Abstract
Context
Type 1 diabetes (T1D) is characterized by high fracture risk, yet little is known regarding diabetes-related mechanisms or risk factors.
Objective
Determine whether glycemic control, advanced glycation end products (AGEs), and microvascular complications are associated with bone turnover markers among older T1D adults.
Design
Cross-sectional.
Setting
Epidemiology of Diabetes Interventions and Complications study (6 of 27 clinical centers).
Participants
232 T1D participants followed for >30 years.
Exposures
Glycemic control ascertained as concurrent and cumulative hemoglobin A1c (HbA1c); kidney function, by estimated glomerular filtration rates (eGFR); and AGEs, by skin intrinsic fluorescence.
Main Outcome Measures
Serum procollagen 1 intact N-terminal propeptide (PINP), bone-specific alkaline phosphatase (bone ALP), serum C-telopeptide (sCTX), tartrate-resistant acid phosphatase 5b (TRACP5b), and sclerostin.
Results
Mean age was 59.6 ± 6.8 years, and 48% were female. In models with HbA1c, eGFR, and AGEs, adjusted for age and sex, higher concurrent HbA1c was associated with lower PINP [β −3.4 pg/mL (95% CI −6.1, −0.7), P = 0.015 for each 1% higher HbA1c]. Lower eGFR was associated with higher PINP [6.9 pg/mL (95% CI 3.8, 10.0), P < 0.0001 for each −20 mL/min/1.73 m2 eGFR], bone ALP [1.0 U/L (95% CI 0.2, 1.9), P = 0.011], sCTX [53.6 pg/mL (95% CI 32.6, 74.6), P < 0.0001], and TRACP5b [0.3 U/L (95% CI 0.1, 0.4), P = 0.002]. However, AGEs were not associated with any bone turnover markers in adjusted models. HbA1c, eGFR, and AGEs were not associated with sclerostin levels.
Conclusions
Among older adults with T1D, poor glycemic control is a risk factor for reduced bone formation, while reduced kidney function is a risk factor for increased bone resorption and formation.
Keywords: bone turnover markers, skin intrinsic fluorescence, advanced glycation end products, estimated glomerular filtration rates, proliferative diabetic retinopathy, diabetic peripheral neuropathy
Fractures, predominantly hip fractures, are a source of considerable morbidity and mortality among older adults (1). Those with type 1 diabetes (T1D) have a 5× higher risk of hip fractures than those without diabetes (2). There is a need to improve our ability to prevent and treat osteoporosis and fractures in this population, especially as life expectancy increases for those with T1D.
Fracture risk in T1D is greater than that accounted for by modestly lower bone mineral density (BMD) (3, 4). Indeed, the magnitude of the BMD deficit explains only 20% of the observed increase in T1D fracture risk (3). Notably, deficits in bone turnover—a key determinant of bone quality that is not captured by BMD—are associated with T1D. Biochemical markers of bone formation and resorption have been reported to be low (5-7); however, the findings are inconsistent (8, 9), and previous studies are mostly reported among middle-aged adults. Moreover, little is known regarding the impact of diabetes-related factors on bone turnover markers (BTMs) in older adults, the group at highest risk of fractures. We hypothesized that poor current and long-term glycemic control, higher levels of advanced glycation end products (AGEs), and presence of microvascular complications have negative effects on BTMs in older adults with T1D.
To investigate these relationships, we measured markers of bone formation and resorption in participants from 6 out of 27 clinical centers in the Epidemiology of Diabetes Interventions and Complications (EDIC) study, the observational follow-up study of the Diabetes Control and Complications Trial (DCCT). These participants have been followed for >30 years, and the mean age of active participants is now 60 years. The aim of our cross-sectional study was to determine whether the diabetes-related risk factors and coexisting microvascular complications have unfavorable associations with BTMs and sclerostin, an osteocyte product that blocks bone formation. Secondarily, we examined whether BTM and sclerostin differed between EDIC participants and the controls without diabetes.
Research Design and Methods
Participants With Type 1 Diabetes
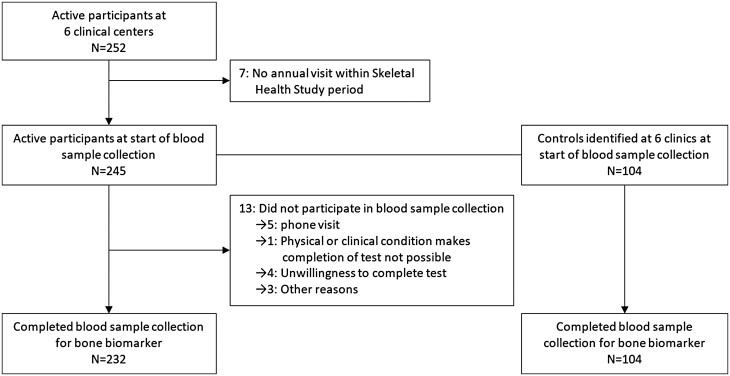
The DCCT/EDIC study has been described in detail elsewhere (10, 11). Briefly, between 1983 and 1989, 1441 participants with T1D aged 13 to 39 years were randomized in the DCCT, a multicenter, controlled clinical trial designed to compare the effects of intensive and conventional diabetes therapy. After a mean follow-up of 6.5 years, the DCCT study ended in 1993 after demonstrating that intensive therapy was highly effective in reducing diabetic microvascular complications. Intensive therapy was recommended for all participants, and participants returned to their own healthcare providers for ongoing diabetes care (12). In 1994, 1375 (96%) of the 1428 surviving DCCT cohort agreed to enroll in the EDIC observational follow-up study. At EDIC years 24-26 (calendar years 2017-2019), 6 of 27 clinical centers had access to high-resolution peripheral quantitative computed tomography scanning. The high-resolution peripheral quantitative computed tomography results will be described in another study. Among these 6 clinics, 252 active participants were invited to participate in the EDIC Skeletal Health Bone Turnover Marker Study, and 232 participants (92% of 252) enrolled (Fig. 1). There were no age, sex, DCCT treatment group, smoking, hemoglobin A1c (HbA1c), or estimated glomerular filtration rate (eGFR) differences at EDIC years 24-26 between the 232 participants and 20 nonparticipants in the Skeletal Health Bone Turnover Marker study.
Figure 1.
Bone biomarkers flow chart in EDIC participants and controls at six clinics.
The study was approved by the institutional review boards of all participating centers, and all participants gave written informed consent.
Controls Without Type 1 Diabetes
As part of the EDIC Skeletal Health study, 104 control participants without diabetes were recruited from the same 6 clinical centers. Controls were recruited from spouses, other family members, or friends of the EDIC participants. Nonspouse controls were matched by age within ±5 years. Controls completed BTM assessments, a self-reported medical history, and medication forms and underwent a general physical exam.
Bone Turnover Markers
The serum samples for participants and controls were collected during years 24-26 of EDIC (calendar years 2017-2019) and with the exception of 5 samples were run within 2 years of collection. The 5 samples, which were from EDIC participants, were run within 26 months of collection. There was no relationship between tartrate-resistant acid phosphatase 5b (TRACP5b) levels and the storage time. Blood was collected from fasting participants into vacutainers without any additives and vacutainers containing EDTA anticoagulant. The vacutainers were centrifuged at 3000 g for 10 minutes, and then serum and EDTA plasma samples were aliquoted and frozen at −80°C at the clinical centers. All aliquots were shipped frozen on dry ice to the EDIC Central Biochemistry Laboratory, located at the Advanced Research and Diagnostic Laboratory at the University of Minnesota. Aliquots were stored at −80°C upon receipt and stored until testing, performed after all specimens were collected.
We measured serum concentrations of two bone formation markers, intact procollagen type 1 N-terminal propeptide (PINP) and bone-specific alkaline phosphatase (bone ALP), and 2 bone resorption markers, serum C-telopeptide (sCTX) and tartrate-resistant acid phosphatase isoform 5b (TRACP5b). PINP was measured in serum using a chemiluminescence assay from IDS (Gaithersburg, MD, USA); the interassay coefficient of variation (CV) is 4.6%. Bone ALP was measured in serum using the bone-specific alkaline phosphatase enzyme immunoassay kit from Quidel (San Diego, CA, USA); the interassay CV is 1.9% at mean concentration 16.9 U/L. sCTX was measured in EDTA plasma using a sandwich immunoassay method from Roche Diagnostics (Indianapolis, IN, USA) on the Cobas e801 module; the interassay CVs are 2.5% at mean concentration 320.6 pg/mL and 2.4% at mean concentration 794.0 pg/mL. TRACP5b was measured in serum using the MicroVue tartrate-resistant acid phosphatase (TRAP) 5b enzyme immunoassay kit from Quidel (San Diego, CA, USA); the interassay CV is 3.0% at mean concentration 2.1 U/L.
As an assessment of the blocking of bone formation, sclerostin was measured in serum using the quantitative sandwich enzyme technique of the enzyme-linked immunosorbent assay Quantikine Human SOST/sclerostin immunoassay kit from R&D Systems (Minneapolis, MN); the interassay CV is 5.0% at mean concentration 105.7 pg/mL.
