Abstract
Context
Autoimmune thyroid disease is the most common endocrine comorbidity in autoimmune Addison disease (AAD), but detailed investigations of prevalence and clinical course are lacking.
Objective
This work aimed to provide comprehensive epidemiological and clinical data on autoimmune thyroid disorders in AAD.
Methods
A nationwide registry-based study including 442 patients with AAD and autoimmune thyroid disease were identified through the Norwegian National Registry of Autoimmune Diseases.
Results
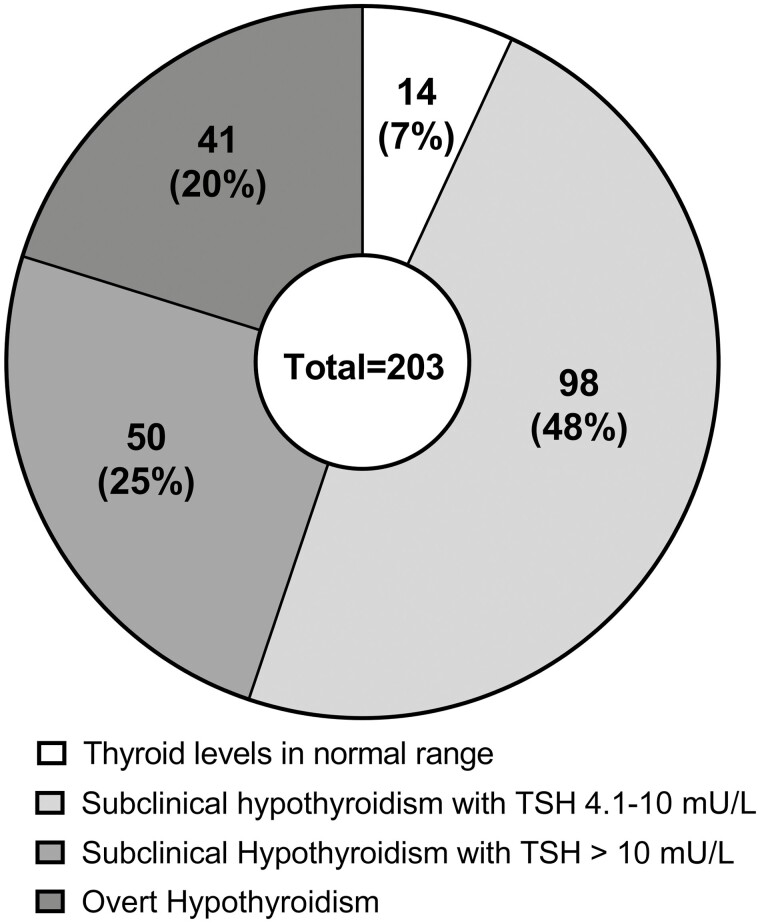
Of 912 registered AAD patients, 442 (48%) were diagnosed with autoimmune thyroid disease. A total of 380 (42%) had autoimmune hypothyroidism. Of the 203 with available thyroid function tests at time of diagnosis, 20% had overt hypothyroidism, 73% had subclinical hypothyroidism, and 7% had thyroid levels in the normal range. Negative thyroid peroxidase antibodies was found in 32%. Ninety-eight percent were treated with levothyroxine, 5% with combination therapy with liothyronine or thyroid extracts, and 1% were observed without treatment. Seventy-eight patients (9%) were diagnosed with Graves disease (GD), of whom 16 (21%) were diagnosed with autoimmune hypothyroidism either before onset or after remission of GD. At the end of follow-up, 33% had normal thyroid hormone levels without antithyroid-drugs or levothyroxine treatment. The remaining had either active disease (5%), had undergone ablative treatment (41%), or had developed autoimmune hypothyroidism (21%).
Conclusion
The true prevalence of hypothyroidism in AAD is lower than reported in the current literature. Careful consideration of the indication to start thyroxin therapy is warranted. Long-term remission rates in GD patients with AAD are comparable to recent reports on long-term follow-up of patients without AAD.
Keywords: autoimmune Addison disease, autoimmune hypothyroidism, Graves disease, autoimmune polyendocrine syndromes
Autoimmune Addison disease (AAD) is associated with a high frequency of organ-specific endocrine and nonendocrine manifestations. More than half of patients have an autoimmune polyendocrine syndrome (1). A report from the Swedish Addison registry found that hypothyroidism was the most frequent concomitant disease, affecting 40% of patients (2). Similar numbers have been reported in 665 patients from the Norwegian registry, which reports that 41% had hypothyroidism and 5.7% hyperthyroidism (3). Italian data on 492 AAD patients reported that 68% had concomitant autoimmune thyroid disorders. In that study thyroid autoimmunity included those with thyroid autoantibodies and an ultrasound pattern indicating thyroiditis, but with normal thyroid function tests. Forty-nine percent of the patients with hypothyroidism had debut before the onset of AAD (4). However, none of these studies gave details on diagnosis and treatment. It is well known that untreated or suboptimal treatment of AAD leads to an increase in thyrotropin (TSH) levels, which can potentially lead to overdiagnosis of hypothyroidism (5, 6). In a previous study from our research group, TSH was elevated in 52% of AAD patients without known hypothyroidism at diagnosis (7). Finally, there are few reports on the combination of AAD and Graves disease (GD). To amend these knowledge gaps, we comprehensively mapped epidemiological and clinical data on autoimmune thyroid disorders in a nationwide AAD registry.
