ABSTRACT
Objectives:
Advances in cancer treatment have led to extended survival, and, as a result, the number of patients with bone metastases is increasing. Activities of daily living (ADL) decrease with bone metastasis and the need for rehabilitation is increasing. This study examined the effects of rehabilitation in patients with bone metastases.
Methods:
We retrospectively reviewed data of cancer patients with bone metastasis who received rehabilitation between 2016 and 2018. Efficacy of rehabilitation was evaluated in 92 patients as the change in the Functional Independence Measure (FIM) score divided by rehabilitation days (FIM change/day) and assessed by different metastatic sites.
Results:
Overall FIM scores significantly improved after rehabilitation. Moreover, FIM change/day improved in patients with pelvic metastases (n=44) more than in patients with other metastatic sites (n=48) (P=0.015). In FIM motor components, improvements in toilet, tub/shower, walk/wheelchair, and stairs were significantly greater in patients with pelvic metastasis than in those with other metastasis sites.
Conclusions:
Rehabilitation improved ADL status to a greater extent in patients with pelvic metastases than in those with other metastasis sites. Patients with pelvic metastases may fear fractures, limiting their ADL, but rehabilitation could eliminate this fear and improve FIM.
Keywords: Functional Independence Measure (FIM), Katagiri score, Mirels score, Pelvic metastases, Spine Instability Neoplastic Score (SINS)
INTRODUCTION
Recent advances in cancer diagnosis, surgery, and the development of molecular targeted agents and immune checkpoint inhibitors have improved the survival of patients with advanced-stage cancers.1,2,3) The adoption of cancer boards to coordinate multidisciplinary therapy has extended the survival rate of cancer patients, and the number of patients with bone metastases has increased accordingly.4) To maintain and improve the activities of daily living (ADL) and quality of life (QOL) of patients, it is necessary to diagnose and treat bone metastases before the occurrence of skeletal-related events (SREs), such as fracture and paralysis. Therefore, early intervention is recommended for cancer rehabilitation. When a patient’s general condition is good, ADL status and QOL are high, and such patients are generally active, meaning that there may be a long-term mechanical load on the metastatic bones. Therefore, prognosis for survival is important for risk assessment of SREs. The Katagiri score is a prognostic prediction method,5) whereas the Functional Independence Measure (FIM) is a representative evaluation method of ADL and is used in many countries.6) Rehabilitation for patients with bone metastases increases the risk of SREs, including pathological fractures and paralysis caused by spinal cord compression. As a result, risk assessment for fractures prior to rehabilitation is necessary. The Spine Instability Neoplastic Score (SINS)7) was developed to assess spinal instability. The Mirels score8) was developed to assess the risk of pathological fracture in long bones with metastases and its scoring components include location, type, and size of the lesion and pain. Higher scores in both of these assessments indicate a greater risk of fracture. However, because the shape of the pelvic bone is complex, there are only a few simple methods for evaluating pelvic metastasis. This makes rehabilitation difficult for patients with pelvic metastases, and the benefits of rehabilitation for these patients have not been well evaluated.
In this study, we collected and analyzed data on cancer patients who received rehabilitation at our hospital, with a special focus on cancer patients with bone metastasis. The aim of the study was to analyze the relationship between rehabilitation effects and patient characteristics based on bone metastasis sites and bone fracture risk scores.
METHODS
Study Design and Subjects
This was a retrospective, descriptive study using a review of clinical records. Ethics approval was obtained from the Research Ethics Board of Kindai University to conduct this study (approval number: 2019–089). The requirement for informed consent was waived by the ethics board. The clinical records of all patients with bone metastases who were admitted to our hospital and received rehabilitation at the rehabilitation department between 2016 and 2018 were reviewed from computerized databases. We reviewed consultation notes, progress notes, operative reports, magnetic resonance imaging (MRI) or computed tomography (CT) scans of the spine for vertebral metastases, whole-body bone scans and X-rays for extraspinal metastases, and abdominal and chest imaging for organ metastases.
Our hospital provides rehabilitation for locomotor, respiratory, cardiovascular, cerebrovascular, and cancer disorders. Physical therapy is performed for almost all diseases, while occupational, speech, and hearing therapy are performed as needed. A rehabilitation plan is prepared according to the characteristics of the disease and is changed according to the condition of the patient. FIM assessment is performed on the first and last day of rehabilitation.
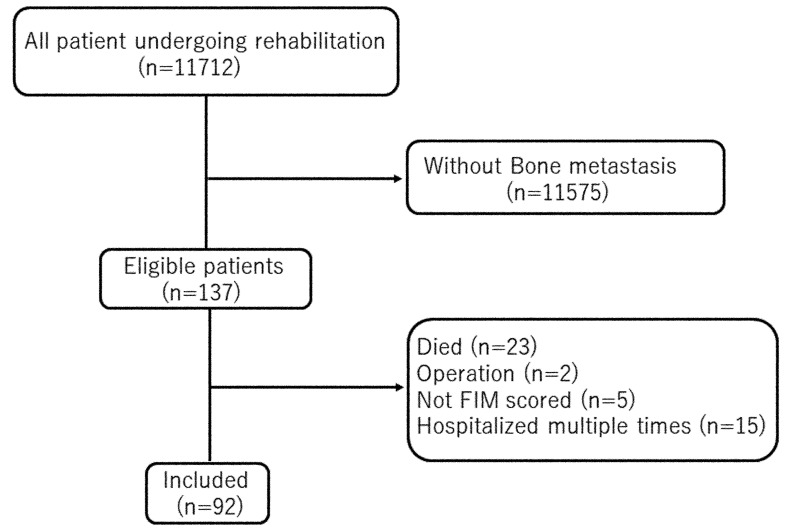
A total of 11,712 patients who received rehabilitation were assessed for study eligibility. Of these, 137 were identified to have bone metastases. Of the remaining 137, patients were excluded from data analyses if their FIM scores were incomplete, if they received surgical therapy, or if they died during the hospital stay. Patients admitted to the hospital multiple times were evaluated only for the first admission after confirmation of bone metastasis. Ninety-two patients were included for evaluation (Fig. 1).
Fig. 1.
Study flowchart of the patient inclusion process.
Data Correction and Measurement
Data were acquired on patient characteristics, including age, gender, length of rehabilitation, bone metastasis, type of primary tumor, and treatment for bone metastases. We obtained FIM scores before and after rehabilitation.6,9) We calculated the SINS and the Mirels score for predicting pathological fracture of the spine and long bones, respectively, as described previously.7,8,10,11) We also calculated the Katagiri score5) as a survival prognosis score.
