Abstract
Context
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) cause less weight loss than expected based on urinary calorie excretion. This may be explained by SGLT2i-induced alterations in central reward and satiety circuits, leading to increased appetite and food intake. Glucagon-like peptide-1 receptor agonists are associated with reduced appetite and body weight, mediated by direct and indirect central nervous system (CNS) effects.
Objective
We investigated the separate and combined effects of dapagliflozin and exenatide on the CNS in participants with obesity and type 2 diabetes.
Methods
This was a 16-week, double-blind, randomized, placebo-controlled trial. Obese participants with type 2 diabetes (n = 64, age 63.5 ± 0.9 years, BMI 31.7 ± 0.6 kg/m2) were randomized (1:1:1:1) to dapagliflozin 10 mg with exenatide-matched placebo, exenatide twice daily 10 µg with dapagliflozin-matched placebo, dapagliflozin and exenatide, or double placebo. Using functional MRI, the effects of treatments on CNS responses to viewing food pictures were assessed after 10 days and 16 weeks of treatment.
Results
After 10 days, dapagliflozin increased, whereas exenatide decreased CNS activation in the left putamen. Combination therapy had no effect on responses to food pictures. After 16 weeks, no changes in CNS activation were observed with dapagliflozin, but CNS activation was reduced with dapagliflozin-exenatide in right amygdala.
Conclusion
The early increase in CNS activation with dapagliflozin may contribute to the discrepancy between observed and expected weight loss. In combination therapy, exenatide blunted the increased CNS activation observed with dapagliflozin. These findings provide further insights into the weight-lowering mechanisms of SGLT2i and GLP-1 receptor agonists.
Keywords: SGLT2 inhibitor, dapagliflozin, GLP-1 receptor agonist, exenatide, functional neuroimaging, central nervous system, satiety and reward circuits, body weight, type 2 diabetes, obesity
The central nervous system (CNS) is the key regulator of food intake, as it receives and integrates a variety of hedonic, environmental, and homeostatic signals to produce an overall response of hunger or satiety. Altered brain reward responses to food stimuli may promote excessive eating, resulting in obesity and eventually type 2 diabetes (1, 2).
Functional magnetic resonance imaging (fMRI) can be used to noninvasively measure patterns of brain activity in response to specific stimuli. fMRI detects the blood-oxygen-level-dependent (BOLD) signal, which reflects changes in deoxyhemoglobin, driven by localized changes in cerebral blood flow and blood oxygenation, which are coupled to underlying neuronal activity (neurovascular coupling) (3). Task-based fMRI identifies brain regions that are functionally involved in specific task performance, for instance, presenting food pictures provides information about regions involved in the process of food evaluation. Although this is a reliable and widely used method, processing these cues involves a complex interconnected circuitry, and it is common that not all regions are simultaneously activated to a similar extent.
Studies using fMRI of the CNS found that compared with lean subjects, obese subjects with and without type 2 diabetes, have increased activation in reward processing areas such as the insula, striatum, and amygdala, in response to visual food cues (4, 5). These increased activations predict less weight loss during weight loss programs and predict future weight (re)gain (6-8).The insula (taste perception, decision making, and interoception), limbic-striatal system (reward motivation), and the prefrontal and orbitofrontal cortex (reward evaluation and decision making) are part of a complex interconnected reward circuitry, and these areas are consistently shown to be involved in response to food cues (5, 9-14)).
Because lifestyle-based weight loss strategies are often not effective in the long term, pharmacological approaches to assist weight loss are important. Two recently introduced drug classes for treatment of type 2 diabetes are sodium-glucose cotransporter-2 inhibitors (SGLT2is) and glucagon-like peptide 1 receptor agonists (GLP-1RAs). Besides their effects on glucose regulation, both therapies are known to reduce body weight (15, 16).
The SGLT2is improve glycemic control in an insulin-independent manner by blocking glucose reabsorption in the renal proximal tubule, thereby enhancing urinary glucose excretion. Urinary glucose excretion induces chronic calorie loss, which leads to body weight reduction. However, the observed weight loss is consistently less than expected from the amount of urinary glucose excretion (17, 18). Around 50 to 85 g glucose per day, equivalent to ∼ 300 kcal/day (1200 kJ/day), is excreted daily in SGLT2i treated patients with type 2 diabetes, which would result in a weight loss of ~7 to 10 kg in a year. However, observed weight loss with SGLT2is is only ~2 to 3 kg (16). As studies showed that acute or chronic treatment with an SGLT2i does not alter energy expenditure (17, 18), the discrepancy between observed and expected weight loss implies that patients increased their energy intake. These findings are supported by studies in animals that showed compensatory hyperphagia in response to SGLT2 inhibition (19, 20), but studies in humans have not been performed. In order to optimize treatment, it is important to investigate this issue in humans and understand the mechanisms whereby SGLT2is might influence appetite regulation and food intake.
In addition to the effects of GLP-1RAs on glucose regulation, they also substantially reduce body weight (15). The weight loss is mainly attributable to suppressed appetite signaling in the brain and increased satiety, which leads to a reduced food intake, via direct and indirect actions on the CNS (13, 21). Due to their effects on the CNS, adding a GLP-1RA to an SGLT2i may have beneficial effects, resulting in reduced food intake and greater reductions in body weight (22). Three large studies showed that when GLP-1RAs are added to SGLT2is, the reductions in body weight loss and glucose were larger than with either therapy alone (23-25). In addition, liraglutide blunted the SGLT2i-induced hyperphagia in rats (20). Therefore, it could be suggested that the possible increased food intake during treatment with SGLT2i, may be prevented by GLP-1RAs via effects on the CNS. However, the effects of adding a GLP-1RA to an SGLT2i on central satiety and reward circuits and food intake in humans are unknown.
We thus investigated if treatment with the SGLT2i dapagliflozin induces alterations in central reward and satiety circuits in response to food-related stimuli in obese patients with type 2 diabetes. In addition, we investigated if adding the GLP-1RA exenatide to dapagliflozin could block this SGLT2i-induced increase in neuronal activity in a priori defined central reward and satiety centers in response to food-related stimuli. Additionally, we investigated the separate and combined effects of dapagliflozin and exenatide on food intake and appetite.
Methods
This randomized, double-blind, placebo-controlled trial was conducted between September 2017 and May 2020 at the Amsterdam University Medical Center, location VUmc, Amsterdam, The Netherlands. The study protocol, protocol amendments, and any other protocol-specific documents were reviewed and approved by local authorities and the ethics review board of the Amsterdam University Medical Center, location VUmc. The study complied with the Declaration of Helsinki and Good Clinical Practice guidelines and was registered at ClinicalTrials.gov (NCT03361098).