Materials and Methods
Patients and Design
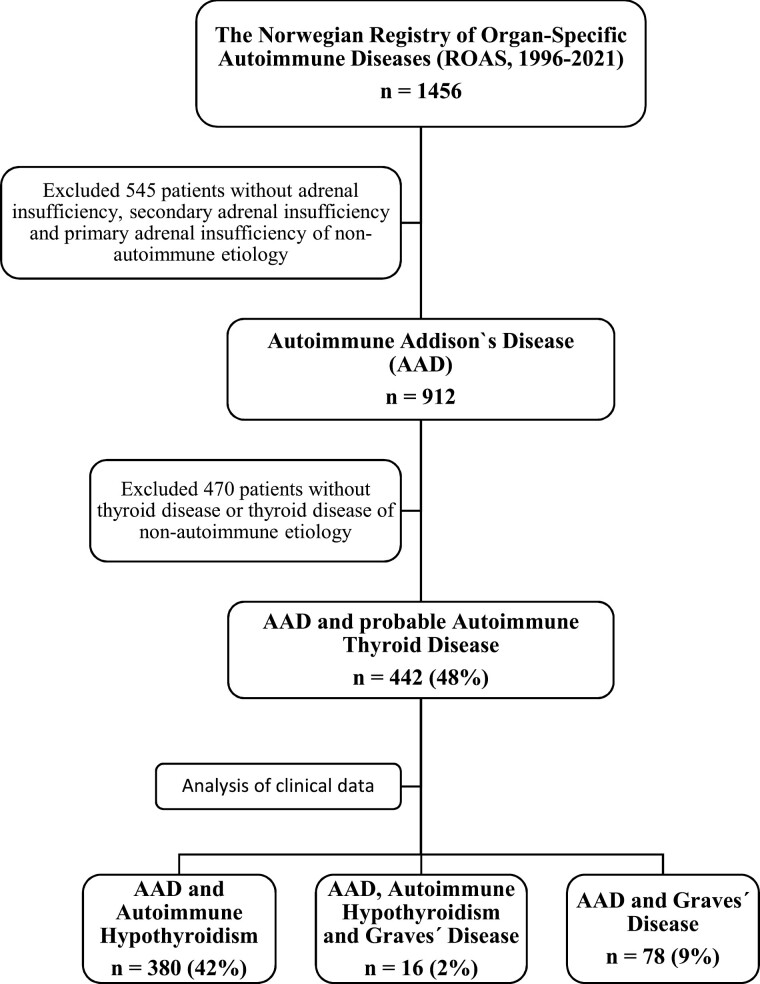
We identified all patients with the combination of AAD and autoimmune thyroid disorder (Hashimoto thyroiditis and/or GD) enrolled in the Norwegian Registry for Organ-specific Autoimmune Diseases (ROAS). ROAS is a national medical research and quality registry for patients with primary adrenal insufficiency (Addison disease), primary ovarian insufficiency, polyendocrine syndromes, and hypoparathyroidism. National coverage for primary adrenal insufficiency is 65% based on the latest national coverage analysis in 2019. Patients with primary adrenal insufficiency and antibodies against 21-hydroxylase or presence of associated autoimmune diseases were classified as having AAD (8). Patients with primary adrenal insufficiency of other causes were excluded. Data from the registry and hospital records were compared with self-reported patient information regarding concomitant thyroid disease collected annually. The present study includes data compiled from the National Registry’s initiation in 1996 until the end of June 2021. During this period, 912 patients with AAD were included, of whom 442 had probable autoimmune thyroid disease. Patients who were found to have nonautoimmune hypothyroidism, for instance after cancer surgery or treatment of nodular disease, were excluded (Fig. 1). Thyroid hormone levels at onset were obtained from 203 of 380 patients (53%) with hypothyroidism. Information on thyroid peroxidase autoantibodies (TPOAbs) was missing for 3 of 380 patients with hypothyroidism. Thyrotropin receptor antibody (TRAb) levels were missing for 17 of 78 patients diagnosed with GD.
Figure 1.
Flowchart of the Autoimmune Addison Disease (AAD) cohort. Flowchart of the inclusion and exclusion criteria of patients from the Norwegian Registry of Organ-specific Autoimmune Diseases (ROAS).
