Abstract
Nonalcoholic fatty liver disease (NAFLD) has gradually become one of the most serious liver diseases threatening human health in the world. Currently, Chinese herbal medicine is a potentially important treatment option for NAFLD, and the development of effective Chinese herbal medicine has a good prospect. Previous studies have suggested that Ficus hirta Vahl. (FV) has various protective effects on the liver. In this study, we investigated the therapeutic outcomes of FV treatment for the liver disease and its underlying mechanism using HepG2 cell lines induced by palmitate (PA) and mouse model fed with high-fat diet (HFD). FV mainly exerts pharmacological effects by mediating lipid metabolism and inflammation. During the lipid metabolism regulation process, CD36, SREBP-1, SCD1, PPAR γ, ACOX1, and CPT1α are the key factors related to the healing effects of FV on NAFLD. During the inflammation process, the downregulation of IL-6, IL-1β, and TNF-α is involved in alleviation of NAFLD. Furthermore, CD36 overexpression promotes lipid abnormal metabolism and inflammation in PA-induced HepG2 cells, while CD36 knockdown and FV supplementation reverse these responses. In addition, FV also modulates gut microbiota composition, such as Allobaculum, Faecalibaculum, and Butyricicoccus in HFD-fed mice. In summary, our findings demonstrated that FV exerted a beneficial preventive and therapeutic effect on NAFLD by improving lipid metabolism and inflammation as well as regulating the structure of gut microbiota, and therefore, FV may be a candidate for the treatment of NAFLD.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) includes a wide range of liver damage, including NAFL (simple steatosis; NAFL), nonalcoholic steatohepatitis (NASH) with inflammation and hepatocyte injury, and advanced fibrosis and cirrhosis [1]. The prevalence of NAFLD in the general population is estimated to be 25% worldwide [2]. The development of NAFLD is closely related to the abnormal lipid metabolism; variations in intracellular cholesterol transport and imbalance of cholesterol homeostasis in NAFLD can cause the accumulation of hepatic free cholesterol [3]. The imbalance between lipid intake and disposal leads to the accumulation of hepatic fat [4]; in addition, NAFLD is often accompanied by a high risk of type 2 diabetes and cardiovascular disease [5]. Furthermore, several studies have demonstrated the close association between gut microbiota and the occurrence and the development of NAFLD. There may be a possible direct association between gut microbiota and inflammation [6]. Therefore, it is generally believed that NAFLD is a liver manifestation of metabolic syndrome (MS) [7]. Nonalcoholic steatohepatitis (NASH) is a subtype of NAFLD and has the potential to progress and result in liver fibrosis, cirrhosis, hepatocellular carcinoma (HCC), and liver transplantation [8]. NASH-related complications bring severe physical, economic, and patient experience pressures to patients, families, and society [9]. Unfortunately, there are limited approaches available for the treatment of NAFLD other than lifestyle changes and reduction of high-calorie, low-fiber diets. Therefore, it is urgent to develop pharmacologically approved treatments for NAFLD [10].
CD36 (cluster of differentiation 36) is a scavenger receptor, which acts as a promoter of transport and uptake of the oxidized low-density lipoprotein (ox-LDL) and long-chain fatty acids [11]. The uptake of fatty acid by the liver may be promoted by fatty acid transport proteins (FATPs) and FAT/CD36 (fatty acid translocase), which have been reported to be higher in obese individuals and NAFLD patients [12]. Oxidized LDL and fatty acid bind to alpha helix at the exposed distal end of the CD36 membrane. CD36 is a lipid-binding pocket containing key Lys164 that initiates downstream signal transduction or promotes fatty acid binding and internalization [13]. Currently, the studies have shown that CD36 is an important factor in liver injury associated with metabolic diseases. In the condition of high expression of CD36, a large amount of fatty acid can be synthesized and then promote the development of fatty liver [14]. The stimulation by palmitate can lead to the elevation of the expression of CD36 in HepG2 cells and the formation of a lipid droplet, which facilitates fatty acid uptake and lipid accumulation [15]. When the liver is exposed to excessive fatty acid for a long period, the function of CD36 will be dysregulated, which may reflect the increased palmitoylation of hepatic CD36. This phenomenon has been reported to be related to NAFLD [16].
Ficus hirta Vahl. is a traditional food and medicinal material in southern China. The water extracts of its roots have been widely used clinically in the treatment of NAFLD [17]. Previous studies have focused on the chemical investigation of the roots of Ficus hirta Vahl. The predominant chemical constituents of Ficus hirta Vahl. mainly include psoralen, bergapten, luteolin, and apigenin [18]. Several studies have shown the pharmacological activities of Ficus hirta Vahl., such as antioxidation [19], anti-inflammation, analgesic, antitussive, antiasthmatic [20], and hepatoprotective [21]. In addition, Ficus hirta Vahl. is effective to prevent the occurrence of alcohol-induced hepatic damage in mice via scavenging free radical inhibiting lipid peroxidation [22]. These results have revealed that extracts of Ficus hirta Vahl. can be used as a candidate drug for liver protection for the treatment of liver diseases.
To clarify the role of Ficus hirta Vahl. (FV) in the treatment of NAFLD, we studied the effects of FV on lipid metabolism and inflammation in NAFLD using the HepG2 cell line induced by PA and the mouse model fed with HFD and determined the therapeutic potential of FV. More importantly, we also found that Ficus hirta Vahl. can alleviate abnormal lipid anabolism and improve inflammation via downregulating the expression of CD36. Meanwhile, Ficus hirta Vahl. relieved liver inflammation in HFD-fed mice by changing gut microbiota component.
2. Materials and Methods
2.1. Materials and Reagents
Ficus hirta Vahl. was bought from Kangmei Pharmaceutical Co., Ltd. (Guangdong, China); palmitic acid (PA, P9697) was bought from Sigma-Aldrich (St. Louis, MO, USA); primary antibodies against IL-6 (DF6078, 1 : 1500), IL-1β (AF5103, 1 : 1500), TNF-α (AF7014, 1 : 500), SREBP-1 (AF6283, 1 : 1500), CPT1α (DF12004, 1 : 1500), ACOX1 (DF12046, 1 : 1500), and GAPDH (AF7012, 1 : 2000) were purchased from Affinity Biosciences Co., Ltd. (Jiangsu, China); antibody against CD36 (18836-1-AP, 1 : 1000) was bought from Proteintech (Wuhan, China); HMGCR (ab171830, 1 : 5000) was purchased from Abcam (Shanghai, China); RIPA buffer and BCA protein assay kit were bought from Beyotime Biotechnology (Shanghai, China); the high-fat diets (HF60), including 60% fat, 20% carbohydrate, and 20% protein, were purchased from Dyets (Dyets Biotechnology Co., Ltd., USA); the normal fat diets including 20 kcal % fat were purchased from Guangdong Medical Laboratory (Guangzhou, China); and D-(+)-glucose (CAS: 50-99-7) and fructose (CAS: 7660-25-5) were bought from Macklin Biochemical Co., Ltd. (Shanghai, China).
2.2. Preparation and Identification of FV
The root of Ficus hirta Vahl. (FV) was extracted by purified water. The components of FV were detected by UPLC-Q/TOF-MS/MS (X500R, AB SCIEX, USA). The sample was separated by an UPLC C18 analytical column with the size of 2.1 mm × 100 mm, I.D. 1.8 μm (ACQUITY UPLC®HSS T3, Waters, USA). In the modified method, the binary gradient mobile phase with water (0.05% acetic acid) was used as mobile phase A, and acetonitrile (ACN) was used as mobile phase B. Supplementary Table 1 shows the time program of the gradient elution. The sample injection volume was 5 μL. Mass spectrometric analysis was conducted by a SCIEX X500R, which was equipped with electrospray ionization (ESI) Turbo V™ ion source, operating in positive and negative ion modes. Supplementary Table 2 lists the parameters in the method. We performed qualitative and relative quantitative analysis using SCIEX O.S. software V 2.0 (AB SCIEX).
2.3. Cell Culture
HepG2 cells (human liver carcinoma cell line) were obtained from Cell Resource Center, Shanghai Institute for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). The obtained HepG2 cells were incubated in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS, Gibco, United States), in which the concentration of penicillin was 100 U/mL and the concentration of streptomycin was 100 U/mL, under the condition of 37°C and 5% CO2. HepG2 cells were incubated with 0.25 mM PA to establish cell model of lipid accumulation and were supplied with different doses of FV extract (high-dose 30 mg/mL, low-dose 15 mg/mL, raw medicinal material content) for 24 h.
2.4. Animals and Experimental Design
C57BL/6J mice (male, eight-week, weight of 18-25 g) were obtained from the Experimental Animal Centre, Guangdong Medical Laboratory (Guangzhou, China). All subjects were acclimated in a 12 h light/dark cycle in pathogen-free (SPF) laboratory in a controlled environment (temperature: 25 ± 2°C, humidity: 50-75%) and fed with a certified laboratory diet ad libitum. The mice were provided with tap water ad libitum. After 2 weeks of habilitation, we randomly divided the mice into 4 groups: the NFD group (n = 8) was fed with a normal fat diet; the HFD group (n = 10) and FV treatment groups (FV-L, FV-H group, n = 10 each group) were fed with high-fat diet and aqueous solution of glucose and fructose for 17 weeks to establish a nonalcoholic fatty liver disease model. The animals in the FV-L and FV-H groups were administered with the water extract of FV at the doses of 5 g/kg and 10 g/kg (raw medicinal material content), respectively. The other groups were administrated with equivalent amount of saline. Body weight was measured once per week. At the end of the experiment, mice were fasted for 18 hours and anesthetized with a 1% pentobarbital (50 mg/kg BW) via intraperitoneal injection. We collected blood samples from the subjects eyeballs and centrifuged the sample at 3000 rpm and 4°C for 15 min to collect serums. In addition, liver tissues of all animals were collected and stored at -80°C for later use. All the animal studies were in accordance with the relevant national legislation and local guidelines on the ethical use of animals. In addition, all the procedures in the study have been approved by the Institutional Animal Care and Use Committee of Jinan University.
2.5. Hematoxylin-Eosin (H&E) and Oil Red O Staining
Oil red O was used to stain the HepG2 cells according to the instruction of Solarbio kits (Cat# G1262, Beijing, China). Three images per sample were taken using an optical microscope (Nikon, Shanghai, China). Then, the tissue sections were performed for H&E staining and oil red O staining according to the instruction of Solarbio kits (Cat# G1262, Beijing, China).
2.6. Biochemical Analysis
The concentration of total cholesterol (TC, A111-1-1), triglyceride (TG, A110-1-1), alanine aminotransferase (ALT, C009-2-1), aspartate aminotransferase (AST, C010-2-1), and low-density lipoprotein cholesterol (LDL-C, A113-1-1) of the mouse serum samples was determined according to the instruction of diagnostic kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.7. Western Blot Analysis
Total protein was extracted from liver tissue and hepatocytes by RIPA lysis, and its concentration was measured by a BCA protein assay kit. Western blot analysis was routinely conducted with primary antibodies against GAPDH, IL-6, IL-1β, TNF-α, SREBP-1α, HMGCR, ACOX1, CD36, and CPT1α. 30 μg protein was placed in each well. An ECL (Affinity Biosciences LTD, Jiangsu, China) was used to detect the protein bands. The band intensity by densitometry for quantification was evaluated using ImageJ 1.48 analysis software and expressed as the mean area density.
