Abstract
Maternal sleep deprivation (MSD) is a global public health problem that affects the physical and mental development of pregnant women and their newborns. The latest research suggests that sleep deprivation (SD) disrupts the gut microbiota, leading to neuroinflammation and psychological disturbances. However, it is unclear whether MSD affects the establishment of gut microbiota and neuroinflammation in the newborns. In the present study, MSD was performed on pregnant Sprague-Dawley rats in the third trimester of pregnancy (gestational days 15–21), after which intestinal contents and brain tissues were collected from offspring at different postnatal days (P1, P7, P14, and P56). Based on microbial profiling, microbial diversity and richness increased in pregnant rats subjected to MSD, as reflected by the significant increase in the phylum Firmicutes. In addition, microbial dysbiosis marked by abundant Firmicutes bacteria was observed in the MSD offspring. Furthermore, quantitative real-time polymerase chain reaction (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA) showed that the expression levels of proinflammatory cytokines interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α) were significantly higher in the MSD offspring at adulthood (P56) than in the control group. Through Spearman correlation analysis, IL-1β and TNF-α were also shown to be positively correlated with Ruminococcus_1 and Ruminococcaceae_UCG-005 at P56, which may determine the microbiota-host interactions in MSD-related neuroinflammation. Collectively, these results indicate that MSD changes maternal gut microbiota and affects the establishment of neonatal gut microbiota, leading to neuroinflammation in MSD offspring. Therefore, understanding the role of gut microbiota during physiological development may provide potential interventions for cognitive dysfunction in MSD-impacted offspring.
Keywords: Maternal sleep deprivation, Gut microbiota, Neuroinflammation, Gut-brain axis, Cognitive function, Firmicutes
INTRODUCTION
Sufficient time and quality of sleep are basic requirements for maintaining a healthy body. Maternal sleep deprivation (MSD) in pregnant women caused by hormonal changes and mental stress has become a critical public health issue (Mindell et al., 2015). According to epidemiological surveys in recent decades, many women live with severe sleep disruption, sleep loss, and sleep disorder symptoms during pregnancy, particularly in late gestation (Du et al., 2021; Mindell et al., 2015; Pien & Schwab, 2004). Studies have shown that MSD can have detrimental consequences in pregnant women and newborns, such as increased maternal risk of gestational hypertension, gestational diabetes, and postpartum depression, as well as increased neonatal risk of premature or overdue delivery and dystocia (Pires et al., 2021).
Daily acclimation handling is often used to induce brief sleep deprivation (SD) in rodents to mimic the status of sleep loss in humans (Vecsey et al., 2013), with such studies revealing the detrimental effects of MSD on offspring development. For instance, MSD can induce long-lasting metabolic perturbations (Harskamp-van Ginkel et al., 2020; Khalyfa et al., 2014), reduce reproductive capability (Alvarenga et al., 2013), increase cardiovascular disease risk (Argeri et al., 2016; Lima et al., 2014; Thomal et al., 2010), and increase aberrant immune responses (Baratta et al., 2020) in offspring. In addition, MSD can also cause neurophysiological disturbances in offspring, such as altered sleep-wake patterns (Aswathy et al., 2018), impaired hippocampal neurogenesis (Zhao et al., 2014), increased neuroinflammation (Zhao et al., 2015), and hostile neuropsychological behaviors (Radhakrishnan et al., 2015). Consistently, our recent studies revealed that MSD induces impairment of synaptic plasticity and cognition in offspring rats by facilitating endocytosis of postsynaptic GluA2-containing α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid receptors (AMPAR) (Peng et al., 2016; Yu et al., 2018).
A growing body of evidence has shown that the gut microbiota plays an indispensable physiological role in the pathogenesis of various human metabolic, immunological, and neurological diseases, suggesting that gut microbiota homeostasis largely determines host health (Li et al., 2016). Importantly, the gut microbiota plays a regulating role in the central nervous system (CNS) via the gut-brain axis, which forms a bidirectional information network between the gut microbiota and brain (Wang & Wang, 2016). Therefore, the gut microbiota is critical for psychological activities such as cognition, sociability, anxiety-like behaviors, and normal stress response, and maintains CNS homeostasis by regulating immunological function and blood-brain barrier integrity (Luczynski et al., 2016). Furthermore, circadian rhythm is intimately correlated with the gut microbiota, with SD impacting the gut microbiota in various ways (Matenchuk et al., 2020). For example, chronic SD (Poroyko et al., 2016) or brief acute SD (El Aidy et al., 2020) can lead to microbial dysbiosis, thereby altering metabolic patterns associated with cognitive capability. A recent study reported that an absence of gut microbiota can suppress the inflammatory response and cognitive impairment induced by SD in germ-free mice (Wang et al., 2021), suggesting a critical role of gut microbiota in sleep regulation and brain function.
Disturbances during either prenatal or postnatal periods may impair the vertical transmission of microbiota and cause microbial dysbiosis in newborns, leading to aberrant behavioral changes and impaired cognitive development (Tochitani, 2021). However, the colonization and development of gut microbiota as well as the mechanism of gut microbiota colonization in neurodevelopment in early life remain unclear. Accordingly, we hypothesized that alterations in gut microbiota from mothers may be augmented in newborns via vertical transmission, leading to neuropsychological impairment. Studying the colonization and development of gut microbiota from sleep-deprived mothers to offspring will improve our understanding of early gut microbiota development and the reasons for impaired neonatal neurodevelopment.
MATERIALS AND METHODS
Animals
Female and male Sprague-Dawley rats were obtained from the Chongqing Medical University Animal Care Centre (Chongqing, China) and mated in the laboratory colony of the Children’s Hospital of Chongqing Medical University. Pregnant rats were housed individually in independent ventilated cages under a controlled temperature (23–25 °C) and 12/12 h light/dark cycle (0730h–1930h), with unlimited access to food and water. On day 9 of gestation, all animals were acclimated by gentle individual handling (5 min/cage/day) for 6 days (Vecsey et al., 2013). On day 15 of gestation, the animals were paired by weight and randomly divided into two groups, including 10 pregnant rats in the MSD group and eight pregnant rats in the normal rearing control group (C). All animal experiments were conducted in accordance with the Chongqing Science and Technology Commission guidelines and approved by the Chongqing Medical University Animal Care Committee (Approval No. CHCMU-IACUC20210114017). Every effort was made to minimize both animal suffering and number of animals used.
