Abstract
Introduction
The 0.19 mg fluocinolone acetonide (FAc) intravitreal implant delivers a continuous intravitreal corticosteroid dose for the treatment of refractory diabetic macular oedema (DMO). The aim of this study was to assess the impact of an FAc intravitreal implant on intraocular pressure (IOP).
Methods
We retrospectively collected anonymised data on the patients’ characteristics, DMO treatment, and IOP and IOP-lowering treatments before and after the FAc intravitreal implant between September 2013 and March 2020 in several European centres.
Results
A total of 221 eyes from 179 patients were included. The mean follow-up duration was 13.4 (± 12.5, range 2.4–33.5) months. Overall, 194 eyes (88.2%) had received an intravitreal dexamethasone injection before the FAc intravitreal implant. For 25 eyes (11.3%) there was a history of glaucoma, and 52 eyes (23.5%) had previous IOP-lowering treatment. Mean IOP before injection was 14.7 (3.4) mmHg and increased to 16.9 (3.7) mmHg 12 months after injection (P < 0.0001). During follow-up, 55 eyes (24.9%) required the addition or initiation of topical IOP-lowering medication, only one patient (0.5%) had laser trabeculoplasty and one patient (0.5%) a minimally invasive glaucoma surgery, and no patient required incisional IOP-lowering surgery.
Conclusion
The FAc intravitreal implant led to substantial IOP elevation. This elevation was monitored most of the time with addition or initiation of topical IOP-lowering medication.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40123-022-00504-z.
Keywords: Diabetic macular oedema, Fluocinolone acetonide, ILUVIEN, Intraocular pressure, Intravitreal corticosteroid, Safety
Key Summary Points
| Why carry out this study? |
| Fluocinolone acetonide intravitreal implant (FAc) has been recently approved as a second-line treatment for diabetic macular oedema. This corticosteroid treatment raises the issue of intraocular pressure elevation. |
| To report changes in intraocular pressure after an FAc intravitreal implant injection for chronic diabetic macular oedema at a European multicentre level. |
| What was learned from the study? |
| FAc intravitreal implant injection led to a predictable and manageable rise in intraocular pressure in 24.4% of patients. |
Introduction
Diabetes mellitus is a common condition with an increasing prevalence, which is expected to reach 693 million patients worldwide by 2045 [1]. Diabetic macular oedema (DMO) is a microangiopathic complication of diabetes mellitus that affects 7.0% of patients with diabetes [2]. DMO is a vision-threatening condition and it is one of the major causes of visual impairment in the working population [3].
The pathogenesis of DMO results from microvascular changes, inflammation, oxidative stress and vascular dysfunction [4]. The main contributing factor is hyperglycaemia, which leads to the production of cytokines and growth factors such as vascular endothelial growth factor (VEGF). These mediators induce endothelial inflammation and breakdown of the blood–retinal barrier with the loss of pericytes and alteration of intercellular junctions. This process leads to fluid accumulation in the macular area and to increased macular thickness [5].
The management of DMO is based on several axes: stringent glycaemic and blood pressure monitoring, focal or grid laser, intravitreal anti-VEGF or corticosteroid injections, and vitreomacular surgery [6–11].
Corticosteroids have an anti-inflammatory effect in addition to their VEGF-inhibiting action [12]. Three molecules are currently available: triamcinolone, dexamethasone and fluocinolone acetonide.
The 190 µg fluocinolone acetonide intravitreal implant (FAc) (ILUVIEN®, Alimera Sciences, Alpharetta, GA, USA) has been approved as a second-line treatment for DMO in patients who have received prior corticosteroids without a clinically significant rise in intraocular pressure (IOP). It delivers an average dose of 0.2 µg/day with an initial dose of approximately 0.25 µg/day, for approximately 36 months [13, 14]
The efficacy of the FAc intravitreal implant has been established; it offers a sustained improvement in visual acuity and a reduction in central macular thickness over time, with an added value of decreasing the number of intravitreal injections compared with intravitreal anti-VEGF or corticosteroid injections [14–16].
The main adverse events after an FAc intravitreal implant are those related to steroids. Cataract is very common and appears on average 6–18 months after the injection [17]. IOP elevation occurs in 7–18% of patients [18, 19], and in 15–37% of cases it may require treatment with IOP-lowering medication, laser trabeculoplasty or IOP-lowering surgery [18, 20–22]
The purpose of this study was to report changes in IOP after an FAc intravitreal implant injection for chronic DMO at a European multicentre level.
Methods
Study Design
We conducted a retrospective, observational, European multicentre study. Data were collected from review of case records of participating centres in France, Germany, Italy and Portugal. This study was conducted in accordance with the Declaration of Helsinki. Local ethics committee approval was given and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statements [23]. We extracted anonymised data from patients’ electronic medical records. The inclusion criterion was consecutive patients undergoing an FAc intravitreal implant injection between 1 September 2013 and 31 December 2020 for chronic DMO, with a minimum follow-up of 12 months after the injection. The exclusion criterion was any other indication for FAc intravitreal implant (uveitis, vein occlusion, post-cataract macular oedema).
