Abstract
Temperature gradient gel electrophoresis (TGGE) is well suited for fingerprinting bacterial communities by separating PCR-amplified fragments of 16S rRNA genes (16S ribosomal DNA [rDNA]). A strategy was developed and was generally applicable for linking 16S rDNA from community fingerprints to pure culture isolates from the same habitat. For this, digoxigenin-labeled polynucleotide probes were generated by PCR, using bands excised from TGGE community fingerprints as a template, and applied in hybridizations with dot blotted 16S rDNA amplified from bacterial isolates. Within 16S rDNA, the hypervariable V6 region, corresponding to positions 984 to 1047 (Escherichia coli 16S rDNA sequence), which is a subset of the region used for TGGE (positions 968 to 1401), best met the criteria of high phylogenetic variability, required for sufficient probe specificity, and closely flanking conserved priming sites for amplification. Removal of flanking conserved bases was necessary to enable the differentiation of closely related species. This was achieved by 5′ exonuclease digestion, terminated by phosphorothioate bonds which were synthesized into the primers. The remaining complementary strand was removed by single-strand-specific digestion. Standard hybridization with truncated probes allowed differentiation of bacteria which differed by only two bases within the probe target site and 1.2% within the complete 16S rDNA. However, a truncated probe, derived from an excised TGGE band of a rhizosphere community, hybridized with three phylogenetically related isolates with identical V6 sequences. Only one of the isolates comigrated with the excised band in TGGE, which was shown to be due to identical sequences, demonstrating the utility of a combined TGGE and V6 probe approach.
Temperature gradient gel electrophoresis (TGGE) and the related technique, denaturing gradient gel electrophoresis, are now frequently applied in microbial ecology to compare the structures of complex microbial communities and to study their dynamics (18). The steps in the procedure are extraction of genomic DNA from environmental samples, amplification of a segment of the 16S rRNA genes (16S ribosomal DNA [rDNA]) in PCR, and electrophoretical separation of PCR products with differing sequences in a denaturing gradient. This allows analysis of many samples, which is essential for studying spatial and temporal variations of microbial community structures in relation to environmental factors, and shifts due to perturbation or experimental treatment. The diversity of complex communities can be explored in a “top-to-bottom” analysis with primer sets of varying phylogenetic specificity (12, 13, 15, 21). Individual bands in the TGGE fingerprints can be assigned to taxa by hybridization with oligonucleotide probes (17, 31), or the sequence can be determined and phylogenetically analyzed (19). However, the ecological role of an organism often cannot be inferred from a comparison of its 16S rRNA sequence to those of known bacteria. The limited sequence database may lack a well-studied closely related reference strain, or the strain may differ in the trait of interest even if the sequences of the 16S rDNA regions used for TGGE are identical. Several groups of organisms have been identified which share almost identical 16S rRNA sequences but in which DNA hybridization is lower than 70% (30).
Thus, a tool is needed to link the bands from community fingerprints to the strains which are present in the environmental sample analyzed. In order to study their properties and autecology, either corresponding cultivated bacterial isolates from the sample must be identified or corresponding unique DNA sequences cloned from DNA of the sample have to be found, which allows the detection and study of the population members in situ (1) or helps in selecting the proper medium for their cultivation (26, 33).
In this study we investigated whether the hypervariable region V6 (20) of the small-subunit rRNA gene could be utilized as a probe target to detect bacterial isolates corresponding to bands of TGGE community fingerprints. A generally applicable method was developed for generating highly specific digoxigenin (DIG)-labeled probes targeting the V6 region (V6 probes) without prior DNA sequence knowledge.
MATERIALS AND METHODS
Rhizosphere samples.
Rhizosphere communities from transgenic and control potato plants were compared. The transgenic plant lines DL4 and DL5 constitutively expressed and secreted T4 lysozyme, which mediates improved resistance to the bacterial soft rot disease (4, 5, 7). A transgenic control line contained the same construct, including the nptII gene but without the T4 lysozyme gene. All transgenic cultivars were derived from variety Désirée. Tubers were provided by K. Düring (BAZ, Quedlinburg, Germany). The potato cultivars were planted in a field located near Quedlinburg in a randomized block design (four cultivars each in eight replicate plots). The soil type was a silt loam. Roots with adhering soil particles were sampled 16 weeks after the plants sprouted (shortly before the potatoes were harvested). Independent samples from eight replicate plots of each cultivar were collected (32 samples). DNA was extracted from bacterial pellets (28) derived by repeated Stomacher blending and differential centrifugation (29).
Bacteria.