Insulin-like growth factor 1 (IGF-1) and its binding proteins were evaluated for their role in mediating associations between risk factors and the 4 BTMs. Insulin-like growth factor 1 (IGF-1) and insulin-like growth factor binding protein 3 (IGFBP-3) were measured in EDTA plasma, each using a sandwich immunoassay method from Roche Diagnostics on the Cobas e801 module. Interassay CVs for IGF-1 are 4.0% at mean concentration 58.7 ng/mL and 4.0% at mean concentration 353 ng/mL; interassay CVs for IGFBP-3 are 3.8% at mean concentration 1013.3 ng/mL and 3.4% at mean concentration 6483.3 ng/mL. Insulin-like growth factor binding protein 1 (IGFBP-1) was measured in serum using the quantitative sandwich enzyme technique of the enzyme-linked immunosorbent assay Quantikine Human IGFBP-1 immunoassay kit from R&D Systems. Interassay CVs for IGFBP-1 are 8.9% at a mean concentration of 11 013 pg/mL.
Calciotropic biomarkers were also assessed. Calcium and phosphorus were measured in serum using colorimetric methods, and albumin was measured in serum using a bromcresol purple method (all Roche Diagnostics) on the Cobas c501 module. Interassay CVs for calcium are 1.3% at mean concentration 8.54 mg/dL and 1.3% at mean concentration 13.73 mg/dL; for albumin are 2.2% at mean concentration 2.68 g/dL and 2.6% at mean concentration 3.90 g/dL; Interassay CVs for phosphorus are 1.9% at mean concentration 3.69 mg/dL and 2.1% at mean concentration 6.84 mg/dL. Intact parathyroid hormone (PTH) was measured in EDTA plasma using a sandwich immunoassay method (Roche Diagnostics) on the Cobas e801 module; the interassay CVs are 2.9% at mean concentration 30.3 pg/mL and 2.5% at mean concentration 176.9 pg/mL. 25-OH vitamin D2 and 25-OH vitamin D3 were measured in serum using liquid chromatography tandem mass spectrometry. Interassay CVs were 5.4% for 25-OH vitamin D3 and 7.5% for 25-OH vitamin D2 at mean concentration 2 ng/mL. 1,25-OH vitamin D was measured in serum on a Liaison XL (DiaSorin Inc, Stillwater, MN, USA) using the Liaison XL 1,25 dihydroxyvitamin D chemiluminescent immunoassay (DiaSorin Inc, Stillwater, MN, USA); interassay CVs are 9.4% at mean concentration 27.3 pg/mL and 10% at mean concentration 107.1 pg/mL.
Evaluations and Risk Factors
Annual EDIC assessments included a self-reported medical history of demographic and behavioral risk factors, current use (within the past year) of bone-related and other medications, menopausal status, and any history of autoimmune disease or fracture. Height, weight, and blood pressure were measured in the annual visit. Calcium and vitamin D intake from diet and supplements were assessed by the Block Food Frequency Screener for calcium and vitamin D (NutritionQuest, Berkeley, CA, USA). HbA1c was measured by high-performance liquid chromatography. The time-weighted cumulative mean HbA1c was calculated with weights proportional to the time-interval between visits during DCCT/EDIC. Skin intrinsic fluorescence (SIF), a measure of AGEs, was obtained once from the skin on the underside of the left forearm near the elbow using the SCOUT DS skin fluorescence spectrometer (VeraLight, Inc, Albuquerque, NM, USA) during EDIC years 16-17 (13, 14).
Proliferative diabetic retinopathy was defined at any time during DCCT/EDIC by neovascularization observed on standardized seven-field fundus photographs (performed every 6-months during DCCT and at specified intervals during EDIC) or by self-reported and/or confirmed scatter photocoagulation (15, 16). Albumin excretion rates (AER) were measured annually during DCCT and on alternate years during EDIC (17). AER was measured from 4-hour urine samples from DCCT baseline to EDIC year 18 and subsequently estimated from spot urine samples, using a validated equation based on urine albumin and creatinine concentrations (18). eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation from serum creatinine measured annually. Reduced eGFR was defined as sustained impaired eGFR (<60 mL/min/1.73 m2 on ≥2 consecutive visits) or kidney failure. Sustained microalbuminuria was defined as AER ≥ 30 mg/24 hours on ≥2 consecutive visits. Macroalbuminuria was defined as AER ≥ 300 mg/24 hours at any time during DCCT/EDIC. Diabetic peripheral neuropathy (DPN), defined as the presence of confirmed clinical neuropathy, was measured during EDIC years 13-14 (19). Cardiovascular autonomic neuropathy (CAN) was measured during EDIC years 16-17 (19).
Statistical Analysis
The frequencies and percentages of categorical variables and means and SDs of continuous variables are reported stratified by EDIC participants and controls. Associations between BTMs and diabetes-related risk factors and complications among EDIC participants with T1D were obtained from linear regression models. The pathway association of bone turnover outcomes with diabetes-related risk factors and complications was prespecified using a directed acyclic diagram. The minimally adjusted models included age, sex, and menopausal status.
The relationship between diabetes-related risk factors and complications with BTM was assessed by entering each variable into the minimally adjusted model separately first and then in combination with either current HbA1c, SIF, or eGFR, to build the final multivariate model. The diabetes-related risk factors included in the final adjusted model were current HbA1c, SIF, and eGFR. For eGFR, an interval of 20 mL/min/1.73 m2 was used because it is approximately equal to 1 SD in this cohort.
The least square means of BTM obtained from linear regression models adjusting for age, sex, and menopausal status were stratified by EDIC participants and controls. For comparisons between 232 participants with T1D and the 104 control subjects, generalized estimating equation models were utilized, with an identity link for continuous variables. P-values were reported from models adjusted for age, sex, and menopausal status and then from models additionally adjusted for eGFR, oral glucocorticoid use, and thiazide diuretic use (20). Generalized estimating equation models used an exchangeable covariance structure to account for the correlation between participants with T1D and the control subjects within matched pairs (21).
All analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC, USA). Results nominally significant at P ≤ 0.05 (2-sided) are cited.
Results
The mean age among the 232 EDIC participants (48% women; 85.7% of women were postmenopausal) who completed bone turnover measurements (Fig. 1) was 59.6 ± 6.8 years, and mean duration of T1D was 37.9 ± 4.8 years (Table 1). Among the 104 controls, 65% were women (60.3% postmenopausal), and the mean age was 60.3 ± 8.2 years. History of any reported fracture was 44% of EDIC participants and 30% of controls. Of note, mean eGFR levels did not differ between T1D and controls, although an eGFR of < 60 mL/min/1.73 m2 was more common in EDIC participants than in controls.
Table 1.
