Abstract
Adult neurogenesis is the creation of new neurons which integrate into the existing neural circuit of the adult brain. Recent evidence suggests that adult hippocampal neurogenesis (AHN) persists throughout life in mammals, including humans. These newborn neurons have been implicated to have a crucial role in brain functions such as learning and memory. Importantly, studies have also found that hippocampal neurogenesis is impaired in neurodegenerative and neuropsychiatric diseases. Alzheimer’s disease (AD) is one of the most common forms of dementia affecting millions of people. Cognitive dysfunction is a common symptom of AD patients and progressive memory loss has been attributed to the degeneration of the hippocampus. Therefore, there has been growing interest in identifying how hippocampal neurogenesis is affected in AD. However, the link between cognitive decline and changes in hippocampal neurogenesis in AD is poorly understood. In this review, we summarized the recent literature on AHN and its impairments in AD.
Keywords: Hippocampal function, Adult hippocampal neurogenesis, Cognitive function, Alzheimer’s disease, Alzheimer’s disease animal models
INTRODUCTION
The mammalian brain is known to generate its full capacity of neurons during their embryonic development stage and perinatal stage (Ming & Song, 2005). This occurs through neurogenesis, the process of generating newborn functional neurons from precursor cells (Kriegstein & Alvarez-Buylla, 2009). However, there are a few regions in the brain that continue to generate newborn cells throughout life. This process is termed adult neurogenesis, which mainly occurs within the hippocampus and the lateral ventricles. Specifically, the subgranular zone (SGZ) of the hippocampus and subventricular zone (SVZ) of the lateral ventricles contain the self-renewing and multipotent neural stem cells responsible for adult neurogenesis (Zhao et al., 2008). The newborn cells in the SGZ generate hippocampal granule cells in the dentate gyrus (DG) while those in the SVZ are found to migrate into the olfactory bulb where they mature into interneurons, especially in rodents (Ming & Song, 2011).
Although the majority of adult born neurons in the hippocampus die (Kempermann et al., 2003), numerous literature points to the fact that they have the potential to synaptically incorporate into the local neural circuit (Alvarez-Buylla & Lim, 2004; Anacker & Hen, 2017; Duan et al., 2008; Gu et al., 2011; Lledo et al., 2006; Toda & Gage, 2018; Vivar et al., 2012). As such, recent research has focused on identifying the hippocampus-engaged functions of these newborn neurons such as cognitive flexibility, learning, memory, pattern separation, and mood (Anacker & Hen, 2017; Gu et al., 2012; Kropff et al., 2015; Miller & Sahay, 2019; Sahay & Hen, 2007; Shors et al., 2001; Yassa et al., 2011). Although adult neurogenesis has been rigorously characterized in rodent brains, the topic remains controversial in human brains (Kempermann et al., 2018; Sorrells et al., 2018). However, recent studies have suggested that adult neurogenesis is impaired in the process of aging and neurodegenerative diseases, such as Alzheimer’s disease (AD) in both humans and animal models (Dennis et al., 2016; Mathews et al., 2017; Moreno-Jiménez et al., 2019) and may lead to cognitive deficits (Mu & Gage, 2011). Therefore, there is an increasing interest in both the fundamental and clinical aspects of adult neurogenesis.
In this review, we focus on reviewing adult hippocampal neurogenesis (AHN), which occurs in the DG, and its functions. We will then shift the topic to AD and discuss recent studies on the mechanistic understanding of AHN in AD. We also summarize the different animal models that are currently used for AD research and their possible application in advancing neurogenesis studies in AD.
HISTORY AND FUNCTION OF AHN
Over the past few decades, the concept of neurogenesis in the adult mammalian brain has been controversial. With continuous efforts in the neuroscience community, it is now widely accepted that the adult brain continues neurogenesis throughout life in certain regions and it can generate functional newborn neurons. In this section, we will review our current understanding of AHN and its functions focusing on neurogenesis in rodents. We will also briefly discuss adult neurogenesis in different animal species.
History and controversy of adult neurogenesis
The earliest evidence of adult neurogenesis dates to the 1960s when Altman and Das used 3H-thymidine labeling to show newborn neurons in the rat cortex and hippocampus (Altman, 1962; Altman & Das, 1965) followed by Kaplan and Hinds who also found similar results in the rat hippocampus and olfactory bulb in 1977 (Kaplan & Hinds, 1977). However, due to the lack of adequate immunocytochemical markers to label these cells and being strongly against the dogma that neural circuits are fixed in mature brains, the observations were constantly dismissed. The first piece of concrete evidence that the new neurons can be functionally integrated into the existing neuronal network, although not in the hippocampus, emerged from Nottebohm’s work in songbirds (Paton & Nottebohm, 1984). This study reported that adult songbirds make new cells as they learn a new song and that these neurons play a role in the memory of the new song. In addition, they showed that the newborn neurons recruited into the existing neural circuit can influence cognitive function. This work was crucial as it helped overturn the criticism and conceptual discrepancies that existed, such as if newborn neurons established connections within the existing neuronal network, this would disturb the already stable network. Nowadays, technological advances such as the use of 5'-bromo-2'-deoxyuridine (BrdU) (Wojtowicz & Kee, 2006), genetic and viral labeling (Enikolopov et al., 2015), and computational network modeling (Aimone, 2016) have allowed adult neurogenesis to settle down as an important field in neuroscience.
The obvious question that follows these observations would be whether adult neurogenesis occurs in adult human brains as well. In 1998, Eriksson et al. (1998) were able to confirm the existence of AHN in the human brain using BrdU labeling and cell-type-specific markers. This observation in the hippocampal DG of human brains was confirmed over the following years from numerous different laboratories (Boldrini et al., 2018; Dennis et al., 2016; Knoth et al., 2010; Kukekov et al., 1999; Mathews et al., 2017; Palmer et al., 1999; Roy et al., 2000; Spalding et al., 2013). However, adult neurogenesis in humans is still controversial as more recent efforts have shown contradicting results where (1) neurogenesis persists in aged humans and (2) neurogenesis declines sharply after infancy and is absent in adults. Spalding et al. (2013) utilized radioactive carbon-13 DNA measurements to provide insight into the cell turnover dynamics and showed that there is substantial neurogenesis throughout life in the human hippocampus. A few years later, Boldrini et al. (2018) suggested that neurogenesis is preserved in humans until almost 79 years of age in the DG followed by a study by Moreno-Jiménez who argued that AHN is robust in healthy human subjects (Moreno-Jiménez et al., 2019). In contrast, Sorrells showed that neurogenesis in the DG declines steeply after birth and is undetectable by adulthood (Sorrells et al., 2018). In addition, recent efforts with single nucleus RNA sequencing have indicated that there is a lack of adult neurogenesis transcriptomic signatures in humans (Franjic et al., 2021). The reasons for these discrepancies are yet to be resolved, although different protocols and quantification methods (e.g. biomarker staining vs. single nucleus RNA sequencing) have been suggested. In addition, the quality of the human brain tissues can depend on the cause of death, postmortem interval, and how long it has been preserved, which could potentially lead to differing results. A recent review paper has extensively compared the differences of the methods used in some of these studies, but more concrete evidence demands novel labeling methods (Kempermann et al., 2018).
The stages of AHN
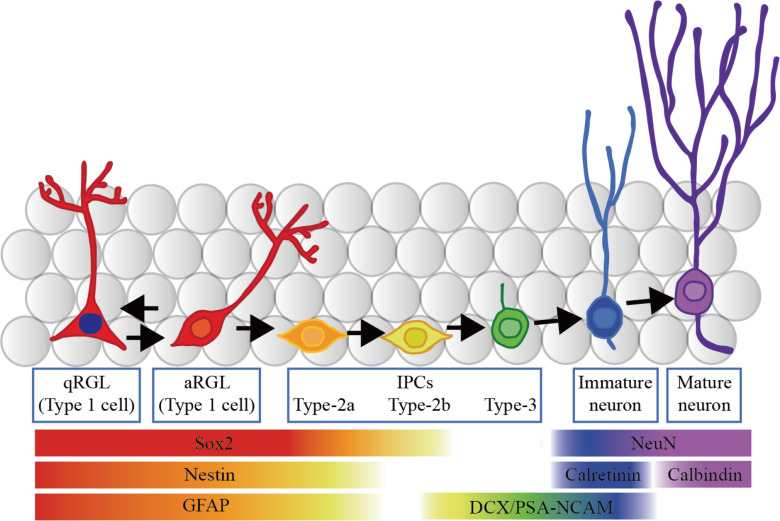
Along with the debate on the existence of adult neurogenesis in human brains, much progress has been made in animal models, especially rodents. One of the advancements from this is our understanding of the different cell types involved throughout the stages of adult neurogenesis in the DG (Figure 1). AHN begins with quiescent neural stem cells (qNSCs), also known as quiescent radial glia-like (qRGL) cells, which are found in the SGZ of the DG, located between the hilus and granule cell layer. As the name suggests, these cells generally have low metabolic activity and are sensitive to the local environment and physiological stimuli (Urbán et al., 2019). When activated, these RGLs are capable of self-renewing themselves and generate intermediate progenitor cells (type-2a, type-2ab, and type-2b cells) (Bonaguidi et al., 2011; Suh et al., 2007). These cells subsequently become immature neurons and finally mature neurons. Eventually, these cells mature into functional granule cells that integrate into the hippocampal circuitry and have important functions (Gonçalves et al., 2016b; Ming & Song, 2011). Differentiating these stages was a challenge in the 1990s. In the past two decades; however, more and more labeling tools have become available (Enikolopov et al., 2015). For example, by using recently-available immunohistochemical markers specific to the stage and type of cell, researchers are able to identify the cell fate and stage of the developing newborn neurons (Figure 1) (see review from Zhang & Jiao ( 2015) for a more detailed outline of biomarkers of neurogenesis). The success with biomarker labeling, together with other tools such as viral labeling and transgenic methods, have greatly advanced the study of adult neurogenesis in the past decade. Nevertheless, basic science research has heavily relied on a few immunohistochemical markers such as doublecortin (DCX) and Ki67, while clinical research still lacks concrete biomarkers to measure AHN. Therefore there has been much effort to identify various biomarkers to identify and utilize AHN as therapeutic targets (see review from Gillotin et al.(2021) for a more detailed review on recent efforts on identifying neurogenesis).
Figure 1.
Schematic of AHN in adult rodent brains
A schematic representation of AHN in the adult rodent brain at the subgranular zone. The most left is the radial glial-like cells, followed by the intermediate progenitor cells and finally, the immature and mature neurons. Important biomarkers of neurogenesis are labeled as well. aRGL, active radial glia-like cell; qRGL, quiescent radial glia-like cell; IPCs, intermediate progenitor cells; DCX, doublecortin; GFAP, glial fibrillary acidic protein; NeuN, neuronal nuclear protein; PSA-NCAM, polysialated form of neural cell adhesion molecule.
Neurogenesis across different animal species
The genesis and circuit integration of new neurons are readily known in the adult brain. However, it remains a challenge to understand the reason why an established neural circuit requires neurogenesis. One idea is to look for the behavioral functions of new neurons in an animal with a simpler neural network than that of humans. Along this journey, although the focus of AHN research has mainly been on rodents and humans, it has indeed been confirmed in many different species. For non-human primates (NHPs), New World monkeys (Gould et al., 1998; Leuner et al., 2007) and old world primates (Gould et al., 1999; Kornack & Rakic, 1999; Perera et al., 2007) have been observed to have AHN although it is thought to decrease significantly after puberty in both species (Jabès et al., 2010). Importantly, a recent study has also provided a single-nucleus transcriptomic atlas of NHPs, allowing us an in-depth view of the age-related changes occurring in different cell types of the primate hippocampus (Zhang et al., 2021a). A huge number of mammals have also been confirmed to display AHN including both wild and domesticated mammals. Some examples include hamsters (Huang et al., 1998), cats (Altman, 1963), dogs (Hwang et al., 2007), moles (Amrein et al., 2014; Peragine et al., 2014), hedgehogs (Alpár et al., 2010), and marsupials (Grabiec et al., 2009; Harman et al., 2003). In contrast, many bat species were found to have very few or absent newborn cells in the adult brain (For a more detailed review, view Amrein (2015)). Interestingly, non-mammalian vertebrates, including fishes, reptiles, amphibians, and birds, were found to have more abundant adult neurogenesis in general (For a more detailed review, view Chapouton et al. (2007)). On the other hand, evidence suggests that cetaceans have a lack of hippocampal neurogenesis (Patzke et al., 2015) and that dolphins also show a lack of neurogenesis in the subventricular zone (Parolisi et al., 2017). Finally, AHN has also been observed in insectivores (Alpár et al., 2010; Bartkowska et al., 2008, 2010).