Evaluation of Instability: SINS, Mirels Score, and Pelvic Metastases in the Acetabulum or Elsewhere
The SINS7) system is based on one clinical factor (pain) and five radiographic parameters (location, bone lesion quality, spinal alignment, vertebral body collapse, and involvement of posterolateral spinal elements). Each component is assigned a score that reflects its contribution to overall instability of the spinal segment. The six individual scores of each component are added for a cumulative score ranging from 0 to 18. A high total score indicates severe instability.10)
The Mirels scoring system8) is based on four characteristics: site of lesion, nature of lesion, size of lesion, and pain. All features were assigned progressive scores ranging from 1 to 3. The three categories for site of the lesion were upper extremity (1), lower extremity (2), and the peritrochanteric area of the femur (3). According to Mirels’ recommendation, prophylactic fixation is highly indicated for a lesion with an overall score of 9 or more. A lesion with an overall score of 7 or less can be managed using radiotherapy and drugs. An overall score of 8 presents a clinical dilemma.11)
For pelvic metastasis, depending on whether acetabular bone metastases were present, we used positron emission tomography/computed tomography (PET/CT), a bone scan, MRI, and CT to investigate the patient.
A Prognosis Prediction Method: Katagiri Score
The Katagiri scoring system5) is based on the six prognostic factors of primary characteristics, visceral metastases, laboratory data, ECOG-PS (Eastern Cooperative Oncology Group Performance Status), previous chemotherapy, and multiple bone metastases. For the primary characteristic category, patients with lung cancer were categorized into two subgroups based on treatment with or without molecular targeted agents, and patients with prostate and breast cancers were categorized according to sensitivity to hormonal therapy. The Katagiri score represents the sum of the scores for each factor and ranges from 0 to 10. The Katagiri scores were categorized into the following three groups: 0–3, high survival rate; 4–6, intermediate survival rate; and 7–10, low survival rate.12)
ADL Evaluation Method: FIM
The FIM score6,9) considers six areas of self-care (eating, grooming, bathing, dressing of upper body, dressing of lower body, and toileting), two of sphincter control (bladder and bowel), three of transfer ability (in a bed, chair, and/or wheelchair; on and off a toilet; and in and out of a tub and/or shower), two of locomotion (walk/wheelchair and stairs), two of communication (comprehension and expression), and three of social cognition (social interaction, problem solving, and memory). The 18 categories are each scored from 1 to 7 for a possible maximum score of 126. Within a category, a score of 1 or 2 indicates complete dependence because the person contributes less than half the energy and requires maximal or complete assistance. A score of 3 to 5 indicates modified dependence, with the person exerting more than half the energy but still needing some assistance. A score of 6 or 7 indicates independence, either with or without use of an assistive device to perform the task, and no helper is needed.13)
Determination of Weight-bearing
Patients with bone metastases first consulted an orthopedic surgeon to determine the level of bed rest required. If necessary, a medical corset was constructed. If the pain was severe, we administered analgesics 30 min before the start of rehabilitation. When there was a metastasis to the acetabulum and pain was experienced during weight-bearing, we stopped further weight-bearing. In cases where a wheelchair was required, the affected limb was allowed to touch the ground during transfer. The patients were switched to bed rehabilitation if the pain persisted after the administration of analgesics.
Data Analysis
Data were analyzed using JMP Version 14.0 (SAS Institute Japan). Descriptive statistics were used for the demographic and clinical data. Continuous variables were described as means (standard deviation, [SD]) for parametric data and medians (interquartile range [IQR]) for nonparametric data. The Shapiro–Wilk test was used to determine if the distribution was normal. The Mann–Whitney U test and Wilcoxon matched-pairs signed-rank test were used for comparisons of nonparametric data. We investigated factors related to changes in each item of the FIM score by performing a univariate analysis along with a Wilcoxon signed-rank test. Spearman’s rank correlation coefficients were used to assess the relationship between length of rehabilitation and FIM score changes. The chi-square test was used for comparisons of the proportions. P <0.05 was considered statistically significant. Because rehabilitation efficacy is related strongly to the duration of rehabilitation,13) FIM efficiency was calculated as the change in FIM score divided by the duration of rehabilitation in days (FIM change/day).
RESULTS
The characteristics of the final study cohort are shown in Table 1. The majority of patients had a bone metastasis lesion at the spine, followed by pelvis and limb metastases. Although most patients received chemotherapy and radiation therapy for treatment of their cancer and bone metastasis, some received only palliative care. As shown in Table 2, the primary tumors were predominantly lung or esophageal cancers, followed by breast cancer, hepatocellular carcinoma, and gastric cancer, with two patients having unknown primary tumors. No fractures occurred during rehabilitation.
Table 1. Baseline characteristics of included patients (n=92).
| Variable | n (%), median [IQR] |
| Patients | |
| Male | 55 (59.8) |
| Female | 37 (40.2) |
| Age, years | 69 [75–61] |
| Duration of rehabilitation, days | 14 [23–8] |
| Bone metastasis | |
| Pelvis | 44 (47.8) |
| Spine | 68 (73.9) |
| Limb | 14 (15.2) |
| Treatment of cancer and bone metastasis | |
| Chemotherapy only | 18 (19.5) |
| Radiation therapy only | 24 (26.1) |
| Chemotherapy and radiation therapy | 41 (44.6) |
| Palliative care only | 9 (9.8) |
| Bone modifying agents | 8 (8.7) |
| SINS | |
| 0–6 | 16 (23.5) |
| 7–12 | 45 (66.1) |
| 13–18 | 7 (10.2) |
| Mirels score | |
| ≤7 | 4 (28.5) |
| 8 | 1 (7.1) |
| ≥9 | 9 (64.2) |
| Pelvic bone metastases in the acetabulum or elsewhere | |
| In the acetabulum | 24 (54.5) |
| Not in the acetabulum | 20 (45.5) |
| Expected prognosis | |
| Katagiri score | |
| 0–3 | 2 (2.1) |
| 4–6 | 42 (45.6) |
| 7–10 | 48 (52.1) |
Data displayed as number (percentage) or median [IQR].
