Abstract
This study is aimed at evaluating five mineral oxides (5MO), mineral trioxide aggregate repair high plasticity (MTA HP), and mineral trioxide aggregate (MTA) in relation to the antimicrobial action over Porphyromonas gingivalis, Porphyromonas endodontalis, Parvimonas micra, Fusobacterium nucleatum, and Prevotella intermedia; the genotoxicity over mouse macrophage (RAW 264.7) and osteoblast (Mg-63) cultures; and the morphological analysis using scanning electron microscopy (SEM) analysis (50 k and ×100 k). Sodium hypochlorite (NaOCl), calcium hydroxide, and saline solution were used as control groups in the different analysis. All data were submitted to a normality test and then analyzed with one-way ANOVA, Tukey, and Kruskal-Wallis and Dunn tests, considering α ≤ 0.05 significance level. It was found that over P. gingivalis and P. endodontalis, there was no a significant difference between the calcium silicate-based cements (CSC) and the control group of saline solution, and only 5MO was similar to the NaOCl group. However, over P. micra, all groups were effective and showed a statistically significant difference compared to the saline solution group. Conversely, none of the groups were effective over F. nucleatum and P. intermedia, except of the NaOCl group. There was a significant difference between 5MO and MTA groups in comparison with NaOCl and MTA HP over osteoblasts and macrophages after 24 hours. SEM images showed small irregular particles interspersed with some elongated needle-like particles and small irregular particles with some larger particles as well as elongated particles. It was concluded that 5MO, MTA, and MTA HP have effective antimicrobial action over P. micra. However, only 5MO is effective over P. gingivalis and P. endodontalis. Besides, 5MO and MTA are not genotoxic over mouse macrophage (RAW 264.7) and osteoblast (Mg-63) cultures.
1. Introduction
Five mineral oxides (5MO) (Golden Yatti LLC, Muscat, Oman) is a calcium silicate-based cement (CSC), it was introduced firstly as a pulp capping material [1]. In the literature, there are three case reports evaluated its efficacy in endodontic perforation and open apex sealing [2–4]. However, only one experimental study, in dogs, reported its efficacy as a direct pulp capping material [5]. On the other hand, mineral trioxide aggregate repair high plasticity (MTA HP) (Angelus, Londrina, PR, Brazil) is a new version of the mineral trioxide aggregate (MTA) (Angelus, Londrina, PR, Brazil) [6], the commonly used CSC [7]. MTA HP was released firstly in 2016, with the proposal of CSC with improved physical properties because of a high plasticity [6].
There is a lack of studies in the literature about the biological properties of 5MO and MTA HP. The antimicrobial action of CSC is an extremely relevant factor to be studied as the success of sealing demands aseptic areas [8]. Besides, the biocompatibility guarantees the efficacy of CSC sealing ability without causing damage to the host tissues [9], being one of the basic requirements in the evaluation of an endodontic material [10]. Lastly, the morphological analysis of these CSC powder plays a major role to understand the nature and porosity of the generated tissues [11].
Therefore, the aim of this study was to evaluate 5MO, MTA HP, and MTA in relation to : (I) the antimicrobial action over Porphyromonas gingivalis, Porphyromonas endodontalis, Parvimonas micra, Fusobacterium nucleatum, and Prevotella intermedia; (II) the genotoxicity over mouse macrophage (RAW 264.7) and osteoblast (Mg-63) cultures; and (III) the morphological analysis. The null hypothesis is that CSCs have no antimicrobial action and are genotoxic.
2. Material and Methods
2.1. Crystal Violet Assay
Five different inocula of P. gingivalis (ATCC 33277), P. endodontalis (ATCC 35406), P. micra (ATCC 23195), F. nucleatum (ATCC 25586), and P. intermedia (ATCC 33563) were prepared and standardized at (1 × 108 CFU/mL) in a spectrophotometer (Visible Spectrophotometer V-5000, Shanghai Metash Instruments Co., Ltd., China) in saline solution (NaCl 0.9%) (Eurofarma, São Paulo, SP, Brazil).
