Abstract
Context
Androgen prohormones such as dehydroepiandrosterone (DHEA) increase in early puberty, peak in the second and third decade, and thereafter decline, independent of menopausal status. Investigators have examined their potential beneficial effects in normal women and those with DHEA-deficient states.
Evidence Acquisition
A review of the literature from 1985 to 2021 on the potential benefits and risks of androgen prohormones in women.
Evidence Synthesis
Studies have examined the potential benefit of DHEA therapy for anti-aging, sexual dysfunction, infertility, metabolic bone health, cognition, and wellbeing in hormone-deficient states such as primary adrenal insufficiency, hypopituitarism, and anorexia as well as administration to normal women across the lifespan.
Conclusions
Data support small benefits in quality of life and mood but not for anxiety or sexual function in women with primary or secondary adrenal insufficiency or anorexia. No consistent beneficial effects of DHEA administration have been observed for menopausal symptoms, sexual function, cognition, or overall wellbeing in normal women. Local administration of DHEA shows benefit in vulvovaginal atrophy. Use of DHEA to improve induction of ovulation response in women with diminished ovarian reserve is not recommended. Risks of high physiologic or pharmacologic use of DHEA include androgenic and estrogenic side effects which are of concern for long-term administration.
Clinical Case
A 49-year-old woman with Addison’s disease who is on low dose estrogen with cyclic progesterone therapy for menopausal symptoms returns for follow-up. She is on a stable glucocorticoid replacement strategy of hydrocortisone 10 mg in the morning and 5 mg in the early afternoon and fludrocortisone 0.05 mg each morning. She has read on the internet that additional therapy with DHEA may help her overall quality of life and libido. She asks whether she should add this therapy to her regimen and at what dose.
Keywords: DHEA, androstenedione, testosterone, hormonal therapies
Physiology of Androgen Prohormones: DHEA and Androstenedione
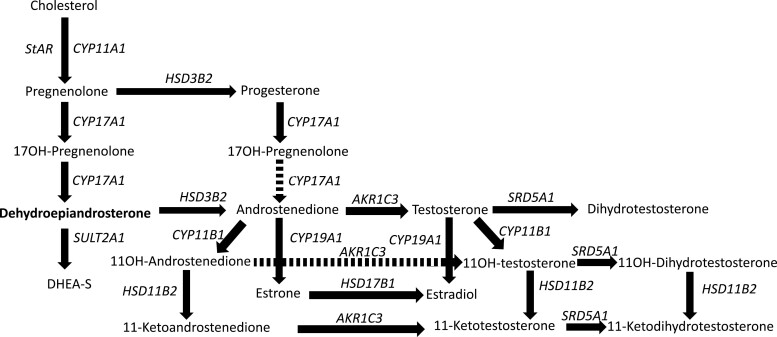
The pathway of adrenal steroidogenesis is reviewed in Fig. 1. Importantly, dehydroepiandrosterone (DHEA), DHEA sulfate (DHEAS), and androstenedione are not themselves androgens but instead prohormones that are converted to testosterone and/or estradiol in target tissues to act on the respective steroid receptors. DHEA was originally extracted from male urine and human plasma in the 1930s and 1950s, respectively, and the sulfated form, DHEAS, was detected in 1944 (reviewed in (1, 2)). Baulieu and coworkers established that DHEAS was the most abundant steroid hormone precursor circulating in plasma (3). In addition, in the brain, DHEA and/or its metabolites may act as neurosteroids via membrane receptors such as gamma aminobutryric acid alpha and N methyl-D aspartate receptors or has been postulated to interact with the peroxisome proliferator activated receptor (PPARα), pregnane X receptor, androstanol, or estrogen receptorβ to have central and metabolic effects (reviewed in (2)). Studies suggested a role for DHEA in the immune system or in improving immune response with aging, but no clinical outcome studies have been reported (4, 5). No specific, high-affinity physiologic DHEA receptor has been identified (2).
Figure 1.
Diagram outlining the major enzymes and steps converting cholesterol to DHEA to DHEAS and to androstenedione to testosterone and estradiol in the adrenal gland. CYP, cytochrome 450; HSD, hydroxysteroid dehydrogenase; SRD, steroid 5a reductase.
DHEA is produced in the zona reticularis of the adrenal gland DHEAS is the most abundant prohormone for steroid hormones is also predominantly made in the adrenal or converted from DHEA in the liver and intestines (6). Whereas DHEA is released in an episodic fashion with a shorter half-life and modulated by stress and exposure to glucocorticoids, DHEAS has a longer half-life and stable levels across the day. In target tissues, DHEA is metabolized to androstenediol, androstenedione, testosterone, dihydrotestosterone, and 17β-estradiol (7). Androstenedione production is derived both from the adrenal gland and the ovary (8). These adrenally produced hormones are precursors for 30% of androgens in men, and 75% in premenopausal and 100% in postmenopausal women (9). DHEA and DHEAS levels increase across adrenarche during the pubertal process before gonadarche and peak in the late 20s to 30s before declining with age independent of menopausal status (9, 10).