As AHN is highly conserved across many animal species, one may speculate that AHN has specific functions in the brain. With these lines of advancements in detecting neurogenesis in different types of animals, one would expect some upcoming achievements in showing the function of new neurons. More importantly, further studies on adult neurogenesis may potentially facilitate our understanding of the niche environment necessary for the viability of the stem cells, and how these environments can control the pace of neurogenesis in certain brain regions of different species. Moreover, interdisciplinary studies between species may provide us with insight on the evolutionary meaning of neurogenesis as the discrepancies between species have been suggested to be linked to adaptation and the functional needs of the species (Kempermann, 2015; Parolisi et al., 2018).
Current evidence showing the functions of neurogenesis
It has been known for a long time now that the hippocampus has a crucial role in memory and learning in humans (Scoville & Milner, 1957). Of the hippocampus, the DG is a unique structure where its dorsal region is thought to be related to cognition and memory, while the ventral region is implicated for mood and stress modulation (Kheirbek et al., 2013; Tannenholz et al., 2014). Moreover, as we have discussed above, the SGZ of the DG is known to be involved in adult neurogenesis. Therefore, it is important to understand the relationship between AHN and hippocampal functions.
Adult neurogenesis is a dynamic process that is sensitive to pharmacological and chemogenetic manipulation, pathological conditions, external stimuli (e.g., exercise and stress) and the local environment (Aimone et al., 2014; Shohayeb et al., 2018; Winner et al., 2011; Winner & Winkler, 2015). As such, together with the desire to understand the fundamentals of biology, there is an increasing interest in the impact these newborn cells have on the existing neural circuitry and the role they have in different brain functions. The hippocampal connectivity has been extensively studied and is now well characterized by the tri-synaptic circuit. The tri-synaptic circuit consists of pyramidal cells in the entorhinal cortex (EC) connecting to the dentate gyrus cells (DGCs) in the DG. The signal is then transferred to the neighboring hippocampal cornu ammonis (CA) 3 followed by the CA1 pyramidal neurons and back to the EC (Amaral & Witter, 1989; Andersen, 1975). Looking more closely at the DG, the DGCs have complex connections with interneurons and CA3 pyramidal neurons (Booker & Vida, 2018; Henze et al., 2000) providing an intricate excitatory and inhibitory balance which play an important role in neural coding and regulating input and output of signals. AHN provides adult-born granule cells that can be integrated into this complex circuitry. Astrocytes (Krzisch et al., 2015; Sultan et al., 2015) and microglia (Rodríguez-Iglesias et al., 2019) have been identified as cell types that can potentially help adult-born DGCs to integrate into the circuit. It has also been discussed that these newborn neurons have different functions and characteristics throughout the process of integrating into the circuitry (Rodríguez-Iglesias et al., 2019). Once successfully integrated into the existing neural circuits, adult born granule cells have unique features that indicate more excitability, heightened plasticity, and a lower threshold for LTP induction (Ge et al., 2007; Gu et al., 2012; Schmidt-Hieber et al., 2004). These features hint that newborn cells potentially play a role in hippocampal functions which include not only learning and memory (Anacker & Hen, 2017; Gu et al., 2012; Hainmueller & Bartos, 2020; Lazarov & Hollands, 2016) but also functions such as anxiety and stress regulation (Surget & Belzung, 2021). Although not reviewed in this paper, it is also important to mention that recent studies have shown that young/immature adult born neurons have a role in learning and memory as well (Baptista & Andrade, 2018; Deng et al., 2010; Dieni et al., 2013; Mongiat et al., 2009).
AHN has been found to be involved in learning and memory which includes cognitive flexibility, emotional memory, spatial navigation, novelty detection and pattern separation (Anacker & Hen, 2017; Gu et al., 2012; Hainmueller & Bartos, 2020; Lazarov & Hollands, 2016). Studies using pharmacological and optogenetic approaches have shown that reduction of newborn cells negatively impacts, or an increase improves, numerous hippocampal-dependent performances including object memory, contextual fear conditioning and extinction learning, spatial learning, pattern separation and forgetting (Akers et al., 2014; Clelland et al., 2009; Danielson et al., 2016; Gao et al., 2018; Gu et al., 2012; Sahay et al., 2011). However, some early studies have found that ablation of new neurons did not affect spatial processing and pattern separation (Groves et al., 2013) or influence subsequent acquisition of spatial memory in the Morris water maze task (Shors et al., 2002). One study even showed that depleting new neurons improved working memory in an 8-arm radial maze (Saxe et al., 2007). These differences may be attributed to the different techniques (e.g. pharmacological vs. chemogenetic vs. optogenetic depletion) used to deplete the new neurons. These techniques may potentially cause off-target effects as it is challenging to selectively disrupt neurogenesis without disrupting the nearby structures. Moreover, behavioral paradigms such as the Morris water maze and contextual fear learning are hippocampus-dependent learning tasks. Therefore, it is also important to examine DG functionality specifically in relation to AHN. Most commonly, pattern separation has been associated with the DG (Bakker et al., 2008; Berron et al., 2016; Leutgeb et al., 2007). Unfortunately, pattern separation studies have also provided us with conflicting results where some have found newborn granule cells to have a critical role in pattern separation (Clelland et al., 2009; Nakashiba et al., 2012; Sahay et al., 2011) while other have not (Swan et al., 2014; Whoolery et al., 2020). Nevertheless, accumulating studies have suggested that the adult born neurons play a significant role in hippocampal-related functions (Toda et al., 2019).
The contradictory conclusion of the role of newborn neurons can also be seen in the emotional tests tackling the function of newborn neurons. For example, studies have shown contradictory evidence on whether AHN has a role in anxiety. In 1999, Lemaire et al. (1999) suggested that stress is closely related to AHN by showing decreased neurogenesis in rats with higher stress induced corticosterone. He also provided evidence that prenatal stress reduced AHN in rats (Lemaire et al., 2000). However, Shors et al. (2002) provided evidence that new neuron depletion does not affect anxiety-like behavior in an elevated plus maze. With different manipulating tools, recent studies have provided a clearer picture of the relationship between anxiety and AHN. One study has found that excessive anxiety leads to a decrease in the number of new neurons (Revest et al., 2009). However, some studies point towards AHN having a role in stress-induced anxiety. Increased AHN reduced anxiety levels measured through behavioral tests in mice (Hill et al., 2015) and AHN was also reported to provide resilience to stress-induced anxiety-like behavior (Anacker et al., 2018; Snyder et al., 2011). Putting all this together, it seems that AHN and anxiety have a bidirectional relationship where one can influence the other. However, further investigation will be needed to understand which comes first. There have also been recent efforts to identify a relationship between AHN and social behavior. Both social recognition memory (Garrett et al., 2015) and stress-induced social avoidance (Lagace et al., 2010) have been shown to be associated with AHN, where mice with lower levels of AHN had problems with remembering previously encountered mice. These lines of evidence suggest that the effects AHN has on social memory and anxiety may not be individual pathways, but rather a complicated and intertwined network that is yet to be fully understood.
Besides these fundamental studies on the function of new neurons, more and more studies allude to a relationship between adult neurogenesis and symptoms observed in patients suffering neuronal degeneration. In a study of neurogenesis using biomarker staining in human AD patients, Tobin et al. (2019) found that a higher number of newly formed neuroblasts was associated with less cognitive impairment based on a correlation analysis. In addition, it has been suggested that decreased AHN is correlated to an increased risk for both AD (Moreno-Jiménez et al., 2019; Tobin et al., 2019) and major depressive disorder (MDD) (Boldrini et al., 2013, 2019; Déry et al., 2013; Kheirbek & Hen, 2011). Moreover, a review by Berger et al. (2020) suggested that AHN could be a potential mediator for an increased risk for AD in those with MDD. Taken together, the potential functions of AHN opens the field to the therapeutic applications for not only aging and neurodegenerative diseases but also for psychiatric diseases including MDD, post-traumatic stress disorder and borderline personality disorder. Therefore, there is an urgent need for thorough investigations of neurogenesis under these pathological conditions. As such, in the next section, we focus on reviewing current updates on the impairment of neurogenesis in AD as an example to discuss neurogenesis under pathological conditions.
NEUROGENESIS IN ALZHEIMER’S DISEASE
Alzheimer’s disease currently affects more than 6 million people in the United States taking its place as the most common type of dementia (2021 Alzheimer's disease facts and figures, 2021). The common indicators of AD are the presence of neurofibrillary tangles (NFTs), amyloid plaques, and synaptic loss (Breijyeh & Karaman, 2020). Specifically, the presence of plaques in the hippocampus, and cerebral cortex has been thought to be the major pathogenic factor for AD. As a result, AD patients have symptoms ranging from inability to form new memories to neuropsychiatric problems such as depression (Lyketsos & Lee, 2004; Lyketsos & Olin, 2002; Lyketsos et al., 2011), psychosis (Jeste & Finkel, 2000; Lyketsos et al., 2011), and sleep disturbances (Ju et al., 2014). Despite the technological advances over the years, a definitive diagnosis of AD can only be made through examination of post-mortem brain tissue for NFTs and amyloid plaques (Terry, 2006). In this section, we aim to review the different hypotheses of AD before summarizing the current understanding of AHN in AD, followed by the current and new drug treatments for AD.
Possible causes of Alzheimer’s disease
The two main hypotheses for the pathogenesis of AD are the cholinergic and the amyloid hypothesis. The cholinergic hypothesis stems from the idea that acetylcholine (ACh), an organic chemical in the brain, plays a big part in cognitive function. In AD patients, cholinergic neurons were found to degenerate and β-amyloid inhibited choline uptake and ACh release (Babic, 1999; Breijyeh & Karaman, 2020). This has been the foundation for many of the current cholinesterase inhibitor treatments for AD patients. The amyloid hypothesis stems from the observation of fibrillar amyloid β (Aβ) peptides (Aβ40 and Aβ42) being deposited and accumulated in the brain tissue (Glenner & Wong, 1984; Masters et al., 1985). These peptides are neurotoxic eventually leading to neuronal cell death and neurodegeneration. Moreover, multiple genes including APP, PSEN1, and PSEN2, which are related to Aβ formation and accumulation (Paroni et al., 2019), have been identified to be mutated in early-onset AD. The amyloid hypothesis has been the driving force for AD research for over 20 years (Hardy & Allsop, 1991; Hardy & Higgins, 1992; Selkoe, 1991). However, with the failure of many recent drugs that have targeted the Aβ plaques (Yiannopoulou et al., 2019), the vascular hypothesis of Alzheimer’s disease has also been gaining interest (De La Torre, 2018; De La Torre & Mussivand, 1993). This idea suggests that reduced cerebral blood flow and cerebral hypoperfusion have implications in the pathogenic pathway of Alzheimer’s disease. This allows us to speculate that patients with vascular risk factors may have a higher risk for AD in the future (Cechetto et al., 2008). Another hypothesis that is gaining more momentum is neuroinflammation and microglia in Alzheimer’s disease. Reactive microglia have been identified to surround amyloid plaques in human AD brains (Mcgeer et al., 1987) and could produce pro-inflammatory cytokines causing neuroinflammation (Del Bo et al., 1995; Hanisch, 2002). With increasing evidence that Aβ, tau and microglia interact in AD patients, research in this area is becoming more popular (for a more detailed review on microglia and AD, view Leng & Edison ( 2021)). Finally, one of the most recent hypotheses is the mitochondria-affiliated Endoplasmic Reticulum (ER) membrane (MAM) hypothesis, which relies on the idea that the mitochondria and the ER interact biologically and physically (Area-Gomez & Schon, 2017). In support of this hypothesis, MAMs have been found to be upregulated in AD patients (Area-Gomez et al., 2012).
As reviewed above, AD is a complex disease and has a multitude of risk factors to consider including, but not limited to, genetics, age and environment (Campdelacreu, 2014). However, we are far from understanding the full picture of AD or finding a definitive cure. Therefore, alternative approaches and aspects of AD pathogenesis that have not been extensively studied, such as AHN, must be examined to gain further insight into understanding AD and ameliorating the symptoms.
