Abstract
Context
Osteoporosis results from disturbances in bone formation and resorption. Recent nonhuman data suggest that the reproductive hormone kisspeptin directly stimulates osteoblast differentiation in vitro and thus could have clinical therapeutic potential. However, the effects of kisspeptin on human bone metabolism are currently unknown.
Objective
To assess the effects of kisspeptin on human bone metabolism in vitro and in vivo.
Methods
In vitro study: of Mono- and cocultures of human osteoblasts and osteoclasts treated with kisspeptin. Clinical study: Randomized, placebo-controlled, double-blind, 2-way crossover clinical study in 26 men investigating the effects of acute kisspeptin administration (90 minutes) on human bone metabolism, with blood sampling every 30 minutes to +90 minutes. Cells for the in vitro study were from 12 male blood donors and 8 patients undergoing hip replacement surgery. Twenty-six healthy eugonadal men (age 26.8 ± 5.8 years) were included in the clinical study. The intervention was Kisspeptin (vs placebo) administration. The main outcome measures were changes in bone parameters and turnover markers.
Results
Incubation with kisspeptin in vitro increased alkaline phosphatase levels in human bone marrow mesenchymal stem cells by 41.1% (P = .0022), and robustly inhibited osteoclastic resorptive activity by up to 53.4% (P < .0001), in a dose-dependent manner. Kisspeptin administration to healthy men increased osteoblast activity, as evidenced by a 20.3% maximal increase in total osteocalcin (P = .021) and 24.3% maximal increase in carboxylated osteocalcin levels (P = .014).
Conclusion
Collectively, these data provide the first human evidence that kisspeptin promotes osteogenic differentiation of osteoblast progenitors and inhibits bone resorption in vitro. Furthermore, kisspeptin acutely increases the bone formation marker osteocalcin but not resorption markers in healthy men, independent of downstream sex steroid levels. Kisspeptin could therefore have clinical therapeutic application in the treatment of osteoporosis.
Keywords: kisspeptin, reproduction, bone metabolism, osteoporosis
Osteoporosis is an escalating global health challenge, with 1 in 2 women and 1 in 5 men over the age of 50 years predicted to suffer an osteoporotic fracture (1, 2). In the United States, approximately 10 million Americans over the age of 50 years suffer from osteoporosis, resulting in 1.5 million osteoporotic fractures and an annual economic cost of over $17 billion (3), with a similar heavy burden in Europe (4). Furthermore, the detrimental impacts on the patient sustaining a fracture are considerable, amounting to the fourth leading cause of chronic disease morbidity in Europe (4). These concerning figures are broadly echoed throughout the world with dramatic future increases in osteoporotic fractures anticipated due to an ageing population (5-7).
Current osteoporosis treatments are generally effective in most, but not all, patients. In addition, they have contraindications and rare but significant much-publicized adverse effects, that limit their recommended duration of use (8-10). Unfortunately, there are no new osteoporosis treatments in late-stage clinical development currently (11). Taken together, there is an urgent need to better understand the regulation of bone remodeling, in order to identify new safe and effective therapeutic targets.
Osteoporosis results from disturbances in the fine balance between bone formation and bone resorption, performed by osteoblasts and osteoclasts, respectively. The most common cause of this disturbance is sex steroid deficiency (predominantly estrogen), although there exist a plethora of alternative secondary causes and risk factors (12). The net result is thinner and disordered bone architecture, which is prone to fracturing. Therefore, improving this bone formation/resorption balance forms the cornerstone for the development of novel therapeutic agents for the prevention and treatment of osteoporosis.
Kisspeptin is a naturally occurring hormone critical for reproduction in men and women (13-17). Furthermore, kisspeptin-based medicines are in clinical development for a range of common reproductive disorders (18-23). It is therefore timely that recent data in rodents demonstrate that kisspeptin administration directly promotes osteoblast differentiation in vitro via the kisspeptin receptor (Kiss1r) expressed on rodent osteoblasts (24). This suggests that kisspeptin may have direct beneficial effects on skeletal homeostasis, independent of its ability to stimulate downstream sex steroid levels via its more established action on the hypothalamic–pituitary–gonadal axis (25). However, there are no data on the direct effects of kisspeptin on human bone metabolism as yet, and so we aimed to investigate this.
We employed RNA sequencing to identify KISS1R expression on human osteoclast precursors and human mature osteoclasts, adding to the previously reported identification of Kiss1r expression on rodent osteoblasts and monocyte-derived osteoclasts (24, 26). We then performed a series of multimodal kisspeptin administration studies both in vitro and clinically (in vivo) to assess the effects of kisspeptin on anabolic and resorptive parameters of bone metabolism, as well as on bone biochemistry in humans, for the first time.
Materials and Methods
Human In Vitro Studies
Generation of osteoblast lineage cells
Human osteoblast lineage cells were obtained from bone specimens from 8 patients who underwent hip replacement surgery (approved by the local ethics committee, S-2011-0114). Each bone specimen was cut into smaller pieces and cleaned with phosphate-buffered saline (PBS) at least 3 times. Five bone pieces were then placed in a 12-well plate well in Dulbecco’s Modified Eagle’s Medium (Invitrogen) containing 1% penicillin/streptomycin, 10% heat-inactivated fetal bovine serum (FBS), 50 µg/mL ascorbic acid, 2 nM L-glutamine, 10–8 M dexamethasone, and 10 mM β-glycerolphosphate. A metal grid was placed on the top of the bone pieces in each well, and cells were incubated at 37°C and 5% CO2. Media was renewed after 7 days initially. Outgrowth cells migrated from the bone pieces after 7 to 10 days. The metal grid and bone pieces were removed after 14 to 20 days, and after approximately 35 days in total the cell outgrowths reached confluency. This validated method is described in further detail elsewhere (27).
Osteoblast differentiation
To evaluate the effects of kisspeptin on osteoblastogenesis, we used a human mesenchymal stem cell (hMSC) line, TERT4-cells (RRID:CVCL_Z017, https://scicrunch.org/resources/Cell%20Lines/source/SCR_013869-1/search?q=CVCL_Z017&l=CVCL_Z017), as previously characterized (28). hMSC-TERT4 cells were grown as described (29), and kisspeptin-54 (Bachem) was added at the start of the experiments, and after 3 and 6 days in accordance with renewal of culture media. The experiments were terminated after 7 days.
Generation of osteoclasts
Human CD14+ monocytes were isolated from buffy coats obtained from 12 anonymous human male blood donors (50-67 years of age). Each buffy coat was diluted 1:1 in PBS and centrifuged through Ficoll–Paque (GE Healthcare) and then suspended twice in 0.5% bovine serum albumin and 2 mM EDTA in PBS. Cells were then purified using BD IMag™ antihuman CD14+ magnetic particles (BD Biosciences) according to the manufacturer’s instructions.
CD14+ monocytes were then seeded in cell culture flasks supplied with Gibco αMEM (Thermo Fisher Scientific) containing 1% penicillin/streptomycin, 10% heat-inactivated FBS (Sigma-Aldrich), and human macrophage colony-stimulating factor (MCSF) 25 ng/mL (R&D Systems) and cultured at 37°C in 5% CO2. Cell culture medium was changed after 2, 5, and 7 days respectively and replaced with α-minimum essential medium (MEM) containing 10% FBS, MCSF, and human receptor activator of nuclear factor κβ ligand (RANKL) (25 ng/mL each) (R&D Systems).
Bone resorption assay
Mature osteoclasts were loosened with Accutase (Sigma-Aldrich) and seeded on cortical bovine bone slices (Boneslices.com) (50 000 cells/bone slice) in 96-well plates in technical replicates of 5 to 6. Bone resorption assays were performed in osteoclast monocultures, or osteoclast and osteoblast cocultures. For osteoclast monocultures, cells were suspended in αMEM containing 10% heat-inactivated FBS, 25 ng/mL MCSF and RANKL and incubated for 40 minutes at 37°C before adding kisspeptin-54 in 4 different concentrations or an equivalent amount of sterile water as control. For osteoclast and osteoblast cocultures, osteoclasts were suspended in αMEM containing 25 ng/mL MCSF and incubated for 4 hours at 37°C. Subsequently, osteoblast lineage cells were loosened with Accutase and suspended in the same cultured media as osteoclasts, before being seeded above the osteoclasts (12 500 osteoblast lineage cells/50 000 osteoclasts). Kisspeptin-54 was added in 3 different concentrations or an equivalent amount of sterile water as control.
