Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in children. Primary-care physicians (PCPs) play a key role in identifying patients requiring specialist referral. In this study, we aim to determine PCPs’ practice patterns for paediatric NAFLD, as knowledge gaps have been reported for adult NAFLD.
Methods
A survey was sent to 60 PCPs in the Eastern Ontario Network from July 2019 to January 2020.
Results
Thirty-seven (62%) PCPs responded to the survey. Twenty-one incorrectly considered the prevalence of paediatric NAFLD to be ≤10%. The majority (35/36) cared for less than five paediatric NAFLD patients. Thirty-four (92%) were only ‘slightly familiar’ or ‘not familiar at all’ with paediatric NAFLD. Only one PCP routinely screens for NAFLD. Only one PCP was aware of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) clinical guidelines for paediatric NAFLD. Twenty-five (68%) correctly selected lifestyle modifications as a treatment option. Lack of confidence in the knowledge of NAFLD was the most common barrier for managing paediatric cases.
Conclusion
The majority of PCPs are not screening for paediatric NAFLD and are not familiar with its clinical spectrum, citing a lack of knowledge regarding NAFLD as the greatest barrier. This may cause delays in diagnosis and a presentation with advanced fibrosis at the time of specialist referral. Dissemination and implementation of clinical guidelines have the potential to improve knowledge and screening rates for NAFLD in children at the primary-care level.
Keywords: Awareness, Nonalcoholic fatty liver disease, Nonalcoholic steatohepatitis, Practice guidelines, Primary care physicians
Graphical Abstract
In recent years, there has been a global increase in the prevalence of nonalcoholic fatty liver disease (NAFLD) which is estimated to be present in ~30% of the general population in North America (1). NAFLD is fatty infiltration of the liver (steatosis) in the absence of significant alcohol, metabolic, genetic diseases, or medications that cause steatosis (2). Its spectrum ranges from benign hepatic steatosis to nonalcoholic steatohepatitis (NASH), which is an inflammatory progressive stage that can lead to cirrhosis and liver failure (2). Although a minority of those with NAFLD (3% to 5%) develop clinically significant liver disease, NAFLD is predicted to be the leading indication for liver transplantation in adults within the next decade due to increasing prevalence (3).
Obesity is strongly associated with NAFLD (4). In 2019, the World Obesity Federation estimated that over 150 million children are obese and this number will rise to 206 million by 2025 (5). Without intervention, obese children and adolescents are five times more likely than nonobese children to be obese in adulthood (6). In 2015, a systematic review and meta-analysis reported that the worldwide prevalence of paediatric NAFLD was 7.6% from general population studies and 34.2% from obesity clinic studies (7). The exact prevalence of paediatric NAFLD in Canada is unknown. Children have been reported to have NAFLD as early as 2 years of age, with NASH-related cirrhosis occurring as early as 8 years (8,9).
Primary care physicians (PCPs) are often the first contact healthcare professionals to manage paediatric patients with NAFLD. PCPs follow patients with NAFLD risk factors such as obesity and diabetes mellitus but may have limited awareness of the prevalence, spectrum, and management of NAFLD in adults (10–13). To address these issues, referral pathways have been developed for PCPs with adult NAFLD patients (14). PCPs play a key role in identifying patients with advanced disease who require a specialist referral, but to our knowledge, there is no information about practice patterns or referral pathways for paediatric NAFLD.
The North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) clinical guidelines for paediatric NAFLD were introduced in 2012 and updated in 2017 (15,16). The target populations are paediatricians and health professionals caring for children. The guidelines include recommendations regarding screening practices, diagnostic tests, treatment modalities, follow-up frequency, and counseling for patients and their families.
This study aimed to determine PCP awareness and attitudes toward paediatric NAFLD and describe whether practices of screening, diagnosis, treatment, and specialist referral practices by PCPs adhere to the NASPGHAN guidelines (16).
METHODS
Study design
A cross-sectional online survey of 60 PCPs in the Eastern Ontario Network (EON) was performed July 2019 through January 2020. EON is a practice-based research network located within the Queen’s University Department of Family Medicine in Kingston, Ontario, Canada. Practice-based research networks, as defined by the Agency for Healthcare Research and Quality, are “groups of primary-care clinicians and practices working together to answer community-based health care questions and translate research findings into practice” (17). EON included 150 PCPs across 14 clinics in Eastern Ontario, Canada. Out of these 150 PCPs, 60 had previously indicated they would be open to participating in research at any given time and were contacted about this online survey.