Outcomes
The primary outcome was the mean IOP change during the 12-month follow-up after the FAc intravitreal implant. Secondary outcomes were (1) a composite endpoint defined as an increase of at least 10 mmHg after the FAc intravitreal implant, or an IOP of 25 mmHg or more at any point during follow-up, or introduction or addition of IOP-lowering treatment (IOP-lowering medication, laser trabeculoplasty or IOP-lowering surgery) [24, 25], (2) predictive factors for IOP elevation or of developing clinically significant IOP elevation.
Data Source and Measurements
We extracted data from participating centres and entered them into a normalising digital template. Datasheets are available upon reasonable request to the corresponding author. We analysed baseline data for each patient: age, sex, injection centre, lens status, DMO duration, previous DMO treatments, history of glaucoma, previous IOP-lowering treatments and baseline IOP before FAc intravitreal implant. The decision to treat the patient with an FAc intravitreal implant was at the clinicians’ discretion. If an off-label indication was retained (pre-existing glaucoma for example), patients were informed of such disposition. We collected information on the FAc intravitreal implantation date, the eye treated and whether the injection was unilateral or bilateral. After the FAc intravitreal injection, we collected IOP measurements at each follow-up visit and recorded the occurrence and date of IOP-lowering medication introduction or addition, laser trabeculoplasty or IOP-lowering surgery. We also collected data on cataract surgery events. IOP was measured via Goldmann applanation (Germany and Portugal) or air-puff tonometry (France and Italy). The same method was used at each visit in the same centre (aside from different methods between centres). It was not corrected by central corneal thickness. Every non-missing assessment collected after FAc intravitreal implant administration was assigned a follow-up visit number as follows: baseline (baseline or day 0), M1 (day 20–50), M3 (day 70–120), M6 (day 145–215), M12 (day 300–420) and at the last visit. If more than one assessment was documented within one visit period, mean IOP values were calculated.
Statistical Analysis
Continuous variables with normal distribution are presented as mean (standard deviation, SD) and non-normally distributed continuous variables are reported as median (interquartile range, IQR). Categorical variables are described as percentage of eyes or of patients. Univariate comparisons between centres were performed using Kruskal–Wallis tests for continuous data and two-tailed Fisher’s exact tests for categorical data. IOP values from M1 to M12 were considered as an outcome in a linear mixed-effect model including crossed random effects for patients and visits [26]. The IOP mean was explained by the following covariates: number of IOP-lowering medications before injection (none, one, or two or more IOP-lowering medications), intravitreal dexamethasone implant injection before the FAc intravitreal implant, age, sex and IOP at baseline (before the injection). Factors associated with a clinically significant IOP elevation at M12 were analysed using generalised logistic regression [27]. The model included the patients as a correlation component and was adjusted for the same covariates as in the linear mixed-effect analysis. The generalised estimation equations (GEE) method [28] was used to estimate odds ratios taking into account the intra-patient correlations.
A P value < 0.05 was considered statistically significant. SAS software version 9.4 (SAS Institute, Cary, NC, USA) was used for all analyses.
Results
Study Population
Overall, 221 eyes from 179 patients were included. Six eyes (five patients) were excluded due to non-DMO injection indication. Patient demographics, baseline characteristics and prior DMO and IOP treatments are presented in Table 1.
Table 1.
Baseline characteristics of the study population before the fluocinolone acetonide intravitreal implant
| Patient characteristics | Patients | France | Germany | Portugal | Italy | P value |
|---|---|---|---|---|---|---|
| n = 179 | n = 110 | n = 41 | n = 22 | n = 6 | ||
| Age (years) | 68.7 (10.9) | 70.0 (10.0) | 62.0 (10.1) | 73.8 (12.56) | 71.0 (5.4) | < 0.001 |
| Sex (male) | 91 (50.8%) | 54 (49.1%) | 23 (56.1%) | 10 (45.5%) | 4 (66.7%) | 0.58 |
