Abstract
Introduction
The post-COVID condition has become a social concern. Although the patient characteristics associated with the development of this condition are partially known, those associated with its persistence have not been identified.
Methods
We conducted a cross-sectional questionnaire-based survey among patients who had recovered from COVID-19 and visited the National Center for Global Health and Medicine between February 2021 and March 2021. Demographic and clinical data, and data regarding the presence and duration of post-COVID conditions were obtained. We identified factors associated with the development and persistence of post-COVID conditions using multivariate logistic and linear regression analyses, respectively.
Results
We analyzed 457 of 526 responses (response rate, 86.9%). The median patient age was 47 years. Of these, 378 patients (84.4%) had mild disease in the acute phase. The number of patients with symptoms at 6 and 12 months after onset or diagnosis was 120 (26.3%) and 40 (8.8%), respectively. Women were at risk of developing fatigue (odds ratio [OR]: 2.03, 95% confidence interval [CI]: 1.31–3.14), dysosmia (OR: 1.91, 95%CI: 1.24–2.93), dysgeusia (OR: 1.56, 95%CI: 1.02–2.39), hair loss (OR: 3.00, 95%CI: 1.77–5.09), and persistence of any symptoms (coefficient: 38.0, 95%CI: 13.3–62.8). Younger age and low body mass index were factors for developing dysosmia (OR: 0.96, 95%CI: 0.94–0.98 and OR: 0.94, 95%CI: 0.89–0.99, respectively) and dysgeusia (OR: 0.98, 95%CI: 0.96–1.00 and OR: 0.93, 95%CI: 0.88–0.98, respectively).
Conclusion
We identified factors involved in the development and persistence of post-COVID conditions. Many patients, even those with mild conditions, experience long-term residual symptoms.
Keywords: COVID-19, Late-onset symptom, On-going symptom, Persistence, Post-COVID condition, Questionnaire-based survey
Abbreviations
- BMI
body mass index
- CI
confidence interval
- COVID-19
Coronavirus disease
- ECMO
extracorporeal membrane oxygenation
- IQR
interquartile range
- NCGM
National Center for Global Health and Medicine
- OR
odds ratio
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
1. Introduction
Coronavirus disease (COVID-19) has become a global pandemic, with more than 440 million infections and more than 6 million deaths worldwide as of March 1, 2022 [1]. After the onset of COVID-19, 76% of patients have prolonged symptoms, lasting more than 6 months [2]. These post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are known as the post-COVID conditions. Previous studies have shown that the frequent symptoms of the post-COVID conditions include fatigue, dyspnea, and dysosmia, and multiple symptoms often overlap and persist [[2], [3], [4]]. Some symptoms of COVID-19, such as hair loss, which are not present at the time of onset, may appear after recovery from the acute condition [4]. Additionally, 5–10% of patients continue to experience moderate-to-severe problems at work and in social and family life even at 8 months after the onset of COVID-19 [5]. The impact of the post-COVID conditions on society is immeasurable. It is important to identify factors involved in the development and persistence of the post-COVID conditions; however, the characteristics of patients who are likely to develop post-COVID conditions are only partially understood [2].
In this study, we explored the factors involved in the development and persistence of post-COVID conditions in a cohort of patients recovering from COVID-19 at a hospital designated for infectious diseases in Japan.
2. Material and methods
This study was designed as a single-center, cross-sectional survey in which a self-reporting, paper-based questionnaire was mailed to eligible patients in April 2021, with two reminders, one after 2 weeks and the other 1 month later. Participation in this survey was voluntary, but not anonymous. Participants who had recovered from COVID-19 were requested to complete and return the questionnaire.
Informed consent was obtained by marking a consent checkbox on the questionnaire. This study was reviewed and approved by the ethics committee of the Center Hospital of the National Center for Global Health and Medicine (NCGM) (NCGM-G-004121-00).
2.1. Participants
The participants recruited in this study were patients who had recovered from COVID-19 and who visited the outpatient service of the Disease Control and Prevention Center in the NCGM from February 2020 to March 2021 to undergo a pre-donation screening test for COVID-19 convalescent plasmapheresis [6]. Most of the participants had received acute-phase treatment for COVID-19 in other hospitals and all participants in this study were Japanese since the screening test was designed only for Japanese patients.
2.2. Questionnaire
We developed the questionnaire through a literature review with reference to previous similar studies [2,3,[7], [8], [9], [10], [11]], findings from our previous study on prolonged and late-onset symptoms of COVID-19 [4], and comprehensive discussions among the authors. We attempted to minimize the number of questions required in order to maximize the response rate. Thirteen patients who had recovered from COVID-19 were included in the pilot study. They provided feedback on the content, clarity, and format of the items, and on whether survey questions were self-explanatory. Minor revisions were made in response to their feedback and the data of these patients were then excluded from the analysis.
2.3. Items investigated
Patient characteristics, information regarding the acute phase of COVID-19, and presence and duration of symptoms related to COVID-19 were investigated (Supplementary material). Disease severity was categorized as follows, according to previous literature [2,12]: 1) mild, no oxygen therapy; 2) moderate, oxygen therapy without mechanical ventilation; 3) severe, mechanical ventilation with or without ECMO. The symptoms related to COVID-19 included fever, fatigue, shortness of breath, joint pain, myalgia, chest pain, cough, abdominal pain, dysosmia (including anosmia), dysgeusia (including ageusia), runny nose, conjunctivitis, headache, sputum, sore throat, diarrhea, nausea, loss of appetite, hair loss, depressed mood, loss of concentration, and memory disturbance.
This information was obtained using a paper-based questionnaire, as it was difficult to obtain this information from the medical records, given that many participants in this study had been treated for the acute phase of COVID-19 at other hospitals.