Characteristics of Epidemiology of Diabetes Interventions and Complications Skeletal Health Bone Turnover study participants and controls
| Participants | Controls | |||
|---|---|---|---|---|
| n | Mean ± SD or n (%) | n | Mean ± SD or n (%) | |
| n | 232 | 104 | ||
| Design | ||||
| Treatment group (intensive) | 232 | 127 (54.7) | n/a | |
| Study cohort (primary prevention) | 232 | 110 (47.4) | n/a | |
| Demographics | ||||
| Attained age, years | 232 | 59.6 ± 6.8 | 104 | 60.3 ± 8.2 |
| Sex, females | 232 | 112 (48.3) | 104 | 68 (65.4) |
| Postmenopausal, yes | 112 | 96 (85.7) | 68 | 41 (60.3) |
| Body mass index, kg/m2 | 232 | 28.6 ± 5.9 | 104 | 27.9 ± 5.0 |
| Current smoker, yes | 231 | 14 (6.1) | 104 | 6 (5.8) |
| Current alcohol use,a yes | 232 | 12 (5.2) | 104 | 6 (5.8) |
| Total calcium from diet and supplements, mg | 232 | 681 ± 525 | 103 | 649 ± 476 |
| Total vitamin D from diet and supplements, IU | 232 | 1336 ± 1943 | 103 | 1101 ± 1680 |
| Current nonleisure activity: strenuous/moderate,b yes | 232 | 126 (54.3) | 104 | 62 (59.6) |
| History of autoimmune disease | ||||
| Celiac disease | 232 | 2 (0.9) | 104 | 0 (0.0) |
| Grave’s disease | 232 | 9 (3.9) | 104 | 2 (1.9) |
| Hashimoto’s disease | 232 | 70 (30.2) | 104 | 7 (6.7) |
| Current medications | ||||
| Osteoporosis treatment | 231 | 4 (1.7) | 104 | 5 (4.8) |
| Anticonvulsants | 231 | 1 (0.4) | 104 | 1 (1.0) |
| Aromatase inhibitors | 231 | 1 (0.4) | 104 | 1 (1.0) |
| Thiazide diuretic | 231 | 33 (14.3) | 104 | 11 (10.6) |
| Loop diuretic | 231 | 11 (4.8) | 104 | 1 (1.0) |
| Oral contraceptives | 111 | 1 (0.9) | 68 | 1 (1.5) |
| Hormone replacement therapy | 112 | 7 (6.3) | 68 | 5 (7.4) |
| Testosterone | 119 | 2 (1.7) | 36 | 0 (0.0) |
| Oral glucocorticoid | 231 | 16 (6.9) | 104 | 2 (1.9) |
| Proton pump inhibitors | 229 | 27 (11.8) | n/a | |
| Thyroid | 229 | 75 (32.8) | 104 | 12 (11.5) |
| Diabetes-related risk factors | ||||
| Duration of T1D, years | 232 | 37.9 ± 4.8 | n/a | |
| Age ofT1D onset, years | 232 | 21.7 ± 7.9 | n/a | |
| Time-weighted mean HbA1c, % | 232 | 8.0 ± 0.9 | n/a | |
| Current HbA1c, % | 232 | 7.8 ± 1.1 | 103 | 5.5 ± 0.3 |
| Normal HbA1c, <5.7% | n/a | 103 | 78 (75.7) | |
| Prediabetes, 5.7%-6.4% | n/a | 103 | 25 (24.3) | |
| eGFR, mL/min/1.73 m2 | 232 | 83.3 ± 20.4 | 103 | 84.1 ± 13.4 |
| eGFR < 60, mL/min/1.73 m2 | 22 (9.5) | 6 (5.8) | ||
| Advanced glycation end productsc | 224 | 22.6 ± 4.4 | n/a | |
| Complications | ||||
| Any proliferative diabetic retinopathy | 232 | 71 (30.6) | n/a | |
| Any sustained eGFR < 60 or ESRD (reduced eGFR) | 232 | 22 (9.5) | n/a | |
| Any sustained AER ≥ 30 mg/24 hours (microalbuminuria) | 232 | 80 (34.5) | n/a | |
| Any AER ≥ 300 mg/24 hours (macroalbuminuria) | 232 | 33 (14.2) | n/a | |
| Cardiovascular autonomic neuropathyd | 224 | 86 (38.4) | n/a | |
| Diabetic peripheral neuropathye | 219 | 71 (32.4) | n/a | |
| History of any fracture | 232 | 102 (44.0) | 104 | 31 (29.8) |
| Primary bone biomarkers | ||||
| PINP, ng/mL | 230 | 53.6 ± 24.7 | 104 | 59.6 ± 21.1 |
| Bone ALP, U/L | 232 | 20.7 ± 6.0 | 104 | 19.9 ± 6.2 |
| sCTX, pg/mL | 232 | 310.2 ± 164.3 | 104 | 434.7 ± 170.9 |
| TRACP5b, U/L | 232 | 3.2 ± 1.3 | 104 | 2.9 ± 1.2 |
| Sclerostin, pg/mL | 232 | 168.4 ± 65.4 | 104 | 112.6 ± 50.4 |
| Other bone biomarkers | ||||
| IGF-1, ng/mL | 232 | 78 ± 27 | 104 | 113 ± 32 |
| IGFBP-1, ng/mL | 231 | 56 ± 51 | 104 | 12 ± 10 |
| IGFBP-3, ng/mL | 232 | 2544 ± 787 | 104 | 3781 ± 874 |
| 25-hydroxyvitamin D, ng/mL | 232 | 35.4 ± 14.0 | 104 | 35.1 ± 13.6 |
| Parathyroid hormone intact, pg/mL | 231 | 40.6 ± 17.1 | 104 | 43.1 ± 13.3 |
Abbreviations: AER, albumin excretion rate; bone ALP, bone-specific alkaline phosphatase; EDIC, Epidemiology of Diabetes Interventions and Complications study; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HbA1c, hemoglobin A1c; IGF-1, insulin-like growth factor 1; IGFBP-1, insulin-like growth factor binding protein 1; IGFBP-3, insulin-like growth factor binding protein 3; n/a, not available; PINP, procollagen 1 intact N-terminal propeptide; sCTX, serum C-telopeptide; T1D, type 1 diabetes; TRACP5b, tartrate-resistant acid phosphatase 5b.
aAverage consumption of any alcohol per day ≥ 27 grams.
bStrenuous/moderate vs sedentary.
cSkin-intrinsic fluorescence, EDIC year 16-17.
dEDIC year 16-17 (2009-2010).
eEDIC year 13-14 (2006-2007).
Table 2 shows the association between each individual risk factor and the BTMs in the models adjusted for age, sex, and menopausal status. There was no association between BMI and BTMs. Higher current HbA1c was associated with lower PINP levels, with a decrease in PINP for each 1% increase in current HbA1c of −3.5 pg/mL (95% CI −6.3, −0.8; P = 0.011) (Table 2). Current HbA1c was not associated with other BTMs. Replacing with or adding time-weighted mean HbA1c did not provide additional explanatory power. Higher AGEs were significantly associated with higher levels of the 4 BTMs (PINP, bone ALP, sCTX, TRACP5b). Lower eGFR was associated with higher levels of the same 4 BTMs. DPN and CAN were associated with higher TRACP5b levels. Diabetes-related risk factors and complications were not associated with sclerostin levels.
Table 2.
Associations between each risk factor and bone turnover markers and sclerostin among Epidemiology of Diabetes Interventions and Complications participants
| PINP, pg/mL | Bone ALP, U/L | sCTX, pg/mL | TRACP5b, U/L | Sclerostin, pg/mL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Individual risk factor | Estimate (95% CI) | P-value | Estimate (95% CI) | P-value | Estimate (95% CI) | P-value | Estimate (95% CI) | P-value | Estimate (95% CI) | P-value |
| Current HbA1c, % | −3.5 (−6.3, −0.8) | 0.011 | −0.04 (−0.72, 0.65) | 0.914 | −12.5 (−31.0, 6.0) | 0.186 | −0.07 (−0.21, 0.07) | 0.342 | −3.2 (−10.6, 4.2) | 0.399 |
| Time-weighted mean HbA1c | −0.6 (−4.0, 2.9) | 0.741 | 0.7 (−0.2, 1.5) | 0.109 | 7.2 (−16.1, 30.4) | 0.543 | 0.08 (−0.09, 0.26) | 0.347 | 3.8 (−5.4, 13.1) | 0.415 |
| Any sustained AER ≥ 30, yes vs no | 1.2 (−5.4, 7.8) | 0.721 | 0.4 (−1.3, 2.0) | 0.653 | 34.4 (−9.9, 78.7) | 0.127 | 0.09 (−0.24, 0.43) | 0.584 | 2.8 (−14.9, 20.6) | 0.753 |
| Any AER ≥ 300, yes vs no | 8.5 (−0.3, 17.4) | 0.059 | 1.4 (−0.8, 3.6) | 0.222 | 86.0 (27.1, 144.9) | 0.004 | 0.36 (−0.08, 0.81) | 0.111 | −7.6 (−31.5, 16.3) | 0.532 |
| eGFR, per −20 mL/min/1.73 m2 | 7.6 (4.6, 10.6) | <0.0001 | 1.2 (0.4, 2.0) | 0.002 | 58.4 (38.3, 78.5) | <0.0001 | 0.3 (0.14, 0.46) | <0.0001 | −3.5 (−12, 5.1) | 0.426 |
| Any sustained eGFR < 60 or ESRD (reduced eGFR), yes vs no | 18.2 (7.9,28.4) | <0.0001 | 3.7 (1.2, 6.3) | 0.005 | 176.8 (109.3, 244.4) | <0.0001 | 0.88 (0.36, 1.41) | 0.001 | 0.2 (−28.2, 28.7) | 0.987 |
| Cardiac autonomic neuropathy,a yes vs no | 5.2 (−1.3, 11.8) | 0.117 | 1.2 (−0.4, 2.8) | 0.150 | 29.3 (−14.2, 72.9) | 0.186 | 0.36 (0.03, 0.68) | 0.032 | 6.6 (−10.7, 24.0) | 0.452 |
| Diabetic peripheral neuropathy,b yes vs no | −0.6 (−7.3, 6.2) | 0.866 | 0.3 (−1.4, 1.9) | 0.754 | 12.1 (−32.9, 57.0) | 0.598 | 0.44 (0.10, 0.78) | 0.011 | 13.2 (−5.2, 31.6) | 0.158 |
| AGEc (SIF), every 5 units | 4.3 (0.6, 7.9) | 0.022 | 1.0 (0.1, 1.9) | 0.034 | 28.6 (3.8, 53.4) | 0.024 | 0.24 (0.05, 0.42) | 0.012 | 5.8 (−4.0, 15.5) | 0.244 |
| Sclerostin, pg/mL | −0.03 (−0.08, 0.02) | 0.209 | −0.013 (−0.025, −0.001) | 0.034 | −0.4 (−0.7, −0.1) | 0.014 | −0.001 (−0.004, 0.001) | 0.328 | ||
| IGF-1, ng/mL, per 1 SD | 1.0 (−2.2, 4.1) | 0.546 | −0.2 (−0.9, 0.6) | 0.690 | 22.6 (1.5, 43.7) | 0.036 | −0.07 (−0.23, 0.09) | 0.408 | −3.1 (−11.6, 5.4) | 0.470 |
| IGFBP-1/1000, ng/mL, per 1 SD | −0.3 (−3.5, 2.8) | 0.828 | −0.2 (−1.0, 0.5) | 0.535 | 10.4 (−10.8, 31.5) | 0.336 | 0.15 (−0.01, 0.31) | 0.061 | 2.8 (−5.6, 11.3) | 0.512 |
| IGFBP-3, ng/mL, per 1 SD | −0.5 (−3.7, 2.7) | 0.763 | −0.2 (−1.0, 0.6) | 0.609 | 32.1 (11.0, 53.2) | 0.003 | 0.05 (−0.11, 0.21) | 0.538 | −9.2 (−17.7, −0.7) | 0.034 |
| 25-hydroxyvitamin D, ng/mL | 0.03 (−0.2, 0.26) | 0.772 | 0.001 (−0.056, 0.058) | 0.978 | 0.6 (−0.9, 2.2) | 0.435 | 0.002 (−0.009, 0.014) | 0.705 | −0.3 (−0.9, 0.3) | 0.357 |
| Parathyroid hormone intact, pg/mL | 0.20 (0.02, 0.38) | 0.033 | 0.09 (0.04, 0.13) | <0.0001 | 2.2 (0.9, 3.4) | <0.0001 | 0.004 (−0.006, 0.013) | 0.429 | −0.4 (−0.9, 0.1) | 0.097 |
Models were adjusted for age, sex, and menopause. Bolded values are P < 0.05.
