Abstract
Objective:
The ocular vascular system plays an important role in preserving the visual function. Alterations in either anatomy or hemodynamics of the eye may have adverse effects on vision. Thus, an imaging approach that can monitor alterations of ocular blood flow of the deep eye vasculature ranging from capillary-level vessels to large supporting vessels would be advantageous for detection of early stage retinal and optic nerve diseases.
Methods:
We propose a super-resolution ultrasound localization microscopy (ULM) technique that can assess both the microvessel and flow velocity of the deep eye with high resolution. Ultrafast plane wave imaging was acquired using an L22–14v linear array on a high frequency Verasonics Vantage system. A robust microbubble localization and tracking technique was applied to reconstruct ULM images. The experiment was first performed on pre-designed flow phantoms in vitro and then tested on a New Zealand white rabbit eye in vivo calibrated to various intraocular pressures (IOP) – 10 mmHg, 30 mmHg and 50 mmHg.
Results:
We demonstrated that retinal/choroidal vessels, central retinal artery, posterior ciliary artery, and vortex vein were all visible at high resolution. In addition, reduction of vascular density and flow velocity were observed with elevated IOPs.
Conclusion:
These results indicate that super-resolution ULM is able to image the deep ocular tissue while maintaining high resolution that is comparable with optical coherence tomography angiography.
Significance:
Capability to detect subtle changes of blood flow may be clinically important in detecting and monitoring eye diseases such as glaucoma.
Keywords: ultrasound localization microscopy, microbubble, ocular vasculature, hemodynamics
I. Introduction
Glaucoma is the leading cause of global irreversible blindness. With an aging population, it is estimated that by 2040 the number of people with glaucoma will reach 111.8 million worldwide [1]. Despite the critical impact of glaucoma on global blindness, the pathophysiology of glaucomatous optic neuropathy is not fully characterized. Elevated intraocular pressure (IOP) is recognized as the primary risk factor for both the onset and progression of glaucomatous optic neuropathy [2]. However, only one-third to one-half of glaucoma patients have elevated IOP at the time of diagnosis [3]. In addition, visual field loss continues in some patients despite corresponding IOPs at normal levels. Consequently, it is clear that IOP-independent factors contribute significantly to the pathogenesis of glaucoma [4].
Most prominently, vascular risk factors, may contribute to the development of glaucoma as a result of retinal ganglion cell loss due to vascular dysregulation or ischemia-reperfusion injury [5, 6]. In 1959, Harrington et al. [7] first suggested that impaired blood flow may cause increased susceptibility to glaucomatous optic neuropathy even in the setting of statistically normal IOPs. Subsequently, Haas et al. [8] demonstrated reduced resistance of the optic nerve head (ONH) to IOP in the setting of vascular insufficiency. In addition, the increased frequency of disc hemorrhages in both primary open-angle glaucoma (POAG) and normal tension glaucoma (NTG) as well as a more recent study demonstrating their arterial origin, appear to be strong indicators pointing toward a vascular component [9]. Overall, evidence suggests that both systemic vascular dysfunction and ocular perfusion deficits of the retina/choroid, ONH, and the retrobulbar vessels in the posterior segment of the eye are associated with localized tissue damage in the pathogenesis of glaucoma [10–12]. Thus, there is a clinical need for imaging modalities to assess the deep ocular vasculature and monitor its early changes with high resolution.
Currently, optical coherence tomography angiography (OCT-A) is the most widely accepted non-invasive approach to access the microvasculature of the retina and choroid [13]. OCT-A has excellent resolution for visualization of the capillary network of the retina under normal circumstances. Swept-source OCT has extended its capability to image choroidal vessels underneath the light-absorbing retinal pigment epithelium, however, OCT-A is still confounded by artifacts and eyes with high refractive errors or long axial lengths, as in myopia. Moreover, visualization of deeper regions (directly underneath the optically opaque scleral tissue) including the laminar cribrosa (LC) and retrobulbar vasculature where vascular dysfunction is most likely to occur, is still limited by OCT-A.
Because the retrobulbar circulation is directly accessible to ultrasound imaging for evaluation of ONH perfusion, color Doppler imaging (CDI) is a potential tool for the evaluation of vascular flow abnormalities related to glaucoma. As a result, some research studies [12] have investigated CDI as an effective method for detection of flow velocity alterations in moderate and advanced glaucoma patients compared to healthy controls. Despite the ability of CDI to obtain quantitative measurements of vascular flow and enhance diagnostic discrimination of glaucomatous pathologies, a key limitation of CDI is its insufficient sensitivity and resolution to resolve small blood vessel signals, especially when small changes occur in the early stage of eye diseases. Furthermore, the acoustic intensity of CDI generally exceeds the U.S Food and Drug Administration (FDA) ophthalmic exposure guidelines, thus further limiting its use (FDA 510k standards for ophthalmic exposure are more stringent than for any other clinical specialty [14]).
Super-resolution ultrasound microvessel imaging, the acoustic analogue to optical sub-diffraction imaging techniques such as fluorescence photo-activated localization microscopy and stochastic optical reconstruction microscopy, has been developed to overcome the same fundamental physical limitations. The basic principle of super-resolution ultrasound microvessel imaging, also known as ultrasound localization microscopy (ULM) relies on the localization and tracking of spatially isolated ultrasound contrast agents – microbubbles (MBs) in the vasculature [15, 16]. Since the average diameter of MBs is considerably smaller than the ultrasound wavelength, the localization of these MBs is able to break the diffraction limit of conventional ultrasound imaging, resulting in an approximately tenfold improvement in resolution while maintaining ultrasound penetration depth. Thus, by providing optical-level imaging resolution at acoustic-level imaging penetration, ULM shows a promising clinical utility for the evaluation of optic nerve and retinal disease.
Ultrafast plane wave imaging and its coherent compounding beamforming have gained traction due to the ability to capture high-quality images without degradation of high frame rate capabilities [17–20]. Compared with conventional ultrasound line-by-line scanning, ultrafast plane wave imaging enables imaging of subjects at thousands of frames per second (fps), permitting the evaluation of a wide range of flow velocities from slow blood flow in small vessels to fast blood flow in major arteries and veins [21]. In addition, the ultrafast frame rate offers more options in terms of advanced signal/imaging processing algorithms. For example, spatiotemporal singular value decomposition (SVD) clutter filtering of ultrafast ultrasound data greatly increases Doppler and functional ultrasound sensitivity to flow in fine vessels [22].
Using ultrafast ultrasound imaging and MB contrast agent, Errico et al. [23] first reconstructed rat brain vasculature with super-resolution. However, long data acquisition time is still one drawback which makes this technology susceptible to motion artifacts. To alleviate this issue, different strategies such as sparsity based image reconstruction [24] and deconvolution based localization [25], have been proposed to achieve super-resolution imaging in a shorter acquisition time using a higher MB concentration while sacrificing the spatial resolution of the reconstructed microvessel image [26]. In our previous study, we successfully applied a deconvolution based image processing approach to achieve microvessel imaging of the posterior segment of the eye with a compromise between imaging resolution and data acquisition time [27]. However, a shorter data acquisition time (to reduce the temporally averaged intensities) and a higher imaging resolution are both preferred for imaging the small scale ocular tissues, which is not achieved by deconvolution based approach. In addition, flow velocity, an important hemodynamic parameter, was lacking. Moreover, only a normal animal model was evaluated, making it difficult to validate the benefit of super-resolution ULM imaging in the diagnosis of eye diseases.
To fill these gaps, we propose a super-resolution ULM technique based on ultrafast plane wave imaging and advanced processing algorithms such as sub-pixel motion registration, MB signal separation, MB pairing and Kalman filter-based MB tracking, that allows robust blood flow velocity imaging of the posterior segment of the eye in vivo at super-resolution. To mimic the conditions seen in glaucoma, we designed and applied the proposed ULM to visualize and quantify changes of vascular density and flow velocity at various levels of IOP (i.e., 10 mmHg, 30 mmHg and 50 mmHg in this study). In doing so, we anticipate to elucidate early vascular abnormalities that may occur prior to the onset of morphological changes.
II. Materials and Methods
A. System setup
The schematic diagram of our experimental setup for imaging the posterior segment of the eye is shown in Fig. 1. A L22–14v linear array probe (Verasonics Inc, Kirkland, WA, USA) operated by a high frequency Verasonics Vantage system (Verasonics Inc, Kirkland, WA, USA) was used. A ‘parametric’ transmit waveform at 15.625 MHz with a two-cycle pulse for each transmit angle was applied in this study. An angle step size of 2° was implemented in 12 angle compounding plane wave imaging with a post-compounded frame rate of 500 Hz. A total of 4 in-phase/quadrature (IQ) datasets were collected for each IOP, and each IQ dataset contained 2000 frames corresponding to 4 seconds of data acquisition. Thus, a total of 16 seconds of data were used to reconstruct the ULM image for each IOP level.
Fig. 1.
