Abstract
Context
Although glycated hemoglobin A1c is currently the best parameter used clinically to assess risk for the development of diabetes complications, it does not provide insight into short-term fluctuations in glucose levels. This review summarizes the relationship between continuous glucose monitoring (CGM)-derived metrics of glycemic variability and diabetes-related complications.
Evidence Acquisition
PubMed and Embase databases were searched from January 1, 2010 to August 22, 2020, using the terms type 1 diabetes, type 2 diabetes, diabetes-related microvascular and macrovascular complications, and measures of glycaemic variability. Exclusion criteria were studies that did not use CGM and studies involving participants who were not diabetic, acutely unwell (post stroke, post surgery), pregnant, or using insulin pumps.
Evidence Synthesis
A total of 1636 records were identified, and 1602 were excluded, leaving 34 publications in the final review. Of the 20 852 total participants, 663 had type 1 diabetes (T1D) and 19 909 had type 2 diabetes (T2D). Glycemic variability and low time in range (TIR) showed associations with all studied microvascular and macrovascular complications of diabetes. Notably, higher TIR was associated with reduced risk of albuminuria, retinopathy, cardiovascular disease mortality, all-cause mortality, and abnormal carotid intima-media thickness. Peripheral neuropathy was predominantly associated with standard deviation of blood glucose levels (SD) and mean amplitude of glycemic excursions (MAGE).
Conclusion
The evidence supports the association between diabetes complications and CGM-derived measures of intraday glycemic variability. TIR emerged as the most consistent measure, supporting its emerging role in clinical practice. More longitudinal studies and trials are required to confirm these associations, particularly for T1D, for which there are limited data.
Keywords: continuous glucose monitoring, diabetes complications, glycemic variability, time-in-range, type 1 diabetes mellitus, type 2 diabetes mellitus
Diabetes is associated with microvascular and macrovascular complications, including nephropathy, retinopathy, neuropathy, and cardiovascular and cerebrovascular disease, all of which contribute to a burgeoning disease burden. The risk of cardiovascular disease mortality and incidence of stroke are 2 to 4 times higher in Americans with diabetes compared to those without diabetes (1). Diabetic nephropathy accounts for 38.6% of new cases of end-stage renal disease, and 11.7% of adults with diabetes reported vision disability due to diabetic retinopathy (2). Globally, up to 75% of all lower-extremity amputations are performed in individuals with diabetes (3). In 2019, diabetes-related costs were estimated to have totaled $760 billion globally and $161.4 billion in Europe, with these figures being primarily composed of preventable hospital-based admissions from diabetes complications (4). Beyond financial cost, diabetes has a major effect on quality of life because of the daily demands of disease self-management, and possible effect of living with diabetes-related complications (5).
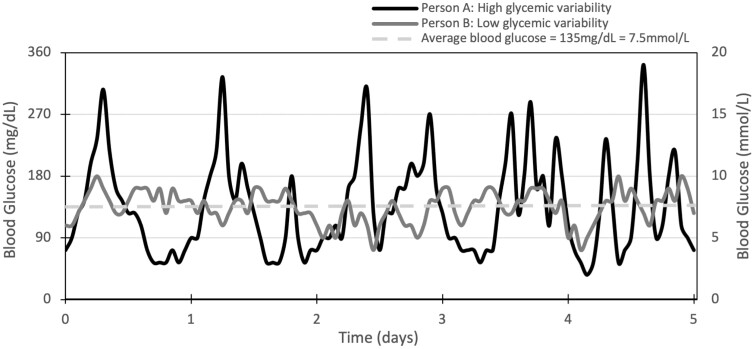
Since the release of findings from the landmark Diabetes Control and Complications Trial (DCCT) and United Kingdom Prospective Diabetes Studies (UKPDS), glycated hemoglobin A1c (HbA1c) has become the gold standard by which to assess the success of glycemic management, and is used to guide clinical decision-making in diabetes management (6). There is a substantial body of evidence linking increases in HbA1c with diabetes-related complications. Yet patients with identical HbA1c values can also have vastly different complications rates—for example, only 11% of the variation in retinopathy risk may be explained by total glycemic exposure (HbA1c and duration of diabetes) in the DCCT cohort (7, 8). Another study found mean HbA1c to be only weakly correlated to the presence and severity of cardiovascular autonomic neuropathy (r = 0.22) (9). In addition, HbA1c measurements may also be influenced by a number of common pathological and physiological factors unrelated to glucose levels such as age, race, iron-deficiency anemia, chronic renal failure, pregnancy, and medications (10). While HbA1c is an integral marker of glycemic exposure over the preceding 8 to 12 weeks, it cannot describe interday or intraday glucose fluctuations. Beck et al (11) showed there can be a wide range of glucose profiles associated with any given HbA1c level. Limitations around HbA1c highlight the need for complementary methods to assess glucose levels in people living with diabetes (Fig. 1).
Figure 1.
High vs low glycemic variability. Glucose profiles of 2 individuals showing identical glycated hemoglobin A1c (6.3%) over a 5-day monitoring period but vastly different variability.
Continuous glucose monitoring (CGM) devices test interstitial glucose levels every 5 minutes and can record and store these data. Since the initial introduction of CGM into clinical practice in 2000 (12), the accuracy and sophistication of these devices has progressively increased. Over recent years, the use of CGM has become more widespread. In the United States their use in people with type 1 diabetes (T1D) has increased from approximately 7% in 2011 to 28% in 2017, according to the Type 1 Diabetes Exchange Clinic Network encompassing 81 diabetes centers (13). In Australia, CGM is used by 79% of people younger than 21 years. In 2017 the Advanced Technologies and Treatments for Diabetes (ATTD) Congress came to an international consensus that mean glucose, glucose management indicator (GMI), glycemic variability, TIR, time above range (TAR), and time below range (TBR) were the primary measurable outcomes of CGM; and that they should be measured for more than 14 days with at least 10 days of valid data (14). Glycemic variability itself can be measured by numerous formulas using CGM data, including SD, mean amplitude of glycemic excursions (MAGE), coefficient of variation for glucose (CV), high blood glucose index (HBGI), low blood glucose index (LBGI), area under the curve hypoglycemia, and continuous overall net glycemic action (CONGA). These metrics are summarized in Table 1. The congress recommends the use of CGM data to complement HbA1c monitoring in a wide range of people with diabetes (14).
Table 1.
Continuous glucose monitoring metrics
| CGM metric | Description |
|---|---|
| TIR | Proportion of time spent with blood glucose levels within 3.9 to 10 mM. For most patients, a TIR of > 70% is an accepted target |
| TBR | Proportion of time spent with blood glucose levels below this range, with recommendations for < 4% of time spent with blood glucose levels 3.8 to 3.0 mM (level 1 TBR), and < 1% of time with blood glucose levels < 3.0 mM (level 2 TBR) |
| TAR | Proportion of time spent with blood glucose levels above this range, with recommendations for < 25% of time with blood glucose levels 10.1 to 13.9 mM (level 1 TAR), and < 5% of time > 13.9 mM (level 2 TAR) |
| SD | Measure of variation of all glucose measurements |
| MAGE | Measure of magnitudes of glycemic excursions (high and low) that exceed 1 SD from mean |
| CV | CV = (SD)/(mean glucose) × 100. CV < 36 is recommended (15) |
| CONGA | Combined measurement of timing and magnitude of blood glucose level fluctuations at specified time periods |
| GMI | Estimate of HbA1c based on average glucose. Formerly known as estimated A1c |
Abbreviations: CGM, continuous glucose monitoring; CONGA, continuous overall net glycemic action; CV, coefficient of variation for glucose; GMI, glucose management indicator; HbA1c, glycated hemoglobin A1c; MAGE, mean amplitude of glycemic excursions, SD, SD of blood glucose levels; TAR, time above range; TBR, time below range; TIR, time in range.
CGM-derived outcomes are strongly correlated to HbA1c and thus indirectly with diabetes-related complications by inference. Beck et al (18) found an r = –0.73 correlation between HbA1c and TIR70-180. Hirsch et al (19) and Vigersky et al (20) correlated HbA1c with TIR at r = –0.75 and r = –0.84, respectively. However, given the recency of the advent of CGM as a clinical and research tool, there is less evidence directly linking CGM metrics to these complications in comparison with HbA1c. This literature review therefore aims to amalgamate, summarize, and assess the existing evidence directly linking CGM-derived metrics with diabetes-related complications.
Materials and Methods
A systematic literature search of PubMed and Embase was performed August 22, 2020, to identify studies demonstrating direct links between CGM-derived metrics of glycemic management, and diabetes-related complications. The search strategy is detailed in Table 2.
Table 2.
Search strategy
| Criteria | Terms included |
|---|---|
| 1 | “Complications” OR “Microvascular” OR “Macrovascular” OR “Nephropathy” OR “Diabetic Kidney Disease” OR “End-Stage Kidney Disease” OR “End-stage renal disease” OR “Chronic Kidney Disease” OR Neuropathy OR Retinopathy OR “Eye disease” OR “CVD” OR “Cardiovascular disease” OR “Stroke” OR “CAD” OR “Coronary Artery Disease” OR “Cardiovascular Autonomic Neuropathy” OR “CAN” |
| 2 | “Target Range” OR “Time In Range” OR “TIR” OR “Glucose Variability” OR “GV” OR “Time Below Range” OR “TBR” OR “Time Above Range” OR “TAR” |
| 3 | “Diabetes Mellitus” OR “T1DM” OR “T2DM” OR “Type 1 Diabetes” OR “Type 2 Diabetes” OR “Diabetes Mellitus, Type 1” OR “Diabetes Mellitus, Type 2” |
| 4 | 1 AND 2 AND 3 |
| 5 | Filters: English AND from 2010 (inclusive) to present |
Inclusion Criteria
Only studies from January 1, 2010 (inclusive) were considered because of the recent rapid development and usage of CGM, and the ATTD consensus on standardized CGM-derived metrics since 2017 (14). Data applicable to T1D and type 2 diabetes (T2D) and Latent Autoimmune Diabetes in Adults (LADA) were included to comprehensively capture all microvascular and macrovascular diabetes-related complications. Furthermore, markers of diabetes complications (eg, carotid intima-media thickness [CIMT] as a proxy for the development of macrovascular disease), were also included for the same reason (21, 22). Included studies were in humans of all ages and that were printed in English. Study types included were those presenting primary data.
