Abstract
Context
Whether fibroblast growth factor-23 (FGF23) and α-Klotho are associated with fractures, especially in chronic kidney disease (CKD), remains controversial.
Objective
We evaluated how FGF23, α-Klotho, and traditional mineral parameters predict fractures in individuals with and without early CKD.
Methods
We conducted a stratified case-cohort analysis using CARTaGENE, a population-based survey from Quebec, Canada. Individuals aged 40 to 69 years were selected according to outcome and CKD status (non-CKD: eGFR > 60 mL/min/1.73 m2; CKD stage 3: eGFR 30-60 mL/min/1.73 m2]). Baseline levels of c-terminal FGF23 (cFGF23), α-Klotho, parathyroid hormone (PTH), phosphate, and calcium were analyzed for associations with osteoporotic fracture incidence from recruitment (2009-2010) through March 2016. Adjusted Cox models were used, and predictors were treated linearly or flexibly using splines.
Results
A total of 312 patients (159 non-CKD; 153 CKD) were included; 98 had ≥ 1 fracture at any site during a median follow up of 70 months. Compared with non-CKD, CKD patients had increased levels of cFGF23 but similar levels of α-Klotho. cFGF23 was linearly associated with increased fracture incidence (adjusted HR = 1.81 [1.71, 1.93] per doubling for all participants). The association of α-Klotho with fracture followed a U-curve (overall P = 0.019) but was attenuated by adjustment for potential mediators (bone mineral density, phosphate, PTH). PTH and phosphate also had U-shaped associations with fracture. Associations were mostly similar between non-CKD and CKD. Adjustment for cFGF23 strongly attenuated the association between CKD status and fractures.
Conclusion
cFGF23 is associated linearly with fracture incidence while α-Klotho, PTH, and phosphate levels have a U-shaped association.
Keywords: chronic kidney disease, fracture, FGF23, α-Klotho, parathyroid hormone
Osteoporotic fractures are a major source of morbidity and mortality in aging populations (1-4). In the past decades, chronic kidney disease (CKD) has been increasingly recognized as a major risk factor for bone disease. Indeed, CKD is associated with abnormalities in mineral metabolism that lead to distinctly impaired bone phenotypes (5, 6). While terminal stages of CKD have been traditionally associated with increased fracture risk, more recent studies have identified earlier stages of CKD, affecting a much larger proportion of individuals, as a novel population with high fracture incidence (7-9).
The fibroblast growth factor 23 (FGF23) is secreted by osteocytes and exerts a phosphaturic effect by decreasing the expression of the sodium-phosphate cotransporter (10-12). It also suppresses the renal hydroxylation of vitamin D (13). Congenital and acquired states of FGF23 excess (such as autosomal dominant hypophosphatemic rickets [ADHR] and tumor-induced osteomalacia) are associated with osteopenia (14-16). Since early CKD is associated with increased circulating levels of FGF23 as a compensatory response to high phosphate levels (17, 18), a role of FGF23 in bone abnormalities of CKD has been proposed. As such, FGF23 was shown to inhibit the Wnt/β-catenin pathway and contribute to bone loss in a rodent model of CKD (19). The enzyme α-Klotho exists in a membrane-bound form and acts as the co-receptor of FGF23 on kidney cells (20). It also exists in a circulating form that is involved in phosphaturia by decreasing NaPi-2a cotransporters and TRPV5 channels in the kidney (21). In contrast to FGF23, circulating levels of α-Klotho decrease with the progression of CKD (22, 23). Furthermore, α-Klotho deletions are associated with decreased bone mass in rodent models (24, 25).
Although increased FGF23 and decreased α-Klotho levels have been linked to bone abnormalities in animal models, their association with clinical bone outcomes remains controversial. While some authors reported an association between FGF23 levels and increased fracture risk in CKD (26-28) and non-CKD populations (29), others did not observe such associations (30, 31). Similarly, inconsistent results were obtained from the few clinical studies evaluating the relationship between α-Klotho levels and fracture (26, 27, 32).
In this study, we primarily aimed at evaluating the association between FGF23-α-Klotho levels and fracture in patients with or without CKD and to compare their association with traditional mineral metabolism parameters through a stratified case-cohort analysis of the CARTaGENE (CAG) cohort. Our secondary objective was to evaluate and compare the association of FGF23, α-Klotho, and traditional mineral parameters with bone mineral density at baseline.
Methods
Design, Data Sources, and Population
We conducted a case-cohort study using data and biological samples from the population-based survey CARTaGENE (CAG; https://cartagene.qc.ca/fr), details of which have been previously published (8, 33). Briefly, CAG aimed to evaluate the association of genetic factors with chronic diseases in a representative sample of Quebec Province (Canada). It recruited individuals aged 40 to 69 years in 2009 and 2010. It excluded individuals living in nursing homes, correctional facilities, or First Nation Reserves. Participants answered a health questionnaire, undertook physical measurements, and had biological (blood and urine) samples taken during the recruitment visit. They provided consent for follow-up with provincial medico-administrative databases. Use of CAG, including follow-up data, was concordant to the Helsinki Declaration and was authorized by local ethics committees and provincial authorities.
All participants were eligible for inclusion into the case-cohort sample, except for patients (1) without plasma samples at baseline (either by nonattendance to the blood draw appointment or if samples were lost); (2) with estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2 (only 17 individuals), renal replacement therapy, kidney transplant; or (3) with teriparatide use. Patients using bisphosphonates were not excluded. We then selected participants according to a case-cohort design stratified for CKD status (Fig. 1) (34). Case-cohort designs allow the evaluation of associations of costly predictors (such as FGF23 and α-Klotho) and rare outcomes (such as fracture) by selecting patients who had the outcome (cases) and a random sample of patients from the original population (cohort), regardless of the occurrence of the outcome. Selection of cases and cohort patients can be stratified for a variable of interest (eGFR), which allows subgroup analyses with appropriate statistical power in each stratum. Compared to case-control designs, case-cohort designs generate a subsample of patients (cohort) representative of the original population which can be used for the evaluation of multiple outcomes (35). Practically, all participants who had an incident fracture in the CKD stratum (cases) were selected. An equal number of non-CKD cases were randomly selected. Finally, an equivalent number of CKD and non-CKD participants (regardless of their outcome status) were randomly selected to form the cohort group. As such, the stratified element of the design allowed for an almost equal number of non-CKD and CKD individuals in both cases and cohort groups. Nevertheless, the process yielded a slight difference between the CKD and non-CKD groups since some patients were selected by chance as both case and cohort in the CKD group.
Figure 1.
Study flowchart. The number of patients included in the CKD group is lower than the sum of case and cohort patients since 7 patients were selected both as case and cohort. Abbreviation: eGFR, estimated glomerular filtration rate.