Cell cultures were incubated for 72 hours at 37°C and 5% CO2 before conditioned media was removed and experiments terminated by adding sterile water to the bone slices. Bone slices were then cleaned and scraped with a cotton stick to remove cells. To visualize resorption excavations, the bone slices were colored with toluidine blue staining (Sigma-Aldrich) and stored at room temperature. Osteoclastic resorptive activity was assessed by microscopic evaluation of the percentage eroded surface per bone surface, subdivided into round excavations (pits) and elongated excavations (trenches), as previously described (30). Microscopic evaluation of osteoclastic resorptive activity was performed using a 100-point counting grid placed in the ocular and a ×10 of a BX53 microscope (Olympus), by validated blinded and randomized systematic count (31).
Metabolic activity
Cell viability in the mature osteoclasts on completion of bone resorptive activity assessment, was evaluated by osteoclast metabolic activity using CellTiter-Blue (Promega), according to the manufacturer’s instructions. CellTiter-Blue reagent was added to the osteoclasts in conditioned medium and incubated for 30 minutes at 37°C. The resulting mix was then transferred to a black 96-well plate and shaken for 10 seconds. Metabolic activity was then measured by fluorescence using a microplate reader (Synergy HT, BioTek) at 560 nm excitation and 590 nm emission.
RNA sequencing
Human osteoclast precursors and osteoclasts, differentiated as described above, were harvested in TRIzol (Thermo Fisher) in 4 different stages of osteoclastogenesis dependent on days of RANKL stimulation: (1) –2 days (CD14+ monocytes), (2) 0 days, (3) 3 days, and (4) 7 days (mature osteoclasts). After 7 days of RANKL stimulation, cells were microscopically assessed for the presence of multiple nuclei (≥2 nuclei/cell) before being lysed for RNA sequencing. A corresponding batch of cells simultaneously differentiated, were seeded onto bovine bone slices as described above, to validate resorptive activity of the mature osteoclasts.
RNA was purified using Econo Spin columns (Epoch Life Sciences). RNA sequencing was performed according to manufacturer’s instructions (TruSeq 2, Illumina) using 2 µg of RNA for preparation of cDNA libraries. Sequencing reads were mapped to the human genome (hg19) using STAR (32), and tag counts were summarized at the gene level using HOMER (33). Differential gene expression was analyzed using DESeq2 (34) and gene ontology analyzed using goseq (34).
Alkaline phosphatase activity
Alkaline phosphatase (ALP) activity was measured in osteoclast and osteoblast cocultures, osteoblast monocultures and hMSC-TERT4 cells. Osteoclast and osteoblast cocultures were performed as described above. For osteoblast monocultures, osteoblast lineage cells were loosened with Accutase and seeded on cortical bovine bone slices (12 500 cells/bone slice) in 96-well plates suspended in αMEM containing 25 ng/mL MCSF. Kisspeptin-54 was added in 3 different concentrations and an equivalent amount of water was used as control. Cells were incubated for 72 hours at 37°C and 5% CO2.
ALP activity was used as an osteoblast activity marker (osteoblast monocultures and in osteoblast/osteoclast cocultures) or as a marker of osteoblastogenesis (hMSC-TERT4 cells), by colorimetric assay using 50 mM 4-nitrophenyl phosphate disodium salt (Calbiochem/Sigma) measured at absorbance 405 nm.
Human Clinical Study
Approval
This study was performed in accordance with the latest version of the Declaration of Helsinki. All participants provided written informed consent prior to inclusion in the study. The research protocol was approved by the UK Riverside Research Ethics Committee (REC ref: 17/LO/1504).
Study participants and sample size
Participants were recruited via online and print advertisements and invited to a medical screening appointment. During this screening appointment, participants underwent detailed medical history and clinical examination (including endocrine assessment). In addition, blood tests were performed to confirm health status and exclude a related abnormality. These included full blood count, bone profile, vitamin D, parathyroid hormone, renal function, liver function, thyroid hormone profile, luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone, sex hormone–binding globulin, and glucose measurement. Individuals were excluded based on the following criteria: body mass index less than 18.5 or greater than 27.5 kg/m2; history or evidence of any medical (eg, hyperparathyroidism) or psychological condition; use of any prescription, recreational, or investigational drug within the preceding 2 months; blood donation within 3 months of participation; abnormal eating behavior; history of cancer. Supplement use was not an exclusion criterion (2 participants were taking regular multivitamin supplements).
A power calculation identified that 24 participants would provide 80% power to detect a 15% change in anabolic bone turnover. To account for possible study withdrawal, 26 participants entered the study, who all completed. This sample size also compares favorably with previous studies of an acute hormonal intervention and bone turnover markers (35, 36).
Study visits
We performed a randomized, double-blinded, placebo-controlled, 2-way crossover clinical study. The 26 participants (mean ± SD; age 26.8 ± 5.8 years, range 18-36 years; body mass index 23.8 ± 2.3 kg/m2, range 19.9-27.3 kg/m2) attended 2 study visits each, 1 for kisspeptin administration and 1 for rate-matched placebo with the visit order randomized (randomizer.org). This was a within-participant design study, where participants therefore acted as their own controls to minimize variability and maximize power.
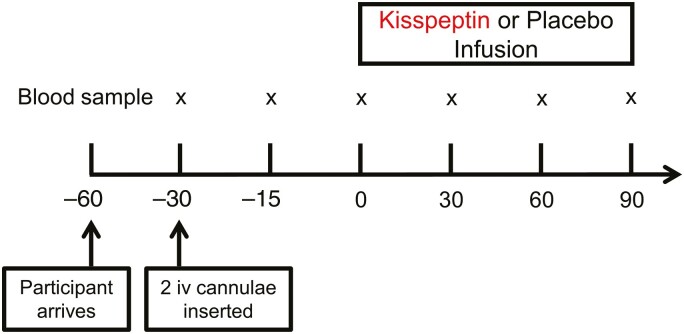
Participants were instructed to refrain from strenuous activity, alcohol, tobacco, and caffeine from the day preceding their study visits. In addition, they were instructed to fast from 22:00 the night preceding each study visit (ensuring a preceding fast of at least 10 hours). All study visits commenced in the morning to control for circadian changes in hormones. Study visits were scheduled to occur a minimum of 1 week apart, as per our previous work, to ensure full washout between visits. After a 30-minute period of rest following arrival, 2 intravenous cannulae were inserted (1 in each arm; 1 for blood sampling and 1 for intravenous administration of kisspeptin or placebo). Blood samples were drawn at 30-minute intervals to +90 minutes (Fig. 1). At T = 0 minutes, a 90-minute intravenous infusion of kisspeptin-54 (1 nmol/kg/hour in gelofusine; B. Braun) or placebo (gelofusine, at matched rate) was commenced. This dosing regimen of kisspeptin was selected based on our previous work (18, 37), to ensure steady-state levels of circulating kisspeptin from 30 minutes onward, while avoiding any increase in downstream testosterone, which has previously been observed following longer periods of kisspeptin administration in humans (38). Participants remained fasted and recumbent throughout the study.
Figure 1.
Experimental protocol for human in vivo clinical study. Twenty-six healthy men participated in a randomized, double-blind, 2-way crossover, placebo-controlled study. Each participant attended 2 study visits; 1 for intravenous (iv) administration of kisspeptin (1 nmol/kg/hour) and 1 for intravenous administration of rate-matched placebo (vehicle), in random order. Blood samples were taken every 30 minutes (x). Participants remained fasted and supine throughout. n = 26 healthy eugonadal men.
Biochemical analyses
Plasma and serum samples were collected as per the assay manufacturer’s guidelines and were stored at –20°C until analysis. Plasma kisspeptin levels were measured using an established manual radioimmunoassay (RRID:AB_2905628, https://scicrunch.org/resources/Any/search?q=AB_2905628&l=AB_2905628) (15), with intra- and interassay coefficients of variation of 8.2% and 10.2%, respectively (39). LH (RRID:AB_2813909, https://scicrunch.org/resources/Any/search?q=AB_2813909&l=AB_2813909), FSH (RRID:AB_2813910, https://scicrunch.org/resources/Any/search?q=AB_2813910&l=AB_2813910), and testosterone (RRID:AB_2848165, https://scicrunch.org/resources/Any/search?q=AB_2848165&l=AB_2848165) levels were analyzed using automated chemiluminescent immunoassays (Abbott Diagnostics). The intra- and interassay coefficients of variation were as follows: LH 4.1% and 2.7%; FSH 4.1% and 3.0%; testosterone 4.2% and 2.8%. Analytical sensitivities were 0.5 IU/L (LH), 0.05 IU/L (FSH), and 2 nmol/L (testosterone).