Biofilms of each inocula were incubated in 96-well microplates (TPP, Trasadingen, Switzerland) at 37°C for 7 days in anaerobic conditions, with replacement of the culture medium every 48 hours. Later, the biofilm measurement test (crystal violet) was performed. Two independent experiments were carried out, with 5 repetitions each, totaling n = 10 for each group.
The groups of this study were as follows: (I) saline solution (negative control group) (Eurofarma, São Paulo, SP, Brazil), (II) 2.5% sodium hypochlorite (NaOCl) (Biodynamics, Ibiporã, PR, Brazil) (positive control group), (III) 5MO (Golden Yatti LLC, Muscat, Oman), (IV) MTA HP (Angelus, Londrina, PR, Brazil), and (V) MTA, white version (Angelus, Londrina, PR, Brazil).
To manipulate the CSCs, a sterilized glass plate and a flexible spatula number 24 were used considering the proportion of (3× powder: 1× liquid) according to the manufacturers' protocol. After 24-hours contact with the groups, 200 μL/well of methanol was added for 20 min to fix the biofilm, then the methanol was removed, and the plates were incubated at 37°C for 24 h. Afterwards, 200 μL/well of 1% crystal violet (V/V) (Synth, Diadema, SP, Brazil) was added for 5 min, the dye was removed, and washes with sterile physiological solution (Eurofarma, São Paulo, SP, Brazil) and 33% acetic acid (V/V) (Synth, Diadema, SP, Brazil) were performed. The plates were read in a microplate reader (BIO-TEK Instruments, Highland Park, Winooski, VT, USA), and the optical densities were converted into the biofilm biomass, using the formula:
%Reduction in biomass = (OD − Treated Group × 100)/(Mean OD Control Group).
2.2. Genotoxicity Analysis
In this study, cultures of mouse macrophages (RAW 264.7) (Rio de Janeiro Cell Bank-APABCAM–RJ, Brazil) and osteoblasts (MG-63) (Rio de Janeiro Cell Bank-APABCAM–RJ, Brazil) were used. The cells were grown in Dulbecco's Modified Eagle Medium (DMEM) (LGC Biotechnology, Cotia, Brazil) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, New York, USA), incubated at 37°C, with atmospheric humidity, with 5% CO2 using cell culture flasks (TPP, Zollstrasse, Switzerland).
The culture medium was changed every 48 h until a state of subconfluence of the cells was observed, and the cells were transferred to another cell flask. The cells were transferred to a falcon-type tube where they were centrifuged for 5 min at 2000 rpm. To quantify the number of viable cells, the Trypan blue (0.4%, Sigma-Aldrich, St. Louis, MO, USA) exclusion test was performed. The cells were cultivated in 96-well microplates, 200 μl of DMEM medium was added and supplemented with 10% FBS containing 2 × 104 viable cells. These plates were incubated (37°C, 5% CO2) for 24 hours for cell adhesion. Then, the supernatant was discarded, and the cells were washed with PBS. The incubation period was 5 min and 24 hours. The number of wells was equal to 10 for all groups.
To prepare CSCs, they were manipulated in a 24-well plate following the manufacturer's proportions and remained in the bottom of the well to set. Ca(OH)2 (Biodynamics Chemicals and Pharmaceuticals LTDA, Paraná, Brazil) was added in this test, as a positive control group. Ca(OH)2 powder was manipulated using the proportion (1 : 1) (powder: liquid). After the materials have set, each well containing the CSCs was filled with 2 mL of culture medium (DMEM) supplemented with 10% fetal bovine serum, and the plates were incubated at 37°C for 24 h with 5% CO2. By this form, a conditioned DMEM was obtained of each material, in which 100 μL per well of the conditioned DMEM of each group was applied.