| Bilateral injection | 42 (23.5%) | 31 (28.2%) | 5 (12.2%) | 6 (27.3%) | 0 | < 0.01 |
| Eye characteristics | Patients | France | Germany | Portugal | Italy | P value |
|---|---|---|---|---|---|---|
| n = 221 | n = 141 | n = 46 | n = 28 | n = 6 | ||
| Lens status (pseudophakic) | 185 (83.7%) | 132 (93.6%) | 20 (43.5%) | 27 (96.4%) | 6 (100%) | < 0.001 |
| DMO duration (months) | 17 (6–50) | 29 (14–66) | 19 (12–28) | 6 (5–7) | 4 (3–7) | < 0.001 |
| Previous DMO treatments | ||||||
| Intravitreal anti-VEGF | 194 (87.8%) | 123 (87.2%) | 45 (97.8%) | 20 (71.4%) | 6 (100%) | < 0.01 |
| Dexamethasone implant | 195 (88.2%) | 141 (100%) | 25 (54.3%) | 23 (82.1%) | 6 (100%) | < 0.001 |
| Intravitreal triamcinolone | 47 (21.3%) | 22 (15.6%) | 2 (4.3%) | 23 (82.1%) | 0 | < 0.001 |
| Focal or grid laser | 43 (19.5%) | 9 (6.4%) | 11 (23.9%) | 23 (82.1%) | 0 | < 0.001 |
| Previous IOP-lowering medication | 0.012 | |||||
| None | 169 (76.5%) | 114 (80.8%) | 33 (71.8%) | 16 (57.2%) | 6 (100%) | |
| Single therapy | 28 (12.7%) | 17 (12.1%) | 8 (17.4%) | 3 (10.7%) | 0 | |
| Dual therapy | 21 (9.5%) | 9 (6.4%) | 3 (6.5%) | 9 (32.1%) | 0 | |
| Triple therapy or more | 3 (1.3%) | 1 (0.7%) | 2 (4.3%) | 0 | 0 | |
| History of glaucoma | 25 (11.3%) | 11 (7.8%) | 13 (28.2%) | 1 (3.6%) | 0 | < 0.01 |
| Previous laser trabeculoplasty | 1 (0.5%) | 1 (0.9%) | 0 | 0 | 0 | 1 |
| Previous IOP-lowering surgery | 1 (0.5%) | 0 | 0 | 1 (4.5%) | 0 | 0.15 |
Continuous variables are displayed as mean (SD) for normal distribution and median (IQR) for non-normal distribution. Categorical variables are displayed as number (percentage) of patients or eyes when required
DMO diabetic macular oedema, IOP intraocular pressure, VEGF vascular endothelial growth factor
In the overall population, the mean age was 68.7 (10.4) years with 91 men (50.8%). Most patients were pseudophakic (83.7%). The median duration of DMO was 16 months (6–50). Prior to the FAc intravitreal implantation, 194 eyes (87.8%) had received one or more intravitreal anti-VEGF injections and 195 eyes (88.2%) had intravitreal dexamethasone implant injection. Overall, 25 eyes (11.3%) had a history of glaucoma and 52 eyes (23.5%) were already under IOP-lowering treatment at baseline. One eye (0.5%) had previously undergone trabeculectomy. A total of 42 patients (23.5%) received a bilateral injection. The mean follow-up duration was 13.4 (± 12.5, range 2.4–33.5).
Detailed data by geographical origin are summarised in Table 1. The mean age in the German centre was 62.0 (10.2) years vs 70.0–73.8 years in other centres (P < 0.001). Only 43.5% of patients were pseudophakic in the German centre vs 93.6% or more in the other centres (P < 0.001). Regarding the history of DMO treatments, only 71.4% of Portuguese patients had previous intravitreal anti-VEGF injections vs 87.2% or more in the other centres (P < 0.01) and only 54.3% of the German patients had previous intravitreal dexamethasone implant vs 82.1% or more in the other centres (P < 0.001). Before the FAc intravitreal implantation, 28.2% of patients had a history of glaucoma in the German centre vs 7.8% or less in the other centres (P < 0.01). In the Portuguese centre, 42.8% of patients had previous IOP-lowering medication vs 28.2% in the German centre, 19.2% in the French centre and none in the Italian centre (P = 0.012).
IOP Changes and Management
The mean IOP in the overall study population before injection was 14.7 (3.4) mmHg. At 12 months after the FAc intravitreal implantation, it increased to 16.9 (4.7) mmHg (n = 95) (P < 0.001). The changes in IOP during follow-up are presented in Fig. 1.
Fig. 1.
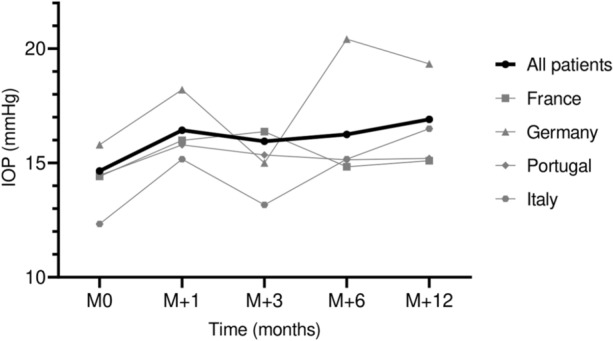
Mean intraocular pressure changes over time by country. IOP intraocular pressure
Over time in the overall study population, 55 eyes (24.9%) required an addition or initiation of IOP-lowering medication, with a mean time of 7.9 (7.4) months after the FAc intravitreal implantation. The proportion of single vs dual therapy or more was 11.0% (25 eyes) and 14.0% (31 eyes), respectively. One eye (0.5%) underwent minimally invasive glaucoma surgery during follow-up and one eye (0.5%) was treated by laser trabeculoplasty. No patient underwent incisional IOP-lowering surgery. The results are presented in Table 2. The IOP distribution and time points in the present study are presented in the Supplementary Material.
Table 2.