MSD and sample collection
MSD was performed in the third trimester of pregnancy (gestational days 15–21) by gentle handling for 6 h per day (1200h–1800h) as described previously (Peng et al., 2016; Radhakrishnan et al., 2015; Vecsey et al., 2009; Yu et al., 2018). Briefly, pregnant rats were kept awake by gently tapping on the outer surface of the cage, lightly rattling the wire cage lid, removing the cage cover to gently pat the rats as they moved freely in the cage, or rummaging through the bed of wood shavings. Maternal fecal samples (C-M: n=10; MSD-M: n=8) were collected before delivery on day 21 of gestation. The pups were separately sacrificed on postnatal day 1 (C-P1:n=11; MSD-P1: n=8), day 7 (C-P7: n=11; MSD-P7: n=8), day 14 (C-P14: n=11; MSD-P14: n=8), and day 56 (C-P56: n=11; MSD-P56: n=8). Cecum and colon contents as well as brain tissues were collected on a sterile clean bench, immediately frozen with liquid nitrogen, and stored at −80 °C for subsequent analysis (Figure 1).
Figure 1.
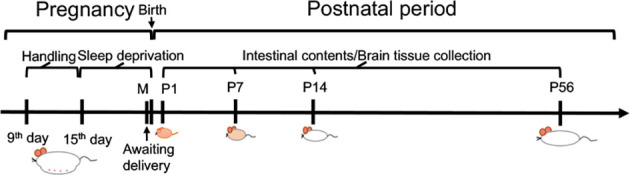
Experimental design
Pregnant rats were subjected to MSD for 6 h per day by acclimation to gentle handling in third trimester of pregnancy (gestational days 15–21). Fecal samples of mother rats (M) and intestinal contents and brain tissues of offspring were collected at different postnatal days (P1, P7, P14, and P56).
Microbiota profiling
DNA from collected samples was extracted according to the manufacturer’s instructions using the EZNA® Stool DNA Kit (Omega, USA). Total DNA was eluted in 50 μL of elution buffer and stored at −80 °C until polymerase chain reaction (PCR) using primers Bakt_341F (CCTACGGGNGGCWGCAG) and Bakt_805R (GACTACHVGGGTATCTAATCC) (Herlemann et al., 2011) obtained from LC-Bio Technology Co., Ltd (China). The PCR products were purified by AMPure XT beads (Beckman Coulter Genomics, USA) and quantified by Qubit (Invitrogen, USA). The amplicon pools were prepared for sequencing, and the size and quantity of the amplicon library were measured using an Agilent 2100 Bioanalyzer (Agilent, USA) and a Library Quantification Kit for Illumina (Kapa Biosciences, USA), respectively. The NovaSeq PE250 platform was used for high-throughput sequencing of the V3–V4 hypervariable region of the prokaryotic 16S rRNA gene.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from the offspring brain tissue samples collected at P1, P7, and P56 (n=6 per group) to determine the expression of the microglia-originated proinflammatory factors interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) using a Bioteke RNA Extraction Kit (Bioteke, Beijing, China) following the manufacturer’s protocols. RNA was subjected to reverse transcription using a Takara PrimeScriptTM RT Reagent Kit (Takara Bio, China). qRT-PCR was performed for 40 cycles (95 °C for 2 min, 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 20 s) using TB Green Premix Ex TaqTM Ⅱ (Takara Bio, China) and Bio-Rad CFX ConnectTM Real-Time System (Bio-Rad, USA). Primer sequences from the reference research included: IL-1β (forward: TGAGGCTGACAGACCCCAAAAGAT, reverse: GCTCCACGGGCAAGACATAGGTAG); IL-6 (forward: AGCCACTGCCTTCCCTACTTCA, reverse: GCCATTGCACAACTCTTTTCTCA); TNF-α (forward: TGGCGTGTTCATCCGTTCTCTACC, reverse: CCCGCAATCCAGGCCACTACTT); and β-actin (forward: GTCCACCCGCGAGTACAACCTTCT, reverse: TCCTTCTGACCCATACCCACCATC) (Roque et al., 2016). The data were normalized and analyzed using the 2–∆∆Ct method. Each sample was run in triplicate.
Enzyme-linked immunosorbent assay (ELISA)
Offspring brain tissues were collected at P1, P7, and P56 (n=5 per group). Residual blood was washed away, and the tissue was then homogenized in 1×PBS (100 mg tissue/1 mL PBS). After performing two freeze-thaw cycles to break the cell membranes, the homogenates were centrifuged at 5 000 g for 5 min at 4 °C. The supernatant was collected and the concentrations of IL-6, IL-1β, and TNF-α were measured using an ELISA Kit (Cusabio, Wuhan, China) according to the manufacturer’s instructions.
Bioinformatics and statistical analysis
The Illumina NovaSeq platform (provided by LC-Bio Technology, China) was used for sequencing following the manufacturer’s guidelines. Briefly, the resulting paired-end reads were assigned to samples based on their unique barcodes, truncated by cutting the barcodes and primer sequences, and then merged using FLASH software. High-quality clean tags were then obtained by filtering raw reads under specific conditions using fqtrim (v0.9.4), and chimeric sequences were identified and deleted using Vsearch (v2.3.4). After dereplication using DADA2, we obtained the feature table and feature sequence. Sequence alignment of species annotation was performed by BLAST with the SILVA and NT-16S databases. Alpha and beta diversities were calculated and analyzed using QIIME2, and alpha diversity indices were reduced by relevant integral multiples to present them in the same graph (Observed OTU and Chao1 indices were divided by 1 000, Shannon index was divided by 10, Simpson index was left unprocessed). Relative abundances other than the Wilcoxon test (only phylum and genus with relative abundances greater than 0.1% were included, P<0.05 for significance) were used to describe bacterial taxonomy, with the top 20 bacterial genera shown in the graphs in descending order. ELISA results were analyzed by one-way analysis of variance (ANOVA) with treatment (group) as the between-subjects factor and presented as mean±standard deviation (SD). Spearman correlation analysis was performed using IBM SPSS Statistics (v23.0), with the top 30 bacterial genera in descending order. Graphs were drawn by R package (v3.5.2), Prism (v9.2.0), and OmicStudio tools (https://www.omicstudio.cn/tool).