Participants were contacted via fax and email to participate. Three reminders were sent 8 weeks apart each. No compensation was provided for survey completion. The study was approved by the Research Ethics Board at Queen’s University (File no: 6026798).
Survey development and data collection
A 28-item questionnaire was developed to assess the awareness, attitudes, and practice patterns of paediatric NAFLD. The questions and response options were developed using a literature review of previously published NAFLD awareness questionnaires in the adult population. The paediatric NASPGHAN guidelines were also used to guide the questionnaire and determine important, high yield topics within paediatric NAFLD. Participants were asked about paediatric NAFLD in their practices, along with their general awareness, knowledge, and self-reported familiarity with paediatric NAFLD (Supplementary Appendix).
The questionnaire was administered using Qualtrics Survey Software. The usability and functionality of the questionnaire on Qualtrics were tested by members of the research team before reaching out to potential participants.
Statistics
Descriptive statistics were employed for data presentation: percentages, medians, mean, and ranges for continuous variables, and categorical variables are reported as count and numeric proportions.
RESULTS
Thirty-seven of 60 PCPs (62%; 27 females and 10 males) completed the survey. One participant only answered the demographic-related questions and was removed from the final analysis. The ages of respondents ranged from 25 years old to greater than 65 years old. The majority (78%) of participants were affiliated with an academic institution, but practice locations and settings differed (Table 1). Years in practice ranged from less than five to more than 20.
Table 1.
Practice characteristics of PCPs
| Practice setting* | |
|---|---|
| Hospital | 4 (11%) |
| Clinic | 10 (28%) |
| Group | 30 (83%) |
| Solo private | 1 (3%) |
| Other | 2 (6%) |
| Practice location | |
| Urban | 17 (47%) |
| Sub-urban | 8 (22%) |
| Rural | 9 (25%) |
| Mixed | 2 (6%) |
| Years in practice | |
| <5 | 10 (28%) |
| 5–10 | 9 (25%) |
| 11–15 | 9 (25%) |
| >20 | 8 (22%) |
| Affiliated w/ academic institution | |
| Yes | 28 (78%) |
| No | 8 (22%) |
*Respondents were able to choose more than one practice setting.
Practices
Thirty-five respondents (97%) indicated that they see less than five patients for paediatric NAFLD annually, while one indicated that they see more than 20. Only one regularly screens children for NAFLD. The pre-existing conditions that would prompt them to screen for NAFLD were obesity (81%), diabetes mellitus (69%), elevated liver aspartate transaminase (AST)/alanine transaminase (ALT) (69%), dyslipidemia (44%), hypertension (39%), polycystic ovarain syndrome (33%), sleep apnea (31%), and hypotension (25%). Three participants (8%) were unsure of who they should screen.
Liver enzymes measurement and abdominal ultrasound were reported as the most common screening workups for NAFLD by 30/36 (83%) and 27/36 (75%), respectively.
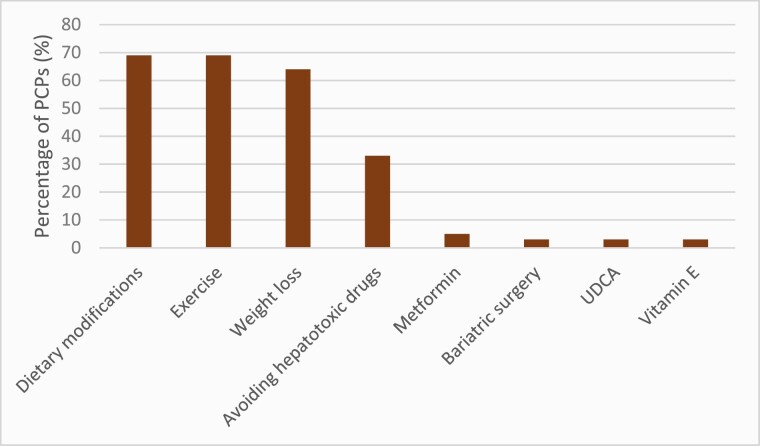
When asked about current treatment modalities for paediatric NAFLD, participants chose dietary modifications (69%), any form of exercise (69%), weight loss (64%), avoiding hepatotoxic drugs (33%), metformin (5%), bariatric surgery (3%), ursodeoxycholic acid (3%), and vitamin E (3%). Eight participants (22%) were unsure of which treatment to offer (Figure 1).
