Abstract
Context
Subclinical thyroid dysfunction and anemia are common disorders, and both have increasing prevalence with advancing age.
Objective
The aim of this study was to assess whether levothyroxine treatment leads to a rise in hemoglobin levels in older persons with subclinical hypothyroidism.
Methods
This preplanned combined analysis of 2 randomized controlled trials included community-dwelling persons aged 65 years and older with subclinical hypothyroidism who were randomly assigned to levothyroxine or placebo treatment. The levothyroxine dose was periodically titrated aiming at thyroid stimulating hormone (TSH) level within the reference range, with mock titrations in the placebo group. The main outcome measure was the change in hemoglobin level after 12 months.
Results
Analyses included 669 participants (placebo n = 337, levothyroxine n = 332) with a median age of 75 years (range, 65-97) and mean baseline hemoglobin of 13.8 ± 1.3 g/dL. Although levothyroxine treatment resulted in a reduction in TSH from baseline after 12 months of follow-up compared with placebo, the change in hemoglobin level was not different between the levothyroxine and the placebo groups (−0.03 g/dL [95% CI, −0.16 to 0.11]). Similar results were found in stratified analyses including sex, age, or TSH levels. No difference in change of hemoglobin levels after 12 months was identified in 69 participants with anemia at baseline (−0.33 g/dL [95% CI, −0.87 to 0.21]).
Conclusion
In persons aged 65 years and older with subclinical hypothyroidism, treatment with levothyroxine does not lead to a rise in hemoglobin levels, regardless of the presence of anemia.
Keywords: thyroid, anemia, RCT, subclinical hypothyroidism, older adults
Subclinical thyroid dysfunction and anemia are common disorders, and both have increasing prevalence with advancing age (1-4). The symptoms of both subclinical hypothyroidism and anemia are frequently nonspecific and of similar nature (e.g., fatigue, malaise, shortness of breath, and exercise intolerance).
A number of studies have suggested a potential causal relationship between thyroid dysfunction and hematopoiesis (5-8). Lower hemoglobin levels have been observed in persons with (subclinical) hypothyroidism compared with their euthyroid counterparts and small cohort studies have shown increases in hemoglobin and erythropoietin levels after treatment of overt hypothyroidism (8-17).
We reported earlier the results of an individual participant data meta-analysis using data from 16 independent observational cohort studies including more than 42 000 participants. In participants with both overt and subclinical hypothyroidism, we demonstrated a higher anemia prevalence (18). In addition, reduced thyroid function at baseline was associated with an increased risk of anemia during follow-up. To date, however, there is a lack of experimental evidence of the effect of levothyroxine treatment on hemoglobin levels in older persons with subclinical hypothyroidism.
The aim of this study was to assess whether levothyroxine treatment for subclinical hypothyroidism leads to a rise in hemoglobin levels in older persons. We performed a preplanned secondary outcome analysis of 2 randomized controlled trials on levothyroxine treatment for subclinical hypothyroidism in older persons.
Methods
The current preplanned combined analysis uses data from the TRUST (Thyroid hormone Replacement for Untreated older adults with Subclinical hypothyroidism Trial) trial (19, 20) and the IEMO 80-plus Thyroid trial (21). Both trials were randomized, double-blind, placebo-controlled parallel group trials investigating the potential multimodal effects of levothyroxine treatment for persons with subclinical hypothyroidism aged 65 years and older, and aged 80 years and older, respectively. The identical design, study processes, and infrastructure allowed for a preplanned combined analysis. Consequently, the combined cohorts are presented and analyzed as a single study group throughout the manuscript.
The full study protocols and statistical analysis plans for the TRUST Thyroid trial (19, 20) and the IEMO 80-plus Thyroid trial (21) have been published previously. In short, persons aged 65 years and older (TRUST Thyroid trial) or 80 years and older (IEMO 80-plus Thyroid trial) with a diagnosis of subclinical hypothyroidism, defined as elevated thyrotropin (thyroid stimulating hormone; TSH) levels (≥ 4.6 and ≤ 19.9 mIU/L), measured on at least 2 occasions with an interval between >3 months and 3 years apart, and free thyroxine (fT4) within normal local laboratory ranges, were enrolled. Exclusion criteria included, but were not limited to, the use of levothyroxine, antithyroid medication, clinical diagnosis of dementia, recent hospitalization for major illness, and terminal illness (20). Participants were randomized in a 1:1 ratio to levothyroxine or placebo.
The study medication consisted of levothyroxine sodium tablets and matching placebo tablets taken orally daily. The intervention group started with 50 micrograms daily (or 25 micrograms for participants with body weight < 50 kg or a history of coronary heart disease) and the control group with a matching placebo for 6 to 8 weeks. Two optional up- or down-titrations after 6- to 8-week intervals, and one at 12 months of follow-up ensured adequate treatment while avoiding potential overtreatment, mirroring routine clinical practice. An adaptive mock titration schedule was applied for the placebo group. Participants, general practitioners (GPs) and study personnel remained blinded to treatment allocation and thyroid function test results throughout the study. Ethics approval was obtained from the medical ethics committees and competent authorities in the Netherlands, United Kingdom, Switzerland, and Ireland. Written informed consent was obtained from all participants.
Study Outcome
The predetermined outcome of the present analysis was the change from baseline hemoglobin levels after 12 months of treatment. Hemoglobin data was analyzed from samples collected as part of an ancillary biobank procedure. Hemoglobin (Hb) levels were measured as part of a complete blood count in automated analysis systems in EDTA anticoagulated blood samples and measured within 4 hours of sample collection (the Netherlands: Sysmex XN10/20; Scotland: Sysmex XN10; Switzerland: Siemens Advia; Ireland: Advia 2120i until October 2015, and afterwards Sysmex XN-9000), at baseline and after 12 months. Anemia was defined according to the World Health Organization criteria (Hb < 12 g/dL for women and Hb < 13 g/dL for men) and anemia severity as mild (women: 11.0 ≤ Hb ≤ 11.9 g/dL, men: 11.0 ≤ Hb ≤ 12.9 g/dL), moderate (all: 8.0 ≤ Hb ≤ 10.9 g/dL) or severe (all: Hb < 8.0 g/dL) (22).