Exclusion Criteria
This review is designed to evaluate CGM, by which most devices measure glucose levels every 5 minutes. Studies using less frequent measurement regimens, such as the 7-point glucose profiles used in the DCCT, were excluded, as recent studies have suggested these glucose profiles are too infrequent to portray actual glycemic variability (23, 24). Baghurst et al (25) demonstrated that markers of acute glycemic variability, such as SD and MAGE, became unreliable if measured more than 2 to 4 hours and 1 hour apart, respectively.
The metrics used to evaluate glycemic management were those proposed at the ATTD Congress (14): TIR, TAR, TBR, CV, and GMI. All markers of intraday glycemic variability were included, which is the domain that distinguishes CGM from other glucose-monitoring regimens. Consequently, metrics not reliant on CGM, such as visit-to-visit glucose variability or HbA1c variability, were excluded. Acutely unwell populations, for example postsurgery or poststroke patients, were excluded because of potentially altered glycemic management through mechanisms such as altered cortisol release (26). Studies in pregnant populations were also excluded (27). Publications involving insulin pump use were excluded because their effects on glycemic management may have altered the development of diabetes complications above the effect of CGM alone.
Data Extraction and Synthesis
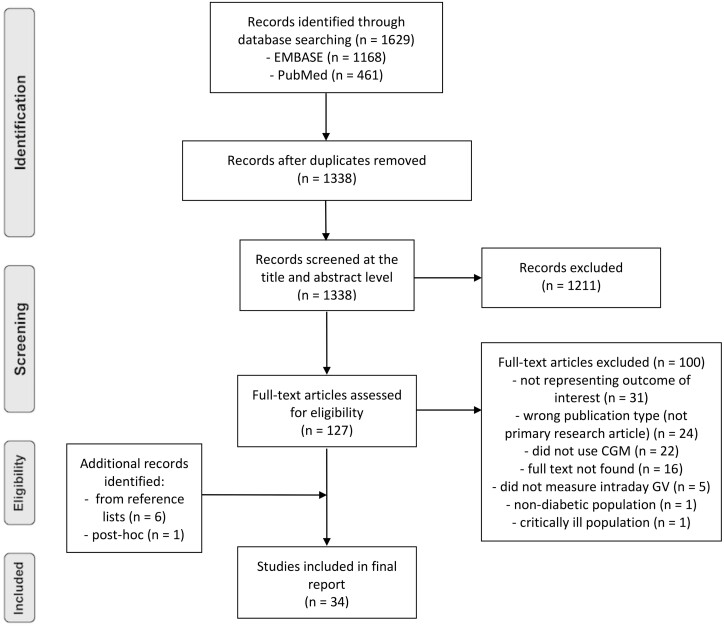
A total of 1629 records were identified and exported to EndNote X9. Duplications were removed, and 1338 publications were screened by title and abstract (Fig. 2). The majority of papers excluded did not represent the outcome of interest. A total of 127 full-text papers were screened according to the defined criteria, and 27 met these criteria. A further 7 papers that met the criteria were identified: 6 via hand-searching reference lists, and 1 post hoc. The literature search identified conference abstracts that were subsequently hand-searched and included provided there was full-text availability. Data were then classified into T1D and T2D, and extracted into purpose-built tables.
Figure 2.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart.
Of the 34 included papers, 30 used cross-sectional study designs and 4 were longitudinal cohort studies. Nine studies involved participants with T1D, 22 with T2D, and 3 had mixed populations. Of the 20 852 total participants, 663 had T1D, 19 909 had T2D, 192 had LADA, and 88 were control participants without diabetes. Thirteen papers were from China, 9 from Europe, 4 from South Korea, 3 from Japan and the United States, and 2 from Australia. In addition, one multinational study included data from the United States, Europe, and Cameroon.
Results
Microvascular Complications
One study involving 32 participants addressed microvascular complications as a whole, showing associations with glycaemic variability (Table 3).
Table 3.
Microvascular complications results
| Study | Study size, No. | Population + age, y | Diabetes duration, y | Mean HbA1c, % | Duration of CGM trace, d | Findings |
|---|---|---|---|---|---|---|
| Šoupal et al (2014) (28) cross-sectional | 32 | T1D | 19.5 ± 5.5 | 8.6 ± 0.9 | 12-14 | Presence of any microvascular complication associated with |
| 41.5 ± 11.5 | ||||||
| • SD: OR = 7.5 (1.83-52.08), P < .01b | ||||||
| • MAGE: OR = 2.83 (1.3-8.17), P = .01b | ||||||
| • CV: 0.43 ± 0.06 vs 0.38 ± 0.08, P = .03a |
Values expressed as mean ± SD.
Abbreviations: CGM, continuous glucose monitoring; CV, coefficient of variation for glucose; HbA1c, glycated hemoglobin A1c; MAGE, mean amplitude of glycemic excursions; SD, SD of blood glucose levels; T1D, type 1 diabetes.
a Univariable analysis.
b Multivariable analysis.
Nephropathy
Six studies addressed nephropathy, involving 1563 participants (Table 4). Four of the 5 studies investigating albuminuria demonstrated statistically significant associations with glycemic variability. The larger studies (n = 866 and n = 281) found these associations with TIR. One study associated reduced eGFR (estimated glomerular filtration rate) with high SD.
Table 4.
Nephropathy results
| Study | Study size, No. | Population + age, y | Diabetes duration, y | Mean HbA1c, % | Duration of CGM trace, d | Findings |
|---|---|---|---|---|---|---|
| Šoupal et al (2014) (28) cross-sectional | 32 | T1D | 19.5 ± 5.5 | 8.6 ± 0.9 | 12-14 | Microalbuminuria associated with higher |
| 41.5 ± 11.5 | • SD: 4.3 ± 0.5 vs 3.6 ± 0.8 mmol/L, P = .04a | |||||
| • CV: 0.46 ± 0.1 vs 0.39 ± 0.1 mmol/L, P = .02a | ||||||
| • MAGE: 7.5 ± 0.9 vs 6.1 ± 1.2 mmol/L, P = .01a | ||||||
| Jin et al (2015) (29) cross-sectional | 173 | T2D | 10.9 (6-16) | 8.2 ± 3.7 | 3 | Macroalbuminuria associated with higher |
| 56.7 ± 8.4 | • SD: OR = 1.04 ± 0.04, P = .03b | |||||
| • MAGE: OR = 1.01 ± 0.01, P = .04a | ||||||
| Kuroda et al (2020) (30) longitudinal | 281 | T2D | 13 (7-23) | 6.9 (6.5-7.5) | 10 | Albumin-creatinine ratio associated with reduced TIR: β = –0.10, P = .04b |
| 68 (62-71) | ||||||
| Magri et al (2018) (31) cross-sectional | 121 | T2D | 3 (2-5) | 6.8 (6.3-7.6) | 3 | Albuminuria not associated with TBR, TIR, or TAR |
| 64 (57-68) | ||||||
| Yokota et al (2019) (32) cross-sectional | 100 | T2D | 10 (0.1-42) | 8.5 ± 1.9 | 3 | Lower eGFR associated with high (≥ 35.9) SD: 66.2 ± 22.8 vs 78.8 ± 25.9, P = .01a |
| 60 ± 14 | ||||||
| Yoo et al (2020) (33) cross-sectional | 866 | T2D | 13.1 ± 8.6 | 8.2 ± 1.5 | 3 | Albuminuria risk associated with |
| 58.5 ± 10.3 | • 10% lower TIR: OR = 0.94 (0.88-0.99), P = .04b | |||||
| • 10% higher TAR > 180 mg/dL: OR = 1.07 (1.01-1.19), P = .03b | ||||||
| • 10% higher TAR > 250 mg/dL: OR = 1.10 (1.01-1.20), P = .03b |
Values expressed as mean ± SD or median (interquartile range).
Abbreviations: CGM, continuous glucose monitoring; CV, coefficient of variation for glucose; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin A1c; MAGE, mean amplitude of glycemic excursions; SD, SD of blood glucose levels; T1D, type 1 diabetes; T2D, type 2 diabetes; TAR, time above range; TBR, time below range; TIR, time in range.
a Univariable analysis.
b Multivariable analysis.
Retinopathy
Six studies addressed retinopathy, involving 6599 participants (Table 5). Four of the 5 studies investigating the presence of established retinopathy demonstrated statistically significant associations with glycemic variability. The larger studies (n = 3262 and n = 3119) found these associations for SD and reduced TIR. Both studies that addressed structural retinal changes in T1D found associations with glycemic variability, particularly LBGI.
Table 5.