Abbreviations: AER, albumin excretion rate; AGE, advanced glycation end product; bone ALP, bone-specific alkaline phosphatase; EDIC, Epidemiology of Diabetes Interventions and Complications study; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HbA1c, hemoglobin A1c; IGF-1, insulin-like growth factor 1; IGFBP-1, insulin-like growth factor binding protein 1; IGFBP-3, insulin-like growth factor binding protein 3; PINP, procollagen 1 intact N-terminal propeptide; sCTX, serum C-telopeptide; SIF, skin intrinsic fluorescence; TRACP5b, tartrate-resistant acid phosphatase 5b.
aEDIC year 16-17 (2009-2010).
bEDIC year 13-14 (2006-2007).
cSkin-intrinsic fluorescence: EDIC year 16-17 (2009-2010).
Table 3 shows the multivariable models for the association between diabetes-related risk factors and BTMs with adjustment for age, sex, and menopausal status and inclusion of current HbA1c, eGFR, and AGEs simultaneously. Current HbA1c remained negatively associated with lower PINP [−3.4 pg/mL (95% CI −6.1, −0.7), P = 0.015 for each 1% higher HbA1c, independent of the possible mediating effects of AGEs and kidney disease] (Table 3). Of note, current HbA1c remained associated with lower PINP levels with adjustment for eGFR without AGEs [−3.2 pg/ml (95% CI −5.9, −0.5), P = 0.020] or with adjustment for AGEs without eGFR [−3.8 pg/mL (−6.6, −1.0), P = 0.005].
Table 3.
Associations between risk factors and bone turnover markers among Epidemiology of Diabetes Interventions and Complications participants, multivariable model
| Risk factors | PINP, pg/mL | Bone ALP, U/L | sCTX (pg/mL) | TRACP5b (U/L) | ||||
|---|---|---|---|---|---|---|---|---|
| Estimate (95% C.I.) | P-value | Estimate (95% C.I.) | P-value | Estimate (95% C.I.) | P-value | Estimate (95% C.I.) | P-value | |
| Current HbA1c (per 1%) | -3.4 (-6.1, -0.7) | 0.015 | 0.2 (-0.5, 0.8) | 0.669 | -10.1 (-28.3, 8.1) | 0.274 | -0.07 (-0.21, 0.07) | 0.337 |
| eGFR (per -20 mL/min/1.73 m2) | 6.9 (3.8, 10.0) | <0.0001 | 1.0 (0.2, 1.9) | 0.011 | 53.6 (32.6, 74.6) | <0.0001 | 0.3 (0.1, 0.4) | 0.002 |
| AGE (SIF) (per 5 units) | 2.5 (-1.1, 6.1) | 0.176 | 0.7 (-0.3, 1.6) | 0.170 | 13.3 (-11.1, 37.7) | 0.284 | 0.17 (-0.02, 0.35) | 0.086 |
Models were adjusted for age, sex, and menopause. Bolded values are P < 0.05.
Abbreviations: AGE, advanced glycation end products; bone ALP, bone-specific alkaline phosphatase; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; PINP, procollagen 1 intact N-terminal propeptide; sCTX, serum C-telopeptide; SIF, skin intrinsic fluorescence; TRACP5b, tartrate-resistant acid phosphatase 5b.
Associations of AGEs with the 4 BTMs were strengthened by adjustment for current HbA1c but attenuated by adjustment for eGFR. For example, the difference in AGEs for each 5-unit increase with PINP went from 4.3 (95% CI 0.6, 7.9; P = 0.022) to 4.6 (95% CI 1.0, 8.2; P = 0.013) with adjustment for current HbA1c, but fell to 3.4 (95% CI −0.3, 7.0; P = 0.073) with adjustment for eGFR. With adjustment for both variables, the association between AGEs and PINP remained attenuated [2.5 (95% CI −1.1, 6.1), P = 0.176] (Table 3). The associations of AGEs with the other BTMs were similarly attenuated with adjustment for both current HbA1c and eGFR (Tables 2 and 3) primarily by adjustment for eGFR (data not shown).
Of the diabetes microvascular complications, only eGFR was independently associated with the 4 BTMs in multivariable models. In models adjusted for current HbA1c and AGEs, lower eGFR was associated with higher PINP [6.9 pg/mL (95% CI 3.8, 10.0), P < 0.0001 for each −20 mL/min/1.73 m2 eGFR], Bone ALP [1.0 U/L(95% CI 0.2, 1.9), P = 0.011], CTX [53.6 pg/mL (95% CI 32.6, 74.6), P < 0.0001], and TRACP5b [0.3 U/L (95% CI 0.1, 0.4), P = 0.002] (Table 3). The association of eGFR and the 4 BTMs remained statistically significant after adjustments for other complications, including DPN and CAN (data not shown). Associations of eGFR with the BTMs also persisted after adjusting for PTH, with an estimate for PINP of 7.3 pg/mL (95% CI 4.2, 10.5; P < 0.0001), bone ALP of 0.9 U/L (95% CI 0.1, 1.7; P = 0.033), sCTX of 52.9 pg/ml (95% CI 32.1, 73.6; P < 0.0001), and TRACP5b of 0.31 U/L (95% CI 0.2, 0.5; P = 0.0002).
Additional adjustment for use of oral glucocorticoids, proton pump inhibitors, or thiazide diuretics did not substantially alter the associations with concurrent HbA1c, AGEs, or eGFR reported in Table 3 for the 4 BTMs (data not shown).
IGF-1 and its binding proteins IGFBP-1 and IGFBP-3 were not intermediaries in the associations between risk factors and the 4 BTMs. PTH levels were positively associated with, PINP, bone ALP, and sCTX levels, while 25(OH) vitamin D levels were not associated with any BTMs.
Calciotropic biochemistries showed a lower serum calcium in EDIC participants as compared to the controls without diabetes, although the corrected serum calcium, phosphorus, PTH, and vitamin D levels did not differ (Table 4). Markers of bone formation showed no difference in bone-specific alkaline phosphatase but a decrease in PINP in EDIC participants as compared to controls. Markers of bone resorption differed, with lower sCTX and higher TRACP5b levels in EDIC participants as compared to controls without diabetes. The sCTX and TRACP5b differences persisted after adjustment for eGFR (Table 4). Notably, the correlations between sCTX and TRACP5b within EDIC participants (r = 0.64, P < 0.0001) and within controls (r = 0.58, P < 0.0001) were similar. As compared to controls, IGF-1 and IGFBP3 levels were lower, while IGFBP-1 and sclerostin levels were higher in EDIC participants.
Table 4.