Participants
Participants were recruited from our database and by advertisement in local newspapers. Eligible participants were men or postmenopausal women, aged 18-75 years, with a stable body weight (< 5% reported change during the previous 3 months), a body mass index (BMI) > 27 kg/m2, and diagnosed with type 2 diabetes. For the current treatment of diabetes, metformin with or without sulfonylurea derivatives was allowed (stable dose for ≥ 3 months). The glycated hemoglobin (HbA1c) levels for participants treated with metformin monotherapy were 7% to 10% (53-86 mmol/mol) and for metformin plus sulfonylurea 7.5% to 10% (58-86 mmol/mol (25). Exclusion criteria were a history of serious cardiovascular, renal, or liver disease, malignancies (excluding basal cell carcinoma), uncontrolled thyroid disease, the use of any centrally acting agent or oral glucocorticoids, substance abuse, neurological or psychiatric disease including eating disorders and depression, and MRI contraindications. Written informed consent was obtained from all participants before any trial-related activities.
General Experimental Protocol
Participants were randomized 1:1:1:1, performed by an independent trial pharmacist using computer-generated numbers, to dapagliflozin 10 mg with exenatide-matched placebo, exenatide twice daily 10 µg with dapagliflozin-matched placebo, dapagliflozin and exenatide, or placebo dapagliflozin and placebo exenatide. Randomization was stratified by gender and left-handedness. Participants were instructed to inject exenatide (or placebo) twice daily 15 to 30 minutes before breakfast and dinner. Exenatide (or placebo) was initiated at a dose of 5 µg twice daily, followed by a dose increase to 10 µg twice daily after 4 weeks (recommended titration schedule), which was maintained until the end of the study. Participants were instructed to take dapagliflozin (or placebo) 10 mg (recommended dose for treatment with dapagliflozin) once daily at 8 pm during the 16-week treatment period. Adherence was followed up by counting the remaining capsules and injection pens at all visits. To maintain blinding throughout the study, participants were treated in a double-dummy design. There was no difference in appearance between exenatide and placebo injections, or dapagliflozin and placebo tablets. All study medications were provided by AstraZeneca.
Participants underwent 3 fMRI sessions, at baseline, after 10 days (short-term) and after 16 weeks (longer-term) of treatment, and they had 1 safety visit after 8 weeks. On each visit, participants arrived at 8:30 am at the research unit after an overnight fast and participants refrained from exercise, alcohol, and caffeine for 12 hours before the sessions. Sulfonylureas were temporarily discontinued during the 3 days prior to examination, and metformin was discontinued on the day of examination. Exenatide was injected on the morning of the visit around 8:30 AM.
At each visit, blood was drawn 5 to 10 minutes before the start of the MRI protocol. Measurements of anthropometrics were performed and body composition was measured by bioimpedance spectroscopy (ImpediMed). Systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and heart rate (HR) were determined by using an automated oscillometric device (Dinamap, GE Healthcare, Little Chalfont, UK) at the brachial artery of the nondominant arm. Measurements were performed in triplicate at 2-minute intervals by using the mean of the 3 measurements.
fMRI Protocol
The fMRI measurements were performed as described previously (5, 13). Briefly, pictures were presented in 3 runs comprising 6 blocks each: 2 blocks consisting of high-calorie food (sweet and savory), 2 blocks of low-calorie food (fruits and vegetables), and 2 blocks of nonfood items (eg, trees, flowers, rocks, and bricks). Within each block 7 pictures were presented for 2.5 seconds each, separated by a 0.5-second blank screen. Each block was followed by 9 seconds of gray blank screen with a fixation cross. Across each block and session, pictures were matched for shape and color. Participants were instructed to attentively watch each picture. One hour after the scanning session a recognition test was performed. The recognition test consisted of 20 pictures of which participants needed to identify 10 pictures that were previously shown in the scanner.
Imaging data were acquired using a 3.0 Tesla GE Signa HDxt scanner (General Electric, Milwaukee, WI, USA). Structural MRI was obtained using a T1-weighted sequence. fMRI data were acquired using an echo planar imaging T2*-weighted blood oxygenation level–dependent sequence with 40 ascending slices per volume (3 mm thickness, 0 mm gap), which resulted in whole-brain coverage. Functional images were preprocessed with fMRIprep v1.2.3 (26). In short, this included slice-time correction (3dTshift, AFNI 20160207), motion correction (FLIRT), distortion correction (3dQwarp), and automatic removal of motion artifacts using independent component analysis (ICA-AROMA). Data were spatially smoothed (6 mm FWHM) and high pass-filtered (128-second cutoff) using FSL. T1-coregistered volumes were normalized to Montreal Neurological Institute space. Preprocessed data were analyzed in the context of the general linear model with SPM12 (Wellcome Trust Centre for Neuroimaging, London, U.K.). At the first (single-subject) level, high-calorie food, low-calorie food, and nonfood blocks were modeled. To assess CNS activation related to viewing food pictures and, more specifically, their hedonic quality, we computed the following 2 contrasts at each time point: food > nonfood and high-calorie > nonfood, which refer to the activity during viewing food or high-calorie food that is greater compared with viewing nonfood pictures. These first level contrast images were entered into second-level ANOVA. To test our hypotheses, dapagliflozin and dapagliflozin plus exenatide were compared with placebo after 10 days and 16 weeks of treatment. Since there were no differences between the groups at baseline, we did not add baseline activation as covariate. In additional analyses, exenatide was compared with placebo, and dapagliflozin was compared with dapagliflozin-exenatide.
A priori regions of interest (ROIs) were determined based on previous studies (ie, insula [including adjacent opercular cortices], striatum [ie, putamen and caudate nucleus], amygdala, and orbitofrontal cortex), as these regions are consistently shown to be involved in responses to food cues and are part of the central reward circuits (5, 10-14)). First, we explored, using whole-brain analyses, if differences in activation in a priori ROIs were present at an uncorrected P < 0.001. CNS activations were reported as significant when these survived familywise error (FWE) correction for multiple comparisons (PFWE < 0.05) on the voxel level using small volume correction within the predefined ROIs, using 5-mm (for amygdala) or 10-mm (for insula, putamen, caudate nucleus, and orbitofrontal cortex) radius spheres as described previously (5, 13, 27).
At baseline, we performed a structural MRI using a T1 weighted sequence, to detect structural brain changes, and a T2 weighted FLAIR sequence to assess white matter lesions, using the Fazekas score. These sequences were assessed by a clinical radiologist (F.B.).