RESULTS
MSD increases diversity and richness of gut microbiota in mother rats
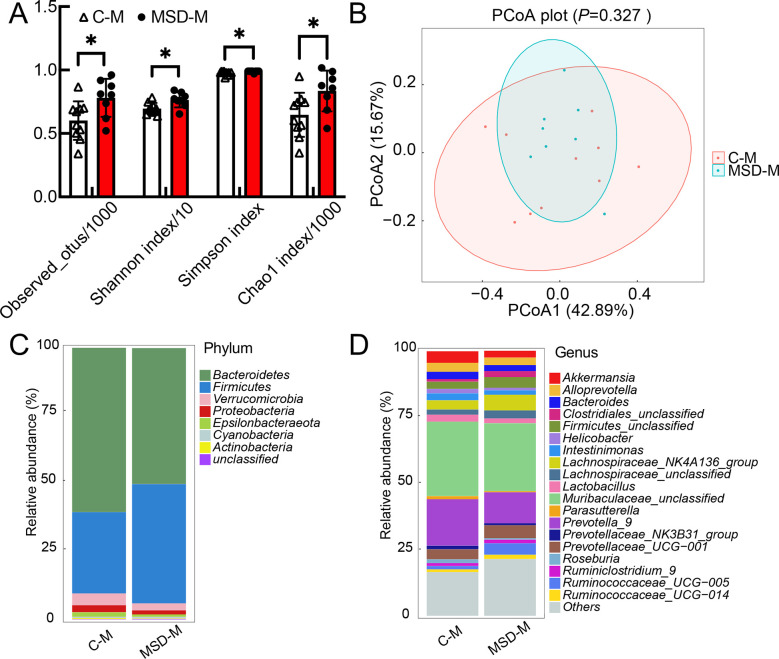
To verify whether MSD in the third trimester altered maternal gut microbiota, we collected maternal fecal samples before delivery on day 21 of gestation. The alpha diversity indices (Observed OTUs, Shannon, Simpson, and Chao1) were determined to compare microbial richness and diversity. Weighted UniFrac principal coordinate analysis (PCoA) of beta diversity was calculated to evaluate microbial community similarity. Compared with the controls (C-M), richness and diversity of gut microbiota increased significantly in the MSD mother rats (MSD-M) (Figure 2A), although microbial composition remained unchanged (Figure 2B). Further analysis revealed that Firmicutes abundance increased in the MSD-M group, while Proteobacteria abundance decreased at the phylum level (Figure 2C). Abundance of genera Ruminococcaceae_UCG-005, Ruminococcus_1, Lachnospiraceae_UCG-001, Robinsoniella, Ruminococcaceae_unclassified, Christensenellaceae_R-7_group Lachnospiraceae_UCG-008, Ruminiclostridium_6, Acetivibrio, and Clostridiales_Family_XIV_Incertae_Sedis_unclassified increased, while abundance of Escherichia-Shigella decreased in the MSD-M group (Figure 2D). These results suggest that MSD in the third trimester increases the diversity and richness of gut microbiota in mother rats, as emphasized by the abundance of bacterial phylum Firmicutes.
Figure 2.
Microbial richness and diversity increased in sleep-deprived pregnant rats
A: Alpha diversity indices increased in both richness and diversity of gut microbiota in MSD group (MSD-M). B: Weighted UniFrac PCoA showed no significant difference in microbial composition between MSD-M and control (C-M) groups. C, D: Relative abundance of top 20 microbial phyla (C) and genera (D) were analyzed to show subtle differences between MSD-M and C-M groups. All OTUs under 1% were classified as “others”. Data are expressed as means±SD, *: P<0.05.
First two weeks of early life are critical for development of gut microbiota
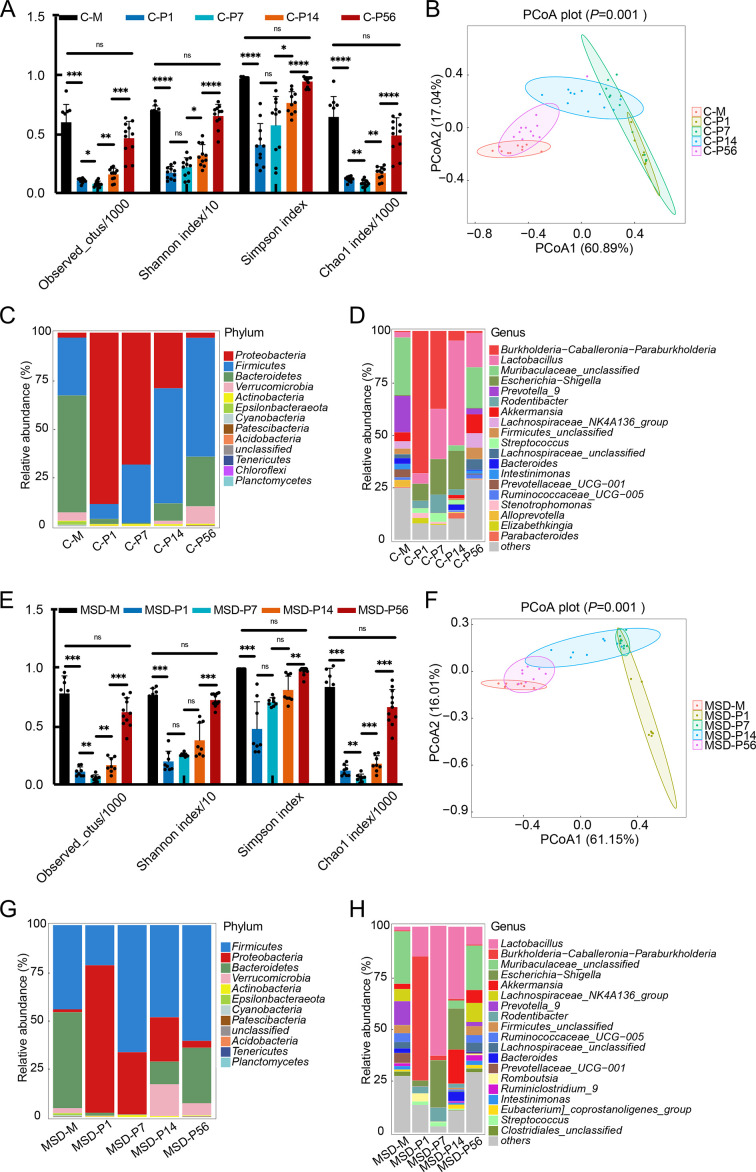
As maternal resident bacteria are a primary source for gut microbiota colonization in offspring during perinatal periods (Francis & Dominguez-Bello, 2019), we next explored the diversity and composition of gut microbiota in MSD offspring. Results showed that microbial diversity and richness steadily increased with bacterial community change in both control (Figure 3A, B) and MSD offspring (Figure 3C, D) during microbial colonization and development. Further analysis of relative abundance of gut microbiota revealed bacterial succession from dam to pup to adult offspring (Figure 3E, F). At the phylum level, Proteobacteria gradually decreased to the maternal level from P1 to P56 in both control and MSD offspring (Figure 3E, G). Firmicutes gradually increased in control offspring (Figure 3E), but not in MSD offspring (Figure 3G). At the genus level, Burkholderia-Caballeronia-Paraburkholderia gradually decreased in the control offspring (Figure 3F), whereas MSD offspring showed a sudden decline at P7 (MSD-P7) and continued low abundance (MSD-P14 and MSD-P56) (Figure 3H). Lactobacillus first increased and then declined in both groups, but with high abundance at P7 (MSD-P7) in MSD offspring (Figure 3F) and P14 (C-P14) in control offspring (Figure 3H).
Figure 3.