Definitions and Clinical Data
Data on sex, age, year of diagnosis, concomitant autoimmune diseases, autoantibody status, and treatment were obtained from the registry. Medical records of all patients, except for 4 with unknown site for follow-up, were validated by an endocrinologist. Presence of autoimmune hypothyroidism, autoimmune hyperthyroidism (GD), type 1 diabetes, B12 deficiency, primary gonadal insufficiency, vitiligo, celiac disease, and alopecia were registered. To verify the diagnosis of autoimmune hypothyroidism we obtained test results on TSH, free thyroxine (FT4) at onset, and TPOAb during the course of the disease, when available. Subclinical hypothyroidism was defined as TSH greater than 4.0 mU/L and FT4 within the reference range, and further divided into mild (TSH 4.1-10 mU/L) and severe (TSH > 10 mU/L) (9). Overt hypothyroidism was defined as TSH greater than and FT4 less than the reference range. We included patients with hyperthyroidism diagnosed with GD by their attending physician and obtained information on TRAb levels, presence of orbitopathy, remission rates, and treatment given if remission was not achieved. Patients who were euthyroid without medication were defined as being in remission from GD. Patients with hypothyroidism after ablative treatment for GD were registered as having GD. Patients who were treated with levothyroxine for autoimmune hypothyroidism before they were diagnosed with GD or developed autoimmune hypothyroidism after GD remission were registered as having both diseases (coexisting diseases).
Autoantibodies
ROAS biobank serum samples are routinely analyzed for 21-hydroxylase autoantibodies using radiobinding ligand assay as described previously (10). TPOAb and TRAb were analyzed in clinical routine at local hospitals throughout the country. Different methods were used during the period 1996 to 2021. Therefore, the local reference range was used to score if autoantibodies were present or absent.
Statistics
Statistical analyses were performed using IBM SPSS Statistics version 26.0 for Windows. We report our categorical data as absolute numbers and percentages and continuous data as medians and range (minimum to maximum).
Ethics
This study was approved by the local ethics committee (REK No. 234967). All participants gave informed consent when they were included in ROAS (REK no. 2013/1504).
Results
Clinical and Epidemiological Characteristics
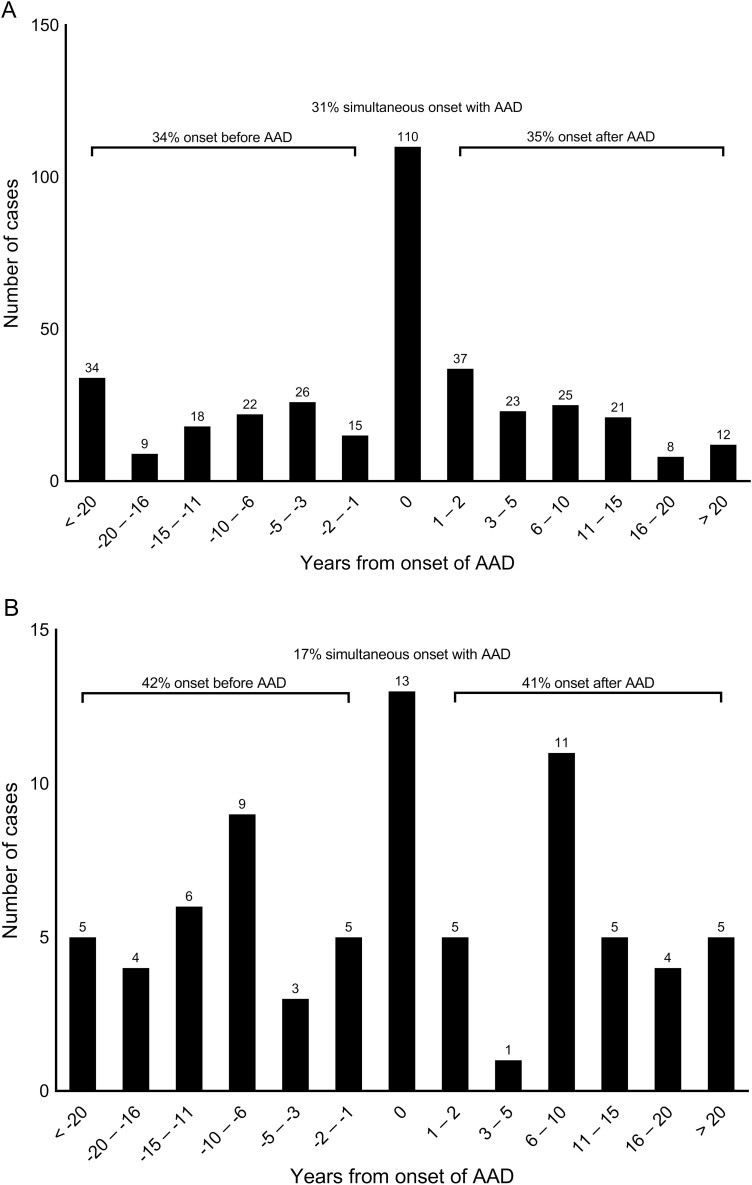
Altogether 442 patients had the combination AAD and autoimmune hypothyroidism or hyperthyroidism (GD), after excluding patients with nonautoimmune primary adrenal insufficiency, hypothyroidism, and hyperthyroidism (see Fig. 1). Sixteen patients were found to have both autoimmune hypothyroidism and hyperthyroidism, which leaves a total prevalence of thyroid disorders in the AAD cohort of 48%. Sex, age at presentation, autoantibody status, and frequency of concomitant autoimmune diseases were recorded (Table 1). Seven (2%) of these patients had autoimmune polyendocrine syndrome type-1; the remaining had autoimmune polyendocrine type-2 (APS-2). The proportions of women were 71% and 77% in the groups with hypothyroidism and GD, respectively. The prevalence of hypothyroidism was 50% among women and 29% among men. Eleven percent of women and 5% of men were diagnosed with GD. GD patients had a median age 33.5 years at the onset of AAD, while patients with hypothyroidism had a median age of 36 years. Thyroid disease had a median onset of 38 years for hypothyroidism and 33 years for GD. The temporal relationship between the onset of AAD and thyroid disease is shown in Fig. 2, demonstrating great diversity in onset (range of 59 years for hypothyroidism and 55 years for hyperthyroidism). Simultaneous onset, defined as diagnosis within the same year, of AAD and thyroid disease was most common in hypothyroidism, occurring in 31% of patients. Median year of diagnosis for AAD was 2000 (1951-2021), which emphasizes that our study represents long-term follow up data.