Ad Libitum Lunch Buffet
After the fMRI sessions, participants were presented a standardized choice buffet to assess energy intake (5). Participants were instructed to eat as much as they liked and were not informed that their choices and intake were monitored. Total intake of energy (kcal), and percentages of kcal derived from fat, protein, and carbohydrates were calculated. Nutritional analysis was performed using the Dutch Food Tables (NEVO; https://www.rivm.nl/nevo/).
Questionnaires
Participants were asked to score their sensation of hunger, fullness, appetite, prospective food consumption, and desire to eat on a 10-point Likert scale before the lunch buffet (fasted) and directly after the lunch buffet (28).
Indirect Calorimetry
Resting energy expenditure and the respiratory quotient were measured by indirect calorimetry with a ventilated-hood system (Quark RMR, COSMED, Rome, Italy). The measurements were performed under standardized conditions; participants were awake in supine position, at complete physical rest and in a thermally neutral environment for 30 minutes. Steady state periods of measurements were selected, where a coefficient of variation of 10% was considered as acceptable. Oxygen consumption and carbon dioxide production was measured, and energy expenditure was calculated using the Weir formula (29).
Statistical Analysis
The sample size was calculated based on previous fMRI studies, addressing activity in comparable CNS circuits involved in satiety and reward regulation. To detect differences in treatment effects between groups of 3% (SD 2.5%) in mean difference in BOLD fMRI signal change, 12-16 participants per group were required for 85% power (after adjustment for multiple comparisons among the 4 groups).
Clinical data were analyzed with the SPSS version 26 and are expressed as mean ± standard error of the mean (SEM) (unless otherwise stated). To test treatment effects vs placebo, linear mixed models were used in the intention to treat population. The endpoint of interest was added as dependent variable, and treatment allocation as independent variable in dummy variables. Visit was added as fixed factor. The intervention-by-visit interaction and a random intercept were included in the model. Additionally, the corresponding baseline values were included as independent variable.
Results
Baseline Characteristics
The study was conducted between September 18, 2017 and March 25, 2020. A total of 106 people were screened, of whom 68 were included (Supplemental Figure 1 (30)). During baseline testing, 4 patients experienced previously unknown MRI-related claustrophobia; 3 of these participants were excluded and replaced, and 1 patient continued treatment, but without fMRI measurements. Two participants dropped out, just before the last test visit, 1 because of personal reasons, the other because of ongoing nausea. The presence of white matter lesions was evenly distributed among the 4 groups. Only 1 patient had severe white matter lesions (Fazekas score 3). Medication adherence for dapagliflozin (or placebo) was 99.4% after 10 days, and 99.0% after 16 weeks. Medication adherence for exenatide (or placebo) was 100% after 10 days, and 97.5% after 16 weeks.
At baseline, clinical characteristics were generally well balanced between the treatment groups [Table 1]. For all participants, the medications used at baseline remained unchanged during the study.
Table 1.
Baseline characteristics
| Dapagliflozin (n = 16) | Exenatide (n = 17) | Dapagliflozin + Exenatide (n = 16) | Placebo (n = 16) | |
|---|---|---|---|---|
| Age, years | 64 (8.4) | 65 (5.8) | 64 (7.4) | 60.9 (7.2) |
| Female, n (%) | 4 (25) | 6 (35.3) | 4 (25) | 4 (25.0) |
| Weight, kg | 97.8 (15.4) | 96.6 (13.3) | 93.6 (13.4) | 99.1 (21.9) |
| BMI, kg/m2 | 31.7 (3.3) | 32.7 (5.1) | 30.9 (3.4) | 31.5 (5.9) |
| Body fat, % | 34.9 (5.5) | 38.6 (8.6) | 34.9 (6.2) | 34.9 (7.4) |
| Waist circumference, cm | 114.1 (9.1) | 109.5 (7.6) | 109.2 (7.9) | 113.8 (18.8) |
| Diabetes duration, years | 8.0 [5.5,13.5] | 10.0 [6,18] | 7.0 [5,12.8] | 9.5 [7,10.5] |
| Fasting glucose, mmol/L | 8.7 (1.5) | 9.9 (1.9) | 10.7 (3.6) | 9.5 (3.0) |
| HbA1c, % | 7.8 (0.6) | 7.9 (0.8) | 8.0 (1.3) | 8.0 (0.95) |
| HbA1c, mmol/mol | 61.3 (6.1) | 65.0 (11.1) | 63.5 (14.5) | 64.7 (11.7) |
| Total cholesterol, mmol/L | 4.2 (0.2) | 4.4 (0.3) | 4.5 (0.3) | 4.1 (0.2) |
| Triglycerides, mmol/L | 1.8 (0.2) | 2.0 (0.2) | 2.9 (0.8) | 2.0 (0.2) |
| SBP, mmHg | 136.4 (10.7) | 132.1 (11.1) | 130.3 (10.8) | 132.6 (13.2) |
| DBP, mmHg | 80.8 (5.8) | 81.0 (7.2) | 79.7 (6.8) | 81.3 (6.8) |
| MAP, mmHg | 99.4 (5.2) | 98.1 (7.7) | 96.6 (7.8) | 98.3 (7.8) |
| HR, bpm | 64.6 (11.1) | 71.0 (9.8) | 71.4 (7.9) | 68.5 (9.6) |
| eGFR, mL/min/1.73 m2 | 83.4 (14.6) | 83.2 (13.7) | 88.8 (10.6) | 87.8 (11.2) |
| Use of, n (%) | ||||
| Metformin | 16 (100) | 17 (100) | 16 (100) | 16 (100) |
| SU derivative | 5 (31.3) | 6 (35.3) | 3 (18.8) | 8 (50.0) |
| Beta blocker | 4 (25.0) | 4 (23.5) | 3 (18.8) | 2 (12.5) |
| Statin | 12 (75.0) | 14 (82.4) | 12 (75.0) | 14 (87.5) |
| Anti-coagulant | 4 (25.0) | 4 (23.5) | 5 (31.3) | 1 (6.3) |
| RAS inhibition | 5 (31.3) | 12 (70.6) | 10 (66.7) | 9 (56.3) |
| ACE inhibitor | 2 (40.0) | 8 (47.1) | 7 (70.0) | 6 (66.7) |
| ARB | 3 (60.0) | 4 (23.5) | 3 (30.0) | 3 (30.0) |
Data are means (± SD) or median [interquartile range] for continuous metrics, and number (percent) for categorical characteristics. Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin-II receptor blocker, BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HR, heart rate (beats per minute); MAP, mean arterial pressure; SBP, systolic blood pressure; SU, sulfonylurea.