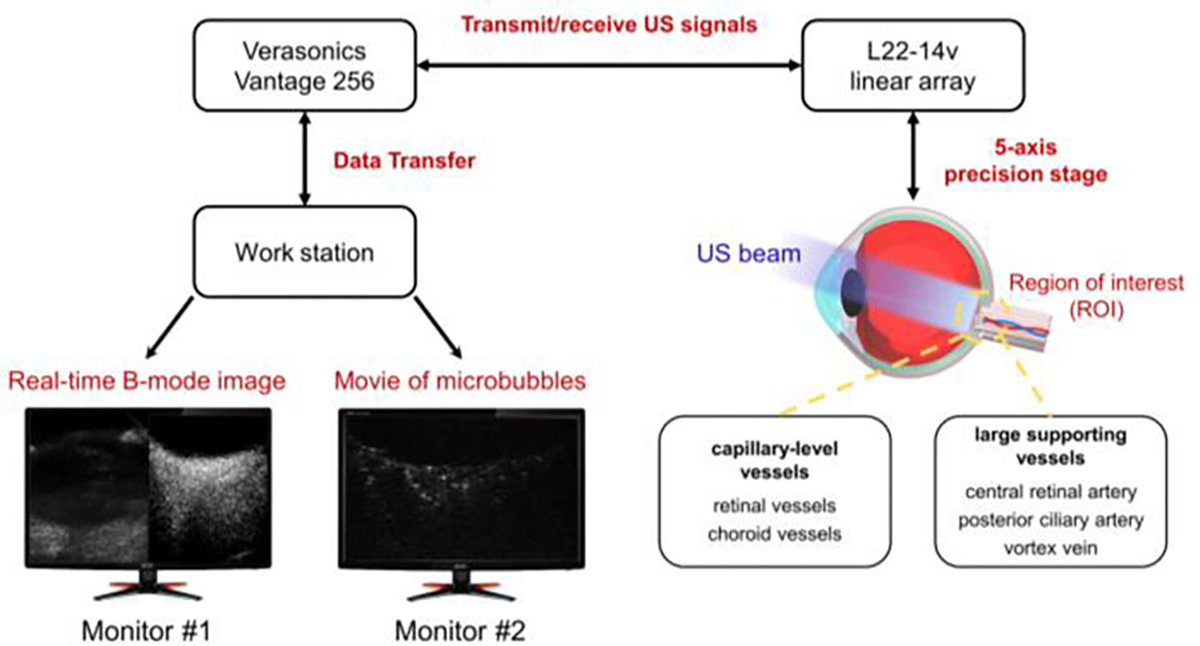
Schematic diagram of the ULM experimental setup. The L22–14v linear array probe with 128 elements was mounted on a manually controlled 5-axis precision stages which was used to find the region of interest. Microbubbles were injected via ear vein access.
Before performing the experiment, we adjusted the transmit voltage from 5 V to 15 V (one-sided voltage) for the purpose of selecting the most suitable voltage to achieve a good MB visualization. Although the U.S FDA guidelines of acoustic intensity only apply to clinical use, we still tried to minimize the acoustic intensity in our study which could be a potential reference for future translational study. Therefore, the acoustic pressure output of the array transducer at the each transmit voltage was measured using a needle hydrophone (HGL-0085, ONDA Co, Sunnyvale, CA, USA) under the plane wave mode.
B. Flow phantom experiment
Before performing the in vivo animal study, we first characterized the performance of our proposed ULM algorithms on a custom-made flow channel phantom via a two-part study. According to the manufacturer’s specifications, the laboratory tube (Dow Silicones Corporation, Midland, MI, USA) used in this study has a 300 μm inner diameter and 640 μm outer diameter. A syringe pump (Model NE-1000, New Era Pump Systems Inc., Farmingdale, NY, USA) was connected to the flow phantom and controlled the flow speeds. Lumason MB suspension (Bracco Diagnostics Inc., Monroe Township, NJ, USA) was diluted with saline solution to approximately 1/500 times the original concentration (1.5 to 5.6 × 108 MBs/mL) to provide a solution with adequately isolated MB signals. The details of each part study design were described below.
For the 1st part, the flow channel phantom was placed at the same 2D ultrasound imaging plane while maintaining an oblique angle of 22.5 degrees with respect to the surface of the ultrasound probe. In order to cover flow speeds in both capillary-level vessels and major large vessels (a large range of flow velocity), we investigated mean flow speeds of 2.5 mm/s, 5 mm/s, 10 mm/s, 50 mm/s and 100 mm/s, respectively.
To further explore the potential bias of flow speed reconstruction generated by out of the imaging plane issue (i.e., the ultrasound imaging plane crossing the flow tube somewhat obliquely), in the 2nd part, we rotated the flow channel phantom with steps of 5 degrees, resulting in tilted angles of 5°, 10°, 15° and 20° across the ultrasound imaging plane, respectively. For the purpose of fair comparison among each angle, we set the flow tube at the same mean flow rate of 10 mm/s. It should be pointed out that the tilted angles in part 2 study (that is x-y horizontal plane) were different from the meaning of 22.5 degree angle in part 1 study (that is x-z cross-sectional plane).
C. Rabbit eye imaging in vivo under different IOPs
An in vivo New Zealand white rabbit (~3.5 kg) experiment was performed with adherence to the guidelines by the University of Southern California Institutional Animal Care and Use Committee (IACUC). Prior to, the rabbit was anesthetized using intramuscular injection of ketamine (35 mg/kg) and xylazine (5 mg/kg). Then, two drops of phenylephrine were applied topically to prevent cornea swelling and decrease discomfort, followed by redosing of anesthesia (2.5% sevoflurane) through a facial mask. After imaging, the rabbit was given buprenorphine for potential pain, and was monitored closely until it regained full consciousness.
Before performing the ultrasound scan, a physician created two ports in the pars plana (vitreous compartment) to establish the IOP control system. Specifically, one port was connected with an IOP sensor (Model SPR-524, Millar Inc, TX, USA) via a 2.3 French gauge (Fr) catheter. Real-time IOP measurements were recorded using a custom-built LabView program, which provided feedback on the IOP. Another port was connected to the phosphate-buffered saline (PBS) bag set at various heights to manipulate the IOP via a 27 gauge needle. In this study, a total of three different IOP levels were investigated to observe the influence on ocular vasculature, including 10 mmHg, 30 mmHg and 50 mmHg.
After we applied ultrasound gel to couple the transducer to the surface of the eye, the imaging planes were carefully placed across the retina/choroid, slightly oblique to a horizontal plane superior to the optic nerve. The real-time conventional color Doppler superimposed on B-mode imaging (FlashDoppler.m in Verasonics script) was first executed to find the target imaging region, as illustrated in Fig. 2. Then, an ultrafast vascular imaging was implemented based on a fast randomized SVD (rSVD) filtering technique [28] to generate a ultrafast power Doppler image with improved sensitivity to small vessels compared with CDI. The purpose of this step was to verify and select the optimal imaging plane where all the retina/choroid, ONH and retrobulbar vasculature are presented within a short time. Next, a bolus of 0.1 mL MBs at the original concentration was intravenously administrated through an ear vein via a 27 gauge syringe, followed by a normal PBS chaser. Right after the injection of MBs, we continued to perform a quick view of the filtered MB sequence/video display based on the rSVD filtering technique every 5 seconds until observing the moving MBs. Finally, 16 seconds of ultrafast plane wave data acquisition was collected and saved for offline post-processing. For each new level of IOP, a fresh bolus of MBs and a normal PBS chaser were used.
Fig. 2.
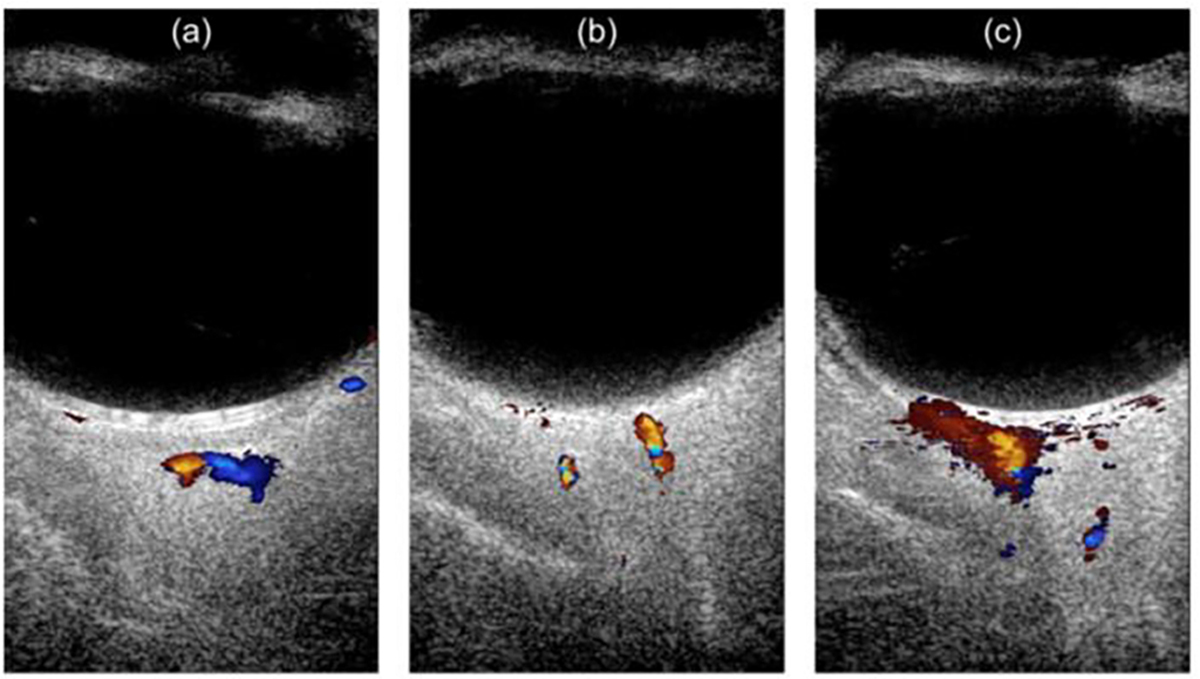
An example of the real-time conventional CDI superimposed on B-mode image on rabbit eye in vivo. (a) conventional CDI at one orientation, (b) conventional CDI at the other orientation, (c) contrast enhanced CDI image using MBs.
D. Post-processing algorithm
MATLAB 2019a software (The MathWorks, Natick, MA, USA) was used in this study to perform the data analysis. For all collected data, the beamformed IQ data were used to obtain a raw B-mode image of each subject. In order to reduce the computational load for the following process, only the selected region of interest (ROI) was fed into the MB localization steps. The details of our implemented ULM processing in one example dataset is illustrated in Fig. 3.
Fig. 3.