Data Collection
Demographic and clinical parameters were collected at baseline using standardized health questionnaires. Medication was collected at recruitment by CAG staff using patients’ medication list. Body mass index (BMI) was measured at baseline. The eGFR was computed at baseline with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula (36) using serum IMDS-calibrated creatinine measurements from a central laboratory. CKD was defined as eGFR below 60 mL/min/1.73 m2 according to Kidney Disease Improving Global Outcomes (KDIGO) guidelines (37). Baseline clinical data were complete except for alcohol consumption (0.7% missing), smoking (0.6%), and BMI (6.3%).
Bone and Mineral Parameters
Circulating levels of cFGF23, α-Klotho, parathyroid hormone (PTH), and phosphate were measured from nonfasting plasma samples collected at baseline and kept at −80 °C. Freeze-thaw cycles were minimized for each sample and the same number of cycles was conducted for each parameter. Commercial enzyme-linked immunosorbent assay (ELISA) kits were used for C-terminal FGF23 (cFGF23; Quidel Corporation, San Diego, CA, USA; intra-assay coefficient of variation [CoV] 1.4% to 2.4%; inter-assay CoV 2.4% to 4.7%) and α-Klotho (Immuno-Biological Laboratories, Minneapolis, MN, USA; intra-assay CoV 2.7% to 3.5%; inter-assay CoV 2.9% to 11.4%) based on previously published comparisons (38, 39). A unique operator (L.C.D.) conducted assays. Levels of intact PTH (IMMULITE 1000; Siemens Healthcare, Erlangen, Germany) and phosphate (Vista; Siemens Healthcare, Erlangen, Germany) were measured using standard clinical methods conducted at the CHU de Quebec – Université Laval laboratory. Calcium levels were measured by CAG at baseline using standard clinical methods at each recruiting center. Data for bone and mineral markers in the case-cohort sample were complete except for PTH (2 patients) and calcium (8 patients).
Outcomes
Our primary outcome was the incidence of fractures at any anatomical site, except for toe, hand, finger, and craniofacial fractures. Incidence of fractures was evaluated in provincial medico-administrative databases with a previously validated algorithm (40). This algorithm has a sensitivity and a positive predictive value above 80% for nonvertebral fractures (40). Fracture incidence was assessed for each patient from recruitment until March 31, 2016. Fracture data was judged complete for all patients since losses to follow-up due to emigration from Quebec are rare (< 1%) (41). Fracture occurrence in the 5 years preceding recruitment was assessed similarly. Death during follow-up was also identified using medico-administrative databases. Our secondary outcome was bone mineral density (BMD) measured at baseline using calcaneal quantitative ultrasound (Lunar Achilles Express; GE Healthcare, Madison, WI, USA) and expressed as the stiffness index (42). Calcaneal BMD data was missing for 5.5% of patients.
Statistical Analysis
Analyses were conducted with R Software 3.5.1 (The R Project for Statistical Computing) using the survey package. P values under 0.05 were considered significant. Missing data in the overall population was addressed with 10 multiply imputed datasets using predictive mean matching (43). Associations were computed in each imputed dataset and combined using Rubin’s rules (44). Since their frequency was negligible (< 3%), missing data for calcium and PTH levels were treated with simple imputation according to previous recommendations (43).
For the principal outcome (fractures), we used Cox models censored at the end of follow-up or death. Each participant in the case-cohort sample was weighted according to its inverse probability of selection using Horvitz-Thompson estimators. Participant weights were further recalibrated with auxiliary variables from the overall cohort using a previously developed methodology (45, 46). This method improves the precision of estimations by using the full extent of available data in the cohort rather than only case-cohort data. Hence, obtained associations more closely approximate the expected associations in the overall CAG population.
Predictors of interest were treated both linearly and flexibly. In linear analyses, we applied a log2 transformation to predictors with a skewed distribution (cFGF23, α-Klotho, PTH; Figure S1 (47)). For flexible analyses, we modeled each predictor using restricted cubic splines with 3 knots at standard locations (43). Effects by CKD status were assessed by adding interaction terms. Unadjusted and adjusted (age, sex, BMI, ethnicity, prior fracture, secondary osteoporosis, alcohol consumption, diabetes, smoking, prevalent cardiovascular disease, and CKD status) models were built. Some parameters (BMD, phosphate, PTH) were treated as exploratory confounders and added separately to models since they could act as mediators in the relationship between cFGF23, α-Klotho and fractures. To maximize the use of all available data in CARTaGENE, adjustment (all predictors except CKD and exploratory confounders) was conducted through a fracture risk score built using all available patients. Such adjustments have previously been found to increase precision without additional bias if correlations between predictors and risk scores are low (which was the case in our cohort) (48). Hence, we were able to account for several confounders and assess interactions while minimizing overfitting. Sensitivity analyses were conducted by: (1) replacing the fracture score built in the overall cohort by a manual adjustment for all variables; (2) omitting weights recalibration using auxiliary variables; (3) excluding participants with missing data for PTH and calcium; (4) adjusting models for bisphosphonate use or hemoglobin levels; (5) using major osteoporotic fractures (MOF; hip, vertebral, distal radius, proximal humerus) as outcome; and (6) using the cFGF23/phosphate ratio as predictor.
Analyses for the secondary outcome (BMD) were conducted using linear regression models with similar consideration for predictors, inverse-probability-weighting, and adjustment scheme as the analyses for the principal outcome. Linear analyses concerning BMD were reported in percent change per percent increase in each parameter.
Results
Population Characteristics
A total of 312 individuals (153 with CKD; 159 without CKD) were selected for this case-cohort study from 18 913 individuals eligible in CARTaGENE (Fig. 1). Median eGFR was 90 mL/min/1.73 m2 (interquartile range [IQR], 79-100) in non-CKD and 55 mL/min/1.73 m2 (IQR, 49-58) in CKD. Population characteristics are displayed in Table 1. Compared with non-CKD patients, CKD patients were older, had a higher prevalence of diabetes, cardiovascular disease, and oral steroids use.
Table 1.