Table 2. Primary lesions of bone metastases (n=92).
| Primary lesion site | n (%) |
| Lung cancer | 30 (32.6) |
| Esophageal cancer | 20 (21.7) |
| Breast cancer | 8 (8.7) |
| Hepatocellular carcinoma | 7 (7.6) |
| Gastric cancer | 4 (4.3) |
| Prostate cancer | 3 (3.3) |
| Pharynx cancer | 2 (2.2) |
| Thyroid cancer | 2 (2.2) |
| Bile duct cancer | 2 (2.2) |
| Renal cell carcinoma | 2 (2.2) |
| Ovarian cancer | 2 (2.2) |
| Uterine cancer | 2 (2.2) |
| Colon cancer | 2 (2.2) |
| Tongue cancer | 1 (1.1) |
| Pancreatic cancer | 1 (1.1) |
| Skin cancer | 1 (1.1) |
| Synovial sarcoma | 1 (1.1) |
| Cancer of unknown primary site | 2 (2.2) |
Appendix 1. . FIM of bone metastasis before rehabilitation.
| FIM before rehabilitation | |||
| Item | With pelvic metaa
n=44 |
Without pelvic metab
n=48 |
P value |
| Self-care | |||
| Eating | 6 [7–5.25] | 6 [7–4] | 0.3 |
| Grooming | 5 [6.75–4] | 4.5 [6–3] | 0.4 |
| Bathing | 3 [4–1] | 2.5 [4–1] | 0.9 |
| Dressing-upper body | 5 [7–3.25] | 4 [5–3] | 0.9 |
| Dressing-lower body | 5 [6–3] | 4 [5–1.25] | 0.1 |
| Toileting | 5 [6–3] | 4 [5.75–1] | 0.1 |
| Sphincter control | |||
| Bladder management | 6 [7–3.25] | 4 [7–1] | 0.9 |
| Bowel management | 6 [7–5] | 4 [7–1] | 0.02 |
| Transfers | |||
| Bed, chair, wheelchair | 5 [5.75–3] | 4 [5–1] | 0.1 |
| Toilet | 5 [5–3] | 4 [5–1] | 0.2 |
| Tub, shower | 2 [5–1] | 1 [4.75–1] | 0.9 |
| Locomotion | |||
| Walk/wheelchair | 1 [5–1] | 1 [5–1] | 0.3 |
| Stairs | 1 [1–1] | 1 [1–1] | 0.7 |
| Motor subtotal score | 55 [67.75–40.5] | 47 [63–25] | 0.1 |
| Communication | |||
| Comprehension | 7 [7–5] | 7 [7–5.25] | 0.9 |
| Expression | 7 [7–5.25] | 7 [7–6] | 0.7 |
| Social cognition | |||
| Social interaction | 7 [7–5.25] | 7 [7–6] | 0.8 |
| Problem solving | 7 [7–5.25] | 7 [7–6] | 0.7 |
| Memory | 7 [7–6] | 7 [7–5.25] | 0.5 |
| Cognitive subtotal score | 35 [35–26.25] | 35 [35–28.25] | 0.9 |
| Total FIM score | 89.5 [102–63.5] | 79.5 [98.75–55.25] | 0.4 |
Data displayed as median [IQR]. P values from Wilcoxon matched-pairs signed-rank test.
a Patients with bone metastases including pelvic metastases.
b Patients with bone metastases except pelvic metastases.
For the SINS, most patients (66.1%) scored 7–12 points, followed by 0–6 points (23.5%) and 13–18 points (10.2%) (Table 1). For the Mirels score, most patients (64.2%) had a score of 9 or above, whereas 28.5% had a score of 7 or below, and 7.1% had a score of 8 (Table 1). Of the pelvic metastases, 54.5% were metastases to the acetabulum and 45.5% were elsewhere.
The distribution of the Katagiri scores is shown in Table 1. There was no significant difference in the distribution between patients with or without pelvic metastasis (P=0.14). Similar results were obtained for spine metastases (P=0.17) and limb metastases (P=0.11) (Chi-squared test).
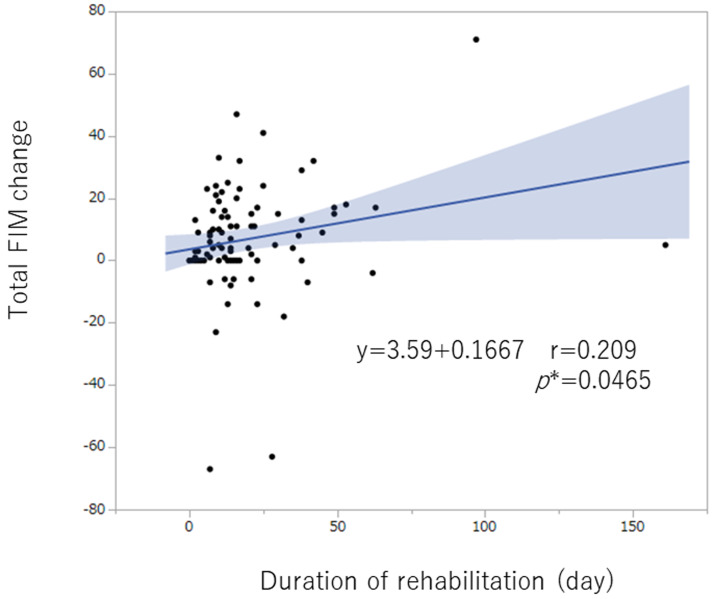
The total FIM score and the motor component of the FIM score improved significantly after rehabilitation, whereas the cognitive component score did not change (Table 3). The duration of rehabilitation (day) correlated significantly with the changes in FIM (Fig. 2). In addition, grooming (r=0.24, P=0.018), dressing-upper body (r=0.41, P<0.0001), dressing-lower body (r=0.23, P=0.028), toileting (r=0.24, P=0.02), and bed/chair/wheelchair transfer (r= 0.22, P=0.03) correlated significantly with the duration of rehabilitation (day). The total FIM scores before rehabilitation (baseline value) were not significantly different between patients with or without pelvic metastases (Appendix 1).
Table 3. FIM scores before rehabilitation and after rehabilitation (n=92).
| Item | Before rehabilitation | After rehabilitation | P value |
| Self-care | |||
| Eating | 6 [7–5] | 7 [7–5] | 0.0043 |
| Grooming | 5 [6–3.25] | 5 [7–4] | 0.0001 |
| Bathing | 3 [4–1] | 4 [5.75–1] | <0.0001 |
| Dressing-upper body | 4 [6–3] | 5 [7–4] | 0.0003 |
| Dressing-lower body | 4 [6–2.25] | 4 [6–3] | 0.0004 |
| Toileting | 4 [6–1.25] | 5 [6–3] | <0.0001 |
| Sphincter control | |||
| Bladder management | 5 [7–1] | 6 [7–2] | 0.0023 |
| Bowel management | 6 [7–2] | 6 [7–3] | 0.0023 |
| Transfers | |||
| Bed, chair, wheelchair | 4 [5–1] | 5 [6–3] | <0.0001 |
| Toilet | 4 [5–1] | 5 [6–2] | <0.0001 |
| Tub, shower | 1 [5–1] | 4 [6–1] | <0.0001 |
| Locomotion | |||
| Walk/wheelchair | 1 [5–1] | 4 [6–1] | <0.0001 |
| Stairs | 1 [1–1] | 1 [5–1] | <0.0001 |
| Motor subtotal score | 51.5 [67–25.7] | 59.5 [78–35.25] | <0.0001 |
| Communication | |||
| Comprehension | 7 [7–5] | 7 [7–5] | 0.419 |
| Expression | 7 [7–6] | 7 [7–6] | 0.415 |
| Social cognition | |||
| Social interaction | 7 [7–6] | 7 [7–5.25] | 0.507 |
| Problem solving | 7 [7–6] | 7 [7–5.25] | 0.507 |
| Memory | 7 [7–6] | 7 [7–5.25] | 0.413 |
| Cognitive subtotal score | 35 [35–28.2] | 35 [35–25.75] | 0.307 |
| Total FIM score | 85.5 [102–57] | 92.5 [112–60.25] | <0.0001 |
Data displayed as median [IQR]. P values from Wilcoxon matched-pairs signed-rank test.