The NaOCl group was used as a negative control group. To verify the viability of the culture, genotoxicity analysis was performed. After the incubation period, the cells were fixed in 100% methanol for 20 min, followed by staining with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma, Missouri, USA), and the dye was removed after 5 min of contact with the cells and then washed with PBS. Micronuclei analysis was performed using a fluorescence microscope (Leica DFC310FX) (Leica Biosystems, Wetzlar, Tokyo, Japan) at ×40 magnification, evaluating 2,000 cells/well.
2.3. Morphological Analysis by Scanning Electron Microscopy
This analysis was performed for powder of each CSC in which a thin layer of powder was dispersed over a polymethylmethacrylate plate mounted on an aluminum stub. All stubs were coated with carbon for electrical conductivity. Samples were visualized by SEM (MIRA3-TESCA, Brno-Kohoutovice, Czech Republic). Images of the different components of the CSC microstructure at different magnifications in electron backscatter mode were captured at magnifications up to ×100 k.
2.4. Statistical Analysis
Data were submitted to a normality test. The data that presented a normal distribution were analyzed with one-way ANOVA and Tukey test. The data that did not present a normal distribution were analyzed with Kruskal-Wallis test and complemented by the Dunn test, considering α ≤ 0.05 significance level using GraphPad Prism 6 (La Jolla, CA, USA).
3. Results
3.1. Crystal Violet Assay
Over P. gingivalis and P. endodontalis, there was no a significant difference between the CSCs and the negative control group (saline solution). Only the 5MO group was similar to the NaOCl group. However, over P. micra, all groups were effective and showed a statistically significant difference when compared to the negative control group (Figure 1).
Figure 1.
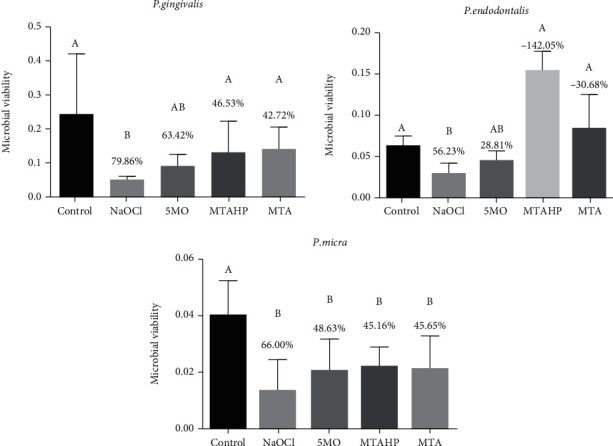
Microbial viability calculated by reflectance and viability reduction (in %) of P. gingivalis, P. endodontalis, and P. micra biofilms by crystal violet assay after treatment with the groups. Different uppercase letters indicate statistical differences.
On the other hand, none of the groups were effective over F. nucleatum and P. intermedia in which none of the groups showed a significant difference when compared to the saline solution group, except of the NaOCl group (Figure 2).
Figure 2.
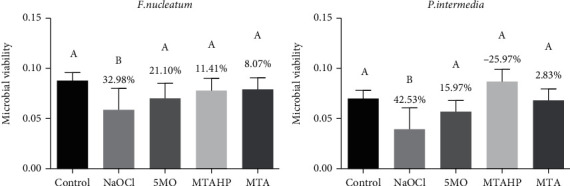
Microbial viability calculated by reflectance and viability reduction (in %) of F. nucleatum and P. intermedia biofilms by crystal violet assay after treatment with the groups. Different uppercase letters indicate statistical differences.
3.2. Genotoxicity Analysis
There was a significant difference between 5MO and MTA groups in comparison with NaOCl and MTA HP over osteoblasts and macrophages after 24 hours. 5MO and MTA groups were similar to the control group (calcium hydroxide) without a significant statistical difference among them (Figure 3).
Figure 3.