2.4. Statistical analysis
The patient characteristics, presence of pneumonia, disease severity, and treatment in the acute phase of COVID-19 were expressed as median and interquartile range (IQR) for continuous variables and as absolute values (n) along with % for categorical variables. The proportion of patients with prolonged symptoms and days since the onset of COVID-19 has been previously described.
Multivariate logistic regression analyses were performed to calculate adjusted odds ratios (ORs) with 95% confidence intervals (CIs) for the development of ongoing/chronic symptoms and late-onset symptoms. The multivariate logistic regression model was derived with adjustments for all potential confounding factors within the participants' characteristics, the presence of pneumonia, disease severity, and treatment in the acute phase of COVID-19 (i.e., age, sex, body mass index [BMI], smoking, high-risk comorbidity, presence of pneumonia, severity, use of antiviral drugs, and use of steroids) according to clinical implications and previous literature [2,12]. Age and BMI were managed as continuous quantitative variables, whereas the other variables were managed as categorical variables.
Subsequently, linear regression analyses were performed to identify the factors associated with the persistence of ongoing/chronic symptoms and late-onset symptoms among patients who developed these symptoms. The dependent variable was the duration of each symptom (days). We included participants' characteristics, presence of pneumonia, disease severity, and treatment in the acute phase of COVID-19 (age, sex, BMI, smoking, high-risk comorbidity, presence of pneumonia, severity, use of antiviral drugs, and use of steroids) as independent variables, according to clinical implications and previous literature [2,12]. Age and BMI were managed as continuous quantitative variables, whereas the other variables were managed as categorical variables.
The level of significance for all statistical tests was set at α = 0.05. Data were analyzed using Stata BE 17.0 (StataCorp, College Station, TX, USA) and R, version 4.0.5 (R Foundation for Statistical Computing; 2018, Vienna, Austria).
3. Results
A self-reporting questionnaire was mailed to 526 patients who had recovered from COVID-19, and 457 responses were obtained (response rate, 86.9%). The demographic and clinical characteristics of the participants are summarized in Table 1 . The median patient age was 47 years, and 50.5% of the patients were women. All participants were Japanese. A total of 245 patients (53.7%) had no underlying medical condition. Overall, 173 patients (40.5%) developed pneumonia. In terms of disease severity, 378 (84.4%), 57 (12.7%), and 13 (2.9%) patients had mild, moderate, and severe disease, respectively. The median number of days from symptom onset or diagnosis of COVID-19 to interview was 248.5 (148.0–363.0) days.
Table 1.
Demographic and clinical characteristics of the participants (n = 457).
| Characteristics | Value |
|---|---|
| Age, median (IQR), years | 47.00 (39.00, 55.00) |
| Female sex, No. (%) | 231 (50.5) |
| Body mass indexa, median (IQR) (2 missing) | 23.3 (21.05, 26.12) |
| Ethnicity, No. (%) | |
| Japanese |
457 (100) |
| Smoking history, No. (%) (1 missing) | |
| Yes | 168 (36.8) |
| Alcohol use, No. (%) (14 missing) | |
| Yes | 376 (82.3) |
| Individual comorbidities, No. (%) | |
| Hypertension | 66 (14.4) |
| Dyslipidemia | 51 (11.2) |
| Diabetes | 28 (6.1) |
| Connective tissue disease | 1 (0.2) |
| COPD | 3 (0.7) |
| Bronchial asthma | 59 (12.9) |
| Myocardial infarction | 0 (0) |
| Solid tumor | 6 (1.3) |
| Immunodeficiency | 0 (0) |
| Chronic kidney disease | 1 (0.2) |
| Others |
82 (17.9) |
| Number of comorbidities (%) | |
| 0 | 245 (53.7) |
| 1 | 148 (32.4) |
| 2 | 49 (10.7) |
| ≧3 | 15 (3.3) |
| High risk comorbiditiesb (%) | |
| Yes | 35 (7.7) |
| History of pregnancy, No. (%) (228 missing) | |
| Yes |
105 (45.9) |
| Acute COVID-19 characteristics, No. (%) | |
| Pneumonia diagnosed (30 missing) | 173 (40.5) |
| Highest severity during clinical course of COVID-19 (9 missing) | |
| Mild | 378 (84.4) |
| Moderate | 57 (12.7) |
| Severe | 13 (2.9) |
| Pharmacological treatments | |
| Antiviral (21 missing) | 84 (19.3) |
| Corticosteroids (47 missing) |
53 (12.9) |
| Timing of the interview (13 missing) | |
| Days since symptom onset or diagnosis of COVID-19, median (IQR) 248.5 (147.5, 358.25) | |
Abbreviations: COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease; ECMO, extracorporeal membrane oxygenation; IMV, invasive mechanical ventilation; IQR, interquartile range.
Calculated as weight in kilograms divided by height in meters squared.
Including history of malignancy, diabetes, chronic kidney disease, COPD [34].
3.1. Classification of symptoms associated with COVID-19
The number of participants experiencing symptoms associated with COVID-19, the number of participants with symptoms lasting for >4 weeks and >12 weeks, and the number of participants whose symptoms developed >4 weeks after the onset of COVID-19 are summarized in Supplementary table. The most common symptoms associated with acute COVID-19 were fever (293 participants, 64.1%), fatigue (292 participants, 64.0%), dysosmia (219 participants, 47.9%), cough (214 participants, 46.8%), and dysgeusia (185 participants, 40.6%). On the other hand, the most common symptom lasting for >4 weeks was dysosmia (104 participants, 22.8%), followed by loss of concentration (95 participants, 20.8%) and fatigue (93 participants, 20.4%). The most common symptom that developed >4 weeks after the onset of COVID-19 was hair loss (58 participants), followed by memory disturbance (17 participants), and depressed mood (16 participants).
