiviral mask increased rapidly above 70 °C, generating a high level of free radicals that disrupted the membrane of nanosized (∼100 nm) virus-like particles and made the masks self-cleaned and reusable. In a similar work [101], molybdenum disulfide (MoS2) nanosheets were used to prepare modified polycotton fabrics with photothermal properties. Upon illumination by sunlight, the nanosheet-modified fabrics rapidly increased surface temperature up to ∼77 °C, making them ideal for sunlight-mediated self-disinfection. Complete self-disinfection of the nanosheet-modified fabric was achieved within 3 min of illumination, making the fabrics favorably reusable upon self-disinfection. Superhydrophobic self-decontaminating N95 respirators, based on polyimide film and AgNPs with plasmonic heating of surface temperature above 80 °C within 1 min of sunlight illumination, were developed [102].
Carbon nanostructures, such as graphene, a two-dimensional atomic layer of hexagonally bonded carbon atoms, showed a possible application in the fabrication of coatings with photothermal effect. A superhydrophobic, photo-sterilized, and reusable mask based on graphene nanosheet-embedded carbon films, quickly heating up to 110 °C under solar illumination and showing prominent photo-sterilize performance, was described in [103].
3.3. Polymer-based antiviral surfaces
3.3.1. Antiviral surfaces based on synthetic polymers
Synthetic polymers act directly on virus infection by contacting with specific sites on the surface of the viral particles. Antiviral coatings of the synthetic polymers are physically adsorbed or chemically attached to surfaces. The first successful antiviral surfaces based on synthetic polymers were developed by the Klibanov group [104], where the ‘non-release’ strategy to render antimicrobial functionality to common materials (plastics, glass, textiles) was proposed. In contrast to the methods releasing antimicrobial agents from the surface, this strategy involves covalent or non-covalent attachment of certain long, moderately hydrophobic polycations to material surfaces, intended to physically damage virus structure as demonstrated for influenza virions. The typical physically adsorbed permanent antiviral coating based on N,N-dodecyl methyl-polycations is presented in Fig. 8 a. In turn, its analog in the form of the grafted brush coating of hydrophobic polycations is depicted in Fig. 8b.
Fig. 8.
Physically adsorbed (a) and grafted brush (b) antiviral coatings of hydrophobic polycations based on N,N-dodecyl methyl-polycations. Hypothetical scheme (c) of the antiviral action of hydrophobic polycation.
Antiviral activity of the hydrophobic polycations was related with the adhesion of viral particles to the surface-immobilized hydrophobic polycation. Additionally, analysis of virus solutions disinfected by polymer coatings revealed significant amount of viral RNA, thereby evidencing that their integrity was compromised allowing the release of their genomic material [105]. The studied virucidal activities did not show discernible correlation with surface properties such as hydrophobicity, charge, protein affinity, roughness, adhesive interactions, and polymer-chain extension lengths. They exhibited a marginal correlation with the surface density of the quaternary ammonium group. The antiviral effectivity of the hydrophobic polycationic against influenza viruses, poliovirus, rotavirus and Herpes simplex viruses (HSVs) 1 and 2 was shown in [106]. The hypothetical scheme of the antiviral action of hydrophobic polycation is demonstrated in Fig. 8c.
In similar works where microfiltration membranes with polyethyleneimine were developed, the inclusion of a cationic polymer could increase viral reduction. Polyethyleneimine exerts an attractive electrostatic interaction with the negatively charged virus and inactivates the virus also over time by causing damage to the virion [107], [108].
Cationic cellulose nanocrystals were modified by surface-initiated atom-transfer radical polymerization of poly(N,N-dimethylaminoethyl methacrylate) and subsequent quaternization of the amino groups of the polymer pendant [109]. The cationic polymer brush-modified cellulose nanocrystals showed a Z-potential of + 38 mV. The electrostatically modified cellulose nanocrystals with high affinity bound cowpea chlorotic mottle virus and norovirus-like particles.
In recent work [110] the novel anti-infective materials based on fluorinated polycationic coating with broad-spectrum anti-pathogenic activity were developed. A fluorinated polycationic coating was synthesized on a negatively charged hydrophilic polyester textile by initiating a one-step initiated chemical vapor deposition of poly(dimethyl amino methyl styrene-co-1H,1H,2H,2Hperfluorodecyl acrylate). The antiviral capacity of the coating was shown using the negatively charged recombinant lentivirus (a single-stranded RNA virus) with enhanced green fluorescence protein (egfp) gene as a virus model. The coating effectively inactivated the virus and showed good biocompatibility with mouse NIH 3 T3 fibroblast cells.
Unfortunately, the above-mentioned methodology and other similar methodologies using synthetic polymers have two essential disadvantages. Firstly, species-specific action of the synthetic polymers and secondly, a limited number of the viral particles that can be tied to the functionalized surfaces.
3.3.2. Antiviral surfaces based on biopolymers
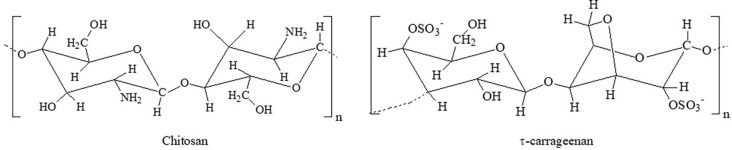
Chitosan (Fig. 9 ) is derived from chitin, the second most abundant polysaccharide after cellulose [111], [112]. Chitosan is composed of 2-acetamido-2-deoxy-D-glucopyranose units linked by a β-(1, 4)-glycosidic bond. It is the most promising renewable polymeric material in recent years [113]. Chitosan has been extensively studied for its ability to form dried films and its inherent antimicrobial activity.
Fig. 9.
Biopolymers with potential to manufacture antiviral coatings.
In work [111], chitosan-based films formed with green tea extract showed effectiveness in reducing the infectivity of murine norovirus (MNV-1). In similar work [114], electrospun nanofibers made of chitosan and functionalized with a quaternary amine could adsorb a non-enveloped model virus, porcine parvovirus (PPV). In work [112], 6-deoxy-6-bromo-N-phthaloyl chitosan with improved antiviral activity was synthesized. An interesting approach was realized in the work [115], where the chitosan–sialyloligosaccharide ionic complex inhibited the attachment of influenza virus to the host cell.
Similarly, as in the case of synthetic polymer coatings, application of the chitosan-based coatings is strongly limited due to species-specific action of these biopolymers, limitation in amount of the viral particles attached to chitosan macromolecules, and, in addition, the release of the chitosan molecules.
Also, other polysaccharides demonstrate antiviral properties. Hyaluronic acid is a non-sulphated glycosaminoglycan, which consists of alternately repeating D-glucuronic acid and N-acetylglucosamine units. There is evidence that hyaluronic acid can interfere with viral replication in vitro. Work [116] provided evidence on the effects of hyaluronic acid on a variety of RNA and DNA viruses, with or without the lipid envelope, characterized by very different replication strategies. The antiviral mechanism(s) of hyaluronic acid probably involves a general/nonspecific host cell-virus interaction at the membrane level, such as virus entry or release, rather than restricted, virus-specific events occurring inside the cell. Different film-forming dispersions based on κ−, ι− (Fig. 9) and λ − carrageenans and green tea extract have been developed as an innovative strategy to guarantee the food safety of blueberries and raspberries [117]. Carrageenan-based coatings applied to berries showed antiviral activity against Murine norovirus (MNV-1) and hepatitis A virus (HAV) strain HM-175/18f.