Impaired neurogenesis in AD
As AD’s most common and devastating symptom is memory loss, the hippocampus is of great interest in the field of AD. From what we reviewed above, one could also speculate that all the potential hypotheses of AD can occur in and significantly impact the hippocampus. We have discussed how the hippocampus is one of the major areas that continues to generate newborn cells throughout life and this AHN has been associated with memory, learning and cognitive function. Therefore, in this section, we will review AHN in AD and how impaired neurogenesis may attribute to cognitive dysfunction in AD mouse models.
Neurogenesis in human AD patients: In the natural process of aging in humans, it has been hypothesized that hippocampal neurogenesis will decline with age as it was observed to decrease in rodents (Ben Abdallah et al., 2010; Gage, 2000; Seib et al., 2013). Indeed, the number of DCX positive cells in human brains was found to decrease with age (Moreno-Jiménez et al., 2019). In addition, Boldrini et al. (2018) also found that there was a decrease in angiogenesis, a smaller quiescent pool, and less neuroplasticity in the aged human brain, indicating reduced, but not absent, neurogenesis. It has also been suggested that the physiological changes that occur with aging are exacerbated in neurodegenerative diseases like AD. Therefore, it has been speculated that adult neurogenesis will decline earlier and more rapidly in AD patients. However, in contrast to the hypothesis, Jin et al. (2004b) reported that there was an increase in neurogenic factors such as DCX, TUC4, and PSA-NCAM in the hippocampus of AD patients. They suggested that there was an increase in AHN in AD patients as a compensatory mechanism to overcome the cell loss that occurs from neurodegeneration. Similarly, Ziabreva et al. (2006) also reported that there was an increase in Nestin-positive stem cells in AD patients. However, it is important to note that the samples are postmortem brains, meaning the medical history of the patients are not completely known, which could provide confounding effects, especially since the sample size for both studies were small. Moreover, the Nestin results from Ziabreva et al. (2006) raised concerns as it has been shown that Nestin expression may be alternatively increased due to reactive astrocytes (Liddelow & Barres, 2017). As such, more recent studies have shown opposite results. Moreno-Jiménez et al. (2019) compared the brains of normal aging patients and AD patients for DCX positive cells. They showed that there was a decrease in the number of immature neurons in AD brains throughout all the ages they examined. Tobin et al. (2019) also found similar results where the number of DCX positive cells and stem cells were decreased in AD patients relative to healthy individuals. They also claimed that DCX positive cells were still visible even in patients over 90 years old. More importantly, this study proposed a correlation between cognitive function and neurogenesis in AD patients. Although a closer examination is needed, this opens the possibility that adult neurogenesis can be used as a measure of cognitive function in AD patients. As such, recent studies have started to focus on in vivo imaging of neurogenesis in rodents through technology such as magnetic resonance imaging (MRI) (Vreys et al., 2010), positron emission tomography (PET) (Rueger et al., 2010), 2-photon calcium imaging (Danielson et al., 2016; Gonçalves et al., 2016a), and gradient index lens imaging (Carrier-Ruiz et al., 2021). These methods will allow us to examine the function of newborn granule cells and the changes they go through in pathologic conditions in an in vivo setting, which can provide further insight on how to utilize AHN as a target for therapeutic interventions.
Available AD animal models: As there are limitations to examining human tissue for research, animal models have been crucial for examining multiple aspects of AD pathogenesis and phenotypes. Unfortunately, it is not possible to directly extrapolate results from animal models to humans due to variability between species (Kempermann et al., 2018) and the difficulty to design proper cognitive tests (Schubiger et al., 2020). Moreover, AHN studies in non-rodent animals have been challenged due to longer life spans and costs. Nevertheless, the information from animal studies are valuable to provide the foundations and direction for further research. Therefore, we will briefly review the different animal models that are widely used in AD research.
Mouse models: Currently, the most used animal model for AD research is incontestably transgenic (Tg) mice. There are generally two different generations of AD mouse models: the first-generation amyloid precursor protein (APP) overexpressing mouse models and the second-generation APP knock-in (APP-KI) mouse models. The first-generation mice commonly overexpress proteins related to familial AD (FAD) mutant APP, presenilin, or a combination of these. These mice have been useful as they mimic the amyloid beta pathology well in the mouse brain. Although the cerebral amyloid accumulation is replicated well, the onset, size and regional distribution of the plaques differ between mouse models (Table 1). Moreover, driving the gene expression beyond physiological levels brings unwanted artifacts and phenotypes such as disrupting nearby genes (Kuro-O et al., 1997; Saito et al., 2016; Verret et al., 2012) or causing hyperactivity (Rodgers et al., 2012). Most importantly, many of the first-generation mice tend to have cognitive impairment before amyloid beta accumulation (Hsiao et al., 1996; Mucke et al., 2000), which is the opposite of our understanding of the disease in humans (Hardy & Allsop, 1991; Serrano-Pozo et al., 2011).
Table 1. Summary of plaque formation, cognitive impairment, and impaired AHN in commonly used AD mouse models.
| Mouse model | Age of plaque formation (Hippocampus, month) | Age of cognitive impairment (month) | AHN change | Primary paper |
| N/A: Not available. | ||||
| J20 | 6 (Hong et al., 2016) | 4 (Cheng et al., 2007;
Wright et al., 2013) |
3 months ↑ (López-Toledano & Shelanski, 2007) No change
(Sun et al., 2009) |
Mucke et al., 2000 |
| 5 months ↓ (López-Toledano & Shelanski, 2007) | ||||
| 12 months ↑ (Jin et al., 2004a) | ||||
| 5xFAD | 6 (Oakley et al., 2006) | 4–5 (Devi & Ohno, 2010;
Oakley et al., 2006) |
2–4 months ↓ (Moon et al., 2014; Zaletel et al., 2018) | Oakley et al., 2006 |
| APPswe/ PS1ΔE9 | 6 (Jankowsky et al., 2004) | 12 (Lalonde et al., 2005; Volianskis et al., 2010) | 2 months ↓ (Demars et al., 2010) | Jankowsky et al., 2004 |
| 6 months ↓ (Verret et al., 2007) | ||||
| 3xTg | 6 (Oddo et al., 2003) | 4 (Billings et al., 2005;
Stover et al., 2015) |
2–4 months ↓ (Hamilton et al., 2015; Rodríguez et al., 2008) | Oddo et al., 2003 |
| 6 months ↓ (Valero et al., 2014) | ||||
| 11, 18 months ↓ (Hamilton et al., 2010) | ||||
| Tg2576 | 6 (Westerman et al., 2002) | 10 (Arendash & King, 2002) | 3 months ↑ proliferation / ↓ Survival (Krezymon et al., 2013) | Hsiao et al., 1996 |
| APP NL-G-F Knock-in | 4 (Saito et al., 2014) | 6 (Saito et al., 2014) | N/A | Saito et al., 2014 |
| AppSAA Knock-in | 4 (Xia et al., 2021) | N/A | N/A | Xia et al., 2021 |
In order to avoid these pitfalls from the APP overexpression paradigm, there has been much struggle to generate human APP-KI mouse models, which would produce more pathogenic Aβ without overexpressing APP. In 2014, a group led by Saido was able to successfully generate APP-KI models (Saito et al., 2014). These mice also showed all the other AD related pathology observed in the first-generation mice, including gliosis, cell loss, and synaptic loss. Moreover, these mice developed plaques before cognitive impairment, solving many of the problems before. More recently, Denali therapeutics released a novel APP-KI line with the Jackson Lab, allowing more access to APP-KI mice for AD research (Xia et al., 2021). However, both generations so far have failed to generate neurofibrillary tangles, which is also an important hallmark of AD in human patients (For a more detailed overview of the APP mouse models, view Sasaguri et al. (2017)).
Rat models: Overall, rats are closer to humans both physiologically and genetically, making them a great model for AD research and more ideal for cognitive tests as they mimic human behavior closer than mice (Bryda, 2013). Unfortunately, due to cost and maintenance limitations, as well as the limited genetic manipulation tools in rats (Charreau et al., 1996; Tesson et al., 2005), most studies with rodents are still conducted with mice.
There are three well characterized rat models developed for AD research (Cohen et al., 2013; Leon et al., 2010; Liu et al., 2008). These rats are also generated from expression of FAD mutations, enabling them to have similar phenotypes and limitations to those of the AD mouse models. However, the TgF344-AD rat (Cohen et al., 2013) has been shown to have NFTs, which gives an advantage over most AD mouse models, which do not express NFTs unless crossed with a mutated Tau mouse model. There have also been efforts to generate APP-KI rats, similar to those of the second-generation AD mouse models (Serneels et al., 2020; Tambini & D'adamio, 2020; Tambini et al., 2019, 2020). However, they have failed to exhibit significant AD pathology. Recently, a group from China was able to successfully generate an APP-KI model that exhibits disease progression similar to humans (Pang et al., 2022). Although there are many aspects of this rat line to be characterized, this advancement in genetic manipulation for AD rats is promising.
NHPs: NHPs are an important animal model for studying AD due to their biological proximity to humans, such as their 100% sequence homology with human AB (Camus et al., 2015). Their larger brains allow for more advanced studies including more detailed imaging and more accessible CSF collection. From the existing literature, the rhesus monkey has been shown to be the most well characterized non-human primate model (For a more detailed review on this topic please view this article (Drummond & Wisniewski, 2017)). However, due to the complexity of developing monkey models, although very promising, it may take years before we could see some dramatic advancements in AD study with this type of model.
Lower order animals: Although the genetic make-up of lower order animals is simplified and there is limited genetic homology to humans, drosophila, Caenorhabditis elegans, and zebrafish have been used as animal models for AD research. Other than the low cost and the easy maintenance of these models, the main advantage is that genetic manipulations are relatively easy in these species, especially with the wide use of the CRISPR technology. As such, these low order animals can be efficiently used for large scale genetic and drug screenings (For detailed information on these different animal models, please refer to these reviews: Drosophila: Giong et al. (2021), Jeon et al. (2020), Tue et al. (2020); C. elegans: Paul et al. (2020), Giong et al. (2021); Zebrafish: Caramillo & Echevarria ( 2017), Giong et al. (2021)).
Adult neurogenesis studies on these AD animal models have mostly been focused on mouse models, which will be discussed in detail in the next section. However, in order to have a better understanding of how AHN is affected in AD, studies in more advanced animal models such as NHPs are needed. Recent efforts with transcriptomic studies have shed more insight onto how AHN may change with aging in multiple different species (Zhang et al., 2021a). Therefore, it will be important to extend these efforts into examining AD animal models to understand the relationship between AHN and AD.
A mechanistic understanding of Impaired AHN in AD: Although it has been challenging to understand AHN in non-rodent AD animals, and the few studies have focused on characterizing the deficits of AHN, we expect more and more mechanistic studies on hippocampal neurogenesis with our advancing understanding of the causes of AD and its impact on the brain network. Having a concrete understanding of how neurogenesis changes in these models will, in turn, facilitate therapeutic developments to rescue altered neurogenesis in AD and potentially alleviate cognitive function. Therefore, in this section, we will dissect the findings on how AHN is affected in AD mouse models, which are relatively more studied than other animal species. We also provide a summary of the phenotypes for the more commonly used AD models in Table 1.
Young mice (2–4 month old): Ki67 is a marker commonly used to measure proliferation of cells and DCX is a marker that predominantly labels the type-3 IPCs and immature neurons in the SGZ. BrdU can be used to measure proliferation or survival based on the time of injection and when the animal is sacrificed. In 2–4 month AD mice, there has been contradictory evidence on how AHN changes in the SGZ depending on the mouse line. The 5xFAD (Moon et al., 2014; Zaletel et al., 2018; Zhen et al., 2017), 3xTg (Hamilton et al., 2015; Rodríguez et al., 2008), APP/PS1/Nestin-GFP (Zeng et al., 2016), APP/PS1 (Demars et al., 2010; Sun et al., 2009; Zhang et al., 2021c), PS-1 knock-in (Wang et al., 2004), and PS-1 P117L (Wen et al., 2004) mouse models have been shown to have decreased AHN. On the other hand, PS-1 A246E (Chevallier et al., 2005) was shown to have increased AHN. Interestingly, there has been conflicting evidence for AHN in the J20 mouse model. One group found an increase in neurogenesis (López-Toledano & Shelanski, 2007) but other studies have found that there was no significant change (Sun et al., 2009; Zhang et al., 2021b) although the morphology and general trend indicated a decrease in AHN. For the Tg2576 mouse model, the proliferation stage of AHN was found to increase but the survival of newborn cells was decreased in 3 months mice (Krezymon et al., 2013).