Microbial diversity and richness increased in both control and MSD offspring
A: Alpha diversity indices steadily increased in diversity and richness of gut microbiota in control offspring. B: Weighted UniFrac PCoA analysis showed significant differences in microbial composition at different postnatal time points in control offspring. C, D: Relative abundances of top 20 microbial phyla (C) and genera (D) were clustered to show changes from mothers to offspring in control group. E: Alpha diversity indices showed a steady increase in diversity and richness of gut microbiota in MSD offspring. F: Weighted UniFrac PCoA showed significant differences in microbial composition at different postnatal time points in MSD offspring. G, H: Relative abundances of top 20 microbial phyla (G) and genera (H) were clustered to show changes from mother rats to offspring in MSD group. Data are expressed as means±SD, *: P<0.05;**: P<0.01;***: P<0.001;****: P<0.0001.
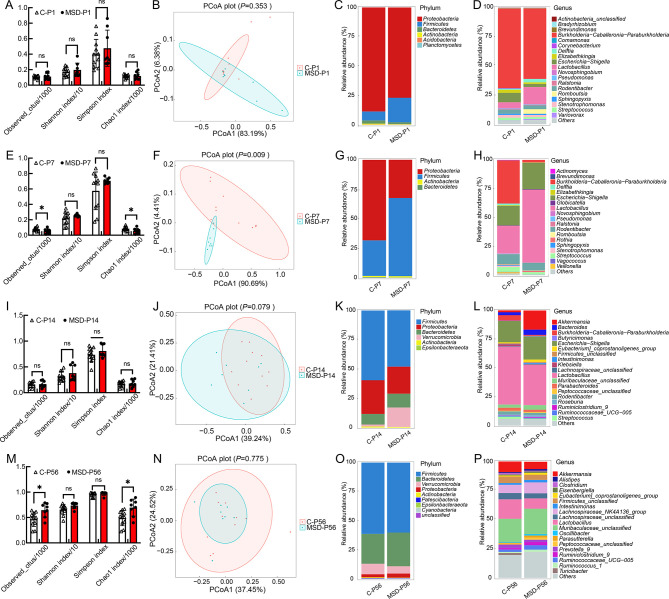
We next explored microbial changes in offspring subjected to MSD at each time point. Results showed that the gut microbiota was already complex at P1 in both the control (C-P1) and MSD (MSD-P1) pups (Figure 4A). Even though no significant difference was found between the microbial communities (Figure4B), there were still hundreds of different bacterial species and high abundance of the phylum Proteobacteria (Figure 4C) and genus Burkholderia-Caballeronia-Paraburkholderia (Figure 4D). Further analysis revealed that no significant differences were observed at the phylum level (Figure 4C), and only the abundance of the genus Romboutsia increased in the MSD-P1 group compared with the C-P1 group (Figure 4D). Notably, marked differences in the microbial community at P7 were observed between groups, with lower richness (Figure 4E) and largely different microbial composition (Figure 4F) in the MSD-P7 group compared to the C-P7 group. At the phylum level, Proteobacteria abundance decreased substantially, whereas Firmicutes abundance increased substantially in the MSD-P7 group (Figure 4G). At the genus level, Lactobacillus abundance increased, whereas Burkholderia-Caballeronia-Paraburkholderia, Sphingopyxis, Stenotrophomonas, Ralstonia, Novosphingobium, Pseudomonas, Brevundimonas, and Delftia abundance decreased in the MSD-P7 group (Figure 4H). However, there were no significant differences in microbial richness, diversity, or composition at P14 between the control (C-P14) and MSD (MSD-P14) groups (Figure 4I, J), although the abundances of the phylum Verrucomicrobia (Figure 4K) and genera Akkermansia and Marvinbryantia increased, while those of Stenotrophomonas and Veillonella decreased in the MSD-P14 group (Figure 4L). At P56, there was no difference in microbial community between the control (C-P56) and MSD (MSD-P56) groups, but the MSD-P56 group showed higher richness (Figure 4M, N). Further analysis revealed no significant differences at the phylum level between groups, with only slight increases in the Ruminococcaceae_unclassified, Fournierella, Acetatifactor, Oscillibacter, Ruminococcaceae_UCG-009, Odoribacter, and UBA1819 genera in the MSD-P56 group (Figure 4O, P).
Figure 4.
Changes in gut microbiota of MSD offspring
Alpha diversity indices showed changes in richness and diversity of microbiota at P1 (A), P7 (E), P14 (I), and P56 (M) in both control and MSD offspring. Weighted UniFrac PCoA showed microbial composition at P1 (B), P7 (F), P14 (J), and P56 (N) in both control and MSD offspring. Relative abundances of top 20 microbial phyla (C, G, K, O) and genera (D, H, L, P) were clustered to show differences between control and MSD offspring. Data are expressed as means±SD,*: P<0.05.
Taken together, these results suggest that gut microbiota colonization occurs during the first 2 weeks of early life, and the microbial community in young adult offspring is similar to that in mother rats. Although the transmission and development of gut microbiota from mothers to offspring were similar in the control and MSD offspring, subtle differences were also found, especially in the highly abundant Firmicutes, which may be related to vertical transmission of microbial dysbiosis from mother to offspring.
Bacterial phylum Firmicutes is dominant in MSD
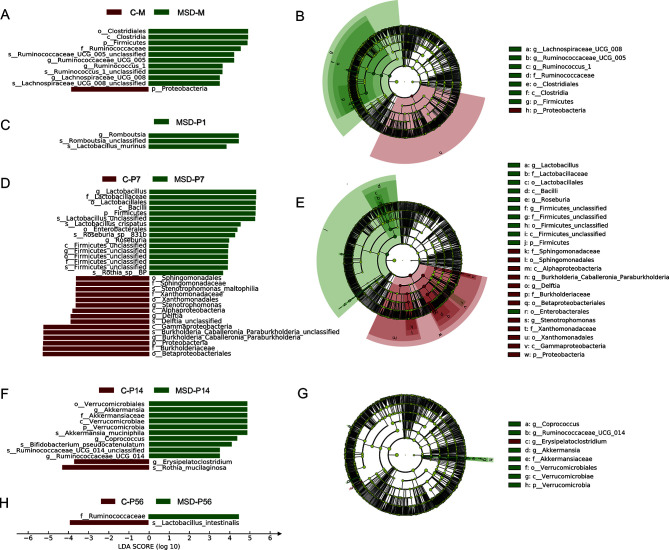
Linear discriminant analysis (LDA) effect size (LEfSe) was used to identify the differentially enriched bacterial taxa associated with MSD, which could provide information about the transmission and development of gut microbiota in offspring. Different bacteria were defined by LDA scores with a cut-off of 3.5. Results showed that MSD-M rats exhibited high levels of the phylum Firmicutes (class Clostridia, order Clostridiales, family Ruminococcaceae, family Lachnospiraceae and genus Ruminococcus), while C-M rats exhibited high levels of the phylum Proteobacteria (Figure 5A, B). At P1 and P7 (Figure 5C–E), the MSD-P1 and MSD-P7 offspring were still dominated by Firmicutes (class Firmicutes_unclassified, class Bacilli, order Lactobacillales, order Firmicutes_unclassified, family Lactobacillaceae, genus lactobacillus, genus Roseburia, and genus Romboutsia), while the control offspring were still dominated by Proteobacteria (family Sphingomonadaceae, family Xanthomonadaceae, family Burkholderiaceae, genus Delftia, genus Stenotrophomonas, and genus Burkholderia_Caballeronia_Paraburkholderia). At P14 (Figure 5F, G), the MSD offspring contained microbial biomarkers of the phylum Verrucomicrobia (class Verrucomicrobiae, order Verrucomicrobiales, family Akkermansiaceae, and genus Akkermansia), while both groups were dominated by microbial biomarkers of Actinobacteria and Firmicutes at P14 and P56 (Figure 5F–H). Thus, MSD-M rats (Figure 5A, B) and their pups, especially MSD-P7 (Figure 5D, E), were dominated by microbial biomarkers mainly found in the phylum Firmicutes. These results suggest that Firmicutes bacteria are strongly associated with MSD, and bacterial biomarkers are likely associated with physical dysfunction in the host.