Peptide-based assemblies form another type of biopolymers that have been intensively developed in the last few years for the fabrication of the antiviral coatings [118]. Three main antiviral effects are applied by different peptide groups: (i) peptides that inhibit virus attachment and virus-cell membrane fusion; (ii) peptides that disrupt the viral envelope; and (iii) peptides that inhibit virus replication by interacting with viral polymerase [119]. Potentially, only the first group of antiviral peptides can be applied in the fabrication of antiviral coatings.
The entry of influenza virus into target cells is possible through receptor-mediated endocytosis, the fusion process mediated by viral hemagglutinin (HA) trimers [119]. Viral entry can be blocked via interaction of antiviral peptide with HA, which commonly interacts with a residue of sialic acid, thus influenza virion cannot be attached to the membrane of a host cell. Jones et al. [120] developed 20 amino acid peptides, derived the fibroblast growth factor signal sequence, demonstrating broad-spectrum activity against human, swine, and avian influenza A H1N1, H2N2, H3N2, H5N1, H5N9, and H7N3 strains and influenza B viruses.
In work [121], Han and coworkers synthesized six peptides that may be bound to the RBD domain of the S protein of SARS-CoV-2 and thus block SARS-CoV-1 binding to host cells. In other work [122], Cao and coworkers designed miniproteins, containing less than 100 amino acid residues, which effectively neutralized SARS-CoV-2 binding to the RBD with lower dissociation constants.
In summary, applications of the biopolymers for fabrication of antiviral surfaces are limited by a few factors, such as the high species-selectivity of the antivirus biopolymers, their relatively low stability, and especially their ability for bio-destruction. The high cost of the production in case of the peptides is also a strong limitation.
3.4. Application of small molecules with receptor inactivation capability for fabrication of antiviral coatings
In general, there are two main methods that can be applied to fabricate an antiviral surface loaded with small molecules. The molecules that act as antiviral agents can be deposited directly on the surface or incorporated into polymeric matrix during polymer processing. The simplest method is the direct physical deposition of small molecules. The effectiveness of this approach is very low due to the rapid reabsorption of antiviral agents [123]. Another similar method is chemical deposition, which allows formation of relatively stable bonds between antivirus agents and the surface, thus prolonging its antiviral activity for an extended period [123].
With a completely different approach, an antiviral agent can be added to a polymer prior to its subsequent procession with a large-scale production technique [123]. This method has numerous limitations, such as the restricted diffusivity of antiviral molecules through the polymeric matrix, the low compatibility between the polymer matrix and antiviral agents, the destruction of antiviral substances during polymer processing, and technological difficulties during polymer processing [123].
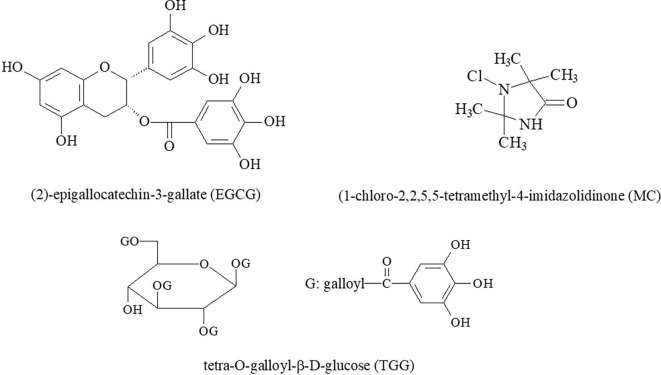
The significant weakness of the application of small molecules for the fabrication of antiviral coatings is their high species-specificity to viruses. For example, small molecules [124] of 1-chloro-2,2,5,5-tetramethyl-4-imidazolidinone (MC, a variety of N-halamine, shown in Fig. 10 ) were coated on non-woven fabrics to remarkably reduce the presence of avian influenza virus in a short period of time. MC molecules at higher concentrations inactivated the virus or disrupted its RNA [124].
Fig. 10.
Small molecules with potential to fabricate antiviral coatings.
Up to our knowledge, only one successful antiviral application of the surfaces loaded with small molecules has been reported [124]. Still, we believe that such applications are possible, for instance using the small antiviral molecules listed below. The main antiviral effect of these surfaces loaded with low molecular components is inhibition of the interactions between viral proteins and cell surfaces.
In work [125], (2)-epigallocatechin-3-gallate (EGCG) (Fig. 10) was identified as an inhibitor of the entry of hepatitis C virus (HCV). EGCG is a flavonoid, present in green tea extract, and belonging to the catechin subclass. This inhibition was not observed for other members of the Flaviviridae family, suggesting a high species-specificity of this molecule. EGCG prevented virus attachment to the cell surface, probably by acting directly on the particle, and had no effect on viral replication and virion secretion.
In work [126], the entry of HIV, hepatitis C virus and other insidious viruses into host cells was inhibited with small molecules of tetra-O-galloyl-β-D-glucose (TGG) (Fig. 10) and luteolin. At this time, TGG and luteolin were not effective against the HIV-luc/VSV pseudotyped virus. Two hydrolyzable tannins, chebulagic acid (CHLA) and punicalagin (PUG), blocked herpes simplex virus type 1 (HSV-1) entry and spread. These compounds inhibited viral glycoprotein interactions with glycosaminoglycans on the cell surface (GAGs) [127]. CHLA and PUG were effective in suppressing infection by human cytomegalovirus (HCMV), hepatitis C virus (HCV), dengue virus (DENV), measles virus (MV) and respiratory syncytial virus (RSV) [128]. For these viruses differences in the roles of interactions with GAGs were concluded [128].
Emodin, an anthraquinone compound derived from the genus Rheum and Polygonum, significantly blocked the interaction of the S protein of SARS-CoV and ACE2 in a dose-dependent manner. It also inhibited the infectivity of S protein-pseudotyped retrovirus to Vero E6 cells [129]. In turn, a naturally occurring terpenoid of Saikosaponin b2 efficiently inhibits the entry of hepatitis C and HCoV-229E virus, including neutralization of virus particles, preventing viral attachment, and inhibiting viral entry/fusion [130], [131].
4. Outlook for the future
Viruses pose a serious threat to human health and society in general, as they are one of the main causes of morbidity and mortality. Despite advances in the development of drugs for antiviral therapy, modern treatment regimens are not able to fully control the course of infections. Untill January 2022, more than 370 million people worldwide have been confirmed infected and more than 5.6 million have died due to SARS-CoV-2. At the same time, the bad consequences of the disease, recognized among the 300 million people who have recovered, include diabetes, hypertension, blood clotting, loss of taste and smell, and others. The last wave of SARS-CoV-2 (the so-called Omicron variant) is affecting more and more children. Therefore, the invention and fabrication of novel high-performance antiviral materials is highly demanded.