Fig. 2.
The duration of rehabilitation (day) correlated significantly with FIM changes. (*P<0.05).
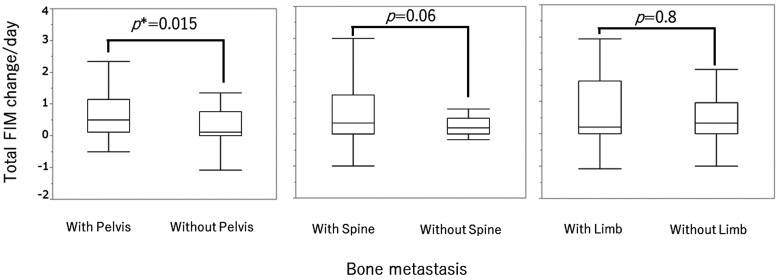
Because the duration of rehabilitation was significantly associated with FIM changes, FIM changes were divided by the number of rehabilitation days (FIM change/day) for further evaluation. The change in total FIM score per day was significantly greater in patients with pelvic metastasis than in those without pelvic metastasis (P=0.015) (Table 4 and Fig. 3).In the motor component of the FIM score, two of the transfer items (toilet and tub/shower) and both locomotion items (walk/wheelchair and stairs) were significantly higher in patients with pelvic metastasis than in those without pelvic metastasis. For the cognitive component of the FIM score, the communication item of expression and the social cognition item of social interaction were significantly higher in patients with pelvic metastasis than in patients without pelvic metastasis. As shown in Table 4, the median and IQR values were similar although the ranges were different. Spine and limb bone metastases did not affect the FIM improvement when corrected for rehabilitation days (Table 4).
Table 4. . FIM change grouped according to the length of rehabilitation in days (FIM change/day).
| Bone metastasis | ||||||||||
| Item | Total n=92 |
With pelvic metaa
n=44 |
Without pelvic metab
n=48 |
P value | With spine meta n=68 |
Without spine meta n=24 |
P value | With limb meta n=14 |
Without limb meta n=78 |
P value |
| Self-care | ||||||||||
| Eating | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0.70 | 0 [0–0] | 0 [0.024–0] | 0.80 | 0 [0–0] | 0 [0–0] | 0.20 |
| Grooming | 0 [0.49–0] | 0 [0.047–0] | 0 [0.051–0] | 0.31 | 0 [0.051–0] | 0 [0.042–0] | 0.70 | 0 [0.013–0] | 0 [0–0] | 0.47 |
| Bathing | 0 [0.009–0] | 0.010 [0.116–0] | 0 [0.07–0] | 0.06 | 0 [0.101–0] | 0 [0.030–0] | 0.10 | 0.07[0.16–0] | 0[0.07–0] | 0.06 |
| Dressing-upper body | 0 [0.059–0] | 0 [0.06–0] | 0 [0.059–0] | 0.79 | 0 [0.052–0] | 0 [0.086–0] | 0.90 | 0[0.08–0] | 0[0.52–0] | 0.94 |
| Dressing-lower body | 0 [0.049–0] | 0 [0.066–0] | 0 [0.025–0] | 0.52 | 0 [0.04–0] | 0 [0.061–0] | 0.90 | 0[0.058–0] | 0[0.52–0] | 0.92 |
| Toileting | 0 [0.47–0] | 0 [0.069–0] | 0 [0.019–0] | 0.101 | 0 [0.044–0] | 0 [0.058–0] | 0.80 | 0[0.08–0] | 0[0.045–0] | 0.33 |
| Sphincter control | ||||||||||
| Bladder management | 0 [0.015–0] | 0 [0.04–0] | 0 [0–0] | 0.79 | 0 [0.03–0] | 0 [0–0] | 0.50 | 0 [0–0] | 0[0.03–0] | 0.80 |
| Bowel management | 0 [0.015–0] | 0 [0.02–0] | 0 [0.015–0] | 0.87 | 0 [0.044–0] | 0 [0–0] | 0.20 | 0 [0–0] | 0[0.03–0] | 0.79 |
| Transfers | ||||||||||
| Bed, chair, wheelchair | 0 [0.079–0] | 0.023 [0.123–0] | 0 [0.061–0] | 0.16 | 0 [0.087–0] | 0 [0.076–0] | 0.66 | 0 [0.83–0] | 0[0.08–0] | 0.62 |
| Toilet | 0 [0.075–0] | 0 [0.119–0] | 0 [0.052–0] | 0.028 | 0 [0.097–0] | 0 [0.0613–0] | 0.36 | 0[0.10–0] | 0[0.07–0] | 0.73 |
| Tub, shower | 0 [0.09–0] | 0 [0.123–0] | 0 [0.045–0] | 0.024 | 0 [0.1137–0] | 0 [0.033–0] | 0.09 | 0[0.09–0] | 0[0.09–0] | 0.57 |
| Locomotion | ||||||||||
| Walk/wheelchair | 0 [0.178–0] | 0.066 [0.23–0] | 0 [0.079–0] | 0.0018 | 0.011 [0.1883–0] | 0 [0.033–0] | 0.13 | 0.02[0.17–0] | 0[0.19–0] | 0.78 |
| Stairs | 0 [0.0941–0] | 0.287 [0.287–0] | 0 [0–0] | 0.0059 | 0 [0.16–0] | 0 [0–0] | 0.03 | 0[0.41–0] | 0[0.08–0] | 0.14 |
| Motor subtotal score | 0.29 [1.081–0] | 0.51 [1.185–0.112] | 0.133 [1.0219–0] | 0.031 | 0.208 [0.714–0] | 0.35 [1.114–0] | 0.17 | 0.21[1.31–0] | 0.3[1.07–0] | 0.81 |
| Communication | ||||||||||
| Comprehension | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0.06 | 0 [0–0] | 0 [0–0] | 0.78 | 0 [0–0] | 0 [0–0] | 0.31 |
| Expression | 0 [0–0] | 0 [0–0] [−0.026, 0.363] | 0 [0–0] [−0.22, 0.3] | 0.019 | 0 [0–0] | 0 [0–0] | 0.80 | 0 [0–0] | 0 [0–0] | 0.98 |
| Social cognition | ||||||||||
| Social interaction | 0 [0–0] | 0 [0–0] [−0.166, 0.272] | 0 [0–0] [−0.33, 0.66] | 0.016 | 0 [0–0] | 0 [0–0] | 0.81 | 0 [0–0] | 0 [0–0] | 0.93 |
| Problem solving | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0.078 | 0 [0–0] | 0 [0–0] | 0.26 | 0 [0–0] | 0 [0–0] | 0.96 |
| Memory | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0.53 | 0 [0–0] | 0 [0–0] | 0.89 | 0 [0–0] | 0 [0–0] | 0.47 |
| Cognitive subtotal score | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0.181 | 0 [0–0] | 0 [0–0] | 0.65 | 0 [0–0] | 0 [0–0] | 0.45 |
| Total FIM score | 0.319 [1–0] | 0.5 [1.142–0.11] | 0.11 [0.755–0] | 0.015 | 0.344 [1.223–0] | 0.2 [0.5–0] | 0.06 | 0.21 [1.6–0] | 0.33 [0.96–0] | 0.80 |
Data displayed as median [IQR]. Data for Expression and Social interaction include [minimum, maximum]. P values from Wilcoxon matched-pairs signed-rank test.