Additional Variables
Thyroid function tests (TSH and fT4 levels) were performed by certified medical laboratories at each site: (1) the Netherlands: Elecsys 2010 (Roche), Architect (Abbott), UniCel DxL (Beckman), Immulite 2000XPI (Siemens), or Vitros ECoQ (Ortho Clinical Diagnostics); (2) United Kingdom: Architect (Abbott); (3) Switzerland: Elecsys 2010 (Roche); and (4) Ireland: Cobas 8001 or E601 (Roche) or Architect (Abbott) (20, 21). Data for fT4 levels at 12 months were available for a subsample of participants (total n = 115, United Kingdom n = 75, the Netherlands n = 37, Ireland n = 3). Socio-demographic data (age, sex and race), information on lifestyle factors (smoking and alcohol intake), the presence of prior conditions and medication use (prescribed and over-the-counter) were recorded by research nurses during baseline and follow-up visits. Comorbidity was defined as having a history of one or more of the following diagnoses: myocardial infarction, angina, stroke, transient ischemic attacks, heart failure, peripheral vascular disease, revascularization, atrial fibrillation, hypertension, diabetes mellitus, epilepsy, dementia, osteoporosis, or other disease (20, 21).
Statistical Methods
To investigate cross-sectional associations between thyroid hormones and hemoglobin or anemia status, linear mixed effect regression models and logistic regression models were used, adjusting for possible confounders age and sex at baseline. Treatment effects were calculated using linear mixed effect regression models (differences in the change of hemoglobin levels between baseline and at 12 months) and logistic regression models (odds of developing anemia or resolving anemia during follow-up), adjusting for study site, study, treatment dose at randomization, age, sex, smoking status, and alcohol consumption, with treatment (levothyroxine or placebo) as the independent variable, hemoglobin level or anemia (stratified for sex) as the dependent variable, age and alcohol as covariates, and sex and smoking status as fixed factors. All confounding variables were assessed for statistical interaction with all P-for-interactions above the 0.05 significance threshold.
Subgroup analyses were performed, stratified by sex, age (< 80 vs ≥ 80 years), TSH level by quartiles (4.6 to 5.10 mIU/L, 5.11 to 5.76 mIU/L, 5.77 to 6.99 mIU/L, and 7.00 to 19.9 mIU/L), hemoglobin level by quartiles (men: 9.35 to 13.70, 13.71 to 14.50, 14.51 to 15.20, and >15.21 g/dL; women: 9.67 to 12.73, 12.74 to 13.40, 13.41 to 14.02, and >14.02 g/dL) and the presence of anemia at baseline. Differences in median hemoglobin levels at baseline were analyzed across quartiles of TSH and fT4 using Kruskal-Wallis tests. Analyses of the treatment effects were performed in the modified intention-to-treat (mITT) population, based on participants with data available for hemoglobin levels. A per protocol analysis in participants with uninterrupted use of study medication throughout the studies was included as a sensitivity analysis. Analyses were repeated while excluding participants using concomitant anti-anemic medications (iron supplements, parenteral iron preparations, vitamin B12, folic acid, erythropoietin, or other anti-anemic drugs, n = 47). All data analyses were performed with SPSS Statistics Software version 22.0 for Windows (IBM, Armond, NY, USA). A P value less than 0.05 was considered statistically significant.
Results
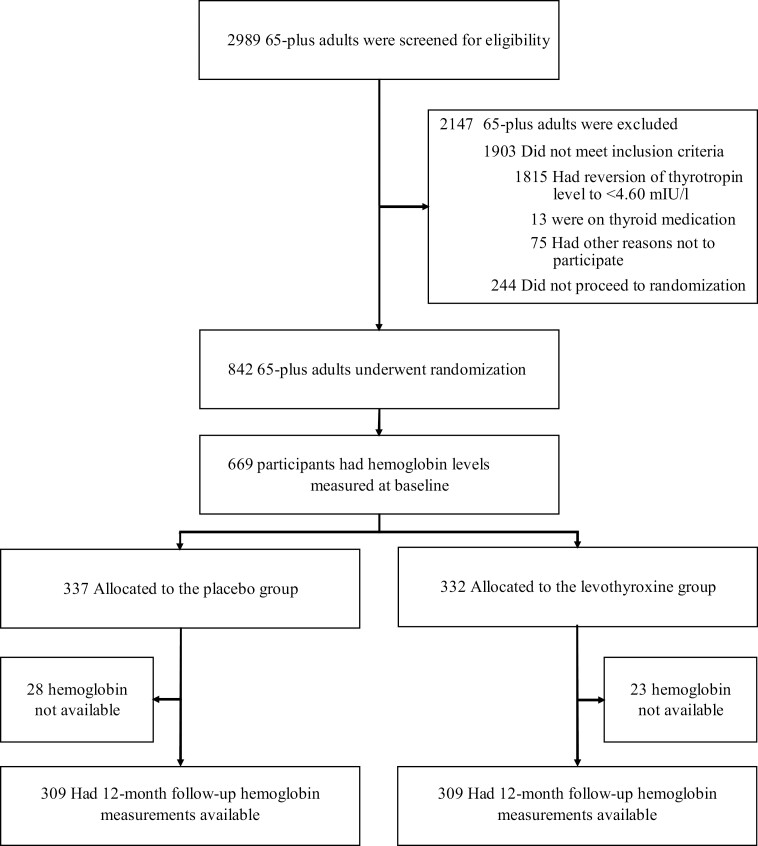
For both studies, 2989 individuals were assessed for eligibility and a total of 842 participants (737 in TRUST, 105 in IEMO) were randomized in the main studies. For the present analysis, 669 participants (592 in TRUST, 77 in IEMO) whose hemoglobin levels were measured in baseline biobank samples were included (placebo n = 337, levothyroxine n = 332, Fig. 1). Table 1 presents the baseline characteristics of the study participants. The median age of the population was 75 years (range, 65-97) in both treatment groups. Men constituted 47.5% and 49.1% of all participants in the placebo and levothyroxine groups, respectively. More than 83% of the study population had one or more comorbid condition. The mean hemoglobin level in the placebo group was 13.8 g/dL (SD 1.3; mean in women 13.4 g/dL [SD 1.1], mean in men 14.3 g/dL [SD 1.3]) and in the levothyroxine group 13.8 g/dL (SD 1.3; mean in women 13.4 g/dL [SD 1.1]; mean in men 14.3 g/dL [SD 1.4]). At baseline, 13.1% of the participants had anemia in the placebo group; 11.4% had anemia in the levothyroxine group. Analysis of the baseline characteristics of the excluded participants that did not have hemoglobin levels available demonstrated minor differences compared with the participants with hemoglobin levels available (Supplemental Table 1) (23).