We classified 17 post-COVID conditions into the following three categories after excluding less frequent symptoms reported in <100 participants (chest pain, abdominal pain, runny nose, conjunctivitis, and nausea): 1) acute symptoms (e.g., fever, headache, loss of appetite, joint pain, sore throat, myalgia, diarrhea, and sputum) that persisted for 4 weeks in <10% of the participants; 2) ongoing/chronic symptoms (e.g., fatigue, dysosmia, cough, dysgeusia, shortness of breath) that persisted for 4 weeks in >10% of the participants, except for late-onset symptoms; and 3) late-onset symptoms (e.g., loss of concentration, depressed mood, hair loss, and memory disturbance) that developed >4 weeks after the onset of COVID-19, in >5% of the participants.
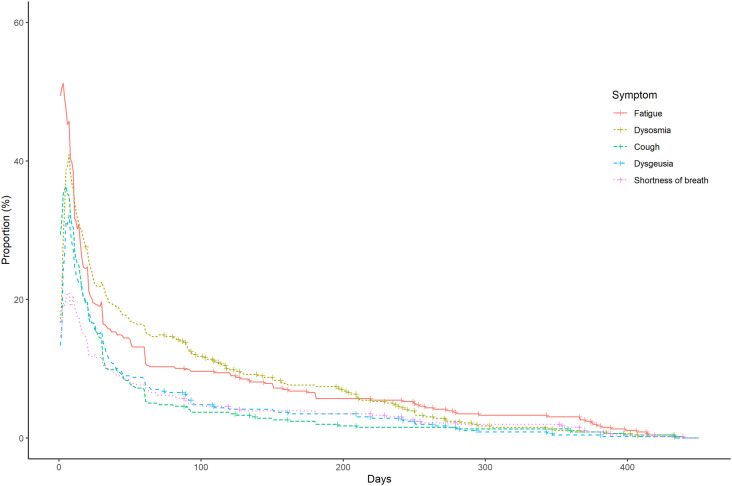
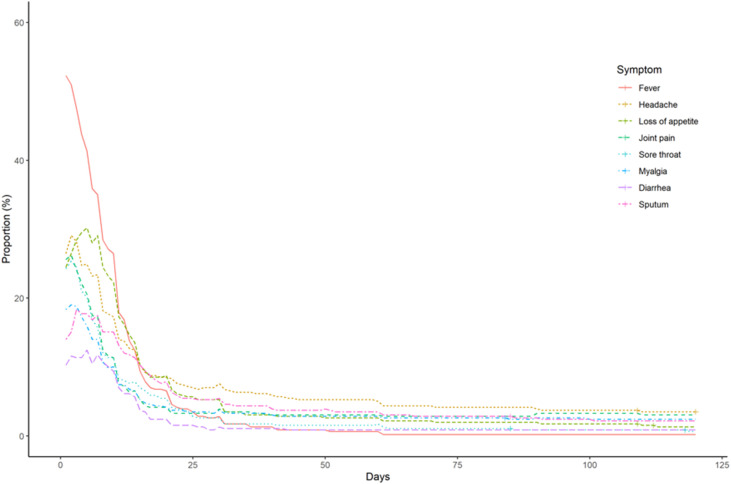
The frequency and duration of acute, ongoing/chronic, and late-onset symptoms are summarized in Supplementary Fig. 1 and Fig. 1, Fig. 2 , respectively. A few participants reported ongoing/chronic symptoms, including dysosmia (n = 35, 7.7%), fatigue (n = 30, 6.6%), shortness of breath (n = 18, 3.9%), dysgeusia (n = 16, 3.5%), and cough (n = 11, 2.4%) at 6 months after symptom onset or diagnosis of COVID-19, and fatigue (n = 14, 3.1%), shortness of breath (n = 7, 1.5%), dysosmia (n = 5, 1.1%), cough (n = 5, 1.1%), and dysgeusia (n = 2, 0.4%) at 12 months after symptom onset or diagnosis of COVID-19.
Fig. 1.
Ongoing/chronic symptoms associated with COVID-19.
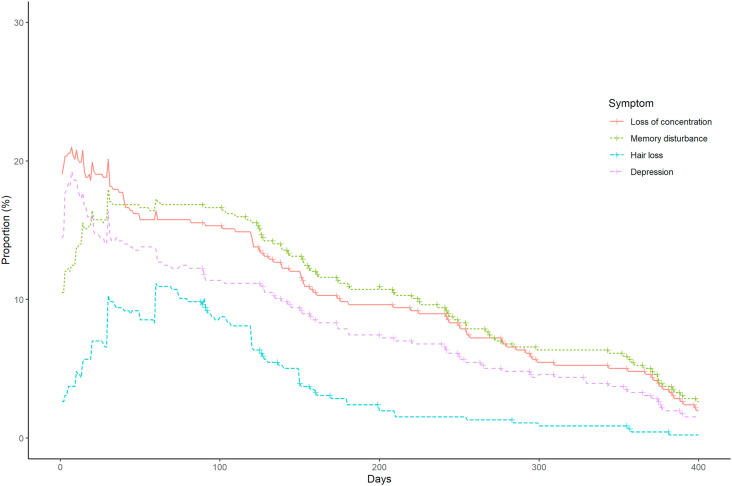
Fig. 2.
Late-onset symptoms associated with COVID-19.
The participant with the longest duration of ongoing/chronic symptoms was a 43-year-old woman who experienced shortness of breath, cough, and fatigue for 439 days. Some participants reported late-onset symptoms, including memory disturbance (n = 52, 11.4%), loss of concentration (n = 45, 9.8%), depressed mood (n = 37, 8.1%), and hair loss (n = 14, 3.1%) at 6 months after symptom onset or diagnosis of COVID-19, and memory disturbance (n = 25, 5.5%), loss of concentration (n = 22, 4.8%), depressed mood (n = 15, 3.3%), and hair loss (n = 2, 0.4%) at 12 months after symptom onset or diagnosis of COVID-19. Among the 89 patients who developed hair loss (14 missing), 83 (93.3%) had diffuse hair loss, and six (6.7%) had patchy hair loss.