Glycemic Control, Anthropometrics, and Resting Energy Expenditure
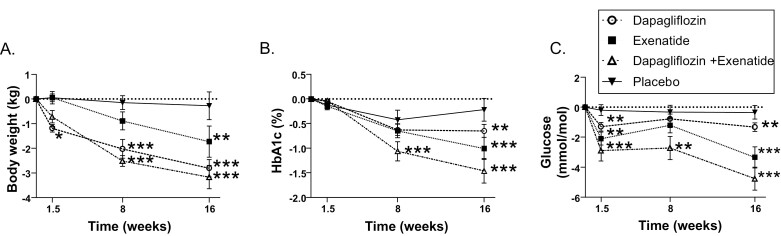
Compared with placebo, 16 weeks of dapagliflozin reduced HbA1c by 0.5 ± 0.19 % (5 mmol/mol; P = 0.009), and exenatide reduced HbA1c by 0.8 ± 0.18% (8.4 mmol/mol; P < 0.001). The combination group showed the largest, almost additive, reduction in HbA1c; −1.2 ± 0.19% (11.9 mmol/mol; P < 0.001) [Fig. 1A].
Figure 1.
Treatment-induced changes in body weight and glucose. Changes in A, body weight; B, HbA1c%; and C, fasting glucose after dapagliflozin (dashed line, white circle), exenatide (dotted line, black square), dapagliflozin and exenatide (dash-dotted line, upward white triangle), and placebo (black line, downward black triangle) after 10 days, 8 weeks, and 16 weeks of treatment. Data points represent mean with SEM. Statistically significant mean differences of treatments compared with placebo corrected for baseline values are indicated as * (P < 0.05), ** (P < 0.01), *** (P < 0.001).
After 10 days of treatment, a reduction in body weight was already observed in the dapagliflozin group compared with placebo (−1.23 ± 0.52 kg; P = 0.02). With the other treatments, no significant changes in body weight were observed after 10 days.
After 16 weeks of treatment compared with placebo, dapagliflozin and exenatide reduced body weight by −2.5 ± 0.5 kg (P < 0.001) and −1.4 ± 0.5 kg (P = 0.008) respectively. After 16 weeks of treatment, weight loss was largest in the combination group (−2.8 ± 0.5 kg; P < 0.001) compared with placebo [Fig. 1B].
Body fat and waist circumference were reduced in all groups after 16 weeks of treatment compared with placebo, with the largest reductions in the combination group (Supplemental Table 1 (30)).
There were no differences in resting energy expenditure or respiratory quotient at all the time points (Supplemental Table 1 (30)).
Ad Libitum Lunch Buffet and Appetite-Related Scores
There was no significant difference in mean energy intake at the ad libitum lunch buffet between the groups after 10 days or 16 weeks of treatment. However, there was a significant increase in carbohydrate intake after 16 weeks in the dapagliflozin group (21.3 ± 9.6 grams; P = 0.029).
After 10 days, compared with placebo, appetite-related scores before the ad libitum lunch buffet tended to increase with dapagliflozin, (+5 points, 10%; P = 0.1). In addition, after 16 weeks of treatment appetite-related scores after lunch were higher in the dapagliflozin group (+4.4 points, 8.8%; P = 0.025) compared with placebo. However, neither exenatide nor dapagliflozin plus exenatide showed any effect on appetite-related scores (Supplemental Table 1 (30)).
CNS Response to Food Pictures
Dapagliflozin
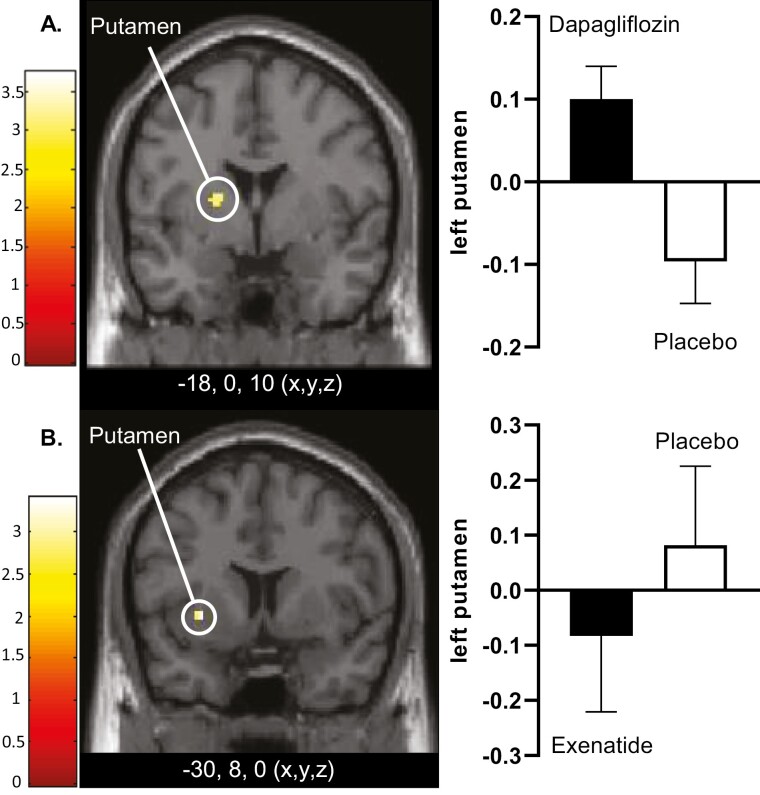
To investigate the effects of an SGLT2i on CNS activation in response to food pictures, dapagliflozin was compared with placebo. After 10 days of treatment, dapagliflozin was associated with increased activity in response to high-calorie food pictures in the left putamen (T = 3.4; P = 0.014) (Fig. 2A, Supplemental Table 2 (30)). After 16 weeks, no significant differences between dapagliflozin and placebo were observed.
Figure 2.
Differences in CNS activation after 10 days of treatment. A, Axial slice showing average differences between dapagliflozin and placebo in left putamen (PFWE < 0.01), T = 3.2) (yellow dot) in response to the viewing of high-calorie vs nonfood pictures. B, Axial slice showing average differences between exenatide and placebo in left putamen (PFWE < 0.05, T = 3.4) (yellow dot) in response to the viewing of high-calorie food pictures vs nonfood pictures. The left side of the axial slices is the left side of the brain. The color scale reflects the T value of the functional activity. Results are presented at the threshold of P < 0.05, familywise error (FWE) corrected for cluster extent. In the graphs, BOLD signal intensity (effect size) is plotted (arbitrary units), mean and SEM. The x,y,z, describes the coordinates of the peak voxel of the observed difference in MNI (Montreal Neurological Institute) space. Abbreviation: BOLD, blood oxygen level–dependent.