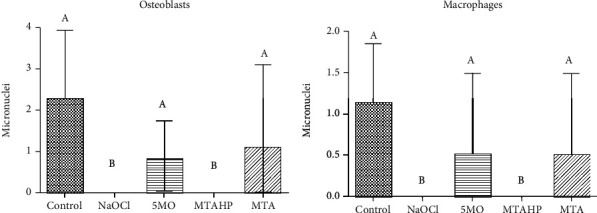
Micronuclei calculated by reflectance of macrophage (RAW 264.7) and osteoblast (Mg-63) cultures after treatment with the groups. Different uppercase letters indicate statistical differences.
3.3. Morphological Analysis by Scanning Electron Microscopy
SEM images showed small irregular particles interspersed with some elongated needle-like particles and small irregular particles with some larger particles as well as elongated particles (50 k and ×100 k) (Figure 4).
Figure 4.
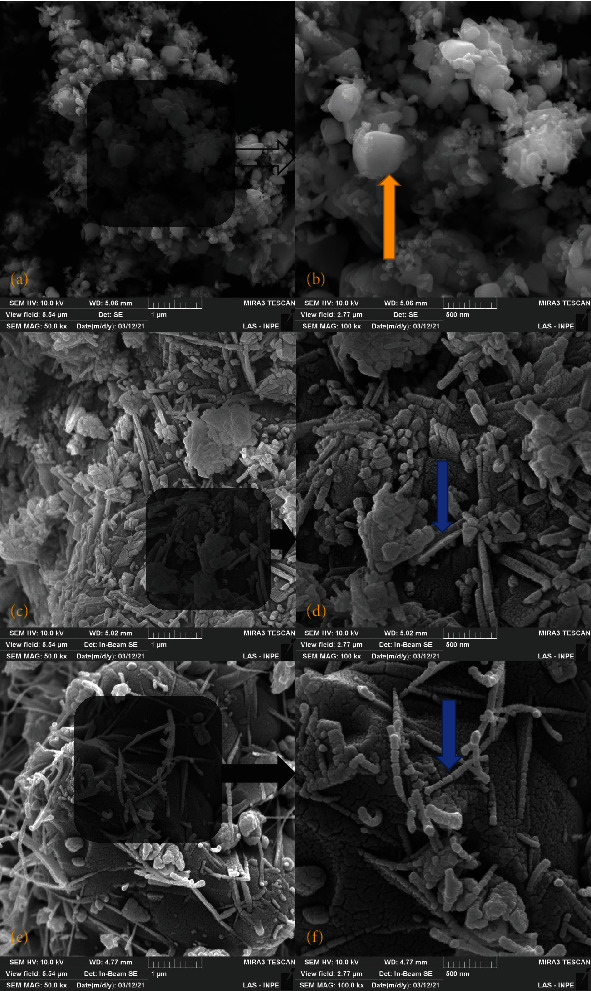
Illustrative images of (a) 5MO powder in ×50 k, (b) 5MO powder in ×100 k, (c) MTA HP powder in ×50 k, (d) MTA HP powder in ×100 k, (e) MTA powder in ×50 k, and (f) MTA powder in ×100 k by scanning electron microscopy. Blue arrow indicates needle-like particles, and golden arrow indicated irregular particles.
4. Discussion
This study was elaborated to evaluate the antimicrobial action, genotoxicity, and the morphological characteristic of three different CSCs including 5MO, MTA HP, and MTA. It was found that these CSCs are effective over some anaerobic bacteria; thus, the null hypothesis was rejected.
In the present study, using crystal violet assay, it was found that MTA and MTA HP were not effective over P. ginigivalis; however, 5MO was effective and has a significant difference when compared to the saline solution group. In the literature, the antimicrobial action of MTA over P. ginigivalis was tested in different studies, and it was not effective after 24, 48, and 72 hours using the inhibition zone test [12, 13].