Table 1.
Clinical and epidemiological characteristics
| Basic demographics | Hypothyroidism | Graves disease |
|---|---|---|
| Prevalence of total AAD cohort, No. (%) | 380/912 (42%) | 78/912 (8.6%) |
| No. of female patients (%) | 271 (71%) | 60 (77%) |
| Age at onset of AAD, median (range) | 36.0 (3-85) | 33.5 (14-64) |
| Age at onset of autoimmune thyroid disease, median (range) | 38.0 (2-87) | 33.0 (9-89) |
| Y between AAD and thyroid disease, median (range) | 0 (–52 to 59) | 1.0 (–55 to 37) |
| Autoantibodies, No. (%) | ||
| 21-Hydroxylase antibodies | 357/377 (95%)a | 75/78 (96%) |
| Thyroid peroxidase antibodies | 256/377 (68%)b | 43/78 (55%) |
| TSH-receptor antibodies | 50/61 (82%)c | |
| Associated autoimmune diseases, No. (%) | ||
| Type 1 diabetes mellitus | 68/380 (18%) | 12/78 (15%) |
| B12 deficiency | 36/380 (9.5%) | 12/78 (15%) |
| Gonadal insufficiency | 26/380 (6.8%) | 4/78 (5.1%) |
| Vitiligo | 51/380 (13%) | 13/78 (17%) |
| Celiac disease | 18/380 (4.7%) | 6/78 (7.7%) |
| Alopecia | 21/380 (5.5%) | 4/78 (5.1%) |
| Coexisting diseased | 16/380 (4.2%) | 16/78 (21%) |
| Total: ≥ 1 associated disease | 187/380 (49%) | 44/78 (56%) |
Abbreviations: AAD, autoimmune Addison disease; TSH, thyrotropin.
a Data missing for 3 patients.
b Data missing for 3 patients.
c Data missing for 17 patients.
d Patients that were diagnosed both with autoimmune hypothyroidism and hyperthyroidism.
Figure 2.
Onset of thyroid disorder. A, Bar chart illustrating the temporal relationship between onset of autoimmune Addison disease (AAD) and hypothyroidism in 361 of 380 patients. Year of diagnosis on the X axis. 0 is defined as simultaneous onset (diagnosis within same year) of AAD and hypothyroidism. For 19 patients, year of diagnosis was missing. B, Bar chart illustrating the temporal relationship between onset of AAD and hyperthyroidism in 76 of 78 patients. Year of diagnosis on the X axis. 0 is defined as simultaneous onset (diagnosis within same year) of AAD and hyperthyroidism. For 2 patients, year of diagnosis was missing.
Diagnosis of Autoimmune Hypothyroidism
Altogether 68% of patients diagnosed with autoimmune hypothyroidism had TPOAbs greater than the reference range (see Table 1). Only 20% had overt hypothyroidism at onset, while the remaining had subclinical hypothyroidism (73%) or thyroid levels in the normal range (7%) when they were diagnosed (Fig. 3). Most patients with overt hypothyroidism were diagnosed simultaneously with AAD. The group that had onset after AAD had the highest proportion of mild subclinical disease, with TSH levels between 4.1 and 10 mU/L (Table 2). The group that had onset before AAD had the highest proportion of TPOAb-negative patients (Table 3). The relationship between TPOAb positivity and the degree of thyroid dysfunction is shown in Table 4. For 177 patients (47%) it was not possible to obtain thyroid hormone levels at onset from hospital records. Many of these were diagnosed before 1995.
Figure 3.
Diagnosis of autoimmune hypothyroidism. The number and percentages of patients with thyroid hormone levels in the normal range, subclinical hypothyroidism, and overt hypothyroidism at diagnosis in 203 of 380 autoimmune Addison disease patients.
Table 2.
Degree of thyroid dysfunction vs temporal relationship between onset of autoimmune Addison disease and hypothyroidism, number (%)
| Onset of hypothyroidism | TSH in normal range | Subclinical hypothyroidism with TSH 4.1-10 mU/L | Subclinical hypothyroidism with TSH > 10 mU/L | Overt hypothyroidism |
|---|---|---|---|---|
| Onset before AAD | 2 (14.3%) | 27 (27.6%) | 12 (24.0%) | 14 (34.1%) |
| Simultaneous onset | 4 (28.6%) | 21 (21.4%) | 25 (50.0%) | 21 (51.2%) |
| Onset after AAD | 8 (57.1%) | 50 (51.0%) | 13 (26.0%) | 6 (14.6%) |
| Total | 14 | 98 | 50 | 41 |
Abbreviations: AAD, autoimmune Addison disease; TSH, thyrotropin.