The extracted bacterial pellets were serially diluted and plated on R2A agar (Difco, Detroit, Mich.). Randomly picked colonies were characterized by their fatty acid profiles, using the Microbial Identification System (MIS) (MIDI Inc., Newark, Del.). Cells of 192 strains, which were selected to represent the diversity of cultivated species, were lysed by freeze-boiling and directly applied to PCR mixtures. Amplification of 16S rDNA fragments, using the primer pair F984GC-R1378 (Table 1), was performed as described previously (12) to compare the migrations of bands from pure cultures to those of the community fingerprints in TGGE. The PCR products were used as targets for Southern blot hybridizations.
TABLE 1.
Primers used in PCR experiments
| Primera | 16S rDNA targetb | Sequence (5′→3′) |
|---|---|---|
| F985PTO | Bacteria; positions 968 to 985 | AACGCGAAGAACCTTACSC |
| R1046PTO | Most bacteria; positions 1046 to 1062 | ACAGCCATGCAGCACCST |
| F971 | Most bacteria; positions 971 to 985 | GCGAAGAACCTTACC |
| R1057 | Many bacteria; positions 1044 to 1057 | CATGCAGCACCTGT |
| F984GC | Bacteria; positions 968 to 984 | CGCCCGGGGCGCGCCCCG GGCGGGGCGGGGGCAC GGGGGG-AACGCGAAG AACCTTACc |
| R1378 | Bacteria; positions 1378 to 1401 | CGGTGTGTACAAGGCCCG GGAACG |
F, forward primer; R, reverse primer; PTO, phosphorothioate bond at position in the sequence marked by a superscript “S.”
Positions according to E. coli 16S rDNA sequence. Positions 1044 to 1045 are phylogenetically less conserved; thus, the primer R1057 is not appropriate for, e.g., Flavobacterium and related genera.
5′ GC clamp (up to the hyphen).
TGGE.
Community fingerprinting of rhizosphere samples by TGGE was carried out as described previously (12). The 16S rDNA fragments (positions 968 to 1401 [Escherichia coli rDNA sequence]) were amplified by PCR from rhizosphere DNA extracts with the primer pair F984GC-R1378. Acid silver staining was used for the routine detection of DNA bands in TGGE gels (24). When DNA was to be recovered from excised bands, staining was performed with SYBR Green I (FMC, Vallensbaek Strand, Denmark).
V6 probes.
Gel pieces containing bands from the TGGE gel were washed with 0.5 ml of Tris-EDTA buffer, frozen at −70°C, and broken into smaller pieces. After centrifugation at 13,000 × g for 30 min at 4°C, 1 μl of the solution extracted from the gel pieces was used in a PCR to reamplify the 16S rDNA fragments with the primer pair F984GC-R1378. The enrichment of the excised band was confirmed by TGGE. The PCR products were ligated into the pGEM-T vector (Promega, Madison, Wis.). Competent cells of E. coli JM109 were transformed as described in the manual of the supplier. Plasmids with the correct insert, as determined by TGGE, were isolated with a plasmid extraction kit (Qiagen, Hilden, Germany). The purified plasmid DNAs were used as templates for preparation of V6 probes by PCR. Two types of V6 probes were prepared. One was amplified with primers F971 and R1057 (Table 1), as described by Smalla et al. (29). The PCR mixture contained DIG-dUTP to label the probes. The other type of V6 probe, intended for removal of flanking conserved bases, was amplified with primers F985PTO and R1046PTO (Table 1), using the same PCR mixture but with the following cycles: one cycle of 5 min at 94°C and 35 cycles of 30 s at 94°C, 30 s at 50°C, and 30 s at 72°C. These primers had a phosphorothioate bond between bases of the 3′ terminus, which was intended to resist cleavage by T7 gene 6 exonuclease, a double-strand-specific 5′-3′ exonuclease (14). The PCR products were purified, using the QIAEX II kit (Qiagen), and digested for 15 min at 37°C with 1 U of T7 gene 6 exonuclease/μl in the supplied buffer (U. S. Biochemicals, Cleveland, Ohio) to remove the primer nucleotides from the PCR product up to the phosphorothioate bond. The products were again purified by QIAEX II for buffer exchange. The remaining single strands (complementary to the primers) were digested by the single-strand-specific mung bean nuclease (16), as recommended by Amersham International (Little Chalfont, England). The size reduction of individual V6 probes compared to untreated aliquots was checked by electrophoresis in a 15% polyacrylamide gel.
Southern hybridizations.