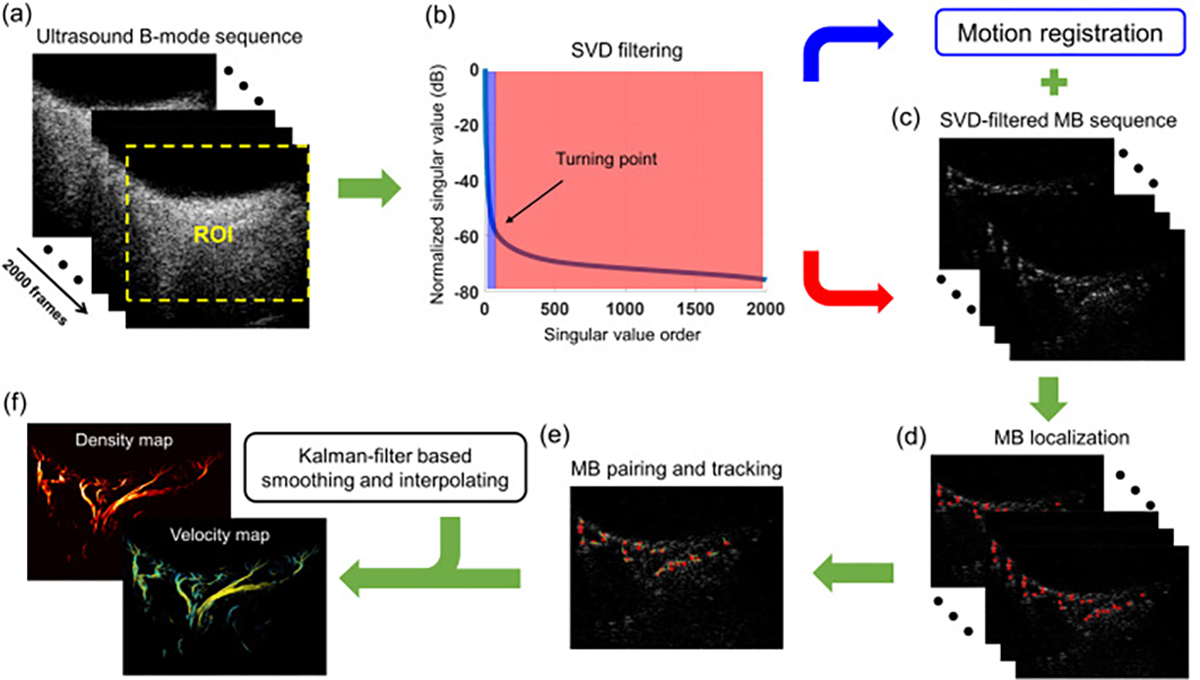
The post-processing steps for super-resolution ULM image. (a) The raw B-mode sequence, (b) SVD filtering for separation of MB signals and background tissue clutters, (c) motion corrected MB sequence, (d) localization of each individual MBs, (e) pairing and tracking of the localized MBs to generate MB moving trajectories, (f) the final reconstructed microvessel image and flow velocity image after applying the kalman-filter based smoothing and interpolating strategy.
A spatiotemporal SVD based clutter filter [22] was used to extract moving MB signals from a stack of ultrasound IQ frames, as indicated in Fig. 3(a). Specifically, each IQ dataset (2000 frames) was reshaped into a 2D Casorati matrix, followed by an SVD calculation. The singular values were sorted in descending order. The tissue signal is mainly represented by the low-order singular values, while the moving MB signals correspond to medium-to-high order singular values. Thus, a threshold cutoff value, identified by finding the turning point of the singular value curve (Fig. 3b) based on the singular value decay rate, was applied to remove low-order singular values that corresponding to tissue signal [29, 30]. The MB signals were then reconstructed by an inverse SVD calculation (Fig. 3c).
The tissue signals were also reconstructed similarly by preserving low-order singular values and rejecting medium-to-high order singular values, which were used for tissue motion estimation. A 2-D phase correlation-based sub-pixel motion estimation algorithm [31] was utilized to measure tissue motion based on the tissue IQ data, which were applied to correct tissue motion in the MB signals (Fig. 3c). Fig. 4 shows the comparison of super-resolution microvessel images with and without motion correction. Contrast-enhanced ultrafast power Doppler images, namely ultrasensitive Doppler images, can be generated by accumulating the power of these MB signals along the temporal dimension (slow-time dimension).
Fig. 4.
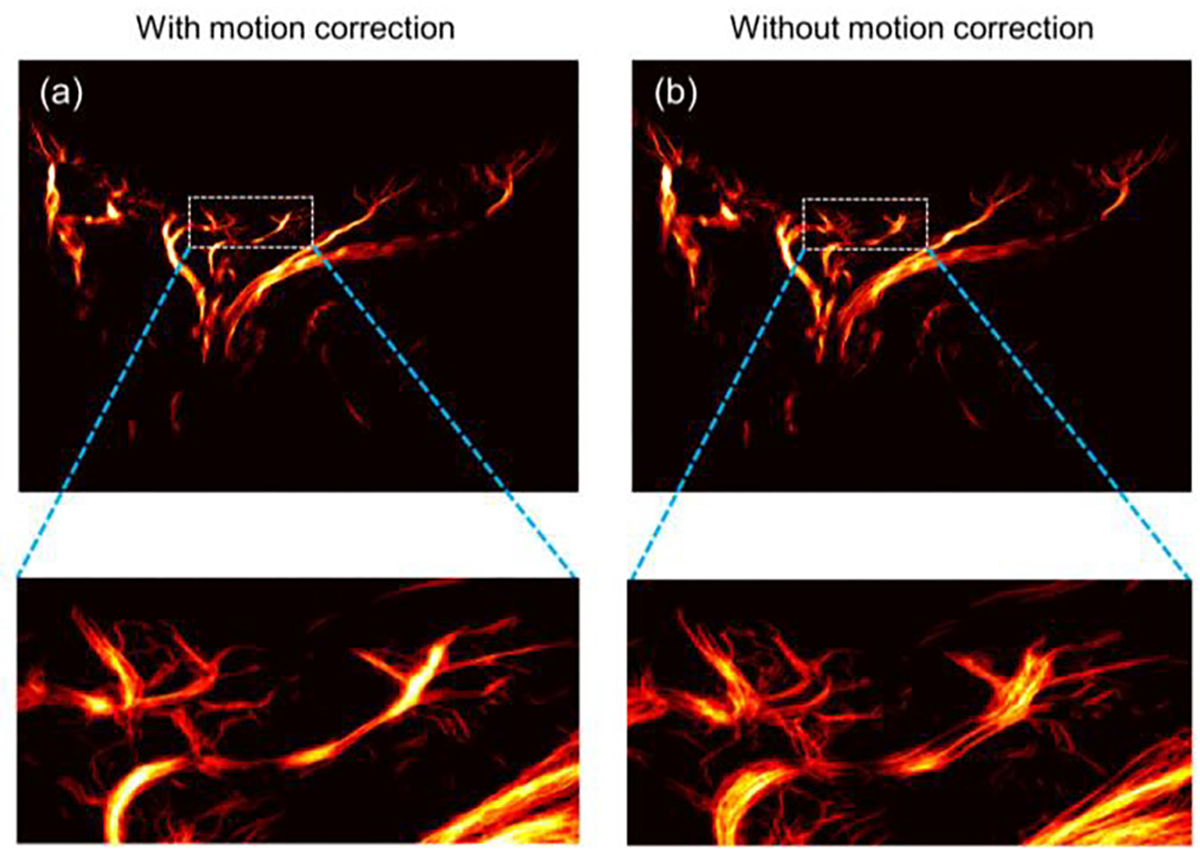
The super-resolution ULM microvessel images (a) with and (b) without motion correction. The bottom images were zoom-in views of the box regions in upper images.
Before MB localization, a noise profile was first derived and applied to equalize the MB signal intensity throughout the field-of-view [32]. Then, the equalized images were spatially interpolated to an axial-lateral pixel resolution of 9.9 μm using a 2-D spline interpolation (which corresponds to roughly lambda/10), followed by an empirical intensity thresholding to remove the noisy background. Next, a 2-D normalized cross-correlation algorithm was performed between the interpolated MB frames and a predefined point-spread function (PSF, that is, 276 μm in axial direction and 365 μm in lateral direction), which was generated from a multivariate Gaussian function with the full-width-half-maximum (FWHM) empirically determined from isolated MBs in the imaging plane. A thresholding was applied to the resulting cross-correlation map to remove pixels with a correlation coefficient < 0.6, and then the MB centroids were localized as the regional peaks of the thresholded cross-correlation map (Fig. 3d). Afterward, the localized MBs were paired frame by frame using a bipartite graph-based pairing algorithm to track the movement of individual MBs over frames (Fig. 3e) [33]. To improve the robustness of tracking, only MBs successfully tracked over ten frames were preserved for microvessel image generation and MB moving speed estimation. Finally, a Kalman-filter-based algorithm was used to further remove noisy MB traces, smooth and interpolate each MB trajectory for better microvessel delineation [34]. All the remaining MB trajectories were accumulated to generate the microvessel image, and the flow speeds of all the MB trajectories were averaged to create the flow velocity image, as indicated in Fig. 3(f).
Since a short ultrasound exposure time is preferred in imaging ocular tissues, the original MB concentration without dilution was applied in this study. Thus, a previously proposed MB signal separation method [35] was utilized here to optimize the localization and tracking performance at relatively high MB concentration. In other words, the algorithm was first applied to derive several subsets of data from the original MB data (Fig. 3a) based on the MB flow speeds and directions via application of various bandpass filters in the temporal direction. More specifically, four 6th order IIR bandpass filters were used (the lower and higher 3-dB frequencies for the four filters were 35 Hz and 105 Hz, 59 Hz and 129 Hz, 93 Hz and 163 Hz, 137 Hz and 250 Hz, respectively), and the positive and negative Doppler frequency components of each filtered data were further separated to generate MB subsets with different flow directions. As a result, eight subsets of data were extracted from the original data. With MB separation, the MB concentration of each data subset was assumed to be spatially sparser, which facilitated accurate MB localization and tracking, especially for regions with high MB concentrations. The same signal processing steps as described above from Fig. 3 (b–f) were applied to each of the data subsets, and the super-resolution microvessel images from all the subsets were combined to generate a final improved microvessel image.
III. Results
A. Acoustic energy selection
A higher transmit voltage may improve signal-to-noise ratio (SNR) at the risk of MB destruction. Based on the empirical analysis of the filtered IQ dataset collected at a range from 5 V to 15 V in our study, a transmitted voltage of 10 V was selected due to good MB visualization. At this voltage, we measured the peak rarefactional pressure of the transmitted pulses with 2 cycle sinusoids at 15.625 MHz transmit frequency using a hydrophone.