Recently the role of 11-oxygenated adrenal prohormones has been investigated for their role in adrenarche and in states of adrenal androgen excess such as premature pubarche, congenital adrenal hyperplasia and adrenal tumors (11, 12). An abundant unconjugated androgen produced by the adrenal glands is 11β-hydroxyandrostenedione. One of its byproducts, 11-ketotestosterone, is produced locally in peripheral tissues; together with its 5α-reduced downstream product, 11-ketodihyrdrotestosterone, they have been shown to be bioactive androgens with potencies similar to testosterone. These 11-oxyandrogens peak in midlife but do not decline with age (11). Their role in adrenal androgen–“deficient” states or effects of supplementation have not been investigated to date.
One of the issues as we review the literature or consider DHEA supplementation is what is the physiologic dose of this hormone in women? The fact that DHEA and other prohormones are currently available over the counter as supplements in the United States or via the internet without FDA oversight for potency or duration of action is a major issue. DHEA is widely available as a dietary supplement in the United States; however, quality control of DHEA or androstenedione has been shown to be inconsistent (13). Therapeutic regimens in clinical trials have usually given DHEA administered in physiologic doses of 25 to 50 mg/day; however, some studies examined pharmacologic dosing up to 1600 mg/day (14). Based upon the pharmacology of DHEA, the supplementation dose should be 25 mg in postmenopausal women and 50 mg in men (15).
Disorders for Which Androgen Prohormones Have Been Administered
The effects of DHEA administration in physiologic to pharmacologic doses has been examined in women with low DHEA levels, namely deficient states such as primary and secondary insufficiency and anorexia, as well as supplementation to those women with normal DHEA levels for various potential health outcomes. This review will distinguish between these interventions and conclude an overall lack of data to support DHEA administration to most women.
DHEA Therapy in DHEA Nondeficient States
Anti-aging Promotion for DHEA
The abundance of the adrenal precursors, and their decline with aging and with exposure to exogenous glucocorticoids has raised the potential for their supplementation as an “anti-aging” prescription (3). Mortola and Yen performed initial studies in 17 women aged 40-70, given DHEAS 50 mg nightly for 3 months in a placebo-controlled, crossover study and reported an 84% increase in physical and psychological wellbeing in women, but no change in libido (16). A 2-fold increase in androstenedione, testosterone, and dihydrotestosterone levels was detected without change in estradiol. Baulieu and colleagues examined the administration of synthetic DHEA at 50 mg daily in 280 older women and men (ages 60-79, 140 each) without adrenal insufficiency for a year in the DHEAge study (17). Increases in testosterone and estradiol were observed in the women at 6 months with 21% of values outside the normal premenopausal range. The androgens decreased by 12 months, whereas estradiol remained consistently increased from baseline (17). The authors postulated an adaptive mechanism with time to limit the potential androgenic side effects long term, but which was not seen with the elevation in estradiol which persisted. These observations are important to consider with any long-term DHEA therapy in postmenopausal women. Improvement in sexual function was reported, but only 25% of the subjects understood the visual analog scale and extent of response was variable at 6 or 12 months (17). The authors posited a potential effect of changes in estradiol on these sexual outcomes in women. No effects on libido or sexual function were observed in men (17). In addition, there were no effects on muscle strength with this year-long intervention in older women aged 60-80 (18). More recently, investigators performed a 2-year, placebo-controlled, randomized, double-blind study in 57 elderly women with low DHEA levels. In the 27 women who received DHEA, there was a consistent increase in both testosterone and estradiol levels, but again no improvement noted in quality of life (19). Thus, these studies and others have argued against the use of DHEA as an anti-aging panacea for women. The potential long-term risks of DHEA administration to increase testosterone and estradiol levels in older women regarding estrogen-dependent malignancies and cardiovascular benefit or risk would also need to be considered.