Osteocalcin levels were analyzed using an established enzyme-linked immunosorbent assay (Takara) for undercarboxylated osteocalcin (RRID:AB_2800334, https://scicrunch.org/resources/Any/search?q=AB_2800334&l=AB_2800334) and carboxylated osteocalcin (RRID:AB_2800333, https://scicrunch.org/resources/Any/search?q=AB_2800333&l=AB_2800333). The intra- and interassay coefficients of variation were as follows: undercarboxylated osteocalcin <6.7% and <9.9%; carboxylated osteocalcin <2.4% and <4.8%; total osteocalcin <8.2% and <10.2%. Limits of quantification were <0.25 ng/mL (carboxylated osteocalcin) and <0.375 ng/mL (total osteocalcin). P1NP levels were analyzed using an established immunoassay (Roche Cobas, RRID:AB_2782967, https://scicrunch.org/resources/Any/search?q=AB_2782967&l=AB_2782967) with intra- and interassay coefficients of variation of 3% and 12.7% respectively (limit of quantification <5 µg/L). CTx levels were analyzed using an established immunoassay (Roche Cobas, RRID:AB_2905599, https://scicrunch.org/resources/Any/search?q=aB_2905599&l=aB_2905599) with intra- and interassay coefficients of variation of 2.5% and 4.2% respectively (limit of quantification 0.01 ng/mL).
Other biochemical parameters were assayed on the standard Abbott Alinity platform with intra- and interassay coefficients of variation as follows: calcium <3% and 2.4%; phosphate <0.6% and 2.6%; parathyroid hormone (PTH) <6.1% and 8.4%; vitamin D <4.5% and 8.3%; total ALP <4.6% and 5.4%; albumin <0.5% and 3.2%; total protein 0.9% and 1.9%; sodium ≤1.5% and 0.9%; potassium <2.7% and 0.7%; creatinine <0.8% and 3.1%.
Statistical Analysis
Human in vitro studies
All statistical analyses (in vitro and clinical study) were performed using GraphPad Prism version 8 (GraphPad Software). Testing for normal distribution for each donor was performed using the Kolmogorov–Smirnov test. For total changes in cell activity, 1-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons tests were applied to the mean of 5 to 6 technical replicates from each donor.
Human clinical study
Baseline characteristics and biochemical changes were normally distributed (Kolmogorov–Smirnov test) and were compared between different study visits utilizing 2-way paired t-tests. Time courses of reproductive hormones (kisspeptin, LH, FSH, and testosterone) and bone turnover markers (osteocalcin, P1NP, and CTx), in response to kisspeptin or placebo administration, were compared using mixed-model analysis of variance. A 2-sided P < .05 was considered statistically significant for all statistical tests (in vitro and clinical study).
Results
Kisspeptin Enhances Osteoblastogenesis In Vitro
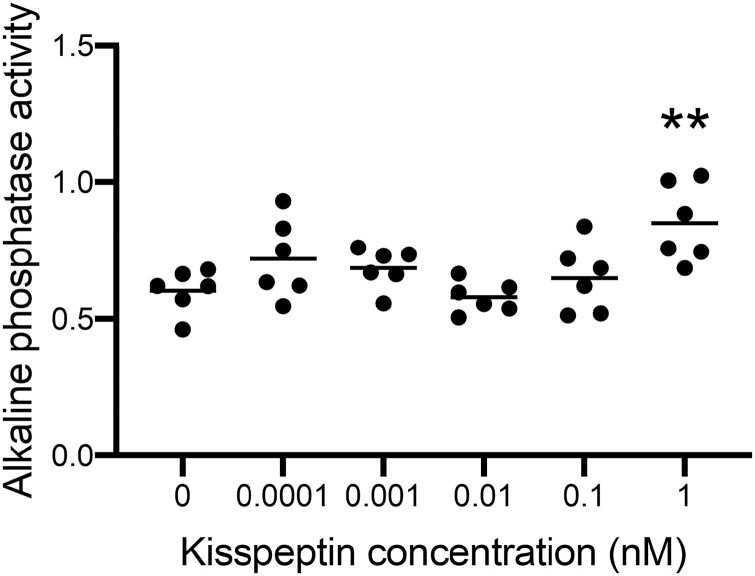
Kiss1 and Kiss1r expression have previously been reported on osteoblasts and their precursors (24, 40, 41). Incubation with kisspeptin has been reported to stimulate osteoblast differentiation via its receptor in murine (fibroblastic) mesenchymal stem cell–like progenitor cells (C3H10T1/2) (24). Here, we assessed the effects of kisspeptin on human osteoblastogenesis using immortalized hMSCs (hMSC-TERT4, generated as described in (28)). Exposure to 1 nM kisspeptin for 7 days induced an increase in ALP activity of 41.1% (P = .0022), indicating enhancement of osteoblastogenesis in this human cell line (Fig. 2).
Figure 2.
Effects of kisspeptin on alkaline phosphatase (ALP) activity in human mesenchymal stem cells. Kisspeptin 1 nM significantly increased ALP activity during osteoblastogenic differentiation of hMSC-TERT4 cells. Presented as mean, each dot indicating a technical replicate, showing an increase in ALP activity at 1 nM compared with control (0) of 41.1% (**P = .0022). Repeated measures 1-way ANOVA with Dunnett’s multiple comparisons test, n = 6.
Kisspeptin Does Not Alter Alkaline Phosphatase Activity in Osteoblast Mono or Cocultures.
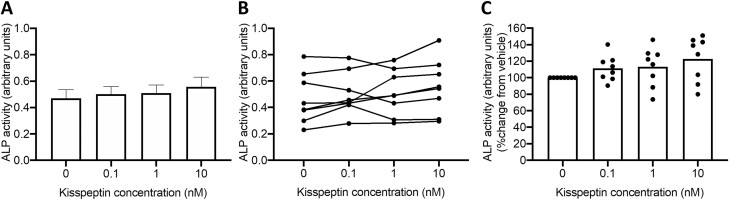
Given the previous findings that kisspeptin can enhance osteoblastogenesis in rodent C3H10T1/2 cells (24) and our human data above, we next assessed whether kisspeptin administration could modulate mature osteoblast activity. We observed that kisspeptin did not alter ALP activity in either osteoblast monoculture (Supplemental Figure 1 (42)) or cocultures (Fig. 3), suggesting a predominant effect on osteoblastogenesis as above.
Figure 3.
Effects of kisspeptin on osteoblast activity in cocultures. Kisspeptin did not alter osteoblast activity measured by alkaline phosphatase (ALP) in osteoblast/osteoclast cocultures. (A) Changes in ALP as mean ± SEM. (B) Effects of kisspeptin on osteoblast activity from each individual experiment connected with a line. Each dot represents the mean of 6 technical replicates. (C) Percentage change in ALP activity compared with control (vehicle) with each dot representing the mean of 6 technical replicates, normalized to control. Repeated measures 1-way ANOVA with Dunnett’s multiple comparisons test, n = 6 technical replicates from 8 osteoclast and 8 osteoblast donors.
The Kisspeptin Receptor (KISS1R) is Expressed During Human Osteoclast Differentiation In Vitro
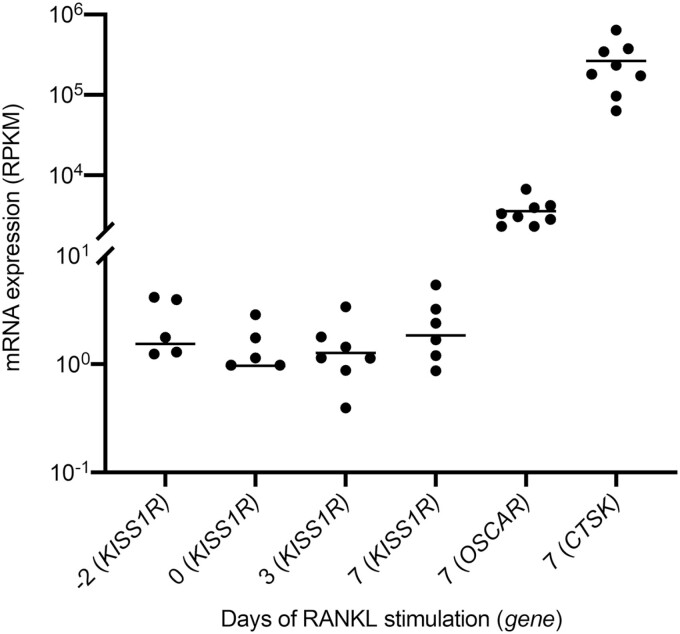
We observed that KISS1R mRNA is detectable throughout the 10-day process of osteoclast differentiation, from CD14+ monocyte to mature human osteoclast in vitro (Fig. 4).
Figure 4.
Expression of KISS1R during human osteoclastogenesis. Gene expression of KISS1R across different stages of human osteoclastogenesis, according to number of days of RANKL (25 ng/mL) stimulation, with osteoclast specific genes, OSCAR and CTSK in mature osteoclasts. KISS1R expression is identified throughout osteoclastogenesis. Each gene is normalized to gene length and the number of reads sequenced per sample. Each dot represents 1 donor. Only expression data within the detectable range is plotted. RPKM: reads per kilobase million, n = 8 donors.