Population characteristics
| Eligible population(n = 18 913) | Case-cohort population (n = 312) | ||||
|---|---|---|---|---|---|
| Non-CKD (n = 159) | CKD (n = 153) | ||||
| Cohort (n = 111) | Cases (n = 48) | Cohort (n = 112) | Cases (n = 48) | ||
| Demography | |||||
| Age, years | 53 (48-61)a | 53 (46-60) | 57 (51-63)b | 64 (59-67) | 60 (55-66)b |
| Sex, female | 9587 (50.7) | 51 (45.9) | 35 (69.2)b | 48 (42.9) | 31 (64.6)b |
| White ethnicity | 16937 (89.6) | 103 (92.8) | 44 (91.7) | 104 (92.9) | 45 (93.8) |
| Clinical parameters | |||||
| eGFR (mL/min/1.73 m2) | 90 (78-99)a | 90 (79-100) | 91 (81-99) | 56 (50-59) | 52 (46-56)b |
| Body mass index (kg/m2) | 27 ± 5 | 28 ± 5 | 28 ± 6 | 30 ± 6 | 29 ± 6 |
| Active smoking | 3593 (19.0) | 20 (18.0) | 4 (8.3) | 14 (12.5) | 16 (33.3)b |
| Weekly alcohol servings | 2 (0-6)a | 3 (1-7) | 1 (0-5)b | 1 (0-3) | 1 (0-5) |
| Prior fracture | 655 (3.5) | 3 (2.7) | 4 (8.3) | 2 (1.8) | 5 (10.4)b |
| Secondary osteoporosis | 2888 (15.3) | 18 (16.2) | 13 (27.1)b | 27 (24.1) | 15 (31.3) |
| Diabetes | 1876 (9.9)a | 10 (9.0) | 4 (8.3) | 25 (22.3) | 8 (16.7) |
| Cardiovascular disease | 1304 (6.9)a | 8 (7.2) | 2 (4.2) | 21 (18.8) | 9 (18.8) |
| Calcaneal QUS stiffness index | 103 ± 19 | 104 ± 17 | 94 ± 20b | 102 ± 19 | 94 ± 20b |
| Medication | |||||
| Glucocorticoids | 136 (0.7)a | 0 (0) | 0 (0) | 3 (2.7) | 3 (6.3) |
| Bisphosphonates | 683 (3.6) | 2 (1.8) | 7 (14.6)b | 9 (8.0) | 7 (14.6) |
| Hormonal replacement therapy | 1351 (3.6) | 8 (7.2) | 8 (16.7) | 7 (6.3) | 3 (6.3) |
| Oral contraceptives | 268 (1.4) | 0 (0) | 1 (2.1) | 0 (0) | 0 (0) |
| Mineral parameters | |||||
| cFGF23, RU/mL | 80 (73-91) | 79 (73-98) | 88 (79-105) | 92 (79-114) | |
| α-Klotho, pg/mL | 677 (565-877) | 713 (583-862) | 662 (543-831) | 651 (498-783) | |
| PTH, ng/L | 17.3 (11.6-23.1) | 14.9 (8.9-23.7) | 19.7 (11.3-27.5) | 20.8 (13.0-28.3) | |
| Phosphate, mmol/L | 1.10 ± 0.21 | 1.07 ± 0.20 | 1.08 ± 0.22 | 1.15 ± 0.28 | |
| Calcium, mmol/L | 2.38 ± 0.10 | 2.38 ± 0.09 | 2.36 ± 0.11 | 2.37 ± 0.13 | |
Continuous variables are presented as means (SD) or medians (interquartile range) according to their distribution. Categorical variables are presented as counts (percentages). Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; PTH, parathyroid hormone. QUS, quantitative ultrasound.
aIndicates a P value < 0.05 for non-CKD vs CKD comparison.
bIndicates a P value < 0.05 for cases vs cohort patients in a given CKD status.
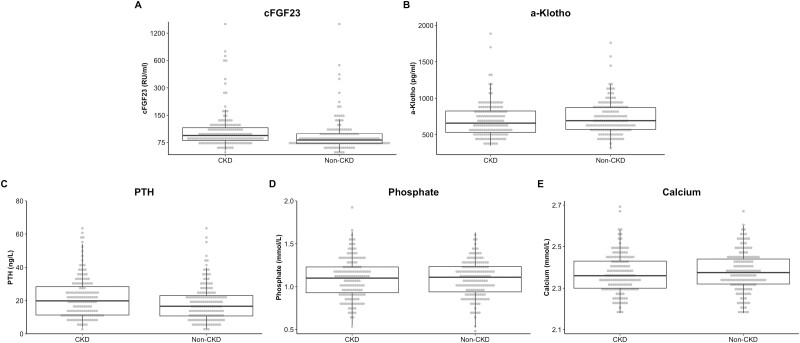
As shown in Fig. 2, patients with CKD had significantly higher levels of cFGF23 (difference = 34.3 [0.9, 67.7] RU/mL; P = 0.045). Similar results were obtained with log-transformed levels of cFGF23 (0.25 [0.10, 0.39]; P = 0.001). No differences were observed for α-Klotho (−12.9 [−74.8, 48.9] pg/mL; P = 0.682), PTH (2.52 [−0.19, 5.24] ng/L; P = 0.079), phosphate (−0.019 [−0.072, 0.034] mmol/L; P = 0.480) and calcium (−0.010 [−0.033, 0.014] mmol/L; P = 0.433). During a median follow-up period of 70 months, 803 fractures occurred in the eligible cohort. From these, we included 48 CKD and 48 non-CKD individuals as cases. Two patients included as cohort for non-CKD suffered fracture during follow-up. Anatomical sites of fractures were distal lower limb (47%), non-major upper limb (13%), forearm (12%), humerus (13%), vertebrae (5%), hip (4%), pelvis (2%), and non-hip femoral (4%). In the overall CARTaGENE cohort, 379 patients (2.0%) died during the follow-up period while 8 patients (2.6%, 1 non-CKD, 7 CKD) died in the sub-cohort.
Figure 2.
Levels of bone and mineral parameters in CKD and non-CKD. Dots represents each individual value stratified by CKD status. Boxes indicate the interquartile range of a parameter, and the bold horizontal line indicates the median. Vertical lines below and above each box indicate the 5th and 95th percentile of each parameter. Bbreviations: CKD, chronic kidney disease; PTH, parathyroid hormone.
Association of cFGF23 With Fracture and BMD
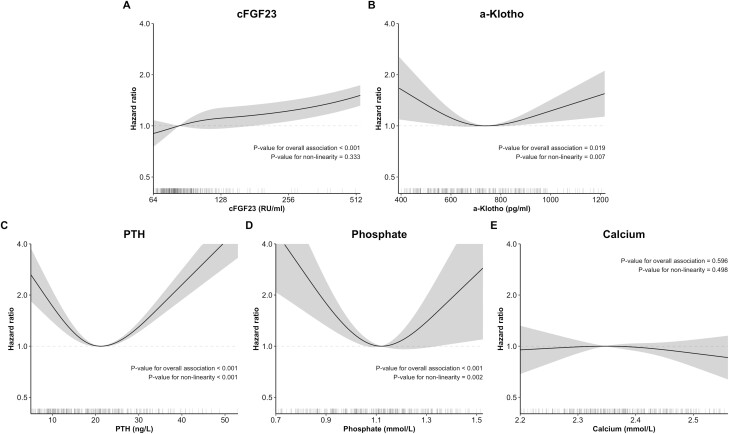
In linear analyses, cFGF23 was associated with increased fracture after adjustment in both non-CKD and CKD patients (Table 2; interaction P = 0.226). In flexible analyses, we observed that FGF23 was associated with fracture following linear curves in the overall case-cohort population (Figure 3; nonlinearity P = 0.333). Stratified analyses revealed linear and visually similar relationships between cFGF23 fracture in non-CKD and CKD (Figure S2 (47)). Similar associations were also observed after further adjustments for PTH, phosphate, and BMD (Table S1, Figures S3 and S4 (47)).