Equal amounts of PCR products (primer pair F984GC-R1378) from cloned 16S rDNA and from a selection of bacterial isolates were blotted from an agarose gel onto an uncharged nylon membrane (25) or with a dot blot device onto Hybond N+ nylon membranes (Amersham) (3). High-stringency hybridization at 62°C, washing, and detection of DNA hybrids was carried out as recommended by Boehringer (Mannheim, Germany), but the hybridization solution contained 5% (wt/vol) blocking powder, 1× SSC (0.15 M NaCl plus 0.015 M sodium citrate), 0.1% (vol/vol) N-laurylsarkosinate, 0.02% (wt/vol) sodium dodecyl sulfate, and 50% (vol/vol) formamide.
DNA sequence analysis.
Selected cloned TGGE bands were sequenced with standard primers SP6 and T7 (IIT GmbH, Bielefeld, Germany). PCR products from 16S rDNA of bacterial rhizosphere isolates were sequenced with primers 536r, 357r, FoxF, and 1385r (DSMZ, Braunschweig, Germany). Construction of consensus sequences, alignments to most-similar database sequences, and matrix calculations of sequence similarities to closely related database sequences were done with ARB software (32) and the supplied database, 6pubmrz97, which was supplemented by 16S rDNA sequences from the GenBank database.
Nucleotide sequence accession numbers.
GenBank accession numbers of the sequences from this study are given in Table 2, except that of the 5′ partial 16S rDNA sequence of strain B3a, which is AF060537.
TABLE 2.
Analysis of partial 16S rRNA gene sequences of excised bands from potato rhizosphere TGGE fingerprints (E. coli positions 985 to 1377)
| Band | Accession no. | Closest database reference (GenBank accession no.) | % Sim-ilarity |
|---|---|---|---|
| B1 | AF060530 | E. carotovora ATCC 15713 (M59149) | 98.7 |
| B2 | AF060531 | Kluyvera cryocrescens (Y07652) | 98.5 |
| B3 | AF060532 | Potato rhizosphere isolate B3a (AF060977) | 100 |
| Enterobacter amnigenus JCM 1237 (AB004749) | 100 | ||
| Enterobacter aerogenes (AJ001237) | 100 | ||
| B4 | AF060533 | Flavobacterium succinicans DSM 4002 (RDP) | 100 |
| B5 | AF060534 | Pseudomonas corrugata ATCC 29736 (D84012) | 100 |
| B6 | AF060535 | Erwinia persicinus ATCC 35998 (U80205) | 98.7 |
| B7 | AF060536 | Cellulomonas cellulans DSM 43879 (X83809) | 95.3 |
RESULTS AND DISCUSSION
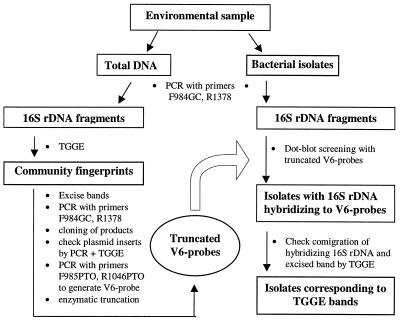
Fingerprinting of bacterial communities by separation of amplified 16S rDNA fragments with TGGE provides the opportunity to compare the community structure features of multiple environmental samples. Although sequencing of bands for analysis of TGGE fingerprints provides insight into the community structure through the phylogenetic affiliations of community members (19), the information about their physiological and ecological traits derived from the partial sequences is often rather limited (11, 22). Thus, a link between bands of TGGE rhizosphere fingerprints and corresponding bacterial isolates from the same habitat was developed, which is based on probes that target a region of the 16S rDNA including the hypervariable region V6. A method was developed to improve the specificity of the V6 probes by removal of phylogenetically conserved nucleotides. The approach is illustrated in Fig. 1.
FIG. 1.
Scheme of the V6 probe approach. Polynucleotide probes that target the hypervariable region V6 were used to screen for bacterial isolates corresponding to bands of community fingerprints derived by electrophoretic separation of PCR-amplified 16S rDNA fragments in a temperature gradient (TGGE).
Development of V6 probes.
In a first attempt, V6 probes were synthesized and DIG labeled, using PCR with the primer pair F971-R1057 (Table 1), which amplified a fragment between E. coli 16S rDNA sequence positions 971 and 1057. The specificities of probes derived from strains of Agrobacterium tumefaciens and Sinorhizobium meliloti were tested with dot blotted 16S rDNAs of six phylogenetically closely related species of the Rhizobiaceae and a selection of species from more distantly related groups. Both probes cross-hybridized only with the 16S rDNA of the closest relative, Agrobacterium rubi or Sinorhizobium fredii, respectively (data not shown), which have identical sequences of the V6 regions (37). A probe from Ralstonia solanacearum slightly cross-hybridized only with the 16S rDNA of Ralstonia eutropha (data not shown). The V6 probe approach was recently applied successfully to prove the identities of rhizobacterial isolates (29) or cloned 16S rDNA from soil (9, 10) with prominent populations of the natural community which had equally migrating bands in denaturing gradients. However, for the dense cluster of Enterobacteriaceae isolates, specificities at the genus level were not achieved (Fig. 2a). Attempts to increase the specificity of the probe by hybridization at a higher temperature (65°C) or by adding an excess of primers to block conserved binding sites resulted in only minor improvements.