Specifically, the negative peak pressure was 205 kPa, corresponding to the mechanical index of 0.052. As a result, the acoustic exposure on ocular tissue in this study is within the FDA 510k standards for ophthalmic exposure, which limits mechanical index to 0.23. In addition, we calculated the spatial peak pulse average intensity (Isppa) of 2.8 W/cm2 and spatial peak temporal average intensity (Ispta) of 2.15 mW/cm2, respectively. All these results show that plane wave intensities implemented here are well below the FDA limits [14] (FDA 510k limits on Isppa and Ispta are 28.0 W/cm2 and 17.0 mW/cm2, respectively).
B. Flow phantom results
Figure 5 shows the reconstructed super-resolution flow velocity images of the flow phantom under various testing conditions. To be specific, the results of part 1 flow phantom study were shown in Fig. 5 (a–e). The reconstructed averaged flow speeds were 2.7 mm/s, 5.4 mm/s, 10.5 mm/s, 50.5 mm/s and 83 mm/s, which were all less than 10% discrepancy of the pre-defined pumping speeds (expect for 83 mm/s flow rate due to overlapped MBs in very high flow speed), indicating that our ULM algorithms can accurately reconstruct the flow speed. In addition, parabolic flow profiles of different flow rates were observed, which is expected from fully-developed flow through a flow channel.
Fig. 5.
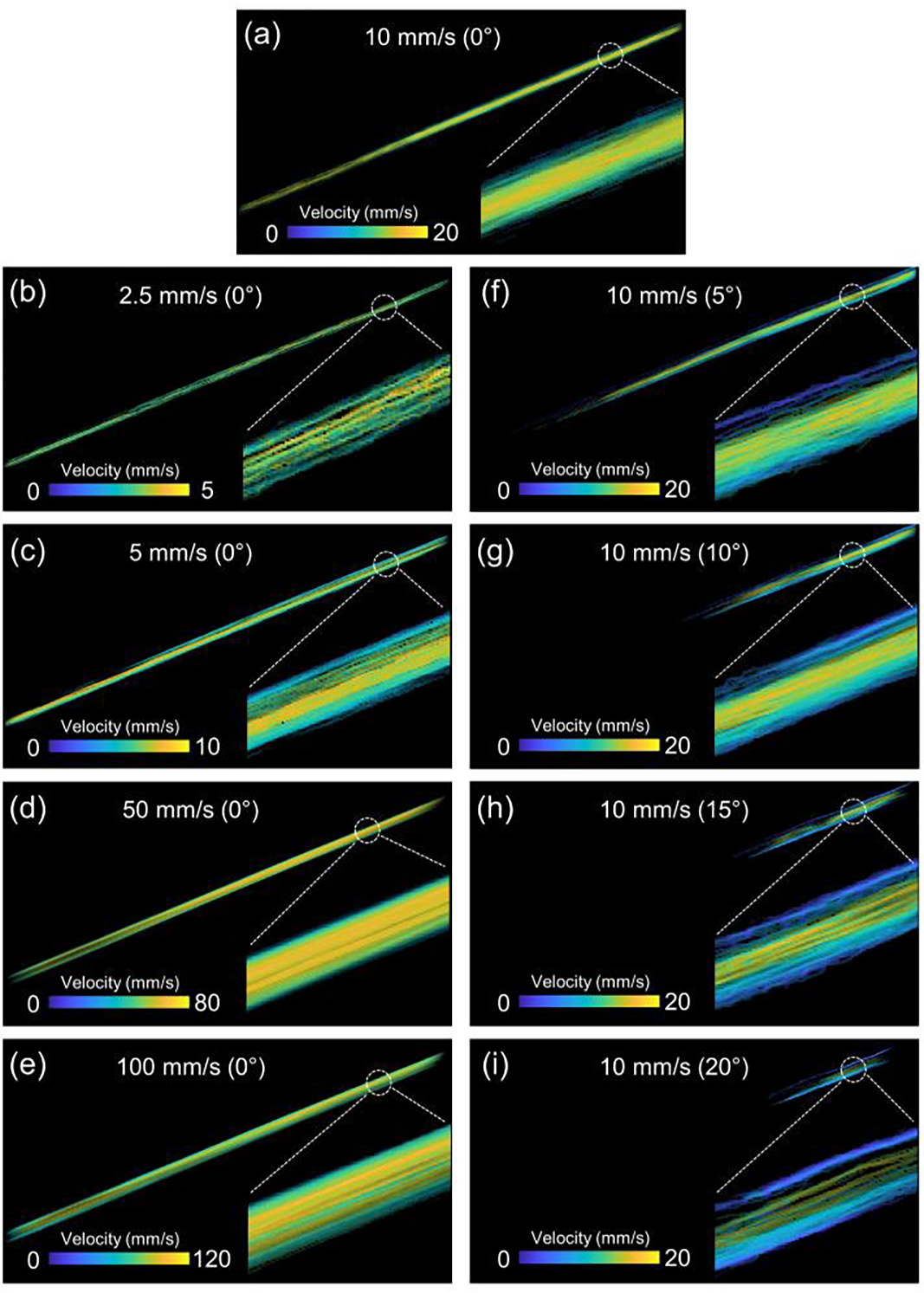
The reconstructed super-resolution flow velocity images of the tube phantom. Various flow profiles at (a) 10 mm/s, (b) 2.5 mm/s, (c) 5 mm/s, (d) 50 mm/s and (e) 100 mm/s where the flow phantom was placed at the same imaging plane. (f-i) the imaging plane crossed the flow channel plane with 5°, 10°, 15° and 20°, respectively. The degrees in the parenthesis represented the angle between the flow channel plane and ultrasound imaging plane. The right-bottom parts of each panel is the zoom-in view of the full scale super-resolution flow velocity images.
To further investigate the potential caused by the out-of-plane artifacts (that is, the flow phantom is not fully within the 2D imaging plane), we performed part 2 flow phantom study. As indicated in Fig. 5(f–i), the calculated averaged flow speed decreased with an increase of the angle between the tube channel plane and imaging plane. In particular, the preset flow speed of the tube channel is a constant 10 mm/s for all angle tests. However, the reconstructed flow speeds decreased from 10.5 mm/s (Fig. 5a) to 9.9 mm/s, 9.7 mm/s, 9.1 mm/s, and 8.4 mm/s with an increase of the angle, respectively.
C. In vivo posterior eye vasculature at normal IOP
Figure 6 shows the gray-scale B-mode images of the posterior segment of the rabbit eye in vivo at different levels of IOP. Because the starting depth of the displayed image is 12 mm away from the surface of the ultrasound probe, the presented B-mode image has an actual imaging depth from 12 mm to 22 mm (which sufficiently captures the posterior segment of the eye).
Fig. 6.
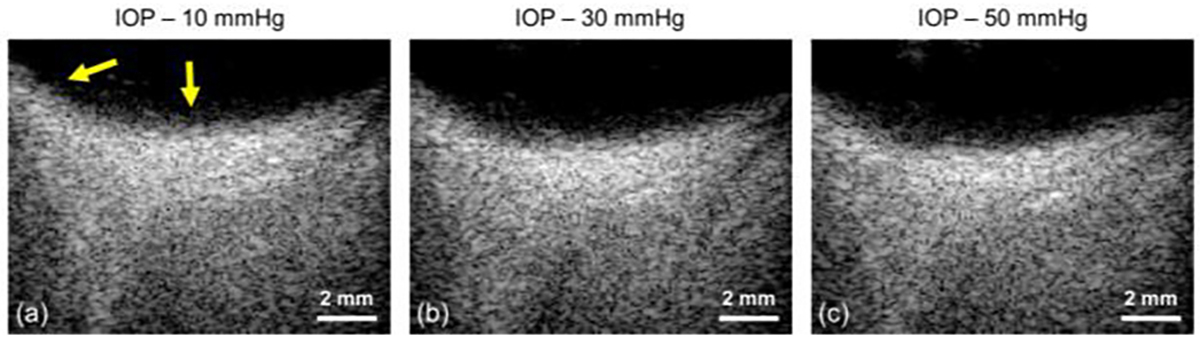
The B-mode images of the posterior segment of the rabbit eye in vivo. Three different IOP levels were investigated, including (a) 10 mmHg, (b) 30 mmHg and (c) 50 mmHg. The starting depth of each image is 12 mm away from the ultrasound transducer surface. The yellow arrow indicated subtle changes of eye positions and curvature with the elevated IOPs.
Figure 7 (a–d) shows the post-processed images of the vasculature data, including the ultrasensitive power Doppler image, super-resolution microvessel image, bi-directionally coded super-resolution microvessel image and super-resolution flow velocity image. Due to the super-resolution characteristics in ULM images, the vascular anatomy of the posterior segment of the eye is clearly depicted, including the posterior ciliary artery (PCA), central retinal artery (CRA) and vortex vein (VV), and the smaller retinal/choroidal branches.
Fig. 7.