Adult mice (5–6 month old): In 5–6 month mice, the 3xTg (Valero et al., 2014), APP/PS1 (Baglietto-Vargas et al., 2017; Verret et al., 2007) and J20 (López-Toledano & Shelanski, 2007) mouse models were shown to have decreased AHN with the former two being more specific for the survival of newborn DGCs and the latter for proliferation. Interestingly though, there was no difference in survival in the J20 mice at this age. However, similar to the young mice, the J20 line showed impaired morphology and maturation (Sun et al., 2009).
Aged mice (10+ month old): By this age, most AD mouse models tend to have widespread plaques in their brains. 3xTg (Hamilton et al., 2010) and PDAPP APPind (Donovan et al., 2006) were found to have significantly decreased AHN, although the latter was suggested to have more abnormal newborn cells in the granule cell layer. However, the J20 line has shown contradictory results as one group found an increase in AHN (Jin et al., 2004a) while another group has shown a decrease at 9 months (Zhang et al., 2021c).
As seen in this section, the overall trend seems to be that AHN is impaired in AD mouse models. However, there are contradictory results between studies and mouse lines. This conflict may be due to technical issues including mouse handling, different protocols, experimental paradigm, and type of markers used (Kempermann et al., 2018). Moreover, it has also been suggested that plaques may induce neurogenesis although the exact timeline for when this happens is not clear (Ermini et al., 2008; Gan et al., 2008). These conflicting results and the possibility for plaque induced neurogenesis highlight the challenges of using the current AD mouse models to study the relationship between AD and AHN. Therefore, there is a need for studies in the improved APP-KI mouse models. In addition, it will be important to have comprehensive studies, such has a monthly analysis of AHN, that handle multiple mouse lines together to examine how AHN changes with age in these AD mouse models. Despite these issues, the ongoing research for AHN in AD is essential to understanding the changes neurodegeneration brings to the adult brain.
IMPAIRED AHN IN AD MOUSE MODELS AND ITS POSSIBLE ASSOCIATION WITH DECLINED COGNITION
Cognitive dysfunction is a main symptom in AD patients and many AD mouse models have also been identified to have similar deficits. However, despite the evidence that AHN is altered in AD mouse models, we lack the knowledge on how the impaired adult neurogenesis directly affects cognitive function. Interestingly, it has been found that the altered AHN occurs before the learning and memory deficits or other common hallmarks like amyloid plaque formation (Li Puma et al., 2021). This allows us to hypothesize that AHN may have a causative role in the cognitive decline of the AD mouse models.
In support of this hypothesis, the APPswe/PS1ΔE9 mouse line shows altered hippocampal circuitry (compromised hippocampal inhibition) and overexcitable hippocampal neurons in response to ablating hippocampal neurogenesis (Hollands et al., 2017). The 5xFAD mouse line also shows that ablation of AHN led to exacerbation of cognitive dysfunction (Choi et al., 2018). On the other hand, the combination of pharmacological stimulation of neurogenesis and physical exercise improved the cognitive deficits and reduced amyloid burden in the 5xFAD mouse line (Choi et al., 2018). Similarly, in the 3xTg-AD mice, exercise alone was sufficient to improve cognitive function (Kim et al., 2019). However, the literature also suggests that the morphology and functional integration of newborn cells in the AD mouse models are abnormal (Krezymon et al., 2013; Richetin et al., 2015; Sun et al., 2009), which could lead to negative consequences in the long term. Therefore, one may also speculate that inhibiting abnormal AHN will improve cognitive function. In contrast to the literature before, Zhang et al. (2021b) reported that ablating adult neural stem cells (aNSC) in the J20 and APP/PS1 mice improved the cognitive and synaptic deficits to normal levels. Another external factor that is known to increase AHN is the use of an enriched environment (EE). EE was found to improve cognitive function in APP/PS1 mice and reduced Aβ load and tau phosphorylation in the hippocampus (Hu et al., 2010; Lazarov et al., 2005; Zhang et al., 2021c).
Additional studies have also supported the hypothesis that AHN improves cognitive function in AD. One study used adult bone marrow-derived mesenchymal stem cell transplantation in APP/PS1 mice to increase AHN, which improved memory and cognitive function (Yan et al., 2014). More recent studies have shown that micro-RNAs from NSCs have a role in AD as well. Micci et al. (2019) reported that micro-RNAs from NSCs improved memory deficits and protected the hippocampus from Aβ and Walgrave et al. (2021) demonstrated that replacement of miR-132, a downregulated micro-RNA in AD, improved AHN and memory deficits.
In conclusion, these studies demonstrate that adult neurogenesis very likely influences cognitive function of AD mouse models especially by increasing healthy adult neurogenesis and ablating abnormal adult neurogenesis. Moreover, many of the studies show that neurogenesis has a role in decreasing Aβ levels, which suggests that neurogenesis may also have a role in clearance or degradation of Aβ. However, there is a lack of consistency between different AD mouse models and the overexpression of APP in the first-generation AD mouse models has consistently been questioned as a factor for influencing the results. Therefore, further confirmation and mechanistic investigation of neurogenesis is required in the newer models such as the APP-KI mice.
CURRENT TREATMENTS FOR AD, POSSIBLY LINKED WITH AHN?
Pharmacological treatments for AD have been focused on treating the symptoms of patients to increase their quality of life. The most commonly used non-disease altering drugs include Donepezil, Galantamine, and Rivastigmine, which are FDA approved cholinesterase inhibitors used for mild to moderate AD. Donepezil treats cognitive function and behavioral functions and is used for mild to moderate cases of AD (Birks & Harvey, 2018; Knowles, 2006; Tan et al., 2014). Galantamine also showed advantages to cognitive function and is used in cases where mild to moderate dementia is present (Tan et al., 2014). Rivastigmine showed effectiveness in patients with mild to moderate dementia, and like the other cholinesterase inhibitors, its main target is cognitive functions (Birks & Evans, 2015; Tan et al., 2014). Unfortunately, our understanding of how cholinesterase inhibitors help relieve the symptoms of AD is yet unclear. However, it is speculated that by preventing the breakdown of acetylcholine, a chemical thought to play a role in memory and thinking, the drugs are improving cognitive function in these patients (Birks, 2006). Nevertheless, the AD brain produces less acetylcholine as the disease progresses, eventually rendering the drugs ineffective.
The drug Memantine is an uncompetitive N-methyl-D-aspartate receptor (NMDAR) modulator/antagonist that is used for moderate to severe AD. Overstimulation of NMDARs causes unusual calcium levels and glutamate overproduction, resulting in a decrease in cognitive function and learning and memory deficits. Memantine blocks the channels of NMDARs preventing the overstimulation of NMDARs, which occurs in AD. Those with moderate to severe Alzheimer's disease who took memantine saw an improvement in their cognitive, functional, and behavioral declines (Tan et al., 2014).
Opposed to the pharmacological treatments described above, Disease-Modifying treatments (DMTs) have shown promising results (Thomas et al., 2021). Aducanumad and Gantenerumab are 2 examples. Aducanumab is a recent antibody treatment that has been FDA approved. It is a human IgG1 anti-Aβ monoclonal antibody that selects for Aβ clusters and removes β-amyloid (Thomas et al., 2021). However, there is still great controversy to whether this drug truly has clinical impact as only a sub-population of clinical trial subjects were found to have improvement (Clinicaltrials.Gov, 2021a, 2021b; Tian et al., 2020). Gantenerumab, another Aβ monoclonal antibody, binds with great attraction towards congregated Aβ. This drug is still in its phase 3 clinical trials and has shown some effects on tau levels (Klein et al., 2019; Ostrowitzki et al., 2017) although it is not certain whether it has clinical efficacy. Another recent drug, ALZ-801 has recently started its phase 3 clinical trials. This drug has been debated to have a higher safety profile for treating AD for its relief of many symptoms that previous medications of AD bring up such as gastrointestinal tract problems. ALZ-801 is a selective anti-oligomer agent that prevents the formation of Aβ42 oligomers (Tolar et al., 2020). However, this drug is an optimized prodrug of tramiprosate, which has been shown to have its clinical efficacy limited to high-risk APOE4 carriers only. Nevertheless, these drugs are very promising in, alleviating some AD symptoms but it remains to be studied whether these drugs can improve the deficits seen with AHN, or whether the alleviation of symptoms requires the participation of AHN. This is especially important as emerging studies have shown that commonly used drugs have an effect on AHN in animal models (Gillotin et al., 2021) and there is also an effort to repurpose pre-existing drugs for AD (Taubes et al., 2021). Moreover, one study showed the genetic and pharmacological stimulation of AHN with the combination of pharmacologically increasing brain-derived neurotrophic factor improved cognition in AD mice (Choi et al., 2018). Therefore, there is a great need for research on how AHN is affected by AD related drugs in both animal models and humans.
CONCLUSION AND PERSPECTIVES
In this review, we first summarized our current understanding of AHN and its functions, focusing on cognitive function and stress. We also described how AHN was preserved across many different species. Next, we examined the different hypotheses of Alzheimer’s disease and the relationship between AHN and AD. We then discussed about the idea that impaired AHN in AD animal models indeed has a role in the declining cognitive function seen in AD. We also pointed out some of the common and new animal models used for AD research, which will potentially advance the studies of neurogenesis impairment in AD. Finally, we touched upon the current treatments and the potential for AHN to be a target for future AD drugs.
Although AHN has been a topic of interest in the field of AD, there is much that is not known and needs extensive investigation. However, the recent advent of APP-KI mice allows us to explore neurogenesis in the context of improved AD mouse models and we hope that these studies will be more transferable to human subjects. As technology continues to rapidly develop, we will also be able to examine neurogenesis more concretely in the human brain and develop more efficient methods to conduct research. As such, it is important to continue our efforts to explore adult neurogenesis in both human AD patients and AD animal models to further our understanding of AD. Most importantly, tackling hippocampal neurogenesis may provide us a window to mitigate the damage from AD and provide novel therapeutic approaches to treat AD. Numerous studies have tried to influence AHN in AD animal models through different approaches including genetic/pharmacological (Choi et al., 2018), dietary (Cao et al., 2009; Lee et al., 2002; Morello et al., 2018; Qin et al., 2006) and behavioral interventions (discussed in section above). However, there is a lingering question of what the optimal time point is for these interventions. Once this question is addressed, it may also be possible to combine multiple treatments to develop novel approaches to treat AD through AHN.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
T.A.K. conceived the review. T.A.K., M.D.S., and K.W. wrote the manuscript with input from all authors. T.A.K., M.D.S., and K.W. prepared the illustration and table. S.G. provided valuable suggestions. All authors read and approved the final version of the manuscript.
Funding Statement
This study was supported by the Medical Scientist Training Program (T32 GM008444) and Mechanistic Study of Declining Hippocampal Neurogenesis in the Aging Brain (R01AG066912 to S.G.)
References
- 1.2021. 2021 Alzheimer's disease facts and figures. Alzheimers & Dement, 17(3): 327–406.