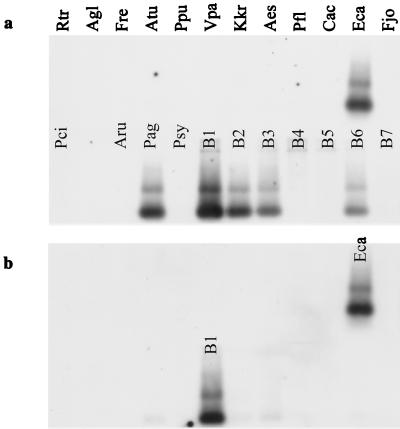
FIG. 2.
Hybridization specificity of the V6 probes derived from the 16S rDNA of band B1 in the TGGE fingerprint of the bacterial rhizosphere community from T4 lysozyme-producing potato line DL4. The blot was hybridized with the nontruncated V6 probe (a) or with the truncated V6 probe (b). Targets on the Southern blot were 16S rRNA gene fragments from rhizobacteria and from bands B1 to B7 of rhizosphere community fingerprints (Rtr, Rathayibacter tritici; Agl, Arthrobacter globiformis; Fre, Flavobacterium resinovorum; Atu, A. tumefaciens; Ppu, Pseudomonas putida; Vpa, Variovorax paradoxus; Kkr, Kokuria kristinae; Aes, Aureobacterium esteroaromaticum; Pfl, Pseudomonas fluorescens; Cac, Comamonas acidovorans; Eca, E. carotovora; Fjo, Flavobacterium johnsoniae; Pci, Pseudomonas cichorii; Aru, A. rubi; Pag, P. agglomerans; Psy, Pseudomonas syringae. See Table 2 for sequence similarities of bands B1 to B7.)
Truncated V6 probes with increased specificities.
One-third of the length of the V6 probes consists of phylogenetically conserved nucleotides which are required for priming the PCR but counteract probe specificity. Thus, a method was developed and applied to remove the conserved parts of the probe, i.e., the incorporated primers and their complements. To achieve this, the primers F985PTO and R1046PTO were synthesized, with a phosphorothioate replacing a phosphate in the bond of the last 2 nucleotides at the 3′ end. After PCR amplification of the fragment from 968 to 1062, the nucleotides of the incorporated primers were removed 5′ to 3′ by exonuclease digestion, but hydrolysis was stopped at the phosphorothioate bond (Fig. 3, lane 2). The remaining complementary single strand could subsequently be eliminated by the single-strand-specific mung bean nuclease (Fig. 3, lane 3).
FIG. 3.
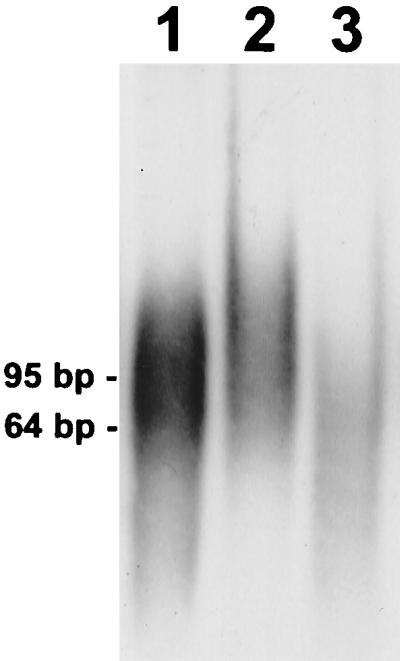
Preparation of a truncated V6 probe. Lane 1 contains the 95-bp PCR product amplified with phosphorothioate primers F985PTO and R1046PTO. On average 11 nucleotides of DIG-dUTP were introduced during PCR (maximum, 33). Lane 2 contains the partially single-stranded probe after hydrolysis of the incorporated primer nucleotides by T7 gene 6 exonuclease. Lane 3 contains the truncated 64-bp V6 probe after removal of the single-stranded primer complements by mung bean nuclease. The probe and intermediate products were separated by polyacrylamide gel electrophoresis, electroblotted, and detected by exposure of the film to chemiluminescence.