Intraocular pressure-related events and cataract surgery after the fluocinolone acetonide implant and time-to-event analyses
| All eyes (n = 221) | France (n = 141) | Germany (n = 46) | Portugal (n = 28) | Italy (n = 6) | |
|---|---|---|---|---|---|
| Emergent IOP-lowering medication | |||||
| None | 166 (75.2%) | 118 (83.7%) | 20 (43.5%) | 23 (82.2%) | 5 (83.3%) |
| Single therapy | 25 (11.3%) | 6 (4.3%) | 16 (34.8%) | 2 (7.1%) | 1 (16.7%) |
| Dual therapy | 24 (10.8%) | 14 (9.9%) | 7 (15.2%) | 3 (10.7%) | 0 |
| Triple therapy or more | 6 (2.7%) | 3 (2.1%) | 3 (6.5%) | 0 | 0 |
| Mean time to event (months) | 7.9 (7.4) | 4.0 (3.5) | 11.7 (8.1) | 2.8 (2.4) | |
| Laser trabeculoplasty | 1 (0.5%) | 1 (0.7%) | 0 | 0 | 0 |
| MIGS | 1 (0.5%) | 1 (0.7%) | 0 | 0 | 0 |
| Incisional IOP-lowering surgery | 0 | 0 | 0 | 0 | 0 |
| Cataract surgery | 23 (10.4%) | 0 | 22 (47.8%) | 1 (3.5%) | 0 |
| Mean time to event (months) | 15.2 (7.6) | 14.7 (7.3) | 27 | ||
Continuous variables are displayed as mean (SD) and time-to-event analyses are presented in months and were not performed for laser trabeculoplasty or MIGS, as the number of events was very small. Categorical variables are displayed as number (percentage)
IOP intraocular pressure, MIGS minimally invasive glaucoma surgery
The incidence of clinically significant IOP elevation during the first 12 months of follow-up was 24.4% (54 eyes). In total, 25 eyes (11.3%) developed IOP of ≥ 25 mmHg and 21 (9.5%) experienced an increase in IOP of ≥ 10 mmHg compared with baseline.
Cataract Surgery
Overall, 22 eyes (10%) benefited from cataract surgery during follow-up, with a mean time to event of 15.2 (7.6) months (Table 1).
Predictive Factors for IOP Elevation After an FAc Intravitreal Implant
In the mixed-model analysis (Table 3), IOP was significantly lower in older patients (P = 0.041). It was also correlated with the baseline IOP, with an increase of 0.57 (0.06) mmHg for each additional mmHg before injection (P < 0.001). Regarding the history of previous corticosteroid injections, the IOP was significantly higher in patients who had not received an intravitreal dexamethasone implant before the FAc intravitreal implant (18.08 mmHg vs 16.41 mmHg, P = 0.009). No statistically significant difference was found for sex or number of IOP-lowering medications before the injection. The risk of developing clinically significant IOP elevation was higher in patients with one IOP-lowering medication at baseline compared to patients without treatment (odds ratio [OR] = 6.03 [2.32, 15.68], P < 0.001) (Table 4). However, no statistically significant association was found for patients with two or more medications before the injection (OR = 1.90 [0.56, 6.42], P = 0.304). Patients with a prior intravitreal dexamethasone implant injection were less likely to develop clinically significant IOP elevation after the FAc intravitreal implant (OR = 0.31 [0.11, 0.89], P = 0.027). Age or sex was not associated with the risk of clinically significant IOP elevation.
Table 3.
Factors associated with intraocular pressure elevation after a fluocinolone acetonide intravitreal implant (mixed-model analysis)
| Fixed effects (reference) | Estimate | SE | P value | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|
| Age (years) | −0.05 | 0.02 | 0.041 | −0.91 | −0.01 |
| Sex (male) | 0.36 | 0.47 | 0.447 | −0.57 | 1.29 |
| IOP before injection (mmHg) | 0.58 | 0.07 | < 0.001 | 0.45 | 0.71 |
| Dexamethasone implant before FAc injection | −1.67 | 0.64 | < 0.001 | −2.92 | −0.41 |
| Previous IOP-lowering medication (none) | |||||
| Single therapy | 0.05 | 0.72 | 0.944 | −1.36 | 1.46 |
| Dual therapy | 1.04 | 0.77 | 0.176 | −0.47 | 2.56 |
| Triple therapy or more | 0.30 | 1.80 | 0.867 | −3.26 | 3.86 |
FAc fluocinolone acetonide, IOP intraocular pressure, SE standard error, 95% CI 95% confidence interval
Table 4.
Risk factors for clinically significant intraocular pressure elevation at 12 months after a fluocinolone acetonide intravitreal implant (generalised logistic regression analysis)
| Parameter (reference) | OR | Lower 95% CI | Upper 95% CI | P value |
|---|---|---|---|---|
| Age | 0.99 | 0.96 | 1.02 | 0.406 |
| Sex (male) | 0.58 | 0.28 | 1.21 | 0.148 |
| IOP before injection | 1.12 | 0.98 | 1.27 | 0.091 |
| Dexamethasone implant before FAc injection | 0.31 | 0.11 | 0.87 | 0.027 |
| Previous IOP-lowering medication (none) | ||||
| Single therapy | 6.04 | 2.32 | 15.69 | < 0.001 |
| Dual therapy or more | 1.90 | 0.56 | 6.42 | 0.304 |
FAc fluocinolone acetonide, IOP intraocular pressure, OR odds ratio, 95% CI 95% confidence interval
Subgroup Analysis
Patients with more IOP-lowering medications before the injection were more likely to have additional IOP-lowering treatments during the 12-month follow-up: 61% (n = 17) of patients with monotherapy before injection and 33% (n = 8) of patients with dual therapy or more vs 18% (n = 30) of patients with no IOP-lowering treatment at baseline (P < 0.001) (Supplementary Material).