Numerous factors are able to affect non-specific viral particle deposition and presence on various surfaces, and the majority of them is related to the physico-chemical properties of the surfaces and viral particles, including long-range electrostatics and shorter-range van der Waals components of free energies as well as their hydrophobic/hydrophilic balances. Traditional approaches using non-specific interactions are not sufficient nowadays to protect surfaces against virus deposition and survival. This is because various viral species have different physico-chemical properties. Therefore, a recently formulated concept of passive and active ‘antiviral surfaces’ has been applied to prevent virus transmission on surfaces, relying on different mechanisms ‘added’ to surfaces with antiviral agents or surface modifications.
Five types of the antiviral mechanisms can be identified for antiviral surfaces, i.e. direct and indirect actions, virus receptor inactivation, photothermal effect and antifouling behavior (for superhydrophobic surfaces). In addition, all antiviral surfaces can be divided into two types, passive and active ones. Passive surfaces prevent virus deposition by applying superhydrophobic coatings. The surface should be antifouling, i.e. repel contaminated droplets or body fluids. Such systems are very promising, because they keep the virus encapsulated and suppress surface contaminants. Obviously, such materials need to be improved to have additional functions, such as temperature- or pH-responsivity, controlled morphology, etc. As an example, in works [132], [133], [134], [135] temperature-responsive hydrophobic poly(cholesteryl methacrylate) or poly(butyl methacrylate) coatings have been developed that are potentially suitable for fabrication of superhydrophobic sensitive passive antiviral coatings.
Active antiviral surfaces contain agents engaged in antiviral activity, such as metals, synthetic or natural polymers, and small molecules. Active antiviral materials that contain metals such as silver, copper, zinc, and titanium have great practical application because they are not extremely toxic, they do not pollute the environment, and they are not expensive for application at the industrial scale. The future of metal-based coatings is in the combination of metallic nanoparticles with a polymer matrix. Produced in this manner hybrid antiviral coatings should possess multifunctional properties. For instance, nanocomposite coatings based on non-cytotoxic, temperature-responsive and antibacterial POEGMA with silver nanoparticles have recently been constructed [33]. We believe that this and similar systems might be applied to fabricate self-cleaning antiviral coatings [136]. Another approach to improve the efficiency of metal-based antiviral coatings includes the acceleration of their antiviral activity with the parallel application of a few metal types.
The main disadvantage of the coatings based on synthetic polymers is limited amount of viral particles that can be destroyed. During the antiviral action, the coatings are fouled by the parts of destroyed viruses and bacteria, resulting in the reduced disinfection of microorganisms. This problem can be solved by incorporation of polymers with self-cleaning function, e.g., activated at tuned temperature or pH, such as the well-known poly(4-vinylpiridine) [137]. Although biopolymers are low-toxic and originate from natural sources, their spectrum of action is narrow, they have relatively low stability, and high ability for bio-destruction. To overcome these disadvantages, the coating based on biopolymers might be combined with different inclusions to improve antiviral properties.
In turn, the main difficulty in fabricating antiviral coatings loaded with small molecules is associated with their common ability to act only on certain types of viruses. Therefore, molecules that target a wider range of viruses are required. It is worth to notice that forming relatively stable bonds between the antivirus agents and the surface is a significant advantage in this application.
Studies on the recently formulated concept of antiviral surfaces have just begun. The properties of antiviral surfaced need to be significantly improved by including additional functions, such as temperature- or pH-responsivity, multifunctionality, non-specific action on different types of the viruses, long-term application, high antiviral efficiency and self-cleaning.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Yurij Stetsyshyn acknowledges funding from the Visiting Professor program of the Jagiellonian University. Yana Shymborska thanks for financial support from the SciMat Priority Research Area budget under the strategic programme Excellence Initiative at the Jagiellonian University (Grant No. U1U/P05/NO/03.43). Ostap Lishchynskyi acknowledges funding from the Special Research Fund (BOF) - Doctoral Scholarships for Candidates from Developing Countries of the Ghent University (Grant No. BOF21/DOS/069).
References
- 1.Guarner J. Three emerging coronaviruses in two decades: the story of SARS, MERS, and now COVID-19. Am. J. Clin. Pathol. 2020;153:420. doi: 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shang Z., Chan S.Y., Liu W.J., Li P., Huang W. Recent insights into emerging coronavirus: SARS-CoV-2. ACS Infect. Dis. 2021;7(6):1369–1388. doi: 10.1021/acsinfecdis.0c00646. [DOI] [PubMed] [Google Scholar]
- 3.Simões M.F., Antunes A. Microbial pathogenicity in space. Pathogens. 2021;10:450. doi: 10.3390/pathogens10040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapshak P. In: Global Virology III: Virology in the 21st Century. Shapshak P., Balaji S., Kangueane P., Chiappelli F., Somboonwit C., Menezes L.J., Sinnott J.T., editors. Springer International Publishing; Cham: 2019. Astrobiology, Artificial Intelligence: Extra-Solar System Investigations; pp. 541–573. [Google Scholar]
- 5.Feng T., Nie C., Peng P., Lu H., Wang T., Li P., Huang W. Nanoagent-based theranostic strategies against human coronaviruses. Nano Res. 2022;15:3323–3337. doi: 10.1007/s12274-021-3949-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chua M.H., Cheng W., Goh S.S., Kong J., Li B., Lim J.Y.C., Mao L., Wang S., Xue K., Yang L., Ye E., Zhang K., Cheong W.C.D., Tan B.H., Li Z., Tan B.H., Loh X.J. Face masks in the new COVID-19 normal: materials, testing, and perspectives. Research. 2020;2020:7286735. doi: 10.34133/2020/7286735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pullangott G., Kannan U., Gayathri S., Kiran D.V., Maliyekkal S.M. A comprehensive review on antimicrobial face masks: An emerging weapon in fighting pandemics. RSC Adv. 2021;11:6544–6576. doi: 10.1039/D0RA10009A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelleïeux S., Bertrand I., Skali-Lami S., Mathieu L., Francius G., Gantzer C. Accumulation of MS2, GA, and Qβ phages on high density polyethylene (HDPE) and drinking water biofilms under flow/non-flow conditions. Water Res. 2012;46:6574–6584. doi: 10.1016/j.watres.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 9.Armanious A., Aeppli M., Jacak R., Refardt D., Sigstam T., Kohn T., Sander M. Viruses at solid–water interfaces: a systematic assessment of interactions driving adsorption. Environ. Sci. Technol. 2016;50:732–743. doi: 10.1021/acs.est.5b04644. [DOI] [PubMed] [Google Scholar]
- 10.Zhu P., Wang Y., Chu H., Wang L. Superhydrophobicity preventing surface contamination as a novel strategy against COVID-19. J. Colloid Interface Sci. 2021;600:613–619. doi: 10.1016/j.jcis.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez L., Li X., Wang J., Nangmenyi G., Economy J., Kuhlenschmidt T.B., Kuhlenschmidt M.S., Nguyen T.H. Adsorption of rotavirus and bacteriophage MS2 using glass fiber coated with hematite nanoparticles. Water Res. 2009;43:5198–5208. doi: 10.1016/j.watres.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 12.da Silva A.K., Kavanagh O.V., Estes M.K., Elimelech M. Adsorption and aggregation properties of norovirus GI and GII virus-like particles demonstrate differing responses to solution chemistry. Environ. Sci. Technol. 2011;45:520–526. doi: 10.1021/es102368d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerba C.P. Applied and theoretical aspects of virus adsorption to surfaces. Adv. Appl. Microbiol. 1984;30:133–168. doi: 10.1016/S0065-2164(08)70054-6. [DOI] [PubMed] [Google Scholar]
- 14.Zerda K.S., Gerba C.P., Hou K.C., Goyal S.M. Adsorption of viruses to charge-modified silica. Appl. Environ. Microbiol. 1985;49:91–95. doi: 10.1128/aem.49.1.91-95.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosa V., Ho D., Sabino-Silva R., Siqueira W.L., Silikas N. Fighting viruses with materials science: Prospects for antivirus surfaces, drug delivery systems and artificial intelligence. Dent. Mater. 2021;37:496–507. doi: 10.1016/j.dental.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maguire-Boyle S.J., Liga M.V., Li Q., Barron A.R. Alumoxane/ferroxane nanoparticles for the removal of viral pathogens: the importance of surface functionality to nanoparticle activity. Nanoscale. 2012;4:5627–5632. doi: 10.1039/C2NR31117H. [DOI] [PubMed] [Google Scholar]
- 17.Moreno-Cerrada D., Rodríguez C., Moreno-Madrid F., Selivanovitch E., Douglas T., de Pablo P.J., Manso Silván M. Loading the dice: The orientation of virus-like particles adsorbed on titanate assisted organosilanized surfaces. Biointerphases. 2019;14(1):011001. doi: 10.1116/1.5077010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das K., Aramini J.M., Ma L.C., Krug R.M., Arnold E. Structures of influenza A proteins and insights into antiviral drug targets. Nat. Struct. Mol. Biol. 2010;17:530–538. doi: 10.1038/nsmb.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.https://viralzone.expasy.org/6 ViralZone, SIB Swiss Institute of Bioinformatics.