Retinopathy results
| Study | Study size, No. | Population + age, y | Diabetes duration, y | Mean HbA1c, % | Duration of CGM trace, d | Findings |
|---|---|---|---|---|---|---|
| Picconi et al (2016) (34) cross-sectional | 37 | T1D | 19.0 ± 10.4 | 7.9 ± 1.1 | 3 | Inner nuclear layer thickness correlated with |
| 41.5 ± 10.0 | • CONGA-1: r = 0.40, P = .03 | |||||
| • CONGA-2: r = 0.39, P = .03 | ||||||
| • CONGA-4: r = 0.41, P = .02 | ||||||
| Retinal nerve fiber layer thickness correlated with LBGI: r = –0.38, P = .03 | ||||||
| Sartore et al (2013) (35) cross-sectional | 68 | T1D, T2D | 15.0 ± 8.3 | 8.1 ± 1.6 | 3 | Retinopathy associated with |
| 48.6 ± 13.8 | • SD: OR = 1.03 (1.01-1.06), P = .01a | |||||
| • CONGA-2: OR = 1.02(1.00-1.04), P = .04a | ||||||
| • HBGI: OR = 1.10 (1.01-1.18), P = .03a | ||||||
| Retinopathy not associated with MAGE: OR = 1.74 (0.69-4.40), P = .24a | ||||||
| Šoupal et al (2016) (28) cross-sectional | 32 | T1D | 19.5 ± 5.5 | 8.6 ± 0.9 | 12-14 | Retinopathy associated with SD: 4.1 ± 0.7 vs 3.5 ± 0.8 mmol/L, P = .03a |
| 41.5 ± 11.5 | ||||||
| Stem et al (2016) (36) cross-sectional | 81 | T1D | 14.0 ± 6.7 | 7.9 ± 1.0 | 5 | Neurodegenerative structural retinal changes were associated with |
| 46.5 ± 16.5 | • LBGI: β = –0.47, P = .02, R2 = 0.28b | |||||
| • Area under curve for hypoglycemia: β = –0.45, P = .02, R2 = 0.26b | ||||||
| Neither presence of retinopathy nor neuroretinal function associated with LBGI or area under curve for hypoglycemia | ||||||
| Lu et al (2018) (37) cross-sectional | 3262 | T2D | 8.1 ± 6.8 | 8.9 ± 2.2 | 3 | Retinopathy severity associated with |
| 60.2 ± 12.0 | • Lower TIR: P < .01a | |||||
| • Lower TIR quartiles: r = –0.15, P < .01a | ||||||
| • SD: P < .01a | ||||||
| • CV: P < .01a | ||||||
| • MAGE: P < 0.01a | ||||||
| Any diabetic retinopathy negatively associated with 10% increase in TIR: OR = 0.92 (0.88-0.96), P < .01b | ||||||
| Mild nonproliferative retinopathy negatively associated with | ||||||
| • 10% increase in TIR: OR = 0.93 (0.87-0.99), P = .02b | ||||||
| • Highest compared to lowest quartile TIR: OR = 0.56 (0.36-0.87), P = .01b | ||||||
| Moderate nonproliferative retinopathy negatively associated with | ||||||
| • 10% increase in TIR: OR = 0.91 (0.84-0.98), P = .01b | ||||||
| • Highest compared to lowest quartile TIR: OR = 0.48 (0.27-0.83), P = .01b | ||||||
| Vision-threatening retinopathy negatively associated with | ||||||
| • 10% increase in TIR: OR = 0.91 (0.85-0.98), P = 0.02b | ||||||
| • Highest compared to lowest quartile TIR: OR = 0.53 (0.30-0.91), P = .02b | ||||||
| Lu et al (2019) (38) cross-sectional | 3119 | T2D, LADA | 7.7 ± 6.3 | 8.9 ± 2.1 | 3 | Retinopathy associated with |
| 57.6 ± 10.1 | • SD: OR = 1.15 (1.03-1.29), P = 0.02b | |||||
| • MAGE: OR = 1.21 (1.11-1.31), P < .01a | ||||||
| • CV: OR = 1.16 (1.07-1.26), P < .01a | ||||||
| Retinopathy associated with increasing quartiles of SD and MAGE in T2D: P < .01 | ||||||
| No significant associations for LADA |
Values expressed as mean ± SD or median (interquartile range).
Abbreviations: CGM, continuous glucose monitoring; CONGA, continuous overall net glycemic action; HbA1c, glycated hemoglobin A1c; LADA, latent autoimmune diabetes of adulthood; LBGI, low blood glucose index; SD, SD of blood glucose levels; HBGI, high blood glucose index; MAGE, mean amplitude of glycemic excursions; T1D, type 1 diabetes; T2D, type 2 diabetes; TIR, time in range.
a Univariable analysis.
b Multivariable analysis.
Neuropathy
Peripheral neuropathy was investigated in 7 studies involving 2247 participants (Table 6). Four papers investigated the presence of peripheral neuropathy, while 3 studied markers of nerve conduction. Glycemic variability markers, particularly SD, MAGE, and reduced TIR, were associated both with the presence of peripheral neuropathy and abnormal nerve conduction across all papers. Cardiac autonomic neuropathy (CAN) was measured in 8 publications, involving 782 participants (Table 7). CAN was associated with glycemic variability in 7 of these publications, but some associations were directly contradicted in 4 publications. Reduced TIR was investigated in 2 studies and found to be associated with CAN in both.
Table 6.
Peripheral neuropathy results
| Study | Study size, No. | Population + age, y | Diabetes duration, y | Mean HbA1c, % | Duration of CGM trace, d | Findings |
|---|---|---|---|---|---|---|
| Kwai et al (2016) (39) cross-sectional | 17 | T1D | Not recorded | 8.1 ± 0.3 | 6 | Multiple measures of abnormal motor and sensory axonal function associated with MAGE |
| 28.6 ± 1.5 | ||||||
| • Super excitability: r = 0.54, P = .04 | ||||||
| • S2 accommodation: r = –0.76, P < .01 | ||||||
| • Minimum current threshold (I/V) slope: r = 0.71, P < .01 | ||||||
| • Strength duration time constant: r = 0.66, P < .01 | ||||||
| • Latency: r = 0.65, P < .01 | ||||||
| Šoupal et al (2014) (28) cross-sectional | 32 | T1D | 19.5 ± 5.5 | 8.6 ± 0.9 | 12-14 | Impaired vibration perception threshold associated with SD: r = 0.51, P < .01 |
| 41.5 ± 11.5 | ||||||
| Kuroda et al (2020) (30) longitudinal | 281 | T2D | 13 (7-23) | 6.9 (6.5-7.5) | 10 | Peripheral neuropathy an explanatory factor for TIR: β = –0.11, P = .03b |
| 68 (62-71) | ||||||
| Li et al (2020) (40) cross-sectional | 740 | T2D | 10.7 ± 7.5 | 8.6 ± 1.9 | 3 | Abnormal nerve conduction study markers negatively associated with highest TIR tertile |
| 60.2 ± 12.8 | ||||||
| • Lower risk of slowing conduction velocity: OR = 0.26(0.18-0.40), P < .01b | ||||||
| • Lower risk of amplitude reduction: OR = 0.60(0.41-0.88), P = .01b | ||||||
| • Higher rate of reduced latency: OR = 1.71(1.16-2.53), P = .01b | ||||||
| Mayeda et al (2020) (41) cross-sectional | 105 | T2D | 19.1 ± 10.0 | 7.8 ± 1.6 | 12 | Michigan Neuropathy Screening Instrument questionnaire score ≥ 2 associated with 10% reduction in TIR: OR = 1.25 (1.02-1.52), P = .03b |
| 67.1 ± 10.0 | ||||||
| Peripheral neuropathy associated with | ||||||
| • TAR: OR = 1.24 (1.03-1.50), P = .02b | ||||||
| • 1% increase in GMI: OR = 1.79 (1.05-3.04), P = .03b | ||||||
| Peripheral neuropathy not associated with 6% increase in CVa | ||||||
| Hu et al (2018) (42) cross-sectional | 982 | T2D | 5.2 (4.2-8.0) | 9.9 ± 1.3 | 3 | Peripheral neuropathy associated with |
| 55.1 ± 10.9 | • SD: OR = 3.71 (2.61-5.28), P < .01a | |||||
| • MAGE: OR = 4.57 (3.48-6.10), P < .01b | ||||||
| Xu et al (2014) (43) cross-sectional | 90 | T2D | 5.5 (2-8.5) | 6.5 ± 0.4 | 3 | Peripheral neuropathy associated with: |
| 59.3 ± 7.5 | • SD: OR = 2.95 (1.55-5.61), P < .01a | |||||
| • MAGE: OR = 2.05 (1.36-3.09), P < .01b |
Values expressed as mean ± SD or median (interquartile range).
Abbreviations: CGM, continuous glucose monitoring; CV, coefficient of variation for glucose; GMI, Glucose Management Index; HbA1c, glycated hemoglobin A1c; MAGE, mean amplitude of glycemic excursions; SD, SD of blood glucose levels; T1D, type 1 diabetes; T2D, type 2 diabetes; TAR, time above range; TIR, time in range;
a Univariable analysis.
b Multivariable analysis.
Table 7.