Cognition
Because of the known deterioration in cognition with aging, several studies have examined the effects of supplementation on cognitive outcomes. Wolf and coworkers examined effects of DHEA after a stressor to examine its potential role as a neurocognitive protector (20). After 2 weeks of treatment, placebo group performance deteriorated significantly on a test of selective attention following a psychosocial stressor (P < .05), while deterioration was not evident in the DHEA group (P = .85). DHEA was associated with significant impairment on a visual memory recall test (P < .01) following the stressor. No significant effects were found on a third cognitive task. In contrast, Nair et al enrolled 57 women with low DHEAS levels in a 24-month study and no significant changes in quality of life measures were found (19). In 2008, von Muhlen administered DHEA for 1 year and showed no significant benefit on cognition performance in 225 healthy older people (21). Reduced performance in a visual memory recall test observed in 1 trial and a significant drop-out rate in favor of placebo emerged in another trial (21). Work by our group in a small study to understand how DHEA alters steroid hormone levels across the day demonstrated that after administration of DHEA to postmenopausal women, androgens increased transiently followed by estradiol levels, with a different impact on whichever specific cognitive outcome measure was randomly assessed across the day (22). Estrogen levels were positively and androgen levels negatively associated with measures of recognition memory; however, the associations were reversed in perceptual identification tests (22). These results suggested that estrogens produced a positive effect on recognition memory, while androgens produced a negative effect. This pattern reversed in perceptual identification, with estrogens producing a negative effect and androgens producing a positive effect. The metabolism of DHEA across the day may explain its complex effects on cognition. The effects of DHEA supplementation may be direct on neurocognitive targets or indirect via metabolism to androgen or estrogen targets (2). In addition, the ability of specific cognitive measures to detect specific effects of DHEA administration may be complex, and individually variable. A Cochrane meta-analysis of 5 validated studies concluded there were no consistent effects of DHEA supplementation on cognitive outcomes (23). Thus, collectively, we have no data to support DHEA supplementation to older women for cognitive benefit.
What About Effects of DHEA in the Perimenopause and on Menopausal Symptoms?
Barnhart and coworkers performed a 3-month study in 60 perimenopausal women given DHEA 50 mg daily and demonstrated elevations in prohormone and testosterone levels and a 2-fold increase in estradiol levels (24). They reported no improvement in severity of perimenopausal symptoms, mood, dysphoria, libido, cognition memory, or wellbeing (24). A meta-analysis was performed to examine 16 trials in peri- and postmenopausal women; the quality of the studies was low to moderate. DHEA did not improve the quality of life, did not consistently affect menopausal symptoms and minor effects of sexual function compared with hormonal therapy, and was associated with androgenic side effects such as skin changes, acne, and hirsutism (25).
What About Use of DHEA Supplementation in Deficient States?
Adrenal insufficiency
Both primary adrenal insufficiency or secondary adrenocorticotropin (ACTH) deficiency with hypopituitarism results in long-term low or undetectable DHEA levels (26). In contrast, in isolated gonadal insufficiency, the androgen prohormones are usually normal as the major production of these hormones is the adrenal gland and peripheral conversion.
Initial studies of predominantly premenopausal women aged 23-59 with adrenal insufficiency by Arlt and colleagues (n = 24) given DHEA 50 mg daily or placebo for 4 months demonstrated improved wellbeing and decreased depression and anxiety. In addition, they noted an increased frequency of sexual thoughts, sexual interest, and satisfaction (27). Others administered micronizable DHEA 50 mg/day to 24 women aged 26-69 with Addison’s disease in a 3-month, crossover study. They observed a significant improvement in in self-esteem and fatigue in the evening; however, they found no consistent effect on cognition or sexual arousal or function (28). Studies in 19 women with adrenal insufficiency given a lower dose of 25 mg of DHEA daily which restored levels to premenopausal range reported no improvements in wellbeing or sexual function and yet a high rate of side effects, including sweat odor and scalp itching, even with this lower dose (29). Gurnell and coworkers administered DHEA 50 mg daily to 32 women with adrenal insufficiency for a longer term 12 months and noted no improvement in fatigue, cognitive or sexual function (30). In addition, high DHEAS levels were observed in women with some androgenic side effects. Thus these data overall do not support a consistent beneficial effect of DHEA administration to women with adrenal insufficiency.
Studies in women with hypopituitarism reported variable outcomes in quality of life, sexual function, or improved mood. Administration of DHEA 25 mg to adolescent girls with central ACTH deficiency increased pubic hair and improved wellbeing (31). However, Johannsson and coworkers administered DHEA in an age-based dosing of 20 mg to 50 mg daily to 38 women with hypopituitarism for 6 months in an open-label, crossover design and observed no significant improvement in quality of life, and sexual interest or activity (32). In later studies, authors asked whether adding DHEA to growth hormone therapy was beneficial. DHEA administered at 50 mg daily for 4 months in a double-blind, placebo-controlled, crossover study did not improve quality of life (33).
A recent meta-analysis of 10 studies summarized that DHEA administration in women with primary or secondary adrenal insufficiency results in only small improvements in quality of life and mood (depression) but no significant consistent effects on anxiety or sexual function (34). DHEA at a dose of 50 mg to premenopausal women with hypopituitarism women restores androgen prohormones and testosterone to normal or often high levels. The role of variable estrogen replacement therapy in these studies may have impacted the variability in the results.
Anorexia nervosa
There are profound effects on the endocrine system in women with anorexia (35). Investigators examined the effects of DHEA 50 mg daily for potential beneficial effects in premenopausal women with anorexia on wellbeing and bone density. In 30 women randomized to DHEA 50 mg daily compared with standard hormonal therapy with an oral contraceptive for a year, they noted that patients receiving DHEA exhibited improvement on 3 validated psychological instruments (Eating Attitudes Test, Anorexia Nervosa Subtest, and Spielberger Anxiety Inventory) (36). DHEA administration also improved bone turnover markers. In another small study, DHEA resulted in resumption of menses in 50% of women via a hypothesized increase in estradiol levels (37). These data again support that DHEA action is via conversion to androgens and estrogens in different target tissues.