Bone turnover markers among Epidemiology of Diabetes Interventions and Complications participants and controls
| Bone turnover markers | Participants | Controls | ||||
|---|---|---|---|---|---|---|
| n | LSM,a 95% CI | n | LSM,a 95% CI | P-valueb | P-valuec | |
| Serum calcium, mg/dL | 221 | 9.0 (9.0, 9.1) | 103 | 9.1 (9.0, 9.2) | 0.025 | 0.018 |
| Albumin, g/dL | 221 | 3.7 (3.6, 3.7) | 103 | 3.9 (3.9, 4.0) | <0.0001 | <0.0001 |
| Corrected serum calcium, mg/dL | 221 | 9.3 (9.2, 9.4) | 103 | 9.2 (9.1, 9.3) | 0.104 | 0.153 |
| Serum phosphorus, mg/dL | 219 | 3.6 (3.5, 3.6) | 102 | 3.5 (3.5, 3.6) | 0.803 | 0.883 |
| Parathyroid hormone intact, pg/mL | 231 | 42.3 (39.2, 45.5) | 104 | 43.3 (40.8, 45.9) | 0.628 | 0.500 |
| 25-hydroxyvitamin D, ng/mL | 232 | 35.5 (33.3, 37.6) | 104 | 34.7 (32.3, 37.1) | 0.639 | 0.826 |
| 1,25-dihydroxyvitamin D, pg/mL | 225 | 43.5 (41.6, 45.5) | 101 | 45.5 (43.3, 47.7) | 0.182 | 0.193 |
| PINP, ng/mL | 230 | 52.7 (48.9, 56.5) | 104 | 59.1 (55.2, 63.0) | 0.020 | 0.030 |
| Bone ALP, U/L | 232 | 20.6 (19.6, 21.6) | 104 | 19.9 (18.6, 21.1) | 0.307 | 0.252 |
| sCTX, pg/mL | 232 | 308.7 (280.4, 337.1) | 104 | 434.1 (401.1, 467.1) | <0.0001 | <0.0001 |
| TRACP5b, U/L | 232 | 3.2 (3.0, 3.4) | 104 | 2.8 (2.6, 3.0) | 0.004 | 0.003 |
| Sclerostin, pg/mL | 232 | 164.5 (155.0, 173.9) | 104 | 112.1 (102.8, 121.3) | <0.0001 | <0.0001 |
| IGF-1, ng/mL | 232 | 74.9 (70.9, 79.0) | 104 | 113.0 (107.3, 118.6) | <0.0001 | <0.0001 |
| IGFBP-1/1000, ng/mL | 231 | 59.3 (52.2, 66.5) | 104 | 12.3 (10.2, 14.5) | <0.0001 | <0.0001 |
| IGFBP-3, ng/mL | 232 | 2550.2 (2426.5, 2673.8) | 104 | 3786.6 (3629.0, 3944.2) | <0.0001 | <0.0001 |
Abbreviations: bone ALP, bone-specific alkaline phosphatase; EDIC, Epidemiology of Diabetes Interventions and Complications study; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; IGF-1, insulin-like growth factor 1; IGFBP-1, insulin-like growth factor binding protein 1; IGFBP-3, insulin-like growth factor binding protein 3; LSM, least square mean; PINP, procollagen 1 intact N-terminal propeptide; sCTX, serum C-telopeptide; TRACP5b, tartrate-resistant acid phosphatase 5b.
aAdjusted for age, sex and menopause.
bCompared between EDIC participants and control and was based on generalized linear models with repeated measures of pairs data adjusting for age, sex, and menopause.
cCompared between EDIC participants and controls and was based on generalized linear models with repeated measures of pairs data adjusting for age, sex, menopause, eGFR, and the medication use of oral glucocorticoid and thiazide diuretic.
Discussion
In older individuals with T1D, we found that worse current glycemic control is an independent risk factor for reduced bone formation and that lower eGFR is an independent risk factor for increased bone resorption and formation. A higher level of AGEs is also a risk factor for increased bone resorption and formation, but this relationship is not independent of reduced kidney function defined by eGFR. This is one of the few studies focused on bone turnover in older adults with T1D, a group at high risk of fracture. Notably, this is the first study to implicate poor kidney function as a risk factor for increased bone turnover in T1D, independent of glycemic control.
Our results indicate that worse current glycemic control is associated with lower bone formation. Cumulative glycemic exposure was exceptionally well ascertained in DCCT/EDIC, but only current HbA1c—not time-weighted mean HbA1c—was associated with PINP. In addition, when we adjusted for skin AGEs and kidney function, the association between current HbA1c and PINP persisted. Together, these results suggest a short-term effect of glycemia on bone formation. This is consistent with prior T1D data in young and middle-aged adults showing that higher glucose levels are associated with lower markers of bone formation (5, 9, 22, 23). The relationship might be explained by a toxic effect of hyperglycemia on osteoblasts (24, 25). In vitro data show that hyperglycemia inhibits the osteogenic differentiation of mesenchymal stem cells (26) and the osteoblast response to mechanical loading (27, 28). A negative effect of hyperglycemia on bone formation is also found in healthy adults, with increases in glucose levels leading to suppression of bone turnover (29).
Kidney disease is a known risk factor for increased bone turnover in broader populations (30), yet in T1D, prior studies have generally not included individuals with reduced eGFR (either <60 (5) or <30 mL/min/1.73 m2 (22, 31)). In our report, which includes participants with a broad range of eGFR, eGFR but not albuminuria or other diabetes complications (neuropathy, retinopathy) was strongly associated with all measures of bone turnover.
The mechanism that underlies the relationship between renal function and BTMs is unclear. Long-term hyperglycemia might be contributory, perhaps through indirect delayed effects on bone cells, yet it is unlikely to be the main factor, given that time-weighted HbA1c did not relate to BTMs. Secondary hyperparathyroidism is also unlikely to be the only mediator because the association between eGFR and BTMs persisted after adjusting for PTH. Alternatively, increased oxidative stress in chronic kidney disease (32) might be causal. Accumulation of reactive oxygen species in chronic kidney disease triggers an inflammatory cascade, including recruitment of macrophages and the production of cytokines such as nuclear factor kappa B and transforming growth factor beta (33), which might stimulate BTMs (34).
Our data also show that AGEs are a risk factor for increased bone turnover in T1D. Other T1D studies measuring BTMs have not measured (5, 9, 22, 23, 31, 35-38) or reported (39) a relationship between AGEs and BTMs. AGEs, such as poor kidney function, might have a proinflammatory effect that is linked with increased BTMs. When AGEs interact with their receptor, RAGE, a signaling cascade is activated (40), which generates reactive oxygen species (41), nuclear factor kappa B (42), interleukin 6, tumor necrosis factor alpha (43) and receptor activator of nuclear factor kappa-Β ligand (44). This proinflammatory bone microenvironment likely stimulates bone resorption (45) and, perhaps via coupling, increases bone formation. When we included current HbA1c, the association between AGEs and BTMs persisted, suggesting that factors other than recent hyperglycemia are mediating the effects of AGEs on BTMs. Importantly, when we included eGFR, both with and without current HbA1c, the association between AGEs and BTMs was attenuated, suggesting that reduced kidney function accounts in part for the effects of AGEs on BTMs.
Our finding that diabetes-related factors did not relate to sclerostin levels was unexpected. A study in middle aged T1D participants (age 43.4 ± 9 years) reported a positive association between HbA1c and sclerostin (31). Our lack of associations might be due to the older age in EDIC; sclerostin levels increase with age and higher values might have obscured a relationship with HbA1c. We also did not find that IGF-1 was an intermediary in the associations between diabetes-related factors and BTMs. Low IGF-1 levels in T1D (46) are hypothesized to reduce bone formation (47). Our lack of association was perhaps due to the older age and longer duration of T1D in this cohort.
In comparing EDIC participants to controls, we found that PINP levels were lower in EDIC participants. This is consistent with other studies, including a prior study of middle age adults (mean 45.6 years) in which PINP was also shown to be lower in T1D vs controls (5).
We found that the bone resorption marker sCTX was lower in T1D, while TRACP5b, in contrast, was higher in T1D as compared to controls. Other studies are heterogenous, showing that sCTX is either decreased (5, 22, 36) or no different (31, 35) in middle-aged individuals with T1D and that TRACP5b is elevated in T1D adults (37) but not different in T1D children (48). In the largest meta-analysis of BTMs in diabetes, there was no difference in TRAP levels between T1D and controls (8). One possible explanation for our conflicting sCTX and TRAP results might be that sCTX and TRAP reflect different aspects of bone resorption. Specifically, reduced sCTX, a degradation product of type I collagen, which is considered the more robust marker of bone resorption (49), likely represents a decrease in osteoclastic activity, while increased TRACP5b, an osteoclast enzyme, perhaps reflects a compensatory rise in osteoclast number but not activity. There is precedent for this idea with studies of odanacatib, a cathepsin K inhibitor which inhibits a lysosomal protein in osteoclasts, that show a decrease in sCTX with a concomitant increase in TRACP5b (50).
Strengths and Limitations
A major strength of this study is the well-designed phenotyping of the DCCT/EDIC cohort. The rigorous characterization and relatively large size of the cohort allowed for investigation of the impact of distinct diabetes-related factors on BTMs. The cohort also included T1D patients in whom little is known about BTMs, namely older adults and those across a range of renal function.