Figure 5.
Dominant bacteria in MSD offspring at different postnatal time points
Linear discriminant analysis (LDA) effect size (LEfSe) showed different abundant bacterial taxa in mother rats (A) and offspring at P1 (C), P7 (D), P14 (F), and P56 (H). Different bacteria were defined by LDA scores with a cut-off of 3.5. Pie diagram summarized phylogenetic changes in mother rats (B) and their offspring at P7 (E) and P14 (G).
MSD increases neuroinflammation in offspring
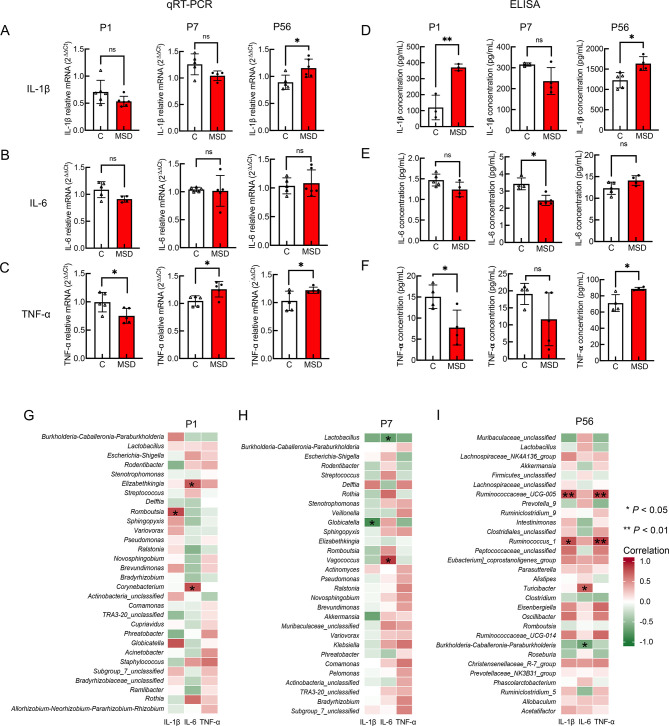
The gut microbiota exerts protective effects for the development of the host immune system (Hajela et al., 2015), and microbial dysbiosis leads to fluctuations in inflammatory cytokines in the brain (Chassaing & Gewirtz, 2014; Li et al., 2020). Thus, we performed qRT-PCR to assess the expression of proinflammatory cytokines IL-6, IL-1β, and TNF-α at P1, P7, and P56. Compared with the control offspring, the expression of IL-1β increased at P56 (P=0.020), but not at P1 (P=0.100) or P7 (P=0.087) (Figure 6A). No differences were observed in the expression of IL-6 between the two groups at the different time points (P1, P=0.064; P7, P=0.845; P56, P=0.699; Figure 6B). Furthermore, compared with the control offspring, the expression of TNF-α increased at P1 (P=0.038) and P56 (P=0.049), but decreased at P7 (P=0.023) (Figure 6C). To further confirm the qRT-PCR and ELISA results, we measured the proinflammatory cytokines in the brains of MSD offspring. Results showed that, compared with control offspring, the concentration of IL-1β increased in the MSD offspring at P1 (MSD-P1; P=0.006) and at P56 (MSD-P56; P=0.012), but not at P7 (MSD-P7; P=0.093) (Figure 6D). Furthermore, the concentration of IL-6 decreased in the MSD offspring at P7 (MSD-P7; P=0.003), but not at P1 (MSD-P1; P=0.070) or P56 (MSD-P56; P=0.077) compared with the control offspring (Figure 6E). The concentration of TNF-α increased in the MSD offspring at P1 (MSD-P1; P=0.026) and at P56 (MSD-P56; P=0.016), but not at P7 (MSD-P7; P=0.117) compared with control offspring (Figure 6F). Overall, the concentration of proinflammatory cytokines increased in the brains of young adult MSD offspring, although no significance was found at several time points, suggesting that neuroinflammation is activated in MSD offspring.
Figure 6.
MSD increased proinflammatory cytokines in brains of MSD offspring
A–C: mRNA expression of proinflammatory cytokines IL-1β (A), IL-6 (B), and TNF-α (C) in brains of MSD offspring measured by qRT-PCR. D–F: Proinflammatory cytokines IL-1β (D), IL-6 (E), and TNF-α (F) in brains measured by ELISA. G–I: Spearman heat-map showed correlations among top 30 microbial genera and proinflammatory cytokines IL-1β, IL-6, and TNF-α at P1 (G), P7 (I), and P56 (H). Data are expressed as means±SD, *: P<0.05;**: P<0.01.
To further explore the relationship between gut microbiota and neuroinflammation, Spearman correlation analysis was performed to determine the correlations between the top 30 bacterial genera and concentrations of IL-1β, IL-6, and TNF-α at each time point (Figure 6G, I). Results showed that IL-1β was positively correlated with bacterial genera in the phylum Firmicutes at P1 (genus Romboutsia) and P56 (genera Ruminococcaceae_UCG_005 and Ruminococcus_1), but negatively correlated at P7 (genus Globicatella). TNF-α was positively correlated with the genera Ruminococcaceae_UCG-005 and Ruminococcus_1 at P56. IL-6 was associated with the phylum Firmicutes, showing positive correlation with Vagococcus (P7) and Turicibacter (P56) and negative correlation with Lactobacillus (P7). These results suggest that activation of neuroinflammation is positively correlated with offspring abundance of bacterial genera in Firmicutes, which is also a biomarker associated with MSD, as shown in LEfSe analysis.