Table 3.
Thyroid peroxidase antibody status vs temporal relationship between onset of autoimmune Addison disease and hypothyroidism, number (%)
| Onset of hypothyroidism | TPOAb-positive No. (%) | TPOAb-negative No. (%) | Total No. |
|---|---|---|---|
| Onset before AAD | 81 (64.3%) | 45 (35.7%) | 126 |
| Simultaneous onset | 80 (72.7%) | 30 (27.3%) | 110 |
| Onset after AAD | 86 (69.4%) | 38 (30.6%) | 124 |
Abbreviations: AAD, autoimmune Addison disease; TPOAb, thyroid peroxidase antibody.
Table 4.
Degree of thyroid dysfunction vs thyroid peroxidase antibody status in 203 of 380 patients with available thyroid function tests
| TSH at onset | TPOAb-positive, No. (%) | TPOAb-negative, No. (%) | Total No. (%) |
|---|---|---|---|
| TSH in normal range | 11 (78.6%) | 3 (21.4%) | 14 (7%) |
| Subclinical hypothyroidism with TSH 4.1-10 mU/L | 74 (75.5%) | 24 (24.5%) | 98 (48%) |
| Subclinical hypothyroidism with TSH > 10 mU/L | 42 (84.0%) | 8 (16.0%) | 50 (25%) |
| Overt hypothyroidism | 33 (80.5%) | 8 (19.5%) | 41 (20%) |
Abbreviations: TPOAb, thyroid peroxidase antibody; TSH, thyrotropin.
Replacement Therapy for Hypothyroidism
Most of the patients used levothyroxine in monotherapy. Fourteen patients (4%) used combination therapy with liothyronine and 4 patients (1%) used thyroid extracts. Observation without treatment was unusual (1.5%). For 3 patients (0.8%) there was no available information on treatment.
Treatment and Remission Rates for Graves Disease
Remission without antithyroid drugs (ATDs) or thyroxine treatment was achieved in 33% of the GD patients at the end of follow-up. The remaining had active disease, had undergone ablative treatment, or had developed autoimmune hypothyroidism. Thirty-eight (49%) patients had experienced a single episode of GD, whereas 21 (27%) had an undulating course with 2 or more episodes. Nineteen patients (24%) had received ATD treatment on 2 or more occasions. For 19 (24%) patients, the number of episodes was not stated in the medical record. Nineteen percent received radioiodine therapy, and 22% were treated with thyroidectomy. Sixteen percent experienced orbitopathy.
Associated Autoimmunity
Associated autoimmunity was diagnosed in 49% of patients with hypothyroidism. The most common comorbidity was type 1 diabetes (18%), followed by vitiligo (13%) and B12 deficiency (9.5%). Primary gonadal insufficiency was diagnosed in 7% of all patients with hypothyroidism. Among women with AAD and hypothyroidism, 9% had gonadal insufficiency. GD patients had associated autoimmunity in 56% of the cases. Twenty-one percent (16/78) were treated for autoimmune hypothyroidism, either before they were diagnosed with GD or after remission. B12 deficiency and type 1 diabetes were diagnosed in 15% each, 17% had vitiligo, and 7.7% had celiac disease (see Table 1).
Discussion
Scrutiny of one of the largest cohort of patients with AAD reported to date confirms earlier observations that autoimmune thyroid disease is the most commonly diagnosed comorbidity, affecting about 50% of patients. However, surprisingly few had overt hypothyroidism and a sizable portion had no documented hypothyroidism. GD was also common and remission rates were lower than what has previously been reported in Norway (11).
The prevalence we found of hypothyroidism and hyperthyroidism is in alignment with a previous study from our registry and 2 large cohort studies from Sweden and Italy (2-4). However, the present study is unique because it includes more patients and reports thyroid hormone levels at the onset of hypothyroidism. Surprisingly, we found that only 20% of patients had overt hypothyroidism at onset and that a considerable share was TPOAb negative or had mild subclinical disease. These findings indicate that clinicians tend to overdiagnose hypothyroidism and have a low threshold for starting treatment. The fact that the patient already had an organ-specific autoimmune disease might have lowered the bar for diagnosis and treatment.
The female-to-male ratio and age at onset in our study is comparable to previous reports (2-4). Specifically, 71% and 77% in the groups with hypothyroidism and hyperthyroidism were women, respectively. The onset of AAD was usually in young adulthood, but with an age range of 3 to 85 years. The GD cohort had a younger age of onset of AAD compared to those with hypothyroidism, raising the question whether GD patients represent a more severe phenotype of APS-2 or whether hyperthyroidism hastens or unmasks the diagnosis of AAD more than is the case for hypothyroidism.