Our study has a number of limitations. Serum BTMs are not direct tissue measures of bone turnover. However, direct tissue turnover measures are difficult to obtain because of the invasive nature of bone biopsies, as evidenced by the paucity of reports in T1D (51, 52). Future histomorphometric assessment may provide further insight on the diabetes-related risk factors identified here and their impact on bone turnover in aging individuals with T1D. Further limitations relate to the AGE measurements. SIF was measured 8 years before the bone turnover measures, but since AGEs are irreversible (53), this would only underestimate the association with BTMs. Moreover, serum AGEs and serum receptor for AGE levels were not measured, although these measures might not accurately reflect bone collagen levels. In addition, AGE skin immunofluorescence is not a direct measurement of AGEs, although direct measures such as skin or bone biopsies are generally not a viable alternative for large observational studies. Skin immunofluorescence is known to correlate with skin biopsy levels of specific AGEs (54), and cadaver data suggest a correlation between AGEs by skin biopsy and skin immunofluorescence (55). Preliminary data show a correlation between skin immunofluorescence and direct bone AGEs by measurement of total fluorescent AGE by fluorescence spectrometry (56). Skin intrinsic fluorescence does not measure nonfluorescent AGEs, which might be more important for bone turnover than fluorescent AGEs, but their levels correlate with fluorescent AGEs (57). Assays of specific AGEs in serum or urine could provide a direct measurement but only provide a snapshot of AGE levels. The tissue levels identified with skin immunofluorescence may be more relevant than circulating levels in assessing associations with bone turnover. Finally, EDIC participants might have been healthier than the general T1D population, although this would only lead to underestimation of the differences we found between T1D and controls.
Conclusion
In conclusion, among older adults with T1D, factors related to diabetes are associated with alterations in BTMs. In particular, worse current glycemic control is associated with reduced bone formation, while reduced kidney function defined by eGFR is associated with increased BTMs. Prospective studies are needed to assess whether measures to improve glycemic control promote bone formation in the short term, while measures to preserve kidney function prevent excessive long-term bone turnover. Increased formation and prevention of excess turnover may potentially prevent bone loss and ultimately modify fracture risk in this population.
Acknowledgments
DCCT/EDIC Research Group
Study Chairpersons – D.M. Nathan (chair), B. Zinman (vice-chair); Past: O. Crofford; Deceased: S. Genuth; Editor, EDIC Publications – D. M. Nathan.
Clinical Centers
Case Western Reserve University – Current: R. Gubitosi-Klug, L. Mayer, J. Wood, D. Miller, A. Nayate, M. Novak, S. Pendegast, L. Singerman, D. Weiss, H. Zegarra; Past: E. Brown, P. Crawford, M. Palmert, P. Pugsley, J. Quin, S. Smith-Brewer; Deceased: W. Dahms, S. Genuth, J. McConnell.
Weill Cornell Medical College – Current: N. S. Gregory, R. Hanna, R. Chan, S. Kiss, A. Orlin, M. Rubin; Past: S. Barron, B. Bosco, D. Brillon, S. Chang, A. Dwoskin, M. Heinemann, L. Jovanovic, M. E. Lackaye, T. Lee, B. Levy, V. Reppucci, M. Richardson; Deceased: R. Campbell.
Henry Ford Health System – Current: A. Bhan, J. K. Jones, D. Kruger, P.A. Edwards, H. Remtema; Past: E. Angus, A. Galprin, M. McLellan, A. Thomas; Deceased: J.D. Carey, F. Whitehouse.
International Diabetes Center – Current: R. Bergenstal, S. Dunnigan, M. Johnson, A. Carlson; Past: R. Birk, P. Callahan, G. Castle, R. Cuddihy, M. Franz, D. Freking, L. Gill, J. Gott, K. Gunyou, P. Hollander, D. Kendall, J. Laechelt, S. List, W. Mestrezat, J. Nelson, B. Olson, N. Rude, M. Spencer, L. Thomas; Deceased: D. Etzwiler, K. Morgan.
Joslin Diabetes Center – Current: L. P. Aiello, E. Golden, P. Arrigg, R. Beaser, L. Bestourous, J. Cavallerano, R. Cavicchi, O. Ganda, O. Hamdy, T. Murtha, D. Schlossman, S. Shah, G. Sharuk, P. Silva, P. Silver, M. Stockman, J. Sun, E. Weimann; Past: V. Asuquo, A. Jacobson, R. Kirby, L. Rand, J. Rosenzwieg, H. Wolpert.
Massachusetts General Hospital – Current: D. M. Nathan, M. E. Larkin, M. Cayford, A. deManbey, L. Gurry, J. Heier, A. Joseph, F. Leandre, K. Martin, C. Shah, C. Stevens, N. Thangthaeng; Past: E. Anderson, H. Bode, S. Brink, M. Christofi, C. Cornish, D. Cros, S. Crowell, L. Delahanty, K. Folino, S. Fritz, C. Gauthier-Kelly, J. Godine, C. Haggan, K. Hansen, P. Lou, J. Lynch, C. McKitrick, D. Moore, D. Norman, M. Ong, E. Ryan, C. Taylor, D. Zimbler.
Mayo Clinic – Current: A. Vella, A. Zipse, A. Barkmeier; Past: B. French, M. Haymond, J. Mortenson, J. Pach, R. Rizza, L. Schmidt, W. F. Schwenk, F. J. Service, R. Woodwick, G. Ziegler; Deceased: R. Colligan, A. Lucas, B. Zimmerman.
Medical University of South Carolina – Current: H. Karanchi, L. Spillers, J. Fernandes, K. Hermayer, S. Kwon, K. Lee, M. Lopes-Virella, T. Lyons, M. Nutaitis; Past: A. Blevins, M. Bracey, S. Caulder, J. Colwell, S. Elsing, A. Farr, D. Lee, P. Lindsey, L. Luttrell, R. Mayfield, J. Parker, N. Patel, C. Pittman, J. Selby, J. Soule, M. Szpiech, T. Thompson, D. Wood, S. Yacoub-Wasef.
Northwestern University – Current: A. Wallia, M. Hartmuller, S. Ajroud-Driss, P. Astelford, A. Degillio, M. Gill, L. Jampol, C. Johnson, L. Kaminski, N. Leloudes, A. Lyon, R. Mirza, D. Ryan, E. Simjanoski, Z. Strugula; Past: D. Adelman, S. Colson, M. Molitch, B. Schaefer.
University of California, San Diego – Current: S. Mudaliar, G. Lorenzi, O. Kolterman, M. Goldbaum; Past: T. Clark, M. Giotta, I. Grant, K. Jones, R. Lyon, M. Prince, R. Reed, M. Swenson; Deceased: G. Friedenberg.
University of Iowa – Current: W. I. Sivitz, B. Vittetoe, J. Kramer; Past: M. Bayless, C. Fountain, R. Hoffman, J. MacIndoe, N. Olson, H. Schrott, L. Snetselaar, T. Weingeist, R. Zeitler.
University of Maryland – Current: R. Miller, S. Johnsonbaugh; Past: M. Carney, D. Counts, T. Donner, J. Gordon, M. Hebdon, R. Hemady, B. Jones, A. Kowarski, R. Liss, S. Mendley, D. Ostrowski, M. Patronas, P. Salemi, S. Steidl.
University of Michigan – Current: W. H. Herman, R. Pop-Busui, C. L. Martin, P. Lee, J. W. Albers, E. L. Feldman; Past: N. Burkhart, D. A. Greene, T. Sandford, M.J. Stevens; Deceased: J. Floyd.
University of Minnesota – Current: J. Bantle, M. Rhodes, D. Koozekanani, S. Montezuma, J. Terry; Past: N. Flaherty, F. Goetz, C. Kwong, L. McKenzie, M. Mech, J. Olson, B. Rogness, T. Strand, R. Warhol, N. Wimmergren.
University of Missouri – Current: D. Goldstein, D. Hainsworth, S. Hitt; Deceased: J. Giangiacomo.
University of New Mexico – Current: D. S. Schade, J. L. Canady, R. B. Avery, M. R. Burge, J. E. Chapin, A. Das, L. H. Ketai; Past: D. Hornbeck, C. Johannes, J. Rich, M. L. Schluter.
University of Pennsylvania – Current: M. Schutta, P. A. Bourne, A. Brucker; Past: S. Braunstein, B. J. Maschak-Carey, S. Schwartz; Deceased: L. Baker.
University of Pittsburgh – Current: T. Orchard, L. Cimino, D. Rubinstein; Past: D. Becker, B. Doft, D. Finegold, K. Kelly, L. Lobes, N. Silvers, T. Songer, D. Steinberg, L. Steranchak, J. Wesche; Deceased: A. Drash.
University of South Florida – Current: J. I. Malone, A. Morrison, M. L. Bernal, P. R. Pavan; Past: L. Babbione, T. J. DeClue, N. Grove, D. McMillan, H. Solc, E. A. Tanaka, J. Vaccaro-Kish.
University of Tennessee – Current: S. Dagogo-Jack, C. Wigley, S. Huddleston, A. Patel; Past: M. Bryer-Ash, E. Chaum, A. Iannacone, H. Lambeth, D. Meyer, S. Moser, M. B. Murphy, H. Ricks, S. Schussler, S. Yoser; Deceased: A. Kitabchi.
University of Texas – Current: P. Raskin, S. Strowig, Y-G. He, E. Mendelson, R. L. Ufret-Vincenty; Past: M. Basco; Deceased: S. Cercone.
University of Toronto – Current: B. A. Perkins, B. Zinman, A. Barnie, N. Bakshi, M. Brent, R. Devenyi, K. Koushan, M. Mandelcorn, F. Perdikaris, L. Tuason; Past: D. Daneman, R. Ehrlich, S. Ferguson, A. Gordon, K. Perlman, S. Rogers.