DISCUSSION
Sleep loss has become a global concern in modern society, especially during pregnancy (Hutchison et al., 2012) due to a series of complicated factors such as physiological status (Chien & Chen, 2015), psychological concerns (Jiang et al., 2021) and living conditions (Nam et al., 2018). Both pregnant women and their babies can suffer severe consequences caused by MSD (Pires et al., 2021). Evidence accumulated from recent studies suggests that MSD can cause metabolic disorders, decrease reproductive capacity, and increase cardiovascular disease risk, as well as abnormal immune response and cognitive impairment in offspring (Alvarenga et al., 2013; Argeri et al., 2016; Baratta et al., 2020; Harskamp-van Ginkel et al., 2020; Khalyfa et al., 2014; Lima et al., 2014; Peng et al., 2016; Thomal et al., 2010; Yu et al., 2018). In addition, the gut microbiota plays an important role in neuropsychological development in newborns (Tochitani, 2021), suggesting that MSD may affect the cognitive development of offspring via the gut-brain axis. In the present study, we found that both gut microbiota richness and diversity increased after MSD in mother rats (Figure 2A). These results are contradictory to findings in nonpregnant SD animals (Gao et al., 2019; Maki et al., 2020). These discrepancies between previous studies and our work need to be resolved but may be at least partially explained by pregnancy, which impacts hormonal changes and metabolic patterns (Qi et al., 2021). Indeed, pregnancy is associated with profound alterations in the gut microbiota (Crusell et al., 2018; Grech et al., 2021), particularly in the third trimester, which may lead to metabolic, immunological, and hormonal changes necessary to support a healthy pregnancy and fetal growth (Koren et al., 2012). These changes in hormone levels, such as estrogen and progesterone, may also affect the microbial community, accompanied by unique inflammatory and immunological changes (García-Gómez et al., 2013; Nuriel-Ohayon et al., 2019).
Accumulating evidence indicates that the gut microbiota provides protective effects for the development of the host immune system (Hajela et al., 2015), and microbial dysbiosis can lead to fluctuations in inflammatory cytokines in the brain (Chassaing & Gewirtz, 2014; Li et al., 2020). Studies have revealed that fecal transplantation with healthy gut microbiota can alleviate symptoms caused by stress and reduce aberrant inflammatory responses (Rao et al., 2021). Although disrupted sleep is also associated with an increase in proinflammatory biomarkers (Okun & Coussons-Read, 2007) and abundance of the phylum Firmicutes (Benedict et al., 2016; Poroyko et al., 2016), the relationship between MSD and gut microbiota development in offspring is unknown. We found that bacteria in the phylum Firmicutes (such as classClostridia, order Clostridiales, family Ruminococcaceae, family Lachnospiraceae, and family Lactobacillaceae) were highly abundant in the MSD mothers and their offspring. With the increase in Firmicutes, the Firmicutes to Bacteroidetes (FB) ratio increased in the MSD group, especially in MSD-P7. An increase in the FB ratio is associated with abnormal lipid metabolism and higher body mass index (John & Mullin, 2016; Koliada et al., 2017). Combined with previous study on the increase in the FB ratio in young adults with insufficient sleep (Benedict et al., 2016), we speculate that MSD-induced microbial dysbiosis in offspring may lead to changes in metabolism and immune response through microbiota-host interactions, thereby affecting physiological functions. Indeed, we revealed a significantly altered microbial community in MSD-P7 offspring, which may be related to the transfer of microbial dysbiosis from MSD mothers to their offspring.
Dysregulation of microglial activation may also contribute to MSD-induced neuroinflammation and cognitive impairment in young offspring (Zhao et al., 2014, 2015). However, how MSD causes such dysregulation in offspring is not clear. In the present study, we found that microbial dysbiosis in MSD mothers may be passed to their offspring, which may disrupt interactions between the microbiota and microglia through the gut-brain axis, subsequently leading to aberrant neuroinflammation. IL-6, IL-1β, and TNF-α are proinflammatory biomarkers of microglia, which play critical roles in regulating brain homeostasis and maturation of neural circuits. Thus, we performed qRT-PCR and ELISA to evaluate the state of neuroinflammation in MSD offspring. Consistent with previous research (Zhao et al., 2015), we found that MSD increased the expression and concentration of IL-1β and TNF-α in young adult offspring (MSD-P56). Interestingly, low concentrations of IL-6, IL-1β, and TNF-α were observed in early life (P1 and P7) of the MSD group (Figure 6D, E). One possible explanation is that the immature immune system was incapable of generating enough proinflammatory cytokines due to microbial dysbiosis in early life (MSD-P1 and MSD-P7) as gut microbiota homeostasis is very important for the development and maturation of the host immune system and immune regulation (Goyal et al., 2021; Yoo et al., 2020; Zhao & Elson, 2018). In addition, some proinflammatory cytokines may be obtained from the mother through the blood-placental barrier during the prenatal period (Fricke et al., 2018). For example, a higher concentration of IL-1β was observed although IL-1β mRNA expression remained unchanged in the MSD group at P1. In addition, Spearman correlation analysis revealed that the phylum Firmicutes played an important role in neuroinflammation in the MSD offspring (Figure 6G–I), indicating a close relationship between proinflammatory cytokines and gut microbiota.
In summary, MSD increased microbial richness and diversity in mother rats, with high abundance of bacteria in the phylum Firmicutes, and induced microbial community disturbance and neuroinflammation in offspring. Combined with recent research (Peng et al., 2016; Yu et al., 2018; Zhao et al., 2014, 2015), these findings suggest that MSD may cause cognitive disability and aberrant inflammatory response in offspring by disrupting gut microbiota development and the gut-brain axis. Although our findings provide potential interventions for the clinical treatment of cognitive dysfunction in MSD offspring, we only detected a correlation between gut microbiota and neuroinflammation. Thus, further studies, such as fecal transplantation and metabolic profiling, should be conducted to determine the underlying mechanisms of these neurophysiological and behavioral phenotypes in offspring subjected to MSD.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Z.Y.Y. and Z.F.D. conceived the study and wrote the manuscript. Z.Y.Y. performed MSD. Z.Y.Y., X.H.L., and Q.X. collected the intestinal contents and brain tissues. Z.Y.Y. and L.Z. performed biochemical assay. Z.Y.Y., W.T.H., and D.X.L. analyzed the data. All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We are grateful to other Dong laboratory members for technical support and helpful suggestions.