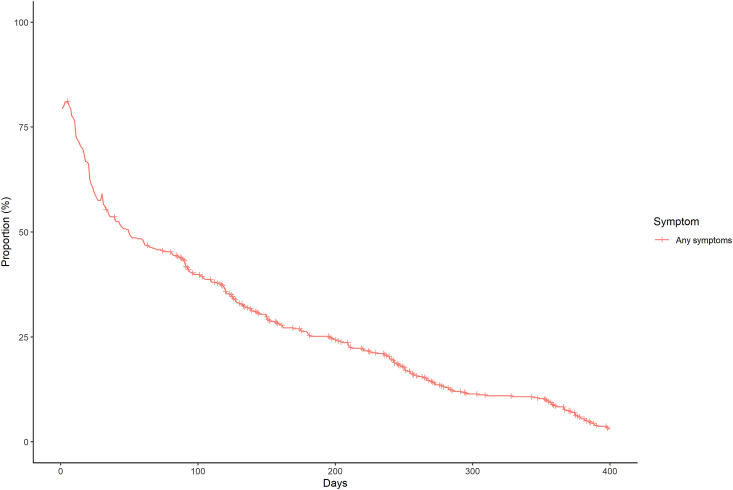
The frequency and duration of at least one symptom are shown in Fig. 3 (any symptom included acute, ongoing/chronic, and late-onset symptoms). The number of participants with at least one symptom at 6 months and 12 months after symptom onset or diagnosis of COVID-19 were 120 (26.3%) and 40 (8.8%), respectively.
Fig. 3.
Proportion of participants who had at least one symptom associated with COVID-19.
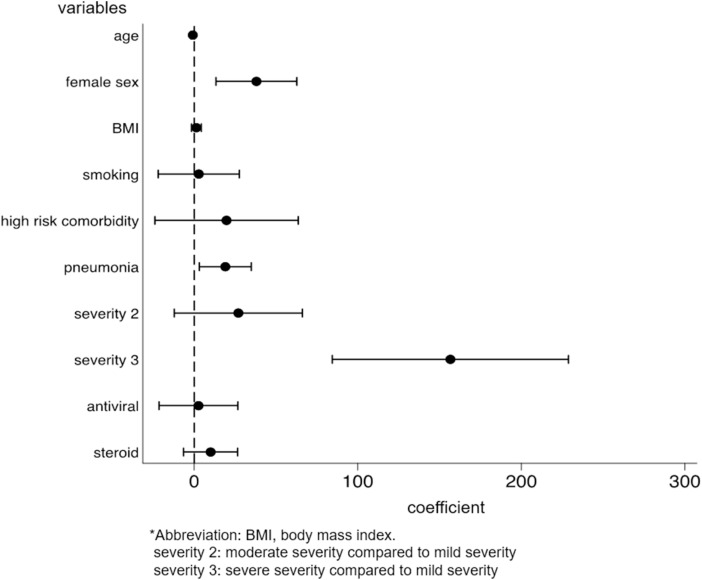
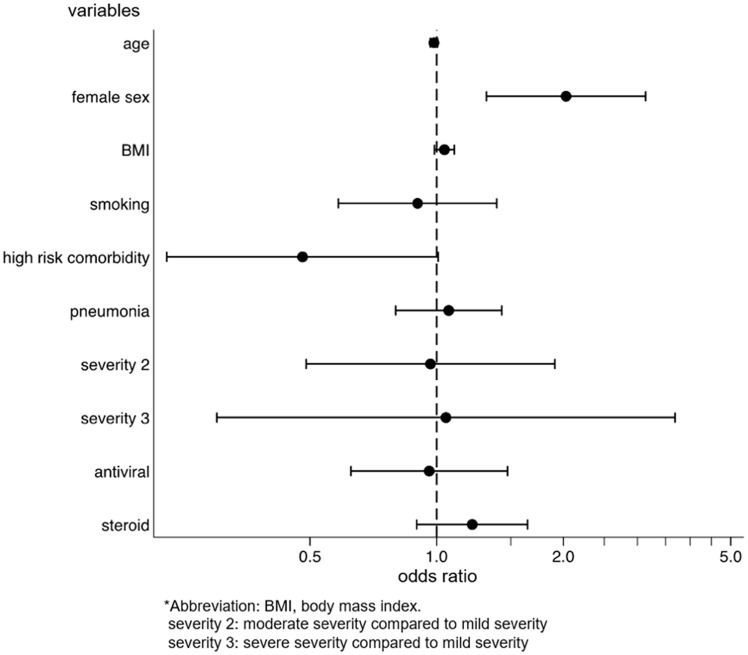
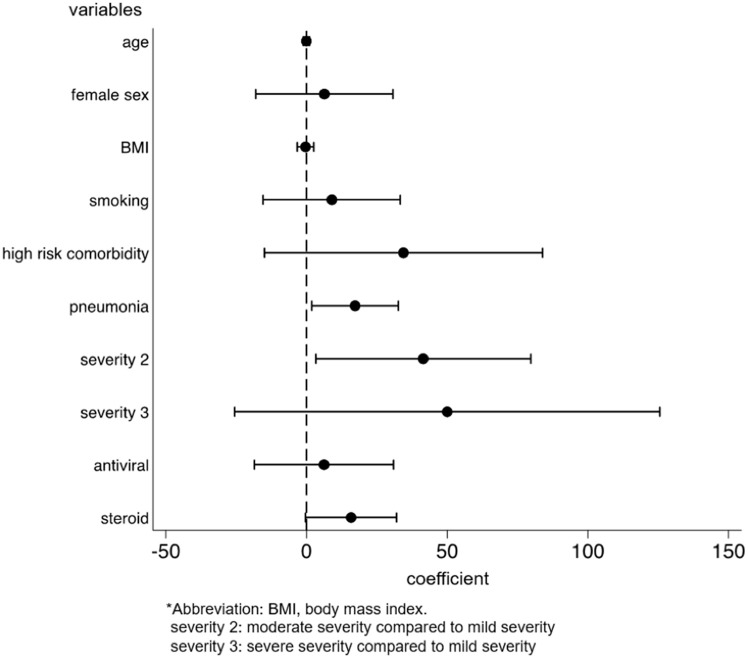
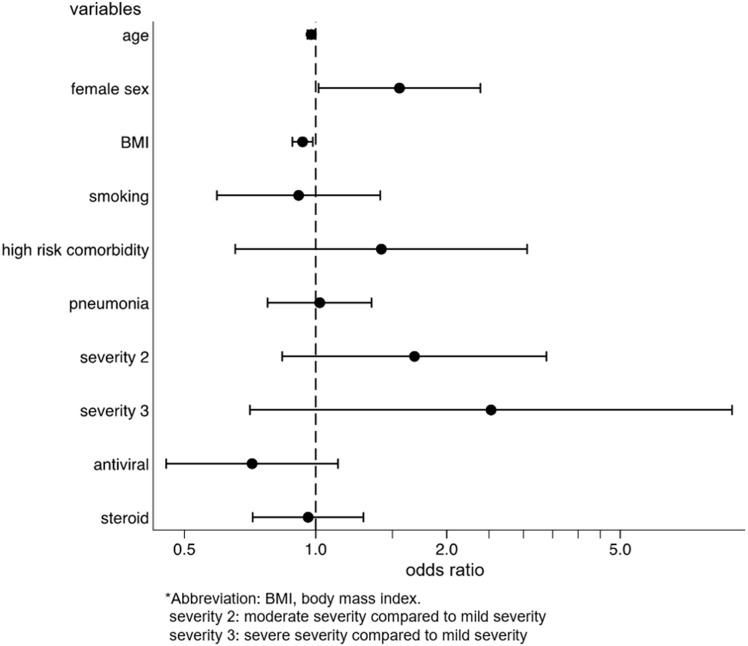
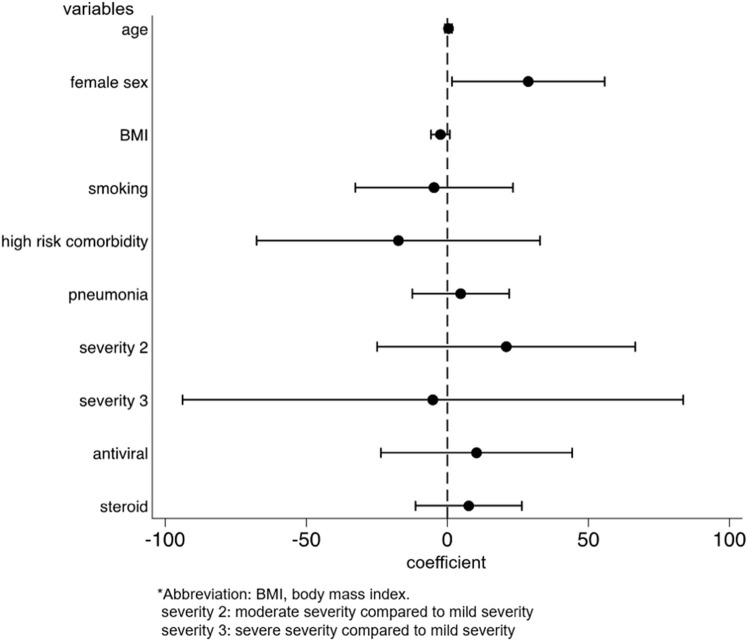
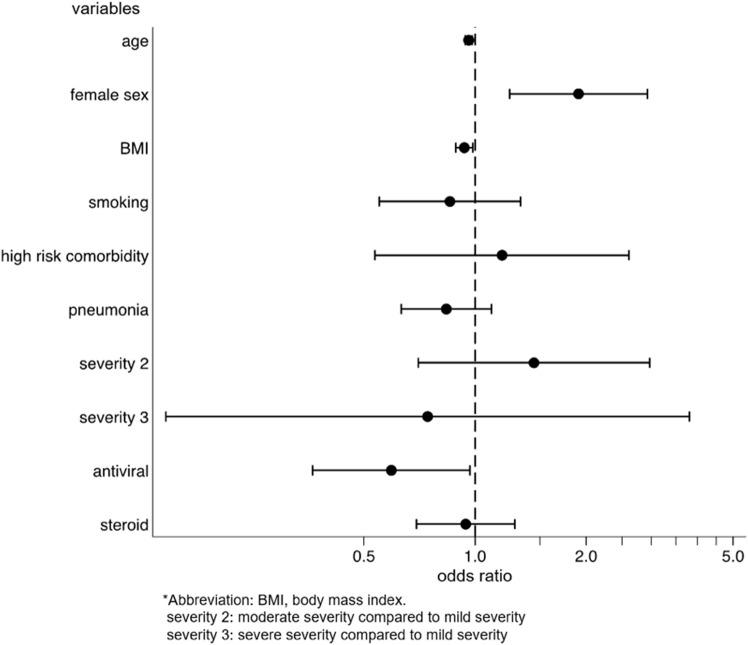
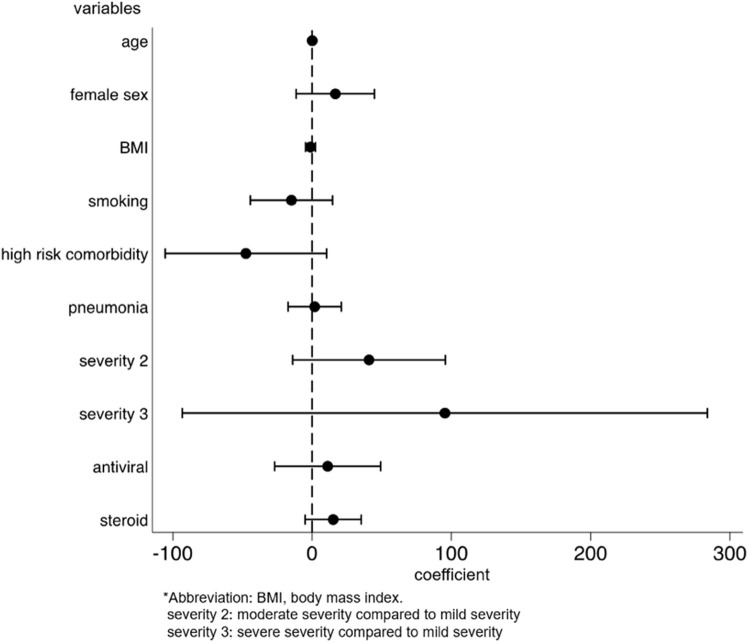
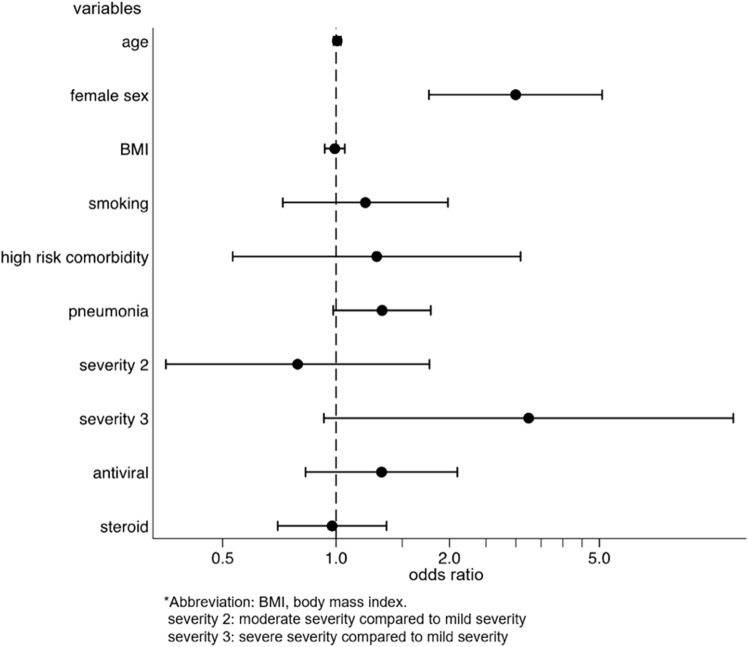
We identified the factors for the development and persistence of common symptoms, such as fatigue, dysgeusia, dysosmia, and hair loss. After multivariable adjustment, development of fatigue was associated with female sex (OR: 2.03, p = 0.001, 95%CI: 1.31–3.14), while the persistence of fatigue among patients with fatigue was associated with being diagnosed with pneumonia (coefficient 17.3, p = 0.028, 95%CI: 1.92–32.6) and