Figure 1.
Recruitment, randomization, and patient flow of the participants.
Table 1.
Baseline characteristics of the study participants
| Placebo group | Levothyroxine group | |
|---|---|---|
| N | 337 | 332 |
| Socio-demographic characteristics: | ||
| Age, median (range) | 75 (65-97) | 75 (65-93) |
| Male, no. (%) | 160 (47.5) | 163 (49.1) |
| MMSE, median (IQR) | 29 (28-30) | 29 (27-29) |
| Barthel Index, mean (SD) | 19.6 (0.9) | 19.7 (0.9) |
| Instrumental activities of daily living score, mean (SD) | 13.6 (1.1) | 13.7 (0.8) |
| Handgrip strength, kg | 27.6 (11.1) | 28.3 (10.6) |
| Body mass index, kg/m2 | 27.6 (4.4) | 28.1 (5.2) |
| Systolic blood pressure, mmHg | 143.3 (19.3) | 141.6 (19.0) |
| Diastolic blood pressure, mmHg | 75.4 (11.9) | 74.1 (11.6) |
| Current smoker, no. (%) | 24 (7.1) | 25 (7.5) |
| Current alcohol user, no. (%)a | 213 (63.2) | 187 (56.3) |
| Comorbidities, no. (%)b | 280 (83.1) | 282 (84.9) |
| 0-1 | 161 (47.8) | 144 (43.4) |
| 2-4 | 160 (47.5) | 167 (50.3) |
| 4+ | 16 (4.7) | 21 (6.3) |
| Caucasian, no. (%) | 331 (98.2) | 328 (98.8) |
| Concurrent anti-anemic medication use, no. (%)c | 21 (6.2) | 26 (7.8) |
| Thyroid function tests | ||
| TSH, median (IQR), mIU/L | 5.8 (5.1 - 6.8) | 5.8 (5.1 - 7.1) |
| fT4, pmol/L | 13.4 (1.9) | 13.4 (2.0) |
| Hematological parameters | ||
| Hemoglobin, g/dLd | 13.8 (1.3) | 13.8 (1.3) |
| Men | 14.3 (1.3) | 14.3 (1.4) |
| Women | 13.4 (1.1) | 13.4 (1.1) |
| Anemia status, no. (%)e | ||
| No anemia | 293 (86.9) | 294 (88.6) |
| Mild anemia | 36 (10.7) | 31 (9.3) |
| Moderate anemia | 8 (2.4) | 7 (2.1) |
| Severe anemia | 0 (0) | 0 (0) |
Continuous data are presented as mean (SD) and, if stated otherwise, as median (IQR); categorical data are presented as number (percentage).
Abbreviations: fT4, free thyroxine; IQR, interquartile range; MMSE, Mini-Mental State Exam; TSH, thyrotropin (thyroid stimulating hormone);
aDefined as an average of one or more self-reported consumed units of alcohol per week.
bComorbidity was defined as having a history of one or more of the following: myocardial infarction, angina, stroke, transient ischemic attacks, heart failure, peripheral vascular disease, revascularization, atrial fibrillation, hypertension, diabetes mellitus, epilepsy, dementia, osteoporosis, or other.
cConcurrent medication use was defined as using one or more of the following medications: iron supplements, parenteral iron preparations, vitamin B12, folic acid, erythropoietin, or other anti-anemic drugs.
dConversion factor of hemoglobin from g/dL to mmol/L = value*0.6206.
eAnemia defined by World Health Organization categories: women hemoglobin [Hb] < 12.0 g/dL, men Hb < 13.0 g/dL. Anemia severity was graded as: mild (women: 11.0 ≤ Hb ≤ 11.9 g/dL, men: 11.0 ≤ Hb ≤ 12.9 g/dL), moderate (all: 8.0 ≤ Hb ≤ 10.9 g/dL), severe (all: Hb < 8.0 g/dL).