Table 2.
Association of cFGF23, α-Klotho, and mineral parameters with fracture incidence at any site
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
| Overall (n = 312) | Non-CKD (n = 159) | CKD (n = 153) | Interaction P | ||
| cFGF23 (per doubling) | 1.81 (1.71, 1.93)a | 1.99 (1.84, 2.14)a | 2.03 (1.87, 2.19)a | 1.73 (1.35, 2.21)a | 0.226 |
| α-Klotho (per doubling) | 0.68 (0.51, 0.89)a | 1.04 (0.91, 1.20) | 1.11 (0.96, 1.29) | 0.60 (0.33, 1.09) | 0.050 |
| PTH (per doubling) | 0.80 (0.71, 0.89)a | 1.10 (0.99, 1.23) | 1.00 (0.91, 1.11) | 1.69 (1.12, 2.55)a | 0.015 |
| Phosphate (per 0.1 mmol/L) | 1.04 (0.99, 1.09) | 0.94 (0.90, 0.98)a | 0.92 (0.88, 0.96)a | 1.12 (0.94, 1.32) | 0.031 |
| Calcium (per 0.1 mmol/L) | 1.07 (0.99, 1.15) | 0.95 (0.88, 1.03) | 0.96 (0.88, 1.04) | 0.94 (0.55, 1.60) | 0.938 |
Associations are presented as hazard ratios (95% CI). Hazard ratios and confidence intervals were generated in 10 multiply imputed datasets and combined using Rubin’s rules. The adjusted model includes a risk score for fractures built in the overall cohort (including age [treated flexibly with restricted cubic splines], sex, ethnicity, body mass index [treated flexibly with restricted cubic splines], prior fracture at any anatomical site, active smoking, diabetes, prevalent cardiovascular disease, alcohol consumption [treated flexibly with restricted cubic splines], secondary osteoporosis) and chronic kidney disease status.
Abbreviations: CKD, chronic kidney disease; PTH, parathyroid hormone.
aIndicates P value for association < 0.05.
Figure 3.
Association of cFGF23, α-Klotho, and mineral parameters with fracture incidence. Dark lines represent associations with fracture incidence as hazard ratios compared to the point of lowest (or highest) risk for each parameter. They are adjusted for a fracture risk score (including age [treated flexibly with restricted cubic splines], sex, ethnicity, body mass index [treated flexibly with restricted cubic splines], prior fracture at any anatomical site, active smoking, diabetes, prevalent cardiovascular disease, alcohol consumption [treated flexibly with restricted cubic splines], secondary osteoporosis) and chronic kidney status. Shaded areas represent the 95% CI at each level. Associations and confidence intervals were generated from 10 multiply imputed datasets using Rubin’s rules. Lines below each plot represent the distribution of parameters for each patient. The x-axis for cFGF23 is displayed on the log scale. The y-axis is displayed on the log scale. Abbreviation: PTH, Parathyroid hormone.
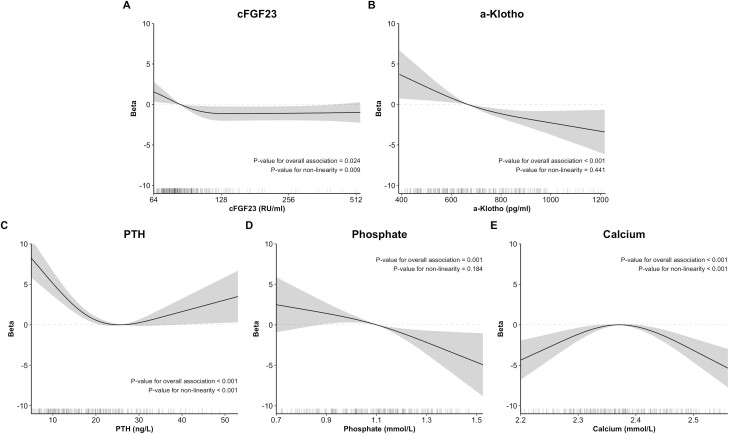
There was no association between cFGF23 and BMD in linear analyses (Table 3). However, there was a nonlinear association between cFGF23 and BMD (Figure 4; nonlinearity P value = 0.009) in which increasing cFGF23 levels were initially associated with lower BMD. This association was observed in both non-CKD and CKD individuals (Figure S5 (47); interaction P value = 0.763)
Table 3.
Association of cFGF23, α-Klotho, and mineral parameters with bone mineral density measured by calcaneal ultrasound
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
| Overall (n = 312) | Non-CKD (n = 159) | CKD (n = 153) | Interaction P | ||
| cFGF23 | 1.46 (−1.29, 4.21) | −0.71 (−2.57, 1.15) | −0.93 (−3.19, 1.34) | 1.01 (−3.23, 5.25) | 0.452 |
| α-Klotho | −3.91 (−9.26, 1.43) | −7.05 (−10.5, −3.64)a | −7.56 (−11.20, −3.91)a | −2.68 (−4.02, 9.39) | 0.012 |
| PTH | −1.69 (−3.70, 0.32) | −4.69 (−5.73, −3.64)a | −4.72 (−5.84, −3.61)a | −2.39 (−5.80, 1.01) | 0.204 |
| Phosphate | −8.87 (−11.72, −6.00)a | −7.60 (−11.67, −3.52)a | −7.60 (−12.03, −3.18)a | −8.18 (−20.16, 3.80) | 0.931 |
| Calcium | −30.28 (−47.20, −13.36)a | −13.49 (−33.26, 6.28) | −13.51 (−34.05, 7.03) | −5.63 (−54.04, 42.77) | 0.749 |
Bone density was measured using calcaneal quantitative ultrasound and stiffness index was used for these analyses. Associations are presented as beta (95% CI) per percent increase. Associations and confidence intervals were generated in 10 multiply imputed datasets and combined using Rubin’s rules. The adjusted model includes an adjustment score for stiffness index built in the overall cohort (including age [treated flexibly with restricted cubic splines], sex, ethnicity, body mass index [treated flexibly with restricted cubic splines], prior fracture at any anatomical site, active smoking, diabetes, prevalent cardiovascular disease, alcohol consumption [treated flexibly with restricted cubic splines], secondary osteoporosis) and chronic kidney disease status.
Abbreviations: CKD, chronic kidney disease; PTH, parathyroid hormone.
aIndicates P value for association < 0.05.
Figure 4.
Association of cFGF23, α-Klotho, and mineral parameters with bone density measured by calcaneal ultrasound. Dark lines represent associations with bone density (measured as calcaneal QUS stiffness index) as betas compared to the point of lowest risk, highest risk, or the median for each parameter. They are adjusted for a bone density score (including age [treated flexibly with restricted cubic splines], sex, ethnicity, body mass index [treated flexibly with restricted cubic splines], prior fracture at any anatomical site, active smoking, diabetes, prevalent cardiovascular disease, alcohol consumption [treated flexibly with restricted cubic splines], secondary osteoporosis), and chronic kidney status. Shaded areas represent the 95% CI at each level. Associations and confidence intervals were generated from 10 multiply imputed datasets using Rubin’s rules. Lines below each plot represent the distribution of parameters for each patient. Abbreviations: PTH, parathyroid hormone; QUS, quantitative ultrasound.