2.8. Determination of Inflammatory Cytokine Levels in Serum
The levels of IL-6 (EMC004), IL-1β (EMC001b), and TNF-α (EMC102a) in each mouse serum sample were determined by ELISA kits, which were produced by Neobioscience (Shenzhen, China).
2.9. RT-qPCR Analysis
The gene expression of HepG2 cells and mouse liver tissues was performed by RT-qPCR. Trizol regent was used to isolate the total RNA of the HepG2 cells and liver tissue, and HiScript II Q RT SuperMix for qPCR (cat no. R223-01, Vazyme, Nanjing, China) was used to reverse transcribe them into cDNA. The synthesized cDNA was used as a template and quantified using the BioEasy Master Mix Kit (cat no. BSB25L1B, SYBR Green, High ROX) and a real-time PCR detection system (Line Gene 9600 Plus, Bioer Technology, China). Supplementary Table 3 lists the human and mouse primer sequences for quantitative real-time PCR.
2.10. RNA-seq, KEGG Analysis, and Gene Set Enrichment Analysis
The transcriptome sequencing was performed by Novogene (Beijing, China). After generating clusters, we sequenced the library preparations on the Illumina Novaseq 6000 platform.
In order to analyze Kyoto Encyclopedia of Genes and Genomes (KEGG) as well as Gene Ontology (GO) biological process, the crossover differentially expressed genes (DEGs, P < 0.05 and |log2FC| > 2.0) were submitted into the Web-based gene set analysis toolkit (WebGestalt, http://www.webgestalt.org/option.php) [23]. Then, the Gene Set Enrichment Analysis (GSEA) was conducted on the Java GSEA platform. We calculated the fold change of gene expression, and the gene list was generated according to the change of |log2FC|. The genes involved in each KEGG pathway were denoted as a gene set. Then, a ranked list and a “gene set” permutation type of the gene set was generated. P < 0.05 was set as the cutoff criterion.
2.11. Cell Transfection
The plasmid overexpressing CD36 was designed and built by iGene Biotechnology Co., Ltd. (Guangzhou, China) to generate a CD36-overexpressed (CD36 OE) cell line, and an empty vector was used as a control. siCD36 was synthesized by RIBOBIO Co., Ltd. (Guangzhou, China). The experiment of cell transfection was performed. The efficiency of knockdown and overexpression of CD36 were confirmed by western blot analysis and RT-qPCR.
2.12. 16S rRNA Sequencing
Frozen mouse fecal samples were used to characterize the gut microbiota. The genomic DNA was extracted and used to amplify the V3–V4 region of the 16S rRNA genes. First, the amplicons were purified and then combined in equal amounts for sequencing library preparation and Miseq sequencing analysis. The PE reads were obtained from Miseq sequencing, and then, they were spliced based on the overlapping relationship. Next, the sequence quality was simultaneously controlled and filtered. The samples were distinguished and then went through OTU cluster analysis and species taxonomy analysis. Based on the taxonomic information, the community structure can be statistically analyzed at different taxonomic levels. Multiple samples were used in various statistical and visual analyses to study the community composition and phylogenetic information, such as multivariate analysis and significance of differences tests. In addition, we performed Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis to further study the biological signaling pathways in the gut microbiota.
2.13. Spearman's Analysis
The correlation between the 8 genera of microbiota and 4 signaling pathways was analyzed using Spearman's correlation coefficient (r). In the correlation analysis, P < 0.05 was considered as the significant criterion.
2.14. Statistical Analysis
The data was expressed as the mean ± standard deviations (SD). Furthermore, statistical analysis was implemented using GraphPad Prism 5.0 software (GraphPad Software, Inc.; San Diego, CA, USA). The statistical analyses in this study including one-way ANOVA and Tukey's post hoc test were used to compare multiple groups. In the statistical test, P < 0.05 was considered statistically significant.
3. Results
3.1. Identification of Bioactive Components for FV Extract
The total ion chromatogram (TIC) of FV was investigated by UPLC-Q/TOF-MS/MS. The results are shown in Figure 1. The components were characterized by matching with SCIEX high-resolution MS/MS database or ChemSpider online database. The results identified a total of 54 chemical constituents in FV. Among them, 23 ingredients were determined in positive ion mode, while 31 ingredients were determined in negative ion mode. The FV extract contained 11 types of coumarins, 11 types of flavonoids, 5 types of carboxylic acids, 3 types of terpenes, 6 types of aldehydes, and other types of compounds. The detailed information is shown in Table 1.
Figure 1.
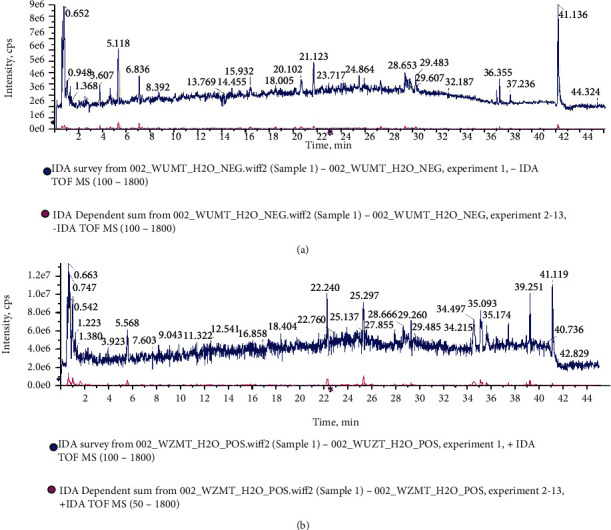
UPLC-Q/TOF-MS/MS analysis. The total ion chromatogram (TIC) of FV of by UPLC-Q/TOF-MS/MS in the (a) negative and (b) positive ion modes.
Table 1.
Compounds identified in FV prescription by UPLC-Q/TOF-MS.
| No. | Retention time (min) | Molecular formula | [M+H]+/-(m/z) (mass error) (ppm) | Intensity | Identification |
|---|---|---|---|---|---|
| 1 | 0.6 | C6H9N3O2 | 156.077(1.7) [M+H]+ | 131400 | Histidine |
| 2 | 0.65 | C5H9NO2 | 116.0707(0.7) [M+H]+ | 731900 | Proline |
| 3 | 0.67 | C7H7NO2 | 138.0553(2.6) [M+H]+ | 692800 | Trigonelline |
| 4 | 0.68 | C7H12O6 | 191.0559(-0.9) [M+H]− | 1043000 | Quinic acid |
| 5 | 0.77 | C4H6O5 | 133.0139(-2.5) [M+H]− | 1208000 | L-Malic acid |
| 6 | 0.94 | C6H8O7 | 191.019(-3.6) [M+H]− | 3592000 | Citric acid |
| 7 | 0.95 | C5H5N5 | 136.0621(2.4) [M+H]+ | 500200 | Adenine |
| 8 | 1.05 | C6H5NO2 | 124.0391(-1.9) [M+H]+ | 46600 | Nicotinic acid |
| 9 | 1.1 | C8H11NO3 | 170.0813(0.5) [M+H]+ | 53320 | Vitamin B6 |
| 10 | 1.2 | C9H12N2O6 | 243.062(-1.1) [M+H]− | 1073000 | Uridine |
| 11 | 1.28 | C4H6O4 | 117.0203(8.6) [M+H]− | 162400 | Amber acid |
| 12 | 1.4 | C6H13NO2 | 132.102(0.4) [M+H]+ | 24570 | Leucine |
| 13 | 1.65 | C10H15NO | 166.1225(-0.7) [M+H]+ | 4021000 | Hordenine |
| 14 | 2.06 | C10H13N5O5 | 284.0993(1.3) [M+H]+ | 115600 | Guanosine |
| 15 | 2.27 | C10H13N5O4 | 266.0892(-0.9) [M+H]− | 172100 | Adenosine |
| 16 | 2.52 | C9H11NO2 | 164.0716(-0.4) [M+H]− | 144900 | Phenprobamate |
| 17 | 2.78 | C6H6O3 | 127.0393(2.3) [M+H]+ | 170000 | 5-Hydroxymethylfurfural |
| 18 | 3.93 | C17H26O11 | 407.1546(-0.6) [M+H]+ | 408900 | 8-O-Acetylharpagide |
| 19 | 5.15 | C21H18O12 | 461.0723(-0.5) [M+H]− | 14410 | Luteolin-7-O-β-D-glucuronide |
| 20 | 5.24 | C7H6O3 | 137.0244(-0.2) [M+H]− | 602000 | Protocatechuic aldehyde |
| 21 | 7.38 | C15H14O6 | 289.0716(-0.6) [M+H]− | 351700 | Catechin |
| 22 | 7.5 | C7H6O2 | 121.0305(8.1) [M+H]− | 495700 | p-Hydroxybenzaldehyde |
| 23 | 8.19 | C8H8O2 | 137.0602(3.8) [M+H]+ | 347200 | Anisaldehyde |
| 24 | 8.46 | C8H8O4 | 167.035(0) [M+H]− | 250800 | Vanillic acid |
| 25 | 8.53 | C9H6O4 | 177.0195(0.7) [M+H]− | 134600 | Esculetin |
| 26 | 10.31 | C8H8O2 | 135.0448(-2.5) [M+H]− | 321000 | 4′-Hydroxyacetophenone |
| 27 | 12.48 | C9H6O3 | 161.0238(-3.7) [M+H]− | 794100 | 4-Hydroxycoumarin |
| 28 | 12.51 | C9H8O3 | 163.04(-0.3) [M+H]− | 163500 | p-Coumaric acid |
| 29 | 12.67 | C9H10O4 | 183.0653(0.5) [M+H]+ | 160400 | Syringaldehyde |
| 30 | 12.8 | C21H20O12 | 463.0875(-0.6) [M+H]− | 27330 | Quercetin-3′-O-glucoside |
| 31 | 12.9 | C9H6O3 | 163.039(0.2) [M+H]+ | 204600 | 7-Hydroxycoumarin |
| 32 | 12.96 | C10H10O4 | 193.0504(-0.9) [M+H]− | 284200 | Ferulic acid |
| 33 | 13.07 | C10H10O4 | 195.0659(3.4) [M+H]+ | 113500 | Isoferulic acid |
| 34 | 13.89 | C10H8O3 | 175.04(-0.3) [M+H]− | 192700 | 7-Methoxycoumarin |
| 35 | 14.63 | C15H12O7 | 303.0509(-0.4) [M+H]− | 248600 | Dihydroquercetin |
| 36 | 16.14 | C27H30O15 | 595.1647(-1.7) [M+H]+ | 48030 | Glucosylvitexin |
| 37 | 18.02 | C21H20O10 | 431.098(-0.8) [M+H]− | 1645000 | Vitexin |
| 38 | 18.34 | C15H12O6 | 287.0562(0.4) [M+H]− | 335100 | Aromadendrin |
| 39 | 19.51 | C26H28O14 | 563.1411(0.9) [M+H]− | 237000 | Schaftoside |
| 40 | 21.9 | C20H17NO4 | 336.123(0) [M+H]+ | 39780 | Berberine |
| 41 | 22.12 | C11H6O4 | 201.0194(0.4) [M+H]− | 726600 | Xanthotoxol |
| 42 | 22.25 | C11H6O3 | 187.0388(-0.9) [M+H]+ | 36910000 | Psoralen |
| 43 | 24.45 | C15H12O5 | 271.0611(-0.4) [M+H]− | 777300 | Naringenin |
| 44 | 25.31 | C12H8O4 | 217.0496(0.1) [M+H]+ | 20270000 | Bergapten |
| 45 | 25.32 | C15H10O6 | 285.0402(-0.9) [M+H]− | 2446000 | Luteolin |
| 46 | 26.65 | C15H10O5 | 269.0455(-0.3) [M+H]− | 6317000 | Apigenin |
| 47 | 27.92 | C15H22O2 | 235.1694(0.7) [M+H]+ | 1094000 | Curcumenol |
| 48 | 29.08 | C14H14O3 | 229.087(-0.2) [M+H]− | 476900 | Demethylsuberosin |
| 49 | 29.25 | C15H24O2 | 237.1848(-0.5) [M+H]+ | 2528000 | Curdione |
| 50 | 29.59 | C16H14O4 | 269.082(0.2) [M+H]− | 654000 | Isoimperatorin |
| 51 | 30.3 | C15H20O2 | 233.1541(2.3) [M+H]+ | 145700 | Isoalantolactone |
| 52 | 35.37 | C16H30O2 | 253.2174(0.4) [M+H]− | 175800 | Sclareol glycol |
| 53 | 35.57 | C33H40N2O9 | 609.2803(-0.6) [M+H]+ | 113300 | Reserpine |
| 54 | 35.75 | C18H32O2 | 279.2332(1) [M+H]− | 337800 | Linoleic acid |
3.2. Amelioration of Lipid Accumulation and Inflammation by FV In Vitro
We used oil red O staining to explore the effect of FV on lipid homeostasis in PA-induced HepG2 cells. As shown in Figure 2(a), PA caused a significant increase in the number of lipid droplets in HepG2 cells, while FV reversed it in a dose-dependent manner; the oil red O score is showed in Figure 2(b). Furthermore, western blot and RT-qPCR were performed to determine the protein and mRNA expression levels of biomarkers related to lipogenesis and inflammation in HepG2 cells. The results demonstrated that after FV treatment, the expression levels of HMGCR (Figures 2(f) and 2(l)), SREBP-1 (Figure 2(l)), FABP1 (Figure 2(c)), SCD1 (Figure 2(d)), CD36 (Figures 2(e) and 2(l)), and ACACA (Figure 2(g)) were remarkably suppressed while the levels of key enzymes regulating fatty acid oxidation (ACOX1 and CPT1α) (Figure 2(l)) were increased. Meanwhile, we found that FV treatment significantly ameliorated inflammation in the HepG2 cell line induced by PA by decreasing the level of proinflammatory factor, including IL-1β (Figures 2(i) and 2(k)), IL-6 (Figure 2(k)), TNF-α (Figure 2(k)), and CCL5 (Figure 2(h)). These results suggested that FV might restore PA-induced lipogenesis and inflammation responses.