Figure 1.
Initial management practices of paediatric NAFLD by PCP respondents.
Fifty-eight per cent of respondents have referred less than 1% of paediatric patients to a gastroenterology specialist for NAFLD. One respondent stated that they “have not yet referred as no cases although I have patients who likely should have been screened.” Only one participant was aware of the NASPGHAN clinical guidelines.
Knowledge
The most common answer to the estimated prevalence of paediatric NAFLD was between 1% and 10% (58% of respondents). Out of seven true/false questions regarding NAFLD, the average score was 54% (median of answers correct: 4/7) (Table 2). When asked which diet was the best for paediatric patients with NAFLD, 23 participants (64%) were unsure. Seventeen participants (47%) correctly indicated a liver biopsy as the gold standard for diagnosis of NASH, while 16 (44%) were unsure and three (8%) incorrectly specified MRI or ultrasound. Seven participants (19%) were unsure of the indications for a liver biopsy in children with NAFLD.
Table 2.
PCPs’ knowledge of paediatric NAFLD
| Correctly identified an association between NAFLD and metabolic syndrome | |
|---|---|
| Yes | 31 (86%) |
| No | 0 (0%) |
| Unsure | 5 (14%) |
| Correctly identified that NAFLD can lead to cirrhosis | |
| Yes | 30 (83%) |
| No | 0 (0%) |
| Unsure | 6 (17%) |
| Correctly identified that NASH means inflammation | |
| Yes | 28 (78%) |
| No | 1 (3%) |
| Unsure | 7 (19%) |
| Correctly identified that NASH does not mean cirrhosis | |
| Yes | 28 (78%) |
| No | 1 (3%) |
| Unsure | 7 |
| Correctly identified that NASH does not mean fibrosis | |
| Yes | 11 (31%) |
| No | 5 (14%) |
| Unsure | 10 (28%) |
| Correctly identified NASH as a more severe condition | |
| Yes | 18 (50%) |
| No | 2 (6%) |
| Unsure | 16 (44%) |
| Correctly identified that NASH is not associated with excess alcohol intake | |
| Yes | 28 (78%) |
| No | 2 (6%) |
| Unsure | 6 |
NAFLD Nonalcoholic fatty liver disease; NASH Nonalcoholic steatohepatitis; PCP Primary care physician.
Attitudes
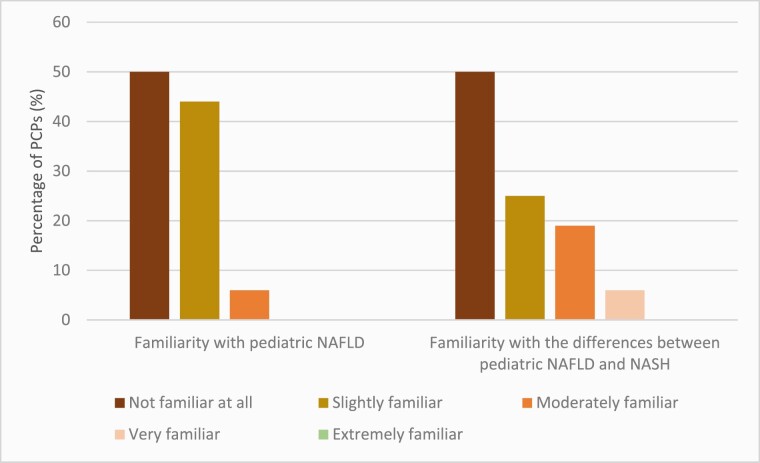
Eighteen (50%) participants indicated that they were ‘not familiar at all’ with paediatric NAFLD or the differences between paediatric NAFLD and NASH (Figure 2). When asked if they consider NAFLD an important paediatric health problem, 11 (31%) said yes, 6 (17%) said no, and 19 were unsure (53%).