a Patients with bone metastases including pelvic metastases.
b Patients with bone metastases except pelvic metastases.
Fig. 3.
Comparison of total FIM change/day in those with pelvic metastases and those without (left). With pelvis: patients with bone metastases, including pelvic metastases. Without pelvis: patients with bone metastases, except pelvic metastases. (*P<0.05). Comparisons are also shown for patients with and without spine metastases (middle) and for patients with and without limb metastases (right).
There were no significant differences between the SINS, Mirels score, and FIM items (P≥0.05). Of the patients with pelvic metastases, those with acetabular metastases had significant improvements in dressing the upper body (0.03 [0.08–0]) than those without metastases (0.007 [0–0]) (P=0.047).
The changes in FIM items of dressing-upper body, dressing-lower body, and social interaction were significantly lower in patients with a high Katagiri score (7–10 points) than in those with a low score (0–3 points or 4–6 points). The median and IQRs were similar for these data but the ranges were different (Table 5).
Table 5. FIM change/day in Katagiri score categories.
| Katagiri score | |||
| 0–3 and 4–6 points | 7–10 points | ||
| Item | n=43 | n=49 | P value |
| Self-care | |||
| Eating | 0 [0.02–0] | 0 [0–0] | 0.07 |
| Grooming | 0 [0.09–0] | 0 [0–0] | 0.04 |
| Bathing | 0 [0.08–0] | 0 [0–0.1] | 0.36 |
| Dressing-upper body | 0.02 [0.08–0] | 0 [0–0] | 0.011 |
| Dressing-lower body | 0 [0.08–0] | 0 [0–0] | 0.004 |
| Toileting | 0 [0.05–0] | 0 [0.019–0] | 0.14 |
| Sphincter control | |||
| Bladder management | 0 [0.03–0] | 0 [0–0] | 0.36 |
| Bowel management | 0 [0.027–0] | 0 [0–0] | 0.64 |
| Transfers | |||
| Bed, chair, wheelchair | 0.02 [0.09–0] | 0 [0.061–0] | 0.09 |
| Toilet | 0.018 [0.09–0] | 0 [0.061–0] | 0.15 |
| Tub, shower | 0 [0.09–0] | 0 [0.1–0] | 0.48 |
| Locomotion | |||
| Walk/wheelchair | 0.02 [0.1–0] | 0 [0.23–0] | 0.9 |
| Stairs | 0 [0.12–0] | 0 [0–0] | 0.05 |
| Motor subtotal score | 0.36 [1.07–0] | 0.18 [1.112–0] | 0.39 |
| Communication | |||
| Comprehension | 0 [0–0] | 0 [0–0] | 0.07 |
| Expression | 0 [0–0] | 0 [0–0] | 0.38 |
| Social cognition | |||
| Social interaction | 0 [0–0] [−0.14, 0.2] | 0 [0–0] [−0.3, 0.6] | 0.021 |
| Problem solving | 0 [0–0] | 0 [0–0] | 0.1 |
| Memory | 0 [0–0] | 0 [0–0] | 0.7 |
| Cognitive subtotal score | 0 [0–0] | 0 [0–0] | 0.21 |
| Total FIM score | 0.34 [1–0] | 0.26 [1.10–0] | 0.47 |
Data displayed as median [IQR]. Data for Social interaction include [minimum, maximum]. P values from Wilcoxon matched-pairs signed-rank test.
DISCUSSION
The results of this study showed that FIM scores were improved by rehabilitation in patients with bone metastasis. Notably, improvements in FIM change per day were greater in patients with a pelvic metastasis than those with metastases in the spine or limbs. This result suggests that patients with pelvic metastases may receive more benefit from rehabilitation therapy. With the recent progress in cancer treatment, patients experience a longer survival period, and, concomitantly, the number of patients with metastatic bone tumors is increasing.14) ADL status is decreased in patients with bone metastasis, and rehabilitation is required to improve patient QOL. We suspect that the effectiveness of rehabilitation in patients with metastatic bone tumors may differ depending on the site of bone metastasis and the general condition of the patient. This study examined the effects of rehabilitation in patients with metastatic bone tumors. Evaluation was based on the metastatic site, the Mirels score, SINS, the presence or absence of acetabulum metastases, the Katagiri score, and changes in FIM between admission and discharge based on the number of rehabilitation days (FIM change/day). We found a significantly higher FIM change/day in patients with pelvic metastasis than in those with metastases at other sites, especially in the FIM items of transfer and locomotion. Other FIM items, expression and social interaction, also showed a significantly greater FIM change/day in patients with pelvic metastasis. In this study the effectiveness of rehabilitation was evaluated by examining changes in the FIM index before and after rehabilitation. Not many studies have used the FIM items for this purpose and we consider it is possible to determine the specific effects of rehabilitation by using this scoring system.