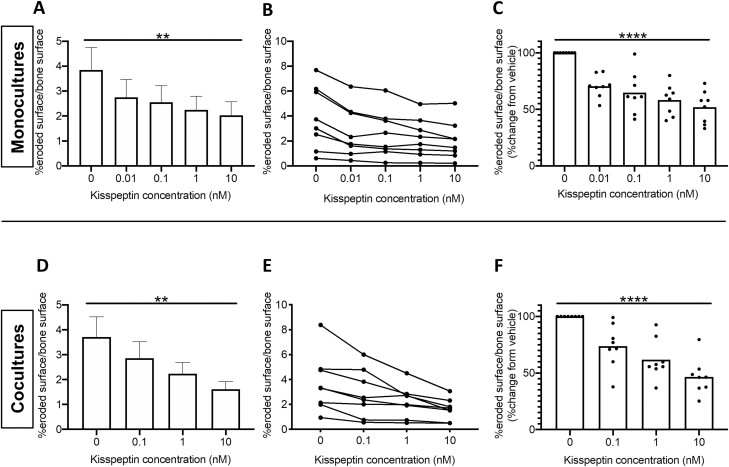
Kisspeptin Inhibits Bone Resorption by Osteoclasts in Mono- and Cocultures In Vitro
Having identified the presence of KISS1R mRNA in human osteoclasts above, we then proceeded to investigate if kisspeptin administration could modulate osteoclast activity. Indeed, we observed that kisspeptin exerted a potent dose-dependent decrease in osteoclast activity (assessed by blinded counting of eroded bovine bone slices as previously described (31, 43, 44)) (Fig. 5A-5C). This inhibitory effect was consistent across osteoclasts from all 8 human male donors ranging from 29.6% (0.01 nM kisspeptin) to 48.1% osteoclast inhibition at the highest dose (10 nM kisspeptin, P < .0001) and was observed clearly on microscopy of both osteoclast mono- and cocultures (Fig. 6). In keeping with this, kisspeptin administration induced a dose-dependent decrease in osteoclast metabolic activity following incubation for 72 hours (Supplemental Figure 2, P < .0001 (42)).
Figure 5.
Effects of kisspeptin on osteoclast activity. Kisspeptin dose-dependently decreased osteoclast activity in osteoclast monocultures (A-C), and osteoclast/osteoblast cocultures (D-F). (A, D) Changes in percentage eroded surface per bone surface from different osteoclast donors, as mean ± SEM (n = 8). (B, E) Effects of kisspeptin on osteoclast activity from each experiment connected with a line. Each dot represents the mean of 6 technical replicates per donor (B, n = 8; E, n = 8 [osteoclast donors], n = 8 [osteoblast donors]). (C, F) Percentage change in osteoclast activity compared with control with each dot representing the mean of 6 technical replicates per donor normalized to control (n as for B, E). A (**P = .0019), D (**P = .0052), repeated measures 1-way ANOVA and Dunnett’s multiple comparisons test. C (****P < .0001), F (0 vs 10 nM, 53.4% suppression, ****P < .0001), repeated measures 1-way ANOVA.
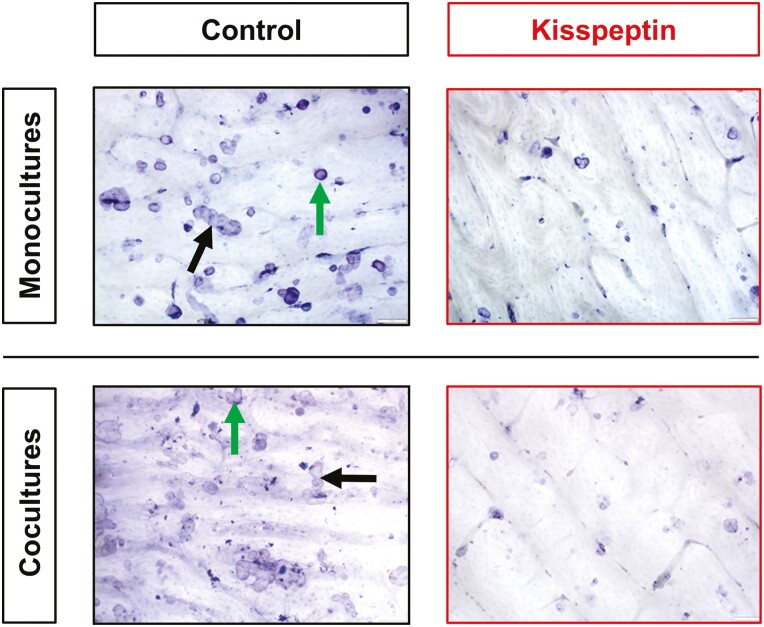
Figure 6.
Representative microscopy images demonstrating osteoclast inhibitory effects of kisspeptin (10 nM) administration. Bone slices are white with resorption cavities seen as purple coloring with distinct edges. Pits are depicted by round cavities (green arrows), whereas trenches by elongated cavities (black arrows).
Having identified this potent antiresorptive effect of kisspeptin on osteoclast activity in monocultures, we next assessed whether this inhibitory effect was also present in osteoclast/osteoblast cocultures, which more closely represent the in vivo bone remodeling environment. Concordant with our findings in monocultures, kisspeptin administration dose-dependently and robustly inhibited osteoclast activity in cocultures ranging from 26.2% (0.1 nM kisspeptin) to 53.4% (10 nM kisspeptin, P < .0001) (Fig. 5D-5F).
Collectively, the identification of KISS1R mRNA in human bone cells, the enhancement of osteoblastogenesis by kisspeptin, and the potent direct antiresorptive effect of kisspeptin on osteoclast activity in vitro, identify a potentially beneficial kisspeptin-induced uncoupling of bone remodeling in human bone cells for the first time. Therefore, we next investigated if this novel and beneficial effect on bone remodeling could be induced in living humans by kisspeptin administration.
Kisspeptin Administration Stimulates Osteoblast Activity in Healthy Humans In Vivo
We next investigated if the observed in vitro effects on bone metabolism above, could be translated into humans. We performed a randomized, double-blinded, placebo-controlled, 2-way crossover study in 26 healthy men (age 26.8 ± 5.8 years, body mass index 23.8 ± 2.3 kg/m2) who attended 2 study visits (1 for kisspeptin and 1 for placebo administration in blinded random order), following an overnight fast (Fig. 1). This acute time-course and kisspeptin dose was selected so as to avoid later downstream increases in sex steroids known to occur after much longer kisspeptin administration (38).
Effects of kisspeptin on bone formation markers
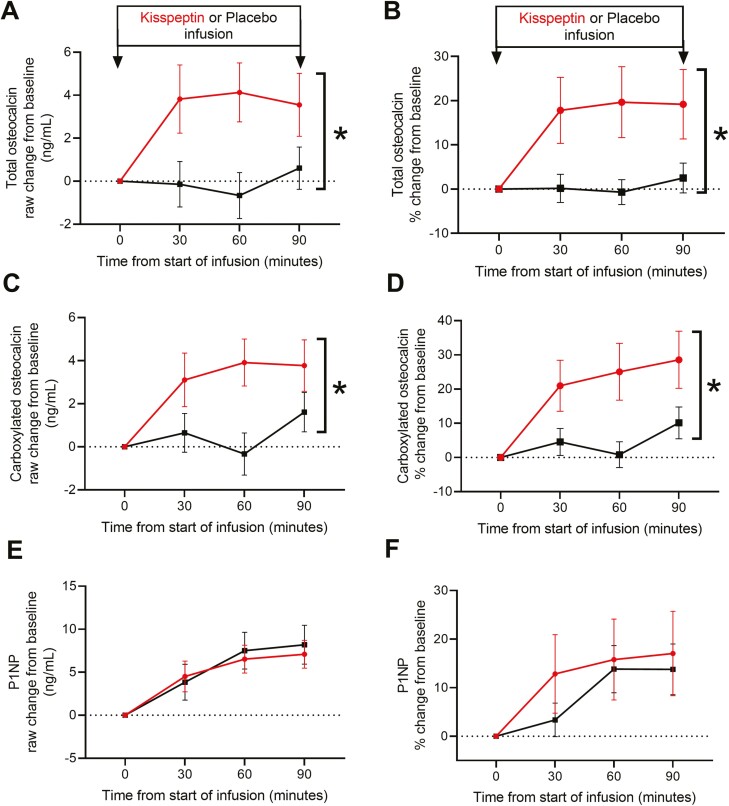
Importantly, baseline bone turnover marker levels and biochemistry (including calcium, phosphate, parathyroid hormone, vitamin D, ALP, albumin, protein, sodium, potassium, and creatinine) were similar at the commencement of each study visit for the 26 healthy participants (Table 1). Subsequently, kisspeptin administration elicited a maximal 20.3% increase in total osteocalcin levels (P = .021), an established marker of bone formation, during this acute experimental time-course, compared with placebo (Fig. 7A and 7B). Within total osteocalcin, we also assessed the carboxylated form (Gla osteocalcin), which predominates in bone remodeling. Here, we observed a similarly striking 24.3% maximal increase in carboxylated osteocalcin levels during kisspeptin administration, compared with placebo (P = .014) (Fig. 7C and 7D). Kisspeptin administration had no significant effect on circulating P1NP levels during this acute time-course (Fig. 7E and 7F).