- 20.M. Lazniewski W. K. Dawson, T. Szczepinska, D. Plewczynski, The structural variability of the influenza A hemagglutinin receptor-binding site, Briefings Funct. Genomics 17 (2018) 415–427. https://doi: 10.1093/bfgp/elx042. [DOI] [PMC free article] [PubMed]
- 21.Byrd-Leotis L., Cummings R.D., Steinhauer D.A. The interplay between the host receptor and Influenza virus hemagglutinin and neuraminidase. Int. J. Mol. Sci. 2017;18:1541. doi: 10.3390/ijms18071541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.https://viralzone.expasy.org/764 ViralZone, SIB Swiss Institute of Bioinformatics.
- 23.Finkelstein M.T., Mermelstein A.G., Miller E.P., Seth P.C., Stancofski E.-S.-D., Fera D. Structural analysis of neutralizing epitopes of the SARS-CoV-2 spike to guide therapy and vaccine design strategies. Viruses. 2021;13:134. doi: 10.3390/v13010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Z., Ostrikov K.K. Future antiviral surfaces: Lessons from COVID-19 pandemic. Sustainable Mater. Technol. 2020;25:e00203. [Google Scholar]
- 25.Banerjee I., Pangule R.C., Kane R.S. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011;23:690–718. doi: 10.1002/adma.201001215. [DOI] [PubMed] [Google Scholar]
- 26.Huang H., Fan C., Li M., Nie H.L., Wang F.B., Wang H., Wang R., Xia J., Zheng X., Zuo X., Huang J. COVID-19: a call for physical scientists and engineers. ACS Nano. 2020;14:3747–3754. doi: 10.1021/acsnano.0c02618. [DOI] [PubMed] [Google Scholar]
- 27.Cohen N., Dotan A., Dodiuk H., Kenig S. Superhydrophobic coatings and their durability. Mater. Manuf. Processes. 2016;31:1143–1155. doi: 10.1080/10426914.2015.1090600. [DOI] [Google Scholar]
- 28.Hooda A., Goyat M.S., Pandey J.K., Kumar A., Gupta R. A review on fundamentals, constraints and fabrication techniques of superhydrophobic coatings. Prog. Org. Coat. 2020;142 doi: 10.1016/j.porgcoat.2020.105557. [DOI] [Google Scholar]
- 29.Meguid S.A., Elzaabalawy A. Potential of combating transmission of COVID-19 using novel self-cleaning superhydrophobic surfaces: part I—protection strategies against fomites. Int. J. Mech. Mater. Des. 2020;16:423–431. doi: 10.1007/s10999-020-09513-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W., Wang Y., Tang X., Yuen T.T.T., Han X., Li J., Huanga N., Chan J.F.W., Chu H., Wang L. Liquid repellency enabled antipathogen coatings. Mater. Today Bio. 2021;12 doi: 10.1016/j.mtbio.2021.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stetsyshyn Y., Awsiuk K., Kusnezh V., Raczkowska J., Jany B., Kostruba A., Harhay K., Ohar H., Lishchynskyi O., Shymborska Y., Kryvenchuk Y., Krok F., Budkowski A. Shape-Controlled synthesis of silver nanoparticles in temperature-responsive grafted polymer brushes for optical applications. Appl. Surf. Sci. 2019;463:1124–1133. doi: 10.1016/j.apsusc.2018.09.033. [DOI] [Google Scholar]
- 32.Raczkowska J., Stetsyshyn Y., Awsiuk K., Brzychczy-Włoch M., Gosiewski T., Jany B., Lishchynskyi O., Shymborska Y., Nastyshyn S., Bernasik A., Ohar H., Krok F., Ochońska D., Kostruba A., Budkowski A. “Command” surfaces with thermo-switchable antibacterial activity. Mater. Sci. Eng., C. 2019;103:109806. doi: 10.1016/j.msec.2019.109806. [DOI] [PubMed] [Google Scholar]
- 33.Nastyshyn S., Raczkowska J., Stetsyshyn Y., Orzechowska B., Bernasik A., Shymborska Y., Brzychczy-Włoch M., Gosiewski T., Lishchynskyi O., Ohar H., Ochońska D., Awsiuk K., Budkowski A. Non-cytotoxic, temperature-responsive and antibacterial POEGMA based nanocomposite coatings with silver nanoparticles. RSC Adv. 2020;10(17):10155–10166. doi: 10.1039/c9ra10874b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zezin A.A., Zharikov A.A., Emel’yanov A.I., Pozdnyakov A.S., Prozorova G.F., Abramchuk S.S., Zezina E.A. One-Pot Preparation of Metal–Polymer Nanocomposites in Irradiated Aqueous Solutions of 1-Vinyl-1,2,4-triazole and Silver Ions. Polymers. 2021;13(23):4235. doi: 10.3390/polym13234235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shevtsova T., Cavallaro G., Lazzara G., Milioto S., Donchak V., Harhay K., Korolko S., Budkowski A., Stetsyshyn Y. Temperature-responsive hybrid nanomaterials based on modified halloysite nanotubes uploaded with silver nanoparticles. Colloids Surf. A. 2022;641 doi: 10.1016/j.colsurfa.2022.128525. [DOI] [Google Scholar]
- 36.Singh R., Gautam N., Mishra A., Gupta R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011;43:246. doi: 10.4103/0253-7613.81505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rakowska P.D., Tiddia M., Faruqui N., Bankier C., Pei Y., Pollard A.J., Zhang J., Gilmore I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021;2:1–19. doi: 10.1038/s43246-021-00153-y. [DOI] [Google Scholar]