Funding Statement
This work was supported by the National Natural Science Foundation of China (82071395, 91749116), Natural Science Foundation of Chongqing (cstc2021ycjh-bgzxm0186, cstc2020jcyj-zdxmX0004), Science and Technology Research Program of Chongqing Municipal Education Commission (KJZD-K201900403), and Innovation Research Group at Institutions of Higher Education in Chongqing (CXQTP19034)
References
- 1.Alvarenga TA, Aguiar MFP, Mazaro-Costa R, Tufik S, Andersen ML Effects of sleep deprivation during pregnancy on the reproductive capability of the offspring. Fertility and Sterility. 2013;100(6):1752–1757. doi: 10.1016/j.fertnstert.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Argeri R, Nishi EE, Volpini RA, Palma BD, Tufik S, Gomes GN Sleep restriction during pregnancy and its effects on blood pressure and renal function among female offspring. Physiological Reports. 2016;4(16):e12888. doi: 10.14814/phy2.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aswathy BS, Kumar VM, Gulia KK The effects of rapid eye movement sleep deprivation during late pregnancy on newborns' sleep. Journal of Sleep Research. 2018;27(2):197–205. doi: 10.1111/jsr.12564. [DOI] [PubMed] [Google Scholar]
- 4.Baratta AM, Kanyuch NR, Cole CA, Valafar H, Deslauriers J, Pocivavsek A Acute sleep deprivation during pregnancy in rats: rapid elevation of placental and fetal inflammation and kynurenic acid. Neurobiology of Stress. 2020;12:100204. doi: 10.1016/j.ynstr.2019.100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benedict C, Vogel H, Jonas W, Woting A, Blaut M, Schürmann A, et al Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Molecular Metabolism. 2016;5(12):1175–1186. doi: 10.1016/j.molmet.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chassaing B, Gewirtz AT Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicologic Pathology. 2014;42(1):49–53. doi: 10.1177/0192623313508481. [DOI] [PubMed] [Google Scholar]
- 7.Chien MY, Chen HC Poor sleep quality is independently associated with physical disability in older adults. Journal of Clinical Sleep Medicine. 2015;11(3):225–232. doi: 10.5664/jcsm.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crusell MKW, Hansen TH, Nielsen T, Allin KH, Rühlemann MC, Damm P, et al Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome. 2018;6(1):89. doi: 10.1186/s40168-018-0472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du M, Liu J, Han N, Zhao ZL, Yang J, Xu XR, et al Maternal sleep quality during early pregnancy, risk factors and its impact on pregnancy outcomes: a prospective cohort study. Sleep Medicine. 2021;79:11–18. doi: 10.1016/j.sleep.2020.12.040. [DOI] [PubMed] [Google Scholar]
- 10.El Aidy S, Bolsius YG, Raven F, Havekes R A brief period of sleep deprivation leads to subtle changes in mouse gut microbiota. Journal of Sleep Research. 2020;29(6):e12920. doi: 10.1111/jsr.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis AP, Dominguez-Bello MG Early-life microbiota perturbations and behavioral effects. Trends in Microbiology. 2019;27(7):567–569. doi: 10.1016/j.tim.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Fricke EM, Elgin TG, Gong HY, Reese J, Gibson-Corley KN, Weiss RM, et al Lipopolysaccharide-induced maternal inflammation induces direct placental injury without alteration in placental blood flow and induces a secondary fetal intestinal injury that persists into adulthood. American Journal of Reproductive Immunology. 2018;79(5):e12816. doi: 10.1111/aji.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao T, Wang ZX, Dong YL, Cao J, Lin RT, Wang XT, et al Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. Journal of Pineal Research. 2019;67(1):e12574. doi: 10.1111/jpi.12574. [DOI] [PubMed] [Google Scholar]
- 14.García-Gómez E, González-Pedrajo B, Camacho-Arroyo I Role of sex steroid hormones in bacterial-host interactions. BioMed Research International. 2013;2013:928290. doi: 10.1155/2013/928290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyal D, Ali SA, Singh RK Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer's disease. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2021;106:110112. doi: 10.1016/j.pnpbp.2020.110112. [DOI] [PubMed] [Google Scholar]
- 16.Grech A, Collins CE, Holmes A, Lal R, Duncanson K, Taylor R, et al Maternal exposures and the infant gut microbiome: a systematic review with meta-analysis. Gut Microbes. 2021;13(1):1897210. doi: 10.1080/19490976.2021.1897210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajela N, Ramakrishna BS, Nair GB, Abraham P, Gopalan S, Ganguly NK Gut microbiome, gut function, and probiotics: implications for health. Indian Journal of Gastroenterology. 2015;34(2):93–107. doi: 10.1007/s12664-015-0547-6. [DOI] [PubMed] [Google Scholar]
- 18.Harskamp-van Ginkel MW, Ierodiakonou D, Margetaki K, Vafeiadi M, Karachaliou M, Kogevinas M, et al Gestational sleep deprivation is associated with higher offspring body mass index and blood pressure. Sleep. 2020;43(12):zsaa110. doi: 10.1093/sleep/zsaa110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herlemann DP, Labrenz M, Jürgens K, Bertilsson S, Waniek JJ, Andersson AF Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. The ISME Journal. 2011;5(10):1571–1579. doi: 10.1038/ismej.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchison BL, Stone PR, Mccowan LM, Stewart AW, Thompson JM, Mitchell EA A postal survey of maternal sleep in late pregnancy. BMC Pregnancy and Childbirth. 2012;12:144. doi: 10.1186/1471-2393-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Y, Li PX, Zhong L, Liu BY, Gao XY, Ning L, et al The influence of changes in work stressors and coping resources on sleep disturbances: evidence from the OHSPIW cohort study. Sleep. 2021;44(8):zsab039. doi: 10.1093/sleep/zsab039. [DOI] [PubMed] [Google Scholar]
- 22.John GK, Mullin GE The gut microbiome and obesity. Current Oncology Reports. 2016;18(7):45. doi: 10.1007/s11912-016-0528-7. [DOI] [PubMed] [Google Scholar]
- 23.Khalyfa A, Mutskov V, Carreras A, Khalyfa AA, Hakim F, Gozal D Sleep fragmentation during late gestation induces metabolic perturbations and epigenetic changes in adiponectin gene expression in male adult offspring mice. Diabetes. 2014;63(10):3230–3241. doi: 10.2337/db14-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, et al Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population . BMC Microbiology. 2017;17(1):120. doi: 10.1186/s12866-017-1027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, et al Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150(3):470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li DT, Wang P, Wang PP, Hu XS, Chen F The gut microbiota: a treasure for human health. Biotechnology Advances. 2016;34(7):1210–1224. doi: 10.1016/j.biotechadv.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Li YY, Zhang B, Zhou Y, Wang DM, Liu XC, Li L, et al Gut microbiota changes and their relationship with inflammation in patients with acute and chronic insomnia. Nature and Science of Sleep. 