Cardiac autonomic neuropathy results
| Study | Study size, No. | Population + age, y | Diabetes duration, y | Mean HbA1c, % | Duration of CGM trace, d | Findings |
|---|---|---|---|---|---|---|
| Jun et al (2019) (44) cross-sectional | 80 | T1D | 10.1 ± 7.3 | 8.2 ± 1.7 | 3 | CAN associated with |
| 39.9 ± 14.0 | • Reduced TIR: 40.0 (26.3-53.2) vs 57.0 (41.1-72.2), P < .01a | |||||
| • TBR: 5.1 (0.0-15.7) vs 1.7 (0.0-4.6), P = 0.01a | ||||||
| • SD: OR = 1.05 (1.02-1.09), P = .01b | ||||||
| • MAGE: OR = 1.02 (1.01-1.03), P = .02b | ||||||
| • CV: OR = 1.11 (1.05-1.18), P < .01b | ||||||
| • LBGI: OR = 1.29 (1.11-1.49), P < .01b | ||||||
| • HBGI: OR = 1.23 (1.05-1.43), P = .01b | ||||||
| • Log(TIR + 1): OR = 0.08 (0.01-0.58), P = .03b | ||||||
| • Log(TBR + 1): OR = 15.1 (3.33-68.57), P < .01b | ||||||
| • Log(TBR < 54 mg/dL + 1): OR = 38.6 (6.35-234.7), P < .01b | ||||||
| Nyiraty et al (2018) (45) cross-sectional | 20 | T1D | 17.5 ± 2.5 | 8.1 ± 0.2 | 6 | CAN severity associated with SD: r = 0.49, P < .05b |
| 39.5 ± 3.4 | Presence of CAN was not associated with SD, MAGE or CONGAa | |||||
| Di Flaviani et al (2010) (46) cross-sectional | 26 | T2D | 4.4 ± 4.8 | 6.7 ± 1.3 | 1 | Abnormal sympathovagal balance (increased LF/HF ratio) associated with MAGE only at nighttime: r = 0.40, P = .04a |
| 59.2 ± 10.6 | ||||||
| Guo et al (2020) (47) cross-sectional | 349 | T2D | 6 (2-12) | 9.2 ± 2.3 | 3 | CAN severity associated with SD: P < .01a |
| 53.1 ± 12.9 | Manifest CAN negatively associated with TIR: OR = 0.97 (0.95-0.98), P < .01b | |||||
| Severe CAN negatively associated with TIR: OR = 0.94 (0.91-0.98), P < .01b | ||||||
| Jun et al (2015) (48) cross-sectional | 110 | T2D | 12.8 ± 7.1 | 7.9 ± 1.0 | 3 | CAN associated with |
| 58.1 ± 8.4 | • SD: OR = 1.04 (1.01-1.07), P < .01a | |||||
| • CV: OR = 1.07 (1.01-1.13), P = .03b | ||||||
| No association with MAGE: OR = 1.01 (0.99-1.02), P = .06a | ||||||
| Kalopita et al (2014) (49) cross-sectional | 50 | T2D | 5.5 (2.0-9.3) | 7.1 ± 3.3 | 1 | CAN, as measured by abnormal indices of heart rate variability on ECG, not associated with SD or MAGE |
| 58.4 ± 9.9 | ||||||
| Matsutani et al (2018) (50) longitudinal | 57 | T2D | 11.5 ± 9.6 | 7.3 ± 1.0 | 3 | Baroreflex sensitivity associated with |
| 67.2 ± 7.7 | • CV: β = –0.31, P = .03b | |||||
| • SD: r = –0.37, P = .01a | ||||||
| Xu et al (2016) (51) cross-sectional | 90 | T2D | Not recorded | 9.3 ± 2.1 | 3 | CAN associated with MAGE: OR = 1.73 (1.01-2.73), P = .02b |
| 46.7 ± 10.0 | CAN not associated with CV |
Values expressed as mean ± SD or median (interquartile range).
Abbreviations: CAN, cardiac autonomic neuropathy; CGM, continuous glucose monitoring; CONGA, continuous overall net glycemic action; CV, coefficient of variation for glucose; ECG, electrocardiography; HbA1c, glycated hemoglobin A1c; HBGI, high blood glucose index; LBGI, low blood glucose index; MAGE, mean amplitude of glycemic excursions; SD, SD of blood glucose levels; T1D, type 1 diabetes; T2D, type 2 diabetes; TBR, time below range; TIR, time in range.
a Univariable analysis.
b Multivariable analysis.
Macrovascular Disease
Thirteen studies addressed different aspects of macrovascular disease, which encompasses cardiovascular, cerebrovascular, and peripheral vascular disease, involving 10 206 participants: 412 with T1D and 9794 with T2D (Tables 8 and 9). Four papers investigated the presence of established macrovascular disease in which MAGE and reduced TIR were the predominant associations. The large (n = 6225) prospective cohort study identified TIR as an association for cardiovascular disease mortality and all-cause mortality. Glycemic variability was associated with abnormal echocardiography finding in two studies. Three studies used angiography to measure coronary artery disease in which glycemic variability, particularly MAGE, was an association. Cardiovascular disease risk factors were associated with glycemic variability in 1 out of 2 studies. Endothelial function was investigated in 1 paper and generally was not statistically significantly correlated with glycemic variability. CIMT was evaluated as a well-established proxy for cardiovascular and cerebrovascular disease (21, 22) in 4 publications. Associations with MAGE and SD were varied, but associations with TIR and TBR were unopposed.
Table 8.
Macrovascular disease results
| Study | Study size, No. | Population + age, y | Diabetes duration, y | Mean HbA1c, % | Duration of CGM trace, d | Findings |
|---|---|---|---|---|---|---|
| Borg et al (2011) (52) longitudinal | 427 | T1D, T2D | Not recorded | 6.8 ± 1.3 | > 2 d, 4 separate times | Cardiovascular disease risk factors (lipid profile, blood pressure, CRP) not associated with SD, MAGE, or CONGA |
| 46 ± 14 | ||||||
| Peña et al (2012) (53) cross-sectional | 52 | T1D | 5.5 ± 4 | 8.9 (6.7-14) | 2 | Endothelial function, measured by low-mediated dilatation, inversely correlated with LBGI: r = –0.30, P = .03 |
| 14 (2.7) | ||||||
| Not significantly associated with | ||||||
| • SD: r = 0.16, P > .05a | ||||||
| • MAGE: r = –0.06, P > .05a | ||||||
| • CONGA-1: r = –0.04, P > .05a | ||||||
| • CONGA-4: r = 0.04, P > .05a | ||||||
| • CONGA-8: r = –0.05, P > .05a | ||||||
| Snell-Bergeon et al (2010) (54) cross-sectional | 75 | T1D | 29 ± 8 | 7.4 ± 0.9 | 5 | Coronary artery calcium associated with |
| 42 ± 9 | • TAR: OR = 5.5 (1.3-22.6), P = .02b | |||||
| • Time-out-of-range: OR = 5.7 (1.3-24.9), P = .02b | ||||||
| • SD in men only: OR = 4.7 (1.1-19.7), P = 0.03b | ||||||
| Log coronary artery calcification score associated with | ||||||
| • Time out of range: r = 0.41, P = .03b | ||||||
| • TAR: r = 0.47, P = .01b | ||||||
| Di Flaviani et al (2010) (46) cross-sectional | 26 | T2D | 4.4 ± 4.8 | 6.7 ± 1.3 | 1 | Left ventricular mass index correlated with CONGA-2: r = 0.55, P = .01a |
| 59.2 ± 10.6 | ||||||
| Lu et al (2020) (55) longitudinal | 6,225 | T2D | 9.7 ± 7.4 | 8.9 ± 2.2 | 3 | Cardiovascular disease mortality associated with |
| 61.7 ± 11.9 | • TIR 71%-85%: HR = 1.35 (0.90-2.04), P = .02b | |||||
| • TIR 51%-70%: HR = 1.47 (0.99-2.19), P = .02b | ||||||
| • TIR < 50%: HR = 1.85 (1.25-2.72), P = .02b | ||||||
| • 10% decrease in TIR: HR = 1.05 (1.00–1.11), P = .02b | ||||||
| All-cause mortality associated with | ||||||
| • TIR 71%-85%: HR = 1.23 (0.98-1.55), P < .01b | ||||||
| • TIR 51%-70%: HR = 1.30 (1.04-1.63), P < .01b | ||||||
| • TIR < 50%: HR = 1.83 (1.48-2.28), P < .01b | ||||||
| • 10% decrease in TIR: HR = 1.08 (1.05-1.12), P < .01b | ||||||
| Magri et al (2018) (31) cross-sectional | 121 | T2D | 3 (2-5) | 6.8 (6.3-7.6) | 3 | Macrovascular disease associated with TBR: OR = 1.12 (1.01-1.23), P = .02b |
| 64 (57-68) | ||||||
| Macrovascular disease not associated with TIR: P = 0.63b or TAR: P = .39b | ||||||
| Su et al (2011) (56) cross-sectional | 344 | T2D | 6.1 ± 6.2 | 7.6 ± 1.5 | 3 | Coronary artery disease associated with |
| 63.9 ± 9.0 | • MAGE: 3.7 ± 1.4 vs 3.2 ± 1.2 mmol/L, P < .01a | |||||
| • MAGE ≥ 3.4 mmol/L: OR = 2.61 (1.41-4.83), P < .01b | ||||||
| Gensini score (measure of coronary artery disease severity) correlated with MAGE: R2 = 0.19, r = 0.28, P < .01b | ||||||
| Tang et al (2016) (57) cross-sectional | 240 | T2D | 5.7 ± 6.2 | 6.1 ± 0.9 | 3 | Framingham risk score (10-y cardiovascular disease risk) correlated with |
| 51.9 ± 8.0 | ||||||
| • SD: r = 0.51, P < .01 | ||||||
| • MAGE: r = 0.49, P < .01 | ||||||
| Framingham risk score > 20% (high 10-y cardiovascular disease risk) associated with | ||||||
| • SD: OR = 1.22, P = .04a | ||||||
| • MAGE: OR = 1.62 (1.20-2.32), P < .01b | ||||||
| Yokota et al (2019) (32) cross-sectional | 100 | T2D | 10 (0.1-42) | 8.5 ± 1.9 | 3 | Reduced left ventricular diastolic function associated with high (≥ 35.9 mg/dL) SD: OR = 3.67 (1.02-13.22), P < .05b |
| 60 ± 14 | ||||||
| Zhang et al (2013) (58) cross-sectional | 148 |
T2D 59.6 ± 7.0 |
Not recorded | 7.2 ± 1.3 | 3 | Cardiovascular complications associated with |
| • MAGE: 4.0 (3.3-4.8) vs. 2.6 (1.9-3.5), P < .01a | ||||||
| • SD: 2.0 ± 0.8 vs 0.1.5 ± 0.4, P < .05a | ||||||
| SYNTAX scores (a complete angiography scoring system) statistically significantly correlated to MAGE: r = 0.52, P = .01b | ||||||
| Coronary intima-media thickness correlated with MAGE: r = 0.46, P < .01 |
Values expressed as mean ± SD or median (interquartile range).