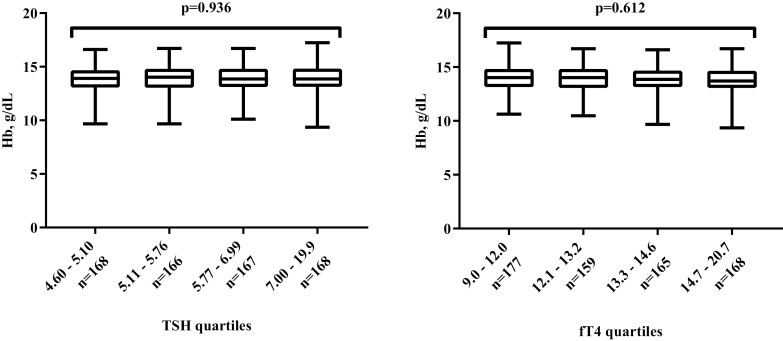
Table 2 shows the relation between thyroid function and hematological parameters at baseline. No association was observed between TSH or fT4 levels and hemoglobin levels in univariable and multivariable regression models. Although a small association between higher fT4 and the odds of having anemia was observed at baseline (odds ratio [OR] 1.13; 95% CI, 1.01 to 1.26; P = 0.041), in the multivariable models’ higher levels of TSH or fT4 were not associated with increased odds of having anemia. Median hemoglobin levels at baseline were comparable across quartiles of TSH and fT4 (Fig. 2).
Table 2.
Baseline associations between thyroid function and hematologic parameters in 669 older participants with subclinical hypothyroidism
| Estimate | 95% CI | P value | |
|---|---|---|---|
| Thyroid function and hemoglobin | Beta | ||
| TSH | |||
| Univariable | −0.01 | −0.06 to 0.04 | 0.649 |
| Multivariable | −0.01 | −0.06 to 0.04 | 0.736 |
| fT4 | |||
| Univariable | −0.05 | −0.10 to 0.00 | 0.065 |
| Multivariable | −0.03 | −0.07 to 0.02 | 0.267 |
| Thyroid function and anemia (n = 82) | Odds ratio | ||
| TSH | |||
| Univariable | 1.04 | 0.93 to 1.16 | 0.523 |
| Multivariable | 1.03 | 0.91 to 1.17 | 0.595 |
| fT4 | |||
| Univariable | 1.13 | 1.01 to 1.26 | 0.041 |
| Multivariable | 1.08 | 0.96 to 1.22 | 0.200 |
Units: TSH mIU/L; fT4 pmol/L. Betas and corresponding confidence intervals were calculated using linear mixed effect regression models and represent the difference in hemoglobin level (g/dL) per unit increase in TSH (mIU/L) or fT4 (pmol/L), Odds ratios with corresponding confidence intervals were calculated using logistic regression models, per unit increase in TSH or fT4. Multivariable models were adjusted for possible confounders age and sex.
Figure 2.
Baseline Hb levels per quartiles of TSH and fT4. Boxplots represent the median, interquartile range, minimum, and maximum in Hb levels per quartile of TSH (mIU/L) and fT4 (pmol/L). Difference in medians were evaluated using the Kruskal-Wallis test.
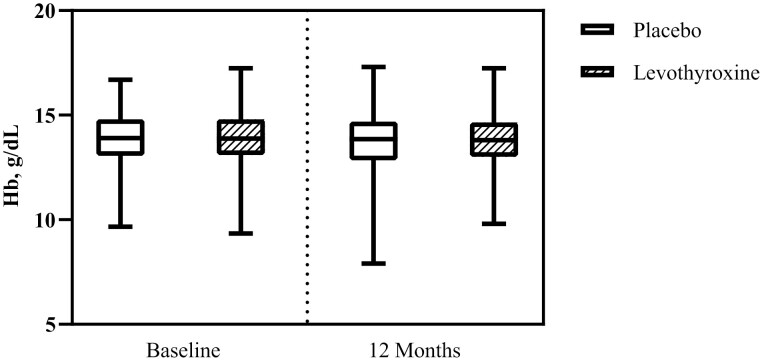
Although significant reductions in TSH (−1.98 mIU/L; 95% CI, −2.30 to −1.66; P < 0.001) and increases in fT4 (2.33; 95% CI, 1.87 to 2.80; P < 0.001) over 12 months of follow-up were observed in the levothyroxine group compared with the placebo group, no differences between the groups were observed in the change in hemoglobin levels after 12 months of follow-up (between-group difference −0.03 g/dL; 95% CI, −0.16 to 0.11; P = 0.703, Fig. 3).
Figure 3.
Levothyroxine treatment effect on Hb levels after 12 months of treatment. Boxplots represent the median, interquartile range, minimum, and maximum in Hb levels. Treatment effect −0.03 g/dL (95% CI, −0.16 to 0.11). Treatment effect (95% CI) was calculated using linear mixed effect regression models adjusting for study site, study, treatment dose at randomization, age, and sex and represents the additional change in hemoglobin level after 12 months of treatment with levothyroxine, compared with placebo.
Results were not substantially different in the per protocol analysis for participants that remained on the assigned treatment after 12 months and had no major protocol violations (hemoglobin treatment effect −0.05 g/dL; 95% CI, −0.19 to 0.10; P = 0.530). Similar results were also found when subgroups were stratified by sex, age, quartiles of TSH levels, or quartiles of hemoglobin levels in men and women. No difference in change of hemoglobin levels after 12 months was identified in 69 participants with anemia at baseline (−0.33 g/dL [95% CI, −0.87 to 0.21]) or in nonanemic participants at baseline (0.01 g/dL [95% CI, −0.12 to 0.15]). (Table 3).
Table 3.