Discussion
The efficacy of controlling chronic DMO after FAc intravitreal implantation has been widely studied across Europe [18, 29–33]. Moreover, international treatment guidelines and consensus on the management of IOP in patients with DMO after FAc intravitreal implantation have been recently published [34, 35]. However, few studies other than pivotal reports have focused on its impact on IOP [20, 22]. In our study, IOP-related adverse events are reported in a European multicentre real-world design.
The safety results regarding IOP-related adverse events in this study are consistent with known side effects of corticosteroid injection [21, 36]. The mean IOP remained within the normal range (< 21 mmHg) during the first year after treatment, with a substantial proportion of cases of clinically significant IOP elevation (24.4%). IOP elevation was successfully controlled with topical treatment in most cases. Only one patient needed laser trabeculoplasty, one had a minimally invasive glaucoma surgery and no patient had incisional IOP-lowering surgery. FAc intravitreal implant was more likely to induce clinically significant IOP elevation in patients previously treated with IOP-lowering medications. Patients with more IOP-lowering medications before the FAc intravitreal implant were more likely to have additional IOP-lowering treatments during the 12-month follow-up.
These results are different from those of the FAME studies, where 18.4% of patients had an increase in IOP of ≥ 30 mmHg and 38.4% of patients received IOP-lowering treatment after injection, 1.3% had laser trabeculoplasty and 4.8% had IOP-lowering surgery [20]. Selection bias could explain these striking differences. The overall population of this study was different from that of the FAME trials, where patients with a history of elevated IOP were excluded [16]. Instead, a quarter of our patients presented with previous IOP-lowering treatment and/or history of glaucoma. Participants without a previous intravitreal dexamethasone injection were not excluded in this study, in order to reflect the different European practices. On the contrary, patients with a history of IOP elevation after a corticosteroid injection or a steroid injection during the 3 months before the FAc intravitreal implant were excluded from the FAME trials.
Other retrospective studies found similar results to this study. In the Medisoft study [18], the mean IOP remained < 21 mmHg during the 3-year follow-up. In the ILUVIEN Registry Safety Study (IRISS) [22], the mean IOP remained below 21 mmHg during the 24-month follow-up and the mean IOP increase was 1.9 mmHg. Elevated IOP of > 25 mmHg occurred in 19.1% of cases after the FAc intravitreal implant and IOP elevation was treated with IOP-lowering medication in 23.3% of the patients, with laser trabeculoplasty in 0.3% and with surgery in 2.0%. In the Retro-IDEAL study [29], the mean IOP rose from 15.8 mmHg at baseline to 18.2 mmHg at 12 months and 22.2% of patients had an increase in IOP of ≥ 10 mmHg. IOP elevation was managed with IOP-lowering medication in 27.2% of patients and 3.7% of patients needed IOP-lowering surgery.
Two phase 4 prospective studies have been conducted [19, 33]. Both excluded patients with a history of IOP-related events, but in neither case did IOP elevation require laser or surgical treatment, unlike in the FAME study.
Clinically significant IOP elevation may be more relevant than mean IOP for assessing the potential risk of steroid-induced glaucoma [37]. In this study, clinically significant IOP elevation was defined according to the SAFODEX study (IOP ≥ 25 mmHg or increase ≥ 10 mmHg compared with baseline IOP) [25]. Comparisons between different studies are hampered due to the lack of standard criteria to describe clinically significant IOP elevation with varying criteria being used [22, 29, 33]. We conducted an additional analysis of clinically significant IOP elevation using another definition for clinically significant IOP elevation (IOP increase of at least 6 mmHg to an IOP of more than 21 mmHg) and found similar results (data not shown).
Regarding predictive factors, an increased risk of elevated IOP was found for younger patients. Some studies have reported similar results with other intraocular corticosteroid treatments [25, 38]. We also found that a higher IOP value before injection was correlated with a higher IOP during follow-up. Patients with an IOP above or close to 21 mmHg before FAc intravitreal implant should be monitored more closely, as other authors have also suggested this association [39]. Moreover, the risk of elevated IOP and of clinically significant IOP elevation was lower for patients who benefited from a previous intravitreal dexamethasone injection before the FAc intravitreal implant. The pathophysiology underlying steroid-induced IOP elevation is not fully understood. Recent studies suggest that corticosteroids induce accumulation of extracellular matrix material in the trabecular meshwork, leading to an increased outflow resistance of the aqueous humour [37, 40]. These results confirm the benefit of a steroid IOP-response test before the FAc intravitreal implant, in accordance with previous studies [36, 41].
In Europe, the FAc intravitreal implant is indicated for the treatment of vision impairment associated with chronic DMO considered insufficiently responsive to available therapies. It is contraindicated in the presence of pre-existing glaucoma [42]. However, the Food and Drug Administration (FDA) guidelines are more restrictive and indicate the FAc intravitreal implant for patients with DMO who have been previously treated with corticosteroids and did not have a clinically significant rise in IOP [43]. In this study, we noted differences in several baseline characteristics between countries. France, Italy and Portugal seemed to follow the American guidelines, with a large majority of patients who had previous intravitreal dexamethasone or triamcinolone injections. On the other hand, most German patients were corticosteroid-naïve before the FAc intravitreal implant. This could be a potential selection bias that reflects local or national practices.