The specificities of the V6 probes increased dramatically after truncation. The nontruncated V6 probe from TGGE band B1 hybridized with all 16S rDNA sequences related to the Enterobacteriaceae (Fig. 2a), i.e., 16S rDNA of isolates of Erwinia carotovora and Pantoea agglomerans and cloned TGGE bands B1, B2, B3, and B6 (sequence affiliations are shown in Table 2). In contrast, the truncated V6 probe was observed to be specific for E. carotovora 16S rDNA (Fig. 2b). Weak cross-hybridization was detected with fragments B2 and B3, which had sequence similarities of 96.2 or 95.4% with B1 and 10- or 8-bp differences from the probe derived from B1, respectively. The truncated V6 probe of fragment B3 cross-hybridized with neither fragment B2, which had six mismatches with the probe, nor the other sequences tested (data not shown). The sequences of B2 and B3 were 97.2% similar. The results of the sequence analyses of the excised TGGE bands are given in Table 2.
Cloned 16S rDNAs of 15 phylogenetically closely related members of the β subdivision of the class Proteobacteria were hybridized to a truncated V6 probe derived from one of the clones to determine the number of nucleotide mismatches, which prevent hybridization of truncated V6 probes. The sequences differed from that of the probe within the target region by 2, 3, 6, 10, or 17 nucleotides. The only clone which produced a hybridization signal was the one from which the probe originated (data not shown). The most similar target sequence had A or T at E. coli 16S rDNA sequence positions 1001 and 1039 instead of G or C in the probe. Thus, the truncated V6 probe hybridization stringency was sufficient to distinguish targets differing by only 2 nucleotides. The similarity between the two complete 16S rDNAs was 98.8%, which indicates that differentiation on the species level may well be possible (30). The theoretical increase of specificity due to the truncation of the V6 probe was predicted by the equation of Baldino et al. (2), which accounts for the percentage of mismatches. It estimated a 1.5-fold-stronger destabilizing effect of each probe-target mismatch for the truncated V6 probe compared to the nontruncated probe. Moreover, nucleotide mismatches are relatively less clustered in hybrids with truncated V6 probes, which increases their destabilizing effects (8).
Rhizosphere.
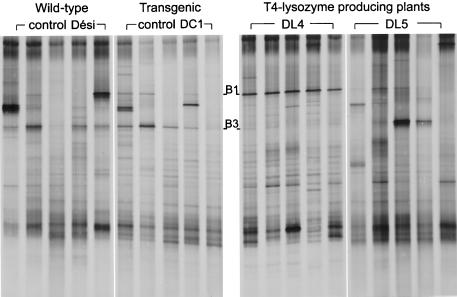
We investigated whether truncated V6 probes derived from bands in TGGE fingerprints of bacterial rhizosphere communities could be used to screen for the corresponding bacterial strains isolated from the same habitat. Community fingerprinting by TGGE separation of amplified 16S rDNA fragments was applied to compare bacterial rhizosphere communities of transgenic T4 lysozyme-producing potato plants, a transgenic control and the wild type. In Fig. 4, TGGE patterns from five of the eight rhizosphere samples of each plant line are shown. In most of the patterns from one of the T4 lysozyme-producing plant lines, DL4, band B1 was much stronger than in the other patterns. In only one of the eight samples of DL4 was band B3 stronger than B1 (not shown). The bands B1 and B3 were excised from the TGGE gel, reamplified, subcloned, and used to generate truncated V6 probes. The probes were hybridized to the dot blotted 16S rDNAs of 192 strains, representing the diversity of cultivated bacteria from the potato rhizosphere.
FIG. 4.
Fingerprints of bacterial rhizosphere communities by TGGE separation of amplified 16S rDNA fragments. Dési, wild-type potato variety Désirée; DL4 and DL5, T4 lysozyme-expressing potato lines; DC1, transgenic control with nptII gene but no T4 lysozyme gene. Truncated V6 probes from bands B1 and B3 were used to screen for corresponding bacterial isolates from the potato rhizosphere.