moderate disease severity, as compared to mild severity (coefficient 41.5, p = 0.033, 95%CI: 3.33–79.7) (Supplementary Figs. 2a and 2b). The risk of developing dysgeusia was associated with female sex (OR: 1.56, p = 0.042, 95%CI: 1.02–2.39) and was inversely associated with age (OR: 0.98, p = 0.015, 95%CI: 0.96–1.00) and body mass index (BMI) (OR: 0.93, p = 0.012, 95%CI: 0.88–0.98). Persistence of dysgeusia among patients with dysgeusia was associated with the female sex (coefficient 28.7, p = 0.038, 95%CI: 1.65–55.7) (Supplementary Figs. 3a and 3b). The risk of developing dysosmia was also associated with female sex (OR: 1.91, p = 0.003, 95%CI: 1.24–2.93) and was inversely associated with age (OR: 0.96, p < 0.001, 95%CI: 0.94–0.98), BMI (OR: 0.94, p = 0.014, 95%CI: 0.89–0.99), and antiviral drug use (OR: 0.59, p = 0.037, 95%CI: 0.36–0.97). No factors were associated with the persistence of dysosmia (Supplementary Figs. 4a and 4b). The risk of developing hair loss was associated with the female sex (OR, 3.00; p < 0.001; 95%CI, 1.77–5.09) (Supplementary Fig. 5). Persistence of any symptom was associated with female sex (coefficient 38.0, p = 0.003, 95%CI: 13.3–62.8), being diagnosed with pneumonia (coefficient 19.0, p = 0.019, 95%CI: 3.11–35.0), and severe disease, as compared to mild disease (coefficient 157, p < 0.001, 95%CI: 84.4–229) (Fig. 4 ).
Fig. 4.
Factors for persistence of any symptoms associated with COVID-19.
4. Discussion
This cross-sectional questionnaire survey is one of the few studies that have identified factors involved in both the development and persistence of the post-COVID-19 condition. We also examined the duration of post-COVID conditions in detail by surveying participants who had recovered from COVID-19 for a median period of 248.5 days.
Importantly, we found that female sex was a risk factor for developing multiple symptoms, such as fatigue, dysosmia, dysgeusia and hair loss, and for persistence of dysgeusia and any symptoms associated with COVID-19. Female sex has been recognized as a risk factor for post-COVID conditions in a number of previous studies [2,13,14]. Women are more prone to multiple symptoms and are more likely to experience fatigue, sleep disorders, and mental symptoms [14]. However, according to a previous study [15], women are more likely to report symptoms than men, constituting a reporting bias.