Figure 2.
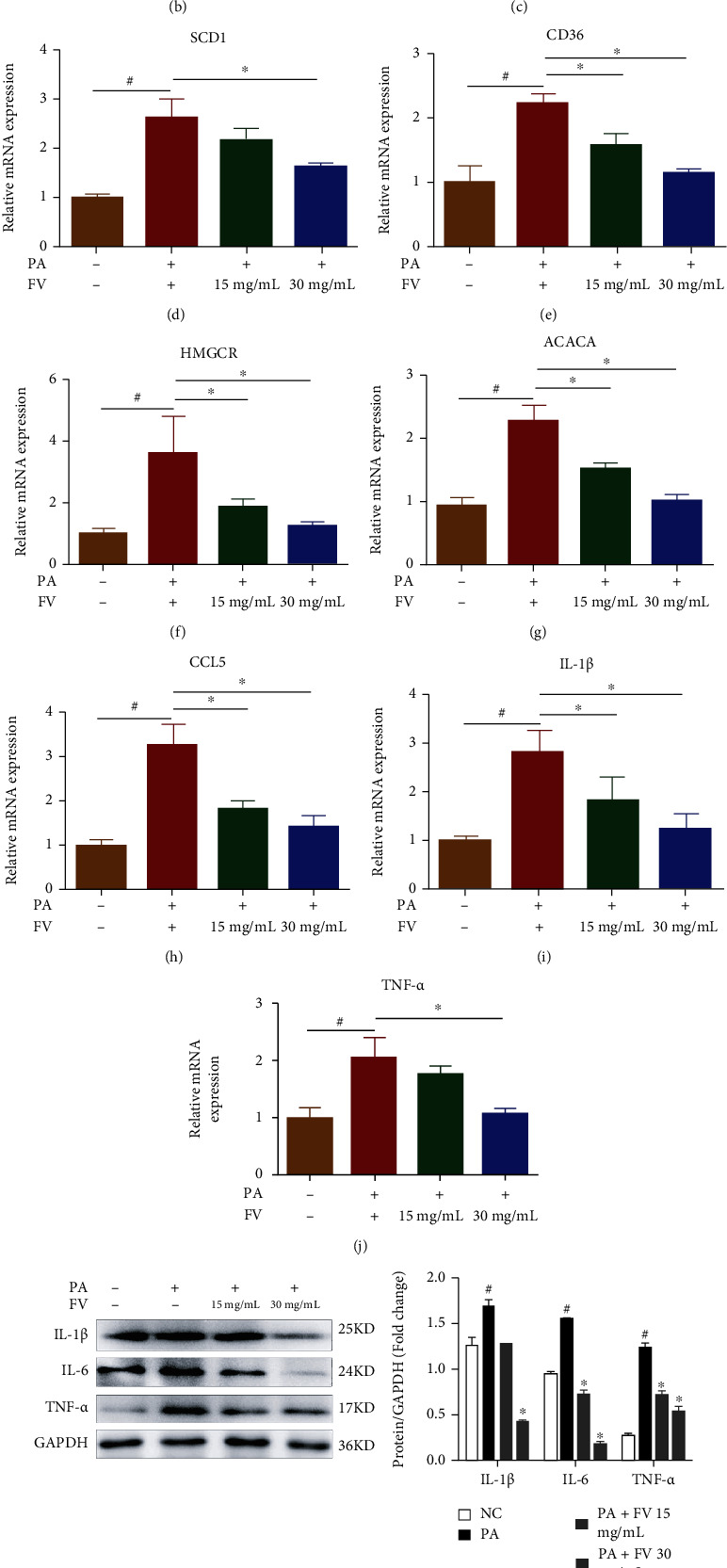
Amelioration of lipid accumulation in PA-induced HepG2 cells by FV. (a) Oil red O was used to measure the level of lipid accumulation (magnification 100x, scale bar = 250 μm). (b) The oil red O-positive area was analyzed and quantified. (c–j) The relative mRNA expression levels of FABP1, SCD1, CD36, HMGCR, ACACA, CCL5, IL-1β, and TNF-α were determined by qRT-PCR. (k) The proinflammatory factor protein levels of IL-1β, IL-6, and TNF-α. (l) The protein levels related to lipid metabolism of SREBP-1, ACOX1, CD36, CPT1α, and HMGCR were analyzed by western blotting, and the relative ratios were calculated and expressed as the mean ± SD; n = 3. #P < 0.05 means that the difference between the NC group and the PA group is significant. ∗P < 0.05 means that the difference between the PA group and the FV (30 mg/mL) group or the FV (15 mg/mL) group is significant.
3.3. Attenuation of Hepatic Steatosis and Serum Lipid Levels in HFD-Fed Mice by FV
A mouse model fed with HFD was utilized to further explore the beneficial effects of FV. As shown in Figures 3(a) and 3(c), HFD-fed mice exhibited an increased body weight and a higher liver index, and a 17-week FV administration alleviated these gains. Compared with normal diet mice, HFD-fed mice showed liver enlargement and discoloration, which were much improved by the FV administration (Figure 3(b)).
Figure 3.
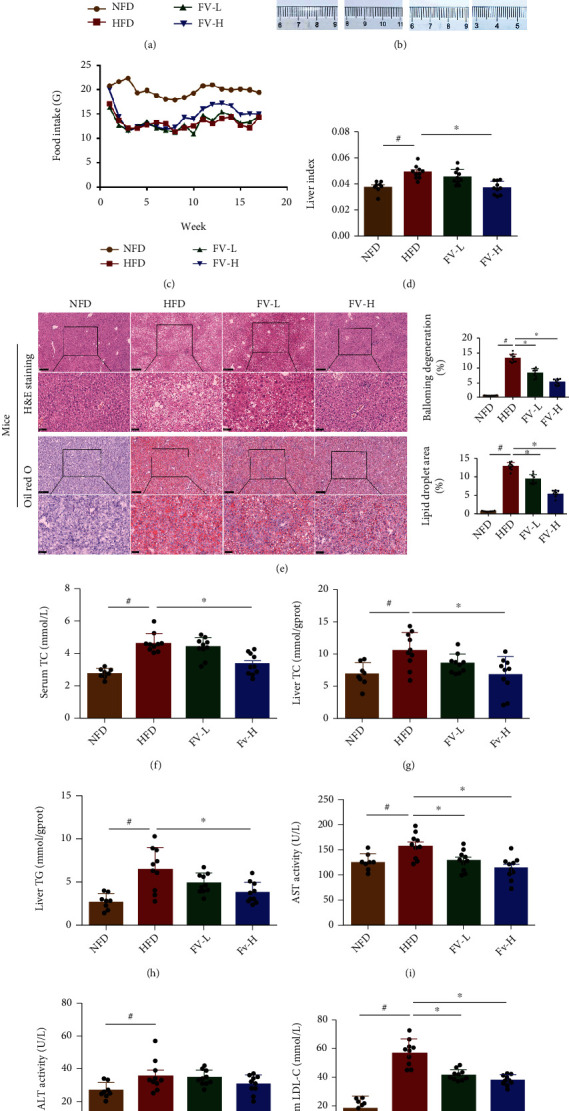
Effects of FV on accumulation of liver fat and lipid levels in serum in HFD-fed mice. (a) Body weight, (b) macroscopic observation of livers in subjects in different groups, (c) weekly food intake per mouse in each group, (d) liver index, (e) light microscopic H&E image and oil red O staining image of liver tissues of subjects in different groups (x100 original magnification, scale bar = 250 μm; 400x original magnification, scale bar = 50 μm), H&E score and oil red O score, (f) serum TC, (g) liver TC, (h) liver TG, (i) serum AST, (j) ALT, and (k) serum LDL-C. The data are expressed in the format of mean ± SD (n = 8, 10, 10). #P < 0.05 means there is a significant difference between the NFD group and the HFD group. ∗P < 0.05 means the difference between the HFD group and the FV-L group or the FV-H group is significant. NFD: normal fat diet group; HFD: high-fat diet group; FV-L group: high-fat diet with FV administration at dose of 5 g medicinal materials/kg body weight; FV-H group: high-fat diet with FV at dose of 10 g medicinal materials/kg body weight.
To evaluate the extent of liver injury in mice, we performed H&E staining and measured transaminase level in mouse serum. The liver tissue of mice in the HFD group showed obvious changes in morphology, including extensive cell necrosis, loss of hepatic structure, and a large amount of inflammatory cell infiltration. However, compared with the HFD group, the prophylactic use of FV obviously rescued the injured area in a dose-dependent manner (Figure 3(d)). In addition, compared to the mice fed with high-fat diet, the serum ALT and AST levels in the FV treatment group were decreased (Figures 3(i) and 3(h)).