The truncated V6 probe derived from TGGE band B1 specifically hybridized with the 16S rDNA of four isolates. All were identified by fatty acid methyl ester-MIS analysis as E. carotovora subsp. carotovora. This was in agreement with the identification by sequence analysis of band B1. Three of the isolates had 16S rDNA fragments which migrated as band B1 in TGGE. Evidence from band intensities in TGGE community fingerprints showed that these bacteria represented the most abundant population associated with the roots of old DL4-type potato plants and were much less abundant in the rhizosphere of the wild-type plants. The affiliation of this isolate with those E. carotovora strains that might cause soft rot (23) may be of agronomic interest. It is unclear whether the growth of E. carotovora is an indirect effect of the T4 lysozyme, which had a 20- to 70%-higher expression level in DL4 than in DL5 (6), or whether, e.g., somaclonal variation caused a weakening of the cultivar DL4, which had a significantly reduced stem length, smaller leaves, and a lower root mass compared to those of the other cultivars. The other T4 lysozyme-producing potato line, DL5, did not show the prominent E. carotovora band and thus might be a better candidate for further field tests. The fragment of one E. carotovora isolate migrated slightly differently from band B1, demonstrating that this isolate was not identical to the dominant population in the rhizosphere of potato type DL4. Also, the type strain of E. carotovora subsp. carotovora, DSM 30168, could be differentiated by TGGE from the four isolates.
Although the specificity of the truncated V6 probe from TGGE band B1 was sufficient to identify E. carotovora sequences, the truncated V6 probe from band B3 hybridized with 16S rDNA of three bacterial isolates which were affiliated with different species as determined by fatty acid profiling and partial 16S rDNA sequence analysis (Table 3). All were closely related to members of the genus Enterobacter (Table 3). To verify whether the correct targets were detected by the truncated V6 probe, the 16S rDNAs of the three isolates were partially sequenced. The V6 regions of all three isolates were identical to the V6 probe sequence, showing the high stringency of the hybridization. However, the strains could be differentiated by subsequent TGGE analysis based on differences in 16S rDNA regions V7 and V8. Only the 16S rDNA fragment of strain B3a was identical in electrophoretic mobility to band B3, and DNA sequencing, in fact, revealed sequence identity. The different migrations of the 16S rDNA fragments of strains B3b and B3c could be explained by five or seven base substitutions, respectively, of A to G or C to T in positions 1244 to 1367. Sequencing demonstrated that phylogenetically very similar strains were detected by the truncated V6 probe. The strains could be differentiated by TGGE analysis because the separation in TGGE is mainly caused by sequence differences in melting domains distant from the nonmelting GC-clamped end of the TGGE fragment. The V6 region is located next to the GC clamp. Thus, TGGE analysis complements the V6 probe approach. As little as one base difference could be detected by TGGE (27). However, screening of many strains by TGGE is not practical. In addition, fragments with largely different DNA sequences may have similar migration distances in TGGE (15, 35). These could easily be differentiated with probes.
TABLE 3.
Identification of potato rhizosphere isolates with 16S rRNA genes that hybridized with the truncated V6 probe derived from band B3 of the rhizosphere TGGE fingerprint
| Rhizosphere isolate | Identification by duplicate fatty acid profiles (similarity in database TSBA 3.9 of the MIS) | Identification by 16S rRNA sequence analysis (% similarity) |
|---|---|---|
| B3a | Enterobacter intermedius (0.71) or Enterobacter amnigenus (0.59) | E. amnigenus JCM 1253 (99.9) |
| B3b | Enterobacter agglomerans (0.97/0.95) | E. amnigenus JCM 1253 (98.7) |
| B3c | Actinobacillus lignieresii (0.44/0.43) | Enterobacter sp. strain HCB (98.1) |
Polynucleotide probes targeting rRNA genes were also applied by Trebesius et al. (34). They used a variable region of the 23S rRNA as a target site, but as not all parts of the ca. 220 nucleotides were hypervariable, specificity at the species level could be achieved only by tedious optimization of the hybridization stringency for a Pseudomonas stutzeri-specific probe. The hypervariable region V6 is probably the optimal target within the 16S rDNA to generate polynucleotide probes which are specific at standard hybridization conditions. This was evident from a profile of the positional phylogenetic conservation of the 16S rDNA. It was constructed from the recently published quantitative map of nucleotide substitution rates (36), using calculated 45-mer average substitution rates. The V6 region had the highest average phylogenetic variability and, in contrast to other variable regions, it was closely flanked by conserved sites which are suitable for the annealing of eubacterial primers. Amplification of other highly variable regions (i.e., V2 and V3) by PCR with eubacterial primers leads to products which also contain moderately variable nucleotide stretches, counteracting probe specificity.
A possible aid for the analysis of bacteria, when they cannot be cultivated, is to use the V6 probe approach to identify a clone containing a complete 16S rRNA gene (or larger fragments) which corresponds to the TGGE band of interest. In our work, E. coli JM109 hosts, containing multicopy pGEM-T plasmids with complete 16S rDNA inserts, could be grown and lysed in microtiter plates and directly applied to dot blots for hybridization analysis. The additional sequence information can be useful for designing specific oligonucleotide probes to be used for in situ detection (1). The direct applicability of truncated V6 probes for identification of individual cells remains to be tested.