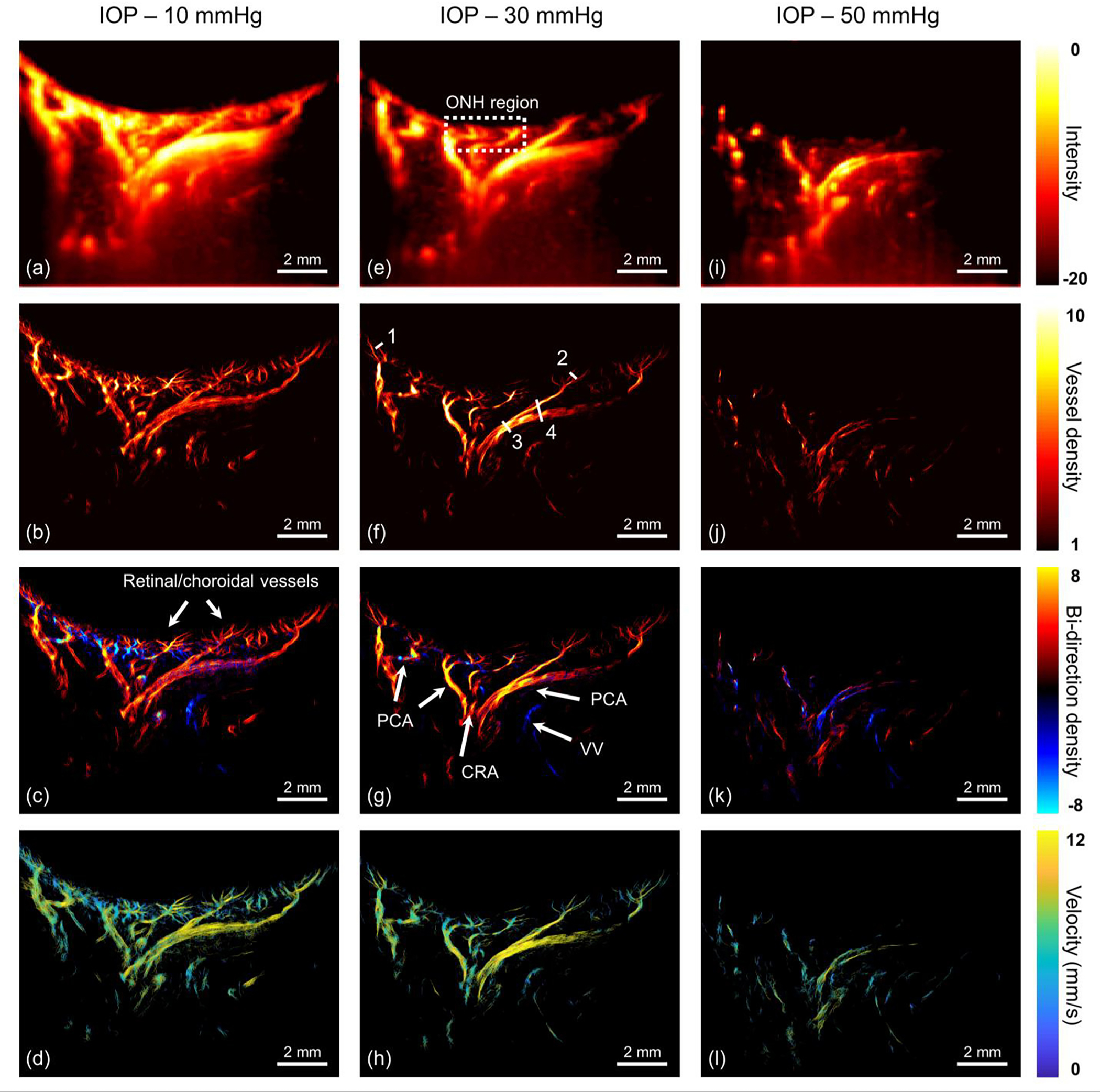
In vivo visualization of the eye vasculature network under different IOPs. (a-d) 10 mmHg, (e-h) 30 mmHg and (i-l) 50 mmHg. From the 1st row to 4th row, the corresponding images are ultrasensitive power Doppler image, super-resolution microvessel image, super-resolution bi-directional microvessel image and super-resolution flow velocity image, respectively. The dash box refers to the optic nerve head region. All solid arrows represent reconstructed vessels ranging from capillary-level vessel to major supporting vessels, including retinal/choroidal vessels, central retinal artery (CRA), posterior ciliary artery (PCA) and vortex veins (VV). Four line markers in (f) were used to calculate the detectable resolution and the resolved distance. Note: dynamic range of (i) is [−12 0] for the better visualization purpose.
Compared with conventional CDI which only captures large vessels, ultrasensitive power Doppler image based on ultrafast plane wave imaging offers vasculature information with greater sensitivity - more vessel branches, such as the CRA and PCA, are visualized. However, its resolution remains insufficient to capture the retinal and choroidal vessels at the capillary-level, and vessel locations and boundaries are difficult to decipher in this mode. In the super-resolution microvessel image, multiple vessel branches were seen in the retina/choroid regions, which is consistent with the normal physiologic anatomy. With regard to the vital ONH region indicated by the white rectangle in Fig. 7(e), a convergence of multiple arteries following the direction of the optic nerve sheath, including the CRA and PCAs. Thus, the vessel distribution in the vicinity of the ONH is relatively abundant. Moreover, some other PCA vessels are distributed along the orbit, in a circular shape.
In order to distinguish the artery or vein of the ocular vascular system, bi-directional microvessel imaging was defined. More specifically, arteries were mapped with a red color indicating flow direction toward the ultrasound probe. In contrast, a blue color was used to represent the veins that go away from the ultrasound probe. However, due to the limitations of the 1D linear array, the arrangement of artery and vein at the ONH cannot be fully visualized as seen in the 2D cross-sectional imaging plane shown in the Fig. 7(c).
It is well established that ocular blood flow is a significant contributing factor in glaucoma [36–38]. Thus, we further reconstructed the super-resolution flow velocity image via the calculation of the moving speed of each MB in the microvessels. As shown in Fig. 7(d), the retinal/choroidal vessels have a relatively small mean flow velocity of 7.1 mm/s. With regard to the major supporting arteries, mean flow velocity of the PCA and CRA were measured to be 8.6 mm/s and 8 mm/s, respectively. In comparison, the average flow velocity of the prominent VV is 7.1 mm/s.
D. In vivo posterior eye vasculature at elevated IOPs
Fig. 7(e–h) and (i–l) shows the ultrasensitive power Doppler image and the reconstructed super-resolution ULM images at IOPs of 30 mmHg and 50 mmHg, respectively. Compared with the vascular anatomy at 10 mmHg, it was observed that the majority of vessel structures remained the same with IOP elevation, but vessel diameters either decreased or dropped out. To fairly compare the quantification of ultrasensitive power Doppler images with our super-resolution microvessel images, we defined the vascular density based on the proportion of the existing vessel area over the entire selected ROI. As shown in Table 1 and Fig. 8(a), in the same selected ROIs, there was an inverse relationship between vascular density and IOP. Super-resolution microvessel images provided better quantification of vascular density than ultrasensitive power Doppler imaging due to its enhanced resolution. For example, ultrasensitive power Doppler image is barely able to distinguish changes in vascular density in ROI_3 and ROI_4. Using ultrasensitive power Doppler image exclusively, the retinal and choroidal vessel branches at the same level of IOP were not distinguishable, making it difficult to observe the subtle variations of vascular density.
TABLE 1.
The vascular density (%) of different selected regions (defined in Fig. 8) in ultrasensitive power Doppler image.
| ROI_1 | ROI_2 | ROI_3 | ROI_4 | |
|---|---|---|---|---|
|
| ||||
| 10 mmHg | 70.4 | 92.1 | 100 | 93.7 |
| 30 mmHg | 28.8 | 79.7 | 100 | 89.5 |
| 50 mmHg | 0.6 | 30.7 | 97.7 | 88.4 |
Note: the vascular density is calculating by thresholding the intensity of background noise in each ultrasensitive power Doppler image.
Fig. 8.
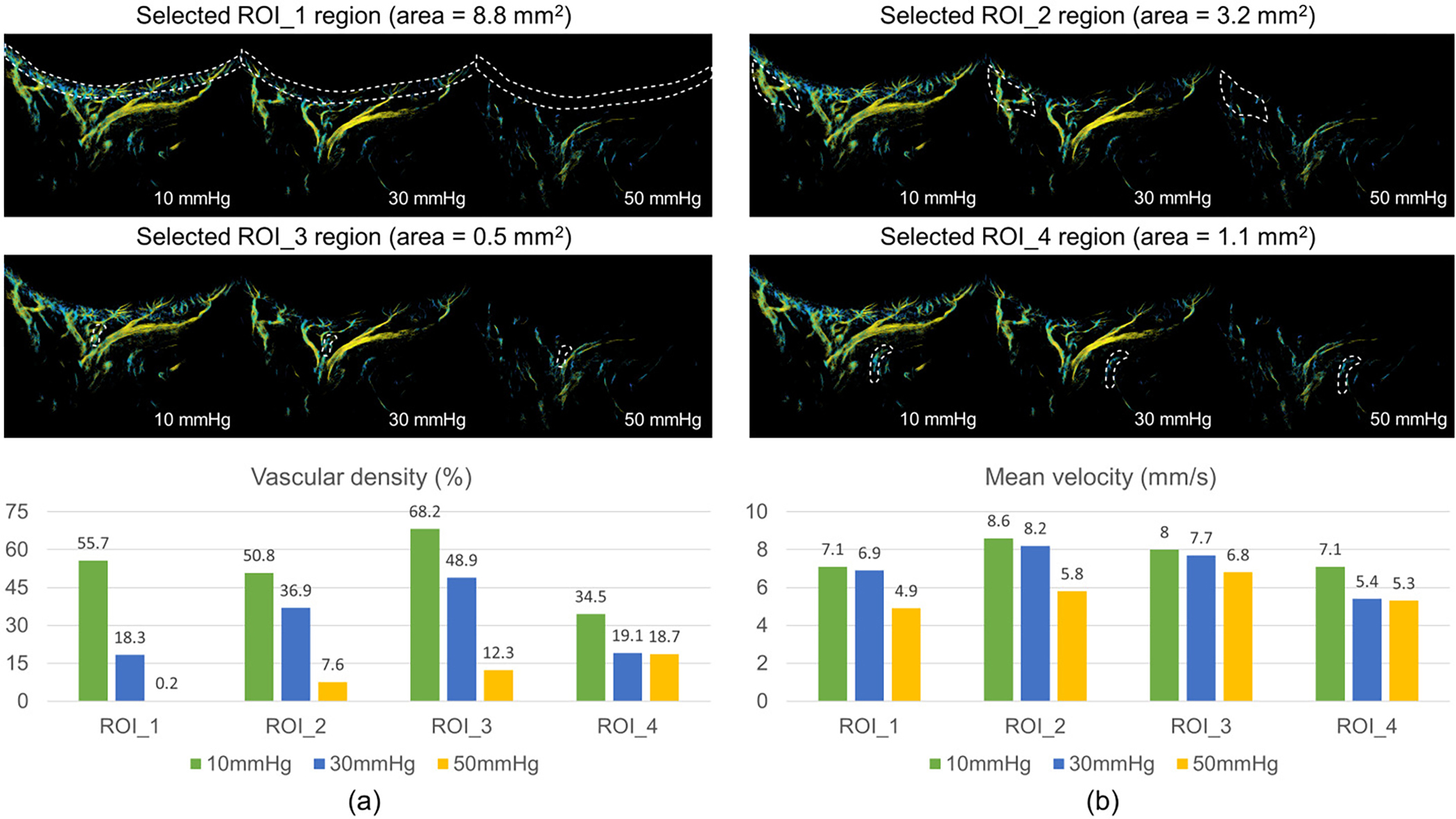
The measured vascular density (a) and mean flow velocity (b) on selected ROIs. A total of four ROIs were investigated in this study. ROI_1 is where retinal/choroidal vessels are dominant; ROI_2, ROI_3 and ROI_4 are large supporting vessels, corresponding to PCA, CRA and VV, respectively. The white dash lines describe the selected regions used to calculate the mean flow velocity and vascular density.