Changes in TSH, fT4, and hemoglobin levels in relevant subgroups in older participants with subclinical hypothyroidism on levothyroxine treatment, compared with placebo
| Baseline | 12-month follow-up | ||||||
|---|---|---|---|---|---|---|---|
| N | Placebo | Levothyroxine | Placebo | Levothyroxine | Treatment effect (95% CI)a | P value | |
| Effect on thyroid hormone levels b | |||||||
| TSH, mIU/Lb | 660 | 6.3 (1.7) | 6.5 (2.1) | 5.5 (2.5) | 3.6 (2.1) | −1.98 (−2.30 to −1.66) | <0.001 |
| fT4, pmol/L b | 177 | 13.4 (1.9) | 13.4 (2.0) | 12.4 (1.7) | 14.7 (2.8) | 2.33 (1.87 to 2.80) | <0.001 |
| Effect on hemoglobin levels | |||||||
| Sex | |||||||
| Men | 298 | 14.3 (1.3) | 14.3 (1.4) | 14.2 (1.5) | 14.2 (1.5) | −0.05 (−0.26 to 0.15) | 0.599 |
| Women | 320 | 13.4 (1.1) | 13.4 (1.1) | 13.4 (1.3) | 13.4 (1.1) | 0.01 (−0.16 to 0.17) | 0.950 |
| Age | |||||||
| < 80 years | 466 | 14.0 (1.2) | 13.9 (1.3) | 14.0 (1.3) | 13.8 (1.3) | −0.06 (−0.20 to 0.08) | 0.412 |
| ≥ 80 years | 152 | 13.4 (1.4) | 13.6 (1.4) | 13.3 (1.5) | 13.5 (1.5) | 0.10 (−0.23 to 0.44) | 0.540 |
| TSH | |||||||
| 4.60 – 5.10 | 157 | 13.8 (1.2) | 13.8 (1.3) | 13.6 (1.3) | 13.6 (1.4) | −0.09 (−0.32 to 0.13) | 0.418 |
| 5.11 – 5.76 | 152 | 13.8 (1.4) | 13.9 (1.2) | 13.8 (1.7) | 13.9 (1.3) | −0.03 (−0.35 to 0.29) | 0.863 |
| 5.77 – 6.99 | 158 | 13.9 (1.2) | 13.8 (1.3) | 13.8 (1.5) | 13.9 (1.4) | 0.16 (−0.08 to 0.41) | 0.188 |
| > 7.0 | 151 | 13.8 (1.3) | 13.8 (1.5) | 13.8 (1.2) | 13.8 (1.3) | −0.13 (−0.40 to 0.14) | 0.336 |
| Hemoglobin quartiles split by sex | |||||||
| Men | |||||||
| 9.35 – 13.70 | 67 | 12.4 (0.8) | 12.3 (1.1) | 12.5 (1.2) | 12.5 (1.4) | −0.26 (−0.95 to 0.44) | 0.455 |
| 13.71 – 14.50 | 93 | 14.2 (0.3) | 14.1 (0.3) | 14.3 (0.7) | 14.0 (0.7) | −0.20 (−0.70 to 0.30) | 0.419 |
| 14.51 – 15.20 | 60 | 15.0 (0.2) | 14.9 (0.2) | 14.4 (1.5) | 14.8 (0.7) | −0.09 (−0.52 to 0.34) | 0.680 |
| 15.21 – 17.24 | 73 | 15.8 (0.4) | 15.8 (0.5) | 15.5 (0.8) | 15.4 (1.1) | 0.03 (−0.30 to 0.35) | 0.877 |
| Women | |||||||
| 9.67 – 12.73 | 79 | 11.9 (0.8) | 12.1 (0.5) | 12.0 (1.3) | 12.1 (0.9) | 0.04 (−0.35 to 0.43) | 0.842 |
| 12.74 – 13.40 | 84 | 13.1 (0.2) | 13.2 (0.2) | 13.0 (0.8) | 13.3 (0.6) | 0.02 (−0.21 to 0.25) | 0.858 |
| 13.41 – 14.02 | 76 | 13.8 (0.2) | 13.7 (0.2) | 13.8 (0.5) | 13.7 (0.7) | −0.19 (−0.55 to 0.18) | 0.317 |
| 14.03 – 16.30 | 81 | 14.7 (0.4) | 14.7 (0.6) | 14.6 (0.9) | 14.4 (0.8) | −0.13 (−0.55 to 0.29) | 0.540 |
| Anemia at baseline | |||||||
| No anemia | 549 | 14.1 (1.0) | 14.1 (1.1) | 14.0 (1.2) | 14.0 (1.2) | 0.01 (−0.12 to 0.15) | 0.886 |
| Anemia | 69 | 11.6 (0.8) | 11.6 (0.8) | 11.9 (1.3) | 11.8 (1.2) | −0.33 (−0.87 to 0.21) | 0.233 |
aTreatment effects were calculated using linear mixed effect regression models adjusting for study site, study, treatment dose at randomization, age and sex and represent the additional change in hemoglobin level (g/dL) after 12 months of treatment with levothyroxine, compared with placebo. In stratified analysis the stratifying variable was not an adjusting variable for that analysis.
bTreatment effects represent the additional change in TSH (mIU/L) or fT4 (pmol/L) after 12 months of treatment with levothyroxine, compared with placebo. Anemia defined by World Health Organization categories: Women hemoglobin (Hb) < 12.0 g/dL, men Hb < 13.0 g/dL.
In the 618 (92.4%) participants for whom hemoglobin measurements were available at 12 months, levothyroxine treatment was not associated with decreased odds of developing anemia (placebo n = 17/272, levothyroxine n = 19/277, OR 1.16 [95% CI, 0.59 to 2.29], P = 0.675) or increased odds of resolution of anemia (placebo n = 11/37, levothyroxine n = 7/32, OR 1.80 [95% CI, 0.54 to 5.93], P = 0.337). Similar results were found in sensitivity analyses restricted to participants with anemia without anti-anemic medication (data not shown).
No confounding variables in any of the models demonstrated significant statistical interaction (data not shown).
Discussion
In this combined analysis of 2 randomized trials of older adults with a diagnosis of subclinical hypothyroidism, levothyroxine treatment for 12 months was not associated with an increase in hemoglobin levels. Additionally, no changes in hemoglobin levels were observed in relevant subgroup analyses including sex, age, and baseline hemoglobin levels. No baseline associations were identified between TSH and hemoglobin levels or the presence of anemia. A clinically insignificant increased odds of having anemia at baseline was identified for fT4 in the univariable analysis, which was no longer present when correcting for the influence of sex and age, and it is regarded as a chance finding.