Abbreviations: CGM, continuous glucose monitoring; CONGA, continuous overall net glycemic action; CRP, C-reactive protein; HBGI, high blood glucose index; LBGI, low blood glucose index; MAGE, mean amplitude of glycemic excursions; SD, SD of blood glucose levels; T1D, type 1 diabetes; T2D, type 2 diabetes; TAR, time above range; TBR, time below range; TIR, time in range.
a Univariable analysis.
b Multivariable analysis.
Table 9.
Carotid intima-media thickness results
| Study | Study size, No. | Population + age, y | Diabetes duration, y | Mean HbA1c, % | Duration of CGM trace, d | Findings |
|---|---|---|---|---|---|---|
| Cesana et al (2013) (59) cross-sectional | 17 | T1D | 13.6 ± 8.8 | 7.7 ± 1.2 | 1 | CIMT not correlated with SD or MAGE |
| 40.7 ± 7.5 | ||||||
| Lu et al (2020) (60) cross-sectional | 2,215 | T2D | 8.5 ± 6.7 | 8.9 ± 2.1 | 3 | Abnormal (≥ 1 mm) CIMT associated with |
| 60.4 ± 11.5 | • SD: 2.3 ± 0.9 vs 2.5 ± 0.9, P = .01a | |||||
| • MAGE: 5.8 ± 2.5 vs 6.3 ± 2.7, P = .01a | ||||||
| • Lower TIR: 66.4 ± 23.5 vs 60.7 ± 24.9, P < .01a | ||||||
| Abnormal CIMT not associated with CV: P = .33a | ||||||
| Abnormal CIMT negatively associated with 10% higher TIR: OR = 0.94 (0.88-1.00), P = .04b | ||||||
| Magri et al (2018) (31) cross-sectional | 121 | T2D | 3 (2-5) | 6.8 (6.3-7.6) | 3 | Abnormal CIMT associated with TBR: OR = 1.09 (1.00-1.19), P = .04b |
| 64 (57-68) | ||||||
| Mo et al (2013) (61) cross-sectional | 216 | T2D | 9 (5-13.3) | 8.3 ± 1.7 | 3 | Intracranial/cervical artery stenosis severity, measured by magnetic resonance angiography, not associated with SD or MAGE |
| 63 ± 10 | ||||||
| In participants without existing plaques found on magnetic resonance angiography, CIMT correlated with | ||||||
| • SD: standardized β = 0.34, P = .01, R2 = 0.31b | ||||||
| • MAGE: standardized β = 0.32, P = .01, R2 = 0.27b | ||||||
| In those with existing atherosclerotic plaque, CIMT not correlated with SD or MAGE |
Values expressed as mean ± SD or median (interquartile range).
Abbreviations: CGM, continuous glucose monitoring; CIMT, carotid intima-media thickness; CV, coefficient of variation for glucose; HbA1c, glycated hemoglobin A1c; MAGE, mean amplitude of glycemic excursions; SD, SD of blood glucose levels; T1D, type 1 diabetes; T2D, type 2 diabetes; TBR, time below range; TIR, time in range;.
a Univariable analysis.
b Multivariable analysis.
Glycemic Variability Metrics and Glycated Hemoglobin A1c
Overall, 22 out of 34 studies investigated the associations between CGM metrics and diabetes complications after adjusting for HbA1c (Table 10). Most (n = 19) showed various CGM metrics (namely MAGE, TIR, CV, and TBR) remained associated with diabetes complications after adjusting for HbA1c. Five studies found a glycemic variability metric to lose significance after adjustment for HbA1c.
Table 10.
Glycemic variability metrics and glycated hemoglobin A1c
| CGM marker | No. of papers showing associations of glucose metrics with diabetes complications after adjusting for HbA1c | No. of papers in which statistical significance was lost after adjusting for HbA1c |
|---|---|---|
| SD | 7 | 3 |
| MAGE | 7 | 1 |
| TIR (and time-out-of-range) | 5 | 1 |
| CV | 3 | 0 |
| TBR (and AUC TBR) | 2 | 0 |
| LBGI | 2 | 0 |
| CONGA-2 | 0 | 2 |
| HBGI | 1 | 1 |
| TAR | 1 | 0 |
Abbreviations: AUC, area under the curve; CGM, continuous glucose monitoring; CONGA, continuous overall net glycemic action; CV, coefficient of variation for glucose; HbA1c, glycated hemoglobin A1c; HBGI, high blood glucose index; LGBI, low blood glucose index; MAGE, mean amplitude of glycemic excursions; SD, SD of blood glucose levels; TAR, time above range; TBR, time below range; TIR, time in range.
Complete findings from each individual study can be found in Tables S4 (62).
Discussion
This review of 34 publications totaling 20 852 participants, which investigated the associations between 6 different domains of diabetes complications against more than 10 different markers of intraday glycemic variability, demonstrated associations with all included diabetes complications. Glycemic variability, particularly low TIR and high SD, was almost unanimously associated with nephropathy, retinopathy, and peripheral neuropathy. Associations with CAN were present but varied. Glycemic variability was also associated with the presence of cardiovascular disease, cardiovascular disease mortality, all-cause mortality, and CIMT, as well as echocardiography and angiography abnormalities.
Of the CGM metrics recommended at the ATTD Congress, TIR was identified as the single most important CGM metric as it provides the most clinically practical information in 11 studies involving 14 319 participants (14). In nephropathy, TIR and glycemic variability metrics were strongly supported except for one cross-sectional study. The evidence for TIR and glycemic variability metrics in retinopathy was particularly strong given the study sizes (n = 6381) (37, 38). Glycemic variability was also associated with all severities of retinopathy, including preclinical neuroretinal abnormalities. Benbow et al (63) found peripheral neuropathy to be the most common complication of diabetes, having a major effect on quality of life, and CAN is a particularly strong risk factor for mortality in T1D (64, 65). Overall, the evidence suggests CAN is associated with decreased TIR, but not glycemic variability; but in peripheral neuropathy both glycemic variability—particularly MAGE—and lower TIR were supported as risk factors. These results highlight the importance of measuring these intraday parameters.
Approximately three-quarters of individuals with diabetes die from a cardiovascular cause (66), and a diagnosis of diabetes may be as much a risk factor for poor cardiovascular disease outcomes as having coronary artery disease itself (67), even in T1D (68). Most evidence supported lower TIR as a risk factor for macrovascular disease. The largest study was of longitudinal study design and importantly provided strong evidence for TIR as a protective factor for cardiovascular disease mortality (55). The second largest was a cross-sectional study that also showed strong correlations between TIR and CIMT. Only one cross-sectional study that measured the presence of overt macrovascular disease found no statistically significant association with TIR.
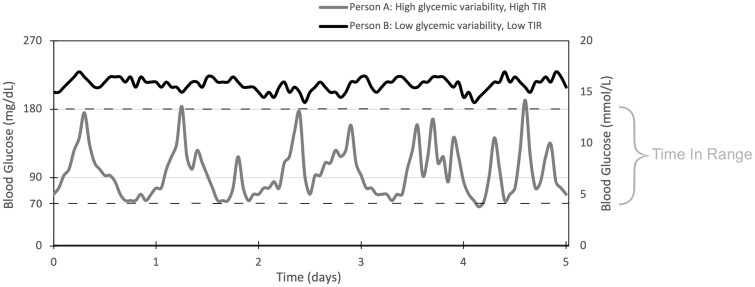
Glycemic variability showed mixed results for macrovascular disease and tended to relate more to microvascular complications. The ATTD Congress decided on CV as the consensus marker of glycemic variability (14), as SD is flawed in that it is significantly influenced by mean glucose. Yet CV seemed to have the least statistically significant associations with complications of diabetes, although it was measured only in 12 studies. While TIR and CV are emerging as popular CGM metrics, the relationship between the two should also be elucidated as it is possible to have a highly variable CGM trace with a high TIR, and conversely a minimally variable trace with low TIR (Fig. 3). Three studies investigated this in T2D, finding TIR remaining associated with albuminuria, retinopathy, and CAN after adjustments for glycemic variability metrics such as SD, MAGE, and CV (33, 37, 47). No studies examined the association of glycemic variability metrics after adjustment for TIR. Further research is required to clarify the nature of the relationship between TIR and CV with overt diabetes-related complications. Ideally these would involve large-scale longitudinal studies or randomized controlled trials (RCTs) with consistent CGM data that are long enough to capture adequate numbers of outcome events. However, such studies would be expensive and difficult to standardize for confounders such as exercise, physical activity, and treatment. Rather than individually randomized trials, evidence from CGM data using artificial intelligence may assist in interpreting observational data from individuals using CGM in collaboration with producers of CGM.