- 38.Delumeau L.-V., Asgarimoghaddam H., Alkie T., Jones A.J.B., Lum S., Mistry K., Aucoin M.G., DeWitte-Orr S., Musselman K.P. Effectiveness of antiviral metal and metal oxide thin-film coatings against human coronavirus 229E. APL Mater. 2021;9(11):111114. doi: 10.1063/5.0056138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sreekanth T.V.M., Nagajyothi P.C., Muthuraman P., Enkhtaivan G., Vattikuti S.V.P., Tettey C.O., Kim D.H., Shim J., Yoo K. Ultra-sonication-assisted silver nanoparticles using Panax ginseng root extract and their anti-cancer and antiviral activities. J. Photochem. Photobiol., B. 2018;188:6–11. doi: 10.1016/j.jphotobiol.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Sharma V., Kaushik S., Pandit P., Dhull D., Yadav J.P., Kaushik S. Green synthesis of silver nanoparticles from medicinal plants and evaluation of their antiviral potential against chikungunya virus. Appl. Microbial. Biotechnol. 2019;103:881–891. doi: 10.1007/s00253-018-9488-1. [DOI] [PubMed] [Google Scholar]
- 41.El-Sheekh M.M., Shabaan M.T., Hassan L., Morsi H.H. Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herps Simplex (HSV-1) virus in vitro using cell-line culture technique. Int. J. Environ. Health Res. 2022;32:616–627. doi: 10.1080/09603123.2020.1789946. [DOI] [PubMed] [Google Scholar]
- 42.Lara H.H., Ayala-Nuñez N.V., Ixtepan-Turrent L., Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010;8:1–10. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elechiguerra J.L., Burt J.L., Morones J.R., Camacho-Bragado A., Gao X., Lara H.H., Yacaman M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005;3(1) doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogers J.V., Parkinson C.V., Choi Y.W., Speshock J.L., Hussain S.M. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res. Lett. 2008;3:129–133. doi: 10.1007/s11671-008-9128-2. [DOI] [Google Scholar]
- 45.Fayaz A.M., Ao Z., Girilal M., Chen L., Xiao X., Kalaichelvan P.T., Yao X. Inactivation of microbial infectiousness by silver nanoparticles-coated condom: a new approach to inhibit HIV-and HSV-transmitted infection. Int. J. Nanomedicine. 2012;7:5007. doi: 10.2147/IJN.S34973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galdiero S., Falanga A., Vitiello M., Cantisani M., Marra V., Galdiero M. Silver nanoparticles as potential antiviral agents. Molecules. 2011;16:8894–8918. doi: 10.3390/molecules16108894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeremiah S.S., Miyakawa K., Morita T., Yamaoka Y., Ryo A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020;533:195–200. doi: 10.1016/j.bbrc.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar A., Nath K., Parekh Y., Enayathullah M.G., Bokara K.K., Sinhamahapatra A. Antimicrobial silver nanoparticle-photodeposited fabrics for SARS-CoV-2 destruction. Colloid Interface Sci. Commun. 2021;45 doi: 10.1016/j.colcom.2021.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balagna C., Perero S., Percivalle E., Nepita E.V., Ferraris M. Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceramics. 2020;1 doi: 10.1016/j.oceram.2020.100006. [DOI] [Google Scholar]
- 50.Merkl P., Long S., McInerney G.M., Sotiriou G.A. Antiviral activity of silver, copper oxide and zinc oxide nanoparticle coatings against SARS-CoV-2. Nanomaterials. 2021;11:1312. doi: 10.3390/nano11051312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pilaquinga F., Morey J., Torres M., Seqqat R., Pina M.D.L.N. Silver nanoparticles as a potential treatment against SARS-CoV-2: A review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021;13:e1707. doi: 10.1002/wnan.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiang D.X., Chen Q., Pang L., Zheng C.L. Inhibitory effects of silver nanoparticles on H1N1 influenza A virus in vitro. J. Virol. Methods. 2011;178:137–142. doi: 10.1016/j.jviromet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Borrego B., Lorenzo G., Mota-Morales J.D., Almanza-Reyes H., Mateos F., López-Gil E., la Losa N., Burmistrov V.A., Pestryakov A.N., Brun A., Bogdanchikova N. Potential application of silver nanoparticles to control the infectivity of Rift Valley fever virus in vitro and in vivo, Nanomedicine: Nanotechnology. Biol. Med. 2016;12:1185–1192. doi: 10.1016/j.nano.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 54.Park S., Ko Y.S., Lee S.J., Lee C., Woo K., Ko G. Inactivation of influenza A virus via exposure to silver nanoparticle-decorated silica hybrid composites. Environ. Sci. Pollut. Res. 2018;25:27021–27030. doi: 10.1007/s11356-018-2620-z. [DOI] [PubMed] [Google Scholar]
- 55.S. Jiang, Y. He, Patent No. 2 384 497. https://patentimages.storage.googleapis.com/06/28/92/2fe0d65c3acad4/ES2384497T3.pdf, 2012 (accessed 5 July 2012).