As shown in oil red O staining, the accumulations of lipid droplets in hepatocytes were significantly severe in the HFD group, which were reduced in the liver of mice with FV administration (Figure 3(d)). Furthermore, compared with the HFD group, the increased levels of TC and TG in the liver of mice with FV treatment were much recovered (Figures 3(f) and 3(g)); similarly, the increased level of TC and LDL-C in serum was also removed by FV treatment (Figures 3(e) and 3(j)).
3.4. Attenuates Lipogenesis and Inflammation in the Liver of HFD-Fed Mice by FV
To analyze the influencing mechanisms of FV on the accumulation of lipid, we further studied the beneficial effects of FV in the in vivo model. Compared with HFD-fed mice, FV treatment significantly reduced the mRNA expression of Srebp-1, Acaca, Hmgcr, Fabp1, Pparγ, and Cd36. Meanwhile, FV treatment led to the increase (Figures 4(a)–4(h)) in the level of key enzymes related to the regulation of fatty acid oxidation (Pparα and Cpt1α) (Figures 4(c) and 4(f)). It is worth noting that compared with the mice fed with HFD, FV treatment caused the reduction of the expressions of lipid metabolism-related proteins, which included SREBP-1, HMGCR, and CD36. However, ACOX1 and CPT1α levels in the livers of animals with NAFLD were increased after FV treatment (Figure 4(i)).
Figure 4.
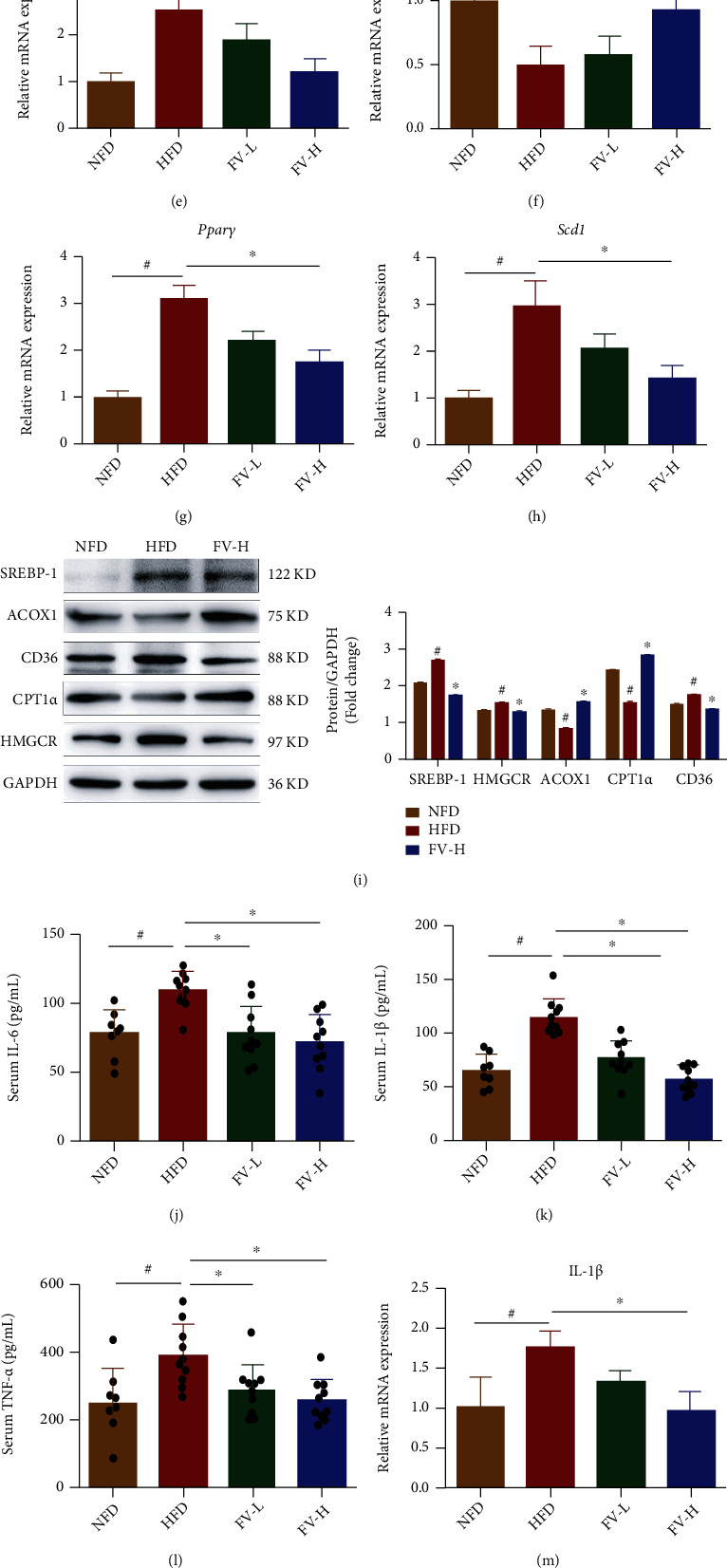
Effects of FV on liver lipogenesis-related markers in the mice fed with HFD. (a–h) The relative mRNA expression levels of Acaca, Cd36, Cpt1α, Srebp-1, Hmgcr, Pparα, Pparγ, and Scd1 were determined by qRT-PCR. (i) The lipid metabolism relevant protein levels of SREBP-1, ACOX1, CD36, CPT1α, and HMGCR were analyzed by western blotting, and the relative ratios were calculated. (j–l) Serum IL-6, IL-1β, and TNF-α were identified by ELISA kits. (m, n) The relative mRNA expression levels of IL-1β, TNF-α, and Ccl5 were identified by qRT-PCR. (p) The proinflammatory factor protein levels of IL-1β, IL-6, and TNF-α were analyzed by western blotting, and their relative ratios were calculated. The densitometry was obtained by averaging repeated experiments results, normalized to GAPDH. The data are expressed as the mean ± SD, n = 3. #P < 0.05 means there is significant difference between the NFD group and the HFD group. ∗P < 0.05 means the difference between the HFD group and the FV-L group or the FV-H group is significant.
ELISA results showed that compared with HFD-fed mice, FV significantly downregulated the levels of IL-6, IL-1β, and TNF-α in serum (Figures 4(j)–4(l)). As shown in Figure 4(p), the upregulated proteins of TNF-α, IL-6, and IL-1β in the liver tissues in the mice fed with HFD were also suppressed by FV administration. In addition, the mRNA expression of Tnf-α, Il-1β, and Ccl5 in liver tissues was successively suppressed by FV administration (Figures 4(m)–4(o)). These data indicated that FV partially alleviated the hepatocyte damage by reducing inflammation in serum and hepatocytes.
3.5. FV Regulated Gene Expression and Signaling Pathways in the Liver of HFD-Fed Mice
In order to systematically investigate the potential mechanism of FV on NAFLD mice, we conducted transcriptome analysis through RNA sequencing of liver tissues in the mice fed with NFD and the mice fed with HFD with or without FV treatment.
The results from principal component analysis (PCA) indicated that the gene expression profile of mice from the FV group clustered with the NFD group but separated from the HFD group (Figure 5(a)). The volcano plots demonstrated that the differentially expressed genes (DEGs) in the HFD group underwent significant changes compared to those in the NFD group or high-fat diet treated with FV assumption group (Figure 5(b)). Compared with the mouse group fed with HFD, there were 394 DEGs in the mice fed with NFD and 261 in mouse group treated with FV. Next, we also studied the therapeutic effect of FV on NAFLD's main signaling pathways. GO biological process and KEGG enrichment analyses were conducted using WebGestalt. Figure 5(c) shows the top 10 KEGG pathways which have the most significant false discovery rate (FDR) and P value, including PPAR signaling pathway, fatty acid elongation, and biosynthesis of unsaturated fatty acids. According to GO enrichment analysis results, the 10 most significantly enriched GOBP terms are listed in Figure 5(d) (P < 0.05). The results suggested that DEGs participated in the regulation of metabolic processes for lipid, small molecule, unsaturated fatty acid, etc.
Figure 5.
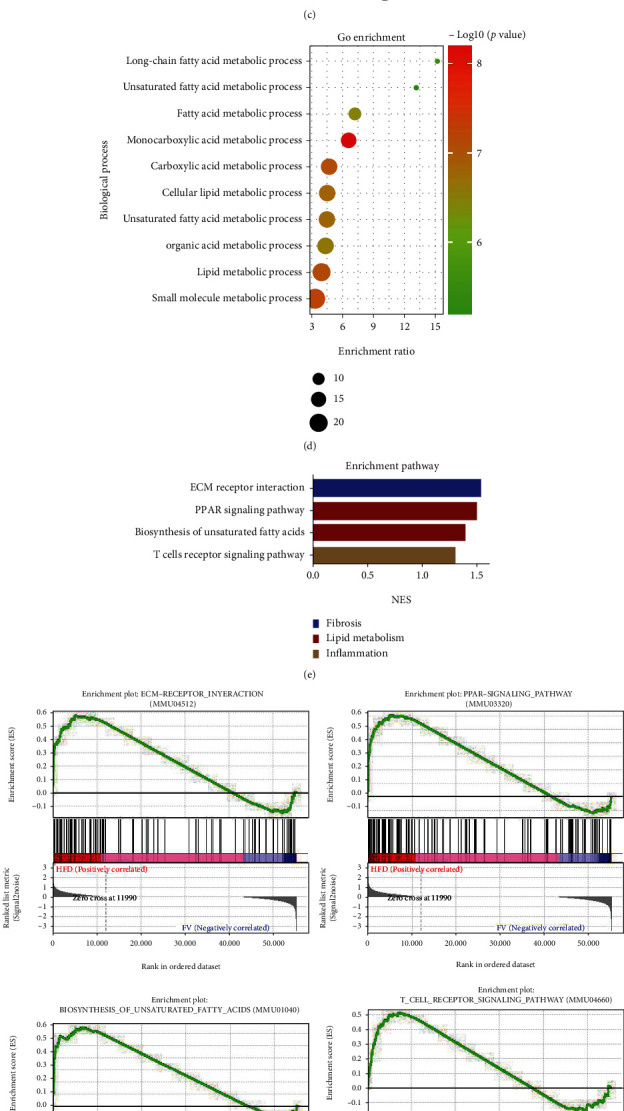
Key targets and pathway between the NFD group, the HFD group, and the FV-H group revealed by RNA-seq analyses. (a) PCA of the RNA-seq data from the NFD group, the HFD group, and the FV-H group. (b) Volcano plot indicating the DEGs (red, upregulated genes; green, downregulated genes) from three groups (HFD group vs. NFD group or FV-H group). (c) The KEGG pathway enrichment analysis of crossover DEGs. (d) The GO enrichment analysis of crossover DEGs. (e, f) GSEA pathway enrichment analysis of pathways that are related to inflammation, lipid metabolism, and fibrosis. (g) Heat map of gene expression profiles related to lipid metabolism, fibrosis, and inflammation based on RNA-seq dataset. n = 3 in each group.
In addition, the GSEA pathway enrichment results indicated that the cellular signaling pathways related to inflammation (such as the T cell receptor signaling pathways), lipid metabolism (such as the PPAR signaling pathway and biosynthesis of unsaturated fatty acids), and fibrosis (such as the extracellular matrix (ECM) receptor interaction) were enriched and significantly downregulated by FV treatment (Figures 5(e) and 5(f)). The results of RNA-seq showed that lipid metabolism-related genes, such as CD36, had significantly different expressions between the HFD-fed mice treated with FV and the mice without FV treatment. This indicated that the CD36 differential gene may be one of the primary responsive targets that cause the FV to affect HFD-fed mice (Figure 5(g)). Therefore, we hypothesized that potential targets of FV might be present in the CD36 gene of the lipid metabolism signaling pathway. The following experiments were conducted to confirm this hypothesis.