Association of α-Klotho With Fracture and BMD
α-Klotho was not significantly associated with fracture in linear analyses (Table 2). However, flexible analyses showed a U-shaped curve with a nadir at 742 pg/mL (Figure 3; P = 0.009). This association was slightly attenuated after adjustment for PTH and more strongly after adjustment for BMD and phosphate (Figure S3 (47)). There was no significant interaction with CKD in stratified analyses (Figure S2 (47); interaction P value = 0.160).
In linear and flexible analyses, α-Klotho was associated with decreasing BMD in the overall case-cohort population and in non-CKD individuals (Table 3 and Fig. 4). In CKD, α-Klotho was not associated with BMD in linear analyses but followed an inverted U-shaped relationship in stratified flexible analyses (Figure S5 (47); interaction P value < 0.001).
Association of Traditional Mineral Parameters With Fracture and BMD
PTH was not linearly associated with fracture incidence in the overall case-cohort population and in non-CKD individuals (Table 2). However, it was significantly associated with increased fracture incidence in CKD (interaction P value = 0.015). Flexible analyses showed a U-curve with a nadir at 21 ng/L (Fig. 3; P < 0.001). This association was observed in both non-CKD and CKD (Figure S2 (47); interaction P value = 0.501).
Phosphate was linearly associated with decreased fracture incidence in the overall case-cohort population and in non-CKD individuals (Table 2). Phosphate was associated with fractures in flexible analyses following a U-shaped curve (Figure 3; P < 0.001) with a nadir at 1.12 mmol/L. This association was similar in non-CKD and CKD (Figure S2; interaction P value = 0.461). Calcium was not associated with fracture incidence in linear and flexible analyses for the overall case-cohort population (Table 2 and Fig. 3). However, it was associated with fractures following a U-shaped curve in CKD (Figure S2 (47); interaction P value < 0.001).
With regard to BMD, both PTH and phosphate were linearly associated with BMD in non-CKD individuals (Table 3). In flexible analyses, PTH was associated with BMD following a U-shaped curve, while phosphate followed a downward linear curve (Fig. 4). Calcium showed an inverted U-shaped curve in non-CKD and a U-shaped curve in CKD (Figure S5 (47); interaction P value = 0.010).
Association of CKD With Fracture Incidence
As previously reported (8), CKD was associated with increased fracture incidence after adjustment for demographic and clinical confounders (hazard ratio [HR] = 1.37 [1.02, 1.83]). The association was attenuated after further adjustments for cFGF23 (HR = 1.08 [0.80, 1.46]) or PTH (HR = 1.20 [0.89, 1.63]). In contrast, it was barely attenuated after adjustments for α-Klotho (HR = 1.38 [0.99, 1.93]), phosphate (HR = 1.40 [1.00, 1.97]), or calcium levels (HR = 1.34 [0.98, 1.83]).
Sensitivity Analyses
As shown in Table S2 (47), exclusion of patients with missing data for calcium or PTH did not alter the results. Analyses conducted without recalibrating participants’ weights or with manual adjustment for all confounders did not consistently influence association estimates. However, these 2 analyses increased the confidence interval widths as expected. Adjusting analyses for bisphosphonate use or hemoglobin levels yielded results similar to the principal analysis. When MOFs were used as outcome (Table 4), the linear associations of cFGF23 (in global, non-CKD, and CKD populations), α-Klotho (in CKD) and PTH (in global and non-CKD populations) were amplified. In contrast, the association of phosphate with reduced fractures in the global and non-CKD populations was not seen with MOF. Finally, results for the cFGF23/phosphate ratio in the global (adjusted HR = 2.08 [1.90, 2.28]), non-CKD (HR = 2.16 [1.93, 2.41]), and CKD (HR = 1.64 [1.12, 2.40]) populations were similar to the results for cFGF23 alone.
Table 4.
Association of cFGF23, α-Klotho, and mineral parameters with major osteoporotic fracture
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
| Overall (n = 312) | Non-CKD (n = 159) | CKD (n = 153) | Interaction P | ||
| cFGF23 (per doubling) | 2.19 (1.91, 2.51)a | 2.34 (2.04, 2.68)a | 2.42 (2.09, 2.79)a | 1.84 (1.51, 2.24)a | 0.019 |
| α-Klotho (per doubling) | 0.53 (0.33, 0.87)a | 1.07 (0.81, 1.42) | 1.29 (0.97, 1.71) | 0.30 (0.09, 0.97)a | 0.018 |
| PTH (per doubling) | 1.01 (0.75, 1.38) | 1.95 (1.35, 2.82)a | 2.09 (1.39, 3.15)a | 0.95 (0.50, 1.79) | 0.036 |
| Phosphate (per 0.1 mmol/L) | 1.16 (1.04, 1.29)a | 1.01 (0.93, 1.08) | 1.00 (0.93, 1.07) | 1.12 (0.85, 1.47) | 0.431 |
| Calcium (per 0.1 mmol/L) | 1.10 (0.95, 1.28) | 1.01 (0.88, 1.17) | 1.02 (0.89, 1.19) | 0.88 (0.38, 2.02) | 0.720 |
Associations are presented as hazard ratios (95% CI). Hazard ratios and confidence intervals were generated in 10 multiply imputed datasets and combined using Rubin’s rules. The adjusted model includes a risk score for fractures built in the overall cohort (including age [treated flexibly with restricted cubic splines], sex, ethnicity, body mass index [treated flexibly with restricted cubic splines], prior fracture at any anatomical site, active smoking, diabetes, prevalent cardiovascular disease, alcohol consumption [treated flexibly with restricted cubic splines], secondary osteoporosis), and chronic kidney disease status.
Abbreviation: CKD, chronic kidney disease; PTH, parathyroid hormone.
aIndicates P value for association < 0.05.
Discussion
In this case-cohort study, cFGF23 was associated with decreased BMD at baseline and increased fracture incidence in both non-CKD and early CKD individuals. In contrast, α-Klotho, PTH, and phosphate levels had mostly U-shaped relationships with fractures.