Other Areas Where DHEA Supplementation Has Been Promoted
Sexual dysfunction
In a systematic review and meta-analysis of 23 trials in 1188 postmenopausal women, DHEA therapy was not associated with an improvement in libido or other sexual function outcomes when compared with placebo (38). The Endocrine Society guidelines and recent international guidelines agree on the lack of data supporting the use of DHEA for sexual dysfunction (39-41).
Mood
Based upon initial reports of improvement in mood with administration of DHEA in those with adrenal insufficiency, authors have postulated that the effects on wellbeing may be via the direct effects in the brain where DHEA may act as a neurosteroid (2). Some population studies, however, have demonstrated a higher prevalence of major depressive disorders in women with high prohormone and androgen levels such as those with polycystic ovarian syndrome or congenital adrenal hyperplasia (42). A recent analysis of androgen precursors and androgen levels in 1659 women with mood disorders found no correlation of onset, remission, or recurrence of depression with androgen or sex hormone–binding globulin levels (43). However, many earlier studies examined giving DHEA for mood disorders. A recent meta-analysis of all studies where DHEA was administered for depression concluded that there may be a minor effect of DHEA administration; however, all the evidence was of low quality and thus it would be difficult to support the use of DHEA therapy to women with depression (44).
Metabolic bone health
Administration of DHEA in older adults was shown to have a sex-specific effect on bone mineral density (45, 46). Many of the small studies where DHEA was administered for wellbeing or other targets across the lifespan found variable effects on bone quality and density (47). Jankowski and coworkers examined pooled data from 295 women and 290 men aged 55 or older given DHEA or placebo for 12 months (45). Women showed increases in DHEAS, testosterone estradiol, and insulin-like growth factor 1, whereas men showed increases in DHEAS, estradiol and insulin-like growth factor 1. Women demonstrated a small increase in lumbar spine bone mineral density (1.0 ± 3.4%) and trochanter (0.5 ± 3.8%) and maintained total hip, whereas men showed no benefit on bone but a decrease in fat mass (45). This group and others examining effects in women with anorexia supported the conclusion that any bone effect of DHEA is via effects on changes in estrogen (36, 37, 46). Importantly, the effect size for DHEA therapy on the bone in women was significantly less than estrogen therapy or FDA-approved osteoporosis medications. No data are available concerning effects of DHEA supplementation on fracture risk.
Metabolic effects
Pharmacologic dosing of 100 mg daily demonstrated a decrease in fat mass and increase in strength in men, but not women (48). Decreases in serum leptin, but no effect on fasting glucose insulin, were observed; the authors concluded no significant effect on carbohydrate metabolism body composition or exercise capacity, and this is supported by others (48, 49). More recently, Nair and coworkers examined a 2-year intervention with DHEA 50 mg daily and showed no effects on body composition or peak volume of oxygen consumed per minute, muscle strength, or insulin sensitivity (19). Based upon data suggesting DHEA may act centrally on PPARα receptors related to fat oxidation and fat deposition (50), Villareal and Holloszy asked whether DHEA therapy would alter body composition as assessed using magnetic resonance imaging, a more sensitive measure (51). Twenty-eight women aged 71 (65-78) were assigned to receive DHEA 50 mg for 6 months. Similar to other reports in the literature, both testosterone and estradiol levels increased in the experimental group. DHEA supplementation decreased bodyweight (–0.9 compared with placebo +0.6 kg), associated with a decrease in visceral fat mass (10.2%) and subcutaneous fat (6%). They observed lower insulin levels and unchanged glucose, resulting in an increase in the insulin sensitivity index. The authors suggest an improvement in the risk or severity of metabolic syndrome associated with abdominal obesity. Although intriguing, the authors admit the changes observed after DHEA supplementation may be related to changes in estrogen after DHEA supplementation in women, and thus the risk/benefit of long-term administration would need to be considered.
Genitourinary symptoms
Genitourinary syndrome of the menopause affects 27% to 84% of postmenopausal women and can significantly impair health, sexual function, and quality of life (52). Initial interest in the use of DHEA for vaginal dryness was proposed by Labrie et al in 1997 when he administered a 10% DHEA cream to 14 women aged 60-70 and noted a vaginal estrogenic effect on the maturation index but no adverse effects on the atrophic endometrium (53). However, no measurements of DHEAS or other hormones were included. This observation started the interest in the safety and efficacy of synthetic DHEA, prasterone, for potential benefit with hypothesized fewer androgenic or estrogenic effects. Labrie and coworkers demonstrated the effectiveness of DHEA 0.50%, 6.5 mg of prasterone, on symptoms and signs of vaginal dryness (54). The question clinically, however, is whether DHEA is better or safer than nonhormonal therapies or low-dose topical or systemic estrogen therapy (55). Recent guidelines suggest initial use of nonhormonal agents such as vaginal lubricants and moisturizers, and then if not effective consideration of low-dose topical estrogen, DHEA, or the mixed agonist/antagonist ospemifene (56). No large head-to-head studies have been performed. Long-term studies on the endometrial safety of vaginal estrogen, vaginal DHEA, and ospemifene are lacking.