3.6. CD36: A Potential Target of FV for Ameliorating Lipid Accumulation
We confirmed that FV treatment restored the level of CD36 in the liver of mice, which was significantly elevated by high-fat diet daily. To evaluate the functional role of CD36 in the pathological process of lipid accumulation, we generated plasmid overexpressing CD36 in HepG2 cells (Figure 6(c)) and observed that the overexpression of CD36 significantly increased the lipid accumulation through oil red O staining and TG level; in addition, the overexpression of CD36 was obviously reversed by FV administration (Figures 6(d) and 6(f)). Moreover, after the overexpression of CD36, the levels of PPARγ, SREBP-1, and HMGCR were upregulated and were significantly suppressed by FV treatment (Figures 6(h) and 6(j)). Also, after the overexpression of CD36, the expression of ACOX1 related to fatty acid oxidation was decreased in HepG2 cells and was reversed by FV treatment (Figure 6(j)). We also utilized siRNA to knock down the mRNA level of CD36 in HepG2 hepatocytes (Figure 6(a)). Through oil red O staining and TG level (Figures 6(b) and 6(e)), we observed that siRNAs targeting CD36 significantly attenuated PA-induced lipid accumulation, which was consistent with the role of FV. In addition, lipid metabolism-related proteins, including PPARγ, SCD1, ACACA, SREBP-1, and HMGCR, were downregulated by the FV+siCD36 group (Figures 6(g) and 6(i)), while the expression of ACOX1 was upregulated by siCD36 and FV.
Figure 6.
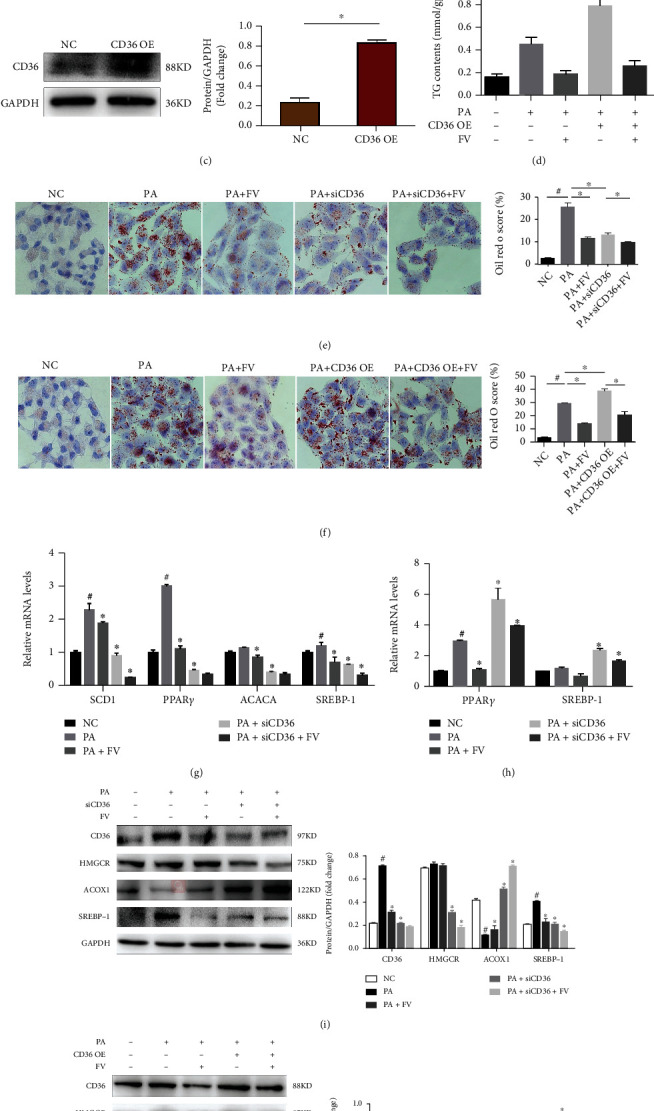
Alleviation of lipid accumulation and inflammation by FV through regulating CD36 in HepG2 cells with PA inducement. (a, c) CD36 protein levels after CD36 knockdown or CD36 overexpression and quantitative analysis. (b, d) TG contents of HepG2 cells in different treatment groups. (e, f) Oil red O to examine the accumulation level of lipid in HepG2 cells and their quantified scores (magnification 100x, scale bar = 250 μm). (g) The mRNA levels of SCD1, PPAR γ, ACACA, SREBP-1, IL-1β, and CCL5 in siCD36 HepG2 cells with or without FV administration under the condition of PA inducement. (h) The mRNA levels of PPAR γ, SREBP-1, IL-1β, and TNF-α in CD36 OE HepG2 cells with or without FV administration under the condition of PA inducement. (i) The protein levels of CD36, HMGCR, ACOX1, and SREBP-1 in siCD36 HepG2 cells. (j) The protein levels of CD36, HMGCR, ACOX1, and SREBP-1 in CD36 OE HepG2 cells. n = 3, ∗P < 0.05 vs. NC group for (a, c); #P < 0.05 vs. NC group, ∗P < 0.05 vs. PA group or PA+siCD36 group/PA+CD36 OE group (b–j).
Considering the important role of chronic inflammation in NAFLD, we evaluated the influence of CD36 on the inflammatory response. As shown in Figure 6(h), CD36 overexpression marked an increase in the mRNA expression of IL-1β and TNF-α, which were ameliorated by FV treatment, while the mRNA expression of IL-1β and CCL5 was obviously suppressed by CD36 knockdown (Figure 6(g)).
3.7. Modulation of Gut Microbiota Composition in HFD-Fed Mice by FV
In order to investigate the effects of FV treatment on the gut microbiota, we used 16S rRNA genetic sequencing to study the composition of the microbiota in mice. From the PCoA results for the gut microbiota, we found that the NFD group, the HFD group, and the FV-H group were clustered into three isolated groups, and the values for the FV-H group were clustered between the other groups (Figure 7(a)). Moreover, the relative abundance of bacteria at the phylum level in the three groups was calculated and shown in Figure 7(b). From the figure, the amount of Firmicutes of mice in the HFD group is higher and the amount of Bacteroidetes is lower. In addition, the Firmicutes/Bacteroidetes ratio in the HFD group was higher than that in the NFD group, but lower in the FV-H treatment group (Figure 7(c)). In obese individuals, the Firmicutes/Bacteroidetes ratio in gut microbiota is usually higher [24]. Therefore, the reduction of Firmicutes/Bacteroidetes ratio in the FV-H group indicates that FV administration can reverse this parameter of obesity.
Figure 7.
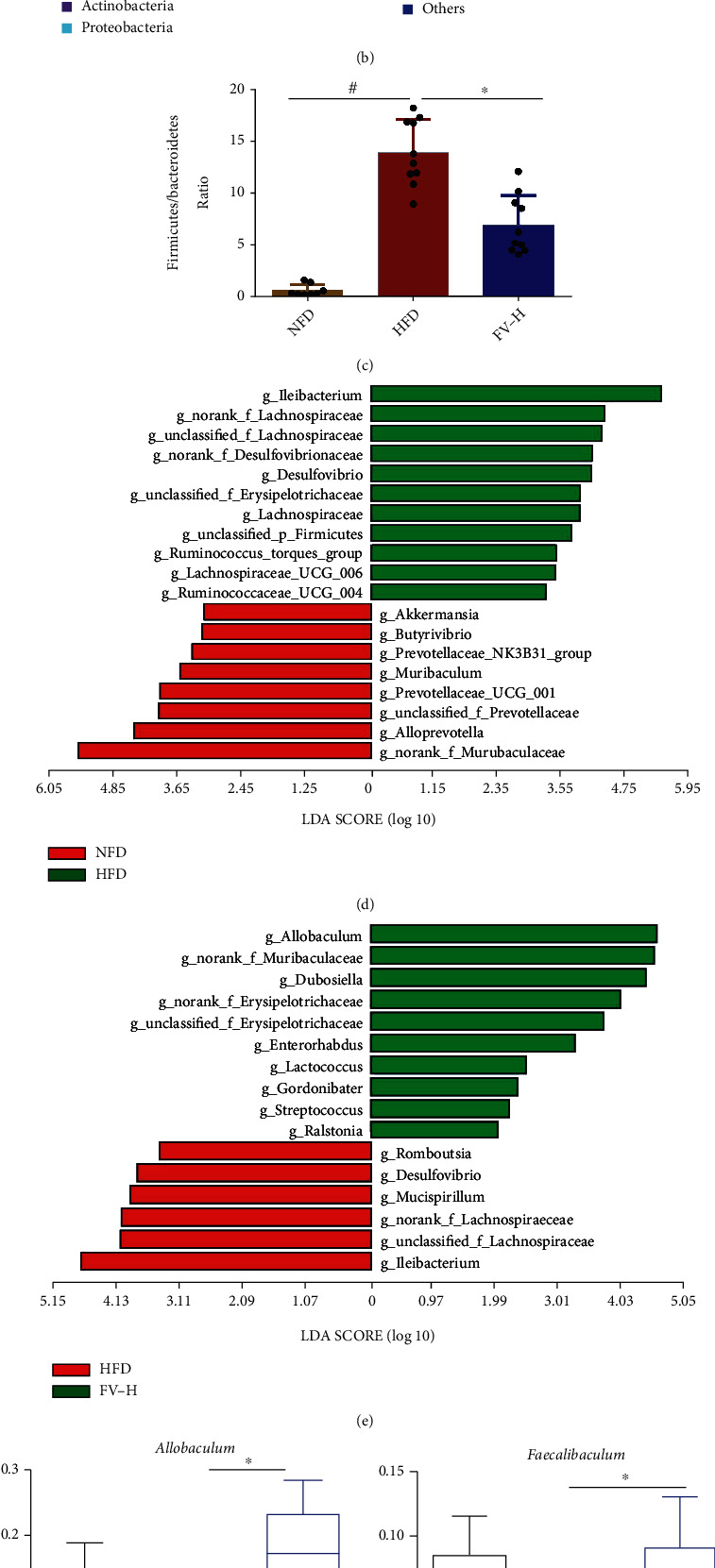
Diversity of gut microbiota in mouse models induced by different diets. (a) PCoA result structure of each group. (b) Taxonomic distribution of bacterial communities of NAFLD mouse fecal samples at the phylum level. (c) Firmicutes/Bacteroidetes ratio at the phylum level. (d) LDA score of differentially abundant taxa between the NFD group and the HFD group. (e) LDA score of differentially abundant taxa between the HFD group and the FV-H group. (f) Relative abundance of g_Allobaculum, g_Faecalibaculum, g_Lactococcus, g_norank_f_Muribaculaceae, g_Ileibacterium, g_Lachnospiraceae_UCG-006, g_Desulfovibrio, and g_Ruminococcus_torques_group at the genus levels. n = 8, 10, 10; #P < 0.05 vs. the NFD group, ∗P < 0.05 vs. the HFD group.