Our findings concerning cFGF23 are in line with previous animal studies in which increased expression of cFGF23 was shown to be detrimental on bone (19, 49). They are also concordant with clinical results from Ribeiro et al, who studied 126 patients with diabetic nephropathy and found that c-terminal FGF23 levels were also associated with increased fractures (27). In contrast, others observed an association between intact FGF23 levels and increased fracture only in CKD patients (28) or in patients with eGFR above median (71 mL/min/1.73 m2) (29). Finally, 2 other studies did not observe associations between FGF23 (intact for 1 study and c-terminal for the other) and fracture incidence or any interaction between FGF23 and CKD status or eGFR (30, 31). Our study differs from these since we: (1) specifically built our case-cohort design to conduct CKD stratified analyses; (2) studied middle-aged patients; (3) used splines in both non-CKD and CKD groups; and (4) used 2 novel methodological strategies to maximize statistical power based on the full scope of available data. Another difference between our study and some of the previous ones is the use of the c-terminal FGF23, which is less prone to preanalytical instability, circadian cycles, and week-to-week variation (38). Nevertheless, since similar associations between FGF23 and fractures were seen with both intact and c-terminal FGF23, the type of assay used does not seem to play a key role in the detection of the FGF23-fracture relationship. The predominant effect of inflammation and iron deficiency on c-terminal rather than intact FGF23 may also influence the relationships between various FGF23 assays and fracture (50). While direct markers of inflammation and iron deficiency were not available in our cohort, adjustment for hemoglobin levels did not influence our findings.
We also showed that only FGF23 levels were different between non-CKD and CKD individuals, which is probably explained by the mild level of renal disease (mostly stage 3A) in our CKD group (median eGFR = 55 mL/min/1.73 m2). This finding is coherent with past studies having shown the early increase of FGF23 in CKD (18). While an age-related eGFR decline may explain part of the CKD status in our cohort, the young age of our CKD group (median 60 to 64 years) suggests that a substantial proportion of these patients had intrinsic renal disease (the expected eGFR at this age is 80 mL/min/1.73 m2) (51). The distribution of cFGF23 could also have influenced the difference in cFGF23 between non-CKD and CKD. Indeed, a few patients with high levels (most of them CKD) may have skewed the mean difference between the groups. Nevertheless, we observed a similar difference after a log-transformation, suggesting a real difference in cFGF23 levels despite normalization of its distribution.
Interestingly, we found that adjustments for cFGF23 strongly attenuated the relationship between CKD and fracture incidence, in contrast to α-Klotho and the other traditional mineral parameters. Additionally, the association between cFGF23 and fractures was not attenuated by further adjustments for PTH, phosphate and BMD, which suggests that this relationship may be independent from these parameters. While these findings highlight a potential role of FGF23 as a biomarker of fracture risk in patients with early stages of CKD, our findings should not be extrapolated to patients with more advanced stages of CKD.
Two studies previously investigated the association of α-Klotho with fractures in nondialysis CKD patients and led to contrasting results. While Ribeiro et al observed an inverse U-shaped relationship between α-Klotho levels and fracture (27), Chalhoub et al did not observe any association in patients from the Health ABC cohort (aged 70-79 years) (32). Our study differs from these studies at several levels, including the use of a population-based cohort, nonlinear analyses with splines, and of interactions with CKD status. In contrast, they used the same ELISA assay to measure α-Klotho levels. Although this assay is the most commonly used one and was shown to perform better than others (notably concerning plasma-serum correlation and intra/inter-assay variation), low agreement between different assays and potential cross-reactivity with other proteins should be recognized as limitations for the use of α-Klotho ELISA assays (39).
Conflicting results concerning α-Klotho have been obtained in animal models. Indeed, while global deletions of α-Klotho have been associated with osteoporosis (24, 25), bone-specific deletions have been associated with increased bone mass (52). Interestingly, we further explored the relationship of PTH and phosphate with α-Klotho by adjusting our models with these 2 factors and found that they attenuated the α-Klotho-fracture association. These results suggest that the association between α-Klotho levels and fracture may be partially mediated by phosphate and PTH levels.
In this study, we also observed that PTH and phosphate were associated with fracture at low and high levels. While PTH levels were not associated with fracture in several studies of the general population (53-55), increased PTH levels were recently associated with fracture in a cohort of patients with CKD stages 3 and 4 (56). Furthermore, while primary hyperparathyroidism is known to increase fracture risk (57), whether hypoparathyroidism is associated with such an increase remains controversial (58, 59). In contrast, the relationship between phosphate and fracture has been less studied. Obando et al studied 2 population-based cohorts (including patients with CKD) and reported that phosphate levels were linearly associated with increased fracture risk in both non-CKD and CKD (60). Another study did not observe a linear association between phosphate levels and fracture in their analysis of the Cardiovascular Health Study (54). Methodological discrepancies, notably the use of restricted cubic splines, may explain the U-shaped relationship findings between phosphate and fractures in our study. Taken together, this body of evidence suggest that high levels of phosphate and PTH, even at “normal values”, can be associated with increased fractures. However, further studies will be needed to elucidate the relationship between low levels of these parameters and fracture, which is suggested by this study.
Our work has several strengths: (1) We conducted a stratified case-cohort study specifically designed to investigate the associations of novel and traditional mineral parameters with fracture according to CKD status; (2) We used statistical methods to maximize the use of available data and enhance precision of our estimates—sensitivity analyses were also used to assess the influence of these statistical methods; (3) We investigated a population-based cohort of middle-aged adults which are often understudied; (4) We assessed 2 bone markers with ELISA kits that were chosen according their previously reported accuracy (38, 39); (5) Finally, we conducted linear and flexible analyses using splines to better characterize the studied associations.
This study also has limitations: (1) Our results cannot be applied to more advanced CKD individuals; (2) Analyses for specific fracture sites were not conducted due to the limited number of fractures in CKD; (3) BMD was measured by quantitative calcaneal ultrasound rather than traditional dual-energy x-ray absorptiometry (DXA). Although this modality uses a completely different mechanism than DXA to measure BMD, the stiffness index it generates has been correlated with DXA at multiple sites and associated with fractures in several studies (42, 61-65); (4) PTH levels were assessed on previously frozen plasma samples. Nevertheless, freeze-thaw cycles were minimized, and we ensured that the time between collection and measurement of each sample was similar. Likewise, to minimize analytical concerns, we only measured C-terminal FGF23 (38); (5) Only one blood measurement was conducted for each individual so longitudinal variation could not be evaluated; (6) Measurements were conducted in a nonfasting state; which could have influenced PTH and phosphate levels; and (7) Data on other potential confounders such as proteinuria, iron status, markers of inflammation, vitamin D, and its metabolites were not available.
In conclusion, we showed that cFGF23 levels are associated with fractures in middle-aged adults with and without early CKD. We also revealed that α-Klotho and some traditional mineral parameters are associated with fracture following U-shaped curves even inside their “physiological” values. Finally, our work identifies cFGF23 as a potential biomarker of increased fracture incidence in early CKD individuals. Taken together, these findings provide crucial insight into the clinical role of FGF23-α-Klotho axis in the development of bone abnormalities. Further studies will be necessary to elucidate the relevance of the observed U-shaped relationships.