Table 1.
Baseline (t = 0 at each study visit) reproductive hormones, bone turnover markers, bone, and renal biochemistry did not differ at commencement of placebo compared with kisspeptin visit
| Analyte | Placebo | Kisspeptin | P-value |
|---|---|---|---|
| Reproductive hormones | |||
| Kisspeptin (pmol/L) | 31.3 ± 3.2 | 36.5 ± 1.7 | .16 |
| LH (IU/L) | 2.6 ± 0.2 | 2.6 ± 0.2 | .82 |
| FSH (IU/L) | 2.3 ± 0.2 | 2.4 ± 0.2 | .28 |
| Testosterone (nmol/L) | 20.7 ± 1.0 | 20.4 ± 1.0 | .69 |
| Bone turnover markers | |||
| Gla OC (ng/mL) | 21.0 ± 1.6 | 19.0 ± 1.8 | .24 |
| Total OC (ng/mL) | 30.8 ± 2.1 | 28.0 ± 2.3 | .19 |
| P1NP (ng/mL) | 72.8 ± 8.4 | 71.1 ± 7.6 | .64 |
| CTx (ng/mL) | 0.55 ± 0.05 | 0.55 ± 0.06 | .99 |
| Bone biochemistry | |||
| Adjusted calcium (mmol/L) | 2.3 ± 0.01 | 2.3 ± 0.01 | .13 |
| Phosphate (mmol/L) | 1.1 ± 0.03 | 1.1 ± 0.02 | .76 |
| PTH (pmol/L) | 3.8 ± 0.2 | 3.3 ± 0.3 | .08 |
| Vitamin D (nmol/L) | 50.6 ± 5.4 | 50.4 ± 5.0 | .83 |
| Total ALP (u/L) | 56.4 ± 2.3 | 57.4 ± 2.2 | .69 |
| Albumin (g/L) | 44.3 ± 0.5 | 44.2 ± 0.5 | >.99 |
| Total Protein (g/L) | 71.0 ± 1.1 | 70.1 ± 1.0 | .34 |
| Renal biochemistry | |||
| Sodium (mmol/L) | 140.4 ± 0.4 | 140.4 ± 0.5 | .84 |
| Potassium (mmol/L) | 4.5 ± 0.1 | 4.4 ± 0.1 | .55 |
| Creatinine (μmol/L) | 98.3 ± 2.7 | 94.5 ± 3.1 | .16 |
Data distributed normally and presented as mean ± SEM, 2-way paired t-test, n = 26 healthy men.
Figure 7.
Acute effects of kisspeptin administration on markers of bone formation in humans. (A) Kisspeptin administration stimulated an acute increase in total osteocalcin levels compared to placebo, as depicted by absolute raw (*P = .013) and (B) percentage change from baseline (*P = .021, maximal increase 20.3% above placebo). (C) Kisspeptin administration stimulated an acute increase in carboxylated osteocalcin levels compared with placebo, as depicted by absolute raw (*P = .015) and (D) percentage change from baseline (*P = .014, maximal increase 24.3% above placebo). (E,F) Kisspeptin administration had no acute effect on circulating P1NP levels. Data shown as mean ± SEM, *P < .05, mixed-model analysis of variance, n = 26 healthy men.
Effects of kisspeptin on bone resorption markers
Kisspeptin administration had no significant effect on circulating CTx levels during this acute time-course (Supplemental Figure 3 (42)).
Effects of kisspeptin on bone biochemical parameters
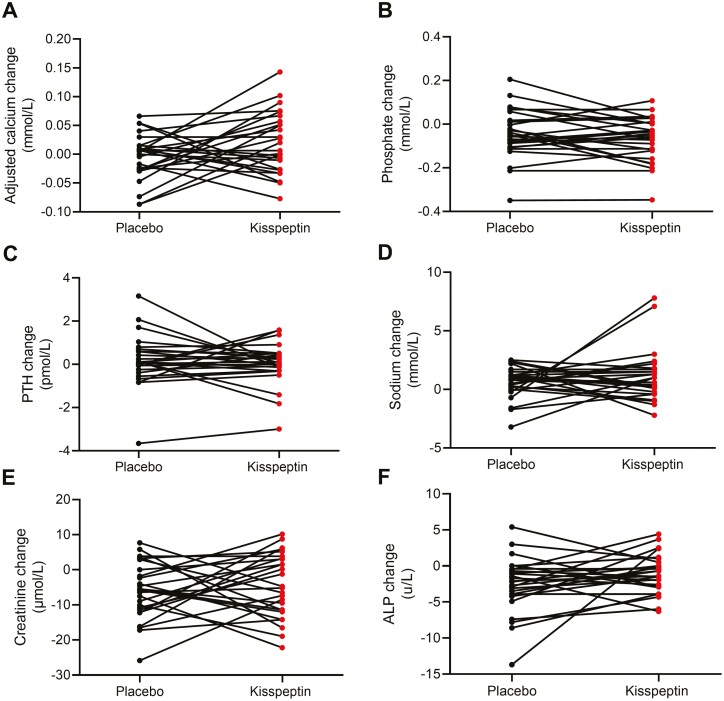
Circulating calcium, phosphate, PTH, sodium, creatinine, and ALP levels were unaltered by kisspeptin administration (Fig. 8).
Figure 8.
Acute effects of kisspeptin administration on circulating biochemical parameters in healthy men. No change in adjusted calcium (A), phosphate (B), parathyroid hormone (PTH, C), sodium (D), creatinine (E), and alkaline phosphatase (ALP, F) levels during kisspeptin administration compared to placebo. Two-way paired t-tests, n = 26 healthy men.
Effects of kisspeptin on downstream reproductive hormones
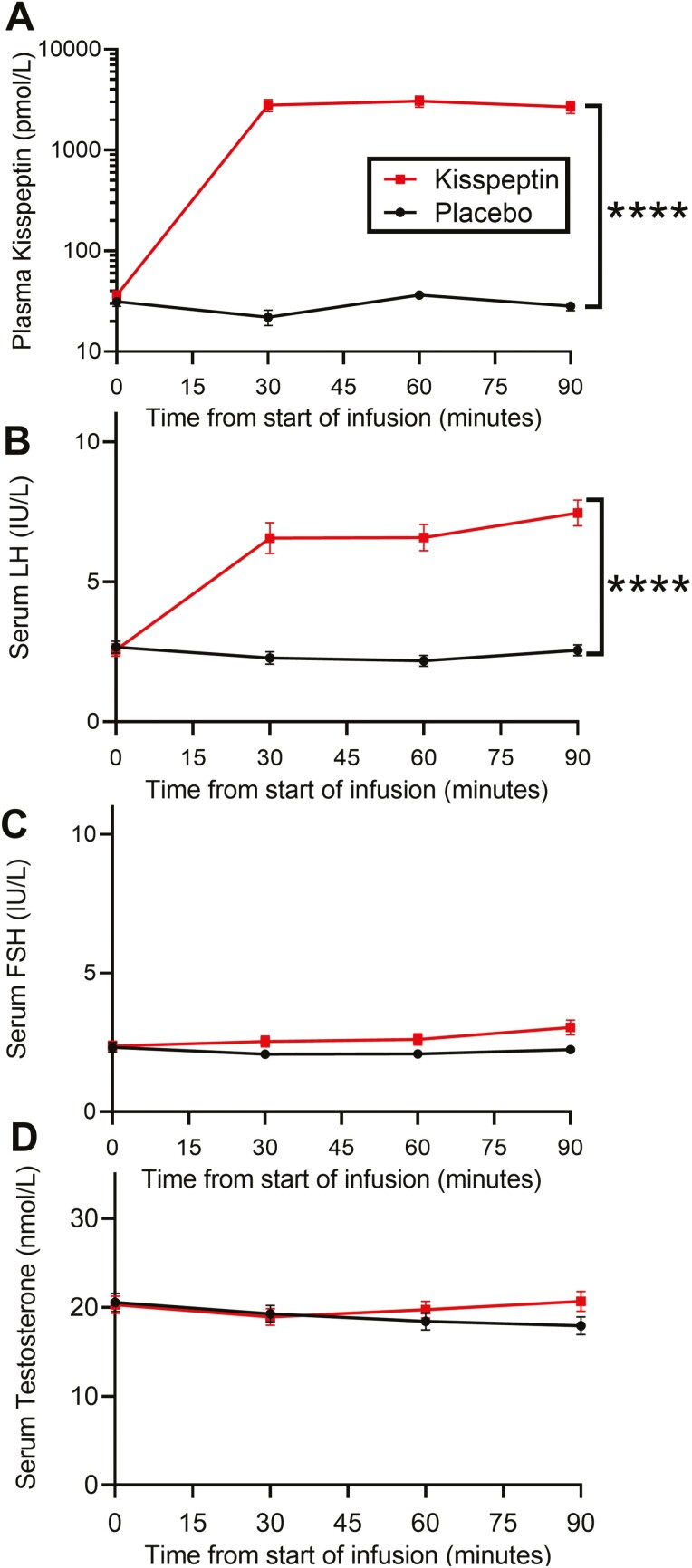
Gonadotropin and testosterone levels were similar at baseline between study visits for the 26 healthy participants (Table 1). As expected, kisspeptin administration elicited a significant increase in circulating kisspeptin levels (P < .0001, Fig. 9A) and LH levels (P < .0001, Fig. 9B) compared with placebo, confirming the bioactivity of this dose of kisspeptin. Importantly, there were no significant changes in downstream FSH or testosterone levels (Fig. 9C and 9D), thereby excluding changes in these downstream hormones as possible confounders. These reproductive hormone data are in keeping with our previous work using this administration protocol (18, 45, 46).