ACKNOWLEDGMENTS
We are grateful to Bert Engelen and the team of workgroup Smalla for helpful discussions and cooperation, Henrike Westphal for excellent technical assistance, and Ed Moore (GBF, Braunschweig) for reading the manuscript. We thank Stefan Weidner (University of Bielefeld) for providing some of the clones and their sequences.
This work was supported by BMBF grant 0311295.
REFERENCES
- 1.Amann R I, Ludwig W, Schleifer K H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldino F, Jr, Chesselet M-F, Lewis M E. High-resolution in situ hybridization histochemistry. Methods Enzymol. 1989;168:761–777. doi: 10.1016/0076-6879(89)68057-3. [DOI] [PubMed] [Google Scholar]
- 3.Brown T. Dot and slot blotting of DNA. In: Ausubel F M, Brent R, Kingston R E, et al., editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1993. pp. 2.9.15–2.9.20. [DOI] [PubMed] [Google Scholar]
- 4.Düring K. A plant transformation vector with a minimal T-DNA. Transgen Res. 1994;3:138–140. doi: 10.1007/BF01974093. [DOI] [PubMed] [Google Scholar]
- 5.Düring K. Genetic engineering for resistance to bacteria in transgenic plants by introduction of foreign genes. Mol Breeding. 1996;2:297–305. [Google Scholar]
- 6.Düring, K., and A. Mahn. Personal communication.
- 7.Düring K, Porsch P, Fladung M, Lörz H. Transgenic potato plants resistant to the phytopathogenic bacterium Erwinia carotovora. Plant J. 1993;3:587–598. [Google Scholar]
- 8.Dyson N J. Immobilization of nucleic acids and hybridization analysis. In: Brown T A, editor. Essential molecular biology. New York, N.Y: Oxford University Press; 1991. pp. 111–156. [Google Scholar]
- 9.Felske A, Akkermans A D L. Prominent occurrence of ribosomes from an uncultured bacterium of the Verrucomicrobiales cluster in grassland soils. Lett Appl Microbiol. 1998;26:219–223. doi: 10.1046/j.1472-765x.1998.00322.x. [DOI] [PubMed] [Google Scholar]
- 10.Felske A, Rheims H, Wolterink A, Stackebrandt E, Akkermans A D L. Ribosome analysis reveals prominent activity of an uncultured member of the class Actinobacteria in grassland soils. Microbiology. 1997;143:2983–2989. doi: 10.1099/00221287-143-9-2983. [DOI] [PubMed] [Google Scholar]
- 11.Fox G E, Wisotzkey J D, Jurtshuk P., Jr How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int J Syst Bacteriol. 1992;42:166–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]
- 12.Heuer H, Krsek M, Baker P, Smalla K, Wellington E M H. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microbiol. 1997;63:3233–3241. doi: 10.1128/aem.63.8.3233-3241.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heuer H, Smalla K. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) for studying soil microbial communities. In: van Elsas J D, Wellington E M H, Trevors J T, editors. Modern soil microbiology. New York, N.Y: Marcel Dekker; 1997. pp. 353–373. [Google Scholar]
- 14.Kerr C, Sandowski P D. Gene 6 exonuclease of bacteriophage T7. I. Purification and properties of the enzyme. J Biol Chem. 1972;247:305–310. [PubMed] [Google Scholar]
- 15.Kowalchuk G A, Stephen J R, De Boer W, Prosser J I, Embley T M, Woldendorp J W. Analysis of ammonia-oxidizing bacteria of the β subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl Environ Microbiol. 1997;63:1489–1497. doi: 10.1128/aem.63.4.1489-1497.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroeker W D, Kowalski D, Laskowski M. Mung bean nuclease. I. Terminally directed hydrolysis of native DNA. Biochemistry. 1976;15:4463–4467. doi: 10.1021/bi00665a020. [DOI] [PubMed] [Google Scholar]
- 17.Manz W, Amann R, Ludwig W, Wagner M, Schleifer K H. Phylogenetic oligonucleotide probes for the major subclasses of proteobacteria: problems and solutions. Adv Microb Ecol. 1992;15:593–600. [Google Scholar]
- 18.Muyzer G, Smalla K. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Leeuwenhoek. 1998;73:127–141. doi: 10.1023/a:1000669317571. [DOI] [PubMed] [Google Scholar]
- 19.Muyzer G, Teske A, Wirsen C O, Jannasch H W. Phylogenetic relationship of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch Microbiol. 1995;164:165–172. doi: 10.1007/BF02529967. [DOI] [PubMed] [Google Scholar]