Subsequently, in order to determine the changes in specific bacterial taxa after the intervention of FV supplementation, we used the linear discriminant analysis (LDA) effect size (LEfSe) to identify the difference in the fecal microbiota composition between the HFD group and the FV-H group. At the genus level, the LDA score was used to analyze specific taxa in different test groups of the mice (Figures 7(d) and 7(e)). Compared with the NFD group, the abundance of g_Ileibacterium, g_Lachnospiraceae_UCG-006, g__Ruminococcus_UCG_004, and g_Lachnoclostridium (Figure 7(d)) in feces of HFD-fed mice was increased, which has been reported to be associated with liver and colon inflammation and relevant mouse or human diseases, including metabolic syndrome, gastrointestinal injury, and immune system disinfection [25, 26]. The same result was obtained in the abundance of g_Desulfovibrio, which is a key producer of endotoxins in animal models of obesity (Figure 7(l)) [27]. The FV supplementation significantly reduced these genera (Figure 7(e)). Correspondingly, the abundance of g_Allobaculum, g_Faecalibaculum, g_norank_f_Muribaculaceae, and g_Butyricicoccus was increased by FV treatment in HFD-fed mice (Figures 7(f)–7(i)). However, the abundance of g_Ileibacterium, g_Lachnospiraceae_UCG-006, and g_Ruminococcus_torques_group was decreased by FV treatment in HFD-fed mice (Figures 7(j), 7(k), and 7(m)). The bacterium of g_Allobaculum, g_Faecalibaculum, and g_Butyricicoccus is acted as the producer of short-chain fatty acids (SCFAs) in the gut, such as formic acid, acetic acid, propionic acid, butyric acid, and valerate acid. More and more studies have emphasized on SCFAs' alleviating effects on inflammation and protective effects on gut barrier function [28]. It has been proved that supplementing animals with butyrate can reduce liver fat accumulation and liver inflammation [29]. The g_norank_f_Muribaculaceae also showed a strong negative correlation with several obesity-related indicators in mice with a high-fat diet [30].
3.8. Correlations among the Gut Microbiota, Signaling Pathways, and Metabolic Parameters
In order to study the relationship between the fecal microbiota and the liver pathological condition, we compared 8 bacterial genera and 4 signaling pathways with 10 metabolic parameters using correlation analyses. The results indicated that Muribaculaceae had significantly negative correlation with the level of liver index, body weight, serum TC, and liver TG. And Allobaculum was negatively correlated with liver index, body weight, serum IL-6, and serum TNF-α. In addition, Faecalibaculum had an obviously negative correlation with liver index, serum IL-1β, and AST, while Ruminococcus_torques_group was positively correlated with these parameters. Moreover, Lachnospiraceae_UCG_006, Desulfovibrio, and Ileibacterium were positively correlated with liver index, liver weight, body weight, serum TC, IL-6, IL-1β, and TNF-α (Figure 8(a)).
Figure 8.
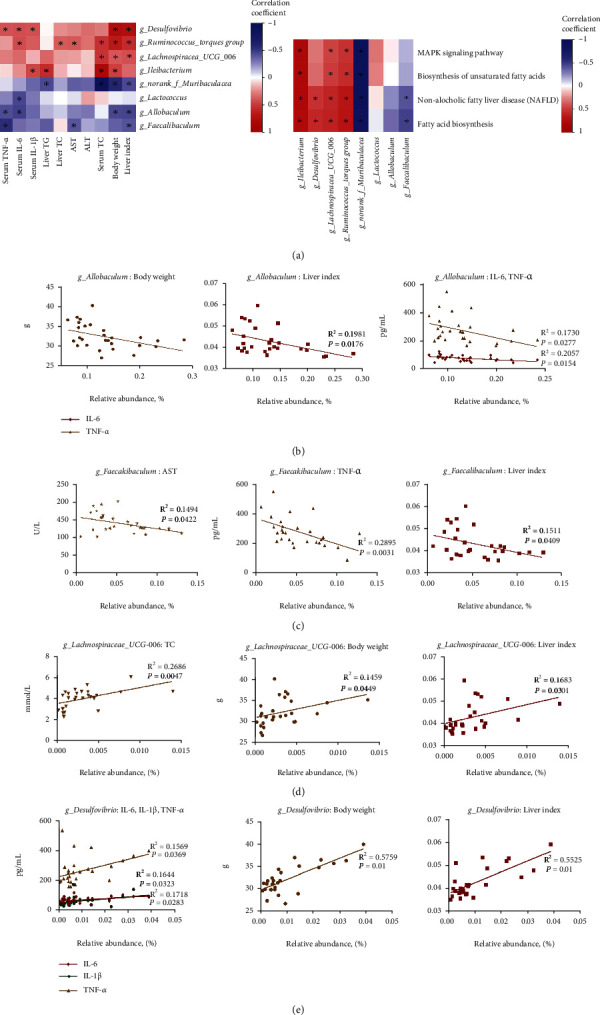
Correlation analyses between metabolic parameters and genera microbiota. (a) Spearman correlation heat map between 8 genera microbiota and 10 metabolic parameters; Spearman correlation heat map between 8 genera microbiota and 4 signaling pathways. (b–e) Linear regression analyses between metabolic parameters and genera microbiota. Significant correlations were marked by ∗P < 0.05.
Consistently, the results also demonstrated that beneficial bacterium was negatively correlated with the 4 signaling pathways, including NAFLD pathway, MAPK signaling pathway, fatty acid biosynthesis, and biosynthesis of unsaturated fatty acid. In particular, Muribaculaceae was significantly negatively correlated with all signaling pathways, and Faecalibaculum was positively negatively correlated with NAFLD and fatty acid biosynthesis signaling pathway. However, Ileibacterium, Lachnospiraceae_UCG_006, and Ruminococcus_torques_group were significantly positively correlated with all signaling pathways, and Desulfovibrio was significantly positively correlated with NAFLD and fatty acid biosynthesis signaling pathways (Figure 8(a)). As shown in Figures 6(b) and 6(c), Allobaculum had a negative linear correlation with IL-6, TNF-a, body weight, and liver index (P < 0.05); Faecalibaculum had a negative linear correlation with AST, TNF-α, and liver index (P < 0.05). Moreover, Figures 6(d) and 6(e) show that Lachnospiraceae_UCG_006 had a positive linear correlation with TC, body weight, and liver index (P < 0.05), while Desulfovibrio had a positive linear correlation with IL-1β, IL-6, TNF-α, body weight, and liver index (P < 0.05).
4. Discussions
The UPLC-Q/TOF-MS/MS identification result of Ficus hirta Vahl. showed 54 compounds, including apigenin, luteolin, psoralen, vitexin, and bergamot lactone. Previous studies have found that apigenin can regulate hepatocyte lipid metabolism and oxidative stress by adjusting PPARγ [31], Nrf2 [31], and XO/NLRP3 pathways [32], thereby attenuating HFD-induced NAFLD. Psoralen is one of the main components of FV, which has the ability to relieve lipid accumulation in PA-induced primary hepatocyte model of NAFLD through downregulating the intracellular content of TC and TG [33]. Simultaneously, the 8-methoxypsoralen, a vitamin D receptor ligand with a promising antisteatosis action, can relieve the symptoms of NAFLD by binding to vitamin D receptor [27]. Furthermore, other ingredients, such as naringenin [34], luteolin [35], and bergapten [36], can also improve NAFLD by relieving liver inflammation or regulating lipid metabolism. Therefore, we speculate that FV may play the role of treating NAFLD through the above components.
In this study, our results proved that natural herbs Ficus hirta Vahl. can prevent and treat NAFLD using in vitro and in vivo models. In comparison with the mice in the HFD group, FV administration alleviated obesity, ameliorated the accumulation of lipid, and attenuated liver inflammation and lipogenesis in HFD-fed mice. Moreover, we firstly discovered that CD36 as a potential target of FV for NAFLD and then FV exerted pharmacologic effects against NAFLD partly by reducing the expression of CD36 to improve lipid metabolism and inflammation. Meanwhile, we revealed that the regulation of the gut microbiota structure by FV supplementation can improve liver inflammation in HFD-fed mice. These results have demonstrated that FV may be used as a candidate to treat lipid metabolism including NAFLD and inflammatory disorders in the future (Figure 9).
Figure 9.
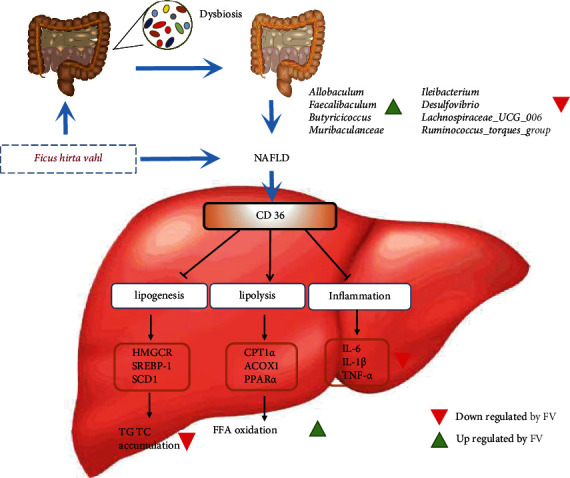
The speculative mechanism of Ficus hirta Vahl. for treatment of NAFLD in HFD-fed mice. FV alleviates nonalcoholic fatty liver disease through adjusting lipid metabolism and inflammation via downregulation of CD36 and regulating the structure of gut microbiota.
Accumulation of lipid in hepatocyte is an important indicator for the pathogenesis of NAFLD [37]. In hepatocytes, fatty acids are mainly stored and transported in the form of TG [38]. However, TG can be excessively accumulated in hepatocytes in NAFLD due to the metabolic disorder of fatty acid [39]. Our data have indicated that compared with NFD-fed mice, TG contents of liver tissue increased obviously in HFD-fed mice, as shown in Figure 3(h), which is consistent with the above results. In contrast, FV supplementation alleviated this symptom in a dose-dependent manner, which indicated that FV was a great contributor to suppress the TG accumulation. Besides, the outcome of oil red O staining revealed that FV supplementation remarkably suppressed the formation of lipid droplet in liver tissue of HFD fed mice.
To provide further insights into the role of FV in hepatic lipid metabolism, we studied the expression of genes related to lipid metabolism using both in vivo and in vitro models. SREBP-1 and its downstream proteins involving ACACA and SCD1 are important transcription factors that regulate the synthesis of fatty acid. CPT1α, ACOX1, and PPARα are associated with fatty acid oxidation, and CD36 and FABP1 are related to lipid uptake. Previous study suggested that dioscin can help ameliorate NAFLD by inhibiting the expression of ACACA, SCD1, and SREBP1 of the liver in HFD-fed mice [40], which is in agreement with our results on the effect of FV treatment. Additionally, we found that after FV treatment, the expression of CPT1α and PPARα in mice was significantly upregulated. These findings were similar to previous studies [41]. All the outcomes above confirmed that FV contributed to lipid metabolism.