- 2.Aimone JB Computational modeling of adult neurogenesis. Cold Spring Harbor Perspectives in Biology. 2016;8(4):a018960. doi: 10.1101/cshperspect.a018960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH Regulation and function of adult neurogenesis: from genes to cognition. Physiological Reviews. 2014;94(4):991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, et al Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344(6184):598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- 5.Alpár A, Künzle H, Gärtner U, Popkova Y, Bauer U, Grosche J, et al Slow age-dependent decline of doublecortin expression and BrdU labeling in the forebrain from lesser hedgehog tenrecs. Brain Research. 2010;1330:9–19. doi: 10.1016/j.brainres.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Altman J Are new neurons formed in the brains of adult mammals? Science. 1962;135(3509):1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- 7.Altman J Autoradiographic investigation of cell proliferation in the brains of rats and cats. The Anatomical Record. 1963;145(4):573–591. doi: 10.1002/ar.1091450409. [DOI] [PubMed] [Google Scholar]
- 8.Altman J, Das GD Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. The Journal of Comparative Neurology. 1965;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez-Buylla A, Lim DA For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41(5):683–686. doi: 10.1016/S0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 10.Amaral DG, Witter MP The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31(3):571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- 11.Amrein I Adult hippocampal neurogenesis in natural populations of mammals. Cold Spring Harbor Perspectives in Biology. 2015;7(5):a021295. doi: 10.1101/cshperspect.a021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amrein I, Becker AS, Engler S, Huang SH, Müller J, Slomianka L, et al Adult neurogenesis and its anatomical context in the hippocampus of three mole-rat species. Frontiers in Neuroanatomy. 2014;8:39. doi: 10.3389/fnana.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anacker C, Hen R Adult hippocampal neurogenesis and cognitive flexibility-linking memory and mood. Nature Reviews Neuroscience. 2017;18(6):335–346. doi: 10.1038/nrn.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, et al Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature. 2018;559(7712):98–102. doi: 10.1038/s41586-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen P. 1975. Organization of hippocampal neurons and their interconnections. In: Isaacson RL, Pribram KH. The Hippocampus. Boston: Springer, 155–175.
- 16.Area-Gomez E, Del Carmen Lara Castillo M, Tambini MD, Guardia-Laguarta C, De Groof AJC, Madra M, et al Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. The EMBO Journal. 2012;31(21):4106–4123. doi: 10.1038/emboj.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Area-Gomez E, Schon EA On the pathogenesis of Alzheimer's disease: the MAM hypothesis. The FASEB Journal. 2017;31(3):864–867. doi: 10.1096/fj.201601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arendash GW, King DL Intra- and intertask relationships in a behavioral test battery given to Tg2576 transgenic mice and controls. Physiology & Behavior. 2002;75(5):643–652. doi: 10.1016/s0031-9384(02)00640-6. [DOI] [PubMed] [Google Scholar]
- 19.Babic T The cholinergic hypothesis of Alzheimer's disease: a review of progress. Journal of Neurology, Neurosurgery & Psychiatry. 1999;67(4):558. doi: 10.1136/jnnp.67.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baglietto-Vargas D, Sánchez-Mejias E, Navarro V, Jimenez S, Trujillo-Estrada L, Gómez-Arboledas A, et al Dual roles of Aβ in proliferative processes in an amyloidogenic model of Alzheimer's disease. Scientific Reports. 2017;7(1):10085. doi: 10.1038/s41598-017-10353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakker A, Kirwan CB, Miller M, Stark CEL Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319(5870):1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baptista P, Andrade JP Adult hippocampal neurogenesis: regulation and possible functional and clinical correlates. Frontiers in Neuroanatomy. 2018;12:44. doi: 10.3389/fnana.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartkowska K, Djavadian RL, Taylor JRE, Turlejski K Generation recruitment and death of brain cells throughout the life cycle of Sorex shrews (Lipotyphla) . European Journal of Neuroscience. 2008;27(7):1710–1721. doi: 10.1111/j.1460-9568.2008.06133.x. [DOI] [PubMed] [Google Scholar]
- 24.Bartkowska K, Turlejski K, Grabiec M, Ghazaryan A, Yavruoyan E, Djavadian RL Adult neurogenesis in the hedgehog (Erinaceus concolor) and mole (Talpa europaea) . Brain, Behavior and Evolution. 2010;76(2):128–143. doi: 10.1159/000320944. [DOI] [PubMed] [Google Scholar]
- 25.Ben Abdallah NMB, Slomianka L, Vyssotski AL, Lipp HP Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiology of Aging. 2010;31(1):151–161. doi: 10.1016/j.neurobiolaging.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Berger T, Lee H, Young AH, Aarsland D, Thuret S Adult hippocampal neurogenesis in major depressive disorder and Alzheimer's disease. Trends in Molecular Medicine. 2020;26(9):803–818. doi: 10.1016/j.molmed.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Berron D, Schütze H, Maass A, Cardenas-Blanco A, Kuijf HJ, Kumaran D, et al Strong evidence for pattern separation in human dentate gyrus. Journal of Neuroscience. 2016;36(29):7569–7579. doi: 10.1523/JNEUROSCI.0518-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Billings LM, Oddo S, Green KN, Mcgaugh JL, Laferla FM Intraneuronal Aβ causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45(5):675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 29.Birks J Cholinesterase inhibitors for Alzheimer's disease. The Cochrane Database of Systematic Reviews. 2006;(1):CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birks JS, Evans JG Rivastigmine for Alzheimer's disease. The Cochrane Database of Systematic Reviews. 2015;(4):CD001191. doi: 10.1002/14651858.CD001191.pub3. [DOI] [PubMed] [Google Scholar]
- 31.Birks JS, Harvey RJ Donepezil for dementia due to Alzheimer's disease. The Cochrane Database of Systematic Reviews. 2018;6(6):CD001190. [Google Scholar]
- 32.Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, et al Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22(4):589–599.e5. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boldrini M, Galfalvy H, Dwork AJ, Rosoklija GB, Trencevska-Ivanovska I, Pavlovski G, et al Resilience is associated with larger dentate gyrus, while suicide decedents with major depressive disorder have fewer granule neurons. Biological Psychiatry. 2019;85(10):850–862. doi: 10.1016/j.biopsych.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, et al Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 2013;38(6):1068–1077. doi: 10.1038/npp.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, et al In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145(7):1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Booker SA, Vida I Morphological diversity and connectivity of hippocampal interneurons. Cell and Tissue Research. 2018;373(3):619–641. doi: 10.1007/s00441-018-2882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breijyeh Z, Karaman R Comprehensive review on Alzheimer’s disease: causes and treatment. Molecules. 2020;25(24):5789. doi: 10.3390/molecules25245789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bryda EC The mighty mouse: the impact of rodents on advances in biomedical research. Missouri Medicine. 2013;110(3):207–211. [PMC free article] [PubMed] [Google Scholar]
- 39.Campdelacreu J Parkinson disease and Alzheimer disease: environmental risk factors. Neurologia. 2014;29(9):541–549. doi: 10.1016/j.nrl.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Camus S, Ko WKD, Pioli E, Bezard E Why bother using non-human primate models of cognitive disorders in translational research? Neurobiology of Learning and Memory. 2015;124:123–129. doi: 10.1016/j.nlm.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Cao CH, Cirrito JR, Lin XY, Wang L, Verges DK, Dickson A, et al Caffeine suppresses amyloid-β levels in plasma and brain of Alzheimer's disease transgenic mice. Journal of Alzheimer's Disease. 2009;17(3):681–697. doi: 10.3233/JAD-2009-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caramillo EM, Echevarria DJ. 2017. Alzheimer's disease in the zebrafish: where can we take it?. Behavioural Pharmacology, 28(2 and 3-Spec Issue): 179–186.
- 43.Carrier-Ruiz A, Sugaya Y, Kumar D, Vergara P, Koyanagi I, Srinivasan S, et al Calcium imaging of adult-born neurons in freely moving mice. STAR Protocols. 2021;2(1):100238. doi: 10.1016/j.xpro.2020.100238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cechetto DF, Hachinski V, Whitehead SN Vascular risk factors and Alzheimer's disease. Expert Review of Neurotherapeutics. 2008;8(5):743–750. doi: 10.1586/14737175.8.5.743. [DOI] [PubMed] [Google Scholar]
- 45.Chapouton P, Jagasia R, Bally-Cuif L Adult neurogenesis in non-mammalian vertebrates. BioEssays. 2007;29(8):745–757. doi: 10.1002/bies.20615. [DOI] [PubMed] [Google Scholar]
- 46.Charreau B, Tesson L, Soulillou JP, Pourcel C, Anegon I Transgenesis in rats: technical aspects and models. Transgenic Research. 1996;5(4):223–234. doi: 10.1007/BF01972876. [DOI] [PubMed] [Google Scholar]
- 47.Cheng IH, Scearce-Levie K, Legleiter J, Palop JJ, Gerstein H, Bien-Ly N, et al Accelerating amyloid-β fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. Journal of Biological Chemistry. 2007;282(33):23818–23828. doi: 10.1074/jbc.M701078200. [DOI] [PubMed] [Google Scholar]
- 48.Chevallier NL, Soriano S, Kang DE, Masliah E, Hu G, Koo EH Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation. The American Journal of Pathology. 2005;167(1):151–159. doi: 10.1016/S0002-9440(10)62962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, et al Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer's mouse model. Science. 2018;361(6406):eaan8821. doi: 10.1126/science.aan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clelland CD, Choi M, Romberg C, Clemenson GD Jr, Fragniere A, Tyers P, et al A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clinicaltrials. gov. 2021a. 221AD301 Phase 3 Study of Aducanumab (BIIB037) in Early Alzheimer's Disease (ENGAGE). Clinical Trials.
- 52.Clinicaltrials. gov. 2021b. 221AD302 Phase 3 Study of Aducanumab (BIIB037) in Early Alzheimer's Disease (EMERGE). Clinical Trials.