Bone metastases are common in patients with cancer of the prostate, breast, lung, or kidney. These four primary sites account for 80% of bone metastases.15) In this study, lung cancer (32.6%) and esophageal cancer (21.7%) were the most common primary lesions. Spinal metastasis was the most frequent (73.9%), followed by metastasis in the pelvis (47.8%). Only a few hospitals perform operations for esophageal cancer near our hospital. In addition, there are many cases with various complications that have a high surgical risk. For the above reasons, we consider that there are many cases of bone metastasis treated in our hospital whose primary origin was esophageal cancer.
The Rizzoli Archived Metastatic Lesion Institute reported that pelvic metastasis occurred in 833 of 4431 cases (18.8%).16) The reason for the dissociation of this proportion was considered to be that this study targeted patients who were not indicated for surgery. Formerly, management of bone metastasis was not considered as important as it is today because it was deemed palliative and not associated with prognosis.4) However, over the past few decades, advances in chemotherapy, immunotherapy, and hormone and radiation therapy have significantly improved life expectancy in patients with metastatic cancer.17) As a result, this has led to an increase in the number of patients at risk of developing bone metastases and experiencing pathological fractures.18) In the current study, surgery was not indicated in any patient with pelvic metastases, with chemotherapy and palliative care provided instead. Determining whether it is acceptable for patients with pelvic metastases to bear weight is often difficult because of the lack of an evaluation method, particularly in those with acetabular metastases. As a consequence, these patients require more reliable rehabilitative treatment.
Rehabilitation in patients with spinal metastases improves pain, analgesic use, and depression.19) Rehabilitation in patients with metastatic spinal cord tumors also significantly improves the FIM and prolongs survival.13) However, no study to date has explored the effectiveness of rehabilitation for those with pelvic bone metastases. In our study, four of the FIM motor items and two of the FIM cognitive items, as reported by FIM change/day, were significantly greater in patients with pelvic bone metastases than in those with other bone metastases. We observed improvements not only in physical parameters, but also in psychological ones, based on the FIM score in patients with pelvic metastases.
SREs are complications associated with bone metastases, such as bone pain, hypercalcemia, pathological fractures, and spinal cord compression.20) These complications affect rehabilitation efficacy. A previous study showed that emotional functioning significantly declined after the occurrence of an SRE.21) Additionally, acetabular metastasis led to a worsened ECOG-PS.22) Of the different kinds of SREs, pathological fractures are estimated to occur in 9%–29% of patients with bone metastases.23,24) Pathological fractures worsen the patient’s ADL status and reduce the survival rate.25,26) Therefore, this may cause patients to be fearful of fractures and limit their daily activities. In addition, unnecessary rest instructions from doctors regarding the primary site may increase these limitations. In the current study, significant improvement was observed in psychological parameters of FIM change/day for patients with pelvic metastases. This indicates that transfer and locomotion training by rehabilitation staff may help remove fear and improve ADL status.
Using scoring methods to classify patients’ baseline functional abilities is important prior to safely initiating rehabilitation for those with bone metastases. Prognosis prediction and risk assessment of pathological fractures and paralysis due to spinal compression can clarify the goals of rehabilitation for each patient and maximize the effect of rehabilitative treatment. Prognosis prediction methods include the Katagiri score, Tomita score, and Tokuhashi score.5,27,28) In this study, the Katagiri score was used and correlated to the improvement of selected components of the FIM achieved through rehabilitation, including dressing-upper body, dressing-lower body, and social interaction. This finding should be noted as an improvement in FIM items in patients with a high survival rate. SINS is an evaluation for predicting the risk of pathological fractures and paralysis due to spinal cord compression in patients with bone metastases,7) whereas the Mirels score is used to evaluate the risk of pathological fracture caused by metastases in long bones.8) In recent years, analysis for bone metastases has been conducted using CT-based structural rigidity analysis,29,30,31) finite element analysis methods,32,33,34)18F-fluorodeoxyglucose PET/CT,35) and parametric response mapping,36) but these are not widely available because they require specialized equipment, software, and expertise to implement.37) For pelvic metastasis, anatomical classification can be made using the Enneking classification.38) This system divides the pelvis into four different zones. Lesions in zone 2 are associated with an increased risk of mechanical failure, because they correlate to a progressive destruction of the hip joint. However, the Enneking classification is used mainly for surgical treatment. Recently, maximum 2D diameter was identified as a significant prognostic factor for pelvic metastatic prostate cancer patients.39) Given that pelvic metastases often occur in multiple sites, an indication for surgery is unlikely. Therefore, in many cases, detailed examinations such as CT or MRI focusing on the pelvis have not been performed, and even in the current study pelvic metastases were not classified in detail. Therefore, it was only possible to classify individual patients depending on whether there were bone metastases in the acetabulum based on different imaging examinations. Those with acetabular metastases had significantly improved dressing of the upper body than those without metastases. This finding is presumed to be the result of intensive upper limb training through rehabilitation because of the difficulty of bearing weight on the lower limbs.
It is assumed that patients may not perform ADL for fear of pathological fractures, and, for this reason, rehabilitation staff may not be able to perform active rehabilitation. The findings of this study suggest that rehabilitation is effective even when surgery is not indicated for pelvic metastasis. In addition, no pathological fractures occurred during rehabilitation in our study cohort. However, there are only a small number of reports on the site of pelvic metastasis and the effect of rehabilitation, with no clear consensus on this relationship. A report confirming the relationship between detailed classification of pelvic metastases and the effects of rehabilitation could lead to safer rehabilitation programs. Pelvic bone metastases are a growing concern in the field of rehabilitation. Rehabilitation is considered minimally invasive care that can improve ADL status and QOL. Every patient needs careful evaluation and staging prior to starting rehabilitation. Ultimately, we believe that ADL status could be improved by evaluating pelvic metastases and performing appropriate rehabilitation treatment.
A major strength of this study is that the changes in FIM were examined item by item. To the best of our knowledge, this is the first time that such an analysis has been reported. In particular, improvements in the FIM motor and cognitive items for those with pelvic metastases were the main outcomes of active rehabilitation. These results suggest that it is effective to incorporate toilet and bath transfer training and walking and stair training into rehabilitation plans, instead of giving up attempts to improve ADL status because of the presence of pelvic metastasis. Our findings also suggested that it is important for therapists to interact and communicate with patients during rehabilitation. This study makes it possible to optimize rehabilitation planning.
This study has several limitations. First, we could not categorize patients by a single metastatic site because multiple bone metastases were often present; therefore, it was not possible to evaluate rehabilitation effects for patients with only pelvic metastases. Second, in many cases, detailed examination of pelvic metastasis was not performed, and it was not possible to evaluate FIM by the specific pelvic metastasis site. Instead, we classified whether bone metastases were present in the acetabulum. Third, the study was unable to assess psychological changes caused by rehabilitation treatment using indices such as the Quick Inventory of Depressive Symptomatology or the Beck Depression Inventory-Second Edition.