University of Washington – Current: I. Hirsch, R. Fahlstrom, L. Van Ottingham, I. H. de Boer, L. Olmos de Koo; Past: S. Catton, J. Ginsberg, J. Kinyoun, J. Palmer.
University of Western Ontario – Current: C. McDonald, M. Driscoll, J. Bylsma, T. Sheidow; Past: W. Brown, C. Canny, P. Colby, S. Debrabandere, J. Dupre, J. Harth, I. Hramiak, M. Jenner, J. Mahon, D. Nicolle, N. W. Rodger, T. Smith.
Vanderbilt University – Current: M. May, J. Lipps Hagan, T. Adkins, A. Agarwal, C. Lovell; Past: S. Feman, R. Lorenz, R. Ramker; Deceased: L. Survant.
Washington University, St. Louis – Current: N. H. White, L. Levandoski; Deceased: I. Boniuk, J. Santiago.
Yale University – Current: W. Tamborlane, P. Gatcomb, K. Stoessel; Past: J. Ahern, K. Fong, P. Ossorio, P. Ramos.
Albert Einstein – Past: J. Brown-Friday, J. Crandall, H. Engel, S. Engel, H. Martinez, M. Phillips, M. Reid, H. Shamoon, J. Sheindlin.
Clinical Coordinating Center
Case Western Reserve University – Current: R. Gubitosi-Klug, L. Mayer, C. Beck, K. Farrell, P. Gaston; Past: S. Genuth, M. Palmert, J. Quin, R. Trail; Deceased: W. Dahms.
Data Coordinating Center
George Washington University, The Biostatistics Center – J. Lachin, I. Bebu, B. Braffett, J-Y. Backlund, L. Diminick, L. El ghormli, X. Gao, D. Kenny, K. Klumpp, M-H. Lin, V. Trapani; Past: K. Anderson, K. Chan, P. Cleary, A. Determan, L. Dews, W. Hsu, P. McGee, H. Pan, B. Petty, D. Rosenberg, B. Rutledge, W. Sun, S. Villavicencio, N. Younes; Deceased: C. Williams.
National Institute of Diabetes and Digestive and Kidney Disease
National Institute of Diabetes and Digestive and Kidney Disease Program Office – E. Leschek; Past: C. Cowie, C. Siebert.
EDIC Core Central Units
Central Biochemistry Laboratory (University of Minnesota) – M. Steffes, A. Karger, J. Seegmiller, V. Arends; Past: J. Bucksa, B. Chavers, A. Killeen, M. Nowicki, A. Saenger.
Central ECG Reading Unit (Wake Forest School of Medicine) – Y. Pokharel, M. Barr, C. Campbell, S. Hensley, J. Hu, L. Keasler, Y. Li, T. Taylor, Z. M. Zhang; Past: R. Prineas, E. Z. Soliman.
Central Ophthalmologic Reading Unit (University of Wisconsin) – B. Blodi, R. Danis, D. Lawrence, H. Wabers; Past: M. Burger, M. Davis, J. Dingledine, V. Gama, S. Gangaputra, L. Hubbard, S. Neill, R. Sussman.
Central Neuropsychological Reading Unit (NYU Winthrop Hospital, University of Pittsburgh) – A. Jacobson, C. Ryan, D. Saporito; Past: B. Burzuk, E. Cupelli, M. Geckle, D. Sandstrom, F. Thoma, T. Williams, T. Woodfill.
The authors acknowledge skeletal health working group members: Ann V Schwartz, Jye-Yu C. Backlund, Ian De Boer, Mishaela Rubin, Annette Barnie, Naina Sinha Gregory, Amisha Wallia, Barbara H. Braffett, Rose Gubitosi-Klug, David J. Kenny, Valerie Arends, Andrew J. Burghardt, Thomas M. Link, Galateia J. Kazakia, Amisha Wallia, and Ming-Hui Lin.
Financial Support
The DCCT/EDIC has been supported by cooperative agreement grants (1982-1993, 2012-2017, 2017-2022), and contracts (1982-2012) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Disease (current grant numbers U01 DK094176 and U01 DK094157) and through support by the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the General Clinical Research Centers Program (1993-2007), and Clinical Translational Science Center Program (2006-present), Bethesda, Maryland, USA. Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA, USA), Animas (Westchester, PA, USA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY, USA), Becton Dickinson (Franklin Lakes, NJ, USA), Eli Lilly (Indianapolis, IN, USA), Extend Nutrition (St. Louis, MO, USA), Insulet Corporation (Bedford, MA, USA), Lifescan (Milpitas, CA, USA), Medtronic Diabetes (Minneapolis, MN, USA), Nipro Home Diagnostics (Ft. Lauderdale, FL, USA), Nova Diabetes Care (Billerica, MA, USA), Omron (Shelton, CT, USA), Perrigo Diabetes Care (Allegan, MI, USA), Roche Diabetes Care (Indianapolis, IN, USA), and Sanofi-Aventis (Bridgewater, NJ, USA).
Disclosure Summary
None of the authors report a conflict of interest.
Author Contributions
M.R. wrote the manuscript. J-Y.C.B. conducted the statistical analyses. J-Y.C.B. and V.A. wrote sections of the manuscript, reviewed, and edited the manuscript; I.H.d.B., R.G-K., A.W., N.S.G., A.B., A.J.B., J.M.L., B.H.B, and A.V.S. reviewed and edited the manuscript. B.H.B. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data Availability
Data collected for the DCCT/EDIC study through June 30, 2017 are available to the public through the NIDDK Repository (https://repository.niddk.nih.gov/studies/edic/). Data collected in the current cycle (July 2017-June 2022) will be available within 2 years after the end of the funding cycle.
References
- 1. Pike C, Birnbaum HG, Schiller M, Sharma H, Burge R, Edgell ET. Direct and indirect costs of non-vertebral fracture patients with osteoporosis in the US. PharmacoEcon. 2010;28(5):395-409. [DOI] [PubMed] [Google Scholar]
- 2. Vilaca T, Schini M, Harnan S, et al. The risk of hip and non-vertebral fractures in type 1 and type 2 diabetes: a systematic review and meta-analysis update. Bone. 2020;137:115457. [DOI] [PubMed] [Google Scholar]
- 3. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int. 2007;18(4):427-444. [DOI] [PubMed] [Google Scholar]
- 4. Shah VN, Harrall KK, Shah CS, et al. Bone mineral density at femoral neck and lumbar spine in adults with type 1 diabetes: a meta-analysis and review of the literature. Osteoporos Int. 2017;28(9):2601-2610. [DOI] [PubMed] [Google Scholar]
- 5. Shanbhogue VV, Hansen S, Frost M, et al. Bone geometry, volumetric density, microarchitecture, and estimated bone strength assessed by HR-pQCT in adult patients with type 1 diabetes mellitus. J Bone Miner Res. 2015;30(12):2188-2199. [DOI] [PubMed] [Google Scholar]
- 6. Starup-Linde J, Vestergaard P. Biochemical bone turnover markers in diabetes mellitus—a systematic review. Bone. 2016; 82:69-78. [DOI] [PubMed] [Google Scholar]
- 7. Bouillon R, Bex M, Van Herck E, et al. Influence of age, sex, and insulin on osteoblast function: osteoblast dysfunction in diabetes mellitus. J Clin Endocrinol Metab. 1995;80(4):1194-1202. [DOI] [PubMed] [Google Scholar]
- 8. Hygum K, Starup-Linde J, Harslof T, Vestergaard P, Langdahl BL. Mechanisms in endocrinology: diabetes mellitus, a state of low bone turnover—a systematic review and meta-analysis. Eur J Endocrinol. 2017;176(3):R137-R157. [DOI] [PubMed] [Google Scholar]
- 9. Abdalrahaman N, McComb C, Foster JE, et al. Deficits in trabecular bone microarchitecture in young women with type 1 diabetes mellitus. J Bone Miner Res. 2015;30(8): 1386-1393. [DOI] [PubMed] [Google Scholar]
- 10. The Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. The DCCT Research Group. Diabetes. 1986;35(5):530-545. [PubMed] [Google Scholar]
- 11. Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22(1):99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diabetes C, Complications Trial Research G, Nathan DM, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14): 977-986. [DOI] [PubMed] [Google Scholar]
- 13. Orchard TJ, Lyons TJ, Cleary PA, et al. The association of skin intrinsic fluorescence with type 1 diabetes complications in the DCCT/EDIC study. Diabetes Care. 2013;36(10):3146-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cleary PA, Braffett BH, Orchard T, et al. Clinical and technical factors associated with skin intrinsic fluorescence in subjects with type 1 diabetes from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Technol Ther. 2013;15(6):466-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):766-785. [PubMed] [Google Scholar]
- 16. Hainsworth DP, Bebu I, Aiello LP, et al. ; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Risk factors for retinopathy in type 1 diabetes: the DCCT/EDIC study. Diabetes Care. 2019;42(5):875-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Boer IH, Group DER. Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):24-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Younes N, Cleary PA, Steffes MW, et al. ; DCCT/EDIC Research Group. Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Clin J Am Soc Nephrol. 2010;5(7):1235-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Braffett BH, Gubitosi-Klug RA, Albers JW, et al. ; DCCT/EDIC Research Group. Risk factors for diabetic peripheral neuropathy and cardiovascular autonomic neuropathy in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes. 2020;69(5):1000-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lachin J. Biostatistical Methods: The Assessment of Relative Risks. 2nd ed. Wiley; 2010. [Google Scholar]