Figure 9.
Effects of peripheral kisspeptin administration (intravenous 1 nmol/kg/hour) on circulating reproductive hormone levels in humans. Kisspeptin administration increased circulating levels of (A) kisspeptin (****P < .0001) and (B) luteinizing hormone (LH, ****P < .0001), compared with placebo. (B) Kisspeptin administration had no effect on follicle stimulating hormone (FSH) or (C) testosterone levels. Data shown as mean ± SEM, ****P < .0001, mixed-model analysis of variance, n = 26 healthy men.
Discussion
Since the first identification of the crucial role for kisspeptin in reproductive hormone control, there has emerged a plethora of literature describing its role in other diverse processes from metabolism to behavior (21, 47-51). As such, given the immense energy expenditure of human bone remodeling and its close interplay with reproductive hormones (25), it is unsurprising to find a link emerging between kisspeptin and bone. Indeed recent pivotal work has identified a neuroskeletal axis, whereby inhibiting estrogen signaling in hypothalamic kisspeptin neurons promotes dense strong bones in rodents, independent of circulating estradiol levels (52). However, the direct effects of kisspeptin on human bone cells remain unknown, until now.
To this end, our data suggest that kisspeptin administration may beneficially uncouple bone turnover acutely in humans independently of downstream sex steroids, and so warrants further study as a potential therapeutic target for clinical disorders of bone metabolism, particularly osteoporosis.
Nonhuman, preclinical work has previously identified that kisspeptin exposure induces osteoblast differentiation of murine mesenchymal stem cell–like osteoprogenitor cells, by activating the kisspeptin receptor upon osteoblasts, to trigger NFATc4-mediated BMP2 expression and activation (24). Indeed, kisspeptin and its cognate receptor are known to be expressed in hMSCs, osteoblast-like cells, and osteogenic precursor cells (40, 41). Here, we translate these findings into humans for the first time and observe that kisspeptin enhances the osteogenic differentiation of hMSC-TERT4 cells in vitro and that acute kisspeptin administration to healthy men increases the osteoanabolic bone marker, osteocalcin. Taken together, these data suggest an osteoanabolic effect of kisspeptin administration in humans.
It is interesting to note that although we observed an osteogenic effect of kisspeptin of similar magnitude to that seen with the established osteoporosis anabolic therapy teriparatide in vitro (53), mature osteoblast activity (measured by ALP) was unaltered in vitro. This suggests that the effects of kisspeptin in humans are predominantly to generate more mature osteoblasts (osteoblastogenesis) rather than increase the activity of already mature osteoblasts. Interestingly, the osteocalcin rise in the human clinical study indicates enhanced activity in fully differentiated osteoblasts by kisspeptin in vivo. Given this, it is plausible that the effect on mature osteoblast activity is not detectable in vitro, but this requires further study as a small change over a long duration could result in effects on bone.
Our human clinical study demonstrated that acute kisspeptin administration elicited a marked increase of up to 20.3% and 24.3% in circulating total and carboxylated osteocalcin levels, respectively. Interestingly, similar magnitude increases in osteocalcin are observed with short-term teriparatide (PTH 1-34) administration (54), with further increases seen in more long-term administration (55), ultimately resulting in increased bone mass and strength. Of note, both kisspeptin and PTH have cognate G-protein–coupled receptors in bone, with genomic and nongenomic downstream effects (56-59). The rapidity of the osteocalcin rise in the current human acute study suggests nongenomic expedited release of osteocalcin from intracellular vesicles (60). However, further studies examining the precise acute and chronic signaling mechanisms for kisspeptin in bone are warranted, to investigate this further. In this human clinical study, we also measured an additional anabolic bone turnover marker, P1NP. Unlike with osteocalcin, we did not observe any significant change in this short 90-minute timeframe. This is in keeping with data showing that acute interventions to bone can impact osteocalcin earlier than P1NP levels (61), and so a longer timeframe may be needed to identify a possible later rise in P1NP. In addition, it is feasible that kisspeptin may have a role predominantly in bone matrix regulation acutely, as evidenced by the acute changes in osteocalcin but not P1NP levels (62), which requires further investigation.
Regarding longer-term studies, it is important to note that we chose this acute 90-minute exposure to kisspeptin in the human clinical study, so as to avoid subsequent elevations in downstream sex steroids that would confound our results. In addition, human observational studies have suggested a role for FSH in bone metabolism (although no human interventional study has shown this as yet (25)). The acute kisspeptin exposure in our study did not elicit a significant increase in downstream FSH and testosterone levels throughout this acute time-course, removing this as a potential additional confounder. Although kisspeptin stimulated an increase in LH levels, as expected, evidence suggests that LH itself does not have a significant direct effect on bone (25).
In terms of bone resorption, we identified that the kisspeptin receptor is present on human osteoclasts. Our finding that kisspeptin exposure potently and dose-dependently inhibited osteoclast activity by up to 53.4% in vitro, proposes an antiresorptive action for kisspeptin, alongside its aforementioned anabolic action. Of key clinical relevance, our antiresorptive findings for kisspeptin compare favorably to a study investigating the effects of zoledronic acid, a current osteoporosis treatment, on osteoclast activity. Indeed, we observed a similar level of osteoclast inhibition in using 10 nM kisspeptin compared with the expected osteoclast inhibition following a standard 5 mg of zoledronic acid treatment (63).
Although we did not observe an effect of kisspeptin on the circulating antiresorptive marker CTx in our 90-minute human infusions, it is likely that this timeframe was too short to detect this, as the in vitro findings were consistent among multiple different donors and after a much longer duration (72 hours) of kisspeptin exposure. Indeed, kisspeptin administration to humans may have predominantly anabolic effects acutely (as per the acute infusion clinical data) but more antiresorptive effects when the duration of exposure is extended (as per the longer exposure in vitro data). Furthermore, there are inherent methodological differences in studying bone resorption by systematic microscopy vs circulating bone markers, as the former provides a more direct assessment of osteoclastic resorptive activity. This, therefore, warrants further study with more prolonged chronic kisspeptin administration studies in humans.
The strengths of this study lie in the multimodal approach employed with both in vitro and in vivo human clinical study, as well as the novelty of being the first kisspeptin–bone study in a large cohort of healthy humans. We designed the acute clinical study to ensure the data were not confounded by later changes in downstream reproductive hormones, while our in vitro work was inherently free from this possible confounder. In addition, we used a range of bone turnover markers and comprehensively assessed related biochemical parameters.
Regarding limitations, even though the in vitro studies suggest a strong antiresorptive effect of kisspeptin, the method is limited by its in vitro nature when interpreting the results in a clinical perspective, although similar results have been observed with zoledronic acid in vitro, which permits some cautious clinical interpretation (63). An additional limitation in the clinical study is the fact that the participants were healthy young men, and so the results are not necessarily generalizable to other populations. For example, patients with osteoporosis tend to be older than those in the current clinical study and may therefore have age-related declines in osteoblastogenic potential (64, 65), although this age-related decline is not a consistent finding in the literature (66). Nonetheless, it is reassuring that the currently available osteoanabolic, teriparatide, has highly beneficial bone effects even in patients >80 years old (67). Clinical studies are therefore warranted to establish the effects of kisspeptin on bone metabolism at different ages and in patients with osteoporosis. It is also important to note that the acute clinical study would not have detected bone effects that may become apparent with more prolonged (chronic) kisspeptin administration, although longer sampling could be confounded by changes in downstream reproductive hormone levels. Therefore, future studies examining kisspeptin-based formulations, administration routes, doses, and dosing intervals will be key to capitalize on or avoid downstream reproductive hormone changes depending on the gonadal status of the patient cohort, while maintaining a beneficial bone effect.