These findings are in contrast with some earlier observational studies (8-10, 13-18), but in line with a few prospective cohort studies and a recent systematic review (24, 25). In some (meta-analyses of) observational studies, associations between hemoglobin and levothyroxine treatment are found in patients with subclinical hypothyroidism, but these are lacking in our experimental study. This paradoxical discrepancy is not fully understood. One hypothetical explanation may be that despite using data from the largest randomized controlled trials (RCTs) to date on levothyroxine treatment for subclinical hypothyroidism in community-dwelling older adults with 12 months of follow-up, the analyses may still have suffered from a lack of power. Alternatively, hypothetical explanations for the observational-experimental paradox may include an overestimation of exposure-outcome associations in observational studies, publication bias for more pronounced observational results, or increased heterogeneity in observational research that introduces confounding (ie, wider spectra of inclusion criteria, comorbidities, disease severity, or treatment modalities). Still, a more likely explanation for the lack of experimental association could be that subclinical hypothyroidism and anemia may not actually be causally related. For instance, in an earlier meta-analysis of observational data, a clear association was found between subclinical hypothyroidism and anemia cross-sectionally. This association remained in the longitudinal analyses, hinting at possible causal pathways, although less pronounced (18). In order to determine whether any potential associations were indeed causal in nature, experimental data were required. The results from this study, however, do not support the causal hypothesis. In line with earlier results from the TRUST and IEMO trials, in which no beneficial effects of levothyroxine treatment were demonstrated for a range of clinically relevant outcomes, including thyroid-specific and generic quality of life, grip strength, blood pressure, and body mass index (19, 26), the lack of a beneficial effect on hemoglobin levels is an added finding suggesting a limited clinical value of treating subclinical hypothyroidism with levothyroxine monotherapy in older persons.
Interestingly, Christ-Crain and colleagues observed increases in erythropoietin levels upon levothyroxine treatment in a small placebo-controlled RCT of women (mean age 59 years [SD 1]) with subclinical hypothyroidism, while hemoglobin levels and hematocrit remained unchanged after 48 weeks of treatment (27). One may hypothesize that effects of thyroid function on hemoglobin levels may only become apparent in those with overt hypothyroidism or severe anemia (5-7, 13, 28-30). Indeed, a number of studies have shown a beneficial effect of thyroid hormone treatment in patients with overt hypothyroidism on erythropoietin levels (14, 16, 17). Only one prior published experimental study evaluated change in hemoglobin levels in subclinically hypothyroid patients with iron-deficiency after levothyroxine supplementation (31). However, in this study participants were randomized to simultaneous levothyroxine and iron supplementation for the treatment group and compared with iron supplementation alone for the control group. To our knowledge, the current study is the first to investigate the effect of levothyroxine supplementation alone on hemoglobin levels in older adults with subclinical hypothyroidism. The absence of change in hemoglobin levels in our study—even in those with the lowest hemoglobin levels—may suggest that in older persons with subclinical hypothyroidism hematopoietic processes are quite robust to changes such as levothyroxine treatment.
The present study used data from the largest RCTs to date on levothyroxine treatment for subclinical hypothyroidism in community-dwelling older adults with 12 months of follow-up, but some limitations must be acknowledged. First, despite the sample size of these RCTs, few participants (n = 21) had a baseline TSH level of more than 10 mIU/L, that is, the upper end of the subclinical hypothyroid spectrum, in which a few earlier studies identified additional risks of unwanted health effects (2, 32, 33). The majority of participants in our RCTs had mild subclinical hypothyroidism (TSH between 4.6 and 10.0 mIU/L). Second, the number of participants with anemia at baseline was rather low, leading to insufficient power to study the effects of levothyroxine on hemoglobin in those with anemia. However, an absence of treatment effect was consistent across stratifications based on quartiles of hemoglobin levels at baseline. Some degree of missing data is not uncommon for RCTs in adults aged 65 years and over (eg, due to errors in laboratory measurements, consent withdrawal from additional blood analyses, measurements outside of the predetermined protocol window, withdrawal from follow-up, or death). Minor differences in baseline characteristics between the trial participants with hemoglobin measurements available and those without hemoglobin measurements can be identified, but it is not to be expected that any treatment effects would be different if these participants had been included. Moreover, any missing data are believed to be missing at random across the treatment groups, so as not to have unbalanced the analyses. Third, fT4 levels were not routinely measured at 12 months and were only available in a subset of participants. Still, these results illustrate that, apart from persistently lowering TSH, levothyroxine treatment did result in a significant increase in fT4. The lack of effect of levothyroxine on hemoglobin levels in this study can therefore not be explained by a lack of increase in thyroid hormone function. Fourth, additional markers of erythropoiesis or other potential causes of anemia such as ferritin, iron, folate, vitamin B12, or kidney function, were not available, restricting further exploration of underlying pathophysiological mechanisms. Fifth, although sensitivity analyses were performed excluding those using anti-anemic medication such as iron, vitamin B12, or erythropoietin, no information was available on blood transfusions or venesection.
In conclusion, treatment with levothyroxine does not improve hemoglobin levels in individuals aged 65 years and older with a diagnosis of subclinical hypothyroidism. Whether anemia in patients with more marked hypothyroidism is responsive to treatment with levothyroxine needs further experimental studies.