Still, these predictive factors can help us identify the profiles of patients who are less at risk of developing elevated IOP after an FAc intravitreal implant and thereby allow for better selection of patients eligible for this steroid treatment.
IOP was not correlated with the number of IOP-lowering medications before injection. However, a subgroup analysis based on prior IOP treatment suggests a higher risk of IOP-lowering medication addition in patients already under treatment before the injection, which is consistent with previous studies [18]. The greater addition of IOP-lowering medications may have limited the increase in IOP in these patients. Altogether, these results show the value of selecting patients eligible for FAc treatment based on their history of IOP-related events [18].
Cataract formation is a well-known side effect of steroid treatment and leads to reversible visual acuity impairment [17]. In our study, almost all phakic patients before the FAc intravitreal implant underwent cataract surgery during follow-up. We noted that a majority of patients from the German centre were phakic before the injection, whereas in other centres almost all patients were pseudophakic. Indeed, two recent studies pointed out the benefit of performing cataract surgery after the FAc intravitreal implant [30, 44]. They showed that a lasting steroid effect at the time of the cataract surgery may prevent pseudophakic cystoid macular oedema.
This study has several strengths and limitations. We followed an observational multicentre and European study design. Our data are representative of the variety of European real-world practices. The main strength of our study is also its large number of patients with IOP-focused data, compared with other smaller studies [44]. We acknowledge several limitations. First, we did not collect any anatomical or functional data to evaluate the impact of IOP elevation on the optic nerve (visual field or retinal nerve fiber layer thickness). To our knowledge, very few studies have investigated optic nerve damage in steroid-induced IOP elevation after an FAc intravitreal implant [22, 45]. In future studies, we should monitor the optic nerve head structure and function to follow the recommendations for the management of elevated IOP after an FAc intravitreal implant [39, 46]. Second, our follow-up period was short compared to the duration of the FAc effectiveness, which lasts up to 36 months. Other studies have shown a mean delay to IOP-related events of 14–18 months after injection [18]. Third, due to its retrospective design, the follow-up was not controlled, and approximately 50% of the eyes had no IOP measurements at months 3 and 12, which may have led to an inaccurate assessment of the IOP. Fourth, no specific protocol for IOP measurement was defined due to the retrospective design of the study. IOP was measured via Goldmann applanation or air-puff tonometry; this methodological limitation may have introduced significant measurement bias. Fifth, results should not be extrapolated to any other indication for FAc intravitreal implant (uveitis, central retinal vein occlusion). Sixth, mean IOP may not be the best outcome for assessing IOP-related events [47]. We presented results from European centres; hence, we acknowledge the limitations of applying these findings to other non-European populations such as patients of African descent. Finally, we did not record data on the number of previous intravitreal injections or retreatment after the FAc intravitreal implant, which could be a confounding factor affecting IOP changes.
Conclusions
In conclusion, this study that focused on IOP-related adverse events provides further evidence of the good safety profile of FAc intravitreal implants. These implants lead to a predictable and manageable rise in IOP, especially in patients with no history of IOP-related events. Younger patients and patients with high pre-FAc IOP values should be monitored closely. Moreover, previous intravitreal dexamethasone implantation seems to be a good strategy for patient selection to minimise IOP-related adverse events. The clinical relevance of these findings warrants further studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The journal’s Rapid Service Fee was funded by the authors.
Medical Writing, Editorial, and Other Assistance
We would like to thank the CFSR for sharing their data.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization, Catherine Creuzot-Garcher, Louis Arnould, Stephanie Baillif; methodology, Abderrahmane Bourredjem; software, Abderrahmane Bourredjem.; validation, Pierre-Henry Gabrielle, Sarah Lebrize; formal analysis, Sarah Lebrize, Abderrahmane Bourredjem; investigation, Catharina Busch, Matus Rehak, Pascale Massin, Breda B Joao, Marco Lupidi, Cesare Mariotti, Mahmoud Hamza, Alice Grise Dulac; writing—original draft preparation, Sarah Lebrize, Louis Arnould.; writing—review and editing, Catherine Creuzot-Garcher.
Disclosures
Baillif S.: Novartis, Bayer, Allergan, Horus. Creuzot-Garcher C.: Allergan, Bayer, Horus, Novartis, Roche, Thea. Massin P.: Novartis, Bayer, Allergan, Horus, Thea. The following authors have nothing to disclose: Arnould L., Barbosa-Breda J., Bourredjem A., Busch C., Gabrielle PH., Grise-Dulac A., Hamza M., Lebrize S., Lupidi M., Mariotti C., Rehak M.