- 53.Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, Spivak I, et al A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric aβ, and frank neuronal loss. Journal of Neuroscience. 2013;33(15):6245–6256. doi: 10.1523/JNEUROSCI.3672-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danielson NB, Kaifosh P, Zaremba JD, Lovett-Barron M, Tsai J, Denny CA, et al Distinct contribution of adult-born hippocampal granule cells to context encoding. Neuron. 2016;90(1):101–112. doi: 10.1016/j.neuron.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De La Torre J The vascular hypothesis of Alzheimer's disease: a key to preclinical prediction of dementia using neuroimaging. Journal of Alzheimer's Disease. 2018;63(1):35–52. doi: 10.3233/JAD-180004. [DOI] [PubMed] [Google Scholar]
- 56.De La Torre JC, Mussivand T Can disturbed brain microcirculation cause Alzheimer's disease? Neurological Research. 1993;15(3):146–153. doi: 10.1080/01616412.1993.11740127. [DOI] [PubMed] [Google Scholar]
- 57.Del Bo R, Angeretti N, Lucca E, De Simoni MG, Forloni G Reciprocal control of inflammatory cytokines, IL-1 and IL-6, and β-amyloid production in cultures. Neuroscience Letters. 1995;188(1):70–74. doi: 10.1016/0304-3940(95)11384-9. [DOI] [PubMed] [Google Scholar]
- 58.Demars M, Hu YS, Gadadhar A, Lazarov O Impaired neurogenesis is an early event in the etiology of familial Alzheimer's disease in transgenic mice. Journal of Neuroscience Research. 2010;88(10):2103–2117. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deng W, Aimone JB, Gage FH New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nature Reviews Neuroscience. 2010;11(5):339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dennis CV, Suh LS, Rodriguez ML, Kril JJ, Sutherland GT Human adult neurogenesis across the ages: an immunohistochemical study. Neuropathology and Applied Neurobiology. 2016;42(7):621–638. doi: 10.1111/nan.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Déry N, Pilgrim M, Gibala M, Gillen J, Wojtowicz JM, Macqueen G, et al Adult hippocampal neurogenesis reduces memory interference in humans: opposing effects of aerobic exercise and depression. Frontiers in Neuroscience. 2013;7:66. doi: 10.3389/fnins.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Devi L, Ohno M Phospho-eIF2α level is important for determining abilities of BACE1 reduction to rescue cholinergic neurodegeneration and memory defects in 5XFAD mice. PLoS One. 2010;5(9):e12974. doi: 10.1371/journal.pone.0012974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dieni CV, Nietz AK, Panichi R, Wadiche JI, Overstreet-Wadiche L Distinct determinants of sparse activation during granule cell maturation. Journal of Neuroscience. 2013;33(49):19131–19142. doi: 10.1523/JNEUROSCI.2289-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer's disease. The Journal of Comparative Neurology. 2006;495(1):70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- 65.Drummond E, Wisniewski T Alzheimer's disease: experimental models and reality. Acta Neuropathologica. 2017;133(2):155–175. doi: 10.1007/s00401-016-1662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duan X, Kang E, Liu CY, Ming GL, Song HJ Development of neural stem cell in the adult brain. Current Opinion in Neurobiology. 2008;18(1):108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Enikolopov G, Overstreet-Wadiche L, Ge SY Viral and transgenic reporters and genetic analysis of adult neurogenesis. Cold Spring Harbor Perspectives in Biology. 2015;7(8):a018804. doi: 10.1101/cshperspect.a018804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 69.Ermini FV, Grathwohl S, Radde R, Yamaguchi M, Staufenbiel M, Palmer TD, et al Neurogenesis and alterations of neural stem cells in mouse models of cerebral amyloidosis. The American Journal of Pathology. 2008;172(6):1520–1528. doi: 10.2353/ajpath.2008.060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Franjic D, Skarica M, Ma SJ, Arellano JI, Tebbenkamp ATN, Choi J, et al Transcriptomic taxonomy and neurogenic trajectories of adult human, macaque, and pig hippocampal and entorhinal cells. Neuron. 2021;110(3):452–469.e14. doi: 10.1016/j.neuron.2021.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gage FH Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 72.Gan L, Qiao SH, Lan X, Chi LY, Luo C, Lien L, et al Neurogenic responses to amyloid-beta plaques in the brain of Alzheimer's disease-like transgenic (pPDGF-APPSw, Ind) mice . Neurobiology of Disease. 2008;29(1):71–80. doi: 10.1016/j.nbd.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao AJ, Xia F, Guskjolen AJ, Ramsaran AI, Santoro A, Josselyn SA, et al Elevation of hippocampal neurogenesis induces a temporally graded pattern of forgetting of contextual fear memories. Journal of Neuroscience. 2018;38(13):3190–3198. doi: 10.1523/JNEUROSCI.3126-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garrett L, Zhang JZ, Zimprich A, Niedermeier KM, Fuchs H, Gailus-Durner V, et al Conditional reduction of adult born doublecortin-positive neurons reversibly impairs selective behaviors. Frontiers in Behavioral Neuroscience. 2015;9:302. doi: 10.3389/fnbeh.2015.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ge SY, Yang CH, Hsu KS, Ming GL, Song HJ A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54(4):559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gillotin S, Sahni V, Lepko T, Hanspal MA, Swartz JE, Alexopoulou Z, et al Targeting impaired adult hippocampal neurogenesis in ageing by leveraging intrinsic mechanisms regulating Neural Stem Cell activity. Ageing Research Reviews. 2021;71:101447. doi: 10.1016/j.arr.2021.101447. [DOI] [PubMed] [Google Scholar]
- 77.Giong HK, Subramanian M, Yu K, Lee JS Non-rodent genetic animal models for studying tauopathy: review of Drosophila, zebrafish, and C. elegans models . International Journal of Molecular Sciences. 2021;22(16):8465. doi: 10.3390/ijms22168465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Glenner GG, Wong CW Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochemical and Biophysical Research Communications. 1984;120(3):885–890. doi: 10.1016/S0006-291X(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 79.Gonçalves JT, Bloyd CW, Shtrahman M, Johnston ST, Schafer ST, Parylak SL, et al In vivo imaging of dendritic pruning in dentate granule cells . Nature Neuroscience. 2016a;19(6):788–791. doi: 10.1038/nn.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gonçalves JT, Schafer ST, Gage FH Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell. 2016b;167(4):897–914. doi: 10.1016/j.cell.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 81.Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E Hippocampal neurogenesis in adult old world primates. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(9):5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gould E, Tanapat P, Mcewen BS, Flügge G, Fuchs E Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(6):3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grabiec M, Turlejski K, Djavadian RL The partial 5-HT1A receptor agonist buspirone enhances neurogenesis in the opossum (Monodelphis domestica) . European Neuropsychopharmacology. 2009;19(6):431–439. doi: 10.1016/j.euroneuro.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 84.Groves JO, Leslie I, Huang GJ, Mchugh SB, Taylor A, Mott R, et al Ablating adult neurogenesis in the rat has no effect on spatial processing: evidence from a novel pharmacogenetic model. PLoS Genetics. 2013;9(9):e1003718. doi: 10.1371/journal.pgen.1003718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, et al Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nature Neuroscience. 2012;15(12):1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gu Y, Janoschka S, Ge SY Studying the integration of adult-born neurons. Journal of Visualized Experiments. 2011;(49):2548. doi: 10.3791/2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hainmueller T, Bartos M Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nature Reviews Neuroscience. 2020;21(3):153–168. doi: 10.1038/s41583-019-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hamilton LK, Aumont A, Julien C, Vadnais A, Calon F, Fernandes KJL Widespread deficits in adult neurogenesis precede plaque and tangle formation in the 3xTg mouse model of Alzheimer's disease. European Journal of Neuroscience. 2010;32(6):905–920. doi: 10.1111/j.1460-9568.2010.07379.x. [DOI] [PubMed] [Google Scholar]
- 89.Hamilton LK, Dufresne M, Joppé SE, Petryszyn S, Aumont A, Calon F, et al Aberrant lipid metabolism in the forebrain niche suppresses adult neural stem cell proliferation in an animal model of alzheimer's disease. Cell Stem Cell. 2015;17(4):397–411. doi: 10.1016/j.stem.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 90.Hanisch UK Microglia as a source and target of cytokines. Glia. 2002;40(2):140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 91.Hardy J, Allsop D Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends in Pharmacological Sciences. 1991;12(10):383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 92.Hardy JA, Higgins GA Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 93.Harman A, Meyer P, Ahmat A Neurogenesis in the hippocampus of an adult marsupial. Brain, Behavior and Evolution. 2003;62(1):1–12. doi: 10.1159/000071955. [DOI] [PubMed] [Google Scholar]
- 94.Henze DA, Urban NN, Barrionuevo G The multifarious hippocampal mossy fiber pathway: a review. Neuroscience. 2000;98(3):407–427. doi: 10.1016/S0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- 95.Hill AS, Sahay A, Hen R Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology. 2015;40(10):2368–2378. doi: 10.1038/npp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hollands C, Tobin MK, Hsu M, Musaraca K, Yu TS, Mishra R, et al Depletion of adult neurogenesis exacerbates cognitive deficits in Alzheimer's disease by compromising hippocampal inhibition. Molecular Neurodegeneration. 2017;12(1):64. doi: 10.1186/s13024-017-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li SM, Ramakrishnan S, et al Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352(6286):712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, et al Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–103. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 99.Hu YS, Xu P, Pigino G, Brady ST, Larson J, Lazarov O Complex environment experience rescues impaired neurogenesis, enhances synaptic plasticity, and attenuates neuropathology in familial Alzheimer's disease-linked APPswe/PS1ΔE9 mice. The FASEB Journal. 2010;24(6):1667–1681. doi: 10.1096/fj.09-136945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang L, DeVries GJ, Bittman EL Photoperiod regulates neuronal bromodeoxyuridine labeling in the brain of a seasonally breeding mammal. Journal of Neurobiology. 1998;36(3):410–420. doi: 10.1002/(SICI)1097-4695(19980905)36:3<410::AID-NEU8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 101.Hwang IK, Yoo KY, Li H, Choi JH, Kwon YG, Ahn Y, et al Differences in doublecortin immunoreactivity and protein levels in the hippocampal dentate gyrus between adult and aged dogs. Neurochemical Research. 2007;32(9):1604–1609. doi: 10.1007/s11064-007-9366-1. [DOI] [PubMed] [Google Scholar]
- 102.Jabès A, Lavenex PB, Amaral DG, Lavenex P Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus. European Journal of Neuroscience. 2010;31(2):273–285. doi: 10.1111/j.1460-9568.2009.07061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, et al Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific γ secretase . Human Molecular Genetics. 2004;13(2):159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- 104.Jeon Y, Lee JH, Choi B, Won SY, Cho KS Genetic dissection of alzheimer's disease using Drosophila models . International Journal of Molecular Sciences. 2020;21(3):884. doi: 10.3390/ijms21030884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jeste DV, Finkel SI Psychosis of Alzheimer's disease and related dementias. Diagnostic criteria for a distinct syndrome. The American Journal of Geriatric Psychiatry. 2000;8(1):29–34. doi: 10.1097/00019442-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 106.Jin KL, Galvan V, Xie L, Mao XO, Gorostiza OF, Bredesen DE, Greenberg DA Enhanced neurogenesis in Alzheimer's disease transgenic (PDGF-APPSw, Ind) mice . Proceedings of the National Academy of Sciences of the United States of America. 2004a;101(36):13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jin KL, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, et al Increased hippocampal neurogenesis in Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2004b;101(1):343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ju YES, Lucey BP, Holtzman DM Sleep and Alzheimer disease pathology—a bidirectional relationship. Nature Reviews Neurology. 2014;10(2):115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kaplan MS, Hinds JW Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197(4308):1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 110.Kempermann G Adult neurogenesis: an evolutionary perspective. Cold Spring Harbor Perspectivers in Biology. 2015;8(2):a018986. doi: 10.1101/cshperspect.a018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kempermann G, Gage FH, Aigner L, Song HJ, Curtis MA, Thuret S, et al Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018;23(1):25–30. doi: 10.1016/j.stem.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130(2):391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 113.Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, et al Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77(5):955–968. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kheirbek MA, Hen R Dorsal vs ventral hippocampal neurogenesis: implications for cognition and mood . Neuropsychopharmacology. 2011;36(1):373–374. doi: 10.1038/npp.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim D, Cho J, Kang H Protective effect of exercise training against the progression of Alzheimer's disease in 3xTg-AD mice. Behavioural Brain Research. 2019;374:112105. doi: 10.1016/j.bbr.2019.112105. [DOI] [PubMed] [Google Scholar]
- 116.Klein G, Delmar P, Voyle N, Rehal S, Hofmann C, Abi-Saab D, et al Gantenerumab reduces amyloid-β plaques in patients with prodromal to moderate Alzheimer's disease: a PET substudy interim analysis. Alzheimer's Research & Therapy. 2019;11(1):101. doi: 10.1186/s13195-019-0559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, et al Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5(1):e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Knowles J Donepezil in Alzheimer's disease: an evidence-based review of its impact on clinical and economic outcomes. Core Evidence. 2006;1(3):195–219. [PMC free article] [PubMed] [Google Scholar]
- 119.Kornack DR, Rakic P Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(10):5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Krezymon A, Richetin K, Halley H, Roybon L, Lassalle JM, Francès B, et al Modifications of hippocampal circuits and early disruption of adult neurogenesis in the tg2576 mouse model of Alzheimer's disease. PLoS One. 2013;8(9):e76497. doi: 10.1371/journal.pone.0076497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kriegstein A, Alvarez-Buylla A The glial nature of embryonic and adult neural stem cells. Annual Review of Neuroscience. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kropff E, Yang SM, Schinder AF Dynamic role of adult-born dentate granule cells in memory processing. Current Opinion in Neurobiology. 2015;35:21–26. doi: 10.1016/j.conb.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Krzisch M, Temprana SG, Mongiat LA, Armida J, Schmutz V, Virtanen MA, et al Pre-existing astrocytes form functional perisynaptic processes on neurons generated in the adult hippocampus. Brain Structure and Function. 2015;220(4):2027–2042. doi: 10.1007/s00429-014-0768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, et al Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Experimental Neurology. 1999;156(2):333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- 125.Kuro-O M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al Mutation of the mouse klotho gene leads to a syndrome resembling ageing . Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 126.Lagace DC, Donovan MH, Decarolis NA, Farnbauch LA, Malhotra S, Berton O, et al Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(9):4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lalonde R, Kim HD, Maxwell JA, Fukuchi K Exploratory activity and spatial learning in 12-month-old APP695SWE/co+PS1/ΔE9 mice with amyloid plaques . Neuroscience Letters. 2005;390(2):87–92. doi: 10.1016/j.neulet.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 128.Lazarov O, Hollands C. 2016. Hippocampal neurogenesis: learning to remember. Progress in Neurobiology, 138–140: 1–18.