Glossary
Abbreviations
- BMD
bone mineral density
- BMI
body mass index
- cFGF23
C-terminal fibroblast growth factor 23
- CKD
chronic kidney disease
- CoV
coefficient of variation
- DXA
dual-energy x-ray absorptiometry
- eGFR
estimated glomerular filtration rate
- ELISA
enzyme-linked immunosorbent assay
- FGF23
fibroblast growth factor 23
- HR
hazard ratio
- KDIGO
Kidney Disease: Improving Global Outcomes
- MOF
major osteoporotic fracture
- PTH
parathyroid hormone
Financial Support
L.C.D. holds a past Master’s scholarship from the Canadian Institutes of Health Research (CIHR), Fonds de Recherche du Québec Santé (FRQS) and Laval University Faculty of Medicine. A.S. holds a PhD scholarship from the CIHR and the Societé Québécoise d’hypertension artérielle. F.M.W. holds a scholarship from FRQS (32661) and past scholarship form the KRESCENT (150006) program from CIHR, Canadian Society of Nephrology and Kidney Foundation of Canada; he is also supported by the Department of Medicine and the Fondation du CHU de Québec from Université Laval.
Disclosures
Louis-Charles Desbiens, Aboubacar Sidibé, Roth-Visal Ung, and Fabrice Mac-Way declare that they have no conflict of interest.
Data Availability
CARTaGENE data (https://cartagene.qc.ca/fr) was used under license. Restrictions apply to its availability to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Brenneman SK, Barrett-Connor E, Sajjan S, Markson LE, Siris ES. Impact of recent fracture on health-related quality of life in postmenopausal women. J Bone Miner Res. 2006;21(6): 809-816. [DOI] [PubMed] [Google Scholar]
- 2. Hallberg I, Rosenqvist AM, Kartous L, Lofman O, Wahlstrom O, Toss G. Health-related quality of life after osteoporotic fractures. Osteoporos Int. 2004;15(10):834-841. [DOI] [PubMed] [Google Scholar]
- 3. Morin S, Lix LM, Azimaee M, Metge C, Caetano P, Leslie WD. Mortality rates after incident non-traumatic fractures in older men and women. Osteoporos Int. 2011;22(9):2439-2448. [DOI] [PubMed] [Google Scholar]
- 4. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. [DOI] [PubMed] [Google Scholar]
- 5. et al. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;(113):S1-130. [DOI] [PubMed] [Google Scholar]
- 6. Mac Way F, Lessard M, Lafage-Proust MH. Pathophysiology of chronic kidney disease-mineral and bone disorder. Joint Bone Spine. 2012;79(6):544-549. [DOI] [PubMed] [Google Scholar]
- 7. Sidibe A, Auguste D, Desbiens LC, et al. Fracture risk in dialysis and kidney transplanted patients: a systematic review. JBMR Plus. 2019;3(1):45-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Desbiens LC, Goupil R, Madore F, Mac-Way F. Incidence of fractures in middle-aged individuals with early chronic kidney disease: a population-based analysis of CARTaGENE. Nephrol Dial Transplant. 2020;35(10):1712-1721. [DOI] [PubMed] [Google Scholar]
- 9. Vilaca T, Salam S, Schini M, et al. Risks of hip and nonvertebral fractures in patients with CKD G3a-G5D: a systematic review and meta-analysis. Am J Kidney Dis. 2020;76(4):521-532. [DOI] [PubMed] [Google Scholar]
- 10. Yoshiko Y, Wang H, Minamizaki T, et al. Mineralized tissue cells are a principal source of FGF23. Bone. 2007;40(6):1565-1573. [DOI] [PubMed] [Google Scholar]
- 11. Gattineni J, Bates C, Twombley K, et al. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol. 2009;297(2):F282-F291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baum M, Schiavi S, Dwarakanath V, Quigley R. Effect of fibroblast growth factor-23 on phosphate transport in proximal tubules. Kidney Int. 2005;68(3):1148-1153. [DOI] [PubMed] [Google Scholar]
- 13. Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429-435. [DOI] [PubMed] [Google Scholar]
- 14. White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60(6):2079-2086. [DOI] [PubMed] [Google Scholar]
- 15. Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278(39):37419-37426. [DOI] [PubMed] [Google Scholar]
- 16. Shimada T, Mizutani S, Muto T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98(11):6500-6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64(6):2272-2279. [DOI] [PubMed] [Google Scholar]
- 18. Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carrillo-Lopez N, Panizo S, Alonso-Montes C, et al. Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int. 2016;90(1):77-89. [DOI] [PubMed] [Google Scholar]
- 20. Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770-774. [DOI] [PubMed] [Google Scholar]
- 21. Hum JM, O’Bryan L, Smith RC, White KE. Novel functions of circulating Klotho. Bone. 2017;100:36-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu MC, Shi M, Zhang J, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24(9):3438-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pavik I, Jaeger P, Ebner L, et al. Secreted Klotho and FGF23 in chronic kidney disease stage 1 to 5: a sequence suggested from a cross-sectional study. Nephrol Dial Transplant. 2013;28(2):352-359. [DOI] [PubMed] [Google Scholar]
- 24. Kawaguchi H, Manabe N, Miyaura C, Chikuda H, Nakamura K, Kuro-o M. Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J Clin Invest. 1999;104(3):229-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45-51. [DOI] [PubMed] [Google Scholar]
- 26. Desbiens LC, Sidibe A, Ung RV, et al. FGF23-klotho axis, bone fractures, and arterial stiffness in dialysis: a case-control study. Osteoporos Int. 2018;29(10):2345-2353. [DOI] [PubMed] [Google Scholar]
- 27. Ribeiro AL, Mendes F, Carias E, et al. FGF23-klotho axis as predictive factors of fractures in type 2 diabetics with early chronic kidney disease. J Diabetes Complications. 2019:107476. doi:10.1016/j.jdiacomp.2019.107476 [DOI] [PubMed] [Google Scholar]
- 28. Lane NE, Parimi N, Corr M, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group . Association of serum fibroblast growth factor 23 (FGF23) and incident fractures in older men: the Osteoporotic Fractures in Men (MrOS) study. J Bone Miner Res. 2013;28(11):2325-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mirza MA, Karlsson MK, Mellstrom D, et al. Serum fibroblast growth factor-23 (FGF-23) and fracture risk in elderly men. J Bone Miner Res. 2011;26(4):857-864. [DOI] [PubMed] [Google Scholar]
- 30. Jovanovich A, Buzkova P, Chonchol M, et al. Fibroblast growth factor 23, bone mineral density, and risk of hip fracture among older adults: the cardiovascular health study. J Clin Endocrinol Metab. 2013;98(8):3323-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Isakova T, Cai X, Lee J, et al. Associations of FGF23 with change in bone mineral density and fracture risk in older individuals. J Bone Miner Res. 2016;31(4):742-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chalhoub D, Marques E, Meirelles O, et al. Association of serum Klotho with loss of bone mineral density and fracture risk in older adults. J Am Geriatr Soc. 2016;64(12):e304-e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Awadalla P, Boileau C, Payette Y, et al. Cohort profile of the CARTaGENE study: Quebec’s population-based biobank for public health and personalized genomics. Int J Epidemiol. 2013;42(5):1285-1299. [DOI] [PubMed] [Google Scholar]