Figure 2.
Respondents’ self-reported familiarity with paediatric NAFLD and its spectrum.
Lack of confidence in their knowledge of NAFLD was the most common answer (81%) for barriers to managing paediatric NAFLD. Other barriers to management that were indicated include time constraints (25%), not comfortable discussing with the patient (8%), lack of compliance by the patient (28%), outside the scope of PCP responsibility (6%), other issues take precedence (19%), and cost of evaluation/ treatment (28%).
DISCUSSION
Due to the silent nature of NAFLD, diagnosis is often delayed, potentially leading to more advanced and potentially irreversible disease at diagnosis (18). Approximately 25% of children with NAFLD have evidence of the progressive subtype and advanced fibrosis was reported at the time of diagnostic liver biopsy in nearly one in seven children (19,20). In our study, PCPs described low rates of diagnosis and referral of paediatric NAFLD, which is likely due to low screening rates related to a self-reported knowledge gap. Heightened awareness among PCPs (potentially through dissemination of clinical guidelines) and the creation of a clinical referral pathway between PCPs and paediatric specialists should be priorities.
Almost one in seven children and youth in Canada are obese (21). The estimated incidence of new diagnoses of type 2 diabetes in Canadian children has been estimated to be 1.54 per 100,000 annually (22). Given the links between obesity, diabetes mellitus, and NAFLD, early identification of NAFLD in children with these diagnoses is of utmost importance (23). Although Polanco-Briceno et al. described limited awareness and familiarity with adult NAFLD and NASH in PCPs and specialists in the United States (12), to our knowledge, no similar study has been conducted with paediatric NAFLD.
In our study, 58% of the respondents correctly predicted the prevalence of paediatric NAFLD by choosing 1% to 10% but the prevalence can be upwards of 30% depending on the at-risk population (7). A recent study from the United States described a startling 62% increase in the incidence of paediatric NAFLD from 2009 to 2018 (24). Almost all respondents (97%) report that they manage less than five paediatric NAFLD patients annually but they may manage others that are yet to be diagnosed. Referral rates are low as reported in studies of adult NAFLD (10,11,25).
Despite their knowledge that NAFLD can lead to cirrhosis, only one-third of the PCP participants believed that NAFLD is an important paediatric health concern. The majority correctly identified the association between NAFLD and metabolic syndrome and indicated that obesity, diabetes mellitus, and accidental findings of increased liver enzymes would prompt them to screen for NAFLD. In our study, the majority of PCPs chose liver enzymes and ultrasound as their screening method for NAFLD. As per the NASPGHAN clinical guidelines, paediatric patients with risk factors for NAFLD should be screened using ALT levels and followed if they are higher than double the sex/age-specific upper limits of normal for greater than 3 months (16). Ultrasound (chosen by 75% of respondents), is not recommended by NASPGHAN for screening due to inadequate specificity and sensitivity (16). Low screening results in our study may be explained by the self-reported lack of familiarity with the spectrum of NAFLD, including NASH, in over half of the PCP respondents.
Liver biopsy is the gold standard for diagnosis of NASH, which was correctly indicated by 47% of PCPs in our study. The other half either responded incorrectly with MRI or ultrasound, or indicated that they were unsure, suggesting the need for improved knowledge dissemination. A liver biopsy should be considered in children who have increased risk of NASH and/or advanced fibrosis (e.g., higher ALT [>80 U/L], AST/ALT >1, splenomegaly, panhypopituitarism and type 2 diabetes) (16). It may also be helpful in excluding other/concomitant causes of chronic liver diseases or before starting any treatment.
The NASPGHAN guidelines indicate that the cornerstone of management in paediatric patients with NAFLD is lifestyle modification, which may lead to reductions in both hepatic and total visceral fat (16). Of the survey respondents, 64% would suggest weight loss as a method to manage paediatric NAFLD, and 69% would suggest dietary modifications to their patients. However, when asked which diet was the best for paediatric patients with NAFLD, 23 participants (64%) were unsure. This is not surprising since there is no high-quality data for a definitive diet (26,27). Current evidence suggests that as long as weight loss is achieved, low-carbohydrate or low-fat diets may benefit liver outcomes such as ALT levels and intrahepatic triglycerides (28).