On the basis of super-resolution ultrasound images, the vascular density of retinal/choroidal vessels seen was reduced from 55.7% to 18.3% to 0.2% with increasing IOP in a selected ROI (ROI_1 in Fig. 8). The major supporting retrobulbar vessels, such as the CRA and PCA, also demonstrated a similar tendency toward vascular density reduction. At 50 mmHg, almost all the small vessels were no longer visible in the ULM image. It should be noted that these tiny vessels were still present in the eye, however, the moving MBs inside capillaries could not be detected by the ultrasound system due to reduced vessel diameter.
In addition to vascular density, the mean velocity in the posterior eye vasculature was calculated at elevated IOPs. Fig. 8(b) shows the changes in mean velocity of the retinal/choroidal region, PCA, CRA and VV (corresponding to ROI 1 to 4) at elevated IOPs. In general, the averaged flow velocity was reduced in all regions. The capillary (ROI_1) and the arteries (ROI_2 and ROI_3) experience a larger drop in velocity when IOP rises from 30 mmHg to 50 mmHg as opposed to 10 mmHg to 30 mmHg.
In order to quantitatively demonstrate the capability of our super-resolution ULM approach to resolve the fine vascular network of the posterior eye ranging from dozens of microns to hundreds of microns, we selected four line markers in the reconstructed ULM image of Fig. 7(f) to calculate the detectable resolution and the resolved distance. As displayed in Fig. 9, the vessels with a FWHM ranging from 20 μm to 330 μm can be detected.
Fig. 9.
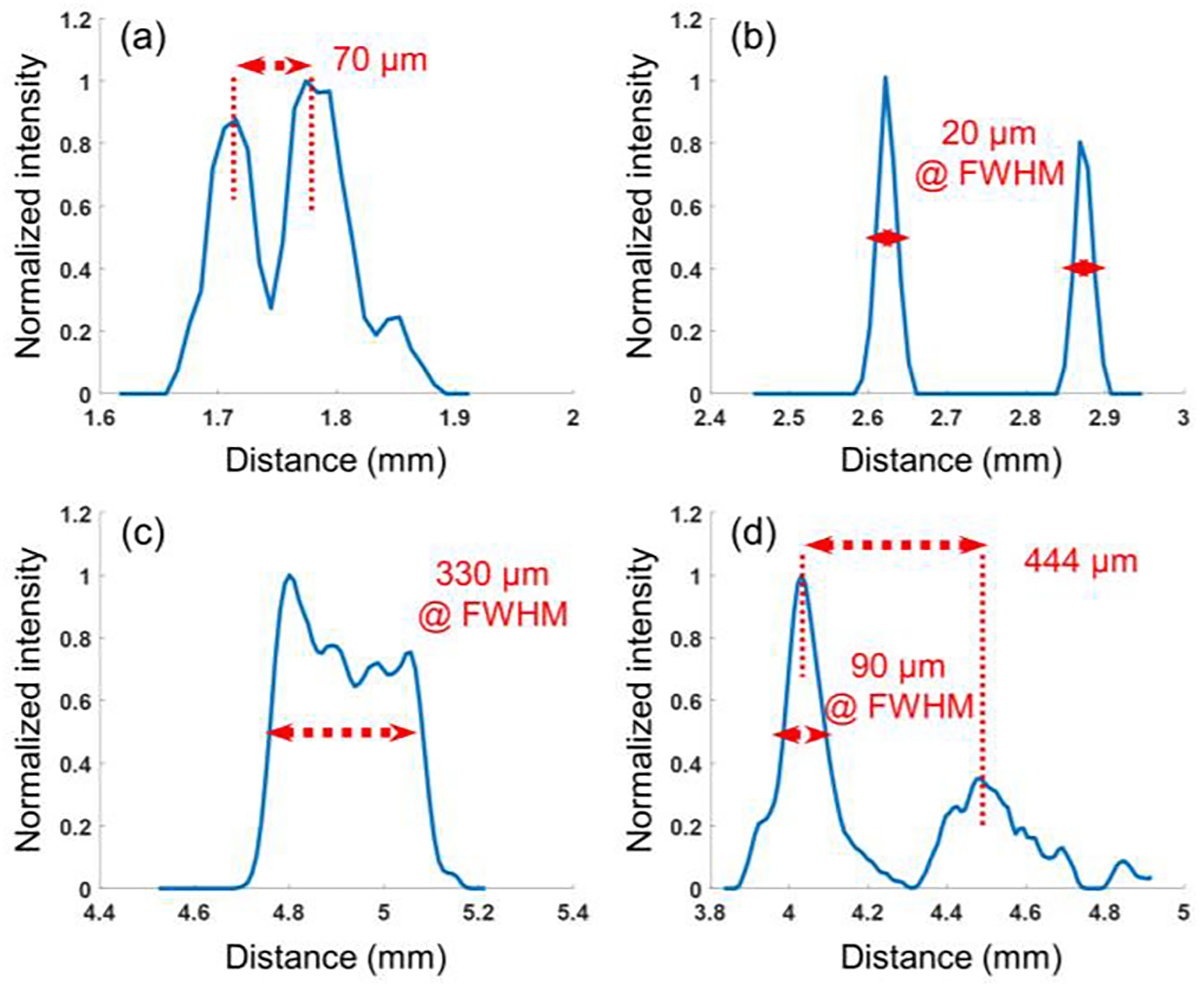
1D cross-section profiles of four line markers indicated in Fig.7 (f).
IV. Discussion
Owing to the metabolic requirements of the retina, the ocular vascular system has a significant impact on visual function. Although OCT-A is the most commonly used technique for investigating the ocular vasculature at high spatial resolution, it is limited by tissue opacities in the sclera, cornea, and crystalline lens (when cataract is present). It is further confounded by eyes with high refractive errors as well. As a result, a suitable technique to image the major vascular regions of the eye, including the deep retinal/choroidal vessels and retrobulbar vasculature beyond the sclera, with sufficiently high resolution is still lacking. Thus, we propose ULM technology for visualizing the deep ocular vasculature with high resolution, which could potentially provide important diagnostic information for retinal and optic nerve disease, especially in early stages prior to the onset of other functional or structural changes.
Acoustic energy exposure is an important consideration when examining the ocular tissue. Based on a previous ophthalmic ultrasound study, it was shown that plane wave Doppler generally has the lowest intensity and mechanical index compared to conventional focused mode and Doppler mode [39]. In general, a higher acoustic energy is able to provide higher SNR but also has a greater risk of MB destruction. Therefore, in this study, acoustic outputs at several excitation voltages were tested. Based on visual inspection of video display of flowing MBs in each spatiotemporal filtered IQ dataset, 10 V was empirically selected. Under such a low transmit voltage, both mechanical index and intensities of this study are within the FDA limitations. These results demonstrate that plane wave imaging with the presence of MBs is suitable for ophthalmic study.
In recent years, many MB localization approaches such as centroid localization [23], curve fitting localization [40], or image deconvolution [27], have been proposed for super-resolved microvessel imaging. In terms of reconstruction accuracy and computational costs, the image deconvolution method has shown superior temporal resolution, while the localization method contributes better spatial resolution. In order to obtain both the high spatial and temporal resolution requirements for ocular tissues, we applied a MB signal separation method [35] based on a clinically standard MB concentration to achieve super-resolution ULM with a relatively short acquisition time. Since eye movement is unavoidable during image acquisition, motion artifacts caused by eye movement or cardiac pulsatile motion should be accounted for when reconstructing the fine scale of ocular vasculature. In this study, a cross-correlation based motion correction algorithm [31] was applied to overcome vessel mismatch. The results in Fig. 4 demonstrate the efficacy of this important motion correction strategy.
Owing to the limitations of current imaging modalities, previous in vivo imaging techniques such as CDI were limited to the assessment of ocular blood flow vessels in low resolution. To demonstrate the advantages of mapping the ophthalmic vasculature using the ULM technique over conventional CDI, we first imaged the posterior segment of the rabbit eye at a normal IOP (10 mmHg) in vivo. As expected, the ULM image provides finer imaging resolution than either CDI or ultrasensitive power Doppler imaging of the eye. As an illustration, upon the request of the oxygen and nutrition of the photoreceptor, the choroid is primarily a vascular structure nourishing the outer one-third of retina through multiple blood flow branches, which is apparently visible in ULM images. Such a super-resolution ULM technique revealed fine retinal/choroidal vessels at a size of 10 to 20 μm, offering a comparable spatial resolution to OCT-A.