Betterle and colleagues (4) found that a considerable proportion of AAD patients with concomitant hypothyroidism and hyperthyroidism were diagnosed before the onset of AAD, but their study used a broader definition of autoimmune thyroid disease by including individuals with normal thyroid function and TPOAbs or signs of thyroiditis by ultrasound. In our study, the onset of hypothyroidism was evenly distributed before, concurrently with, and after the diagnosis of AAD. Intriguingly, the majority of patients with overt hypothyroidism were diagnosed simultaneously with AAD. For patients with hyperthyroidism, we found a greater diversity in time of presentation. Simultaneous presentation was less common and 42% had onset before AAD. The latter difference may be because the symptoms of hyperthyroidism are more specific and diagnostic delay less common than for hypothyroidism. A large proportion of patients in our study presented with thyroid disease after the onset of AAD, with a range of up to 55 years. The latter supports that annual, lifelong screening for thyroid disease should be maintained.
TPOAb predicts progression to overt hypothyroidism in patients with subclinical disease (12). Dalin and colleagues (2) found presence of TPOAbs in 57% of all AAD patients. We found an unexpectedly large proportion of patients with hypothyroidism without TPOAbs (32%), indicating misdiagnosis. According to the existing literature, high serum TPOAb concentrations are present in 90% of patients with Hashimoto thyroiditis (13). TPOAb levels decline over time in most patients, but rarely become negative (14). Furthermore, half of the patients with hypothyroidism diagnosed after the onset of AAD had only mildly elevated TSH, and 25% of these lacked TPOAbs. AAD patients have higher TSH levels when glucocorticoids are withheld or intake is delayed (5).Taken together, these observations indicate that autoimmune hypothyroidism is overdiagnosed among AAD patients, and that even a mild elevation of TSH triggers levothyroxine treatment.
Very few patients were observed without treatment, indicating that even if the treatment is started on an uncertain indication, it is rarely reevaluated and stopped. Levothyroxine is one of the most commonly prescribed drugs in the world and often leads to lifelong replacement therapy. A recent systematic review and meta-analysis found that up to one-third of patients remained euthyroid after thyroid hormone discontinuation, with a higher proportion of patients with an initial diagnosis of subclinical hypothyroidism compared to overt hypothyroidism (15). Our data indicate that more patients can be observed without treatment, especially if TPOAb is negative. However, the number of individuals observed without treatment may be underestimated because of the possibility that those with untreated subclinical disease were not registered as such.
International guidelines state that if TSH is in the range of 4.1 to 10 mU/L and the patient has symptoms suggestive of hypothyroidism, clinicians can consider treatment with levothyroxine. However, the relation between symptoms and TSH levels remains unclear. The question of starting treatment is especially challenging in AAD patients, who often experience fatigue and reduced quality of life due to adrenal insufficiency (16), and are themselves eager to start treatment. Thus, the threshold to start treatment is probably lower than what would be the situation in a non-AAD patient. We suggest that clinicians should optimize the treatment of adrenal insufficiency and observe slightly elevated TSH levels over time before they consider levothyroxine treatment. Similarly, clinicians should consider cortisol deficiency in non-AAD patients with mild subclinical hypothyroidism and high symptom burden. This is clinically important since initiation of levothyroxine in untreated AAD patients could lead to an adrenal crisis (17). Taken together, there seems to be a considerable risk of overprescription of levothyroxine in AAD patients.
Nine percent of our AAD patients were diagnosed with GD. TRAb is a sensitive biomarker for GD, but in our study a statistically significant percentage (18%) was TRAb negative at onset. For 17 patients TRAb at onset was not obtainable because of diagnosis several decades ago. A meta-analysis of 21 studies showed that the overall pooled sensitivity of the TRAb concentration in GD measured with second- and third-generation assays was 97% (18). Part of the reason for the high proportion of TRAb-negative patients may be misdiagnosis. Of the 11 TRAb-negative patients, 4 had spontaneous remission or only short-term use of ATD, and 2 developed hypothyroidism, which indicates that they might have had thyroiditis with leakage of thyroid hormones. Clinicians probably have a lower threshold for diagnosing autoimmune hyperthyroidism in patients with AAD. Thus, the prevalence of GD in our cohort may be overestimated.
The predominant first-line therapy for GD is ATDs. The overall remission rates after a course of ATD has geographical differences and varies between 30% and 60%. A previous Norwegian study found that 52% of patients were in remission 2 years after withdrawal of ATD (11). In our study, normal thyroid status in the longer term was achieved by only one-third of patients. If we exclude patients with uncertain diagnosis, the rate would have been even lower. Certain risk factors have been associated with GD relapse, such as young age (< 40 years), smoking, large goiter size, and high TRAb or FT4 and free 3,5,3′-triiodothyronine at onset (19). Our AAD patients had onset of GD in young adulthood, with a median age of 33 years, which can represent a risk factor for poor response to ATD. In addition, CTLA-4, PTPN22 C/T polymorphism, and human leukocyte antigen subtypes DQB1*02, DQA1*05, and DRB1*03 have been found to be independent predictors for GD recurrence (20, 21), factors that are also associated with AAD (22-25). However, most reported clinical outcomes after treatment of GD have been obtained with short-term follow-up. Our study represents long-term follow-up data, with onset of GD in the time period 1945 to 2021. Our findings are similar to those of a Swedish study that reported that normal thyroid hormone status without thyroxine supplementation was achieved in 36% with 6 to 10 years of follow-up (26).