While the guidelines do not recommend medications, a small number of respondents selected vitamin E, UDCA, and metformin as treatment modalities.
A majority of the survey respondents expressed a lack of confidence in their knowledge of paediatric NAFLD as the main barrier to managing these patients. Other common responses included lack of time, lack of patient compliance, and cost of evaluation/treatment. ‘Pediatric NAFLD being outside the scope of PCP responsibility’ was the least common response, showing that PCPs are aware of the role they play in identifying, managing, and treating paediatric NAFLD.
Only one of the participants was aware of the NASPGHAN guidelines for paediatric NAFLD. This supports previous studies that found some barriers to following guidelines included lack of awareness, lack of familiarity and agreement, self-efficacy, outcome expectancy, ability to overcome the inertia of previous practice, and ambiguous recommendations (29,30). While some providers may be more familiar with adult guidelines, paediatric diagnostic criteria and management have differences that are necessary to emphasize. In a Canadian study on childhood obesity management, practitioners reported relying on their judgment or adult diagnostic criteria to identify paediatric obesity, due to inaccessibility or unawareness of guidelines (31). This emphasizes the importance of access to paediatric guidelines, as paediatric patients often have different clinical recommendations compared to adults.
One limitation is the low response rate, although it was comparable to most other studies using mailed surveys (13,32,33). Surveys are subject to recall bias. Another limitation is the use of a mostly academic-affiliated, single PCP network, which may limit the generalizability to other geographical locations. It would be of interest to also survey general paediatricians.
In the primary care setting, we have identified barriers to recognizing and managing paediatric NAFLD. We advocate for the continued strong collaboration between PCPs and paediatric gastroenterology specialists, as well as an exploration of how to best implement clinical guidelines and referral pathways into practice. Future work could evaluate successful implementation strategies while emphasizing the role of PCPs in managing paediatric NAFLD.
Supplementary Material
Author contributions: VL-K: Primary author, literature review, manuscript review. RM: Eastern Ontario Network contact, manuscript review. DB: Primary family physician, manuscript review. JAF: manuscript review. MK: corresponding author, study planning, and manuscript review. All authors have given final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: There are no funders to report for this submission.
Potential Conflicts of Interest: JAF reports consulting fees from Gilead Sciences. There are no other disclosures. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34(3):274–85. [DOI] [PubMed] [Google Scholar]
- 2. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55(6):2005–23. [DOI] [PubMed] [Google Scholar]
- 3. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148(3):547–55. [DOI] [PubMed] [Google Scholar]
- 4. Angulo P. Obesity and nonalcoholic fatty liver disease. Nutr Rev 2007;65(6 Pt 2):S57–63. [DOI] [PubMed] [Google Scholar]
- 5. Atlas of Childhood Obesity: World Obesity Federation. 2019. http://s3-eu-west-1.amazonaws.com/wof-files/11996_Childhood_Obesity_Atlas_Report_ART_V2.pdf (Accessed March 31, 2020).
- 6. Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: A systematic review and meta-analysis. Obes Rev 2016;17(2):95–107. [DOI] [PubMed] [Google Scholar]
- 7. Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: A systematic review and meta-analysis. PLoS One 2015;10(10):e0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology 2005;42(3):641–9. [DOI] [PubMed] [Google Scholar]
- 9. Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics 2006;118(4):1388–93. [DOI] [PubMed] [Google Scholar]
- 10. Grattagliano I, D’Ambrosio G, D’Ambrozio G, et al. ; “Steatostop Project” Group . Improving nonalcoholic fatty liver disease management by general practitioners: A critical evaluation and impact of an educational training program. J Gastrointestin Liver Dis 2008;17(4):389–94. [PubMed] [Google Scholar]
- 11. Patel PJ, Banh X, Horsfall LU, et al. Underappreciation of non-alcoholic fatty liver disease by primary care clinicians: Limited awareness of surrogate markers of fibrosis. Intern Med J 2018;48(2):144–51. [DOI] [PubMed] [Google Scholar]
- 12. Polanco-Briceno S, Glass D, Stuntz M, Caze A. Awareness of nonalcoholic steatohepatitis and associated practice patterns of primary care physicians and specialists. BMC Res Notes 2016;9:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Said A, Gagovic V, Malecki K, Givens ML, Nieto FJ. Primary care practitioners survey of non-alcoholic fatty liver disease. Ann Hepatol. 2013;12(5): 758–65. [PubMed]
- 14. Srivastava A, Gailer R, Tanwar S, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol 2019;71(2):371–8. [DOI] [PubMed] [Google Scholar]
- 15. Vajro P, Lenta S, Socha P, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr 2012;54(5):700–13. [DOI] [PubMed] [Google Scholar]
- 16. Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN Clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr 2017;64(2):319–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Primary Care Practice-Based Research Networks Rockville, MD: Agency for Healthcare Research and Quality. 2018. https://www.ahrq.gov/research/findings/factsheets/primary/pbrn/index.html (Accessed December 30, 2020).