Chronic fatigue is the symptom most frequently reported after recovery from acute COVID-19 [3,16,17]. However, its cause, pathogenesis, and the reason for affecting predominantly women are unclear [18]. A cross-sectional analytical study found no association between pro-inflammatory markers and chronic fatigue in patients with COVID-19 [17]. Multiple factors may play a role in the development of post-COVID-19 fatigue [18]. A narrative review explained that congestion of the glymphatic system and the subsequent toxic build-up within the central nervous system, caused by increased resistance to cerebrospinal fluid drainage through the cribriform plate due to olfactory neuron damage, may contribute to post-COVID-19 fatigue [19]. Moreover, negative psychological and social factors associated with the COVID-19 pandemic have also been linked to chronic fatigue [20,21]. Women are more likely to be affected by anxiety, having a depressed mood, and distress [22,23], potentially explaining the more marked chronic fatigue in women than in men. Additionally, direct SARS-CoV-2 infection of skeletal muscles, causing muscle damage and weakness, may contribute to fatigue [24]. Further research is needed to clarify the pathophysiology of chronic fatigue in COVID-19 [17].
Furthermore, we found that younger age and lower BMI were factors for dysgeusia and dysosmia. Our telephonic interviews conducted in Japan also revealed that younger participants, particularly those in their twenties, had a higher incidence of dysosmia and dysgeusia [25]. A possible mechanism of gustatory dysfunction in COVID-19 involves the functional link between taste and smell, whereby gustatory perception is reduced because of antecedent olfactory sensory dysfunction [26]. Long-lasting dysosmia and dysgeusia are critical issues that can affect quality of life, even in young patients. Further research is needed to explore the pathophysiology and identify treatment options.
Our study revealed that the most participants with alopecia had diffuse hair loss. Previous studies have reported that the majority of patients with alopecia after COVID-19 recovery had telogen effluvium [27], a noninflammatory alopecia involving diffuse hair loss, consisting with our findings. In contrast, a few participants in our study had patchy hair loss, which could be explained by alopecia areata after COVID-19 recovery [28,29].
Lastly, we found little effect of antiviral medication or steroids on post-COVID condition symptoms, except for dysosmia. Rehabilitation has been suggested for the treatment of post-COVID conditions; however, no treatment, including vitamin C, has been found to be effective [30]. Therefore, avoiding SARS-CoV-2 infection is the most important measure. Further research on the treatment of post-COVID conditions is needed. On the other hand, vaccination compared to no vaccination was associated with reduced odds of long-duration (≥28 days) symptoms of COVID-19 [31]. This implies that vaccination may shorten the duration of post-COVID conditions, in addition to its effects on preventing COVID-19-related morbidity and mortality. The study participants did not include vaccine recipients because this study included participants before the COVID-19 vaccination introduction in Japan.
Our study also clarified that the number of participants with at least one remaining symptom at 6 months and 12 months after symptom onset or diagnosis of COVID-19 were 120 (26.3%) and 40 (8.8%), respectively. This epidemiological data revealed that most patients with post-COVID conditions recovered over a 1-year period. Longitudinal follow-up surveys are required to understand the natural history of post-COVID conditions better.
Our study had several limitations. First, this study was based on a self-reported questionnaire-based survey, which was subject to various biases, such as selection, volunteer, and recall biases. In particular, it is difficult to evaluate causality using only our results. Moreover, the study was limited to COVID-19 convalescent plasmapheresis patients who underwent the pre-donation screening test. It is unclear whether the results of this study can be applied to all patients recovering from COVID-19. Second, some patients had persistent ongoing or chronic or late-onset symptoms at the time of the survey. In these cases, the actual duration of the symptoms was unclear and it is likely that this study underestimated the duration of these symptoms. Long-term observation is needed to understand the duration of post-COVID conditions better. Third, although a previous study indicated that the severity of acute COVID-19 was a risk factor for developing fatigue [2], severe disease was not a factor associated with developing fatigue in our study. This is partly because most cases in our study had mild disease with few comorbidities, and the number of severe cases was markedly small. Further studies are needed to clarify whether the severity of the illness is a risk factor for the development and persistence of post-COVID conditions. Lastly, given the duration of the study, it was likely that alpha (B.1.1.7) strains were the most common in Japan [32,33], however, it was impossible to determine the type of variant in each patient. The prevalence of the SARS-CoV-2 variants may influence the frequency of post-COVID conditions.
5. Conclusions
In conclusion, our cross-sectional questionnaire survey evaluated patients after recovery from COVID-19, most of whom had had mild disease, for the development and persistence of post-COVID conditions. We found that the woman is an important factor associated with the development of fatigue, dysosmia, dysgeusia, hair loss, and with persistence of dysgeusia and any symptoms associated with COVID-19. Younger age and low BMI were factors for dysosmia and dysgeusia. Additionally, about a quarter of the participants had at least one symptom lasting for more than 6 months, indicating that many patients with COVID-19 suffer from long-term residual symptoms, even in mild cases. Our findings of factors associated with the persistence of fatigue, dysosmia, dysgeusia, or hair loss can help to predict the duration of each symptom, which might reduce patients' anxiety about the duration of persistence of each symptom.
Ethics approval and consent to participate
This study was reviewed and approved by the ethics committee of the Center Hospital of the National Center for Global Health and Medicine (NCGM-G-004121-00). Informed consent was obtained from all the participants. All procedures were performed in accordance with the principles of the Declaration of Helsinki.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Funding
This research was funded by the Emerging/Re-emerging Infectious Diseases Project of Japan from the Japan Agency for Medical Research and Development under Grant Number JP20fk0108416.
Authors' contributions
YM, ST, SM, MT, SK, SS, MA, CK, YO, K Tanaka, M Suzuki, KH, and NO conceptualized the study. YM, ST, SM, SK, and KH designed the study. YM, SM, YS, K Takahashi, and M Sanada conducted the research and investigation. YM, ST, SM, MT, YS, K Takahashi, and M Sanada were responsible for the data curation. YM, ST, and SM conducted the statistical analyses. YM, SM, and MT were major contributors to writing the original draft of the manuscript. SM acquired funds for the study and supervised the project along with NO. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank all the people who participated in our study, “Collection and antibody measurement of convalescent plasma foreseeing the use for COVID-19 treatment.”