CONCLUSION
This study examined the effects of rehabilitation on patients with bone metastases. Patients with pelvic metastases showed a significantly greater improvement in ADL status than patients with other metastasis sites. Patients with pelvic metastases may be afraid of pathological fractures and may limit their ADL. After rehabilitation, the FIM items of transfer, locomotion, communication, and social cognition in patients with pelvic metastases improved significantly. We believe that rehabilitation removed the fear of movement and transfer and improved mental depression. However, patients with pelvic metastases are unlikely to have a surgical indication and are often not evaluated in detail because of this reason. This study demonstrated the effectiveness of rehabilitation in patients with pelvic metastases. To maximize the effect of rehabilitation and to prevent a decrease in ADL status as a consequence of the patient’s fear, there is significant need for confirmation of the relationship between detailed classification of pelvic metastases sites and the effect of rehabilitation. Such an advance would allow rehabilitation to be performed in these patients more safely.
ACKNOWLEDGMENTS
This work was supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (KAKENHI Grant Number 21K11325).
Footnotes
CONFLICTS OF INTEREST: The authors report no conflicts of interest.
REFERENCES
- 1.Bandini M,Pompe RS,Marchioni M,Zaffuto E,Gandaglia G,Fossati N,Cindolo L,Montorsi F,Briganti A,Saad F,Karakiewicz PI: Improved cancer-specific free survival and overall free survival in contemporary metastatic prostate cancer patients: a population-based study. Int Urol Nephrol 2018;50:71–78. 10.1007/s11255-017-1744-2 [DOI] [PubMed] [Google Scholar]
- 2.Maemondo M,Inoue A,Kobayashi K,Sugawara S,Oizumi S,Isobe H,Gemma A,Harada M,Yoshizawa H,Kinoshita I,Fujita Y,Okinaga S,Hirano H,Yoshimori K,Harada T,Ogura T,Ando M,Miyazawa H,Tanaka T,Saijo Y,Hagiwara K,Morita S,Nukiwa T, North-East Japan Study Group: Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–2388. 10.1056/NEJMoa0909530 [DOI] [PubMed] [Google Scholar]
- 3.Robert C,Long GV,Brady B,Dutriaux C,Maio M,Mortier L,Hassel JC,Rutkowski P,McNeil C,Kalinka-Warzocha E,Savage KJ,Hernberg MM,Lebbé C,Charles J,Mihalcioiu C,Chiarion-Sileni V,Mauch C,Cognetti F,Arance A,Schmidt H,Schadendorf D,Gogas H,Lundgren-Eriksson L,Horak C,Sharkey B,Waxman IM,Atkinson V,Ascierto PA: Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–330. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 4.Kimura T: Multidisciplinary approach for bone metastasis: a review. Cancers (Basel) 2018;10:156. 10.3390/cancers10060156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katagiri H,Okada R,Takagi T,Takahashi M,Murata H,Harada H,Nishimura T,Asakura H,Ogawa H: New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med 2014;3:1359–1367. 10.1002/cam4.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keith RA,Granger CV,Hamilton BB,Sherwin FS: The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil 1987;1:6–18. [PubMed] [Google Scholar]
- 7.Fisher CG,DiPaola CP,Ryken TC,Bilsky MH,Shaffrey CI,Berven SH,Harrop JS,Fehlings MG,Boriani S,Chou D,Schmidt MH,Polly DW,Biagini R,Burch S,Dekutoski MB,Ganju A,Gerszten PC,Gokaslan ZL,Groff MW,Liebsch NJ,Mendel E,Okuno SH,Patel S,Rhines LD,Rose PS,Sciubba DM,Sundaresan N,Tomita K,Varga PP,Vialle LR,Vrionis FD,Yamada Y,Fourney DR: A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010;35:E1221–E1229. 10.1097/BRS.0b013e3181e16ae2 [DOI] [PubMed] [Google Scholar]
- 8.Mirels H: Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res 1989;249:256–264. 10.1097/00003086-198912000-00027 [DOI] [PubMed] [Google Scholar]
- 9.Stineman MG,Shea JA,Jette A,Tassoni CJ,Ottenbacher KJ,Fiedler R,Granger CV: The functional independence measure: tests of scaling assumptions, structure, and reliability across 20 diverse impairment categories. Arch Phys Med Rehabil 1996;77:1101–1108. 10.1016/S0003-9993(96)90130-6 [DOI] [PubMed] [Google Scholar]
- 10.Murtaza H,Sullivan CW: Classifications in brief: the spinal instability neoplastic score. Clin Orthop Relat Res 2019;477:2798–2803. 10.1097/CORR.0000000000000923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jawad MU,Scully SP: In brief: classifications in brief: Mirels’ classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res 2010;468:2825–2827. 10.1007/s11999-010-1326-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubota H,Soejima T,Sulaiman NS,Sekii S,Matsumoto Y,Ota Y,Tsujino K,Fujita I,Fujimoto T,Morishita M,Ikegaki J,Matsumoto K,Sasaki R: Predicting the survival of patients with bone metastases treated with radiation therapy: a validation study of the Katagiri scoring system. Radiat Oncol 2019;14:13. 10.1186/s13014-019-1218-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang V,Harvey D,Park Dorsay J,Jiang S,Rathbone MP: Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord 2007;45:671–677. 10.1038/sj.sc.3102024 [DOI] [PubMed] [Google Scholar]
- 14.Bray F,Jemal A,Grey N,Ferlay J,Forman D: Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol 2012;13:790–801. 10.1016/S1470-2045(12)70211-5 [DOI] [PubMed] [Google Scholar]
- 15.Aboulafia AJ,Levine AM,Schmidt D,Aboulafia D: Surgical therapy of bone metastases. Semin Oncol 2007;34:206–214. 10.1053/j.seminoncol.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 16.Picci P,Manfrini M,Fabbri N,Gambarotti M,Vanel D: Atlas of musculoskeletal tumors and tumorlike lesions: the Rizzoli case archive. Springer, Cham, 2014. [Google Scholar]
- 17.Hage WD,Aboulafia AJ,Aboulafia DM: Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop Clin North Am 2000;31:515–528. 10.1016/S0030-5898(05)70171-1 [DOI] [PubMed] [Google Scholar]