High resolution vascular anatomy of the LC and retrobulbar region were observed, despite being blind regions in OCT-A. The ULM imaging results, particularly in the bi-directional microvessel image, also confirmed the orbital vascular anatomy through the depiction of the PCA, CRA and VV. The PCA circulation is the main source of blood supply to the ONH and also supplies the choroid up to the equator, making the PCA the most vital component of the ocular and ONH circulation. Disturbances in the PCA circulation cause a variety of ocular and ONH vascular changes, resulting in varying degrees of visual loss [41]. As shown in our ULM image, the PCA travels along the inferior side of the optic nerve sheath before piercing the posterior sclera near the ONH, and then dividing into many branches to supply the choroid. The CRA which is the sole blood supply to the inner two-thirds of the retina, travels along the interior of the optic nerve until reaching the region of the optic disc. The VV which drain the choroid run posteriorly in the sclera exiting the eye. Considering that the vessel diameter varies significantly along the PCA and CRA, our reconstructed super-resolution ULM successfully imaged vessel diameters ranging from dozens of micron to several hundred microns.
Quantitative parameters such as flow velocity and ocular vascular perfusion were evaluated as well. We demonstrated that the retina/choroid has the lowest flow velocity, which may be important for oxygen and nutrient exchange in the capillaries. The flow velocity of the major supporting arteries, such as the PCA and CRA, was generally faster than capillaries. The mean velocity of the PCA was found to be slightly faster than that of the CRA, which is consistent with previous studies [42, 43]. The mean velocity of veins (i.e., VV in this study) was also slower than the arteries in accordance with past findings [44]. However, in contrast to previous ultrasound studies that examined flow velocity of the PCA, CRA and VV in low resolution, our study effectively visualized the vascular anatomy of the deep posterior eye in vivo with high resolution for the first time. As a result, this method provides the capability to monitor subtle velocity changes in vessels of all sizes, including capillaries and large arteries. These results demonstrate that our super-resolution ULM imaging technique has the ability to provide detailed vascular hemodynamics findings of the entire posterior segment of the eye in fine scale resolution.
Previous literature has demonstrated that glaucoma is associated with decreased ocular perfusion, particularly blood flow in the posterior segment of the eye [38, 45]. In this study, we have investigated changes of vascular density and flow velocity in the posterior eye ranging from capillaries to large supporting arteries located in the LC and retrobulbar regions at elevated IOPs. Our findings were consistent with previous observations – decreased ocular blood flow associated with elevated IOP at which glaucoma is likely to occur. However, our study provided much higher spatial resolution and pixel-level flow velocity mapping. On the basis of the quantification metric of the vascular density, we found that elevated IOP caused the vessel to constrict, resulting in a relatively lower vascular density. There was limited visualization of some constricted vessels at 50 mmHg under super-resolution ULM images. One possible explanation is that the MB signal becomes attenuated inside capillaries due to the constraints in volumetric expansion from vessel walls [46, 47]. Overall, findings suggest that super-resolution ULM imaging may provide additional diagnostic information for ocular diseases associated with subtle microvasculature changes in vascular density and flow velocity.
There are many factors that can influence the accuracy of MB velocity estimation including MB concentration, MB localization error, flow speed, hemodynamic complexity, imaging frame rate / PRF, and the implemented tracking algorithm. In this study, high MB concentration and out-of-plane artifacts of 1D linear array were considered to be the major contributing factors affecting the accuracy of flow velocity estimation. More specifically, we implemented relatively high MB concentration to achieve short data acquisition time for in vivo study. As a result, high MB concentration has the potential to reconstruct images at a relatively low flow speed on large vessels (since the MB tracking algorithm relies on tracing the shortest moving distance within the adjacent imaging frames) compared with previous ocular blood flow measurements of large PCA and CRA vessels (> 100mm/s) in a rabbit model by conventional CDI [48]. In terms of the 2D cross-sectional scanning strategy constrained by the linear array transducer, super-resolution ULM cannot be achieved in the elevational plane and thus the flow velocity measured in this study is a projection from 3D space into the 2D cross-sectional plane, resulting in a lower measured flow speed as demonstrated in Fig. 5.
A shorter data acquisition time is preferred in this study in order to reduce the overall acoustic energy exposure for ocular tissues and alleviate the potential eye motion and high computational cost for post-processing steps. As a result, the velocity reconstruction accuracy on large vessels under high MB concentration remains as a limitation. Finding a good balance between MB concentration (related to data acquisition time) and the accuracy of flow velocity estimation on large vessels is still needed. Another limitation of super-resolution ULM technique is that it only provides the average flow velocity, systolic and diastolic velocity and resistance that typically presented in CDI are not available.
Future directions for our study are threefold. First, more animals and precise IOP studies are needed to demonstrate the reproducibility of the ULM technique and reliability of the vascular assessments. Second, although MBs have been approved by the FDA and are widely used by clinicians to achieve contrast-enhanced ultrasound images of the liver and cardiac structures for many years, the use of MBs is currently not routinely used in ophthalmic ultrasound. Thus, further validation of the safety of MBs in the eye is still needed for the next step toward clinical translation. Lastly, a 2D matrix array, capable of providing volumetric imaging at ultrafast frame rates, would be beneficial to provide more accurate vascular anatomic and hemodynamic information of the eye.
V. Conclusions
In summary, our study demonstrated robust ULM microvessel and flow velocity images of the posterior segment of the eye at super-resolution, especially for deep choroidal and retrobulbar vessels. In addition, we investigated the relationship between elevated IOPs and morphological/functional changes of the ocular vasculature, which may be beneficial for improving clinical diagnosis and monitoring of eye diseases, such as glaucoma. Due to the high resolution and deep penetration depth requirements of diagnostic imaging modalities for clinical purposes, this efficient ultrasound based technology shows promise for future translational study and development as a tool for evaluating previously undetectable vascular changes that occur in eye diseases.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) under grant R01EY026091, R01EY028662, R01EY030126, R01NS111039 and NIH P30EY029220. Unrestricted departmental grant from research to prevent blindness.
Contributor Information
Xuejun Qian, Department of Biomedical Engineering, University of Southern California, Los Angeles, CA 90089, USA, and also with the USC Roski Eye Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, USA..
Chengwu Huang, Department of Radiology, Mayo Clinic College of Medicine and Science, Rochester, MN 55905, USA..
Runze Li, Department of Biomedical Engineering, University of Southern California, Los Angeles, CA 90089, USA, and also with the USC Roski Eye Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, USA..
Brian J. Song, Department of Ophthalmology, University of Southern California, Los Angeles, CA 90033, USA.
Hisham Tchelepi, Department of Radiology, University of Southern California, Los Angeles, CA 90033, USA..
K. Kirk Shung, Department of Biomedical Engineering, University of Southern California, Los Angeles, CA 90089, USA..
Shigao Chen, Department of Radiology, Mayo Clinic College of Medicine and Science, Rochester, MN 55905, USA..
Mark. S. Humayun, Department of Biomedical Engineering, University of Southern California, Los Angeles, CA 90089, USA, and also with the USC Roski Eye Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, USA, and also with USC Ginsburg Institute for Biomedical Therapeutics, University of Southern California, Los Angeles, CA 90033, USA.
Qifa Zhou, Department of Biomedical Engineering, University of Southern California, Los Angeles, CA 90089, USA, and also with the USC Roski Eye Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, USA, and also with USC Ginsburg Institute for Biomedical Therapeutics, University of Southern California, Los Angeles, CA 90033, USA..
VI. Reference
- [1].Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, and Cheng C-Y, “Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis,” Ophthalmology, vol. 121, no. 11, pp. 2081–2090, 2014. [DOI] [PubMed] [Google Scholar]
- [2].Marjanovic I, Milic N, Martinez A, and Benitez-del-Castillo J, “Retrobulbar hemodynamic parameters in open-angle and angle-closure glaucoma patients,” Eye, vol. 26, no. 4, pp. 523–528, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Agarwal R, Gupta SK, Agarwal P, Saxena R, and Agrawal SS, “Current concepts in the pathophysiology of glaucoma,” Indian Journal of Ophthalmology, vol. 57, no. 4, pp. 257, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nakazawa T, “Ocular blood flow and influencing factors for glaucoma,” The Asia-Pacific Journal of Ophthalmology, vol. 5, no. l, pp. 38–44, 2016. [DOI] [PubMed] [Google Scholar]
- [5].Galassi F, Giambene B, and Varriale R, “Systemic vascular dysregulation and retrobulbar hemodynamics in normal-tension glaucoma,” Investigative ophthalmology & visual science, vol. 52, no. 7, pp. 4467–4471, 2011. [DOI] [PubMed] [Google Scholar]
- [6].Yanagi M, Kawasaki R, Wang JJ, Wong TY, Crowston J, and Kiuchi Y, “Vascular risk factors in glaucoma: a review,” Clinical & experimental ophthalmology, vol. 39, no. 3, pp. 252–258, 2011. [DOI] [PubMed] [Google Scholar]
- [7].Harrington DO, “The pathogenesis of the glaucoma field: clinical evidence that circulatory insufficiency in the optic nerve is the primary cause of visual field loss in glaucoma,” American journal of ophthalmology, vol. 47, no. 5, pp. 177–185, 1959. [PubMed] [Google Scholar]