Acknowledgments
The authors would like to thank all the participants who participated in the TRUST Thyroid Trial and IEMO 80-plus Thyroid Trial; the physicians, nurses and administrative staff at each study site; the general practitioners and laboratories that helped to recruit participants; members of the Independent Data Monitoring Committee (Prof. Gary Ford, Prof. Thompson, G. Robinson, Prof. Colin Dayan, and Prof. Kathleen Bennett); members of the study Endpoint Committee (Prof. Peter Langhorne, Prof. J. Wouter Jukema, Dr. Tinh-Hai Collet, Prof. Olaf M. Dekkers, and Dr. Anne Marie O’Flynn); members of the TRUST/IEMO Biobank Committee (Prof. Patricia M. Kearney, Prof. Naveed Satar, Dr. Manuel R. Blum, Dr. Nathalie Schwab, and Dr. Wendy P.J. den Elzen); the staff of Mawdsley-Brooks & Co. (UK) responsible for the logistics of handling and distributing the study medication; Merck KGaA for providing the levothyroxine and matching placebos without recompense; and the staff of the Robertson Centre for Biostatistics for providing the electronic data capture and safeguarding.
Glossary
Abbreviations
- fT4
free thyroxine
- Hb
hemoglobin
- IEMO
IEMO 80-plus thyroid trial, NTR3851
- OR
odds ratio
- TRUST
Thyroid hormone Replacement for Untreated older adults with Subclinical hypothyroidism Trial, NCT01660126
- TSH
thyrotropin (thyroid stimulating hormone)
Grants or Fellowships Supporting the Writing of the Paper
The TRUST Thyroid trial was supported by research grant (278148) from the European Union FP7-HEALTH-2011 program. The IEMO 80-plus Thyroid trial was supported by research grant (627001001) from ZonMw under the ZonMw programme Evidence-based Medicine in Old age. Study medication (levothyroxine and matching placebo) was supplied free of charge by Merck KGaA. The sponsors and funding bodies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Author Contributions
R.S.D.P., S.P.M., D.J.S., and W.P.J.d.E. had full access to the study data and manuscript and take responsibility for the integrity of the work as a whole. Study concept and design: J.G., P.M.K., S.P.M., N.R., D.J.S., R.G.J.W. Acquisition, analysis, and interpretation of the data: R.S.D.P., W.P.J.d.E. Drafting of the manuscript: R.S.D.P., W.P.J.d.E. Critical revision of the manuscript for important intellectual content: R.P., S.P.M., D.J.S., T.Q., N.S., R.G.J.W., P.M.K., V.J.C.M., S.B. Funding European Union, Swiss National Science Foundation, and ZonMw: N.R., O.B., T.H.C., J.G., O.M.D., J.W.J., J.W.A.S., D.v.H. Statistical analysis: R.S.D.P., W.P.J.d.E. Obtained funding: J.G., S.P.M., D.J.S., N.R., R.G.J.W., P.M.K. Administrative, technical, or material support: Supervision: J.G., S.P.M., N.R., D.J.S., R.G.J.W.
Disclosures
The authors have nothing to disclose. The sponsors and funding bodies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
N.R. received research grants from the Swiss National Science Foundation (SNSF 320030-150025 and 320030-172676), the Swiss Heart Foundation and an investigator-driven grant Velux Stiftung (grant 974a). T.C. received research grants from the Swiss National Science Foundation (PZ00P3-167826), the Swiss Society of Endocrinology and Diabetes, the Leenaards Foundation, and the Vontobel Foundation. D.v.H. received research grants from the European Union Horizon 2020 research and innovation programme (Thyrage, 666869). All other authors declare no competing interests.
Declaration of Interests
The TRUST Thyroid trial was supported by research grant (278148) from the European Union FP7-HEALTH-2011 program. Dr. Rodondi is supported to study thyroid dysfunction by research grants from the Swiss National Science Foundation (320030-172676 and 32003B_200606 to Prof. Rodondi). Other financial support for the TRUST trial in Switzerland was supplied by grants from the Swiss Heart Foundation (to Dr. Rodondi), and an investigator-driven grant from Velux Stiftung (974a to Dr. Rodondi). The IEMO 80-plus Thyroid trial was supported by research grant (627001001) from ZonMw under the ZonMw programme Evidence-based Medicine in Old age and by grants from the Swiss National Science Foundation (SNSF 320030-150025 and 320030-172676 to Dr. Rodondi). Study medication (levothyroxin and matching placebo) was supplied free of charge by Merck KGaA. Dr Collet’s research is supported by grants from the Swiss National Science Foundation (PZ00P3-167826), the Swiss Society of Endocrinology and Diabetes, the Leenaards Foundation, and the Vontobel Foundation. The research of Dr. van Heemst is supported by a grant from the European Union Horizon 2020 research and innovation programme (Thyrage, 666869). All other authors declare no competing interests. The sponsors and funding bodies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Clinical Trial Information
TRUST: Clinicaltrials.gov: NCT01660126, https://clinicaltrials.gov/ct2/show/NCT01660126
IEMO: Netherlands Trial Register: NTR3851, https://www.trialregister.nl/trial/3681
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379(9821):1142-1154. [DOI] [PubMed] [Google Scholar]
- 2. Rodondi N, den Elzen WP, Bauer DC, et al. . Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beghe C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):3S-10S. [DOI] [PubMed] [Google Scholar]
- 4. Gaskell H, Derry S, Andrew Moore R, McQuay HJ. Prevalence of anaemia in older persons: systematic review. BMC Geriatr. 2008;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fein HG, Rivlin RS. Anemia in thyroid diseases. Med Clin North Am. 1975;59(5):1133-1145. [DOI] [PubMed] [Google Scholar]