Figure 3.
Time in range (TIR) vs glycemic variability. Glucose profiles of 2 individuals highlighting the difference between glycemic variability and TIR.
Another reason for the lack of definitive outcomes is this review’s strict inclusion criteria of CGM use, as opposed to other less frequent blood glucose level (BGL) measurement regimens. While the accuracy of these infrequent measurement regimens is questionable compared to CGM (23-25), there is a wealth of data that must be considered. The DCCT was a large multicenter RCT spanning 10 years and involving 1440 participants. Reanalysis from Beck et al (69, 70) found that every 10% increase in TIR reduced the risk of retinopathy and microalbuminuria by 64% and 40%, respectively. However, the DCCT commenced in 1982 when therapeutic regimens and technologies were very different compared with today. It did not use CGM, and BGLs were measured only via 7 finger-prick samples collected in 1 day, every 3 months.
Mechanisms Relating Glycemic Variability With Diabetes Complications
Since diabetes complication risk is only partly explained by HbA1c (8), intraday glycemic variability is important to measure in conjunction with average glycemia because of its independent pathogeneses summarized by Nusca et al (16). While chronic sustained hyperglycemia can cause excessive protein glycation, acute hyperglycemic fluctuations may cause increased oxidative stress, inflammation, endothelial dysfunction, and altered gene expression (71-75). One case-control study involving 27 participants with T2D investigated the effect of glycemic variability on endothelial function (measured by flow-mediated dilatation) and oxidative stress (measured by plasma 3-nitrotyrosine and 8-iso-PGF2α urinary excretion rates). They found that oscillations in BGLs resulted in statistically significantly more endothelial dysfunction and oxidative stress than constant glucose levels, even if the average glucose was higher in the stable group. These effects lasted even beyond the return to euglycemia; thus, highlighting the importance of glycemic variability (72). A case-control study confirmed the strong association (r = 0.86) between MAGE and 8-iso-PGF2α urinary excretion rates (75), while another showed that transient hyperglycemia can induce lasting epigenetic changes in the promotor region of NFκB (a proinflammatory gene) in vitro and in mice (73). It is also established that high glycemia variability is associated with more frequent episodes of hypoglycemia, which contributes to a range of adverse effects including cardiovascular morbidity and mortality (76).
A narrative review by Livingstone et al (76) explored the glucose variability hypothesis—the notion that glucose variability contributes additional risk of diabetes complications after adjusting for HbA1c. While they agreed mechanisms for glycemic variability causing complications exist, they concluded that there were insufficient data at the time to substantiate this hypothesis. However, this was based primarily on the DCCT/EDIC (Epidemiology of Diabetes Interventions and Complications), which was greatly limited in that it used 7-point glucose profiles to estimate intraday glycemic variability, rather than using 5-minute data points from CGM. Our data (Table 10) showed that CGM-derived metrics of glycemic variability tended to remain associated with diabetes complications after adjusting for HbA1c (and indeed many other potential confounders). However, only one longitudinal study investigated this association, finding CV was associated with baroreflex sensitivity (CAN) after adjusting for HbA1c. Therefore, further longitudinal data are required to investigate the glucose variability hypothesis.
Clinical Implications
Large-scale longitudinal studies have shown that CGM-users have lower HbA1c levels, less hypoglycemia, and more TIR compared to non-CGM cohorts (13, 77). Thus, it would be expected for CGM use to translate longitudinally to reduced complications risk. CGM offers the unique ability to measure glucose levels in situations such as during sleep or exercise. Some systems have no requirement for daily capillary finger-prick tests, which patients appreciate. A survey of 3461 people with T1D or T2D identified TIR as the second most important of all factors that had a “big impact” on daily life with diabetes, while food choices were number one (78). CGM also alleviates the fear of hypoglycemia, which is a major barrier to exercise, dieting, and intensified treatment regimens (79), allowing for early glycemic management. This early management may be crucial in preventing future complications due to the “legacy effect.” A more than 66 000 person-year follow-up of the UKPDS RCT studied the effects of intensive glycemic management on the development of diabetes complications. It demonstrated that early intensive glycemic management resulted in risk reductions that persisted far beyond the transient HbA1c level differences between groups (80). Importantly, the integration of CGM also allows for more sophisticated use of insulin pump therapy, the gold standard for glycemia management.
Health care systems must also adapt to allow for widespread CGM use. This includes education and training around consensus reporting and interpretation of results, their implications on treatment adjustments, as well as follow-up guidelines (81). The ATTD Congress recommends the use of ambulatory glucose profile reports to aid this interpretation (14). Guidelines for when and how often to use CGM also requires further investigation, as Vigersky et al (82) found that periodic 14-day courses of CGM every 3 months would still adequately inform changes and responses to treatment as well as lasting behavioral changes. This may be aided by further cost-effectiveness analysis, which as it stands is extremely variable with different devices, patterns of use, and populations (83, 84).
Strengths and Limitations
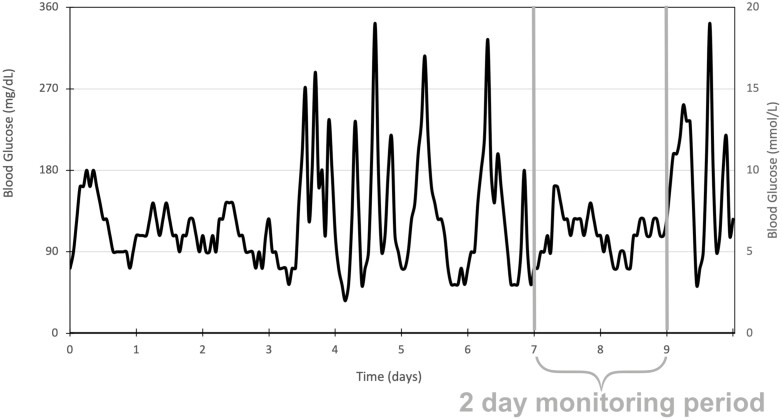
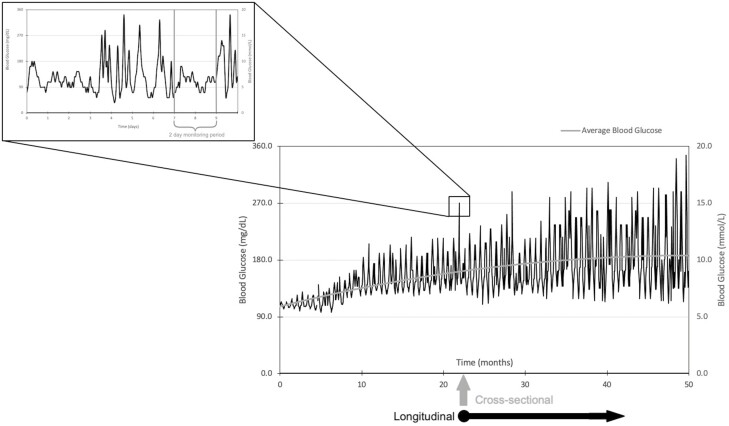
This review had several limitations. First, there was considerable heterogeneity between studies, in the selection of study participants, treatment adjustments, and reporting of data. Thus, a meta-analysis was not possible, making the data more difficult to interpret. Second, 30 out of 34 papers were cross-sectional study designs, meaning that causal relationships between CGM-derived measurements and outcomes cannot be proven. Third, the ATTD Congress suggests longer than 14-day periods of CGM (14). Most studies used only 48 to 72 hours, which may not be representative of long-term control and therefore undermine any associations with long-term complications. This may not be the case in T2D populations, which have less glycemic variability than in T1D, and thus, patterns of glycemia are more reproducible on a day-to-day basis. Shorter monitoring periods in this cohort are therefore more likely to be representative of longer-term patterns of glycemia, which would better correlate with complication development (85). Fourth, some studies included individuals with short diabetes durations, which reduces the likelihood of diabetes-related complications, and therefore underestimates the effect of each marker of glycemic variability on the development of diabetes complications long term. Fifth, there was a distinct lack of data relating to T1D, which is important given the large use of CGM particularly in youth with T1D. While 11 studies involved populations with T1D, this totaled only 663 participants. Sixth, many studies inferred the presence of disease via measuring risk factors or markers of disease rather than definitive outcomes. Finally, while specific populations such as pregnancy were excluded to increase the generalizability and applicability of this review’s findings, this may have led to the exclusion of relevant studies. In particular, publications involving insulin pumps were excluded because their effects on glycemic management may have altered the development of diabetes complications above that of the effect of CGM alone. While this allowed for better isolation of the effects of CGM on diabetes complications, it unrealistically excludes the effect that therapy may have on markers of glycemic variability. Databases such as Web of Science and gray literature were not searched, which may similarly exclude studies. The chief strength of this review includes the number of studies and broad range of complication outcomes included (Figs. 4 and 5).
Figure 4.
Limitation of short continuous glucose monitoring (CGM) periods. Most studies included in this review have CGM periods of 2 to 3 days. This diagram demonstrates how the data from this period may not be representative of the participants’ overall glycemic management.
Figure 5.
Observational vs longitudinal studies. Thirty out of the 34 papers included in this review used cross-sectional study designs. Diabetes complications are the results of years of altered glycemia. This diagram further illustrates how data from a single point in time (as in a cross-sectional study) may misrepresent the preceding months of data that are causative of the disease outcome. Longitudinal studies may be able to provide a more comprehensive analysis of the associations between different metrics of glycemia and the risk of diabetes complications.