- [8].Haas J, "Low tension glaucoma." pp. 153–160. [PubMed] [Google Scholar]
- [9].Chou JC, Cousins CC, Miller JB, Song BJ, Shen LQ, Kass MA, Wiggs JL, and Pasquale LR, “Fundus densitometry findings suggest optic disc hemorrhages in primary open-angle glaucoma have an arterial origin,” American journal of ophthalmology, vol. 187, pp. 108–116, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Garhöfer G, Fuchsjäger-Mayrl G, Vass C, Pemp B, Hommer A, and Schmetterer L, “Retrobulbar blood flow velocities in open angle glaucoma and their association with mean arterial blood pressure,” Investigative ophthalmology & visual science, vol. 51, no. 12, pp. 6652–6657, 2010. [DOI] [PubMed] [Google Scholar]
- [11].Chan KK, Tang F, Tham CC, Young AL, and Cheung CY, “Retinal vasculature in glaucoma: a review,” BMJ open ophthalmology, vol. 1, no. 1, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jimenez-Aragon F, Garcia-Martin E, Larrosa-Lopez R, Artigas-Martín JM, Serai-Moral P, and Pablo LE, “Role of color Doppler imaging in early diagnosis and prediction of progression in glaucoma,” BioMecl research international, vol. 2013, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].De Carlo TE, Romano A, Waheed NK, and Duker JS, “A review of optical coherence tomography angiography (OCTA),” International journal of retina and vitreous, vol. 1, no. 1, pp. 1 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Food, and D. Administration, “Information for manufacturers seeking marketing clearance of diagnostic ultrasound systems and transducers,” Guidance for Industiy and FDA Staff, 2008. [Google Scholar]
- [15].Viessmann O, Eckersley R, Christensen-Jeffries K, Tang M, and Dunsby C, “Acoustic super-resolution with ultrasound and microbubbles,” Physics in Medicine & Biology, vol. 58, no. 18, pp. 6447, 2013. [DOI] [PubMed] [Google Scholar]
- [16].Christensen-Jeffries K, Browning RJ, Tang M-X, Dunsby C, and Eckersley RJ, “In vivo acoustic super-resolution and super-resolved velocity mapping using microbubbles,” IEEE transactions on medical imaging, vol. 34, no. 2, pp. 433–440, 2015. [DOI] [PubMed] [Google Scholar]
- [17].Macé B, Montaldo G, Cohen I, Baulac M, Fink M, and Tanter M, “Functional ultrasound imaging of the brain,” Nature methods, vol. 8, no. 8, pp. 662, 2011. [DOI] [PubMed] [Google Scholar]
- [18].Couture O, Hingot V, Heiles B, Muleki-Seya P, and Tanter M, “Ultrasound localization microscopy and super-resolution: A state of the art,” IEEE transactions on ultrasonics, ferroelectrics, and frequency control, vol. 65, no. 8, pp. 1304–1320, 2018. [DOI] [PubMed] [Google Scholar]
- [19].Montaldo G, Tanter M, Bercoff J, Benech N, and Fink M, “Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography,” IEEE transactions on ultrasonics, ferroelectrics, and frequency control, vol. 56, no. 3, pp. 489–506, 2009. [DOI] [PubMed] [Google Scholar]
- [20].Peng H, Qian X, Mao L, Jiang L, Sun Y, and Zhou Q, “Ultrafast ultrasound imaging in acoustic microbubble trapping,” Applied Physics Letters, vol. 115, no. 20, pp. 203701, 2019. [Google Scholar]
- [21].Bercoff J, Montaldo G, Loupas T, Savery D, Mézière F, Fink M, and Tanter M, “Ultrafast compound Doppler imaging: Providing full blood flow characterization,” IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control, vol. 58, no. 1, pp. 134–147, 2011. [DOI] [PubMed] [Google Scholar]
- [22].Demené C, Deffieux T, Pernot M, Osmanski B-F, Biran V, Gennisson J-L, Sieu L-A, Bergel A, Franqui S, and Correas J-M, “Spatiotemporal clutter filtering of ultrafast ultrasound data highly increases Doppler and fUltrasound sensitivity,” IEEE transactions on medical imaging, vol. 34, no. 11, pp. 2271–2285, 2015. [DOI] [PubMed] [Google Scholar]
- [23].Errico C, Pierre J, Pezet S, Desailly Y, Lenkei Z, Couture O, and Tanter M, “Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging,” Nature, vol. 527, no. 7579, pp. 499, 2015. [DOI] [PubMed] [Google Scholar]
- [24].Bar-Zion A, Tremblay-Darveau C, Solomon O, Adam D, and Eldar YC, “Fast vascular ultrasound imaging with enhanced spatial resolution and background rejection,” IEEE transactions on medical imaging, vol. 36, no. 1, pp. 169–180, 2016. [DOI] [PubMed] [Google Scholar]
- [25].Yu J, Lavery L, and Kim K, “Super-resolution ultrasound imaging method for microvasculature in vivo with a high temporal accuracy,” Scientific reports, vol. 8, no. 1, pp. 13918, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hingot V, Errico C, Heiles B, Rahal L, Tanter M, and Couture O, “Microvascular flow dictates the compromise between spatial resolution and acquisition time in Ultrasound Localization Microscopy,” Scientific reports, vol. 9, no. 1, pp. 1–10, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Qian X, Kang H, Li R, Lu G, Du Z, Shung KK, Humayun MS, and Zhou Q, “In vivo Visualization of Eye Vasculature using Super-resolution Ultrasound Microvessel Imaging,” IEEE Transactions on Biomedical Engineering, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Song P, Trzasko JD, Manduca A, Qiang B, Kadirvel R, Kallmes DF, and Chen S, “Accelerated singular value-based ultrasound blood flow clutter filtering with randomized singular value decomposition and randomized spatial downsampling,” IEEE transactions on ultrasonics, ferroelectrics, and frequency control, vol. 64, no. 4, pp. 706–716, 2017. [DOI] [PubMed] [Google Scholar]
- [29].Huang C, Song P, Gong P, Trzasko JD, Manduca A, and Chen S, “Debiasing-based noise suppression for ultrafast ultrasound microvessel imaging,” IEEE transactions on ultrasonics, ferroelectrics, and frequency control, vol. 66, no. 8, pp. 1281–1291, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Song P, Manduca A, Trzasko JD, and Chen S, “Ultrasound small vessel imaging with block-wise adaptive local clutter filtering,” IEEE transactions on medical imaging, vol. 36, no. 1, pp. 251–262, 2016. [DOI] [PubMed] [Google Scholar]
- [31].Foroosh H, Zerubia JB, and Berthod M, “Extension of phase correlation to subpixel registration,” IEEE transactions on image processing, vol. 11, no. 3, pp. 188–200, 2002. [DOI] [PubMed] [Google Scholar]
- [32].Song P, Manduca A, Trzasko JD, and Chen S, “Noise equalization for ultrafast plane wave microvessel imaging,” IEEE transactions on ultrasonics, ferroelectrics, and frequency control, vol. 64, no. 11, pp. 1776–1781, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Song P, Trzasko JD, Manduca A, Huang R, Kadirvel R, Kallmes DF, and Chen S, “Improved super-resolution ultrasound microvessel imaging with spatiotemporal nonlocal means filtering and bipartite graph-based microbubble tracking,” IEEE transactions on ultrasonics, ferroelectrics, and frequency control, vol. 65, no. 2, pp. 149–167, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tang S, Song P, Trzasko JD, Lowerison M, Huang C, Gong P, Lok U-W, Manduca A, and Chen S, “Kalman Filter–Based Microbubble Tracking for Robust Super-Resolution Ultrasound Microvessel Imaging,” IEEE transactions on ultrasonics, ferroelectrics, and frequency control, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huang C, Lowerison MR, Trzasko JD, Manduca A, Bresler Y, Tang S, Gong P, Lok U-W, Song P, and Chen S, “Short Acquisition Time Super-Resolution Ultrasound Microvessel Imaging via Microbubble Separation,” Scientific reports, vol. 10, no. 1, pp. 1–13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Flammer J, Orgül S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, Renard J-P, and Stefánsson E, “The impact of ocular blood flow in glaucoma,” Progress in retinal and eye research, vol. 21, no. 4, pp. 359–393, 2002. [DOI] [PubMed] [Google Scholar]
- [37].Mohindroo C, Ichhpujani P, and Kumar S, “Current imaging modalities for assessing ocular blood flow in glaucoma,” Journal of current glaucoma practice, vol. 10, no. 3, pp. 104, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hwang JC, Konduru R, Zhang X, Tan O, Francis BA, Varma R, Sehi M, Greenfield DS, Sadda SR, and Huang D, “Relationship among visual field, blood flow, and neural structure measurements in glaucoma,” Investigative ophthalmology & visual science, vol. 53, no. 6, pp. 3020–3026, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Urs R, Ketterling JA, and Silverman RH, “Ultrafast ultrasound imaging of ocular anatomy and blood flow,” Investigative ophthalmology & visual science, vol. 57, no. 8, pp. 3810–3816, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Desailly Y, Couture O, Fink M, and Tanter M, “Sono-activated ultrasound localization microscopy,” Applied Physics Letters, vol. 103, no. 17, pp. 174107, 2013. [Google Scholar]
- [41].Hayreh SS,“Posterior ciliary artery circulation in health and disease the Weisenfeld lecture,” Investigative ophthalmology & visual science, vol. 45, no. 3, pp. 749–757, 2004. [DOI] [PubMed] [Google Scholar]
- [42].Baxter GM, and Williamson T, “Color Doppler imaging of the eye: normal ranges, reproducibility, and observer variation,” Journal of ultrasound in medicine, vol. 14, no. 2, pp. 91–96, 1995. [DOI] [PubMed] [Google Scholar]
- [43].Karami M, Janghorbani M, Dehghani A, and Riahinejad M, “Orbital Doppler evaluation of blood flow velocities in optic neuritis,” Korean Journal of Ophthalmology, vol. 26, no. 2, pp. 116–122, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Urs R, Ketterling JA, Tezel G, and Silverman RH, “Contrast-enhanced plane-wave ultrasound imaging of the rat eye,” Experimental Eye Research, pp. 107986, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zeitz O, Galambos P, Wagenfeld L, Wiermann A, Wlodarsch P, Praga R, Matthiessen ET, Richard G, and Klemm M, “Glaucoma progression is associated with decreased blood flow velocities in the short posterior ciliary artery,” British Journal of Ophthalmology, vol. 90, no. 10, pp. 1245–1248, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lowerison MR, Huang C, Kim Y, Lucien F, Chen S, and Song P, “In Vivo Confocal Imaging of Fluorescently Labelled Microbubbles: Implications for Ultrasound Localization Microscopy,” IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Caskey CF, Stieger SM, Qin S, Dayton PA, and Ferrara KW, “Direct observations of ultrasound microbubble contrast agent interaction with the microvessel wall,” The Journal of the Acoustical Society of America, vol. 122, no. 2, pp. 1191–1200, 2007. [DOI] [PubMed] [Google Scholar]
- [48].Abdallah W, Fawzi A, Patel H, Dagliyan G, Matsuoka N, Grant E, and Humayun M, “Blood velocity measurement in the posterior segment of the rabbit eye using combined spectral Doppler and power Doppler ultrasound,” Graefe’s Archive for Clinical and Experimental Ophthalmology, vol. 248, no. 1, pp. 93–101, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