- 6. Fandrey J, Pagel H, Frede S, Wolff M, Jelkmann W. Thyroid hormones enhance hypoxia-induced erythropoietin production in vitro. Exp Hematol. 1994;22(3):272-277. [PubMed] [Google Scholar]
- 7. Golde DW, Bersch N, Chopra IJ, Cline MJ. Thyroid hormones stimulate erythropoiesis in vitro. Br J Haematol. 1977;37(2):173-177. [DOI] [PubMed] [Google Scholar]
- 8. Das KC, Mukherjee M, Sarkar TK, Dash RJ, Rastogi GK. Erythropoiesis and erythropoietin in hypo- and hyperthyroidism. J Clin Endocrinol Metab. 1975;40(2):211-220. [DOI] [PubMed] [Google Scholar]
- 9. den Elzen WP, de Craen AJ, Mooijaart SP, Gussekloo J. Low thyroid function and anemia in old age: the Leiden 85-plus study. J Am Geriatr Soc. 2015;63(2):407-409. [DOI] [PubMed] [Google Scholar]
- 10. Evans ES, Rosenberg LL, Simpson ME. Erythropoietic response to calorigenic hormones. Endocrinology. 1961;68(3):517-532. [DOI] [PubMed] [Google Scholar]
- 11. Horsley V. The Brown Lectures on Pathology. British Med J. 1885;1(1261):419-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kendrick TS, Payne CJ, Epis MR, et al. . Erythroid defects in TRalpha-/- mice. Blood. 2008;111(6):3245-3248. [DOI] [PubMed] [Google Scholar]
- 13. Kawa MP, Grymula K, Paczkowska E, et al. . Clinical relevance of thyroid dysfunction in human haematopoiesis: biochemical and molecular studies. Eur J Endocrinol. 2010;162(2):295-305. [DOI] [PubMed] [Google Scholar]
- 14. Horton L, Coburn RJ, England JM, Himsworth RL. The haematology of hypothyroidism. Q J Med. 1976;45(177):101-123. [PubMed] [Google Scholar]
- 15. Bremner AP, Feddema P, Joske DJ, et al. . Significant association between thyroid hormones and erythrocyte indices in euthyroid subjects. Clin Endocrinol. 2012;76(2):304-311. [DOI] [PubMed] [Google Scholar]
- 16. Tudhope GR, Wilson GM. Anaemia in hypothyroidism. Incidence, pathogenesis, and response to treatment. Q J Med. 1960;29(4):513-537. [PubMed] [Google Scholar]
- 17. Vitale G, Fatti LM, Prolo S, et al. . Screening for hypothyroidism in older hospitalized patients with anemia: a new insight into an old disease. J Am Geriatr Soc. 2010;58(9):1825-1827. [DOI] [PubMed] [Google Scholar]
- 18. Wopereis DM, Du Puy RS, van Heemst D, et al. . The relation between thyroid function and anemia: a pooled analysis of individual participant data. J Clin Endocrinol Metab. 2018;103(10):3658-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stott DJ, Rodondi N, Kearney PM, et al. . Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;376(26):2534-2544. [DOI] [PubMed] [Google Scholar]
- 20. Stott DJ, Gussekloo J, Kearney PM, et al. . Study protocol; Thyroid hormone Replacement for Untreated older adults with Subclinical hypothyroidism - a randomised placebo controlled Trial (TRUST). BMC Endocr Disord. 2017;17(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Du Puy RS, Postmus I, Stott DJ, et al. . Study protocol: a randomised controlled trial on the clinical effects of levothyroxine treatment for subclinical hypothyroidism in people aged 80 years and over. BMC Endocr Disord. 2018;18(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5-37. [PubMed] [Google Scholar]
- 23. Du Puy RS, Poortvliet RKE, Mooijaart SP, et al. . Supplemental tables from: No effect of levothyroxine on hemoglobin in older adults with subclinical hypothyroidism: pooled results from two RCTs. Posted February 3, 2022. Zenodo. 10.5281/zenodo.5966134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Floriani C, Feller M, Aubert CE, et al. . Thyroid dysfunction and anemia: a prospective cohort study and a systematic review. Thyroid. 2018;28(5):575-582. [DOI] [PubMed] [Google Scholar]
- 25. M’Rabet-Bensalah K, Aubert CE, Coslovsky M, et al. . Thyroid dysfunction and anaemia in a large population-based study. Clin Endocrinol. 2016;84(4):627-631. [DOI] [PubMed] [Google Scholar]
- 26. Mooijaart SP, Du Puy RS, Stott DJ, et al. . Association between levothyroxine treatment and thyroid-related symptoms among adults aged 80 years and older with subclinical hypothyroidism. Jama. 2019;322(20):19771-19711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Christ-Crain M, Meier C, Huber P, Zulewski H, Staub JJ, Muller B. Effect of restoration of euthyroidism on peripheral blood cells and erythropoietin in women with subclinical hypothyroidism. Hormones. 2003;2(4):237-242. [DOI] [PubMed] [Google Scholar]
- 28. Maggio M, De Vita F, Fisichella A, et al. . The Role of the Multiple Hormonal Dysregulation in the Onset of “Anemia of Aging”: Focus on Testosterone, IGF-1, and Thyroid Hormones. Int J Endocrinol. 2015;2015:292574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perrin MC, Blanchet JP, Mouchiroud G. Modulation of human and mouse erythropoiesis by thyroid hormone and retinoic acid: evidence for specific effects at different steps of the erythroid pathway. Hematol Cell Ther. 1997;39(1):19-26. [DOI] [PubMed] [Google Scholar]
- 30. Sullivan PS, McDonald TP. Thyroxine suppresses thrombocytopoiesis and stimulates erythropoiesis in mice. Proc Soc Exp Biol Med. 1992;201(3):271-277. [DOI] [PubMed] [Google Scholar]
- 31. Cinemre H, Bilir C, Gokosmanoglu F, Bahcebasi T. Hematologic effects of levothyroxine in iron-deficient subclinical hypothyroid patients: a randomized, double-blind, controlled study. J Clin Endocrinol Metab. 2009;94(1): 151-156. [DOI] [PubMed] [Google Scholar]
- 32. Gencer B, Collet TH, Virgini V, et al. . Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126(9):1040-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huber G, Staub JJ, Meier C, et al. . Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab. 2002;87(7):3221-3226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.