Dapagliflozin plus Exenatide
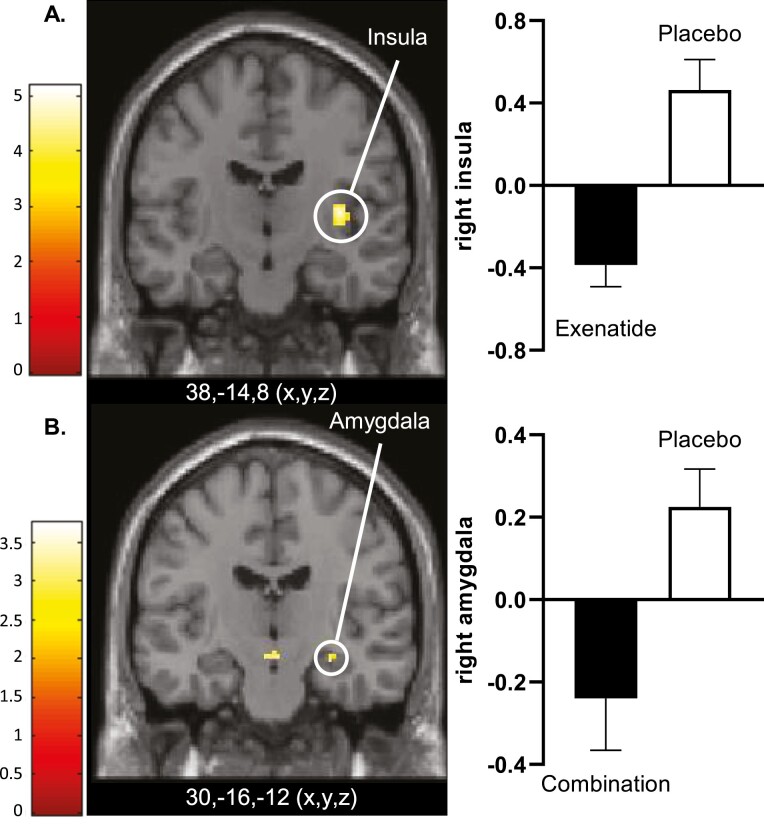
To investigate the effects of combining a GLP-1RA with an SGLT2i on activation in CNS, dapagliflozin plus exenatide was compared with placebo. After 10 days of treatment, no changes in CNS responses were observed in the combination group compared with placebo. After 16 weeks of treatment, dapagliflozin-exenatide treatment was associated with decreased activation in response to food pictures in the right amygdala (T = 3.0; P = 0.032) (Fig. 3B), and trend wise decreased activation in left amygdala (T = 2.8; P = 0.056) compared with placebo. In response to high-calorie food pictures, combination treatment tended to reduce activation in the right insula (T = 3.3; P = 0.07).
Figure 3.
Differences in CNS activation after 16 weeks of treatment. A, Axial slice showing average differences between exenatide and placebo in right insula (PFWE < 0.001, T = 4.7) in response to the viewing of food vs nonfood pictures. B, Axial slice showing average differences between dapagliflozin plus exenatide (combination) and placebo in right amygdala (PFWE < 0.05, T = 2.8) in response to the viewing of food vs nonfood pictures. The left side of the axial slices is the left side of the brain. The color scale reflects the T value of the functional activity. Results are presented at the threshold of P < 0.05, familywise error (FWE) corrected for cluster extent. In the graphs, BOLD signal intensity (effect size) is plotted (arbitrary units), mean and SEM. Abbreviation: BOLD, blood oxygen level–dependent.
Additional Analyses
Exenatide-dapagliflozin vs dapagliflozin
To further explore the effect of combining exenatide with dapagliflozin, dapagliflozin plus exenatide was compared with dapagliflozin therapy. Dapagliflozin-exenatide compared with dapagliflozin tended to decrease activity in response to food and high-calorie food pictures in the right insula (food, T = 3.4; P = 0.052; high-calorie, T = 3.2; P = 0.1).
Exenatide
To corroborate the previously demonstrated GLP-1RA induced changes in CNS activation in response to food pictures, exenatide was compared with placebo. After 10 days of treatment, exenatide treatment was associated with decreased activity in response to high-calorie food pictures in the left putamen (T = 3.6; P = 0.011) (Fig. 2B, Supplemental Table 2 (30)). After 16 weeks, exenatide compared with placebo showed decreased activation in response to food pictures in right insula (T = 5.0; P < 0.001) (Fig. 3A). In addition, exenatide compared with placebo showed reduced activation in bilateral insula (right, T = 4.3; P = 0.003; left, T = 3.8; P = 0.016), and nonsignificantly in right putamen (T = 3.2; P = 0.083), and left rolandic operculum (T = 3.3; P = 0.07) in response to high-calorie food pictures (Supplemental Table 2 (30)).
Correlations between CNS activation and clinical parameters
There were no significant correlations between the changes in brain responses and changes in body weight, fat percentage, HbA1c, glucose, or carbohydrate intake.
Adverse Events
All treatments were generally well tolerated, with slightly more adverse events in the combination group; however, this was not statistically significant (P = 0.11). There was 1 one hypoglycemic event in the exenatide group after 8 weeks of treatment. There were no serious adverse events. Two participants dropped out, 1 patient in the placebo group because of personal reasons, and 1 patient in the combination group because of ongoing nausea (Supplemental Table 3 (30)).
Discussion
Previous studies found that obese people with type 2 diabetes have increased CNS activation in response to viewing food pictures, which may be associated with an increased craving for food (5, 10-14, 31). In this double-blind randomized placebo-controlled trial, we are the first to demonstrate that short-term treatment with dapagliflozin further increased CNS activation in the left putamen in response to the viewing of food pictures. Furthermore, dapagliflozin increased carbohydrate intake and appetite after a meal. Similar to previous studies, we found that exenatide decreased CNS activation in response to viewing food pictures in the putamen after short-term treatment and in bilateral insula after longer-term treatment. Importantly, after adding exenatide to dapagliflozin, the dapagliflozin-induced increase in CNS activation was blunted and even a decreased activation in the amygdala was found after longer-term treatment.
The dapagliflozin-induced increase in CNS activation in the putamen and the increase in carbohydrate intake and appetite scores may explain the discrepancy between the observed and expected weight loss with SGLT2i treatments. The putamen is part of a complex reward circuitry (9). It is engaged in reward processing and conditioning, and activation in the putamen is associated with future weight gain (8, 11).