Conclusion
Recent technological advancements in CGM present an exciting prospect for the future of cost-effective and equitable diabetes management. This literature review aimed to summarize the existing evidence for the direct link between CGM-derived metrics of glycemic management and complications of diabetes. As per the recommendations from the ATTD Congress, TIR was consolidated as an important metric; however, evidence was weaker for CV. While higher glycemic variability and lower TIR tended to be associated with diabetic complications, future research, particularly in the form of longitudinal studies, meta-analyses, and RCTs, are required to better evaluate relationships between these CGM-derived metrics and all diabetes complications, especially in T1D. Future studies should also consider the effect of closed-loop pump therapy on the development of diabetes complications.
Glossary
Abbreviations
- ATTD
Advanced Technologies & Treatments
- BGL
blood glucose level
- CAN
cardiac autonomic neuropathy
- CGM
continuous glucose monitoring
- CIMT
carotid intima-media thickness
- CONGA
continuous overall net glycemic action
- CV
coefficient of variation for glucose
- DCCT
Diabetes Control and Complications Trial
- EDIC
Epidemiology of Diabetes Interventions and Complications
- eGFR
estimated glomerular filtration rate
- GMI
glucose management indicator
- HbA1c
glycated hemoglobin A1c
- HBGI
high blood glucose index
- LADA
latent autoimmune diabetes of adulthood
- LBGI
low blood glucose index
- MAGE
mean amplitude of glycemic excursions
- RCT
randomized controlled trial
- SD
standard deviation of blood glucose levels
- TAR
time above range
- TBR
time below range
- TIR
time in range
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- UKPDS
United Kingdom Prospective Diabetes Studies.
Financial Support
This work received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. M.E.C. is supported by a National Health and Medical Research Council (NHMRC) practitioner fellowship (No. APP1136735).
Author Contributions
M.Y. contributed to the study design, acquisition of data, analysis of data, interpretation of data, drafting the article, revising it for intellectual content and final approval of the version to be published. He is the guarantor of this work. S.J. contributed to the study design, acquisition of data, analysis of data, interpretation of data, drafting the article, revising it for intellectual content and final approval of the version to be published. E.I.E. contributed to study design, acquisition of data, analysis of data, interpretation of data, drafting the article, revising it for intellectual content and final approval of the version to be published. M.E.C. contributed to the interpretation of data, drafting the article, revising it for intellectual content, and final approval of the version to be published. D.O.N. contributed to the interpretation of data, drafting the article, revising it for intellectual content, and final approval of the version to be published.
Conflict of Interests
E.I.E.’s institution receives research funding for unrelated research from Eli Lilly, Novo Nordisk, Bayer, Boehringer, AstraZeneca, and Gilead. E.I.E. and D.O.N. are chief investigators in the NHMRC CTCS–funded trial (No. APP1182464). The other authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88(11):1254-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. US Department of Health and Human Services. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. US Department of Health and Human Services; 2020. Accessed August 22, 2021. https://www.cdc.gov/diabetes/data/statistics-report/index.html [Google Scholar]
- 3. Narres M, Kvitkina T, Claessen H, et al. . Incidence of lower extremity amputations in the diabetic compared with the non-diabetic population: a systematic review. PLoS One. 2017;12(8):e0182081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams R, Karuranga S, Malanda B, et al. . Global and regional estimates and projections of diabetes-related health expenditure: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020;162:108072. [DOI] [PubMed] [Google Scholar]
- 5. Trikkalinou A, Papazafiropoulou AK, Melidonis A. Type 2 diabetes and quality of life. World J Diabetes. 2017;8(4):120-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Diabetes Association. Standards of Medical Care for Patients With Diabetes Mellitus. Diabetes Care. 1989;12(5):365-368. [DOI] [PubMed] [Google Scholar]
- 7. Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial—revisited. Diabetes. 2008;57(4):995-1001. [DOI] [PubMed] [Google Scholar]
- 8. The Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44(8):968-983. [PubMed] [Google Scholar]
- 9. Lai YR, Huang CC, Chiu WC, et al. . HbA1C variability is strongly associated with the severity of cardiovascular autonomic neuropathy in patients with type 2 diabetes after longer diabetes duration. Front Neurosci. 2019;13:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Welsh KJ, Kirkman MS, Sacks DB. Role of glycated proteins in the diagnosis and management of diabetes: research gaps and future directions. Diabetes Care. 2016;39(8):1299-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017;40(8):994-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirsch IB. Introduction: history of glucose monitoring. In: American Diabetes Association, ed. Role of Continuous Glucose Monitoring in Diabetes Treatment. American Diabetes Association; 2018. [PubMed] [Google Scholar]
- 13. Foster NC, Miller K, Dimeglio L, et al. . Marked increases in CGM use has not prevented increases in HbA1c levels in participants in the T1D exchange (T1DX) clinic network. Diabetes. 2018;67(Suppl 1):1689-P. [Google Scholar]
- 14. Battelino T, Danne T, Bergenstal RM, et al. . Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time In Range. Diabetes Care. 2019;42(8):1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monnier L, Colette C, Wojtusciszyn A, et al. . Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care. 2017;40(7):832-838. [DOI] [PubMed] [Google Scholar]
- 16. Nusca A, Tuccinardi D, Albano M, et al. . Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes Metab Res Rev. 2018;34(8):e3047. [DOI] [PubMed] [Google Scholar]
- 17. Service FJ. Glucose variability. Diabetes. 2013;62(5):1398-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beck RW, Bergenstal RM, Cheng P, et al. . The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol. 2019;13(4):614-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirsch IB, Welsh JB, Calhoun P, Puhr S, Walker TC, Price DA. Associations between HbA1c and continuous glucose monitoring-derived glycaemic variables. Diabet Med. 2019;36(12):1637-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vigersky RA, McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther. 2019;21(2):81-85. [DOI] [PubMed] [Google Scholar]
- 21. Amato M, Veglia F, de Faire U, et al. . Carotid plaque-thickness and common carotid IMT show additive value in cardiovascular risk prediction and reclassification. Atherosclerosis. 2017;263:412-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simon A, Megnien JL, Chironi G. The value of carotid intima-media thickness for predicting cardiovascular risk. Arterioscler Thromb Vasc Biol. 2010;30(2):182-185. [DOI] [PubMed] [Google Scholar]
- 23. Patton SR, Midyett LK, Dolan LM, Powers SW. A comparison of average daily risk range scores for young children with type 1 diabetes mellitus using continuous glucose monitoring and self-monitoring data. Diabetes Technol Ther. 2012;14(3):239-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vazeou A. Continuous blood glucose monitoring in diabetes treatment. Diabetes Res Clin Pract. 2011;93(Suppl 1):S125-S130. [DOI] [PubMed] [Google Scholar]
- 25. Baghurst PA, Rodbard D, Cameron FJ. The minimum frequency of glucose measurements from which glycemic variation can be consistently assessed. J Diabetes Sci Technol. 2010;4(6):1382-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lindsberg PJ, Roine RO. Hyperglycemia in acute stroke. Stroke. 2004;35(2):363-364. [DOI] [PubMed] [Google Scholar]
- 27. Alfadhli EM. Gestational diabetes mellitus. Saudi Med J. 2015;36(4):399-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Šoupal J, Škrha J Jr., Fajmon M, et al. . Glycemic variability is higher in type 1 diabetes patients with microvascular complications irrespective of glycemic control. Diabetes Technol Ther. 2014;16(4):198-203. [DOI] [PubMed] [Google Scholar]
- 29. Jin SM, Kim TH, Oh S, et al. . Association between the extent of urinary albumin excretion and glycaemic variability indices measured by continuous glucose monitoring. Diabet Med. 2015;32(2):274-279. [DOI] [PubMed] [Google Scholar]
- 30. Kuroda N, Kusunoki Y, Osugi K, et al. . Hyogo Diabetes Hypoglycemia Cognition Complications (HDHCC) study group . Relationships between time in range, glycemic variability including hypoglycemia and types of diabetes therapy in Japanese patients with type 2 diabetes mellitus: Hyogo Diabetes Hypoglycemia Cognition Complications study. J Diabetes Investig. 2021;12(2):244-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Magri CJ, Mintoff D, Camilleri L, Xuereb RG, Galea J, Fava S. Relationship of hyperglycaemia, hypoglycaemia, and glucose variability to atherosclerotic disease in type 2 diabetes. J Diabetes Res. 2018;2018:7464320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yokota S, Tanaka H, Mochizuki Y, et al. . Association of glycemic variability with left ventricular diastolic function in type 2 diabetes mellitus. Cardiovasc Diabetol. 2019;18(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yoo JH, Choi MS, Ahn J, et al. . Association between continuous glucose monitoring-derived time in range, other core metrics, and albuminuria in type 2 diabetes. Diabetes Technol Ther. 2020;22(10):768-776. [DOI] [PubMed] [Google Scholar]
- 34. Picconi F, Parravano MC, Ylli D, et al. . Role of glycemic variability on retinal neurodegeneration in patients with type 1 diabetes mellitus. Diabetes. 2016;65(Suppl 1):A150-A151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sartore G, Chilelli NC, Burlina S, Lapolla A. Association between glucose variability as assessed by continuous glucose monitoring (CGM) and diabetic retinopathy in type 1 and type 2 diabetes. Acta Diabetol. 2013;50(3):437-442. [DOI] [PubMed] [Google Scholar]
- 36. Stem MS, Dunbar GE, Jackson GR, Farsiu S, Pop-Busui R, Gardner TW. Glucose variability and inner retinal sensory neuropathy in persons with type 1 diabetes mellitus. Eye (Lond). 2016;30(6):825-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lu J, Ma X, Zhou J, et al. . Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41(11):2370-2376. [DOI] [PubMed] [Google Scholar]
- 38. Lu J, Ma X, Zhang L, et al. . Glycemic variability assessed by continuous glucose monitoring and the risk of diabetic retinopathy in latent autoimmune diabetes of the adult and type 2 diabetes. J Diabetes Investig. 2019;10(3):753-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kwai NC, Arnold R, Poynten AM, Krishnan AV. Association between glycemic variability and peripheral nerve dysfunction in type 1 diabetes. Muscle Nerve. 2016;54(5):967-969. [DOI] [PubMed] [Google Scholar]
- 40. Li F, Zhang Y, Li H, et al. . TIR generated by continuous glucose monitoring is associated with peripheral nerve function in type 2 diabetes. Diabetes Res Clin Pract. 2020;166:108289. [DOI] [PubMed] [Google Scholar]
- 41. Mayeda L, Katz R, Ahmad I, et al. . Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res Care. 2020;8(1):e000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hu YM, Zhao LH, Zhang XL, et al. . Association of glycaemic variability evaluated by continuous glucose monitoring with diabetic peripheral neuropathy in type 2 diabetic patients. Endocrine. 2018;60(2):292-300. [DOI] [PubMed] [Google Scholar]
- 43. Xu F, Zhao LH, Su JB, et al. . The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetol Metab Syndr. 2014;6(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jun JE, Lee SE, Lee YB, et al. . Continuous glucose monitoring defined glucose variability is associated with cardiovascular autonomic neuropathy in type 1 diabetes. Diabetes Metab Res Rev. 2019;35(2):e3092. [DOI] [PubMed] [Google Scholar]
- 45. Nyiraty S, Pesei F, Orosz A, et al. . Cardiovascular autonomic neuropathy and glucose variability in patients with type 1 diabetes: is there an association? Front Endocrinol (Lausanne). 2018;9:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Di Flaviani A, Picconi F, Malandrucco I, et al. . Impact of glycemic variability on cardiovascular parameters in type 2 diabetic patients. Paper presented at: Diabetes Conference: 70th Scientific Sessions of the American Diabetes Association; June 2010, Orlando, FL.