- 18. Brunt EM. Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2010;7(4):195–203. [DOI] [PubMed] [Google Scholar]
- 19. Pardee PE, Lavine JE, Schwimmer JB. Diagnosis and treatment of pediatric nonalcoholic steatohepatitis and the implications for bariatric surgery. Semin Pediatr Surg 2009;18(3):144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kistler KD, Molleston J, Unalp A, Abrams SH, Behling C, Schwimmer JB; Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) . Symptoms and quality of life in obese children and adolescents with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2010;31(3):396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rao DP, Kropac E, Do MT, Roberts KC, Jayaraman GC. Childhood overweight and obesity trends in Canada. Health Promot Chronic Dis Prev Can 2016;36(9):194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amed S, Dean HJ, Panagiotopoulos C, et al. Type 2 diabetes, medication-induced diabetes, and monogenic diabetes in Canadian children: A prospective national surveillance study. Diabetes Care 2010;33(4):786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120(Suppl 4):S164–92. [DOI] [PubMed] [Google Scholar]
- 24. Sahota AK, Shapiro WL, Newton KP, Kim ST, Chung J, Schwimmer JB. Incidence of nonalcoholic fatty liver disease in Children: 2009-2018. Pediatrics. 2020;146(6):e20200771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matthias AT, Fernandopulle ANR, Seneviratne SL. Survey on knowledge of non-alcoholic fatty liver disease (NAFLD) among doctors in Sri Lanka: A multicenter study. BMC Res Notes 2018;11(1):556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vos MB, McClain CJ. Nutrition and nonalcoholic fatty liver disease in children. Curr Gastroenterol Rep 2008;10(3):308–15. [DOI] [PubMed] [Google Scholar]
- 27. Tzifi F, Fretzayas A, Chrousos G, Kanaka-Gantenbein C. Non-alcoholic fatty liver infiltration in children: An underdiagnosed evolving disease. Hormones (Athens) 2019;18(3):255–65. [DOI] [PubMed] [Google Scholar]
- 28. Katsagoni CN, Papachristou E, Sidossis A, Sidossis L. Effects of dietary and lifestyle interventions on liver, clinical and metabolic parameters in children and adolescents with non-alcoholic fatty liver disease: A systematic review. Nutrients. 2020;12(9):2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999;282(15):1458–65. [DOI] [PubMed] [Google Scholar]
- 30. Lugtenberg M, Zegers-van Schaick JM, Westert GP, Burgers JS. Why don’t physicians adhere to guideline recommendations in practice? An analysis of barriers among Dutch general practitioners. Implement Sci 2009;4:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He M, Piché L, Clarson CL, Callaghan C, Harris SB. Childhood overweight and obesity management: A national perspective of primary health care providers’ views, practices, perceived barriers and needs. Paediatr Child Health 2010;15(7):419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iacob S, Ester C, Lita M, Ratziu V, Gheorghe L. Real-life perception and practice patterns of NAFLD/NASH in Romania: Results of a survey completed by 102 board-certified Gastroenterologists. J Gastrointestin Liver Dis 2016;25(2):183–9. [DOI] [PubMed] [Google Scholar]
- 33. Wieland AC, Quallick M, Truesdale A, Mettler P, Bambha KM. Identifying practice gaps to optimize medical care for patients with nonalcoholic fatty liver disease. Dig Dis Sci 2013;58(10):2809–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.