- 129.Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VMY, et al Environmental enrichment reduces Aβ levels and amyloid deposition in transgenic mice. Cell. 2005;120(5):701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 130.Lee J, Duan WZ, Mattson MP Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. Journal of Neurochemistry. 2002;82(6):1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 131.Lemaire V, Aurousseau C, Le Moal M, Abrous DN Behavioural trait of reactivity to novelty is related to hippocampal neurogenesis. European Journal of Neuroscience. 1999;11(11):4006–4014. doi: 10.1046/j.1460-9568.1999.00833.x. [DOI] [PubMed] [Google Scholar]
- 132.Lemaire V, Koehl M, Le Moal M, Abrous DN Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Leng FD, Edison P Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nature Reviews Neurology. 2021;17(3):157–172. doi: 10.1038/s41582-020-00435-y. [DOI] [PubMed] [Google Scholar]
- 134.Leon WC, Canneva F, Partridge V, Allard S, Ferretti MT, Dewilde A, et al A novel transgenic rat model with a full Alzheimer's-like amyloid pathology displays pre-plaque intracellular amyloid-β-associated cognitive impairment. Journal of Alzheimer's Disease. 2010;20(1):113–126. doi: 10.3233/JAD-2010-1349. [DOI] [PubMed] [Google Scholar]
- 135.Leuner B, Kozorovitskiy Y, Gross CG, Gould E Diminished adult neurogenesis in the marmoset brain precedes old age. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(43):17169–17173. doi: 10.1073/pnas.0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Leutgeb JK, Leutgeb S, Moser MB, Moser EI Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315(5814):961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 137.Li Puma DD, Piacentini R, Grassi C Does impairment of adult neurogenesis contribute to pathophysiology of alzheimer's disease? A still open question. Frontiers in Molecular Neuroscience. 2021;13:578211. doi: 10.3389/fnmol.2020.578211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liddelow SA, Barres BA Reactive astrocytes: production, function, and therapeutic potential. Immunity. 2017;46(6):957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 139.Liu L, Orozco IJ, Planel E, Wen Y, Bretteville A, Krishnamurthy P, et al A transgenic rat that develops Alzheimer's disease-like amyloid pathology, deficits in synaptic plasticity and cognitive impairment. Neurobiology of Disease. 2008;31(1):46–57. doi: 10.1016/j.nbd.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lledo PM, Alonso M, Grubb MS Adult neurogenesis and functional plasticity in neuronal circuits. Nature Reviews Neuroscience. 2006;7(3):179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 141.López-Toledano MA, Shelanski ML Increased neurogenesis in young transgenic mice overexpressing human APPSw, Ind. Journal of Alzheimer's Disease. 2007;12(3):229–240. doi: 10.3233/JAD-2007-12304. [DOI] [PubMed] [Google Scholar]
- 142.Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, Amatniek J, et al Neuropsychiatric symptoms in Alzheimer's disease. Alzheimer's & Dementia. 2011;7(5):532–539. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lyketsos CG, Lee HB Diagnosis and treatment of depression in Alzheimer's disease. A practical update for the clinician. Dementia and Geriatric Cognitive Disorders. 2004;17(1-2):55–64. doi: 10.1159/000074277. [DOI] [PubMed] [Google Scholar]
- 144.Lyketsos CG, Olin J Depression in Alzheimer's disease: overview and treatment. Biological Psychiatry. 2002;52(3):243–252. doi: 10.1016/S0006-3223(02)01348-3. [DOI] [PubMed] [Google Scholar]
- 145.Masters CL, Simms G, Weinman NA, Multhaup G, Mcdonald BL, Beyreuther K Amyloid plaque core protein in Alzheimer disease and down syndrome. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mathews KJ, Allen KM, Boerrigter D, Ball H, Weickert CS, Double KL Evidence for reduced neurogenesis in the aging human hippocampus despite stable stem cell markers. Aging Cell. 2017;16(5):1195–1199. doi: 10.1111/acel.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mcgeer PL, Itagaki S, Tago H, Mcgeer EG Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neuroscience Letters. 1987;79(1−2):195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- 148.Micci MA, Krishnan B, Bishop E, Zhang WR, Guptarak J, Grant A, et al Hippocampal stem cells promotes synaptic resistance to the dysfunctional impact of amyloid beta oligomers via secreted exosomes. Molecular Neurodegeneration. 2019;14(1):25. doi: 10.1186/s13024-019-0322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miller SM, Sahay A Functions of adult-born neurons in hippocampal memory interference and indexing. Nature Neuroscience. 2019;22(10):1565–1575. doi: 10.1038/s41593-019-0484-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ming GL, Song HJ Adult neurogenesis in the mammalian central nervous system. Annual Review of Neuroscience. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 151.Ming GL, Song HJ Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mongiat LA, Espósito MS, Lombardi G, Schinder AF Reliable activation of immature neurons in the adult hippocampus. PLoS One. 2009;4(4):e5320. doi: 10.1371/journal.pone.0005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Moon M, Cha MY, Mook-Jung I Impaired hippocampal neurogenesis and its enhancement with ghrelin in 5XFAD mice. Journal of Alzheimer's Disease. 2014;41(1):233–241. doi: 10.3233/JAD-132417. [DOI] [PubMed] [Google Scholar]
- 154.Morello M, Landel V, Lacassagne E, Baranger K, Annweiler C, Féron F, et al Vitamin D improves neurogenesis and cognition in a mouse model of Alzheimer’s disease. Molecular Neurobiology. 2018;55(8):6463–6479. doi: 10.1007/s12035-017-0839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, Pallas-Bazarra N, et al Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nature Medicine. 2019;25(4):554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- 156.Mu YL, Gage FH Adult hippocampal neurogenesis and its role in Alzheimer's disease. Molecular Neurodegeneration. 2011;6(1):85. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, et al High-level neuronal expression of aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation . The Journal of Neuroscience. 2000;20(11):4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, et al Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149(1):188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, et al Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. Journal of Neuroscience. 2006;26(40):10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/S0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 161.Ostrowitzki S, Lasser RA, Dorflinger E, Scheltens P, Barkhof F, Nikolcheva T, et al A phase III randomized trial of gantenerumab in prodromal Alzheimer's disease. Alzheimer's Research & Therapy. 2017;9(1):95. doi: 10.1186/s13195-017-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. Journal of Neuroscience. 1999;19(19):8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pang KL, Jiang RC, Zhang W, Yang ZY, Li LL, Shimozawa M, et al An App knock-in rat model for Alzheimer’s disease exhibiting Aβ and tau pathologies, neuronal death and cognitive impairments . Cell Research. 2022;32(2):157–175. doi: 10.1038/s41422-021-00582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Parolisi R, Cozzi B, Bonfanti L Non-neurogenic SVZ-like niche in dolphins, mammals devoid of olfaction. Brain Structure and Function. 2017;222(6):2625–2639. doi: 10.1007/s00429-016-1361-3. [DOI] [PubMed] [Google Scholar]
- 165.Parolisi R, Cozzi B, Bonfanti L Humans and dolphins: decline and fall of adult neurogenesis. Frontiers in Neuroscience. 2018;12:497. doi: 10.3389/fnins.2018.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Paroni G, Bisceglia P, Seripa D Understanding the amyloid hypothesis in Alzheimer's disease. Journal of Alzheimer's Disease. 2019;68(2):493–510. doi: 10.3233/JAD-180802. [DOI] [PubMed] [Google Scholar]
- 167.Paton JA, Nottebohm FN Neurons generated in the adult brain are recruited into functional circuits. Science. 1984;225(4666):1046–1048. doi: 10.1126/science.6474166. [DOI] [PubMed] [Google Scholar]
- 168.Patzke N, Spocter MA, Karlsson KÆ, Bertelsen MF, Haagensen M, Chawana R, et al In contrast to many other mammals, cetaceans have relatively small hippocampi that appear to lack adult neurogenesis. Brain Structure and Function. 2015;220(1):361–383. doi: 10.1007/s00429-013-0660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Paul D, Chipurupalli S, Justin A, Raja K, Mohankumar SK Caenorhabditis elegans as a possible model to screen anti-Alzheimer's therapeutics . Journal of Pharmacological and Toxicological Methods. 2020;106:106932. doi: 10.1016/j.vascn.2020.106932. [DOI] [PubMed] [Google Scholar]
- 170.Peragine DE, Simpson JA, Mooney SJ, Lovern MB, Holmes MM Social regulation of adult neurogenesis in a eusocial mammal. Neuroscience. 2014;268:10–20. doi: 10.1016/j.neuroscience.2014.02.044. [DOI] [PubMed] [Google Scholar]
- 171.Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, et al Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. Journal of Neuroscience. 2007;27(18):4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Qin WP, Yang TL, Ho L, Zhao Z, Wang J, Chen LH, et al Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. Journal of Biological Chemistry. 2006;281(31):21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- 173.Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, et al Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Molecular Psychiatry. 2009;14(10):959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- 174.Richetin K, Leclerc C, Toni N, Gallopin T, Pech S, Roybon L, et al. 2015. Genetic manipulation of adult-born hippocampal neurons rescues memory in a mouse model of Alzheimer's disease. Brain, 138(Pt 2): 440–455.
- 175.Rodgers SP, Born HA, Das P, Jankowsky JL Transgenic APP expression during postnatal development causes persistent locomotor hyperactivity in the adult. Molecular Neurodegeneration. 2012;7(1):28. doi: 10.1186/1750-1326-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rodríguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, Laferla FM, et al Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease. PLoS One. 2008;3(8):e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rodríguez-Iglesias N, Sierra A, Valero J Rewiring of memory circuits: connecting adult newborn neurons with the help of microglia. Frontiers in Cell and Developmental Biology. 2019;7:24. doi: 10.3389/fcell.2019.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, et al In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus . Nature Medicine. 2000;6(3):271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- 179.Rueger MA, Backes H, Walberer M, Neumaier B, Ullrich R, Simard ML, et al Noninvasive imaging of endogenous neural stem cell mobilization in vivo using positron emission tomography . Journal of Neuroscience. 2010;30(18):6454–6460. doi: 10.1523/JNEUROSCI.6092-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sahay A, Hen R Adult hippocampal neurogenesis in depression. Nature Neuroscience. 2007;10(9):1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 181.Sahay A, Scobie KN, Hill AS, O'carroll CM, Kheirbek MA, Burghardt NS, et al Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Saito T, Matsuba Y, Mihira N, Takano J, Nilsson P, Itohara S, et al Single App knock-in mouse models of Alzheimer's disease . Nature Neuroscience. 2014;17(5):661–663. doi: 10.1038/nn.3697. [DOI] [PubMed] [Google Scholar]
- 183.Saito T, Matsuba Y, Yamazaki N, Hashimoto S, Saido TC Calpain activation in alzheimer's model mice is an artifact of APP and presenilin overexpression. Journal of Neuroscience. 2016;36(38):9933–9936. doi: 10.1523/JNEUROSCI.1907-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sasaguri H, Nilsson P, Hashimoto S, Nagata K, Saito T, De Strooper B, et al APP mouse models for Alzheimer's disease preclinical studies. The EMBO Journal. 2017;36(17):2473–2487. doi: 10.15252/embj.201797397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, et al Paradoxical influence of hippocampal neurogenesis on working memory. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(11):4642–4646. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Schmidt-Hieber C, Jonas P, Bischofberger J Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429(6988):184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 187.Schubiger MN, Fichtel C, Burkart JM Validity of cognitive tests for non-human animals: pitfalls and prospects. Frontiers in Psychology. 2020;11:1835. doi: 10.3389/fpsyg.2020.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Scoville WB, Milner B Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Seib DRM, Corsini NS, Ellwanger K, Plaas C, Mateos A, Pitzer C, et al Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell. 2013;12(2):204–214. doi: 10.1016/j.stem.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 190.Selkoe DJ The molecular pathology of Alzheimer's disease. Neuron. 1991;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 191.Serneels L, T'syen D, Perez-Benito L, Theys T, Holt MG, De Strooper B Modeling the β-secretase cleavage site and humanizing amyloid-beta precursor protein in rat and mouse to study Alzheimer's disease. Molecular Neurodegeneration. 2020;15(1):60. doi: 10.1186/s13024-020-00399-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine. 2011;1(1):a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shohayeb B, Diab M, Ahmed M, Ng DCH Factors that influence adult neurogenesis as potential therapy. Translational Neurodegeneration. 2018;7:4. doi: 10.1186/s40035-018-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shors TJ, Miesegaes G, Beylin A, Zhao MR, Rydel T, Gould E Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 195.Shors TJ, Townsend DA, Zhao MR, Kozorovitskiy Y, Gould E Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12(5):578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476(7361):458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi DS, Kelley KW, et al Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555(7696):377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, et al Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Stover KR, Campbell MA, Van Winssen CM, Brown RE Early detection of cognitive deficits in the 3xTg-AD mouse model of Alzheimer's disease. Behavioural Brain Research. 2015;289:29–38. doi: 10.1016/j.bbr.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 200.Suh H, Consiglio A, Ray J, Sawai T, D'amour KA, Gage FH In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus . Cell Stem Cell. 2007;1(5):515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sultan S, Li LY, Moss J, Petrelli F, Cassé F, Gebara E, et al Synaptic Integration of Adult-Born Hippocampal Neurons Is Locally Controlled by Astrocytes. Neuron. 2015;88(5):957–972. doi: 10.1016/j.neuron.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 202.Sun BG, Halabisky B, Zhou YG, Palop JJ, Yu GQ, Mucke L, et al Imbalance between gabaergic and glutamatergic transmission impairs adult neurogenesis in an animal model of Alzheimer's disease. Cell Stem Cell. 2009;5(6):624–633. doi: 10.1016/j.stem.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Surget A, Belzung C. 2021. Adult hippocampal neurogenesis shapes adaptation and improves stress response: a mechanistic and integrative perspective. Molecular Psychiatry,doi: 10.1038/s41380-021-01136-8.