We could not distinguish between direct or indirect effects of dapagliflozin on the CNS. The sodium-glucose cotransporters (including SGLT1 and SGLT2) are active in the intestine and kidney but are also found in the brain (32-34). However, the expression of SGLT2 in brain is low and therefore its physiological function remains questionable (35). Although dapagliflozin has a 1400-fold higher affinity for SGLT2 (36), it could potentially also affect SGLT1. SGLT1 is expressed in neurons throughout the brain, showing high expression in regions that are involved in learning, regulation of feeding behavior, energy expenditure and glucose homeostasis (35). Support for direct effects of SLGT2i on hyperphagia comes from a study in rats that demonstrated that central administration of tofogliflozin, empagliflozin and dapagliflozin increased food intake (20). On the other hand, indirect effects to compensate for the chronic calorie loss, such as metabolic adaptations and afferent neuronal signals, may also play a role. Research to further investigate the exact mechanism of how SGLT2 inhibition affects the CNS is needed.
After longer-term treatment with dapagliflozin, we did not detect an increase in CNS activation in the ROIs anymore. It has previously been shown that weight reduction per se may be associated with decreased CNS activation in areas involved in food motivation and reward in response to food pictures (37). Therefore, weight loss may have blunted dapagliflozin’s enhancing effects on CNS activation after 16 weeks of treatment.
There may also be other reasons for not finding an increase in CNS activation after longer-term treatment. In healthy lean individuals, insulin functions as a negative feedback signal in the regulation of reward-related food intake, resulting in reduced food-cue reactivity. However, in people with obesity, this response to insulin is impaired, reflecting brain insulin resistance (38). A recent study found that, in people with prediabetes, 8 weeks treatment with empagliflozin improved insulin sensitivity in the hypothalamus, and that the improvements in hypothalamic insulin sensitivity mediated the effects on fasting glucose, and the observed reductions in liver fat (39). Therefore, it could be hypothesized that we found no increase in CNS activation in response to food cues after longer-term treatment due to improved insulin sensitivity. This improved insulin sensitivity is unlikely to explain the difference between the observed and expected weight loss with SGLT2i, but adaptive mechanisms may be important after longer-term treatment. Consistent energy loss through the urine may trigger an anabolic response by which enhanced appetite and (carbohydrate) craving partially offset glycosuria and defend body weight (18, 40). For example, compensatory changes in circulating mediators of appetite that encourage weight regain after diet-induced weight loss have been demonstrated after 10 weeks and 1 year (41). It may be hypothesized that the dapagliflozin-induced increase in CNS activation preceded and induced the increase in appetite and carbohydrate intake. However, other adaptive mechanisms (such as changes in hormones/peripheral vagal nerves) may have contributed to the maintenance of the less than expected weight loss.
It is unlikely that differences in glucose levels explain the effects of dapagliflozin. Higher levels of glucose were found to be associated with increased activity in insula, putamen, and prefrontal cortex in response to food pictures, only in obese subjects (42). In the present study, we found an increase in CNS activation in response to food pictures after 10 days of treatment in the dapagliflozin group, but not after 16 weeks. The difference between 10 days and 16 weeks is unlikely to be explained by changes in glucose, as fasting glucose levels were similarly reduced at both time points (−1.6 ± 0.5 mmol/L after 10 days and −1.5 ± 0.5 mmol/L after 16 weeks). In addition, there was no significant correlation between changes in CNS activation and changes in fasting plasma glucose or HbA1c.
After short-term treatment with dapagliflozin plus exenatide, we did not observe dapagliflozin-induced increases in CNS activation, presumably due to activation reducing effects of exenatide. After longer-term treatment, combination therapy even reduced CNS activation in the amygdala and tended to reduce activation in the insula, again suggesting a combined effect (no effect of dapagliflozin and reducing effect of exenatide). The insula receives gustatory and visceral afferents, and it is involved in taste memory and the rewarding aspects of food. The insula modulates the activity of the other brain regions, based on homeostatic signals, and translates these internal signals into subjective feelings such as the urge to eat (9). The amygdala is involved in the association of cues with reward and emotional learning (9). These findings indicate that exenatide has beneficial effects on the CNS responses to food and prevents the increased CNS activation observed with dapagliflozin monotherapy.
The reduced CNS activation in the putamen with exenatide after short-term treatment is in line with short-term effects of GLP-1RA in our previous study (13), in which liraglutide reduced activity primarily in insula and putamen after 10 days, but not after longer-term treatment. In our current study, we also found that exenatide decreased CNS activation in bilateral insula after longer-term treatment. This discrepancy between these results could be explained by differences in study design. In the previous study, we used liraglutide, which is a long-acting GLP-1RA, inducing receptor tachyphylaxis. The short-acting exenatide in the current study induces tachyphylaxis to a lesser extent (43). In addition, liraglutide in the previous study was compared with an active comparator (insulin glargine) and not with placebo, which could also have contributed to the different findings after longer-term treatment. With respect to the effects of exenatide, we have previously demonstrated, using a somatostatin pancreatic pituitary clamp, that the effects of GLP-1RA on the CNS responses to food stimuli were independent of changes in glucose (5).
Different routes of action may be involved in the observed effects of exenatide. As exenatide can cross the blood brain barrier (44, 45), it may exert its effects on the CNS via direct activation of central GLP-1 receptors (46, 47). However, indirect routes of action, involving intestinal vagal afferents, where GLP-1 receptors are present, may also be involved (48, 49).
It could be argued that the exenatide-induced reductions in CNS activation after 16 weeks may be explained by reductions in CNS responses due to body weight reduction (37). However, we did already find a decreasing effect of exenatide before significant weight changes occurred after 10 days of treatment. Also, there was no correlation between CNS activation and change in body weight. These findings therefore suggest that the effects of exenatide are independent of weight changes.
Despite the effects of treatments on the CNS, we observed no overall changes in caloric intake of the ad libitum lunch buffet in all treatment groups. Interestingly, in an exploratory analysis we did find an increase in carbohydrate intake with dapagliflozin treatment. This finding is in line with a previous study in type 1 diabetes patients treated with empagliflozin for 8 weeks in which an increase in carbohydrate intake was observed (50). This finding was also comparable to another study, in which people with prediabetes were treated with empagliflozin for 8 weeks (39). Although the increase in carbohydrate intake in that study was not statistically significant, the effect size was comparable to the current study (39). Only a few studies investigated the effect of SLGT2is on calorie intake, all with different methods of assessing food intake and reporting conflicting results, which makes them difficult to compare (51-53). In the current study, the assessment of energy intake with an ad libitum lunch buffet in a hospital setting, could have increased intake due to a long period of fasting, or decreased intake due to social desirability. Nevertheless, the increase in carbohydrate intake together with changes in CNS responses to visual food cues, and increased appetite scores support the hypothesis that compensatory hyperphagia may explain the difference between observed and expected weight loss with SGLT2i treatment.