- 18.Li S,Peng Y,Weinhandl ED,Blaes AH,Cetin K,Chia VM,Stryker S,Pinzone JJ,Acquavella JF,Arneson TJ: Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol 2012;4:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruff RL,Ruff SS,Wang X: Persistent benefits of rehabilitation on pain and life quality for nonambulatory patients with spinal epidural metastasis. J Rehabil Res Dev 2007;44:271–278. 10.1682/JRRD.2007.01.0006 [DOI] [PubMed] [Google Scholar]
- 20.Tsuzuki S,Park SH,Eber MR,Peters CM,Shiozawa Y: Skeletal complications in cancer patients with bone metastases. Int J Urol 2016;23:825–832. 10.1111/iju.13170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunikane H,Yokota I,Katakami N,Takeda K,Takayama K,Sawa T,Saito H,Harada M,Yokota S,Ando K,Saito Y,Ohashi Y,Eguchi K: Prospective analysis of the association between skeletal-related events and quality of life in patients with advanced lung cancer (CSP-HOR13). Oncol Lett 2019;17:1320–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinoda Y,Sawada R,Yoshikawa F,Oki T,Hirai T,Kobayashi H,Matsudaira K,Oka H,Tanaka S,Kawano H,Haga N: Factors related to the quality of life in patients with bone metastases. Clin Exp Metastasis 2019;36:441–448. 10.1007/s10585-019-09983-0 [DOI] [PubMed] [Google Scholar]
- 23.Aaron AD: Treatment of metastatic adenocarcinoma of the pelvis and the extremities. J Bone Joint Surg 1997;79:917–932. 10.2106/00004623-199706000-00018 [DOI] [PubMed] [Google Scholar]
- 24.Böhm P,Huber J: The surgical treatment of bony metastases of the spine and limbs. J Bone Joint Surg Br 2002;84-B:521–529. 10.1302/0301-620X.84B4.0840521 [DOI] [PubMed] [Google Scholar]
- 25.Hansen BH,Keller J,Laitinen M,Berg P,Skjeldal S,Trovik C,Nilsson J,Walloe A,Kalén A,Wedin R: The Scandinavian Sarcoma Group skeletal metastasis register: survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop Scand 2004;75(sup311):11–15. 10.1080/00016470410001708270 [DOI] [PubMed] [Google Scholar]
- 26.Mavrogenis AF,Pala E,Romagnoli C,Romantini M,Calabro T,Ruggieri P: Survival analysis of patients with femoral metastases. J Surg Oncol 2012;105:135–141. 10.1002/jso.22061 [DOI] [PubMed] [Google Scholar]
- 27.Tokuhashi Y,Matsuzaki H,Oda H,Oshima M,Ryu J: A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005;30:2186–2191. 10.1097/01.brs.0000180401.06919.a5 [DOI] [PubMed] [Google Scholar]
- 28.Tomita K,Kawahara N,Kobayashi T,Yoshida A,Murakami H,Akamaru T: Surgical strategy for spinal metastases. Spine 2001;26:298–306. 10.1097/00007632-200102010-00016 [DOI] [PubMed] [Google Scholar]
- 29.Snyder BD,Cordio MA,Nazarian A,Kwak SD,Chang DJ,Entezari V,Zurakowski D,Parker LM: Noninvasive prediction of fracture risk in patients with metastatic cancer to the spine. Clin Cancer Res 2009;15:7676–7683. 10.1158/1078-0432.CCR-09-0420 [DOI] [PubMed] [Google Scholar]
- 30.Nazarian A,Entezari V,Zurakowski D,Calderon N,Hipp JA,Villa-Camacho JC,Lin PP,Cheung FH,Aboulafia AJ,Turcotte R,Anderson ME,Gebhardt MC,Cheng EY,Terek RM,Yaszemski M,Damron TA,Snyder BD: Treatment planning and fracture prediction in patients with skeletal metastasis with CT-based rigidity analysis. Clin Cancer Res 2015;21:2514–2519. 10.1158/1078-0432.CCR-14-2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damron TA,Nazarian A,Entezari V,Brown C,Grant W,Calderon N,Zurakowski D,Terek RM,Anderson ME,Cheng EY,Aboulafia AJ,Gebhardt MC,Snyder BD: CT-based structural rigidity analysis is more accurate than Mirels scoring for fracture prediction in metastatic femoral lesions. Clin Orthop Relat Res 2016;474:643–651. 10.1007/s11999-015-4453-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sternheim A,Giladi O,Gortzak Y,Drexler M,Salai M,Trabelsi N,Milgrom C,Yosibash Z: Pathological fracture risk assessment in patients with femoral metastases using CT-based finite element methods. A retrospective clinical study. Bone 2018;110:215–220. 10.1016/j.bone.2018.02.011 [DOI] [PubMed] [Google Scholar]
- 33.Roth SE,Mousavi P,Finkelstein J,Chow E,Kreder H,Whyne CM: Metastatic burst fracture risk prediction using biomechanically based equations. Clin Orthop Relat Res 2004;419:83–90. 10.1097/00003086-200402000-00015 [DOI] [PubMed] [Google Scholar]
- 34.Eggermont F,van der Wal G,Westhoff P,Laar A,de Jong M,Rozema T,Kroon HM,Ayu O,Derikx L,Dijkstra S,Verdonschot N,van der Linden Y,Tanck E: Patient-specific finite element computer models improve fracture risk assessments in cancer patients with femoral bone metastases compared to clinical guidelines. Bone 2020;130:115101. 10.1016/j.bone.2019.115101 [DOI] [PubMed] [Google Scholar]
- 35.Ulaner GA,Zindman AM,Zheng J,Kim TW,Healey JH: FDG PET/CT assesses the risk of femoral pathological fractures in patients with metastatic breast cancer. Clin Nucl Med 2017;42:264–270. 10.1097/RLU.0000000000001580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoff BA,Toole M,Yablon C,Ross BD,Luker GD,Van Poznak C,Galbán CJ: Potential for early fracture risk assessment in patients with metastatic bone disease using parametric response mapping of CT images. Tomography 2015;1:98–104. 10.18383/j.tom.2015.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Damron TA,Mann KA: Fracture risk assessment and clinical decision making for patients with metastatic bone disease. J Orthop Res 2020;38:1175–1190. 10.1002/jor.24660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Enneking W,Dunham W,Gebhardt M,Malawar M,Pritchard D: A system for the classification of skeletal resections. Chir Organi Mov 1990;75(Suppl):217–240. [PubMed] [Google Scholar]
- 39.Hayakawa T,Tabata K,Tsumura H,Kawakami S,Katakura T,Hashimoto M,Watanabe Y,Iwamura M,Hasegawa T,Ishiyama H: Size of pelvic bone metastasis as a significant prognostic factor for metastatic prostate cancer patients. Jpn J Radiol 2020;38:993–996. 10.1007/s11604-020-01004-5 [DOI] [PubMed] [Google Scholar]