Different types of MTA were tested over F. nucleatum, P. gingivalis, and P. intermedia in different studies, and it was concluded that MTA (ProRoot) was not effective over these microorganisms and presented no inhibition zone after 24, 48, and 72 hours [12, 14, 15]. However, it is effective in preventing F. nucleatum leakage [16, 17]. In the present study, none of the groups were effective over F. nucleatum and P. intermedia in which none of the groups showed a significant difference compared to the saline solution group, except of the NaOCl group.
In a more recent study, it was found that MTA HP was not effective over P. gingivalis [18], and these outcomes agree with the reported results in the present study, in which MTA HP was not effective over none of the tested anaerobic bacteria except of P. micra. Besides, there is only one study in the literature that evaluated the effect of MTA over P. endodontalis in which the effectivity of MTA was improved with the addition of nitric oxide [19]. According to the same study, the antimicrobial action of the CSC is affected by its setting [19]. In the present study, only 5MO was effective over P. endodontalis.
The endodontic infection is more complex than it was thought. There is an involvement of diverse factors including microorganisms, their byproducts, and growth factors [20]. However, the effective disinfection is not given by the CSCs alone, but by the irrigants, intracanal medications, and the complementary techniques [21–25] highlighting the sealing role of the CSCs [2, 4, 26].
In the literature, it was reported that MTA is not genotoxic when evaluated over mouse lymphoma cells [27], Chinese hamster ovary cells [28], human peripheral lymphocytes [29], murine fibroblasts [30], and human dental pulp stem cells [31] using different concentration of MTA and different evaluation methods, thus, indicating its use as a pulp caping material or a as root canal sealer as in Fillapex MTA [32, 33]. In the present study, MTA was not genotoxic and has a significant difference when compared to the NaOCl group over mouse macrophage (RAW 264.7) and osteoblast (Mg-63) cultures.
To the best of our knowledge, there is no studies in the literature evaluating the genotoxicity of MTA HP and 5MO making the results of the present study pioneers, where 5MO was not genotoxic and has no significant difference when compared to the control group, and MTA HP was genotoxic over mouse macrophage (RAW 264.7) and osteoblast (Mg-63) cultures.
The morphological analysis by SEM showed small irregular particles interspersed with some elongated needle-like particles and small irregular particles with some larger particles as well as elongated particles (50 k and ×100 k) of the tested CSCs in which all these cements favor tissue depositing to from dentin-like tissue bridge [34].
This is an in vitro study; thus, it has limitations to perfectly simulate the clinical conditions. Therefore, clinical studies are indicated to confirm or contradict the outcomes of the present study.
Finally, the CSCs have a finality of promoting tissue repair in interesting areas for apexogensis, apexification, and apicectomy [3–5, 35] or sealing of perforated area [2] and some advantages in vital pulp therapy approaches [36]. Thus, the most expected of these material are not to be genotoxic, and has antimicrobial action, or to not favor the contamination; still, this action may be improved by the use of antimicrobial agents like sodium hypochlorite and others [24, 37, 38] and by complementary technique like photodynamic therapy and passive ultrasonic irrigation [21, 23, 25, 37].
5. Conclusions
5MO has effective antimicrobial action over P. gingivalis, P. endodontalis, and P. micra
MTA and MTA HP have effective antimicrobial action over P. micra
5MO and MTA are not genotoxic over mouse macrophage (RAW 264.7) and osteoblast (Mg-63) cultures
Acknowledgments
This research was supported by a scholarship of the Coordination for the Improvement of Higher Education Personnel (2018/13930-0), FAPESP (2018/26514-4), and PIBIC (2019/03250-4).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The author Maisour Ala Rachi has a patent of five mineral oxides cement. The other authors declare that they have no conflicts of interest.