In order to comprehensively reveal the role of FV in NAFLD and the associated mechanism, we conducted RNA-seq analysis of liver tissues in the HFD-fed mice and corresponding diet mice with FV supplementation. GSEA revealed transcriptional levels involved in significant regulation of lipid metabolism, inflammation, and fibrosis. CD36 is a central regulator for cells metabolism, lipid maintenance, and glucose metabolism. In addition, CD36 transduces signals to mediate its role in inflammation [42] and lipid metabolism, thus accelerating the progression of metabolic diseases including obesity, atherosclerosis, NAFLD, and type 2 diabetes [43]. From the RNA-seq results, FV treatment significantly downregulated the mRNA level of CD36, which may use a feed-forward loop to facilitate the entry of fatty acids, thereby providing positive effects on its own de novo synthesis and functioning as a ligand for PPARγ. PPARγ increases the gene expression of essential proteins that support lipid droplet expansion [44] and it is expressed at low levels in normal liver, whereas increased expression of PPARγ is a common feature of hepatic steatosis [45]. Importantly, PPARγ can activate the transcription of CD36. CD36 silencing ameliorates lipid accumulation and improves hepatic steatosis by restoring the reduction in fatty acid oxidation in vitro [46]. CD36 has been implicated in inflammatory signaling induced by ox-LDL [47]. CD36 can bind to ox-LDL and activate the JNK signaling pathway to induce inflammation; in addition, CD36 can mediate the production of ROS by activating the NLRP3 inflammasome [48]. Our data proved that CD36 intensified the accumulation of the hepatocyte lipid in NAFLD. Interestingly, FV suppressed the expression and activity of CD36, and FV treatment rescued the exacerbated effects of CD36 on lipid metabolism and inflammation. After the HFD-fed mouse group and PA-induced HepG2 cells were treated with FV, the genes related to lipid synthesis, including SREBP-1, ACACA, and SCD1, were apparently decreased, but the genes involved in fatty acid oxidation, such as CPT1α, ACOX1, and PPARα, were significantly elevated. These results indicated that FV could be a potential candidate for NAFLD by attenuating the overaccumulation of lipid.
A few mechanisms have been suggested for the gut microbiome and NAFLD, including the gut microbiome dysbiosis that shifts bacterial components and results in hepatic inflammation. Also, the gut microbiota may produce different metabolites that cause NAFLD susceptibility [49]. In addition, some species of microbes can produce specific enzymes to ferment nutrients into an absorbable form. For example, the conversion of indigestible carbohydrates into SCFAs [50] may have anti-inflammatory and immunomodulatory effects [51]. Otherwise, when bacteria regulate the intestinal permeability, certain species may promote the “leaky gut.” In this case, metabolites related to microbes enter the bloodstream from the gut. Consequently, the body produces cytokines and other mediators to initiate an inflammatory response [52]. Our analysis on the fecal microbiota has shown that FV can improve the gut microbiota dysbiosis in the mice fed with HFD. Also, it is suggested that a high Firmicutes/Bacteroidetes ratio increases the energy uptake and results in obesity because the members of the phylum Firmicutes are more efficient than the members of the Bacteroidetes in helping the host obtain calories from food [53]. However, the relative abundance of bacteria at the phylum level revealed that FV significantly reduced Firmicutes/Bacteroidetes ratio in comparison with the HFD group.
Many studies have aimed at identifying the specific bacteria changes that lead to NAFLD. In our study, after FV administration, at least eight microbiota genera that reside have been changed in the gut. Among them, Allobaculum, Faecalibaculum, and Butyricicoccus have been identified as SCFA-producing bacterium and are inversely associated with different proinflammatory markers. Butyrate is an anti-inflammatory metabolite with the known inhabitation effect on the producing pathway of proinflammatory cytokines [54]. SCFAs inhibit HDAC (histone deacetylase) activity, promote histone acetylation, affect inflammatory response, and contribute to intestinal homeostasis [55]. As bacterial abundance increased sequentially, Ileibacterium, Lachnospiraceae_UCG_006, Desulfovibrio, and Ruminococcus_torqus_group increased with the progression of NAFLD. This phenomenon is in line with the study result on the mouse model that Desulfovibrio is strongly correlated with obesity, metabolic syndrome, and inflammation [56]. Ileibacterium, a novel member of the family Erysipelotrichaceae, was upregulated by LPS induction [57], but its abundance in HFD-fed mice was downregulated by FV treatment. Lachnospiraceae_UCG_006 is the main genus of Lachnospiraceae and has a positive correlation with the pathological characteristics of colitis [58]. These results have indicated that a high-fat diet may lead to imbalance of the gut microecology and activate intestinal pathogenic bacteria to cope with inflammation, and then, the increase in abundance of beneficial bacteria might be caused by the therapeutic effect of the FV supplementation diet.
In the liver, the mitogen-activated protein kinase (MAPK) signaling pathway is important in regulating metabolism [59], as the obesity and the related inflammatory state in insulin-responsive tissues activate the stress-responsive MAPKs, and the hypothesis that MAPKs signaling pathway drives liver metabolic dysfunction has been accepted [60]. In addition, fatty acids accumulate in the liver by hepatocellular uptake and biosynthesis. The metabolic disorders disrupt the balance of hepatic fatty acid metabolism, thus usually causing the accumulation of TG in the liver and NAFLD [38]. Our research results indicated that FV suppressed the MAPK signaling pathway and fatty acid biosynthesis, thereby attenuating the severity state of NAFLD in HFD-fed mice.
5. Conclusion
The results of this study revealed that FV treatment can commendably ameliorate lipid metabolism and hepatic inflammation by regulating CD36 and alleviate the progression of NAFLD by regulating the composition and potential function of the gut microbiota. Ficus hirta Vahl. is an ideal medicine to improve the pathophysiology of diet-induced metabolic diseases and NAFLD.
Acknowledgments
The authors would like to thank the authors of all references. This work was supported by the National Natural Science Foundation of China (Nos. 82074078 and 81803816), Science and Technology Planning Project of Guangdong Province, China (2014A020221063), Natural Science Foundation of Guangdong Province (2021A1515010567), Guangdong Provincial Laboratory of Digestive Cancer Research (No. 2021B1212040006), and Clinical Research Foundation of the Seventh Affiliated Hospital, Sun Yat-sen University (ZSQYLCKYJJ202001).
Abbreviations
- NAFLD:
Nonalcoholic fatty liver disease
- CD36:
Cluster of differentiation 36
- PA:
Palmitic acid
- HFD:
High-fat diet
- TC:
Total cholesterol
- TG:
Total triglyceride
- ALT:
Alanine aminotransferase
- AST:
Aspartate aminotransferase
- LDL-C:
Low-density lipoprotein cholesterol
- H&E:
Hematoxylin and eosin
- ELISA:
Enzyme-linked immunosorbent assay
- IL-6:
Interleukin-6
- IL-1β:
Interleukin-1β
- TNF-α:
Tumor necrosis factor-α
- SREBP-1:
Sterol regulatory element-binding protein 1
- CPT1α:
Recombinant carnitine palmitoyltransferase 1α
- HMGCR:
Recombinant 3-hydroxy-3-methylglutaryl coenzyme A reductase
- ACOX1:
Acyl-coenzyme A oxidase 1
- ACACA:
Acetyl-coenzyme A carboxylase alpha
- FABP1:
Fatty acid-binding protein 1
- PPARα:
Peroxisome proliferator-activated receptor α
- PPARγ:
Peroxisome proliferator-activated receptor γ
- DEGs:
Differentially expressed genes.
Contributor Information
Shijian Xiang, Email: xiangshj3@mail.sysu.edu.cn.
Benjie Zhou, Email: zhoubj23@mail.sysu.edu.cn.
Shasha Li, Email: s19861020@jnu.edu.cn.
Data Availability
Data can be obtained from the corresponding author. Please email at mailto:s19861020@jnu.edu.cn.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
T.Q. and F.Y.Z. performed the experiments and equally contributed to this paper. L.N.J., H.Y.C., F.X., and Y.X.Y. analyzed the data. T.Q. and F.Y.Z. were primarily responsible for writing the manuscript. S.S.L., S.J.X., and B.J.Z. designed the experiments and revised the paper. All authors contributed to manuscript editing and approved the final version.
Supplementary Materials
Supplementary Table 1 shows the time program of the gradient elution in UPLC. Supplementary Table 2 lists the parameters in the mass spectrometric analysis method. Supplementary Table 3 displays human and mouse primer sequences for quantitative real-time PCR.
References
- 1.Eslam M., Newsome P. N., Sarin S. K., et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. Journal of Hepatology . 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z., Anstee Q. M., Marietti M., et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nature Reviews Gastroenterology & Hepatology . 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 3.Li H., Yu X. H., Ou X., Ouyang X. P., Tang C. K. Hepatic cholesterol transport and its role in non-alcoholic fatty liver disease and atherosclerosis. Progress in Lipid Research . 2021;83:p. 101109. doi: 10.1016/j.plipres.2021.101109. [DOI] [PubMed] [Google Scholar]
- 4.Bril F., Barb D., Portillo-Sanchez P., et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology . 2017;65(4):1132–1144. doi: 10.1002/hep.28985. [DOI] [PubMed] [Google Scholar]
- 5.Liang C., Li Y., Bai M., et al. Hypericin attenuates nonalcoholic fatty liver disease and abnormal lipid metabolism via the PKA-mediated AMPK signaling pathway in vitro and in vivo. Pharmacological Research . 2020;153:p. 104657. doi: 10.1016/j.phrs.2020.104657. [DOI] [PubMed] [Google Scholar]
- 6.De Minicis S., Rychlicki C., Agostinelli L., et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology . 2014;59(5):1738–1749. doi: 10.1002/hep.26695. [DOI] [PubMed] [Google Scholar]
- 7.Gaggini M., Carli F., Rosso C., et al. Altered amino acid concentrations in NAFLD: impact of obesity and insulin resistance. Hepatology . 2018;67(1):145–158. doi: 10.1002/hep.29465. [DOI] [PubMed] [Google Scholar]
- 8.Younossi Z. M. Non-alcoholic fatty liver disease - a global public health perspective. Journal of Hepatology . 2019;70(3):531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 9.Younossi Z., Tacke F., Arrese M., et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology . 2019;69(6):2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 10.Saklayen M. G. The global epidemic of the metabolic syndrome. Current Hypertension Reports . 2018;20(2):p. 12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverstein R. L., Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Science Signaling . 2009;2(72):p. re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greco D., Kotronen A., Westerbacka J., et al. Gene expression in human NAFLD. Gastrointestinal and liver physiology . 2008;294(5):G1281–G1287. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 13.Vazquez M. M., Gutierrez M. V., Salvatore S. R., et al. Nitro-oleic acid, a ligand of CD36, reduces cholesterol accumulation by modulating oxidized-LDL uptake and cholesterol efflux in RAW264.7 macrophages. Redox Biology . 2020;36:p. 101591. doi: 10.1016/j.redox.2020.101591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida T., Yokobori T., Saito H., et al. CD36 expression is associated with cancer aggressiveness and energy source in esophageal squamous cell carcinoma. Annals of Surgical Oncology . 2021;28(2):1217–1227. doi: 10.1245/s10434-020-08711-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhao L., Zhang C., Luo X., et al. CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. Journal of Hepatology . 2018;69(3):705–717. doi: 10.1016/j.jhep.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Samovski D., Abumrad N. A. Regulation of lipophagy in NAFLD by cellular metabolism and CD36. Journal of Lipid Research . 2019;60(4):755–757. doi: 10.1194/jlr.C093674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng Y. W., Liu X. Z., Lv Z. C., Peng Y. H. Effects of Ficus hirta Vahl. (Wuzhimaotao) extracts on growth inhibition of HeLa cells. Experimental and toxicologic pathology: official journal of the Gesellschaft fur Toxikologische Pathologie . 2012;64(7-8):743–749. doi: 10.1016/j.etp.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Wan C., Chen C., Li M., Yang Y., Chen M., Chen J. Chemical constituents and antifungal activity of Ficus hirta Vahl. Fruits. Fruits, Plants . 2017;6(4):p. 44. doi: 10.3390/plants6040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi T., Chen Q., He X., et al. Chemical quantification and antioxidant assay of four active components in Ficus hirta root using UPLC-PAD-MS fingerprinting combined with cluster analysis. Chemistry Central Journal . 2013;7(1):p. 115. doi: 10.1186/1752-153X-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou T. N., Tang L. H., Huang S. C., et al. Study on the antitussive and antiasthmatic effects of Radix Fici Hirtae. Zhong yao cai = Zhongyaocai = Journal of Chinese medicinal materials . 2009;32(4):571–574. [PubMed] [Google Scholar]