- 47. Guo Q, Zang P, Xu S, et al. . Time in range, as a novel metric of glycemic control, is reversely associated with presence of diabetic cardiovascular autonomic neuropathy independent of HbA1c in Chinese type 2 diabetes. J Diabetes Res. 2020;2020:5817074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jun JE, Jin SM, Baek J, et al. . The association between glycemic variability and diabetic cardiovascular autonomic neuropathy in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kalopita S, Liatis S, Thomakos P, et al. . Relationship between glucose variability and autonomic nervous system dysfunction in patients with type 2 diabetes. Eur J Intern Med. 2011;22:S44-S45. [Google Scholar]
- 50. Matsutani D, Sakamoto M, Minato S, et al. . Visit-to-visit HbA1c variability is inversely related to baroreflex sensitivity independently of HbA1c value in type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xu W, Zhu Y, Yang X, et al. . Glycemic variability is an important risk factor for cardiovascular autonomic neuropathy in newly diagnosed type 2 diabetic patients. Int J Cardiol. 2016;215:263-268. [DOI] [PubMed] [Google Scholar]
- 52. Borg R, Kuenen JC, Carstensen B, et al. . ADAG Study Group . HbA1(c) and mean blood glucose show stronger associations with cardiovascular disease risk factors than do postprandial glycaemia or glucose variability in persons with diabetes: the A1C-Derived Average Glucose (ADAG) study. Diabetologia. 2011;54(1):69-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Peña AS, Couper JJ, Harrington J, et al. . Hypoglycemia, but not glucose variability, relates to vascular function in children with type 1 diabetes. Diabetes Technol Ther. 2012;14(6):457-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Snell-Bergeon JK, Roman R, Rodbard D, et al. . Glycaemic variability is associated with coronary artery calcium in men with Type 1 diabetes: the Coronary Artery Calcification in Type 1 Diabetes study. Diabet Med. 2010;27(12):1436-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lu J, Wang C, Shen Y, et al. . Time in range in relation to all-cause and cardiovascular mortality in patients with type 2 diabetes: a prospective cohort study. Diabetes Care. 2021;44(2):549-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Su G, Mi S, Tao H, et al. . Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tang X, Li S, Wang Y, et al. . Glycemic variability evaluated by continuous glucose monitoring system is associated with the 10-y cardiovascular risk of diabetic patients with well-controlled HbA1c. Clin Chim Acta. 2016;461:146-150. [DOI] [PubMed] [Google Scholar]
- 58. Zhang X, Xu X, Jiao X, Wu J, Zhou S, Lv X. The effects of glucose fluctuation on the severity of coronary artery disease in type 2 diabetes mellitus. J Diabetes Res. 2013;2013(1):576916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cesana F, Giannattasio C, Nava S, et al. . Impact of blood glucose variability on carotid artery intima media thickness and distensibility in type 1 diabetes mellitus. Blood Press. 2013;22(6):355-361. [DOI] [PubMed] [Google Scholar]
- 60. Lu J, Ma X, Shen Y, et al. . Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther. 2020;22(2):72-78. [DOI] [PubMed] [Google Scholar]
- 61. Mo Y, Zhou J, Li M, et al. . Glycemic variability is associated with subclinical atherosclerosis in Chinese type 2 diabetic patients. Cardiovasc Diabetol. 2013;12(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yapanis M, James S, Craig ME, O’Neal D, Ekinci EI. Data from Complications of Diabetes and Metrics of Glycemic Management Derived From Continuous Glucose Monitoring (S1-4). Figshare. 2022. Deposited March 19, 2022. 10.6084/m9.figshare.19386992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Benbow SJ, Wallymahmed ME, MacFarlane IA. Diabetic peripheral neuropathy and quality of life. QJM. 1998;91(11):733-737. [DOI] [PubMed] [Google Scholar]
- 64. Pop-Busui R, Evans GW, Gerstein HC, et al. . Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33(7):1578-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH;. EURODIAB Prospective Complications Study Group . Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care. 2008;31(7):1360-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hammoud T, Tanguay JF, Bourassa MG. Management of coronary artery disease: therapeutic options in patients with diabetes. J Am Coll Cardiol. 2000;36(2):355-365. [DOI] [PubMed] [Google Scholar]
- 67. Schramm TK, Gislason GH, Køber L, et al. . Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk. Circulation. 2008;117(15):1945-1954. [DOI] [PubMed] [Google Scholar]
- 68. Dabelea D, Kinney G, Snell-Bergeon JK, et al. . Coronary Artery Calcification in Type 1 Diabetes Study . Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003;52(11):2833-2839. [DOI] [PubMed] [Google Scholar]
- 69. Beck RW, Bergenstal RM, Riddlesworth TD, et al. . Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. The Diabetes Control and Complications Trial Research Group. Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Ann Intern Med. 1998;128(7):517-523. [DOI] [PubMed] [Google Scholar]
- 71. Ceriello A, Esposito K, Piconi L, et al. . Glucose “peak” and glucose “spike”: impact on endothelial function and oxidative stress. Diabetes Res Clin Pract. 2008;82(2):262-267. [DOI] [PubMed] [Google Scholar]
- 72. Ceriello A, Esposito K, Piconi L, et al. . Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349-1354. [DOI] [PubMed] [Google Scholar]
- 73. El-Osta A, Brasacchio D, Yao D, et al. . Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205(10):2409-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Monnier L, Colette C. Glycemic variability: should we and can we prevent it? Diabetes Care. 2008;31(Suppl 2):S150-S154. [DOI] [PubMed] [Google Scholar]
- 75. Monnier L, Mas E, Ginet C, et al. . Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681-1687. [DOI] [PubMed] [Google Scholar]
- 76. Livingstone R, Boyle JG, Petrie JR. How tightly controlled do fluctuations in blood glucose levels need to be to reduce the risk of developing complications in people with type 1 diabetes? Diabet Med. 2020;37(4):513-521. [DOI] [PubMed] [Google Scholar]
- 77. Ajjan R, Slattery D, Wright E. Continuous glucose monitoring: a brief review for primary care practitioners. Adv Ther. 2019;36(3):579-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Runge AS, Kennedy L, Brown AS, et al. . Does time-in-range matter? Perspectives from people with diabetes on the success of current therapies and the drivers of improved outcomes. Clin Diabetes. 2018;36(2):112-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in type 2 diabetes. Diabet Med. 2008;25(3):245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589. [DOI] [PubMed] [Google Scholar]
- 81. Pease AJ, Andrikopoulos S, Abraham MB, et al. . Utilisation, access and recommendations regarding technologies for people living with type 1 diabetes: consensus statement of the ADS/ADEA/APEG/ADIPS Working Group. Med J Aust. 2021;215(10):473-478. [DOI] [PubMed] [Google Scholar]
- 82. Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tsuji S, Ishikawa T, Morii Y, et al. . Cost-effectiveness of a continuous glucose monitoring mobile app for patients with type 2 diabetes mellitus: analysis simulation. J Med Internet Res. 2020;22(9):e16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wan W, Skandari MR, Minc A, et al. . Cost-effectiveness of continuous glucose monitoring for adults with type 1 diabetes compared with self-monitoring of blood glucose: the DIAMOND randomized trial. Diabetes Care. 2018;41(6):1227-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Foreman YD, Brouwers MCGJ, van der Kallen CJH, et al. . Glucose variability assessed with continuous glucose monitoring: reliability, reference values, and correlations with established glycemic indices—The Maastricht Study. Diabetes Technol Ther. 2019;22(5):395-403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”