References
- 1.Ala Rachi M. N., Al-Nahlawi T. F., Kouki M. T. New five minerals oxides pulp capping material compared with Dycal. Dental Materials . 2014;30:p. e126. doi: 10.1016/j.dental.2014.08.261. [DOI] [Google Scholar]
- 2.Al-Nahlawi T., Ala Rachi M., Abu H. A. Endodontic perforation closure by five mineral oxides silicate-based cement with/without collagen sponge matrix. International Journal of Dentistry . 2021;2021:8. doi: 10.1155/2021/4683689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orozco E. I. F., Hasna A. A., de Santos Junior M. T., et al. Case report: interdisciplinary management of a complex odontoma with a periapical involvement of superior anterior teeth. F1000Res . 2019;8:p. 1531. doi: 10.12688/f1000research.20337.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu Hasna A., Pereira Santos D., Gavlik de Oliveira T. R., Pinto A. B. A., Pucci C. R., Lage-Marques J. L. Apicoectomy of perforated root canal using bioceramic cement and photodynamic therapy. Journal of Dentistry . 2020;2020:1–8. doi: 10.1155/2020/6677588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darweesh F., Shahawy T., Zaghloul N., Almowaqee M. Impact of a new silicate-based capping material on healing potentiality of traumatically exposed healthy dog’s dental pulp. Egyptian Dental Journal . 2020;66(2):981–989. doi: 10.21608/edj.2020.24580.1042. [DOI] [Google Scholar]
- 6.Silva E. J., Carvalho N. K., Zanon M., et al. Push-out bond strength of MTA HP, a new high-plasticity calcium silicate-based cement. Brazilian Oral Research . 2016;30(1) doi: 10.1590/1807-3107BOR-2016.vol30.0084. [DOI] [PubMed] [Google Scholar]
- 7.Dawood A. E., Parashos P., Wong R. H. K., Reynolds E. C., Manton D. J. Calcium silicate-based cements: composition, properties, and clinical applications. Journal of Investigative and Clinical Dentistry . 2017;8(2):p. 8. doi: 10.1111/jicd.12195. [DOI] [PubMed] [Google Scholar]
- 8.Delboni M. G., Gomes B. P. F. A., Francisco P. A., Teixeira F. B., Drake D. Diversity of _Enterococcus faecalis_ genotypes from multiple oral sites associated with endodontic failure using repetitive sequence-based polymerase chain reaction and arbitrarily primed polymerase chain reaction. Journal of Endodontia . 2017;43(3):377–382. doi: 10.1016/j.joen.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 9.Williams D. F. On the mechanisms of biocompatibility. Biomaterials . 2008;29(20):2941–2953. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Olsson B., Sliwkowski A., Langeland K. Subcutaneous implantation for the biological evaluation of endodontic materials. Journal of Endodontia . 1981;7(8):355–369. doi: 10.1016/S0099-2399(81)80057-X. [DOI] [PubMed] [Google Scholar]
- 11.Lessa F. C. R., Aranha A. M. F., Hebling J., de Souza Costa C. A. Cytotoxic effects of white-MTA and MTA-bio cements on odontoblast-like cells (MDPC-23) Brazilian Dental Journal . 2010;21(1):24–31. doi: 10.1590/S0103-64402010000100004. [DOI] [PubMed] [Google Scholar]
- 12.Odabaş M. E., Cinar C., Akça G., Araz I., Ulusu T., Yücel H. Short-term antimicrobial properties of mineral trioxide aggregate with incorporated silver-zeolite. Dental Traumatology . 2011;27(3):189–194. doi: 10.1111/j.1600-9657.2011.00986.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim R. J.-Y., Kim M.-O., Lee K.-S., Lee D.-Y., Shin J.-H. An _in vitro_ evaluation of the antibacterial properties of three mineral trioxide aggregate (MTA) against five oral bacteria. Archives of Oral Biology . 2015;60(10):1497–1502. doi: 10.1016/j.archoralbio.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Shin M., Chen J.-W., Tsai C.-Y., et al. Cytotoxicity and antimicrobial effects of a new fast-set MTA. BioMed Research International . 2017;2017 doi: 10.1155/2017/2071247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yassen G. H., Huang R., Al-Zain A., Yoshida T., Gregory R. L., Platt J. A. Evaluation of selected properties of a new root repair cement containing surface pre-reacted glass ionomer fillers. Clinical Oral Investigations . 2016;20(8):2139–2148. doi: 10.1007/s00784-016-1715-5. [DOI] [PubMed] [Google Scholar]