- 204.Swan AA, Clutton JE, Chary PK, Cook SG, Liu GG, Drew MR Characterization of the role of adult neurogenesis in touch-screen discrimination learning. Hippocampus. 2014;24(12):1581–1591. doi: 10.1002/hipo.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tambini MD, D'adamio L Knock-in rats with homozygous PSEN1L435F Alzheimer mutation are viable and show selective γ-secretase activity loss causing low Aβ40/42 and high Aβ43 . Journal of Biological Chemistry. 2020;295(21):7442–7451. doi: 10.1074/jbc.RA120.012542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tambini MD, Norris KA, D'adamio L Opposite changes in APP processing and human Aβ levels in rats carrying either a protective or a pathogenic APP mutation. eLife. 2020;9:e52612. doi: 10.7554/eLife.52612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tambini MD, Yao W, D'adamio L Facilitation of glutamate, but not GABA, release in Familial Alzheimer's APP mutant Knock-in rats with increased β-cleavage of APP . Aging Cell. 2019;18(6):e13033. doi: 10.1111/acel.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C, et al Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer's disease: a systematic review and meta-analysis. Journal of Alzheimer's Disease. 2014;41(2):615–631. doi: 10.3233/JAD-132690. [DOI] [PubMed] [Google Scholar]
- 209.Tannenholz L, Jimenez JC, Kheirbek MA Local and regional heterogeneity underlying hippocampal modulation of cognition and mood. Frontiers in Behavioral Neuroscience. 2014;8:147. doi: 10.3389/fnbeh.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Taubes A, Nova P, Zalocusky KA, Kosti I, Bicak M, Zilberter MY, et al Experimental and real-world evidence supporting the computational repurposing of bumetanide for APOE4-related Alzheimer’s disease . Nature Aging. 2021;1(10):932–947. doi: 10.1038/s43587-021-00122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Terry RD Alzheimer's disease and the aging brain. Journal of Geriatric Psychiatry and Neurology. 2006;19(3):125–128. doi: 10.1177/0891988706291079. [DOI] [PubMed] [Google Scholar]
- 212.Tesson L, Cozzi J, Ménoret S, Rémy S, Usal C, Fraichard A, et al Transgenic modifications of the rat genome. Transgenic Research. 2005;14(5):531–546. doi: 10.1007/s11248-005-5077-z. [DOI] [PubMed] [Google Scholar]
- 213.Thomas E, Wasunna-Smith B, Kuruvilla T Aducanumab and disease modifying treatments for Alzheimer's disease. Progress in Neurology and Psychiatry. 2021;25(3):4–6. doi: 10.1002/pnp.711. [DOI] [Google Scholar]
- 214.Tian HKA, Arfaie S, Therriault J, Rosa-Neto P, Gauthier S Lessons learnt from the second generation of anti-amyloid monoclonal antibodies clinical trials. Dementia and Geriatric Cognitive Disorders. 2020;49(4):334–348. doi: 10.1159/000511506. [DOI] [PubMed] [Google Scholar]
- 215.Tobin MK, Musaraca K, Disouky A, Shetti A, Bheri A, Honer WG, et al Human hippocampal neurogenesis persists in aged adults and alzheimer's disease patients. Cell Stem Cell. 2019;24(6):974–982.e3. doi: 10.1016/j.stem.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Toda T, Gage FH Review: adult neurogenesis contributes to hippocampal plasticity. Cell and Tissue Research. 2018;373(3):693–709. doi: 10.1007/s00441-017-2735-4. [DOI] [PubMed] [Google Scholar]
- 217.Toda T, Parylak SL, Linker SB, Gage FH The role of adult hippocampal neurogenesis in brain health and disease. Molecular Psychiatry. 2019;24(1):67–87. doi: 10.1038/s41380-018-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tolar M, Abushakra S, Hey JA, Porsteinsson A, Sabbagh M Aducanumab, gantenerumab, BAN2401, and ALZ-801—the first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimer's Research & Therapy. 2020;12(1):95. doi: 10.1186/s13195-020-00663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tue NT, Dat TQ, Ly LL, Anh VD, Yoshida H Insights from Drosophila melanogaster model of Alzheimer's disease. Frontiers in Bioscience. 2020;25:134–146. doi: 10.2741/4798. [DOI] [PubMed] [Google Scholar]
- 220.Urbán N, Blomfield IM, Guillemot F Quiescence of adult mammalian neural stem cells: a highly regulated rest. Neuron. 2019;104(5):834–848. doi: 10.1016/j.neuron.2019.09.026. [DOI] [PubMed] [Google Scholar]
- 221.Valero J, Mastrella G, Neiva I, Sánchez S, Malva JO Long-term effects of an acute and systemic administration of LPS on adult neurogenesis and spatial memory. Frontiers in Neuroscience. 2014;8:83. doi: 10.3389/fnins.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Verret L, Jankowsky JL, Xu GM, Borchelt DR, Rampon C Alzheimer's-type amyloidosis in transgenic mice impairs survival of newborn neurons derived from adult hippocampal neurogenesis. Journal of Neuroscience. 2007;27(25):6771–6780. doi: 10.1523/JNEUROSCI.5564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Verret L, Mann EO, Hang GB, Barth AMI, Cobos I, Ho K, et al Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149(3):708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Vivar C, Potter MC, Choi J, Lee JY, Stringer TP, Callaway EM, et al Monosynaptic inputs to new neurons in the dentate gyrus. Nature Communications. 2012;3:1107. doi: 10.1038/ncomms2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Volianskis A, Køstner R, Mølgaard M, Hass S, Jensen MS Episodic memory deficits are not related to altered glutamatergic synaptic transmission and plasticity in the CA1 hippocampus of the APPswe/PS1ΔE9-deleted transgenic mice model of β-amyloidosis. Neurobiology of Aging. 2010;31(7):1173–1187. doi: 10.1016/j.neurobiolaging.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 226.Vreys R, Velde GV, Krylychkina O, Vellema M, Verhoye M, Timmermans JP, et al MRI visualization of endogenous neural progenitor cell migration along the RMS in the adult mouse brain: validation of various MPIO labeling strategies. NeuroImage. 2010;49(3):2094–2103. doi: 10.1016/j.neuroimage.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 227.Walgrave H, Balusu S, Snoeck S, Eynden EV, Craessaerts K, Thrupp N, et al Restoring miR-132 expression rescues adult hippocampal neurogenesis and memory deficits in Alzheimer's disease. Cell Stem Cell. 2021;28(10):1805–1821.e8. doi: 10.1016/j.stem.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 228.Wang R, Dineley KT, Sweatt JD, Zheng H Presenilin 1 familial Alzheimer's disease mutation leads to defective associative learning and impaired adult neurogenesis. Neuroscience. 2004;126(2):305–312. doi: 10.1016/j.neuroscience.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 229.Wen PH, Hof PR, Chen XP, Gluck K, Austin G, Younkin SG, et al The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Experimental Neurology. 2004;188(2):224–237. doi: 10.1016/j.expneurol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 230.Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, et al The relationship between Aβ and memory in the Tg2576 mouse model of Alzheimer's disease. Journal of Neuroscience. 2002;22(5):1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Whoolery CW, Yun S, Reynolds RP, Lucero MJ, Soler I, Tran FH, et al Multi-domain cognitive assessment of male mice shows space radiation is not harmful to high-level cognition and actually improves pattern separation. Scientific Reports. 2020;10(1):2737. doi: 10.1038/s41598-020-59419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Winner B, Kohl Z, Gage FH Neurodegenerative disease and adult neurogenesis. European Journal of Neuroscience. 2011;33(6):1139–1151. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- 233.Winner B, Winkler J Adult neurogenesis in neurodegenerative diseases. Cold Spring Harbor Perspectives in Biology. 2015;7(4):a021287. doi: 10.1101/cshperspect.a021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wojtowicz JM, Kee N BrdU assay for neurogenesis in rodents. Nature Protocols. 2006;1(3):1399–1405. doi: 10.1038/nprot.2006.224. [DOI] [PubMed] [Google Scholar]
- 235.Wright AL, Zinn R, Hohensinn B, Konen LM, Beynon SB, Tan RP, et al Neuroinflammation and neuronal loss precede Aβ plaque deposition in the hAPP-J20 mouse model of Alzheimer's disease. PLoS One. 2013;8(4):e59586. doi: 10.1371/journal.pone.0059586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Xia D, Lianoglou S, Sandmann T, Calvert M, Suh JH, Thomsen E, et al. 2021. Fibrillar Aβ causes profound microglial metabolic perturbations in a novel APP knock-in mouse model. bioRxiv,doi: 10.1101/2021.01.19.426731.
- 237.Yan YF, Ma T, Gong K, Ao Q, Zhang XF, Gong YD Adipose-derived mesenchymal stem cell transplantation promotes adult neurogenesis in the brains of Alzheimer's disease mice. Neural Regeneration Research. 2014;9(8):798–805. doi: 10.4103/1673-5374.131596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011;21(9):968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yiannopoulou KG, Anastasiou AI, Zachariou V, Pelidou SH Reasons for failed trials of disease-modifying treatments for alzheimer disease and their contribution in recent research. Biomedicines. 2019;7(4):97. doi: 10.3390/biomedicines7040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zaletel I, Schwirtlich M, Perović M, Jovanović M, Stevanović M, Kanazir S, et al Early impairments of hippocampal neurogenesis in 5xFAD mouse model of Alzheimer's disease are associated with altered expression of SOXB transcription factors. Journal of Alzheimer's Disease. 2018;65(3):963–976. doi: 10.3233/JAD-180277. [DOI] [PubMed] [Google Scholar]
- 241.Zeng Q, Zheng M, Zhang T, He G Hippocampal neurogenesis in the APP/PS1/nestin-GFP triple transgenic mouse model of Alzheimer's disease. Neuroscience. 2016;314:64–74. doi: 10.1016/j.neuroscience.2015.11.054. [DOI] [PubMed] [Google Scholar]
- 242.Zhang H, Li JM, Ren J, Sun SH, Ma S, Zhang WQ, et al Single-nucleus transcriptomic landscape of primate hippocampal aging. Protein & Cell. 2021a;12(9):695–716. doi: 10.1007/s13238-021-00852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhang J, Jiao JW Molecular biomarkers for embryonic and adult neural stem cell and neurogenesis. BioMed Research International. 2015;2015:727542. doi: 10.1155/2015/727542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhang XQ, Mei YF, He Y, Wang DP, Wang J, Wei XJ, et al Ablating adult neural stem cells improves synaptic and cognitive functions in alzheimer models. Stem Cell Reports. 2021b;16(1):89–105. doi: 10.1016/j.stemcr.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhang XQ, Wei XJ, Mei YF, Wang DP, Wang J, Zhang YP, et al Modulating adult neurogenesis affects synaptic plasticity and cognitive functions in mouse models of Alzheimer's disease. Stem Cell Reports. 2021c;16(12):3005–3019. doi: 10.1016/j.stemcr.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhao CM, Deng W, Gage FH Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 247.Zhen JL, Qian YJ, Fu J, Su RJ, An HT, Wang W, et al Deep brain magnetic stimulation promotes neurogenesis and restores cholinergic activity in a transgenic mouse model of Alzheimer’S disease. Frontiers in Neural Circuits. 2017;11:48. doi: 10.3389/fncir.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ziabreva I, Perry E, Perry R, Minger SL, Ekonomou A, Przyborski S, et al Altered neurogenesis in Alzheimer's disease. Journal of Psychosomatic Research. 2006;61(3):311–316. doi: 10.1016/j.jpsychores.2006.07.017. [DOI] [PubMed] [Google Scholar]