- 56.Kang S., Yang M., Hong Z., Zhang L., Huang Z., Chen X., He S., Zhou Z., Zhou Z., Chen Q., Yan Y., Zhang C., Shan H., Chen S. Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharm. Sin. B. 2020;10:1228–1238. doi: 10.1016/j.apsb.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noyce J.O., Michels H., Keevil C.W. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl. Environ. Microbiol. 2007;73:2748–2750. doi: 10.1128/AEM.01139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warnes S.L., Keevil C.W., Samal S.K. Inactivation of norovirus on dry copper alloy surfaces. PLoS ONE. 2013;8(9):e75017. doi: 10.1371/journal.pone.0075017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sunada K., Minoshima M., Hashimoto K. Hashimoto, Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J. Hazard. Mater. 2012;235-236:265–270. doi: 10.1016/j.jhazmat.2012.07.052. [DOI] [PubMed] [Google Scholar]
- 61.Fujimori Y., Sato T., Hayata T., Nagao T., Nakayama M., Nakayama T., Sugamata R., Suzuki K. Novel antiviral characteristics of nanosized copper (I) iodide particles showing inactivation activity against, pandemic H1N1 influenza virus. Appl. Environ. Microbiol. 2009;78(2012):951–955. doi: 10.1128/AEM.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borkow G., Lara H.H., Covington C.Y., Nyamathi A., Gabbay J. Deactivation of human immunodeficiency virus type 1 in medium by copper oxide-containing filters. Antimicrob. Agents Chemother. 2008;52:518–525. doi: 10.1128/AAC.00899-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borkow G., Zhou S.S., Page T., Gabbay J., Tavis J.E. A novel anti-influenza copper oxide containing respiratory face mask. PLoS ONE. 2010;5(6):e11295. doi: 10.1371/journal.pone.0011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hang X., Peng H., Song H., Qi Z., Miao X., Xu W. Antiviral activity of cuprous oxide nanoparticles against Hepatitis C Virus in vitro. J. Virol. Methods. 2015;222:150–157. doi: 10.1016/j.jviromet.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 65.Warnes S.L., Little Z.R., Keevil C.W. Human coronavirus 229E remains infectious on common touch surface materials. MBio. 2015;6:e01697–e01715. doi: 10.1128/mBio.01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hosseini M., Chin A.W., Behzadinasab S., Poon L.L., Ducker W.A. Cupric oxide coating that rapidly reduces infection by SARS-CoV-2 via solids. ACS Appl. Mater. Interfaces. 2021;13:5919–5928. doi: 10.1021/acsami.0c19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Behzadinasab S., Williams M.D., Hosseini M., Poon L.L., Chin A.W., Falkinham A.J.O., III, Ducker W.A. Transparent and Sprayable Surface Coatings that Kill Drug-Resistant Bacteria Within Minutes and Inactivate SARS-CoV-2 Virus. ACS Appl. Mater. Interfaces. 2021;13:54706–54714. doi: 10.1021/acsami.1c15505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jung S., Yang J.-Y., Byeon E.-Y., Kim D.-G., Lee D.-G., Ryoo S., Lee S., Shin C.-W., Jang H.W., Kim H.J., Lee S. Copper-coated polypropylene filter face mask with SARS-COV-2 antiviral ability. Polymers. 2021;13(9):1367. doi: 10.3390/polym13091367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hewawaduge C., Senevirathne A., Jawalagatti V., Kim J.W., Lee J.H. Copper-impregnated three-layer mask efficiently inactivates SARS-CoV2. Environ. Res. 2021;196 doi: 10.1016/j.envres.2021.110947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mantlo E.K., Paessler S., Seregin A., Mitchell A. Luminore coppertouch surface coating effectively inactivates SARS-CoV-2, Ebola virus, and Marburg virus in vitro. Antimicrob. Agents Chemother. 2021;6:e01390–e01420. doi: 10.1128/AAC.01390-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hutasoit N., Kennedy B., Hamilton S., Luttick A., Rashid R.A.R., Palanisamy S. Sars-CoV-2 (COVID-19) inactivation capability of copper-coated touch surface fabricated by cold-spray technology. Manuf. Lett. 2020;25:93–97. doi: 10.1016/j.mfglet.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grass G., Rensing C., Solioz M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011;77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vincent M., Duval R.E., Hartemann P., Engels-Deutsch M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018;124:1032–1046. doi: 10.1111/jam.13681. [DOI] [PubMed] [Google Scholar]
- 75.Govind V., Bharadwaj S., Sai Ganesh M.R., Vishnu J., Shankar K.V., Shankar B., Rajesh R. Antiviral properties of copper and its alloys to inactivate covid-19 virus: a review. Biometals. 2021;34:1217–1235. doi: 10.1007/s10534-021-00339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sagripanti J.L., Routson L.B., Lytle C.D. Virus inactivation by copper or iron ions alone and in the presence of peroxide. Appl. Environ. Microbiol. 1993;59:4374–4376. doi: 10.1128/aem.59.12.4374-4376.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Halioua B., Ziskind B. Harvard University Press; 2005. Medicine in the days of the pharaohs. [Google Scholar]
- 78.Siddiqi K.S., ur Rahman A., Husen A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018;13(1) doi: 10.1186/s11671-018-2532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mishra Y.K., Adelung R., Röhl C., Shukla D., Spors F., Tiwari V. Virostatic potential of micro–nano filopodia-like ZnO structures against herpes simplex virus-1. Antiviral Res. 2011;92:305–312. doi: 10.1016/j.antiviral.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Farouk F., Sgebl R.I. Comparing surface chemical modifications of zinc oxide nanoparticles for modulating their antiviral activity against herpes simplex virus type-1. Int. J. Nanopart. Nanotechnol. 2018;4:21. doi: 10.35840/2631-5084/5521. [DOI] [Google Scholar]
- 81.Mizielińska M., Nawrotek P., Stachurska X., Ordon M., Bartkowiak A. Packaging covered with antiviral and antibacterial coatings based on ZnO nanoparticles supplemented with geraniol and carvacrol. Int. J. Mol. Sci. 2021;22:1717. doi: 10.3390/ijms22041717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ghaffari H., Tavakoli A., Moradi A., Tabarraei A., Bokharaei-Salim F., Zahmatkeshan M., Farahmand M., Javanmard D., Kiani S.J., Esghaei M., Pirhajati-Mahabadi V., Monavari S.H., Ataei-Pirkooh A. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J. Biomed. Sci. 2019;26:1–10. doi: 10.1186/s12929-019-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shirvanimoghaddam K., Akbari M.K., Yadav R., Al-Tamimi A.K., Naebe M. Fight against COVID-19: The case of antiviral surfaces. APL Mater. 2021;9(3):031112. doi: 10.1063/5.0043009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.P. Mlcochova, A. Chadha, T. Hesselhoj, F. Fraternali, J. J. Ramsden, R. K. Gupta, 2020. Extended in vitro inactivation of SARS-CoV-2 by titanium dioxide surface coating. bioRxiv. https://doi.org/10.1101/2020.12.08.415018.
- 85.Akhtar S., Shahzad K., Mushtaq S., Ali I., Rafe M.H., Fazal-ul-Karim S.M. Antibacterial and antiviral potential of colloidal Titanium dioxide (TiO2) nanoparticles suitable for biological applications. Mater. Res. Express. 2019;6 doi: 10.1088/2053-1591/ab3b27. [DOI] [Google Scholar]