- 21. Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121-30. [PubMed] [Google Scholar]
- 22. Neumann T, Samann A, Lodes S, et al. Glycaemic control is positively associated with prevalent fractures but not with bone mineral density in patients with type 1 diabetes. Diabet Med. 2011;28(7):872-875. [DOI] [PubMed] [Google Scholar]
- 23. Danielson KK, Elliott ME, LeCaire T, Binkley N, Palta M. Poor glycemic control is associated with low BMD detected in premenopausal women with type 1 diabetes. Osteoporos Int. 2009;20(6):923-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zayzafoon M, Stell C, Irwin R, McCabe LR. Extracellular glucose influences osteoblast differentiation and c-Jun expression. J Cell Biochem. 2000;79(2):301-310. [DOI] [PubMed] [Google Scholar]
- 25. Botolin S, McCabe LR. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem. 2006;99(2):411-424. [DOI] [PubMed] [Google Scholar]
- 26. Gopalakrishnan V, Vignesh RC, Arunakaran J, Aruldhas MM, Srinivasan N. Effects of glucose and its modulation by insulin and estradiol on BMSC differentiation into osteoblastic lineages. Biochem Cell Biol. 2006;84(1):93-101. [DOI] [PubMed] [Google Scholar]
- 27. Parajuli A, Liu C, Li W, et al. Bone’s responses to mechanical loading are impaired in type 1 diabetes. Bone. 2015;81:152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seref-Ferlengez Z, Maung S, Schaffler MB, Spray DC, Suadicani SO, Thi MM. P2X7R-Panx1 complex impairs bone mechanosignaling under high glucose levels associated with type-1 diabetes. PLoS One. 2016;11(5):e0155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Westberg-Rasmussen S, Starup-Linde J, Hermansen K, Holst JJ, Hartmann B, Vestergaard P, Gregersen S. Differential impact of glucose administered intravenously or orally on bone turnover markers in healthy male subjects. Bone. 2017;97:261-266. [DOI] [PubMed] [Google Scholar]
- 30. Moe S, Drueke T, Cunningham J, et al. Improving Global O. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69(11):1945-1953. [DOI] [PubMed] [Google Scholar]
- 31. Neumann T, Hofbauer LC, Rauner M, et al. Clinical and endocrine correlates of circulating sclerostin levels in patients with type 1 diabetes mellitus. Clin Endocrinol (Oxf). 2014;80(5):649-655. [DOI] [PubMed] [Google Scholar]
- 32. Verma S, Singh P, Khurana S, et al. Implications of oxidative stress in chronic kidney disease: a review on current concepts and therapies. Kidney Res Clin Pract. 2021;40(2):183-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Border WA, Brees D, Noble NA. Transforming growth factor-beta and extracellular matrix deposition in the kidney. Contrib Nephrol. 1994;107:140-145. [DOI] [PubMed] [Google Scholar]
- 34. Adamopoulos IE. Inflammation in bone physiology and pathology. Curr Opin Rheumatol. 2018;30(1):59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alexopoulou O, Jamart J, Devogelaer JP, Brichard S, de Nayer P, Buysschaert M. Bone density and markers of bone remodeling in type 1 male diabetic patients. Diabetes Metab. 2006;32(5 Pt 1):453458. [DOI] [PubMed] [Google Scholar]
- 36. Gennari L, Merlotti D, Valenti R, et al. Circulating sclerostin levels and bone turnover in type 1 and type 2 diabetes. J Clin Endocrinol Metab. 2012;97(5):1737-1744. [DOI] [PubMed] [Google Scholar]
- 37. Olmos JM, Perez-Castrillon JL, Garcia MT, Garrido JC, Amado JA, Gonzalez-Macias J. Bone densitometry and biochemical bone remodeling markers in type 1 diabetes mellitus. Bone Miner. 1994;26(1):1-8. [DOI] [PubMed] [Google Scholar]
- 38. Mastrandrea LD, Wactawski-Wende J, Donahue RP, Hovey KM, Clark A, Quattrin T. Young women with type 1 diabetes have lower bone mineral density that persists over time. Diabetes Care. 2008;31(9):1729-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Neumann T, Lodes S, Kastner B, et al. High serum pentosidine but not esRAGE is associated with prevalent fractures in type 1 diabetes independent of bone mineral density and glycaemic control. Osteoporos Int. 2014;25(5):1527-1533. [DOI] [PubMed] [Google Scholar]
- 40. Davis HM, Essex AL, Valdez S, et al. Short-term pharmacologic RAGE inhibition differentially affects bone and skeletal muscle in middle-aged mice. Bone. 2019;124:89-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yan SF, Ramasamy R, Schmidt AM. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab. 2008;4(5):285-293. [DOI] [PubMed] [Google Scholar]
- 42. Litwinoff E, Hurtado Del Pozo C, Ramasamy R, Schmidt AM. Emerging targets for therapeutic development in diabetes and its complications: the RAGE signaling pathway. Clin Pharmacol Ther. 2015;98(2):135-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen H, Liu W, Wu X, Gou M, Shen J, Wang H. Advanced glycation end products induced IL-6 and VEGF-A production and apoptosis in osteocyte-like MLO-Y4 cells by activating RAGE and ERK1/2, P38 and STAT3 signalling pathways. Int Immunopharmacol. 2017;52:143-149. [DOI] [PubMed] [Google Scholar]
- 44. Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20(3):345-357. [DOI] [PubMed] [Google Scholar]
- 45. Plotkin LI, Essex AL, Davis HM. RAGE signaling in skeletal biology. Curr Osteoporos Rep. 2019;17(1):16-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shapiro MR, Wasserfall CH, McGrail SM, et al. Insulin-like growth factor dysregulation both preceding and following type 1 diabetes diagnosis. Diabetes. 2020;69(3):413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mitchell DM, Caksa S, Joseph T, Bouxsein ML, Misra M. Elevated HbA1c is associated with altered cortical and trabecular microarchitecture in girls with type 1 diabetes. J Clin Endocrinol Metab. 2020;105(4):e1648-e1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Loureiro MB, Ururahy MA, Freire-Neto FP, et al. Low bone mineral density is associated to poor glycemic control and increased OPG expression in children and adolescents with type 1 diabetes. Diabetes Res Clin Pract. 2014;103(3):452-457. [DOI] [PubMed] [Google Scholar]
- 49. Leeming DJ, Alexandersen P, Karsdal MA, Qvist P, Schaller S, Tanko LB. An update on biomarkers of bone turnover and their utility in biomedical research and clinical practice. Eur J Clin Pharmacol. 2006;62(10):781-792. [DOI] [PubMed] [Google Scholar]
- 50. Masarachia PJ, Pennypacker BL, Pickarski M, et al. Odanacatib reduces bone turnover and increases bone mass in the lumbar spine of skeletally mature ovariectomized rhesus monkeys. J Bone Miner Res. 2012;27(3):509-523. [DOI] [PubMed] [Google Scholar]
- 51. Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. Bone loss and bone turnover in diabetes. Diabetes. 1995;44(7):775-782. [DOI] [PubMed] [Google Scholar]
- 52. Armas LA, Akhter MP, Drincic A, Recker RR. Trabecular bone histomorphometry in humans with Type 1 Diabetes Mellitus. Bone. 2012;50(1):91-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci U S A. 1984;81(2):583-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Meerwaldt R, Graaff R, Oomen PH, et al. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47(7):1324-1330. [DOI] [PubMed] [Google Scholar]
- 55. Sell DR, Monnier VM. Structure elucidation of a senescence cross-link from human extracellular matrix: implication of pentoses in the aging process. J Biol Chem. 1989;264(36):21597-21602. [PubMed] [Google Scholar]
- 56. Fan L, Wang B, Sroga GE, Nickolas T, Vashishth D, Rubin MR. Clinical measures of advanced glycation endproducts and bone tissue properties in type 2 diabetes. J Bone Miner Res. 2021;36(suppl 1). https://www.asbmr.org/meetings/annualmeeting/AbstractDetail?aid=17841e08-eeca-4922-bef1-9273ee34b420 [Google Scholar]
- 57. Thomas CJ, Cleland TP, Sroga GE, Vashishth D. Accumulation of carboxymethyl-lysine (CML) in human cortical bone. Bone. 2018;110:128-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data collected for the DCCT/EDIC study through June 30, 2017 are available to the public through the NIDDK Repository (https://repository.niddk.nih.gov/studies/edic/). Data collected in the current cycle (July 2017-June 2022) will be available within 2 years after the end of the funding cycle.