2020;12:895–905. doi: 10.2147/NSS.S271927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lima ILB, Rodrigues AFAC, Bergamaschi CT, Campos RR, Hirata AE, Tufik S, et al Chronic sleep restriction during pregnancy-repercussion on cardiovascular and renal functioning of male offspring. PLoS One. 2014;9(11):e113075. doi: 10.1371/journal.pone.0113075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luczynski P, McVey Neufeld KA, Oriach CS, Clarke G, Dinan TG, Cryan JF Growing up in a bubble: using germ-free animals to assess the influence of the gut microbiota on brain and behavior. International Journal of Neuropsychopharmacology. 2016;19(8):pyw020. doi: 10.1093/ijnp/pyw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maki KA, Burke LA, Calik MW, Watanabe-Chailland M, Sweeney D, Romick-Rosendale LE, et al Sleep fragmentation increases blood pressure and is associated with alterations in the gut microbiome and fecal metabolome in rats. Physiological Genomics. 2020;52(7):280–292. doi: 10.1152/physiolgenomics.00039.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matenchuk BA, Mandhane PJ, Kozyrskyj AL Sleep, circadian rhythm, and gut microbiota. Sleep Medicine Reviews. 2020;53:101340. doi: 10.1016/j.smrv.2020.101340. [DOI] [PubMed] [Google Scholar]
- 32.Mindell JA, Cook RA, Nikolovski J Sleep patterns and sleep disturbances across pregnancy. Sleep Medicine. 2015;16(4):483–488. doi: 10.1016/j.sleep.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Nam S, Whittemore R, Jung S, Latkin C, Kershaw T, Redeker NS Physical neighborhood and social environment, beliefs about sleep, sleep hygiene behaviors, and sleep quality among African Americans. Sleep Health. 2018;4(3):258–264. doi: 10.1016/j.sleh.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nuriel-Ohayon M, Neuman H, Ziv O, Belogolovski A, Barsheshet Y, Bloch N, et al Progesterone increases Bifidobacterium relative abundance during late pregnancy . Cell Reports. 2019;27(3):730–736.e3. doi: 10.1016/j.celrep.2019.03.075. [DOI] [PubMed] [Google Scholar]
- 35.Okun ML, Coussons-Read ME Sleep disruption during pregnancy: how does it influence serum cytokines? Journal of Reproductive Immunology. 2007;73(2):158–165. doi: 10.1016/j.jri.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Peng Y, Wang W, Tan T, He WT, Dong ZF, Wang YT, et al Maternal sleep deprivation at different stages of pregnancy impairs the emotional and cognitive functions, and suppresses hippocampal long-term potentiation in the offspring rats. Molecular Brain. 2016;9:17. doi: 10.1186/s13041-016-0197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pien GW, Schwab RJ Sleep disorders during pregnancy. Sleep. 2004;27(7):1405–1417. doi: 10.1093/sleep/27.7.1405. [DOI] [PubMed] [Google Scholar]
- 38.Pires GN, Benedetto L, Cortese R, Gozal D, Gulia KK, Kumar VM, et al Effects of sleep modulation during pregnancy in the mother and offspring: evidences from preclinical research. Journal of Sleep Research. 2021;30(3):e13135. doi: 10.1111/jsr.13135. [DOI] [PubMed] [Google Scholar]
- 39.Poroyko VA, Carreras A, Khalyfa A, Khalyfa AA, Leone V, Peris E, et al Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in Mice. Scientific Reports. 2016;6:35405. doi: 10.1038/srep35405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qi XY, Yun CY, Pang YL, Qiao J The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes. 2021;13(1):1894070. doi: 10.1080/19490976.2021.1894070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radhakrishnan A, Aswathy BS, Kumar VM, Gulia KK Sleep deprivation during late pregnancy produces hyperactivity and increased risk-taking behavior in offspring. Brain Research. 2015;1596:88–98. doi: 10.1016/j.brainres.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 42.Rao JJ, Qiao Y, Xie RN, Lin L, Jiang J, Wang CM, et al Fecal microbiota transplantation ameliorates stress-induced depression-like behaviors associated with the inhibition of glial and NLRP3 inflammasome in rat brain. Journal of Psychiatric Research. 2021;137:147–157. doi: 10.1016/j.jpsychires.2021.02.057. [DOI] [PubMed] [Google Scholar]
- 43.Roque A, Ochoa-Zarzosa A, Torner L Maternal separation activates microglial cells and induces an inflammatory response in the hippocampus of male rat pups, independently of hypothalamic and peripheral cytokine levels. Brain, Behavior, and Immunity. 2016;55:39–48. doi: 10.1016/j.bbi.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 44.Thomal JT, Palma BD, Ponzio BF, do Carmo Pinho Franco M, Zaladek-Gil F, Fortes ZB, et al Sleep restriction during pregnancy: hypertension and renal abnormalities in young offspring rats. Sleep. 2010;33(10):1357–1362. doi: 10.1093/sleep/33.10.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tochitani S Vertical transmission of gut microbiota: points of action of environmental factors influencing brain development. Neuroscience Research. 2021;168:83–94. doi: 10.1016/j.neures.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, et al Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461(7267):1122–1125. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vecsey CG, Wimmer MEJ, Havekes R, Park AJ, Perron IJ, Meerlo P, et al Daily acclimation handling does not affect hippocampal long-term potentiation or cause chronic sleep deprivation in Mice. Sleep. 2013;36(4):601–607. doi: 10.5665/sleep.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang HX, Wang YP Gut microbiota-brain axis. Chinese Medical Journal. 2016;129(19):2373–2380. doi: 10.4103/0366-6999.190667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Chen WH, Li SX, He ZM, Zhu WL, Ji YB, et al Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Molecular Psychiatry. 2021;26(11):6277–6292. doi: 10.1038/s41380-021-01113-1. [DOI] [PubMed] [Google Scholar]
- 50.Yoo JY, Groer M, Dutra SVO, Sarkar A, Mcskimming DI Gut microbiota and immune system interactions. Microorganisms. 2020;8(10):1587. doi: 10.3390/microorganisms8101587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu YZ, Huang ZL, Dai CF, Du YH, Han HL, Wang YT, et al Facilitated AMPAR endocytosis causally contributes to the maternal sleep deprivation-induced impairments of synaptic plasticity and cognition in the offspring rats. Neuropharmacology. 2018;133:155–162. doi: 10.1016/j.neuropharm.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Q, Elson CO Adaptive immune education by gut microbiota antigens. Immunology. 2018;154(1):28–37. doi: 10.1111/imm.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao QY, Peng C, Wu XH, Chen YB, Wang C, You ZL Maternal sleep deprivation inhibits hippocampal neurogenesis associated with inflammatory response in young offspring rats. Neurobiology of Disease. 2014;68:57–65. doi: 10.1016/j.nbd.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 54.Zhao QY, Xie XF, Fan YH, Zhang JQ, Jiang W, Wu XH, et al Phenotypic dysregulation of microglial activation in young offspring rats with maternal sleep deprivation-induced cognitive impairment. Scientific Reports. 2015;5:9513. doi: 10.1038/srep09513. [DOI] [PMC free article] [PubMed] [Google Scholar]