- 16.Nakata T. T., Bae K. S., Baumgartner J. C. Perforation repair comparing mineral trioxide aggregate and amalgam using an anaerobic bacterial leakage model. Journal of Endodontia . 1998;24(3):184–186. doi: 10.1016/S0099-2399(98)80180-5. [DOI] [PubMed] [Google Scholar]
- 17.Ferris D. M., Baumgartner J. C. Perforation repair comparing two types of mineral trioxide aggregate. Journal of Endodontia . 2004;30(6):422–424. doi: 10.1097/00004770-200406000-00011. [DOI] [PubMed] [Google Scholar]
- 18.ElReash A. A., Hamama H., Eldars W., Lingwei G., Zaen El-Din A. M., Xiaoli X. Antimicrobial activity and pH measurement of calcium silicate cements versus new bioactive resin composite restorative material. BMC Oral Health . 2019;19(1):p. 235. doi: 10.1186/s12903-019-0933-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin J.-H., Ryu J. J., Lee S.-H. Antimicrobial activity and biocompatibility of the mixture of mineral trioxide aggregate and nitric oxide-releasing compound. Journal of Dental Sciences . 2021;16(1):29–36. doi: 10.1016/j.jds.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Almeida Gomes B. P. F., Herrera D. R. Etiologic role of root canal infection in apical periodontitis and its relationship with clinical symptomatology. Brazilian Oral Research . 2018;32(1) doi: 10.1590/1807-3107bor-2018.vol32.0069. [DOI] [PubMed] [Google Scholar]
- 21.Abu Hasna A., Monteiro J. B., Abreu R. T., et al. Effect of passive ultrasonic irrigation over organic tissue of simulated internal root resorption. International Journal of Dentistry . 2021;2021 doi: 10.1155/2021/3130813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasna A. A., de Toledo Ungaro D. M., de Melo A. A. P., et al. Nonsurgical endodontic management of dens invaginatus: a report of two cases. F1000Res . 2019;8:p. 2039. doi: 10.12688/f1000research.21188.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abu Hasna A., Ferrari C. H., Talge Carvalho C. A. Endodontic treatment of a large periapical cyst with the aid of antimicrobial photodynamic therapy - case report. Brazilian Dental Science . 2019;22(4):561–568. doi: 10.14295/bds.2019.v22i4.1745. [DOI] [Google Scholar]
- 24.Carvalho C. A. T., Hasna A. A., Carvalho A. S., et al. Clinical study of sodium hypochlorite, polymyxin B and limewater effect on MMP-3,-8,-9 in apical periodontitis. Brazilian Dental Journal . 2020;31(2):116–121. doi: 10.1590/0103-6440202003081. [DOI] [PubMed] [Google Scholar]
- 25.Hasna A. A., Khoury R. D., Toia C. C., Talge Carvalho C. A., Valera M. C. In vitro evaluation of the antimicrobial effect of N-acetylcysteine and photodynamic therapy on root canals infected with enterococcus faecalis. Iranian Endodontic Journal . 2020;15:236–245. doi: 10.22037/iej.v15i4.26865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lolayekar N., Bhat S. S., Hegde S. Sealing ability of ProRoot MTA and MTA-Angelus simulating a one-step apical barrier technique--an in vitro study. The Journal of Clinical Pediatric Dentistry . 2009;33:305–310. doi: 10.17796/jcpd.33.4.gp472416163h7818. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro D. A., Duarte M. A. H., Matsumoto M. A., Marques M. E. A., Salvadori D. M. F. Biocompatibility in vitro tests of mineral trioxide aggregate and regular and white Portland cements. Journal of Endodontia . 2005;31(8):605–607. doi: 10.1097/01.don.0000153842.06657.e2. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro D. A., Matsumoto M. A., Duarte M. A. H., Marques M. E. A., Salvadori D. M. F. Ex vivo biocompatibility tests of regular and white forms of mineral trioxide aggregate. International Endodontic Journal . 2006;39(1):26–30. doi: 10.1111/j.1365-2591.2005.01043.x. [DOI] [PubMed] [Google Scholar]
- 29.Braz M. G., Camargo E. A., Salvadori D. M. F., Marques M. E. A., Ribeiro D. A. Evaluation of genetic damage in human peripheral lymphocytes exposed to mineral trioxide aggregate and Portland cements. Journal of Oral Rehabilitation . 2006;33(3):234–239. doi: 10.1111/j.1365-2842.2005.01559.x. [DOI] [PubMed] [Google Scholar]
- 30.Zeferino E. G., Bueno C. E. S., Oyama L. M., Ribeiro D. A. Ex vivo assessment of genotoxicity and cytotoxicity in murine fibroblasts exposed to white MTA or white Portland cement with 15% bismuth oxide. International Endodontic Journal . 2010;43(10):843–848. doi: 10.1111/j.1365-2591.2010.01747.x. [DOI] [PubMed] [Google Scholar]
- 31.Victoria-Escandell A., Ibañez-Cabellos J. S., de Cutanda S. B.-S., et al. Cellular responses in human dental pulp stem cells treated with three endodontic materials. Stem Cells International . 2017;2017 doi: 10.1155/2017/8920356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camargo S. E. A., Camargo C. H. R., Hiller K. A., Rode S. M., Schweikl H., Schmalz G. Cytotoxicity and genotoxicity of pulp capping materials in two cell lines. International Endodontic Journal . 2009;42(3):227–237. doi: 10.1111/j.1365-2591.2008.01506.x. [DOI] [PubMed] [Google Scholar]
- 33.Bin C. V., Valera M. C., Camargo S. E. A., et al. Cytotoxicity and genotoxicity of root canal sealers based on mineral trioxide aggregate. Journal of Endodontia . 2012;38(4):495–500. doi: 10.1016/j.joen.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Carvalho C. A. T., Hasna A. A., Carvalho A. S., et al. Calcium silicate-based cements affect the cell viability and the release of TGF-β1 from apical papilla cells. Brazilian Dental Journal . 2021;32(6):1–7. doi: 10.1590/0103-6440202104598. [DOI] [PubMed] [Google Scholar]
- 35.Yan M., Wu J., Yu Y., et al. Mineral trioxide aggregate promotes the odonto/osteogenic differentiation and dentinogenesis of stem cells from apical papilla via nuclear factor kappa B signaling pathway. Journal of Endodontia . 2014;40(5):640–647. doi: 10.1016/j.joen.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 36.Santos J. M., Marques J. A., Diogo P., et al. Influence of preoperative pulp inflammation in the outcome of full pulpotomy using a dog model. Journal of Endodontia . 2021;47(9):1417–1426. doi: 10.1016/j.joen.2021.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Abu Hasna A., Pereira Da Silva L., Pelegrini F. C., Ferreira C. L. R., de Oliveira L. D., Carvalho C. A. T. Effect of sodium hypochlorite solution and gel with/without passive ultrasonic irrigation on Enterococcus faecalis, Escherichia coli and their endotoxins. F1000Res . 2020;9:p. 642. doi: 10.12688/f1000research.24721.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.dos Santos Liberato S. F., da Cruz Vegian M. R., Hasna A. A., et al. Antibiofilm action of Persea americana glycolic extract over Acinetobacter baumannii and absence of toxicity in galleria mellonella. Journal of Complementary and Integrative Medicine . 2022 doi: 10.1515/jcim-2021-0051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