Patients with AAD and thyroid disease have a high incidence of associated autoimmunity. Of particular importance is the risk of developing type 1 diabetes, which appears to be particularly high at 15% to 18%. This emphasizes the importance of patient information about symptoms of hyperglycemia and annual screening with glucose and glycated hemoglobin A1c. Furthermore, we confirm recent observations that the risk of having premature gonadal insufficiency among women with AAD is high, found in 9% of women with hypothyroidism and AAD (27). In comparison, Betterle and colleagues (4) report a prevalence of 14% in their APS-2 patients, but included also those with so-called subclinical ovarian autoimmunity.
Sixteen patients in our cohort were diagnosed both with autoimmune hypothyroidism and hyperthyroidism during their disease course, which indicates that Hashimoto and GD may coexist in the same patient. The latter has previously been described by others (28). High frequency of levothyroxine replacement after GD remission was also recently found in a large GD cohort with long-term follow-up data (26). The occurrence of blocking TRAb is another possibility that could not be excluded in our study.
The reason why some AAD patients develop associated autoimmunity, whereas others do not, remains incompletely understood. In a recently published genome-wide association study there were no differences in genetic associations between those with isolated AAD and those with APS-2. There were also substantial overlaps of associated genetic loci with the most common comorbidities such as autoimmune thyroid disease and type 1 diabetes (25).
There are some limitations to our study. Despite the fact that we here report on one of the largest cohorts to date and provide a comprehensive overview of the combination of AAD and autoimmune thyroid disease, the study was limited by a considerable amount of missing data of both thyroid hormone values and autoantibodies, despite scrutiny of patient records. The use of different assays for measuring thyroid autoantibodies is another important shortcoming, since sensitivity and specificity probably vary among tests.
In summary, even if autoimmune thyroid disease is the most common autoimmune comorbidity in AAD, overt hypothyroidism was seen in only about 20% and GD in about 9% of cases. It is likely that a substantial portion of patients are treated with levothyroxine without having autoimmune thyroid disease. Careful consideration of the indication to start thyroxin therapy is warranted, especially in patients with mild subclinical disease or negative TPOAbs. Finally, we recommend careful monitoring of AAD patients with thyroid disease, in order to detect co-occurring autoimmunity.
Glossary
Abbreviations
- AAD
autoimmune Addison disease
- APS-2
autoimmune polyendocrine syndrome type-2
- ATD
antithyroid drug
- FT4
free thyroxine
- GD
Graves disease
- ROAS
Norwegian Registry for Organ-specific Autoimmune Diseases
- TPOAb
thyroid peroxidase antibody
- TRAb
thyrotropin receptor antibody
- TSH
thyrotropin
Author Contributions
A.E.M.S., G.U., K.L., K.G., T.F., M.S., A.E.T., S.E.H., S.T.S., A.D., H.S., M.L.R., H.L.D.E.S, A.S., S.B., P.M., E.K., Ø.H., L.B., A.P.J., and E.S.H. contributed to collecting clinical data. All authors were involved in writing and critically reviewing the manuscript.
Financial Support
The study was supported by grants from The Regional Health Authorities of Western Norway, Research Council of Norway, Tne Novo Nordisk Foundation and Stiftelsen Kristian Gerhard Jebsen.
Disclosures
The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Husebye ES, Pearce SH, Krone NP, Kämpe O. Adrenal insufficiency. Lancet 2021;397(10274):613-629. [DOI] [PubMed] [Google Scholar]
- 2. Dalin F, Nordling Eriksson G, Dahlqvist P, et al. . Clinical and immunological characteristics of autoimmune Addison disease: a nationwide Swedish multicenter study. J Clin Endocrinol Metab. 2017;102(2):379-389. [DOI] [PubMed] [Google Scholar]
- 3. Erichsen MM, Løvås K, Skinningsrud B, et al. . Clinical, immunological, and genetic features of autoimmune primary adrenal insufficiency: observations from a Norwegian registry. J Clin Endocrinol Metab. 2009;94(12):4882-4890. [DOI] [PubMed] [Google Scholar]
- 4. Betterle C, Scarpa R, Garelli S, et al. . Addison’s disease: a survey on 633 patients in Padova. Eur J Endocrinol. 2013;169(6):773-784. [DOI] [PubMed] [Google Scholar]
- 5. Samuels MH. Effects of variations in physiological cortisol levels on thyrotropin secretion in subjects with adrenal insufficiency: a clinical research center study. J Clin Endocrinol Metab. 2000;85(4):1388-1393. [DOI] [PubMed] [Google Scholar]
- 6. Topliss DJ, White EL, Stockigt JR. Significance of thyrotropin excess in untreated primary adrenal insufficiency. J Clin Endocrinol Metab. 1980;50(1):52-56. [DOI] [PubMed] [Google Scholar]