In the current study, significant weight loss was observed in all active treatment groups, with the largest weight loss in the combination group. These results are comparable to the previous studies (23-25), although weight loss in our combination group was slightly less, possibly because our study was of shorter duration and short-acting exenatide was used.
Although the double-blind, double-dummy, randomized 4-armed design is a major strength of this study, there are some limitations. The study was designed with > 85% power to detect the expected difference between treatment groups in the primary endpoint CNS responses to food pictures. However, for several secondary outcomes, such as appetite and food intake, the sample size was probably too small. The study population consisted of relatively older obese participants with type 2 diabetes, without severe comorbidities. In addition, although we included men and postmenopausal women, (younger) women are relatively underrepresented. Therefore, our findings are limited to this specific population and cannot be generalized to other populations.
In conclusion, the increase in CNS activation in feeding regulation areas in response to food stimuli, after short-term treatment, together with changes in appetite and dietary intake, may have contributed to the discrepancy between the observed and expected weight loss with SGLT2i treatment. In addition, exenatide may mitigate the dapagliflozin-induced increase in CNS activation. These findings provide further insights in the weight-lowering mechanisms of SGLT2i and GLP-1RAs and may lead to further development of treatment strategies for obesity and type 2 diabetes.
Acknowledgments
The authors thank Ton Schweigmann (Department of Radiology and Nuclear Medicine, VU University Medical Center) Renée de Meijer, Jeanette Boerop, and Ingrid Knuffman for their assistance during the test visits, as well as the participants who participated in this study.
Glossary
Abbreviations
- BOLD
blood-oxygen-level-dependent
- CNS
central nervous system
- fMRI
functional magnetic resonance imaging
- FWE
family-wise error
- GLP-1RA
glucagon-like peptide 1 receptor agonist
- HbA1c
glycated hemoglobin A1c
- MRI
magnetic resonance imaging
- ROI
region of interest
- SEM
standard error of the mean
- SGLT2i
sodium glucose cotransporter 2 inhibitor
Financial Support
This work was funded by an investigator-initiated grant from AstraZeneca (ESR-16-11865). The funder had no role in the study design, data analyses or interpretation, or drafting of the manuscript, nor in the decision to submit the manuscript for publication.
Author Contributions
C.C.v.R. designed the study, conducted the experiments, performed the data analysis, and wrote the article. D.J.V. designed the fMRI paradigm, performed the data analysis, and contributed to writing the article. L.v.B. designed the fMRI paradigm and contributed to writing the article. A.S. performed the processing of (functional) MRI scans and contributed to the writing of the article. F.B. performed the analyses of all structural MRI scans and contributed to the writing of the article. M.H.H.K contributed to writing the article. M.N. contributed to writing the article. R.G.IJ. designed the study and the fMRI paradigm, performed the data analysis, and wrote the manuscript. All authors have seen and approved the final version of the manuscript. C.C.v.R. and R.G.IJ. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Clinical Trial Information
ClinicalTrials.gov registration no. NCT03361098
Disclosures
R.G.IJ. is principal investigator of studies sponsored by research grants from AstraZeneca, Eli Lilly & Co., and Novo Nordisk. A.S. was funded by Veni grant (016196153) from the Netherlands Organization for Health Research and Development (ZonMw). Trough M.H.H.K., the Amsterdam University Medical Centers, location VUmc, received research grants from AstraZeneca, Boehringer Ingelheim, Novo Nordisk, and Sanofi-Aventis. M.N. is supported by a personal ZONMW VICI grant 2020 (09150182010020) and received an unrestricted grant from AstraZeneca and serves on the Scientific Advisory Board of Caelus Pharmaceuticals, the Netherlands, and Kaleido, USA. All authors received no personal payments in connection with the abovementioned activities, all payments were directly transferred to the nonprofit Amsterdam UMC. C.C.v.R., D.J.V., and L.v.B. have no conflict of interest.
Data Availability
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement by the corresponding author. Data requests can be submitted by email from 3 months after publication of this report and data will be made accessible for 24 months, with possible extensions considered.
References
- 1. Volkow ND, Wise RA, Baler R. The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci. 2017;18(12):741-752. [DOI] [PubMed] [Google Scholar]
- 2. Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE. The contribution of brain reward circuits to the obesity epidemic. Neurosci Biobehav Rev. 2013;37(9 Pt A):2047-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burger KS, Berner LA. A functional neuroimaging review of obesity, appetitive hormones and ingestive behavior. Physiol Behav. 2014;136:121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Bloemendaal L, RG IJ, Ten Kulve JS, et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63(12):4186-4196. [DOI] [PubMed] [Google Scholar]
- 6. Stice E, Yokum S. Neural vulnerability factors that increase risk for future weight gain. Psychol Bull. 2016;142(5):447-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity (Silver Spring). 2011;19(9):1775-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murdaugh DL, Cox JE, Cook EW 3rd, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59(3):2709-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen J, Papies EK, Barsalou LW. A core eating network and its modulations underlie diverse eating phenomena. Brain Cogn. 2016;110:20-42. [DOI] [PubMed] [Google Scholar]
- 10. Stoeckel LE, Weller RE, Cook EW 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41(2):636-647. [DOI] [PubMed] [Google Scholar]
- 11. Rothemund Y, Preuschhof C, Bohner G, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37(2):410-421. [DOI] [PubMed] [Google Scholar]
- 12. Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117(4):924-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ten Kulve JS, Veltman DJ, van Bloemendaal L, et al. Liraglutide reduces CNS activation in response to visual food cues only after short-term treatment in patients with type 2 diabetes. Diabetes Care. 2016;39(2):214-221. [DOI] [PubMed] [Google Scholar]
- 14. Ten Kulve JS, Veltman DJ, van Bloemendaal L, et al. Endogenous GLP1 and GLP1 analogue alter CNS responses to palatable food consumption. J Endocrinol. 2016;229(1):1-12. [DOI] [PubMed] [Google Scholar]