In the current human study, we administered kisspeptin (exogenously) resulting in circulating kisspeptin levels far higher than those observed endogenously in most settings. However, given the presence of both KISS1 and KISS1R in bone, it would be interesting to also study the possibility of autocrine/paracrine endogenous kisspeptin signaling in bone similar to that observed with kisspeptin in metabolic tissues (68, 69).
Collectively, we provide human in vitro and human clinical evidence for a favorable effect of kisspeptin administration on bone metabolism. Kisspeptin stimulates osteoblastogenesis and potently inhibits osteoclast activity in vitro. Furthermore, kisspeptin administration to healthy men acutely increases the osteoanabolic marker osteocalcin without any change in related biochemical parameters. These favorable bone effects have clinical implications as they suggest a beneficial effect of kisspeptin by uncoupling bone turnover in humans. This warrants further investigation in chronic kisspeptin administration studies and in cohorts with disrupted bone turnover to determine the therapeutic potential of kisspeptin as a novel treatment for osteoporosis and related bone disorders.
Acknowledgments
The authors kindly thank Kent Søe for providing access to bone cells. This work presents independent research supported by the NIHR CRF, BRC at Imperial College Healthcare NHS Trust, and Odense University Hospital Free Research Fund. The Section of Endocrinology and Investigative Medicine is funded by grants from the MRC, NIHR and is supported by the NIHR Biomedical Research Centre Funding Scheme and the NIHR/Imperial Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the funders, NHS, NIHR or the Department of Health.
Glossary
Abbreviations
- ALP
alkaline phosphatase
- ANOVA
analysis of variance
- FBS
fetal bovine serum
- FSH
follicle-stimulating hormone
- hMSC
human mesenchymal stem cell
- LH
luteinizing hormone
- MCSF
human macrophage colony-stimulating factor
- MEM
minimum essential medium
- PBS
phosphate-buffered saline
- PTH
parathyroid hormone
- RANKL
nuclear factor κβ ligand
Financial Support
A.N.C. is supported by the NHS. M.S.H. is supported by Region of Southern Denmark funding (18/17553). L.Y. and E.G.M. are supported by MRC Clinical Research Fellowships (MR/R000484/1 and MR/T006242/1 respectively). M.P. is supported by an NIHR Clinical Lectureship. A.A. is supported by an NIHR Clinical Scientist Award (CS-2018-18-ST2-002). W.S.D. is supported by an NIHR Senior Investigator Award.
Author Contributions
A.N.C., M.S.H, M.K., M.F., and W.S.D. designed the research; A.N.C, M.S.H., A.C., S.C., L.Y., E.M., M.P., M.B., M.K., T.T., P.B., T.T., and A.A. performed the research; A.N.C., M.S.H., L.Y., and A.A. analyzed the data; A.N.C. and M.S.H. wrote the initial draft of the paper. All authors reviewed and edited the manuscript.
Disclosure Summary
The authors declare no potential conflicts of interest relevant to this article.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in the References.
References
- 1. Curtis EM, Moon RJ, Harvey NC, Cooper C. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone. 2017;104:29-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van Staa TP, Dennison EM, Leufkens HGM, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29(6):517-522. [DOI] [PubMed] [Google Scholar]
- 3. US Department of Health and Human Services. Bone health and osteoporosis: A report of the surgeon general. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General, 2004. https://www.ncbi.nlm.nih.gov/books/NBK45513/ [Google Scholar]
- 4. Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European Union: Medical management, epidemiology and economic burden: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kanis J, Odén A, Mccloskey E, Johansson H, Wahl D, Cooper C. A systematic review of hip fracture incidence and probability of fracture worldwide on behalf of the IOF Working Group on Epidemiology and Quality of Life. Osteoporos Int. 2012;23(9):2239-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooper C, Cole Z, Holroyd C, et al. Secular trends in the incidence of hip and other osteoporotic fractures and the IOF CSA Working Group on Fracture Epidemiology Europe PMC Funders Group. Osteoporos Int. 2011;22(5):1277-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abrahamsen B, Skjødt MK, Vestergaard P. Hip fracture rates and time trends in use of anti-osteoporosis medications in Denmark for the period 2005 to 2015: missed opportunities in fracture prevention. Bone. 2019;120:476-481. [DOI] [PubMed] [Google Scholar]
- 8. Rizzoli R, Reginster JY, Boonen S, et al. Adverse reactions and drug-drug interactions in the management of women with postmenopausal osteoporosis. Calcif Tissue Int. 2011;89(2):91-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cipriani C, Pepe J, Minisola S, Lewiecki EM. Adverse effects of media reports on the treatment of osteoporosis. J Endocrinol Invest. 2018;41(12):1359-1364. [DOI] [PubMed] [Google Scholar]
- 10. Pazianas M, Abrahamsen B. Safety of bisphosphonates. Bone. 2011;49(1):103-110. [DOI] [PubMed] [Google Scholar]
- 11. Li SS, He SH, Xie PY, et al. Recent progresses in the treatment of osteoporosis. Front Pharmacol. 2021;12:717065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393(10169):364-376. [DOI] [PubMed] [Google Scholar]
- 13. Tena-Sempere M. Timeline: the role of kisspeptins in reproductive biology. Nat Med. 2008;14(11):1196. [DOI] [PubMed] [Google Scholar]
- 14. Mills EG, Izzi-Engbeaya C, Abbara A, Comninos AN, Dhillo WS. Functions of galanin, spexin and kisspeptin in metabolism, mood and behaviour. Nat Rev Endocrinol. 2021;17(2):97-113. [DOI] [PubMed] [Google Scholar]
- 15. Dhillo WS, Chaudhri OB, Patterson M, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90(12):6609-6615. [DOI] [PubMed] [Google Scholar]
- 16. Dhillo WS, Chaudhri OB, Thompson EL, et al. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab. 2007;92(10):3958-3966. [DOI] [PubMed] [Google Scholar]
- 17. Abreu AP, Toro CA, Song YB, et al. MKRN3 inhibits the reproductive axis through actions in kisspeptin-expressing neurons. J Clin Invest. 2020;130(8):4486-4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Comninos AN, Wall MB, Demetriou L, et al. Kisspeptin modulates sexual and emotional brain processing in humans. J Clin Invest. 2017;127(2):709-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abbara A, Clarke SA, Dhillo WS. Novel concepts for inducing final oocyte maturation in in vitro fertilization treatment. Endocr Rev. 2018;39(5):593-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jayasena CN, Abbara A, Veldhuis JD, et al. Increasing LH pulsatility in women with hypothalamic amenorrhoea using intravenous infusion of Kisspeptin-54. J Clin Endocrinol Metab. 2014;99(6):E953-E961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Navarro VM. Metabolic regulation of kisspeptin — the link between energy balance and reproduction. Nat Rev Endocrinol. 2020;16(8):407-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abbara A, Eng PC, Phylactou M, et al. Kisspeptin receptor agonist has therapeutic potential for female reproductive disorders. J Clin Invest. 2020;130(12):6739-6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Radovick S, Babwah AV. Regulation of pregnancy: evidence for major roles by the uterine and placental Kisspeptin/KISS1R signaling systems. Semin Reprod Med. 2019;37(4):182-190. [DOI] [PubMed] [Google Scholar]
- 24. Son H-E, Kim K-M, Kim E-J, Jang W-G. Kisspeptin-10 (KP-10) stimulates osteoblast differentiation through GPR54-mediated regulation of BMP2 expression and activation. Sci Rep. 2018;8(1):2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mills EG, Yang L, Nielsen MF, Kassem M, Dhillo WS, Comninos AN. The relationship between bone and reproductive hormones beyond estrogens and androgens. Endocr Rev. 2021;42(6):691-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knowles HJ, Cleton-Jansen A-M, Korsching E, Athanasou NA. Hypoxia-inducible factor regulates osteoclast-mediated bone resorption: role of angiopoietin-like 4. FASEB J. 2010;24(12):4648-4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gartland A, Rumney RMH, Dillon JP, Gallagher JA. Isolation and culture of human osteoblasts. Methods Mol Biol. 2012;806:337-55. [DOI] [PubMed] [Google Scholar]
- 28. Simonsen JL, Rosada C, Serakinci N, et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20(6):592-6. [DOI] [PubMed] [Google Scholar]