Infertility
Investigators have examined the potential role of administering testosterone or DHEA to women with unexplained infertility or decreased ovarian reserve with the hypothesis that increase in androgens locally may improve ovarian function and fertility. DHEA is reported to be currently used in 25% of in vitro fertilization clinics without evidence of clear benefit. DHEA was administered in observational studies at a dose of 50 mg daily to women with diminished ovarian reserve and premature ovarian insufficiency with some success (57). These studies, however, were performed without control groups and thus cannot be recommended. Two recent meta-analysis showed conflicting conclusions concerning whether DHEA improved ovarian response in women with diminished ovarian reserve (58, 59). Studies with a placebo-controlled arm showed no benefit.
Adverse Effects of DHEA Administration to Women
Many trials did not provide data on adverse events. Of those that did, androgenic side effects (acne and hirsutism) appeared to be more common with DHEA than placebo, depending on the dose administered and targeted DHEA levels. Side effects were seen in women with adrenal insufficiency, ACTH deficiency, or anorexia. Similar results were reported in a meta-analysis of 28 trials of DHEA therapy in symptomatic postmenopausal women (38). DHEA did not improve quality of life, menopausal symptoms, or sexual function, but did increase androgenic side effects (acne and hirsutism) when compared with placebo or no treatment. Importantly, our patients need to understand that DHEA is metabolized to testosterone and estradiol, and administration would be contraindicated in women with a history of breast cancer or elderly women regarding the risk of estrogens in the cardiovascular system.
Abuse of Androgen Prohormones
Although abuse of androgen prohormones and testosterone have been detailed in men, less is known about the profiles in women athletes using pharmacologic doses of DHEA or other prohormones like androstenedione. Buisson and coworkers administered DHEA at 100 mg daily and detected a specific signature that would alert the authorities in the antidoping evaluation for female athletes. Concentrations of the urinary parameters of the steroid profile were highly impacted by short-term DHEA administration including the androgen epitestosterone (60). The most impacted markers in women were testosterone/epitestosterone and 5αadiol/estradiol, with a detection window of 36 hours for 5αadiol/estradiol. Thus, excess intake of DHEA or androstenedione would be readily detected if taken by athletes.
Novel Research Area for DHEA Administration
With the knowledge that after trauma there is an influx of inflammatory and immune reactions with elevated cortisol in relation to DHEAS levels, Bentley and coworkers have embarked on a prospective, phase II, single-center, cross-sectional, randomized study of DHEA in trauma patients (61). A dose-finding study is underway with sublingual or oral DHEA at 50 to 200 mg for 3 days after trauma. Women aged >50 with femur neck fracture and male and female patients with major trauma aged 16-50 will be recruited. Whether short-term DHEA administration will combat the high cortisol response after trauma is an intriguing question, or conversely whether this dichotomous divergency high cortisol/DHEA that is also observed in anorexia (35) and after injury is somehow protective and should not be altered. We await the results of this and future studies.
Conclusions
Back to our patient. This woman has primary adrenal insufficiency and has concomitant low adrenal prohormone levels. There are conflicting data in the literature concerning the benefit of DHEA in women with adrenal insufficiency at any age. In the premenopausal ages in women with low DHEAS levels, one could argue a trial of DHEA for potential benefits on wellbeing, understanding that the physiologic dose of DHEA in women is somewhere around 25 and not 50 mg/day. In the postmenopausal ages, one must consider the additional impact of conversion to testosterone and estradiol to her breast and bone health and cardiac risks, and discuss the pros and cons of a short-term trial. Long-term supplementation is not well established.
Since the last review of DHEA administration to women published by Davis in the Journal of Clinical Endocrinology & Metabolism in 2011 (47) and subsequent meta-analysis in 2014 (38), there are few new studies and no new substantive data to argue for a re-examination of its use. Yet our patients looking for a panacea for all of their symptoms and deluged by targeted media continue to use/abuse prohormones. For normal women across the lifespan, there are few data to support the administration of DHEA for sexual, cognitive, mood, or overall wellbeing.
Glossary
Abbreviations
- ACTH
adrenocorticotropin
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulfate
- PPARα
peroxisome proliferator activated receptor
Funding
Veterans Affairs Merit Review Award 001 (to M.E.W.), NIH NCI K08CA222620 (to K.K.V.).