- 15. Sun F, Chai S, Li L, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss in patients with type 2 diabetes: a systematic review and network meta-analysis. J Diabetes Res. 2015;2015:157201. doi:10.1155/2015/157201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee PC, Ganguly S, Goh SY. Weight loss associated with sodium-glucose cotransporter-2 inhibition: a review of evidence and underlying mechanisms. Obes Rev. 2018;19(12):1630-1641. [DOI] [PubMed] [Google Scholar]
- 17. Polidori D, Sanghvi A, Seeley RJ, Hall KD. How strongly does appetite counter weight loss? Quantification of the feedback control of human energy intake. Obesity (Silver Spring). 2016;24(11):2289-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1730-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Devenny JJ, Godonis HE, Harvey SJ, Rooney S, Cullen MJ, Pelleymounter MA. Weight loss induced by chronic dapagliflozin treatment is attenuated by compensatory hyperphagia in diet-induced obese (DIO) rats. Obesity (Silver Spring). 2012;20(8):1645-1652. [DOI] [PubMed] [Google Scholar]
- 20. Takeda K, Ono H, Ishikawa K, et al. Central administration of sodium-glucose cotransporter-2 inhibitors increases food intake involving adenosine monophosphate-activated protein kinase phosphorylation in the lateral hypothalamus in healthy rats. BMJ Open Diabetes Res Care. 2021;9(1):e002104. doi:10.1136/bmjdrc-2020-002104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol. 2014;221(1):T1-16. [DOI] [PubMed] [Google Scholar]
- 22. van Baar MJB, van Ruiten CC, Muskiet MHA, van Bloemendaal L, RG IJ, van Raalte DH. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care. 2018;41(8):1543-1556. [DOI] [PubMed] [Google Scholar]
- 23. Frias JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(12):1004-1016. [DOI] [PubMed] [Google Scholar]
- 24. Ludvik B, Frias JP, Tinahones FJ, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(5):370-381. [DOI] [PubMed] [Google Scholar]
- 25. Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356-367. [DOI] [PubMed] [Google Scholar]
- 26. Esteban O, Markiewicz CJ, Blair RW, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16(1):111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. ten Kulve JS, Veltman DJ, van Bloemendaal L, et al. Endogenous GLP-1 mediates postprandial reductions in activation in central reward and satiety areas in patients with type 2 diabetes. Diabetologia. 2015;58(12):2688-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hill AJ, Rogers PJ, Blundell JE. Techniques for the experimental measurement of human eating behaviour and food intake: a practical guide. Int J Obes Relat Metab Disord. 1995;19(6):361-375. [PubMed] [Google Scholar]
- 29. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109(1-2):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Ruiten C, Veltman DJ, Schrantee A, et al. Supplemental Material_DECREASE study.docx. figshare. January 2022. 10.6084/m9.figshare.18053435.v1 [DOI] [Google Scholar]
- 31. Stice E, Burger K. Neural vulnerability factors for obesity. Clin Psychol Rev. 2019;68:38-53. doi:10.1016/j.cpr.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91(2):733-794. [DOI] [PubMed] [Google Scholar]
- 33. Yu AS, Hirayama BA, Timbol G, et al. Regional distribution of SGLT activity in rat brain in vivo. Am J Physiol Cell Physiol. 2013;304(3):C240-C247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chiba Y, Sugiyama Y, Nishi N, Nonaka W, Murakami R, Ueno M. Sodium/glucose cotransporter 2 is expressed in choroid plexus epithelial cells and ependymal cells in human and mouse brains. Neuropathology. 2020;40(5):482-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koepsell H. Glucose transporters in brain in health and disease. Pflugers Arch. 2020;472(9):1299-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Bommel EJ, Muskiet MH, Tonneijck L, Kramer MH, Nieuwdorp M, van Raalte DH. SGLT2 inhibition in the diabetic kidney-from mechanisms to clinical outcome. Clin J Am Soc Nephrol. 2017;12(4):700-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pursey KM, Stanwell P, Callister RJ, Brain K, Collins CE, Burrows TL. Neural responses to visual food cues according to weight status: a systematic review of functional magnetic resonance imaging studies. Front Nutr. 2014;1:7. doi:10.3389/fnut.2014.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kullmann S, Kleinridders A, Small DM, et al. Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet Diabetes Endocrinol. 2020;8(6):524-534. [DOI] [PubMed] [Google Scholar]
- 39. Kullmann S, Hummel J, Wagner R, et al. Empagliflozin improves insulin sensitivity of the hypothalamus in humans with prediabetes: a randomized, double-blind, placebo-controlled, phase 2 trial. Diabetes Care. 2022;45(2):398-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brown E, Wilding JPH, Barber TM, Alam U, Cuthbertson DJ. Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: Mechanistic possibilities. Obes Rev. 2019;20(6):816-828. [DOI] [PubMed] [Google Scholar]
- 41. Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597-1604. [DOI] [PubMed] [Google Scholar]
- 42. Belfort-DeAguiar R, Seo D, Lacadie C, et al. Humans with obesity have disordered brain responses to food images during physiological hyperglycemia. Am J Physiol Endocrinol Metab. 2018;314(5):E522-E529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. 2011;60(5):1561-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord. 2003;27(3):313-318. [DOI] [PubMed] [Google Scholar]
- 45. Hunter K, Holscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. doi:10.1186/1471-2202-13-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32(14):4812-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124(10):4473-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Plamboeck A, Veedfald S, Deacon CF, et al. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am J Physiol Gastrointest Liver Physiol. 2013;304(12):G1117-G1127. [DOI] [PubMed] [Google Scholar]
- 49. Abbott CR, Monteiro M, Small CJ, et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044(1):127-131. [DOI] [PubMed] [Google Scholar]
- 50. Perkins BA, Cherney DZ, Partridge H, et al. Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care. 2014;37(5):1480-1483. [DOI] [PubMed] [Google Scholar]
- 51. Bertran E, Berlie HD, Nixon A, Jaber L. Does dapagliflozin affect energy intake and appetite? A randomized, controlled exploratory study in healthy subjects. Clin Pharmacol Drug Dev. 2019;8(1):119-125. [DOI] [PubMed] [Google Scholar]
- 52. Horie I, Abiru N, Hongo R, et al. Increased sugar intake as a form of compensatory hyperphagia in patients with type 2 diabetes under dapagliflozin treatment. Diabetes Res Clin Pract. 2018;135:178-184. [DOI] [PubMed] [Google Scholar]
- 53. Matsuba I, Kanamori A, Takihata M, et al. Canagliflozin increases calorie intake in type 2 diabetes without changing the energy ratio of the three macronutrients: CANA-K Study. Diabetes Technol Ther. 2020;22(3):228-234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement by the corresponding author. Data requests can be submitted by email from 3 months after publication of this report and data will be made accessible for 24 months, with possible extensions considered.