- 29. Abdallah BM, Haack-Sørensen M, Burns JS, et al. Maintenance of differentiation potential of human bone marrow mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene despite of extensive proliferation. Biochem Biophys Res Commun. 2005;326(3):527-38. [DOI] [PubMed] [Google Scholar]
- 30. Søe K, Delaissé JM. Glucocorticoids maintain human osteoclasts in the active mode of their resorption cycle. J Bone Miner Res. 2010;25(10):2184-92. [DOI] [PubMed] [Google Scholar]
- 31. Boissy P, Andersen TL, Abdallah BM, Kassem M, Plesner T, Delaissé JM. Resveratrol inhibits myeloma cell growth, prevents osteoclast formation, and promotes osteoblast differentiation. Cancer Res. 2005;65(21):9943-52. [DOI] [PubMed] [Google Scholar]
- 32. Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heinz S, Benner C, Spann N, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bergmann NC, Lund A, Gasbjerg LS, et al. Separate and combined effects of GIP and GLP-1 infusions on bone metabolism in overweight men without diabetes. J Clin Endocrinol Metab. 2019;104(7):2953-2960. [DOI] [PubMed] [Google Scholar]
- 36. Christensen MB, Lund AB, Jørgensen NR, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide (GIP) reduces bone resorption in patients with type 2 diabetes. J Endocr Soc. 2020;4(9):bvaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang L, Demetriou L, Wall MB, et al. The effects of kisspeptin on brain response to food images and psychometric parameters of appetite in healthy men. J Clin Endocrinol Metab. 2021;106(4):e1837-e1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jayasena CN, Nijher GMK, Comninos AN, et al. The effects of Kisspeptin-10 on reproductive hormone release show sexual dimorphism in humans. J Clin Endocrinol Metab. 2011;96(12):E1963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abbara A, Al-Memar M, Phylactou M, et al. Performance of plasma kisspeptin as a biomarker for miscarriage improves with gestational age during the first trimester. Fertil Steril. 2021;116(3):809-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang FS, Chen H, Wu ZY, Lin JH. KISS1 expression in osteosarcoma: high in Chinese clinical cases, but lower in cell lines. Asian Pacific J Cancer Prev. 2011;12(12):3229-3234. [PubMed] [Google Scholar]
- 41. Dotterweich J, Tower RJ, Brandl A, et al. The KISS1 receptor as an in vivo microenvironment imaging biomarker of multiple myeloma bone disease. PLoS One. 2016;11(5):e0155087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Comninos AN, Hansen MS, Courtney A, et al. Supplemental Data from: “Acute effects of kisspeptin administration on bone metabolism in healthy men.” Imperial College London Research Data Repository 2022. Deposited March 16, 2022. 10.14469/hpc/10254 [DOI] [PMC free article] [PubMed]
- 43. Merrild DMH, Pirapaharan DC, Andreasen CM, et al. Pit- and trench-forming osteoclasts: a distinction that matters. Bone Res. 2015;3:15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pirapaharan DC, Olesen JB, Andersen TL, et al. Catabolic activity of osteoblast lineage cells contributes to osteoclastic bone resorption in vitro. J Cell Sci. 2019;132(10):jcs229351. [DOI] [PubMed] [Google Scholar]
- 45. Comninos AN, Demetriou L, Wall MB, et al. Modulations of human resting brain connectivity by kisspeptin enhance sexual and emotional functions. JCI Insight. 2018;3(20):e121958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Comninos AN, Yang L, O’Callaghan J, et al. Kisspeptin modulates gamma-aminobutyric acid levels in the human brain. Psychoneuroendocrinology. 2021;129:105244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. León S, Fernadois D, Sull A, et al. Beyond the brain-peripheral kisspeptin signaling is essential for promoting endometrial gland development and function. Sci Rep. 2016;6:29073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Comninos A, Dhillo WS. Emerging roles of Kisspeptin in sexual and emotional brain processing. Neuroendocrinology. 2018;106(2):195-202. [DOI] [PubMed] [Google Scholar]
- 49. Adekunbi DA, Li XF, Lass G, et al. Kisspeptin neurones in the posterodorsal medial amygdala modulate sexual partner preference and anxiety in male mice. J Neuroendocrinol. 2018;30(3):e12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roa J, Barroso A, Ruiz-Pino F, et al. Metabolic regulation of female puberty via hypothalamic AMPK-kisspeptin signaling. Proc Natl Acad Sci USA. 2018;115(45):E10758-E10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cvetković D, Babwah AV, Bhattacharya M. Kisspeptin/KISSIR system in breast cancer. J Cancer. 2013;4(8):653-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Herber CB, Krause WC, Wang L, et al. Estrogen signaling in arcuate Kiss1 neurons suppresses a sex-dependent female circuit promoting dense strong bones. Nat Commun. 2019;10(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. D’Amelio P, Tamone C, Sassi F, et al. Teriparatide increases the maturation of circulating osteoblast precursors. Osteoporos Int. 2012;23(4):1245-1253. [DOI] [PubMed] [Google Scholar]
- 54. de Sousa IO, Diniz ET, Marques TF, Griz L, de Coutinho MAP, Bandeira F. Short-term bone marker responses to teriparatide and strontium ranelate in patients with osteoporosis previously treated with bisphosphonates. Arq Bras Endocrinol Metabol. 2010;54(2):244-249. [DOI] [PubMed] [Google Scholar]
- 55. Finkelstein JS, Wyland JJ, Leder BZ, et al. Effects of teriparatide retreatment in osteoporotic men and women. J Clin Endocrinol Metab. 2009;94(7):2495-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Franssen D, Tena-Sempere M. The kisspeptin receptor: a key G-protein-coupled receptor in the control of the reproductive axis. Best Pract Res Clin Endocrinol Metab. 2018;32(2):107-123. [DOI] [PubMed] [Google Scholar]
- 57. Castaño JP, Martínez-Fuentes AJ, Gutiérrez-Pascual E, Vaudry H, Tena-Sempere M, Malagón MM. Intracellular signaling pathways activated by kisspeptins through GPR54: Do multiple signals underlie function diversity? Peptides. 2009;30(1):10-15. [DOI] [PubMed] [Google Scholar]
- 58. Cheloha RW, Gellman SH, Vilardaga JP, Gardella TJ. PTH receptor-1 signalling – mechanistic insights and therapeutic prospects. Nat Rev Endocrinol. 2015;11(12):712-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jiang D, Franceschi RT, Boules H, Xiao G. Parathyroid hormone induction of the osteocalcin gene: Requirement for an osteoblast-specific element 1 sequence in the promoter and involvement of multiple signaling pathways. J Biol Chem. 2004;279(7):5329-5337. [DOI] [PubMed] [Google Scholar]
- 60. Zoch ML, Clemens TL, Riddle RC. New insights into the biology of osteocalcin. Bone. 2016;82:42-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sugimoto T, Nakamura T, Nakamura Y, Isogai Y, Shiraki M. Profile of changes in bone turnover markers during once-weekly teriparatide administration for 24 weeks in postmenopausal women with osteoporosis. Osteoporos Int. 2014;25(3):1173-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Poundarik AA, Boskey A, Gundberg C, Vashishth D. Biomolecular regulation, composition and nanoarchitecture of bone mineral. Sci Rep. 2018;8(1):1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Møller AMJ, Delaisse JM, Olesen JB, Bechmann T, Madsen JS, Søe K. Zoledronic acid is not equally potent on osteoclasts generated from different individuals. JBMR Plus. 2020;4(11):e10412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Geng S, Zhou S, Glowacki J. Age-related decline in osteoblastogenesis and 1α-hydroxylase/CYP27B1 in human mesenchymal stem cells: stimulation by parathyroid hormone. Aging Cell. 2011;10(6):962-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bethel M, Chitteti BR, Srour EF, Kacena MA. The changing balance between osteoblastogenesis and adipogenesis in aging and its impact on hematopoiesis. Curr Osteoporos Rep. 2013;11(2):99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kowal JM, Möller S, Ali D, Figeac F, Barington T, Schmal H, Kassem M. Identification of a clinical signature predictive of differentiation fate of human bone marrow stromal cells. Stem Cell Res Ther. 2021;12(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Niimi R, Kono T, Nishihara A, et al. Usefulness of daily teriparatide treatment in elderly patients over 80 years of age. Osteoporos Int. 2016;27(5):1869-1874. [DOI] [PubMed] [Google Scholar]
- 68. Wolfe A, Hussain MA.. The Emerging Role(s) for Kisspeptin in Metabolism in Mammals. Front Endocrinol (Lausanne). 2018;9:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bhattacharya M, Babwah AV. Kisspeptin: beyond the brain. Endocrinology. 2015;156(4):1218-1227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in the References.