Footnotes
All authors meet the ICMJE authorship criteria.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jiac.2022.04.025.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
figs6.
figs7.
figs8.
References
- 1.COVID-19 coronavirus pandemic. https://www.worldometers.info/coronavirus Worldometer.
- 2.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carfì A., Bernabei R., Landi F. Gemelli against COVID-19 post-acute care study group. Persistent Symptoms Patients after Acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyazato Y., Morioka S., Tsuzuki S., Akashi M., Osanai Y., Tanaka K., et al. Prolonged and late-onset symptoms of coronavirus disease 2019. Open Forum Infect Dis. 2020:7. doi: 10.1093/ofid/ofaa507. ofaa507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Havervall S., Rosell A., Phillipson M., Mangsbo S.M., Nilsson P., Hober S., et al. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA. 2021;325:2015–2016. doi: 10.1001/jama.2021.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terada M., Kutsuna S., Togano T., Saito S., Kinoshita N., Shimanishi Y., et al. How we secured a COVID-19 convalescent plasma procurement scheme in Japan. Transfusion. 2021;61:1998. doi: 10.1111/trf.16541. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Rio C., Collins L.F., Malani P. Long-term health consequences of COVID-19. JAMA. 2020;324:1723–1724. doi: 10.1001/jama.2020.19719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrigues E., Janvier P., Kherabi Y., Le Bot A., Hamon A., Gouze H., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenforde M.W., Kim S.S., Lindsell C.J., Billig Rose E.B., Shapiro N.I., Files D.C., et al. Symptom duration and factors for delayed return to usual health among outpatients with COVID-19 in a Multistate health care systems Network - United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad I., Rathore F.A. Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang L., Yao Q., Gu X., Wang Q., Ren L., Wang Y., et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C., et al. Attributes and predictors of post-COVID conditions. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang Y.F., Liu T., Yu J.N., Xu X.R., Zahid K.R., Wei Y.C., et al. Half-year follow-up of patients recovering from severe COVID-19: analysis of symptoms and their factors. J Intern Med. 2021;290:444–450. doi: 10.1111/joim.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louie G.H., Ward M.M. Sex disparities in self-reported physical functioning: true differences, reporting bias, or incomplete adjustment for confounding? J Am Geriatr Soc. 2010;58:1117–1122. doi: 10.1111/j.1532-5415.2010.02858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halpin S.J., McIvor C., Whyatt G., Adams A., Harvey O., McLean L., et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93:1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 17.Townsend L., Dyer A.H., Jones K., Dunne J., Mooney A., Gaffney F., et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crook H., Raza S., Nowell J., Young M., Edison P. Post-COVID conditions-mechanisms, factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 19.Wostyn P. COVID-19 and chronic fatigue syndrome: is the worst yet to come? Med Hypotheses. 2021;146:110469. doi: 10.1016/j.mehy.2020.110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgul E., Bener A., Atak M., Akyel S., Aktaş S., Bhugra D., et al. COVID-19 pandemic and psychological fatigue in Turkey. Int J Soc Psychiatr. 2021;67:128–135. doi: 10.1177/0020764020941889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks S.K., Webster R.K., Smith L.E., Woodland L., Wessely S., Greenberg N., et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai J., Ma S., Wang Y., Cai Z., Hu J., Wei N., et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanardo V., Manghina V., Giliberti L., Vettore M., Severino L., Straface G. Psychological impact of COVID-19 quarantine measures in northeastern Italy on mothers in the immediate postpartum period. Int J Gynaecol Obstet. 2020;150:184–188. doi: 10.1002/ijgo.13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrandi P.J., Alway S.E., Mohamed J.S. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol. 1985;129:864–867. doi: 10.1152/japplphysiol.00321.2020. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.COVID-19 registry Japan Disaster management. https://www.bousai.metro.tokyo.lg.jp/_res/projects/default_project/_page_/001/012/970/31kai/2021020407.pdf Cabinet Office.
- 26.Small D.M., Prescott J. Odor/taste integration and the perception of flavor. Exp Brain Res. 2005;166:345–357. doi: 10.1007/s00221-005-2376-9. [DOI] [PubMed] [Google Scholar]
- 27.Moreno-Arrones O.M., Lobato-Berezo A., Gomez-Zubiaur A., Arias-Santiago S., Saceda-Corralo D., Bernardez-Guerra C., et al. SARS-CoV-2-induced telogen effluvium: a multicentric study. J Eur Acad Dermatol Venereol. 2021;35:e181–e183. doi: 10.1111/jdv.17045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capalbo A., Giordano D., Gagliostro N., Balampanos C.G., Persechino F., Orrù F., et al. Alopecia areata in a COVID-19 patient: a case report. Dermatol Ther. 2021;34 doi: 10.1111/dth.14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sgubbi P., Savoia F., Calderoni O., Longo R., Stinchi C., Tabanelli M. Alopecia areata in a patient with SARS-Cov-2 infection. Dermatol Ther. 2020;33 doi: 10.1111/dth.14295. [DOI] [PubMed] [Google Scholar]
- 30.Thomas S., Patel D., Bittel B., Wolski K., Wang Q., Kumar A., et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 Infection: the COVID A to Z Randomized Clinical Trial. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonelli M., Penfold R.S., Merino J., Sudre C.H., Molteni E., Berry S., et al. Factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22:43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ministry of Health, Labour and Welfare Number of domestic cases of variants of SARS-CoV-2 of concern by prefecture (genomic analysis) https://www.mhlw.go.jp/stf/seisakunitsuite/newpage_00054.html
- 33.Centers for Disease Control and Prevention SARS-CoV-2 variant classifications and definitions. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html
- 34.Centers for Disease Control and Prevention Underlying medical conditions associated with high risk for severe COVID-19: information for healthcare providers. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.