Disclosures
The authors declare no potential conflicts of interest.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Lieberman S. An abbreviated account of some aspects of the biochemistry of DHEA, 1934-1995. Ann N Y Acad Sci. 1995;774:1-15. [DOI] [PubMed] [Google Scholar]
- 2. Rutkowski K, Sowa P, Rutkowska-Talipska J, Kuryliszyn-Moskal A, Rutkowski R. Dehydroepiandrosterone (DHEA): hypes and hopes. Drugs. 2014;74(11):1195-1207. [DOI] [PubMed] [Google Scholar]
- 3. Baulieu EE, Corpechot C, Dray F, et al. An adrenal-secreted “androgen”: dehydroisoandrosterone sulfate. its metabolism and a tentative generalization on the metabolism of other steroid conjugates in man. Recent Prog Horm Res. 1965;21:411-500. [PubMed] [Google Scholar]
- 4. Prall SP, Muehlenbein MP. DHEA modulates immune function: a review of evidence. Vitam Horm. 2018;108:125-144. [DOI] [PubMed] [Google Scholar]
- 5. Coles AJ, Thompson S, Cox AL, Curran S, Gurnell EM, Chatterjee VK. Dehydroepiandrosterone replacement in patients with Addison’s disease has a bimodal effect on regulatory (CD4+CD25hi and CD4+FoxP3+) T cells. Eur J Immunol. 2005;35(12):3694-3703. [DOI] [PubMed] [Google Scholar]
- 6. Longcope C. Adrenal and gonadal androgen secretion in normal females. Clin Endocrinol Metab 1986;15(2):213-228. [DOI] [PubMed] [Google Scholar]
- 7. Burger HG. Androgen production in women. Fertil Steril. 2002;77(Suppl 4):S3-S5. [DOI] [PubMed] [Google Scholar]
- 8. Horton R, Tait JF. Androstenedione production and interconversion rates measured in peripheral blood and studies on the possible site of its conversion to testosterone. J Clin Invest. 1966;45(3):301-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Migeon CJ, Keller AR, Lawrence B, Shepard TH, 2nd. Dehydroepiandrosterone and androsterone levels in human plasma: effect of age and sex; day-to-day and diurnal variations. J Clin Endocrinol Metab. 1957;17(9):1051-1062. [DOI] [PubMed] [Google Scholar]
- 10. Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59(3):551-555. [DOI] [PubMed] [Google Scholar]
- 11. Turcu AF, Rege J, Auchus RJ, Rainey WE. 11-Oxygenated androgens in health and disease. Nat Rev Endocrinol. 2020;16(5):284-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schiffer L, Arlt W, Storbeck KH. Intracrine androgen biosynthesis, metabolism and action revisited. Mol Cell Endocrinol. 2018;465:4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thompson RD, Carlson M, Thompson RD, Carlson M. Liquid chromatographic determination of dehydroepiandrosterone (DHEA) in dietary supplement products. J AOAC Int. 2000;83(4):847-857. [PubMed] [Google Scholar]
- 14. Nestler JE, Barlascini CO, Clore JN, Blackard WG. Dehydroepiandrosterone reduces serum low density lipoprotein levels and body fat but does not alter insulin sensitivity in normal men. J Clin Endocrinol Metab. 1988;66(1):57-61. [DOI] [PubMed] [Google Scholar]
- 15. Legrain S, Massien C, Lahlou N, et al. Dehydroepiandrosterone replacement administration: pharmacokinetic and pharmacodynamic studies in healthy elderly subjects. J Clin Endocrinol Metab. 2000;85(9):3208-3217. [DOI] [PubMed] [Google Scholar]
- 16. Mortola JF, Yen SS. The effects of oral dehydroepiandrosterone on endocrine-metabolic parameters in postmenopausal women. J Clin Endocrinol Metab. 1990;71(3):696-704. [DOI] [PubMed] [Google Scholar]
- 17. Baulieu EE, Thomas G, Legrain S, et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci USA. 2000;97(8):4279-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Percheron G, Hogrel JY, Denot-Ledunois S, et al. ; Effect of 1-year oral administration of dehydroepiandrosterone to 60- to 80-year-old individuals on muscle function and cross-sectional area: a double-blind placebo-controlled trial. Arch Intern Med. 2003;163(6):720-727. [DOI] [PubMed] [Google Scholar]
- 19. Nair KS, Rizza RA, O’Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355(16):1647-1659. [DOI] [PubMed] [Google Scholar]
- 20. Wolf OT, Kudielka BM, Hellhammer DH, Hellhammer J, Kirschbaum C. Opposing effects of DHEA replacement in elderly subjects on declarative memory and attention after exposure to a laboratory stressor. Psychoneuroendocrinology 1998;23(6):617-629. [DOI] [PubMed] [Google Scholar]
- 21. von Muhlen D, Laughlin GA, Kritz-Silverstein D, Bergstrom J, Bettencourt R. Effect of dehydroepiandrosterone supplementation on bone mineral density, bone markers, and body composition in older adults: the DAWN trial. Osteoporos Int. 2008;19(5):699-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hirshman E, Wells E, Wierman ME, et al. The effect of dehydroepiandrosterone (DHEA) on recognition memory decision processes and discrimination in postmenopausal women. Psychon Bull Rev. 2003;10(1):125-134. [DOI] [PubMed] [Google Scholar]