Compliance with Ethics Guidelines
The study was conducted according to the guidelines of the Declaration of Helsinki 1964, and approved by the Institutional Review Board of each inclusion centres. As the data were collected retrospectively and patient management was not modified, this study did not require oral and written consent from participants, in compliance with French law (n°2004-806, August 9, 2004). It was conducted in accordance with the law on data protection (n°2004-801, August 6, 2004).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Cho N, Shaw J, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobrin Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14(4):179–183. doi: 10.1080/09286580701396720. [DOI] [PubMed] [Google Scholar]
- 4.Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology. 2015;122(7):1375–1394. doi: 10.1016/j.ophtha.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 2013;34:19–48. doi: 10.1016/j.preteyeres.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 6.ETDRSR Group Photocoagulation for diabetic macular edema. Arch Ophthalmol. 1985;103:1796–1806. doi: 10.1001/archopht.1985.01050120030015. [DOI] [PubMed] [Google Scholar]
- 7.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013–2022. doi: 10.1016/j.ophtha.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 8.Jonas JB, Kreissig I, Söfker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003;121(1):57–61. doi: 10.1001/archopht.121.1.57. [DOI] [PubMed] [Google Scholar]
- 9.Haller JA, Kuppermann BD, Blumenkranz MS, et al. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128(3):289–296. doi: 10.1001/archophthalmol.2010.21. [DOI] [PubMed] [Google Scholar]
- 10.DRCR Network Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology. 2010;117(6):1087–1093.e3. doi: 10.1016/j.ophtha.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA) Ophthalmologica. 2017;237(4):185–222. doi: 10.1159/000458539. [DOI] [PubMed] [Google Scholar]
- 12.Brooks HL, Caballero S, Newell CK, et al. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol. 2004;122(12):1801–1807. doi: 10.1001/archopht.122.12.1801. [DOI] [PubMed] [Google Scholar]
- 13.Campochiaro PA, Nguyen QD, Hafiz G, et al. Aqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implants. Ophthalmology. 2013;120(3):583–587. doi: 10.1016/j.ophtha.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Campochiaro PA, Hafiz G, Shah SM, et al. Sustained ocular delivery of fluocinolone acetonide by an intravitreal insert. Ophthalmology. 2010;117(7):1393–1399.e3. doi: 10.1016/j.ophtha.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125–2132. doi: 10.1016/j.ophtha.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 16.Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626–635. e2. doi: 10.1016/j.ophtha.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Bailey C, Holz F, et al. Long-term outcomes of phakic patients with diabetic macular oedema treated with intravitreal fluocinolone acetonide (FAc) implants. Eye. 2015;29(9):1173–1180. doi: 10.1038/eye.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey C, Chakravarthy U, Lotery A, Menon G, Talks J. Extended real-world experience with the ILUVIEN® (fluocinolone acetonide) implant in the United Kingdom: 3-year results from the Medisoft® audit study. Eye. 2021;31(12):1–7. doi: 10.1038/s41433-021-01542-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massin P, Erginay A, Dupas B, Couturier A, Tadayoni R. Efficacy and safety of sustained-delivery fluocinolone acetonide intravitreal implant in patients with chronic diabetic macular edema insufficiently responsive to available therapies: a real-life study. Clin Ophthalmol. 2016;10:1257–1264. doi: 10.2147/OPTH.S105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parrish RK, Campochiaro PA, Pearson PA, Green K, Traverso CE. Characterization of intraocular pressure increases and management strategies following treatment with fluocinolone acetonide intravitreal implants in the FAME trials. Ophthalmic Surg Lasers Imaging Retina. 2016;47(5):426–435. doi: 10.3928/23258160-20160419-05. [DOI] [PubMed] [Google Scholar]
- 21.Holden SE, Currie CJ, Owens DR. Evaluation of the clinical effectiveness in routine practice of fluocinolone acetonide 190 µg intravitreal implant in people with diabetic macular edema. Curr Med Res Opin. 2017;33(sup2):5–17. doi: 10.1080/03007995.2017.1366645. [DOI] [PubMed] [Google Scholar]
- 22.Chakravarthy U, Taylor SR, Koch FHJ, de Sousa JPC, Bailey C. Changes in intraocular pressure after intravitreal fluocinolone acetonide (ILUVIEN): real-world experience in three European countries. Br J Ophthalmol. 2019;103(8):1072–1077. doi: 10.1136/bjophthalmol-2018-312284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 24.Spaeth GL, de Barros DSM, Fudemberg SJ. Visual loss caused by corticosteroid-induced glaucoma: how to avoid it. Retina. 2009;29(8):1057–1061. doi: 10.1097/IAE.0b013e3181b32cfd. [DOI] [PubMed] [Google Scholar]
- 25.Malclès A, Dot C, Voirin N, et al. Safety of intravitreal dexamethasone implant (Ozurdex): the SAFODEX study. Incidence and risk factors of ocular hypertension. Retina. 2017;37(7):1352–1359. doi: 10.1097/IAE.0000000000001369. [DOI] [PubMed] [Google Scholar]
- 26.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. 2. Springer; 2009. p. 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilbe JM. Logistic regression models. Englewood Cliffs: Chapman & Hall; 2009. [Google Scholar]