- 34. Borgan O, Langholz B, Samuelsen SO, Goldstein L, Pogoda J. Exposure stratified case-cohort designs. Lifetime Data Anal. 2000;6(1):39-58. [DOI] [PubMed] [Google Scholar]
- 35. Cologne J, Preston DL, Imai K, et al. Conventional case-cohort design and analysis for studies of interaction. Int J Epidemiol. 2012;41(4):1174-1186. [DOI] [PubMed] [Google Scholar]
- 36. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levin A, Stevens PE, Bilous RW, et al. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1-150. [Google Scholar]
- 38. Smith ER. The use of fibroblast growth factor 23 testing in patients with kidney disease. Clin J Am Soc Nephrol. 2014;9(7):1283-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heijboer AC, Blankenstein MA, Hoenderop J, de Borst MH, Vervloet MG, consortium N. Laboratory aspects of circulating alpha-Klotho. Nephrol Dial Transplant. 2013;28(9):2283-2287. [DOI] [PubMed] [Google Scholar]
- 40. Jean S, Candas B, Belzile E, et al. Algorithms can be used to identify fragility fracture cases in physician-claims databases. Osteoporos Int. 2012;23(2):483-501. [DOI] [PubMed] [Google Scholar]
- 41. Statistics Canada. Table 17-10-0008-01 estimates of the components of demographic growth, annual. 2017. Accessed February 15, 2020. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000801
- 42. Zagzebski JA, Rossman PJ, Mesina C, Mazess RB, Madsen EL. Ultrasound transmission measurements through the os calcis. Calcif Tissue Int. 1991;49(2):107-111. [DOI] [PubMed] [Google Scholar]
- 43. Harrell F. Regression Modeling Strategies. 2nd ed. Springer International Publishing; 2015. [Google Scholar]
- 44. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley and Sons; 1987. [Google Scholar]
- 45. Breslow NE, Lumley T, Ballantyne CM, Chambless LE, Kulich M. Improved Horvitz-Thompson estimation of model parameters from two-phase stratified samples: applications in epidemiology. Stat Biosci. 2009;1(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Breslow NE, Lumley T, Ballantyne CM, Chambless LE, Kulich M. Using the whole cohort in the analysis of case-cohort data. Am J Epidemiol. 2009;169(11):1398-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Desbiens LC, Sidibe A, Ung RV, Mac-Way F. Data from: FGF23-Klotho axis and fractures in patients without and with early CKD - Supplemental data. 2022. Deposited February 9, 2022. 10.6084/m9.figshare.16641619.v1. [DOI] [PMC free article] [PubMed]
- 48. Arbogast PG, Ray WA. Performance of disease risk scores, propensity scores, and traditional multivariable outcome regression in the presence of multiple confounders. Am J Epidemiol. 2011;174(5):613-620. [DOI] [PubMed] [Google Scholar]
- 49. Wang H, Yoshiko Y, Yamamoto R, et al. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J Bone Miner Res. 2008;23(6):939-948. [DOI] [PubMed] [Google Scholar]
- 50. David V, Martin A, Isakova T, et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016;89(1):135-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Delanaye P, Jager KJ, Bokenkamp A, et al. CKD: a call for an age-adapted definition. J Am Soc Nephrol. 2019;30(10):1785-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Komaba H, Kaludjerovic J, Hu DZ, et al. Klotho expression in osteocytes regulates bone metabolism and controls bone formation. Kidney Int. 2017;92(3):599-611. [DOI] [PubMed] [Google Scholar]
- 53. Barbour KE, Houston DK, Cummings SR, et al. Calciotropic hormones and the risk of hip and nonspine fractures in older adults: the Health ABC Study. J Bone Miner Res. 2012;27(5): 1177-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Robinson-Cohen C, Katz R, Hoofnagle AN, et al. Mineral metabolism markers and the long-term risk of hip fracture: the cardiovascular health study. J Clin Endocrinol Metab. 2011;96(7):2186-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lai JKC, Lucas RM, Clements MS, et al. Hip fracture risk in relation to vitamin D supplementation and serum 25-hydroxyvitamin D levels: a systematic review and meta-analysis of randomised controlled trials and observational studies. BMC Public Health. 2010;10:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Geng S, Kuang Z, Peissig PL, Page D, Maursetter L, Hansen KE. Parathyroid hormone independently predicts fracture, vascular events, and death in patients with stage 3 and 4 chronic kidney disease. Osteoporos Int. 2019;30(10):2019-2025. [DOI] [PubMed] [Google Scholar]
- 57. Narayanan N, Palui R, Merugu C, et al. The risk of fractures in primary hyperparathyroidism: a meta-analysis. JBMR Plus. 2021;5(4):e10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rubin MR. Skeletal manifestations of hypoparathyroidism. Bone. 2019;120:548-555. [DOI] [PubMed] [Google Scholar]
- 59. Pal R, Bhadada SK, Mukherjee S, Banerjee M, Kumar A. Fracture risk in hypoparathyroidism: a systematic review and meta-analysis. Osteoporos Int. 2021;32(11):2145-2153. [DOI] [PubMed] [Google Scholar]
- 60. Campos-Obando N, Koek WNH, Hooker ER, et al. Serum phosphate is associated with fracture risk: the Rotterdam Study and MrOS. J Bone Miner Res. 2017;32(6):1182-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Trimpou P, Bosaeus I, Bengtsson BA, Landin-Wilhelmsen K. High correlation between quantitative ultrasound and DXA during 7 years of follow-up. Eur J Radiol. 2010;73(2): 360-364. [DOI] [PubMed] [Google Scholar]
- 62. Kang C, Speller R. Comparison of ultrasound and dual energy X-ray absorptiometry measurements in the calcaneus. Br J Radiol. 1998;71(848):861-867. [DOI] [PubMed] [Google Scholar]
- 63. McLeod KM, Johnson S, Rasali D, Verma A. Discriminatory performance of the calcaneal quantitative ultrasound and osteoporosis self-assessment tool to select older women for dual-energy X-ray absorptiometry. J Clin Densitom. 2015;18(2):157-164. [DOI] [PubMed] [Google Scholar]
- 64. McCloskey EV, Kanis JA, Oden A, et al. Predictive ability of heel quantitative ultrasound for incident fractures: an individual-level meta-analysis. Osteoporos Int. 2015;26(7):1979-1987. [DOI] [PubMed] [Google Scholar]
- 65. Moayyeri A, Adams JE, Adler RA, et al. Quantitative ultrasound of the heel and fracture risk assessment: an updated meta-analysis. Osteoporos Int. 2012;23(1):143-153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
CARTaGENE data (https://cartagene.qc.ca/fr) was used under license. Restrictions apply to its availability to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.