- 86.Cho M., Chung H., Choi W., Yoon J. Different inactivation behaviors of MS-2 phage and Escherichia coli in TiO2 photocatalytic disinfection. Appl. Environ. Microbiol. 2005;71:270–275. doi: 10.1128/AEM.71.1.270-275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park D., Shahbaz H.M., Kim S.H., Lee M., Lee W., Oh J.W., Lee D.U., Park J. Inactivation efficiency and mechanism of UV-TiO2 photocatalysis against murine norovirus using a solidified agar matrix. Int. J. Food Microbiol. 2016;238:256–264. doi: 10.1016/j.ijfoodmicro.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 88.Hajkova P., Spatenka P., Horsky J., Horska I., Kolouch A. Photocatalytic effect of TiO2 films on viruses and bacteria. Plasma Processes Polym. 2007;4:S397–S401. doi: 10.1002/ppap.200731007. [DOI] [Google Scholar]
- 89.Syngouna V.I., Chrysikopoulos C.V. Inactivation of MS2 bacteriophage by titanium dioxide nanoparticles in the presence of quartz sand with and without ambient light. J. Colloid Interface Sci. 2017;497:117–125. doi: 10.1016/j.jcis.2017.02.059. [DOI] [PubMed] [Google Scholar]
- 90.Zan L., Fa W., Peng T., Gong Z.K. Photocatalysis effect of nanometer TiO2 and TiO2-coated ceramic plate on Hepatitis B virus. J. Photochem. Photobiol., B. 2007;86:165–169. doi: 10.1016/j.jphotobiol.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 91.Sang X., Phan T.G., Sugihara S., Yagyu F., Okitsu S., Maneekarn N., Muller W.E.G., Ushijima H. Photocatalytic inactivation of diarrheal viruses by visible-light-catalytic titanium dioxide. Clin. Lab. 2007;53:413–422. [PubMed] [Google Scholar]
- 92.Skirtach A.G., Antipov A.A., Shchukin D.G., Sukhorukov G.B. Remote activation of capsules containing Ag nanoparticles and IR dye by laser light. Langmuir. 2004;20:6988–6992. doi: 10.1021/la048873k. [DOI] [PubMed] [Google Scholar]
- 93.Shen Y., Skirtach A.G., Seki T., Yagai S., Li H., Möhwald H., Nakanishi T. Assembly of fullerene-carbon nanotubes: temperature indicator for photothermal conversion. J. Am. Chem. Soc. 2010;132:8566–8568. doi: 10.1021/ja1026024. [DOI] [PubMed] [Google Scholar]
- 94.Austin L.A., Mackey M.A., Dreaden E.C., El-Sayed M.A. The optical, photothermal, and facile surface chemical properties of gold and silver nanoparticles in biodiagnostics, therapy, and drug delivery. Arch. Toxicol. 2014;88:1391–1417. doi: 10.1007/s00204-014-1245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.D’Agostino A., Taglietti A., Desando R., Bini M., Patrini M., Dacarro G., Cucca L., Pallavicini P., Grisoli P. Bulk surfaces coated with triangular silver nanoplates: Antibacterial action based on silver release and photo-thermal effect. Nanomaterials. 2017;7:7. doi: 10.3390/nano7010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.D'Agostino A., Taglietti A., Grisoli P., Dacarro G., Cucca L., Patrini M., Pallavicini P. Seed mediated growth of silver nanoplates on glass: Exploiting the bimodal antibacterial effect by near IR photo-thermal action and Ag+ release. RSC Adv. 2016;6:70414–70423. doi: 10.1039/C6RA11608F. [DOI] [Google Scholar]
- 97.Chen J., Ning C., Zhou Z., Yu P., Zhu Y., Tan G., Mao C. Nanomaterials as photothermal therapeutic agents. Prog. Mater Sci. 2019;99:1–26. doi: 10.1016/j.pmatsci.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen C., Kuang Y., Hu L. Challenges and opportunities for solar evaporation. Joule. 2019;3:683–718. doi: 10.1016/j.joule.2018.12.023. [DOI] [Google Scholar]
- 99.Gao M., Zhu L., Peh C.K., Ho G.W. Solar absorber material and system designs for photothermal water vaporization towards clean water and energy production. Energy Environ. Sci. 2019;12:841–864. doi: 10.1039/C8EE01146J. [DOI] [Google Scholar]
- 100.Kumar S., Karmacharya M., Joshi S.R., Gulenko O., Park J., Kim G.H., Cho Y.K. Photoactive antiviral face mask with self-sterilization and reusability. Nano Lett. 2020;21:337–343. doi: 10.1021/acs.nanolett.0c03725. [DOI] [PubMed] [Google Scholar]
- 101.Kumar P., Roy S., Sarkar A., Jaiswal A. Reusable MoS2-modified antibacterial fabrics with photothermal disinfection properties for repurposing of personal protective masks. ACS Appl. Mater. Interfaces. 2021;13:12912–12927. doi: 10.1021/acsami.1c00083. [DOI] [PubMed] [Google Scholar]
- 102.Zhong H., Zhu Z., You P., Lin J., Cheung C.F., Lu V.L., Yan F., Chan C.Y., Li G. Plasmonic and superhydrophobic self-decontaminating N95 respirators. ACS Nano. 2020;14:8846–8854. doi: 10.1021/acsnano.0c03504. [DOI] [PubMed] [Google Scholar]
- 103.Lin Z., Wang Z., Zhang X., Diao D. Superhydrophobic, photo-sterilize, and reusable mask based on graphene nanosheet-embedded carbon (GNEC) film. Nano Res. 2021;14:1110–1115. doi: 10.1007/s12274-020-3158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klibanov A.M. Permanently microbicidal materials coatings. J. Mater. Chem. 2007;17:2479–2482. doi: 10.1039/B702079A. [DOI] [Google Scholar]
- 105.Hsu B.B., Wong S.Y., Hammond P.T., Chen J., Klibanov A.M. Mechanism of inactivation of influenza viruses by immobilized hydrophobic polycations. Proc. Natl. Acad. Sci. 2011;108:61–66. doi: 10.1073/pnas.1017012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Larson A.M., Hsu B.B., Rautaray D., Haldar J., Chen J., Klibanov A.M. Hydrophobic polycationic coatings disinfect poliovirus and rotavirus solutions. Biotechnol. Bioeng. 2011;108:720–723. doi: 10.1002/bit.22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sinclair T.R., Patil A., Raza B.G., Reurink D., van den Hengel S.K., Rutjes S.A., de Roda Husman A.M., Roesink H.D.W., de Vos W.M. Cationically modified membranes using covalent layer-by-layer assembly for antiviral applications in drinking water. J. Membr. Sci. 2019;570-571:494–503. [Google Scholar]
- 108.Sinclair T.R., Robles D., Raza B., van den Hengel S., Rutjes S.A., de Roda Husman A.M., de Grooth J., de Vos W.M., Roesink H.D.W. Virus reduction through microfiltration membranes modified with a cationic polymer for drinking water applications. Colloids Surf., A. 2018;551:33–41. [Google Scholar]
- 109.Rosilo H., McKee J.R., Kontturi E., Koho T., Hytönen V.P., Ikkala O., Kostiainen M.A. Cationic polymer brush-modified cellulose nanocrystals for high-affinity virus binding. Nanoscale. 2014;6:11871–11881. doi: 10.1039/C4NR03584D. [DOI] [PubMed] [Google Scholar]
- 110.Song Q., Zhao R., Liu T., Gao L., Su C., Ye Y., Chan S.Y., Liu X., Wang K., Li P., Huang W. One-step vapor deposition of fluorinated polycationic coating to fabricate antifouling and anti-infective textile against drug-resistant bacteria and viruses. Chem. Eng. J. 2021;418 doi: 10.1016/j.cej.2021.129368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amankwaah C., Li J., Lee J., Pascall M.A. Antimicrobial activity of chitosan-based films enriched with green tea extracts on murine norovirus, Escherichia coli, and Listeria innocua. Int. J. Food Sci. 2020;2020:3941924. doi: 10.1155/2020/3941924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.He X., Xing R., Liu S., Qin Y., Li K., Yu H., Li P. The improved antiviral activities of amino-modified chitosan derivatives on Newcastle virus. Drug Chem. Toxicol. 2021;44:335–340. doi: 10.1080/01480545.2019.1620264. [DOI] [PubMed] [Google Scholar]