- 20.Neefs J-M, Van de Peer Y, Hendriks L, De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1990;18:2237–2242. doi: 10.1093/nar/18.suppl.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nübel U, Garcia-Pichel F, Muyzer G. PCR primers to amplify 16S rRNA genes from Cyanobacteria. Appl Environ Microbiol. 1997;63:3327–3332. doi: 10.1128/aem.63.8.3327-3332.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pace N R. A molecular view of microbial diversity and the biosphere. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. [DOI] [PubMed] [Google Scholar]
- 23.Pishchik V N, Chernyaeva I I, Vorob’ev N I, Lazarev A M. Characteristic features of virulent and avirulent strains of Erwinia carotovora. Microbiology. 1996;65:262–268. [Google Scholar]
- 24.Riesner D, Steger G, Zimmat R, Owens R A, Wagenhöfer M, Hillen W, Vollbach S, Henco K. Temperature-gradient gel electrophoresis of nucleic acids: analysis of conformational transitions, sequence variations, and protein-nucleic acid interactions. Electrophoresis. 1989;10:377–389. doi: 10.1002/elps.1150100516. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Santegoeds C M, Nold S C, Ward D M. Denaturing gradient gel electrophoresis used to monitor the enrichment culture of aerobic chemoorganotrophic bacteria from a hot spring cyanobacterial mat. Appl Environ Microbiol. 1996;62:3922–3928. doi: 10.1128/aem.62.11.3922-3928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheffield V C, Cox R D, Lerman L S, Myers R M. Attachment of a 40-base-pair G+C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci USA. 1989;86:232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smalla K, Cresswell N, Mendoca-Hagler L C, Wolters A, van Elsas J D. Rapid DNA extraction protocol from soil for polymerase chain reaction-mediated amplification. J Appl Bacteriol. 1993;74:78–85. [Google Scholar]
- 29.Smalla K, Wachtendorf U, Heuer H, Liu W-T, Forney L. Analysis of BIOLOG GN substrate utilization patterns by microbial communities. Appl Environ Microbiol. 1998;64:1220–1225. doi: 10.1128/aem.64.4.1220-1225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stackebrandt E, Goebel B M. Taxonomic note: a place for DNA-DNA reassociation and 16S RNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol. 1994;44:846–849. [Google Scholar]
- 31.Stahl D A, Flesher B, Mansfield H R, Montgomery L. Use of phylogenically based hybridization probes for studies of ruminal microbial ecology. Appl Environ Microbiol. 1988;54:1079–1084. doi: 10.1128/aem.54.5.1079-1084.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strunk, O., W. Ludwig, O. Gross, B. Reichel, N. Stuckmann, M. May, B. Nonhoff, M. Lenke, T. Ginhart, A. Vilbig, and R. Westram. 22 October 1998, revision date. [Online.] ARB—a software environment for sequence data. Department of Microbiology, Technical University of Munich, Munich, Germany. http://www.biol.chemie.tu-muenchen.de. [19 January 1999, last date accessed.]
- 33.Teske A, Sigalevich P, Cohen Y, Muyzer G. Molecular identification of bacteria from coculture by denaturing gradient gel electrophoresis of 16S ribosomal DNA fragments as a tool for isolation in pure cultures. Appl Environ Microbiol. 1996;62:4210–4215. doi: 10.1128/aem.62.11.4210-4215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trebesius K, Amann R, Ludwig W, Mühlegger K, Schleifer K H. Identification of whole fixed bacterial cells with nonradioactive 23S rRNA-targeted polynucleotide probes. Appl Environ Microbiol. 1994;60:3228–3235. doi: 10.1128/aem.60.9.3228-3235.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallaeys T, Topp E, Muyzer G, Macheret V, Laguerre G, Rigaud A, Soulas G. Evaluation of denaturing gradient gel electrophoresis in the detection of 16S rDNA sequence variation in rhizobia and methanotrophs. FEMS Microbiol Ecol. 1997;24:279–285. [Google Scholar]
- 36.Van de Peer Y, Chapelle S, De Wachter R. A quantitative map of nucleotide substitution rates in bacterial rRNA. Nucleic Acids Res. 1996;24:3381–3391. doi: 10.1093/nar/24.17.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanagi M, Yamasoto K. Phylogenetic analysis of the family Rhizobiaceae and related bacteria by sequencing of 16S rRNA gene using PCR and DNA sequencer. FEMS Microbiol Lett. 1993;107:115–120. doi: 10.1111/j.1574-6968.1993.tb06014.x. [DOI] [PubMed] [Google Scholar]