- 28.Lipsitz SR, Kim K, Zhao L. Analysis of repeated categorical data using generalized estimating equations. Stat Med. 1994;13(11):1149–1163. doi: 10.1002/sim.4780131106. [DOI] [PubMed] [Google Scholar]
- 29.Augustin AJ, Bopp S, Fechner M, et al. Three-year results from the Retro-IDEAL study: real-world data from diabetic macular edema (DME) patients treated with ILUVIEN® (0.19 mg fluocinolone acetonide implant) Eur J Ophthalmol. 2020;30(2):382–391. doi: 10.1177/1120672119834474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rehak M, Busch C, Unterlauft J-D, Jochmann C, Wiedemann P. Outcomes in diabetic macular edema switched directly or after a dexamethasone implant to a fluocinolone acetonide intravitreal implant following anti-VEGF treatment. Acta Diabetol. 2019;57:469–478. doi: 10.1007/s00592-019-01439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young JF, Walkden A, Stone A, Mahmood S. Clinical effectiveness of intravitreal fluocinolone acetonide (FAc)(ILUVIEN™) in patients with diabetic macular oedema (DMO) refractory to prior therapy: the Manchester experience. Ophthalmol Ther. 2019;8(3):477–484. doi: 10.1007/s40123-019-0197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fusi-Rubiano W, Mukherjee C, Lane M, et al. Treating Diabetic Macular Oedema (DMO): real world UK clinical outcomes for the 0.19 mg Fluocinolone Acetonide intravitreal implant (Iluvien™) at 2 years. BMC Ophthalmol. 2018;18(1):1–7. doi: 10.1186/s12886-018-0726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Figueira J, Henriques J, Amaro M, Rosas V, Alves D, Cunha-Vaz J. A nonrandomized, open-label, multicenter, phase 4 pilot study on the effect and safety of ILUVIEN® in chronic diabetic macular edema patients considered insufficiently responsive to available therapies (RESPOND) Ophthalmic Res. 2017;57(3):166–172. doi: 10.1159/000455235. [DOI] [PubMed] [Google Scholar]
- 34.Kodjikian L, Bandello F, De Smet M, Dot C, Zarranz-Ventura J, Loewenstein A, Sudhalkar S, Bilgic A, Cunha-Vaz J, Dirven W, Behar-Cohen F, Mathis T. Fluocinolone acetonide implant in diabetic macular edema: International experts' panel consensus guidelines and treatment algorithm. Eur J Ophthalmol. 2022 (Online ahead of print). [DOI] [PubMed]
- 35.Goñi FJ, Barton K, Dias JA, Diestelhorst M, Garcia-Feijoo J, Hommer A, Kodjikian L, Nicolò M. Intravitreal corticosteroid implantation in diabetic macular edema: updated European consensus guidance on monitoring and managing intraocular pressure. Ophthalmol Ther. 2022;11:15–34. doi: 10.1007/s40123-021-00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eaton A, Koh SS, Jimenez J, Riemann CD. The USER study: a chart review of patients receiving a 0.2 µg/day fluocinolone acetonide implant for diabetic macular edema. Ophthalmol Ther. 2019;8(1):51–62. doi: 10.1007/s40123-018-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pleyer U, Ursell PG, Rama P. Intraocular pressure effects of common topical steroids for post-cataract inflammation: are they all the same? Ophthalmol Ther. 2013;2(2):55–72. doi: 10.1007/s40123-013-0020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shukla D, Vidhya N, Prasad NM, Mahalakshmi R, Kolluru C, Krishnadas R. Evaluation of patient age as a risk factor for intraocular pressure elevation after intravitreal triamcinolone. Am J Ophthalmol. 2007;144(3):453–454. doi: 10.1016/j.ajo.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 39.Goñi FJ, Stalmans I, Denis P, et al. Elevated intraocular pressure after intravitreal steroid injection in diabetic macular edema: monitoring and management. Ophthalmol Ther. 2016;5(1):47–61. doi: 10.1007/s40123-016-0052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kersey J, Broadway D. Corticosteroid-induced glaucoma: a review of the literature. Eye. 2006;20(4):407–416. doi: 10.1038/sj.eye.6701895. [DOI] [PubMed] [Google Scholar]
- 41.Cicinelli MV, Rosenblatt A, Grosso D, et al. The outcome of fluocinolone acetonide intravitreal implant is predicted by the response to dexamethasone implant in diabetic macular oedema. Eye. 2021;35:3232–3242. doi: 10.1038/s41433-020-01373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Electronic Medicines Compendium. ILUVIEN 190 µg intravitreal implant in applicator. Summary of Product Characteristics; 2019. https://www.medicines.org.uk/emc/medicine/27636. Accessed June 27, 2021.
- 43.Food & Drug Administration. ILUVIEN 0.19 mg New Drug Application (NDA 201923); 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/201923s000lbl.pdf. Accessed June 27, 2021.
- 44.Ulbig M, Wehrmann K, Maier M. Second-line treatment with Iluvien for persistent pre-treated diabetic macular edema. Investig Ophthalmol Vis Sci. 2018;59(9):1898–1898. [Google Scholar]
- 45.Ayar O, Alpay A, Koban Y, et al. The effect of dexamethasone intravitreal implant on retinal nerve fiber layer in patients diagnosed with branch retinal vein occlusion. Curr Eye Res. 2017;42(9):1287–1292. doi: 10.1080/02713683.2017.1313430. [DOI] [PubMed] [Google Scholar]
- 46.Adán A, Cabrera F, Figueroa MS, et al. Clinical-decision criteria to identify recurrent diabetic macular edema patients suitable for fluocinolone acetonide implant therapy (Iluvien®) and follow-up considerations/recommendations. Clin Ophthalmol. 2020;14:2091–2107. doi: 10.2147/OPTH.S252359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlenker M, Kansal V. How mean intraocular pressures are failing patients. Ophthalmol Glaucoma. 2021;4:553–557. doi: 10.1016/j.ogla.2021.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