- 113.Rinaudo M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006;31:603–632. doi: 10.1016/j.progpolymsci.2006.06.001. [DOI] [Google Scholar]
- 114.Bai B., Mi X., Xiang X., Heiden P.A., Heldt C.L. Non-enveloped virus reduction with quaternized chitosan nanofibers containing graphene. Carbohydr. Res. 2013;380:137–142. doi: 10.1016/j.carres.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 115.Cheng S., Zhao H., Xu Y., Yang Y., Lv X., Wu P., Li X. Inhibition of influenza virus infection with chitosan–sialyloligosaccharides ionic complex. Carbohydr. Polym. 2014;107:132–137. doi: 10.1016/j.carbpol.2014.02.048. [DOI] [PubMed] [Google Scholar]
- 116.Cermelli C., Cuoghi A., Scuri M., Bettua C., Neglia R.G., Ardizzoni A., Blasi E., Iannitti T., Palmieri B. In vitro evaluation of antiviral and virucidal activity of a high molecular weight hyaluronic acid. Virol. J. 2011;8:1–8. doi: 10.1186/1743-422X-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Falcó I., Randazzo W., Sánchez G., López-Rubio A., Fabra M.J. On the use of carrageenan matrices for the development of antiviral edible coatings of interest in berries. Food Hydrocolloids. 2019;92:74–85. doi: 10.1016/j.foodhyd.2019.01.039. [DOI] [Google Scholar]
- 118.Hu T., Agazani O., Nir S., Cohen M., Pan S., Reches M. Antiviral Activity of Peptide-Based Assemblies. ACS Appl. Mater. Interfaces. 2021;13:48469–48477. doi: 10.1021/acsami.1c16003. [DOI] [PubMed] [Google Scholar]
- 119.Skalickova S., Heger Z., Krejcova L., Pekarik V., Bastl K., Janda J., Kostolansky F., Vareckova E., Zitka O., Adam V., Kizek R. Perspective of use of antiviral peptides against influenza virus. Viruses. 2015;7:5428–5442. doi: 10.3390/v7102883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jones J.C., Turpin E.A., Bultmann H., Brandt C.R., Schultz-Cherry S. Inhibition of influenza virus infection by a novel antiviral peptide that targets viral attachment to cells. J. Virol. 2006;80:11960–11967. doi: 10.1128/JVI.01678-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Han D.P., Penn-Nicholson A., Cho M.W. Identification of critical determinants on ACE2 for SARS-CoV entry and development of a potent entry inhibitor. Virology. 2006;350:15–25. doi: 10.1016/j.virol.2006.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cao L., Goreshnik I., Coventry B., Case J.B., Miller L., Kozodoy L., Chen R.E., Carter L., Walls A.C., Park Y.J., Strauch E.M., Stewart L., Diamond M.S., Veesler D., Baker D. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science. 2020;370:426–431. doi: 10.1126/science.abd9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de Castro K.C., Costa J.M. Polymeric surfaces with biocidal action: challenges imposed by the SARS-CoV-2, technologies employed, and future perspectives. J. Polym. Res. 2021;28:1–16. doi: 10.1007/s10965-021-02548-4. [DOI] [Google Scholar]
- 124.Ren T., Dormitorio T.V., Qiao M., Huang T.S., Weese J. N-halamine incorporated antimicrobial nonwoven fabrics for use against avian influenza virus. Vet. Microbiol. 2018;218:78–83. doi: 10.1016/j.vetmic.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 125.Calland N., Albecka A., Belouzard S., Wychowski C., Duverlie G., Descamps V., Hober D., Dubuisson J., Rouillé Y., Séron K. (−)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology. 2012;55:720–729. doi: 10.1002/hep.24803. [DOI] [PubMed] [Google Scholar]
- 126.Yi L., Li Z., Yuan K., Qu X., Chen J., Wang G., Zhang H., Luo H., Zhu L., Jiang P., Chen L., Shen Y., Luo M., Zuo G., Hu J., Duan D., Nie Y., Shi X., Wang W., Han Y., Li T., Liu Y., Ding M., Deng H., Xu X. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004;78:11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lin L.T., Chen T.Y., Chung C.Y., Noyce R.S., Grindley T.B., McCormick C., Lin T.C., Wang G.H., Lin C.C., Richardson C.D. Hydrolyzable tannins (chebulagic acid and punicalagin) target viral glycoprotein-glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. J. Virol. 2011;85:4386–4398. doi: 10.1128/JVI.01492-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin L.T., Chen T.Y., Lin S.C., Chung C.Y., Lin T.C., Wang G.H., Anderson R., Lin C.C., Richardson C.D. Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol. 2013;13:1–15. doi: 10.1186/1471-2180-13-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007;74:92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lin L.T., Chung C.Y., Hsu W.C., Chang S.P., Hung T.C., Shields J., Russell R.S., Lin C.C., Li C.F., Yen M.H., Tyrrell D.L.J., Lin C.C., Richardson C.D. Saikosaponin b2 is a naturally occurring terpenoid that efficiently inhibits hepatitis C virus entry. J. Hepatol. 2015;62:541–548. doi: 10.1016/j.jhep.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 131.Cheng P.W., Ng L.T., Chiang L.C., Lin C.C. Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clin. Exp. Pharmacol. Physiol. 2006;33:612–616. doi: 10.1111/j.1440-1681.2006.04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Raczkowska J., Stetsyshyn Y., Awsiuk K., Lekka M., Marzec M., Harhay K., Ohar H., Ostapiv D., Sharan M., Yaremchuk I., Bodnar Y., Budkowski A. Temperature-responsive grafted polymer brushes obtained from renewable sources with potential application as substrates for tissue engineering. Appl. Surf. Sci. 2017;407:546–554. doi: 10.1016/j.apsusc.2017.03.001. [DOI] [Google Scholar]
- 133.Stetsyshyn Y., Raczkowska J., Budkowski A., Awsiuk K., Kostruba A., Nastyshyn S., Harhay K., Lychkovskyy E., Ohar H., Nastishin Y. Cholesterol-based grafted polymer brushes as alignment coating with temperature-tuned anchoring for nematic liquid crystals. Langmuir. 2016;32:11029–11038. doi: 10.1021/acs.langmuir.6b02946. [DOI] [PubMed] [Google Scholar]
- 134.Stetsyshyn Y., Raczkowska J., Lishchynskyi O., Awsiuk K., Zemla J., Dąbczyński P., Kostruba A., Harhay K., Ohar H., Orzechowska B., Panchenko Y., Vankevych Y., Budkowski A. Glass transition in temperature-responsive poly (butyl methacrylate) grafted polymer brushes. Impact of thickness and temperature on wetting, morphology, and cell growth. J. Mater. Chem. B. 2018;6:1613–1621. doi: 10.1039/C8TB00088C. [DOI] [PubMed] [Google Scholar]
- 135.Awsiuk K., Stetsyshyn Y., Raczkowska J., Lishchynskyi O., Dąbczyński P., Kostruba A., Ohar H., Shymborska Y., Nastyshyn S., Budkowski A. Temperature-controlled orientation of proteins on temperature-responsive grafted polymer brushes: poly (butyl methacrylate) vs poly (butyl acrylate): morphology, wetting, and protein adsorption. Biomacromolecules. 2019;20:2185–2197. doi: 10.1021/acs.biomac.9b00030. [DOI] [PubMed] [Google Scholar]
- 136.Stetsyshyn Y., Raczkowska J., Harhay K., Gajos K., Melnyk Y., Dąbczyński P., Shevtsova T., Budkowski A. Temperature-responsive and multi-responsive grafted polymer brushes with transitions based on critical solution temperature: Synthesis, properties, and applications. Colloid Polym. Sci. 2021;299(3):363–383. [Google Scholar]
- 137.Xue Y., Xiao H. Antibacterial/antiviral property and mechanism of dual-functional quaternized pyridinium-type copolymer. Polymers. 2015;7:2290–2303. doi: 10.3390/polym7111514. [DOI] [Google Scholar]