- 21.Cai Q. Y., Chen H. B., Cai S. Q., et al. Effect of roots of Ficus hirta on cocaine-induced hepatotoxicity and active components. China journal of Chinese materia medica . 2007;32(12):1190–1193. [PubMed] [Google Scholar]
- 22.Feng X., Li K., Tan F., et al. Assessment of hepatoprotective potential of Radix Fici Hirtae on alcohol- induced liver injury in Kunming mice. Biochemistry and biophysics reports . 2018;16:69–73. doi: 10.1016/j.bbrep.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao Y., Wang J., Jaehnig E. J., Shi Z., Zhang B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Research . 2019;47(W1):W199–W205. doi: 10.1093/nar/gkz401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hildebrandt M. A., Hoffmann C., Sherrill-Mix S. A., et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology . 2009;137(5):1716–1724.e2. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mailhe M., Ricaboni D., Vitton V., Lagier J. C., Fournier P. E., Raoult D. “ Ileibacterium massiliense” gen. nov., sp. nov., a new bacterial species isolated from human ileum of a patient with Crohn disease. New Microbes New Infect . 2017;17:25–26. doi: 10.1016/j.nmni.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mu H., Zhou Q., Yang R., et al. Naringin attenuates high fat diet induced non-alcoholic fatty liver disease and gut bacterial dysbiosis in mice. Frontiers in Microbiology . 2020;11 doi: 10.3389/fmicb.2020.585066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elhafiz M., Zhao G., Ismail M., et al. Imbalanced insulin substrate-1 and insulin substrate-2 signaling trigger hepatic steatosis in vitamin D deficient rats: 8-methoxypsoralen, a vitamin D receptor ligand with a promising anti-steatotic action. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids . 2020;1865(6):p. 158657. doi: 10.1016/j.bbalip.2020.158657. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X., Zhao Y., Zhang M., et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One . 2012;7(8):p. e42529. doi: 10.1371/journal.pone.0042529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L., Wang R., Wei G. Y., et al. Cryptotanshinone alleviates chemotherapy-induced colitis in mice with colon cancer via regulating fecal-bacteria-related lipid metabolism. Pharmacological Research . 2021;163:p. 105232. doi: 10.1016/j.phrs.2020.105232. [DOI] [PubMed] [Google Scholar]
- 30.Zou J., Shen Y., Chen M., et al. Lizhong decoction ameliorates ulcerative colitis in mice via modulating gut microbiota and its metabolites. Applied Microbiology and Biotechnology . 2020;104(13):5999–6012. doi: 10.1007/s00253-020-10665-1. [DOI] [PubMed] [Google Scholar]
- 31.Feng X., Yu W., Li X., et al. Apigenin, a modulator of PPARγ, attenuates HFD-induced NAFLD by regulating hepatocyte lipid metabolism and oxidative stress via Nrf2 activation. Biochemical Pharmacology . 2017;136:136–149. doi: 10.1016/j.bcp.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Lv Y., Gao X., Luo Y., et al. Apigenin ameliorates HFD-induced NAFLD through regulation of the XO/NLRP3 pathways. The Journal of Nutritional Biochemistry . 2019;71:110–121. doi: 10.1016/j.jnutbio.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Zhou L., Tang J., Yang X., et al. Five constituents in Psoralea corylifolia L. attenuate palmitic acid-induced hepatocyte injury via inhibiting the protein kinase C-alpha/nicotinamide-adenine dinucleotide phosphate oxidase pathway. Frontiers in Pharmacology . 2019;10:p. 1589. doi: 10.3389/fphar.2019.01589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q., Ou Y., Hu G., et al. Naringenin attenuates non-alcoholic fatty liver disease by down-regulating the NLRP3/NF‐κB pathway in mice. British Journal of Pharmacology . 2020;177(8):1806–1821. doi: 10.1111/bph.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun W. L., Yang J. W., Dou H. Y., et al. Anti-inflammatory effect of luteolin is related to the changes in the gut microbiota and contributes to preventing the progression from simple steatosis to nonalcoholic steatohepatitis. Bioorganic Chemistry . 2021;112:p. 104966. doi: 10.1016/j.bioorg.2021.104966. [DOI] [PubMed] [Google Scholar]
- 36.Pattanayak S. P., Bose P., Sunita P., Siddique M. U. M., Lapenna A. Bergapten inhibits liver carcinogenesis by modulating LXR/PI3K/Akt and IDOL/LDLR pathways. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie . 2018;108:297–308. doi: 10.1016/j.biopha.2018.08.145. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka S., Hikita H., Tatsumi T., et al. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology . 2016;64(6):1994–2014. doi: 10.1002/hep.28820. [DOI] [PubMed] [Google Scholar]
- 38.Alves-Bezerra M., Cohen D. E. Triglyceride metabolism in the liver. Comprehensive Physiology . 2017;8(1):1–8. doi: 10.1002/cphy.c170012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verwer B. J., Scheffer P. G., Vermue R. P., Pouwels P. J., Diamant M., Tushuizen M. E. NAFLD is related to post-prandial triglyceride-enrichment of HDL particles in association with endothelial and HDL dysfunction. Liver International . 2020;40(10):2439–2444. doi: 10.1111/liv.14597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao H., Tao X., Xu L., et al. Dioscin alleviates non-alcoholic fatty liver disease through adjusting lipid metabolism via SIRT1/AMPK signaling pathway. Pharmacological Research . 2018;131:51–60. doi: 10.1016/j.phrs.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Q., Kong X., Yuan H., Guan H., Li Y., Niu Y. Mangiferin Improved Palmitate-Induced-Insulin Resistance by Promoting Free Fatty Acid Metabolism in HepG2 and C2C12 Cells via PPARα: Mangiferin Improved Insulin Resistance. Journal Diabetes Research . 2019;2019, article 2052675:13. doi: 10.1155/2019/2052675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navas-Madroñal M., Castelblanco E., Camacho M., et al. Role of the scavenger receptor CD36 in accelerated diabetic atherosclerosis. International Journal of Molecular Sciences . 2020;21(19):p. 7360. doi: 10.3390/ijms21197360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Foll C. Hypothalamic fatty acids and ketone bodies sensing and role of FAT/CD36 in the regulation of food intake. Frontiers in Physiology . 2019;10:p. 1036. doi: 10.3389/fphys.2019.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowe C. E., O'Rahilly S., Rochford J. J. Adipogenesis at a glance. Journal of Cell Science . 2011;124(16):2681–2686. doi: 10.1242/jcs.079699. [DOI] [PubMed] [Google Scholar]
- 45.Choi Y., Song M. J., Jung W. J., et al. Liver-specific deletion of mouse CTCF leads to hepatic steatosis via augmented PPARγ signaling. Gastroenterología y Hepatología . 2021;12(5):1761–1787. doi: 10.1016/j.jcmgh.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y., Yang P., Zhao L., et al. CD36 plays a negative role in the regulation of lipophagy in hepatocytes through an AMPK-dependent pathway[S] Journal of Lipid Research . 2019;60(4):844–855. doi: 10.1194/jlr.M090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bieghs V., van Gorp P. J., Walenbergh S. M., et al. Specific immunization strategies against oxidized low-density lipoprotein: a novel way to reduce nonalcoholic steatohepatitis in mice. Hepatology . 2012;56(3):894–903. doi: 10.1002/hep.25660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu W., Yin Y., Zhou Z., He M., Dai Y. OxLDL-induced IL-1 beta secretion promoting foam cells formation was mainly via CD36 mediated ROS production leading to NLRP3 inflammasome activation. Inflammation Research . 2014;63(1):33–43. doi: 10.1007/s00011-013-0667-3. [DOI] [PubMed] [Google Scholar]
- 49.Safari Z., Gérard P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD) Cellular and molecular life sciences : CMLS . 2019;76(8):1541–1558. doi: 10.1007/s00018-019-03011-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flint H. J., Scott K. P., Duncan S. H., Louis P., Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes . 2012;3(4):289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan J., McKenzie C., Potamitis M., Thorburn A. N., Mackay C. R., Macia L. The role of short-chain fatty acids in health and disease. Advances in Immunology . 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 52.Brandsma E., Kloosterhuis N. J., Koster M., et al. A proinflammatory gut microbiota increases systemic inflammation and accelerates atherosclerosis. Circulation Research . 2019;124(1):94–100. doi: 10.1161/CIRCRESAHA.118.313234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Q., Chen H., Zhang M., Wu T., Liu R. Altered short chain fatty acid profiles induced by dietary fiber intervention regulate AMPK levels and intestinal homeostasis. Food & Function . 2019;10(11):7174–7187. doi: 10.1039/C9FO01465A. [DOI] [PubMed] [Google Scholar]
- 54.Segain J. P., Raingeard de la Blétière D., Bourreille A., et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut . 2000;47(3):397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang P. V., Hao L., Offermanns S., Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proceedings of the National Academy of Sciences of the United States of America . 2014;111(6):2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loy A., Pfann C., Steinberger M., et al. Lifestyle and horizontal gene transfer-mediated evolution of Mucispirillum schaedleri, a core member of the murine gut microbiota. Msystems . 2017;2(1) doi: 10.1128/mSystems.00171-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cox L. M., Sohn J., Tyrrell K. L., et al. Description of two novel members of the family Erysipelotrichaceae: Ileibacterium valens gen. nov., sp. nov. and Dubosiella newyorkensis, gen. nov., sp. nov., from the murine intestine, and emendation to the description of Faecalibaculum rodentium. International Journal of Systematic and Evolutionary Microbiology . 2017;67(5):1247–1254. doi: 10.1099/ijsem.0.001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shao X., Sun C., Tang X., et al. Anti-inflammatory and intestinal microbiota modulation properties of Jinxiang garlic (Allium sativumL.) polysaccharides toward dextran sodium sulfate-induced colitis. Journal of Agricultural and Food Chemistry . 2020;68(44):12295–12309. doi: 10.1021/acs.jafc.0c04773. [DOI] [PubMed] [Google Scholar]
- 59.Manieri E., Sabio G. Stress kinases in the modulation of metabolism and energy balance. Journal of Molecular Endocrinology . 2015;55(2):R11–R22. doi: 10.1530/JME-15-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Samuel V. T., Shulman G. I. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metabolism . 2018;27(1):22–41. doi: 10.1016/j.cmet.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 shows the time program of the gradient elution in UPLC. Supplementary Table 2 lists the parameters in the mass spectrometric analysis method. Supplementary Table 3 displays human and mouse primer sequences for quantitative real-time PCR.
Data Availability Statement
Data can be obtained from the corresponding author. Please email at mailto:s19861020@jnu.edu.cn.