- 23. Grimley Evans J, Malouf R, Huppert F, van Niekerk JK. Dehydroepiandrosterone (DHEA) supplementation for cognitive function in healthy elderly people. Cochrane Database Syst Rev. 6221;2006(4):CD006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barnhart KT, Freeman E, Grisso JA, et al. The effect of dehydroepiandrosterone supplementation to symptomatic perimenopausal women on serum endocrine profiles, lipid parameters, and health-related quality of life. J Clin Endocrinol Metab. 1999;84(11):3896-3902. [DOI] [PubMed] [Google Scholar]
- 25. Scheffers CS, Armstrong S, Cantineau AE, Farquhar C, Jordan V. Dehydroepiandrosterone for women in the peri- or postmenopausal phase. Cochrane Database Syst Rev. 2015;1:CD011066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arlt W. The approach to the adult with newly diagnosed adrenal insufficiency. J Clin Endocrinol Metab. 2009;94(4):1059-1067. [DOI] [PubMed] [Google Scholar]
- 27. Arlt W, Callies F, van Vlijmen JC, et al. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N Engl J Med. 1999;341(14):1013-1020. [DOI] [PubMed] [Google Scholar]
- 28. Hunt PJ, Gurnell EM, Huppert FA, et al. Improvement in mood and fatigue after dehydroepiandrosterone replacement in Addison’s disease in a randomized, double blind trial. J Clin Endocrinol Metab. 2000;85(12):4650-4656. [DOI] [PubMed] [Google Scholar]
- 29. Lovas K, Gebre-Medhin G, Trovik TS, et al. Replacement of dehydroepiandrosterone in adrenal failure: no benefit for subjective health status and sexuality in a 9-month, randomized, parallel group clinical trial. J Clin Endocrinol Metab. 2003;88(3):1112-1118. [DOI] [PubMed] [Google Scholar]
- 30. Gurnell EM, Hunt PJ, Curran SE, et al. Long-term DHEA replacement in primary adrenal insufficiency: a randomized, controlled trial. J Clin Endocrinol Metab. 2008;93(2):400-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Binder G, Weber S, Ehrismann M, et al. ; South German Working Group for Pediatric Endocrinology. Effects of dehydroepiandrosterone therapy on pubic hair growth and psychological well-being in adolescent girls and young women with central adrenal insufficiency: a double-blind, randomized, placebo-controlled phase III trial. J Clin Endocrinol Metab. 2009;94(4):1182-1190. [DOI] [PubMed] [Google Scholar]
- 32. Johannsson G, Burman P, Wiren L, et al. Low dose dehydroepiandrosterone affects behavior in hypopituitary androgen-deficient women: a placebo-controlled trial. J Clin Endocrinol Metab. 2002;87(5):2046-2052. [DOI] [PubMed] [Google Scholar]
- 33. van Thiel SW, Romijn JA, Pereira AM, et al. Effects of dehydroepiandrostenedione, superimposed on growth hormone substitution, on quality of life and insulin-like growth factor I in patients with secondary adrenal insufficiency: a randomized, placebo-controlled, cross-over trial. J Clin Endocrinol Metab. 2005;90(6):3295-3303. [DOI] [PubMed] [Google Scholar]
- 34. Alkatib AA, Cosma M, Elamin MB, et al. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA treatment effects on quality of life in women with adrenal insufficiency. J Clin Endocrinol Metab. 2009;94(10):3676-3681. [DOI] [PubMed] [Google Scholar]
- 35. Misra M, Klibanski A. Endocrine consequences of anorexia nervosa. Lancet Diabetes Endocrinol. 2014;2(7):581-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gordon CM, Grace E, Emans SJ, et al. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002;87(11):4935-4941. [DOI] [PubMed] [Google Scholar]
- 37. Gordon CM, Grace E, Emans SJ, Goodman E, Crawford MH, Leboff MS. Changes in bone turnover markers and menstrual function after short-term oral DHEA in young women with anorexia nervosa. J Bone Miner Res. 1999;14(1):136-145. [DOI] [PubMed] [Google Scholar]
- 38. Elraiyah T, Sonbol MB, Wang Z, et al. Clinical review: the benefits and harms of systemic dehydroepiandrosterone (DHEA) in postmenopausal women with normal adrenal function: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99(10):3536-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wierman ME, Arlt W, Basson R, et al. Androgen therapy in women: a reappraisal: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(10):3489-3510. [DOI] [PubMed] [Google Scholar]
- 40. Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. J Clin Endocrinol Metab. 2019;104(10):4660-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wierman ME, Basson R, Davis SR, et al. Androgen therapy in women: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91(10):3697-3710. [DOI] [PubMed] [Google Scholar]