- 7. Saevik ÅB, Åkerman AK, Grønning K, et al. . Clues for early detection of autoimmune Addison’s disease—myths and realities. J Intern Med. 2018;283(2):190-199. [DOI] [PubMed] [Google Scholar]
- 8. Husebye ES, Allolio B, Arlt W, et al. . Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J Intern Med. 2014;275(2):104-115. [DOI] [PubMed] [Google Scholar]
- 9. Pearce SHS, Brabant G, Duntas LH, et al. . 2013 ETA Guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2(4):215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oftedal BE, Wolff ASB, Bratland E, et al. . Radioimmunoassay for autoantibodies against interferon omega; its use in the diagnosis of autoimmune polyendocrine syndrome type I. Clin Immunol. 2008;129(1):163-169. [DOI] [PubMed] [Google Scholar]
- 11. Nedrebo BG, Holm PI, Uhlving S, et al. . Predictors of outcome and comparison of different drug regimens for the prevention of relapse in patients with Graves’ disease. Eur J Endocrinol. 2002;147(5):583-589. [DOI] [PubMed] [Google Scholar]
- 12. Carlé A, Laurberg P, Knudsen N, et al. . Thyroid peroxidase and thyroglobulin auto-antibodies in patients with newly diagnosed overt hypothyroidism. Autoimmunity. 2006;39(6):497-503. [DOI] [PubMed] [Google Scholar]
- 13. Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003;348(26):2646-2655. [DOI] [PubMed] [Google Scholar]
- 14. Schmidt M, Voell M, Rahlff I, et al. . Long-term follow-up of antithyroid peroxidase antibodies in patients with chronic autoimmune thyroiditis (Hashimoto’s thyroiditis) treated with levothyroxine. Thyroid. 2008;18(7):755-760. [DOI] [PubMed] [Google Scholar]
- 15. Burgos N, Toloza FJK, Singh Ospina NM, et al. . Clinical outcomes after discontinuation of thyroid hormone replacement: a systematic review and meta-analysis. Thyroid. 2021;31(5):740-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bensing S, Hulting AL, Husebye ES, Kämpe O, Løvås K. Management of endocrine disease: epidemiology, quality of life and complications of primary adrenal insufficiency: a review. Eur J Endocrinol. 2016;175(3):R107-R116. [DOI] [PubMed] [Google Scholar]
- 17. Bornstein SR, Allolio B, Arlt W, et al. . Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tozzoli R, Bagnasco M, Giavarina D, Bizzaro N. TSH receptor autoantibody immunoassay in patients with Graves’ disease: improvement of diagnostic accuracy over different generations of methods. Systematic review and meta-analysis. Autoimmun Rev. 2012;12(2):107-113. [DOI] [PubMed] [Google Scholar]
- 19. Shi H, Sheng R, Hu Y, et al. . Risk factors for the relapse of Graves’ disease treated with antithyroid drugs: a systematic review and meta-analysis. Clin Ther. 2020;42(4):662-675.e4. [DOI] [PubMed] [Google Scholar]
- 20. Vos XG, Endert E, Zwinderman AH, Tijssen JG, Wiersinga WM. Predicting the risk of recurrence before the start of antithyroid drug therapy in patients with Graves’ hyperthyroidism. J Clin Endocrinol Metab. 2016;101(4):1381-1389. [DOI] [PubMed] [Google Scholar]
- 21. Tanrikulu S, Erbil Y, Ademoglu E, et al. . The predictive value of CTLA-4 and Tg polymorphisms in the recurrence of Graves’ disease after antithyroid withdrawal. Endocrine. 2006;30(3):377-381. [DOI] [PubMed] [Google Scholar]
- 22. Wolff AS, Mitchell AL, Cordell HJ, et al. . CTLA-4 as a genetic determinant in autoimmune Addison’s disease. Genes Immun. 2015;16(6):430-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Skinningsrud B, Husebye ES, Gervin K, et al. . Mutation screening of PTPN22: association of the 1858T-allele with Addison’s disease. Eur J Hum Genet. 2008;16(8):977-982. [DOI] [PubMed] [Google Scholar]
- 24. Skinningsrud B, Lie BA, Lavant E, et al. . Multiple loci in the HLA complex are associated with Addison’s disease. J Clin Endocrinol Metab. 2011;96(10):E1703-E1708. [DOI] [PubMed] [Google Scholar]
- 25. Eriksson D, Røyrvik EC, Aranda-Guillén M, et al. . GWAS for autoimmune Addison’s disease identifies multiple risk loci and highlights AIRE in disease susceptibility. Nat Commun. 2021;12(1):959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sjölin G, Holmberg M, Törring O, et al. . The long-term outcome of treatment for Graves’ hyperthyroidism. Thyroid. 2019;29(11):1545-1557. [DOI] [PubMed] [Google Scholar]
- 27. Vogt EC, Breivik L, Røyrvik EC, Grytaas M, Husebye ES, Øksnes M. Primary ovarian insufficiency in women with Addison’s disease. J Clin Endocrinol Metab. 2021;106(7):e2656-e2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dasari S, Naha K, Hande M, Vivek G. Hot and cold: coexistent Graves’ disease and Hashimoto’s thyroiditis in a patient with Schmidt’s syndrome. BMJ Case Rep. 2014;2014:bcr2013010432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”