- 42. Costello MF, Misso ML, Balen A, et al. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: assessment and treatment of infertility. Hum Reprod Open 2019;2019(1):hoy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Wit AE, Giltay EJ, de Boer MK, et al. Plasma androgens and the presence and course of depression in a large cohort of women. Transl Psychiatry. 2021;11(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peixoto C, Grande AJ, Mallmann MB, Nardi AE, Cardoso A, Veras AB. Dehydroepiandrosterone (DHEA) for depression: a systematic review and meta-analysis. CNS Neurol Disord Drug Targets. 2018;17(9):706-711. [DOI] [PubMed] [Google Scholar]
- 45. Jankowski CM, Wolfe P, Schmiege SJ, et al. Sex-specific effects of dehydroepiandrosterone (DHEA) on bone mineral density and body composition: A pooled analysis of four clinical trials. Clin Endocrinol (Oxf) 2019;90(2):293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jankowski CM, Gozansky WS, Kittelson JM, Van Pelt RE, Schwartz RS, Kohrt WM. Increases in bone mineral density in response to oral dehydroepiandrosterone replacement in older adults appear to be mediated by serum estrogens. J Clin Endocrinol Metab. 2008;93(12):4767-4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Davis SR, Panjari M, Stanczyk FZ. Clinical review: DHEA replacement for postmenopausal women. J Clin Endocrinol Metab. 2011;96(6):1642-1653. [DOI] [PubMed] [Google Scholar]
- 48. Morales AJ, Haubrich RH, Hwang JY, Asakura H, Yen SS. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol (Oxf) 1998;49(4):421-432. [DOI] [PubMed] [Google Scholar]
- 49. Chen H, Jin Z, Sun C, Santos HO, Kord Varkaneh H. Effects of dehydroepiandrosterone (DHEA) supplementation on cortisol, leptin, adiponectin, and liver enzyme levels: a systematic review and meta-analysis of randomised clinical trials. Int J Clin Pract. 4698;2021:e1. [DOI] [PubMed] [Google Scholar]
- 50. Clark BJ, Prough RA, Klinge CM. Mechanisms of action of dehydroepiandrosterone. Vitam Horm. 2018;108:29-73. [DOI] [PubMed] [Google Scholar]
- 51. Villareal DT, Holloszy JO. Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA 2004;292(18):2243-2248. [DOI] [PubMed] [Google Scholar]
- 52. Portman DJ, Gass ML. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Climacteric. 2014;17(5):557-563. [DOI] [PubMed] [Google Scholar]
- 53. Labrie F, Martel C, Berube R, et al. Intravaginal prasterone (DHEA) provides local action without clinically significant changes in serum concentrations of estrogens or androgens. J Steroid Biochem Mol Biol. 2013;138:359-367. [DOI] [PubMed] [Google Scholar]
- 54. Labrie F, Archer DF, Martel C, Vaillancourt M, Montesino M. Combined data of intravaginal prasterone against vulvovaginal atrophy of menopause. Menopause 2017;24(11):1246-1256. [DOI] [PubMed] [Google Scholar]
- 55. Archer DF, Labrie F, Montesino M, Martel C. Comparison of intravaginal 6.5mg (0.50%) prasterone, 0.3mg conjugated estrogens and 10µg estradiol on symptoms of vulvovaginal atrophy. J Steroid Biochem Mol Biol. 2017;174:1-8. [DOI] [PubMed] [Google Scholar]
- 56. Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from the North American Menopause Society and the International Society for the Study of Women’s Sexual Health. Menopause 2018;25(6):596-608. [DOI] [PubMed] [Google Scholar]
- 57. Gleicher N, Barad DH. Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR). Reprod Biol Endocrinol. 2011;9(9):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Qin JC, Fan L, Qin AP. The effect of dehydroepiandrosterone (DHEA) supplementation on women with diminished ovarian reserve (DOR) in IVF cycle: Evidence from a meta-analysis. J Gynecol Obstet Hum Reprod 2017;46(1):1-7. [DOI] [PubMed] [Google Scholar]
- 59. Schwarze JE, Canales J, Crosby J, Ortega-Hrepich C, Villa S, Pommer R. DHEA use to improve likelihood of IVF/ICSI success in patients with diminished ovarian reserve: a systematic review and meta-analysis. JBRA Assist Reprod 2018;22(4):369-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Buisson C, Frelat C, Privat K, Martinat N, Audran M, Collomp K. Metabolic and isotopic signature of short-term DHEA administration in women: comparison with findings in men. Drug Test Anal. 2018;10(11-12):1744-1754. [DOI] [PubMed] [Google Scholar]
- 61. Bentley C, Potter C, Yakoub KM, et al. A prospective, phase II, single-centre, cross-sectional, randomised study investigating dehydroepiandrosterone supplementation and its profile in trauma: ADaPT. BMJ Open 2021;11(7):e040823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.